Introduction
Gastric cancer (GC) and breast cancer (BC) are two
of the most common malignant cancer types worldwide, particularly
in China. In 2015, GC caused ~498,000 mortalities and 679,000 new
cases (1). Among women ≤45 years of
age, BC remains the leading cause of cancer-related mortality,
followed by lung cancer (1). Due to
cancer recurrence and metastasis, numerous patients fail treatment
despite improvements in GC and BC therapy. In patients with GC and
BC, radiotherapy (RT) serves a crucial role in controlling local
recurrence (2). In the past
decades, great progress has been made to improve the ability to
select appropriate patients, and thus, maximize clinical benefits,
while minimizing toxicity and disease burden (2). However, limitations persist despite
the appropriate use of RT. Intrinsic or acquired radioresistance
and non-specific toxicity limit the efficacy of RT (2). To overcome these shortcomings,
increased efforts have been made to discover an effective
radiosensitizer characterized by higher efficacy and lower
toxicity.
HER2 is upregulated in 13–23% of GC and 15–30% of BC
cases (3–5). HER2 acts as an oncogene in different
cancer types, most likely because amplification of this gene
consistently results in HER2 upregulation and the acquisition of
beneficial properties of malignant cells (6). Moreover, accumulating evidence has
revealed that HER2 is involved in radioresistance (7,8).
Previous studies have reported that HER2-targeting agents,
including trastuzumab, lapatinib and afatinib, could be utilized to
sensitize HER2-overexpressing cancer cells to RT by inhibiting
signaling pathways inducing radioresistance (9–11). The
combination of RT and trastuzumab has been applied in clinical
practice and has been investigated mainly as an adjuvant therapy
(12). The side effects observed
with the concurrent use of trastuzumab and locoregional RT in BC
are acceptable, demonstrating satisfactory outcomes (12); however, longer follow-ups are
required to further identify these effects. The mechanism of HER2
induced-radioresistance in cancer cells also remains unknown.
Previous studies have revealed that HER2 transport from membranes
to nuclei contributes to radioresistance, and the survival of
irradiated HER2-overexpressing cancer cells is decreased after
inhibiting this pathway (13,14).
Not all patients with HER2 overexpression benefit
from trastuzumab. For instance, certain patients develop resistance
to trastuzumab after 1 year, even those who achieved an initial
reaction to this drug (15).
Similarly, primary and acquired resistance to lapatinib, a
reversible dual tyrosine kinase inhibitor (TKI) of EGFR and HER2,
remains a significant clinical problem (16). To further enhance HER2 inhibition,
pyrotinib, a novel irreversible dual (EGFR/HER2) TKI, has been
evaluated for the treatment of HER2-overexpressing cancer types.
Compared with trastuzumab and lapatinib, pyrotinib effectively
overcomes drug resistance triggered by EGFR or HER2 mutations,
which is due to the covalent binding between cysteine residues of
the receptor and electrophilic groups of pyrotinib (17,18).
Currently, enhancing the sensitivity of cancer cells to irradiation
by targeting the HER family has been approved as a novel method to
potentiate the therapeutic efficacy of irradiation (19). Although the antitumor effect of
pyrotinib has been previously reported (20), the role of pyrotinib in sensitizing
HER2-overexpressing cancer cells to irradiation needs to be further
elucidated.
The present study hypothesized that pyrotinib may be
a promising irradiation sensitizer in patients with
HER2-overexpressing GC and BC. The current study aimed to
investigate the radiosensitizing effect of pyrotinib in
HER2-overexpressing GC and BC cell lines, as well as in xenograft
models. Additionally, the potential mechanism involving HER2
nuclear transport was identified.
Materials and methods
Cell culture and reagents
Human GC cell lines (NCI-N87 and MKN28) and human BC
cell lines (SKBR3 and MCF7) were purchased from the Type Culture
Collection of the Chinese Academy of Sciences. All cell lines were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. All cell lines were
authenticated using Short Tandem Repeat profiles. Pyrotinib was
gifted by Hengrui Medicine Co., Ltd. The cytotoxic drugs
fluorouracil, cisplatin, docetaxel and epirubicin were obtained
from Sigma-Aldrich (Merck KGaA). All drugs were dissolved in DMSO
at appropriate concentrations (pyrotinib at 100 mg/ml, fluorouracil
at 100 mM, cisplatin at 10 µM, docetaxel at 100 µM and epirubicin
at 10 µM).
X irradiation
The irradiation of cancer cells and mice was
performed using an RS2000 X-ray Biological Research Irradiator (160
kV; 25 mA; 3-mm copper filter; Rad Source Technologies, Inc.).
Western blotting
After treatment, the whole-cell protein was
extracted by lysing cells with RIPA buffer, which was supplied with
a complete protease inhibitor cocktail (Roche Diagnostics, Inc.).
Nuclear protein was extracted using the Nuclear and Cytoplasmic
Protein Extraction kit (cat. no. P0028; Beyotime Institute of
Biotechnology) following the manufacturer's instructions, at 0,
0.25, 0.5, 1 and 6 h after irradiation. The Enhanced BCA Protein
Assay kit (cat. no. P0010; Beyotime Institute of Biotechnology) was
used to determine protein concentrations. An equal amount of
protein (20 µg) was electrophoresed in 8–10% SDS-PAGE and then
transferred to the PVDF membrane (cat. no. IPVH00010; EMD
Millipore). After blocking with 5% non-fat milk at room temperature
for 1 h, the membranes were incubated with the primary antibody
diluted at 1:1,000 overnight at 4°C. Antibodies against HER2 (cat.
no. 2165), γ-H2A histone family member X (γ-H2AX; cat. no. 9718),
phosphorylated (p)-Akt (cat. no. 4060), Akt (cat. no. 4691), p-MAPK
(cat. no. 4370), MAPK (cat. no. 4695) were purchased from Cell
Signaling Technology, Inc. Primary antibodies against GAPDH (cat.
no. A00227) and Lamin B1 (cat. no. BA1228) were purchased from
Wuhan Boster Biological Technology, Ltd. After washing three times,
each for 10 min, in TBS-0.1% Tween-20, the membranes were incubated
with the secondary antibody (1:5,000; cat. no. A0208; Beyotime
Institute of Biotechnology) labeled with horseradish peroxidase for
1 h at 37°C. The blots were visualized using the Enhanced
Chemiluminescence Detection kit (cat. no. 32209; Thermo Fisher
Scientific, Inc.).
Immunofluorescence
Cancer cells (5×104 cells/ml) were
cultured on slips and fixed with 4% paraformaldehyde for 10–20 min
at room temperature. After washing in PBS, the cells were
permeabilized with 0.1% Triton X-100, blocked using 5% BSA (Gibco;
Thermo Fisher Scientific, Inc.) for 1 h at room temperature and
incubated with primary antibodies overnight at 4°C. Primary
antibodies used were as follows: Anti-HER2 (1:200; cat. no. 2165;
Cell Signaling Technology, Inc.) and γ-H2AX (1:200; cat. no. 9718;
Cell Signaling Technology, Inc.). After washing in PBS three times,
the cells were incubated with FITC-conjugated anti-rabbit secondary
antibody (1:500; cat. no. BA1105; Wuhan Boster Biological
Technology, Ltd.) for 1 h at room temperature in the dark. DAPI
stained for 3 min at room temperature and was used for nuclear
localization, as well as to assess the quality of the experiment.
The slides were washed with PBS until the excess DAPI was removed.
After drying, the slides were sealed with coverslips and mounting
medium. Finally, the cells were visualized with fluorescent
microscope (magnification, ×200).
Cell viability assay
To assess cell viability, cells were seeded in
96-well plates at a density of 5,000 cells per well and cultured
overnight to grow adhering to the wall. Then, the cells were
treated with the indicated concentrations of drugs and/or the
indicated dose of irradiation at 37°C. An increasing dose of
pyrotinib (0, 0.1, 1, 10, 100 and 1,000 µg/ml) was used to examine
the sensitivity of GC and BC cells to pyrotinib. After 48 h, the
original culture media were removed. The cells of each well were
washed with PBS and maintained in fresh culture media with 10% Cell
Counting Kit-8 reagent (CCK-8; cat. no. AR1160; Wuhan Boster
Biological Technology, Ltd.) for another 1 h at 37°C according to
the manufacturer's instructions. The absorbance of viable cells was
measured using a microplate reader at 450 nm. Each treatment was
performed in ≥3 replicate wells.
Clonogenic survival assay
Cancer cells were cultured in 6-well plates at
different densities (100, 200, 600, 2,000 and 6,000 cells/per
well). Following pretreatment with pyrotinib (0.1 µg/ml, 37°C for
24 h), cells were exposed to irradiation at different radiation
doses (0, 2, 4, 6 and 8 Gy). After incubation for 14–20 days, cells
were fixed with anhydrous methanol for 15 min at room temperature
and stained with 0.1% crystal violet for 20–30 min at room
temperature. Colonies counting >50 cells were considered as
surviving clones. The plating efficiency (PE) and surviving
fraction (SF) were calculated as follows: PE=mean colony number in
un-irradiated controls/number of seeded cells. SF=mean colony
number/(number of cells seeded × PE). A Multi-target single-hit
model [S=1-(1-e−D/D0) N] was used to
calculate D0 (the average irradiation dose of lethal
exposure), with the sensitizer enhancement ratio (SER) determined
as follows, SER=D0 of combination
treatment/D0 of irradiation treatment alone.
Measurement of apoptosis
Quantification of cell apoptosis was performed using
the Annexin V-FITC/PI Apoptosis Detection kit (cat. no. KGA105;
Nanjing KeyGen Biotech Co., Ltd.) following the manufacturer's
protocol. After treatment, cells were trypsinized before collection
and suspended in 300 µl binding buffer. Then, 5 µl Annexin V-FITC
and 5 µl PI were added to each sample. The cell samples were
incubated in the dark for 15 min at room temperature, detected
using flow cytometry (BD LSRFortessa; BD Biosciences), and analyzed
using FlowJo software version 10.7 (FlowJO LLC). The apoptotic rate
was calculated as the percentage of early + late apoptotic
cells.
Cell cycle distribution
Cell cycle distribution was detected using the
PI/RNase buffer (cat. no. KGA512; Nanjing KeyGen Biotech Co., Ltd.)
following the manufacturer's protocol. NCI-N87 and SKBR3 cells were
treated with pyrotinib (0.1 µg/ml), irradiation (4 Gy) or the
combination of pyrotinib and irradiation. After treatment, cells
were harvested via trypsinization, washed with PBS and fixed with
70% ethanol for 20 min at 4°C. Then, cell samples were suspended in
RNase buffer with PI, shielded from light for 30 min at room
temperature and analyzed via flow cytometry (BD LSRFortessa; BD
Biosciences) and FlowJo software version 10.7 (FlowJo LLC).
Xenograft models
Animal experiments were performed according to the
guidelines of, and were approved by the Ethical Committee of Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology (permit no. TJ2015A). Female nude (BALB/c nu-nu;
age, 4–6 weeks; weight, 12–15 g) mice were obtained from Charles
River, Ltd., and fed under pathogen-free conditions (temperature
26–28°C; humidty, 40–60%; 10 h light/14 h dark cycle; provided with
food and water by staff). Tumor cells (1×106; 100 µl)
mixed with 100 µl Matrigel were subcutaneously inoculated into the
rear flank. Mice were randomly divided into four groups (6–8 mice
per group): Control, pyrotinib (10 mg/kg/day; intraperitoneal
injection) only, irradiation (10 Gy on day 8) only and a
combination of pyrotinib (10 mg/kg/day; intraperitoneal injection)
and irradiation (10 Gy on day 8), when xenograft tumors grew to
~5-mm in diameter. During tumor treatment with irradiation, the
rest of the body of mouse was shielded using a lead shield. The
body weights of the mice and xenograft tumor volumes were measured
thrice weekly. To calculate the tumor volume, the following
equation was applied: Tumor volume (mm3)=length ×
width2/2. A total of 3 weeks after treatment, the mice
were executed via cervical vertebra dislocation. The mortality of
the mice was verified by cardio-respiratory arrest, absence of
nervous reflexes and muscular flaccidity. The tumors were harvested
for analysis.
Tumor tissues were immersed in 4% paraformaldehyde
for 4 h at room temperature, placed in processing cassettes,
dehydrated via a serial alcohol gradient (50, 70, 85, 95 and 100%)
and embedded in paraffin wax blocks. Then, 5 µm-thick tissue
sections were dewaxed in xylene, rehydrated via decreasing
concentrations of ethanol and washed in PBS. The sections were
stained with hematoxylin for 10 min and eosin for 30 sec both at
room temperature. After staining, sections were dehydrated using
increasing concentrations of ethanol and xylene. The slides were
observed under a light microscope (magnification, ×200).
Statistical analysis
Statistical analyses were performed with the SPSS
software version 23.0 (IBM Corp,). Each experiment in the present
study was performed ≥3 times. Data are presented as the mean ± SD.
Statistical differences between two groups were calculated using a
two-tailed Student's t-test. Tukey's test was used for the
comparison of multiple groups following one-way ANOVA. P<0.05
was considered to indicate a statistically significant
difference.
Results
HER2-overexpressing GC and BC cells
are selectively sensitive to pyrotinib inhibition
To identify HER2-overexpressing GC and BC cells, the
degree of HER2 protein expression was evaluated in two GC cell
lines (NCI-N87 and MKN28) and two BC cell lines (SKBR3 and MCF-7).
Western blotting results demonstrated that NCI-N87 and SKBR3 cells
had upregulated HER2 protein expression, while the remaining cell
lines did not overexpress HER2 protein (Fig. 1A). Furthermore, it was observed that
HER2 was mainly located on cell membranes (Fig. 1B).
To examine the sensitivity of GC and BC cells to
pyrotinib, each cell line was exposed to increasing doses of
pyrotinib. Compared with HER2 non-overexpressing GC and BC cells,
NCI-N87 and SKBR3 cells displayed sensitivity to pyrotinib
(Fig. 1C).
Pyrotinib enhances the
radiosensitivity of HER2-overexpressing GC and BC cells
After treatment for 48 h, pyrotinib induced the
dose-dependent proliferative inhibition of NCI-N87 and SKBR3 cells
(Fig. 1C). Pyrotinib at 0.1 µg/ml
was unable to suppress cell viability significantly, and the
survival rate of cancer cells was >80%. Therefore, the drug
concentration of 0.1 µg/ml was selected for further in vitro
experiments. The combination of irradiation and pyrotinib
significantly inhibited proliferation (Fig. 2A) and clonogenic survival in NCI-N87
and SKBR3 cells (Fig. 2B). Compared
with cells treated with irradiation alone, pretreatment of
pyrotinib decreased colony formation of cancer cells after
irradiation, suggesting that pyrotinib enhanced the
radiosensitivity of NCI-N87 and SKBR3 cells (SER=1.375 and 1.326;
Fig. 2B). As pyrotinib sensitized
HER2-overexpressing GC and BC cells to irradiation, this drug may
function as a promising radiosensitizer in indicated patients, and
its targeted toxicity toward HER2-overexpressing cancer cells may
augment the therapeutic effect.
Pyrotinib augments irradiation
response in HER2-overexpressing tumor xenografts
Based on the in vitro radiosensitizing
effects in HER2-overexressing cancer cells, the potential
irradiation response of pyrotinib was further examined in
vivo. NCI-N87 and SKBR3 cells were used to perform tumor
xenograft experiments in athymic nude mice. Following the
establishment of the xenograft models, different groups of mice
were treated with a single-dose of irradiation (10 Gy) alone,
pyrotinib (10 mg/kg/day) alone or a combination of irradiation and
pyrotinib. The antitumor effect of these treatments was determined
by measuring the tumor volume (Fig.
3). In both NCI-N87 and SKBR3 ×enograft tumors, irradiation
combined with pyrotinib demonstrated a greater antitumor effect
compared with irradiation alone, although treatment with
irradiation or pyrotinib alone treatment also had a significant
inhibitory effect on tumor growth. Moreover, the combination
strategy did not induce any mortality or a significant decrease in
mouse body weight (data not shown), suggesting that pyrotinib may
act as an effective and safe RT sensitizer.
Pyrotinib inhibits irradiation-induced
HER2 nuclear transport
Following HER2 activation, two main cell signaling
pathways, including PI3K/Akt and MEK/MAPK pathways, could be
activated involving in the mitogenic and survival signals (7). To assess their involvement in
pyrotinib-mediated radiosensitization in the HER2-overexpressing GC
and BC cells, the expression levels of p-Akt and MAPK were examined
after irradiation, with or without the pretreatment of pyrotinib.
Western blotting results demonstrated that pyrotinib markedly
suppressed Akt signaling and exerted no effect on MAPK signaling in
irradiated SKBR3 cells. In NCI-N87 cells, pretreatment of pyrotinib
before irradiation presented no notable influences on Akt and MAPK
signaling (Fig. 4A).
Previous studies have reported that the nuclear
transport pathway also contributes to radioresistance, especially
HER2 transport from membranes to nuclei (13,14).
To examine the influence of pyrotinib on irradiation-induced
HER2-nuclear transport, the nuclear protein was extracted at 0,
0.25, 0.5, 1 and 6 h after irradiation, with or without
pretreatment of pyrotinib. The expression of HER2 in nuclear
fraction was notably increased after irradiation, attaining a peak
at 0.5 h after irradiation. In NCI-N87 and SKBR3 cells, treatment
with pyrotinib before irradiation decreased HER2 expression in the
nucleus at different time-points (Fig.
4B). Thus, it was indicated that pyrotinib can decrease the
irradiation-induced HER2 nuclear transport, improving the
radiosensitivity of HER2-overexpressing cells.
Pyrotinib increases apoptosis and G2/M
arrest induced by irradiation in NCI-N87 cells
To determine whether pyrotinib affected
radiosensitivity by inducing apoptosis, flow cytometric analysis
was performed. The irradiation dose of 4 Gy was selected, since the
irradiation of 4 Gy combined with pyrotinib suppressed cell
viability significantly and the survival rate of cancer cells was
modest (Fig. 2A). To evaluate the
occurrence of apoptosis, NCI-N87 and SKBR3 cells were examined at
48 h after irradiation (4 Gy), with or without pyrotinib (0.1
µg/ml), suggesting that the combination treatment significantly
increased the apoptosis of NCI-N87 cells, but not SKBR3 cells, when
compared with the irradiation alone group (Fig. 4C). Treatment with pyrotinib or
irradiation alone induced a significant increase of apoptosis in
SKBR3 cells in comparison with control group. Additionally,
irradiation alone demonstrated a significant increase of apoptosis
in NCI-N87 cells. However, pyrotinib alone failed to induce any
significant changes of apoptosis in NCI-N87 cells.
The effects of pyrotinib and irradiation alone, or
combination on cell cycle progression were examined using flow
cytometry (Fig. 4D). Following
irradiation, G2/M phase cells were increased in the cell
cycle distribution of both two cell lines, compared with the
control group. Moreover, pyrotinib induced in G2/M
arrest after irradiation only in NCI-N87 cells. In SKBR3 cells,
pyrotinib failed to significantly affect irradiation-induced
G2/M arrest.
These findings indicated that pyrotinib promotes
apoptosis and G2/M arrest induced by irradiation, and
this may be the additional mechanism via which pyrotinib could
overcomes resistance to irradiation in HER2-overexpressing GC and
BC.
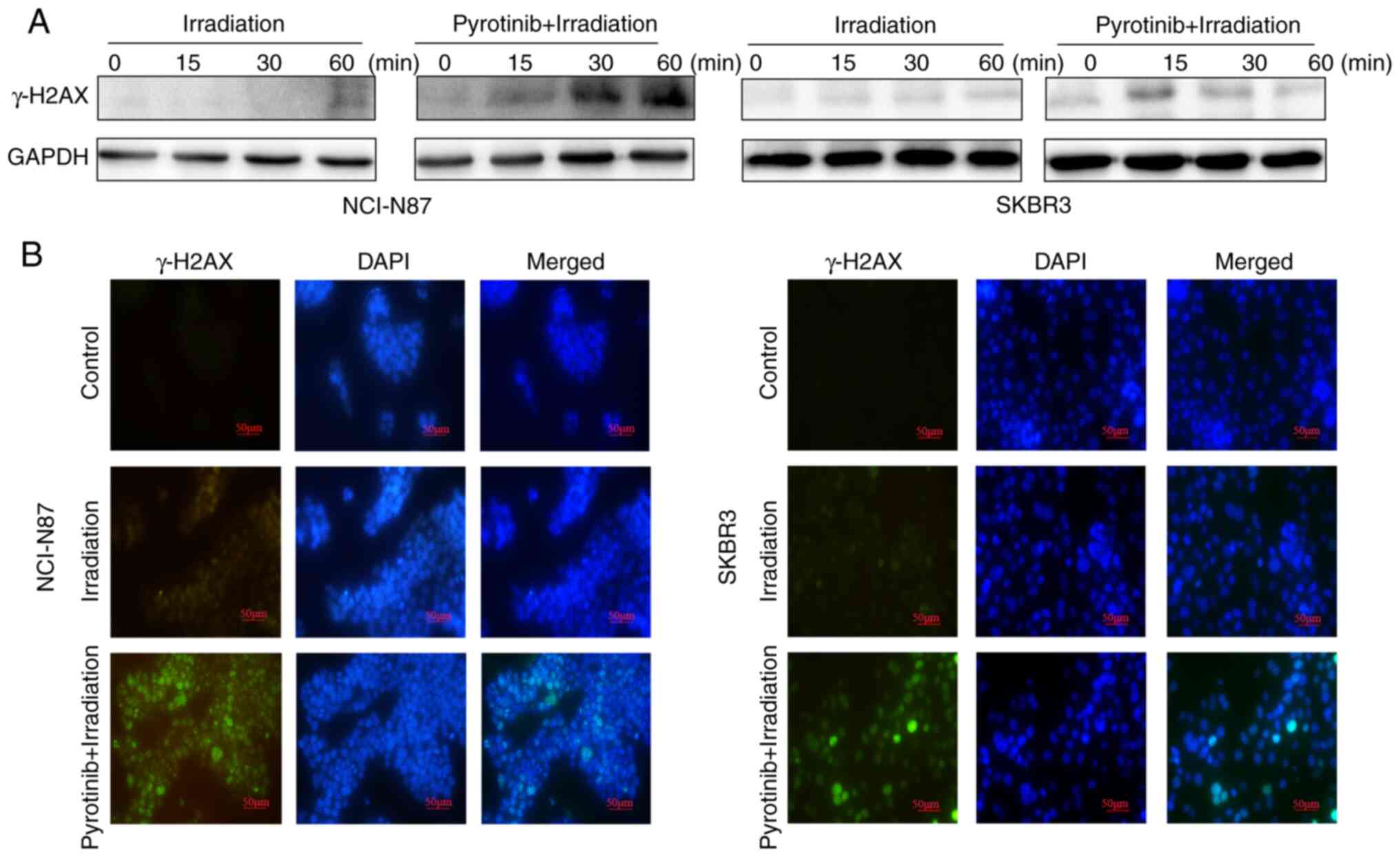
Pyrotinib inhibits DNA double-strand
break (DSB) repair in irradiated HER2-overexpressing GC and BC
cells
To further determine the other potential mechanisms
of pyrotinib-induced radiosensitivity, the effect of this agent on
DSBs repair was investigated. The expression of γ-H2AX, a marker of
DSBs, was assessed in irradiated NCI-N87 and SKBR3 cells, with or
without pretreatment of pyrotinib. Pyrotinib co-treatment markedly
enhanced the expression of γ-H2AX compared with irradiated cells 1
h after irradiation (Fig. 5A and
B). Additionally, γ-H2AX expression was present for a longer
period in NCI-N87 cells compared with in SKBR3 cells. As DSBs
repair is a well-established cause of radioresistance (21), these findings suggested that the
inhibition of DSBs repair may be a common mechanism via which
pyrotinib sensitizes HER2-overexpressing GC and BC cells to
irradiation.
Pyrotinib enhances the cytotoxicity of
docetaxel in HER2-overexpressing GC and BC cells
To investigate the possible antitumor effects of
pyrotinib in combination with docetaxel, fluorouracil, cisplatin or
epirubicin, NCI-N87 and SKBR3 cells were exposed to varying
concentrations of each agent alone, with or without pyrotinib (0.1
µg/ml) for 48 h. The combination experiments demonstrated that
pyrotinib significantly increased the cytotoxicity of docetaxel and
cisplatin in NCI-N87 cells (Fig.
6). While in SKBR3 cells, pyrotinib significantly enhanced the
cytotoxicity of docetaxel and fluorouracil. It was found that
pyrotinib did not increase the cytotoxicity of epirubicin in both
cell lines. Overall, the data indicated that pyrotinib sensitized
both NCI-N87 and SKBR3 cells to docetaxel.
Discussion
The present study demonstrated that pyrotinib
enhanced the radiosensitivity of HER2-overexpressing GC and BC
cells, both in vitro and in vivo. It was suggested
that the major mechanism involved in this process was
pyrotinib-induced inhibition of HER2 nuclear transport.
Additionally, the present findings indicated that pyrotinib was
associated with increased DNA damage induced by irradiation. A
potent capacity of pyrotinib to augment the cytotoxicity of
docetaxel was observed in both GC and BC cell lines. These data
could provide a strategy to improve the response to RT, as well as
to select the most efficient drug combination in individual
patients.
The present results suggested that inhibition of
irradiation-induced HER2 nuclear transport was a crucial mechanism
for pyrotinib-enhanced radiosensitivity. Although the mechanism of
HER2-induced radioresistance remains unknown, increasing evidence,
including data from the current study, reveal the presence and
function of HER2 in the nucleus (13–20,22).
Nuclear HER2 positivity has been identified as an independent
prognostic factor in patients with BC with membrane
HER2-upregulation (23).
Reportedly, irradiation can result in HER2 nuclear transport, which
may contribute to the radioresistance of cancer types with high
HER2 expression (14). In the
present study, nuclear HER2 was decreased with pyrotinb treatment
in irradiated cells.
The response to irradiation is recognized to be
driven by the repair efficacy of irradiation-induced DNA damage, in
which DSBs serve a major role (21). Since EGFR can inhibit DNA damage
(24), the present study
investigated whether pyrotinib suppressed DSB repair in response to
irradiation by detecting γ-H2AX expression. H2AX is rapidly
phosphorylated at the site of DSBs and acts as a damage signaling
protein forming nuclear foci visible using immunofluorescence
(21). Compared with cells treated
with irradiation alone, pyrotinib significantly markedly γ-H2AX
expression in NCI-N87 and SKBR3 cells after irradiation, suggesting
a failure of DSBs repair. The present findings suggested pyrotinib
was associated with increased DNA damage induced by
irradiation.
Cancer cell exposure to irradiation results in the
activation of the HER family, subsequently stimulating downstream
signaling pathways that regulate cellular processes, including
proliferation, apoptosis and cell cycle distribution (25,26).
The key mechanism of irradiation-induced cell death is apoptosis
(25). In the present study,
pyrotinib increased irradiation-induced apoptosis only in NCI-N87
cells. The cell cycle distribution was also analyzed. Compared with
irradiation alone, the combination of irradiation and pyrotinib
substantially enhanced G2/M arrest in NCI-N87 cells. The
enhanced radiosensitivity identified in NCI-N87 cells may be
attributed to increased apoptosis and G2/M arrest, while
this was not observed in SKBR3 cells. Genetic heterogeneity between
the two cell lines may explain these inconsistent results. However,
other factors besides HER2 may participate in the
irradiation-induced apoptosis and cell cycle redistribution in
BC.
Trastuzumab combined with RT has been widely used in
the adjuvant therapy of BC (12,27,28).
The toxicities of RT with concurrent trastuzumab are deemed
acceptable and the outcomes were favorable (27,28). A
clinical trial provided relevant evidence demonstrating the
radiosensitizing effect of trastuzumab in HER2-overexpressing BC
(29). However, tumors expressing
HER2 may exhibit autocrine stimulation of EGFR/HER1 via expression
of one of its numerous ligands including EGF, amphiregulin (AR) and
TGF-α (30). Therefore, this type
of cooperation may result in the activation of additional
intracellular pathways, contributing to tumor progression. In this
respect, pyrotinib, an irreversible dual (EGFR/HER2) TKI, has
displayed high potency in HER2-dependent cancer cells in
vitro and in vivo (20).
Previous investigations have reported that irradiated cells may
activate HER2 and trigger subsequent signal transduction, including
PI3K/Akt and MEK/MAPK pathways (31,32).
The enhanced radiosensitivity induced by trastuzumab has been
attributed mainly to the inhibition of the PI3K/Akt signal pathway,
instead of the MEK/MAPK-mediated signal transduction (11). Consistently, the present study
demonstrated that pyrotinib combined with irradiation markedly
suppressed p-Akt expression in SKBR3 cells. By contrast, in NCI-N87
cells, pyrotinib did not notably inhibit the PI3K/Akt or MEK/MAPK
pathways. Thus, the distinct mechanisms of action and chemical
structures of pyrotinib and trastuzumab may induce diverse signal
transduction pathways in cancer types.
The current findings support the feasibility of RT
combined with pyrotinib in patients with HER2-overexpressing GC and
BC. Indeed, neoadjuvant RT may achieve clinical tumor downstaging
and increase the possibility of R0 resection in patients with local
advanced GC (33–36). Thus, neoadjuvant RT could be
developed as a promising standard treatment for patients with
potentially resectable local advanced GC. Since INT-0116 and ARTIST
studies were published, postoperative RT has also been utilized in
patients with GC (37,38). R1 surgical resection is an
indication for postoperative RT. RT is considered as a crucial
component of BC therapy and has been recommended in various
guidelines (e.g. National Comprehensive Cancer Network; American
Society of Clinical Oncology; European Society for Medical
Oncology; Chinese Society of Clinical Oncology) for >20 years
(39–41). In patients harboring regional lymph
node metastasis or residual tumor tissue in the chest wall after
surgery or systemic therapy, RT can not only decrease the local
recurrence rate but also prolong survival time (42,43).
In the present study, the use of sub-cytotoxicity
concentration of pyrotinib could increase the cytotoxicity induced
by irradiation and the radiosensitivity of tumor tissues, without
enhancing the irradiation dose and side effects of RT. Before
actual clinical applications, further research is required to
assess the feasibility, benefits and adverse reactions of using
pyrotinib as a radiosensitizer. The clinical applications of
pyrotinib are currently under investigation. For instance, studies
into the tolerability, safety and pharmacokinetic properties in
humans have been completed (20).
The first study (clinical trial no. NCT01937689) of pyrotinib in
patients with HER2-positive metastatic BC who previously received
treatment with trastuzumab, or were trastuzumab naïve, reported
that the median progression-free survival was 35.4 and 59.7 weeks
in the 320 and 400 mg dose cohorts, respectively (44). Further investigations of pyrotinib
in patients with HER2-positive metastatic BC are ongoing to collect
data relating to its safety and efficacy. In addition, the present
results suggested that pyrotinib enhanced the cytotoxicity of
docetaxel in NCI-N87 and SKBR3 cells, which may provide a novel
treatment strategy for HER2-overexpressing GC and BC.
Several limitations of the present study should be
mentioned. First, as few GC cell lines overexpress HER2, except
NCI-N87, confirmation of HER2 expression in additional GC cell
lines would be valuable. Second, the effect of RT in combination
with pyrotinib and docetaxel requires further investigation.
In conclusion, to the best of our knowledge, the
present findings are the first to suggest that pyrotinib sensitized
HER2-overexpressing GC and BC cells to irradiation by inhibiting
HER2 nuclear transport. It was identified that DNA damage appeared
to serve a crucial role in pyrotinib-induced sensitization of
cancer cells to irradiation (Fig.
7). The cytotoxicity of docetaxel was enhanced by pyrotinib in
HER2-overexpressing GC and BC cells. However, additional clinical
investigations and associated translational studies are required to
further understand the current findings.
Acknowledgements
Not applicable.
Funding
This work was funded by the National Natural Science
Foundation of China (grants nos. 81472921 and 81372664).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH wrote the original manuscript and analyzed the
data. TH, XL, BW, PP, YD and GH performed the experiments and
partial data analysis. YD and GH revised the original manuscript.
HQ and XY designed and revised the research for critical
intellectual content. Each author made a significant scientific
contribution to the present research and was familiar with the
primary data. All listed authors have read the complete manuscript
and approved the submission and publication of the paper.
Ethics approval and consent to
participate
Experiments in the present study were executed
according to the guidelines of, and were approved by the Ethical
Committee of Tongji Hospital, Tongji Medical College, Huazhong
University of Science and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TKI
|
tyrosine kinase inhibitor
|
|
GC
|
gastric cancer
|
|
BC
|
breast cancer
|
|
RT
|
radiotherapy
|
|
STR
|
Short Tandem Repeat
|
|
PE
|
plating efficiency
|
|
SF
|
surviving fraction
|
|
SER
|
sensitizer enhancement ratio
|
|
DSBs
|
double-strand breaks
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valentini V and Cellini F: Radiotherapy in
gastric cancer: A systematic review of literature and new
perspectives. Expert Rev Anticancer Ther. 7:1379–1393. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez EA and Spano JP: Current and
emerging targeted therapies for metastatic breast cancer. Cancer.
118:3014–3025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
No M, Choi EJ and Kim IA: Targeting HER2
signaling pathway for radiosensitization: Alternative strategy for
therapeutic resistance. Cancer Biol Ther. 8:2351–2361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou J, Zhou Z, Chen X, Zhao R, Yang Z, Wei
N, Ni Q, Feng Y, Yu X, Ma J and Guo X: HER2 reduces breast cancer
radiosensitivity by activating focal adhesion kinase in vitro and
in vivo. Oncotarget. 7:45186–45198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu T, Cho BJ, Choi EJ, Park JM, Kim DH and
Kim IA: Radiosensitizing effect of lapatinib in human epidermal
growth factor receptor 2-positive breast cancer cells. Oncotarget.
7:79089–79100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai YC, Ho PY, Tzen KY, Tuan TF, Liu WL,
Cheng AL, Pu YS and Cheng JC: Synergistic blockade of EGFR and HER2
by new-generation EGFR tyrosine kinase inhibitor enhances radiation
effect in bladder cancer cells. Mol Cancer Ther. 14:810–820. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang K, Lu Y, Jin W, Ang KK, Milas L and
Fan Z: Sensitization of breast cancer cells to radiation by
trastuzumab. Mol Cancer Ther. 2:1113–1120. 2003.PubMed/NCBI
|
|
12
|
Jacob J, Belin L, Pierga JY, Gobillion A,
Vincent-Salomon A, Dendale R, Beuzeboc P, Campana F, Fourquet A and
Kirova YM: Concurrent administration of trastuzumab with
locoregional breast radiotherapy: Long-term results of a
prospective study. Breast Cancer Res Treat. 148:345–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Yu S, Zhuang L, Zheng Z, Chao T
and Fu Q: Caveolin-1 is involved in radiation-induced ERBB2 nuclear
transport in breast cancer cells. J Huazhong Univ Sci Technolog Med
Sci. 32:888–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo B, Yu S, Zhuang L, Xia S, Zhao Z and
Rong L: Induction of ERBB2 nuclear transport after radiation in
breast cancer cells. J Huazhong Univ Sci Technolog Med Sci.
29:350–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: Mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi H, Zhang W, Zhi Q and Jiang M:
Lapatinib resistance in HER2+ cancers: Latest findings
and new concepts on molecular mechanisms. Tumour Biol. Oct
10–2016.(Epub ahead of print). doi: 10.1007/s13277-016-5467-2.
View Article : Google Scholar
|
|
17
|
Canonici A, Gijsen M, Mullooly M, Bennett
R, Bouguern N, Pedersen K, O'Brien NA, Roxanis I, Li JL, Bridge E,
et al: Neratinib overcomes trastuzumab resistance in HER2 amplified
breast cancer. Oncotarget. 4:1592–1605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Y, Li L, Zhang G, Wan H, Yang C, Diao
X, Chen X, Zhang L and Zhong D: Metabolic characterization of
pyrotinib in humans by ultra-performance liquid
chromatography/quadrupole time-of-flight mass spectrometry. J
Chromatogr B Analyt Technol Biomed Life Sci. 1033-1034:117–127.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sartor CI: Epidermal growth factor family
receptors and inhibitors: Radiation response modulators. Semin
Radiat Oncol. 13:22–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Yang C, Wan H, Zhang G, Feng J and
Zhang L, Chen X, Zhong D, Lou L, Tao W and Zhang L: Discovery and
development of pyrotinib: A novel irreversible EGFR/HER2 dual
tyrosine kinase inhibitor with favorable safety profiles for the
treatment of breast cancer. Eur J Pharm Sci. 110:51–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khanna KK and Jackson SP: DNA
double-strand breaks: Signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cordo Russo RI, Béguelin W, Díaz Flaqué
MC, Proietti CJ, Venturutti L, Galigniana N, Tkach M, Guzmán P, Roa
JC, O'Brien NA, et al: Targeting ErbB-2 nuclear localization and
function inhibits breast cancer growth and overcomes trastuzumab
resistance. Oncogene. 34:3413–3428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schillaci R, Guzmán P, Cayrol F, Beguelin
W, Diaz Flaque MC, Proietti CJ, Pineda V, Palazzi J, Frahm I,
Charreau EH, et al: Clinical relevance of ErbB-2/HER2 nuclear
expression in breast cancer. BMC Cancer. 12:742012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang SM and Harari PM: Modulation of
radiation response after epidermal growth factor receptor blockade
in squamous cell carcinomas: Inhibition of damage repair, cell
cycle kinetics, and tumor angiogenesis. Clin Cancer Res.
6:2166–2174. 2000.PubMed/NCBI
|
|
25
|
Ethier SP and Lawrence TS: Epidermal
growth factor receptor signaling and response of cancer cells to
ionizing radiation. J Natl Cancer Inst. 93:890–891. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dent P, Yacoub A, Contessa J, Caron R,
Amorino G, Valerie K, Hagan MP, Grant S and Schmidt-Ullrich R:
Stress and radiation-induced activation of multiple intracellular
signaling pathways. Radiat Res. 159:283–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Halyard MY, Pisansky TM, Dueck AC, Suman
V, Pierce L, Solin L, Marks L, Davidson N, Martino S, Kaufman P, et
al: Radiotherapy and adjuvant trastuzumab in operable breast
cancer: Tolerability and adverse event data from the NCCTG Phase
III Trial N9831. J Clin Oncol. 27:2638–2644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belkacémi Y, Gligorov J, Ozsahin M,
Marsiglia H, De Lafontan B, Laharie-Mineur H, Aimard L, Antoine EC,
Cutuli B, Namer M and Azria D: Concurrent trastuzumab with adjuvant
radiotherapy in HER2-positive breast cancer patients: Acute
toxicity analyses from the French multicentric study. Ann Oncol.
19:1110–1116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horton JK, Halle J, Ferraro M, Carey L,
Moore DT, Ollila D and Sartor CI: Radiosensitization of
chemotherapy-refractory, locally advanced or locally recurrent
breast cancer with trastuzumab: A phase II trial. Int J Radiat
Oncol Biol Phys. 76:998–1004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: Receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Contessa JN, Hampton J, Lammering G,
Mikkelsen RB, Dent P, Valerie K and Schmidt-Ullrich RK: Ionizing
radiation activates Erb-B receptor dependent Akt and p70 S6 kinase
signaling in carcinoma cells. Oncogene. 21:4032–4041. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bowers G, Reardon D, Hewitt T, Dent P,
Mikkelsen RB, Valerie K, Lammering G, Amir C and Schmidt-Ullrich
RK: The relative role of ErbB1-4 receptor tyrosine kinases in
radiation signal transduction responses of human carcinoma cells.
Oncogene. 20:1388–1397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trip AK, Poppema BJ, van Berge Henegouwen
MI, Siemerink E, Beukema JC, Verheij M, Plukker JT, Richel DJ,
Hulshof MC, van Sandick JW, et al: Preoperative chemoradiotherapy
in locally advanced gastric cancer, a phase I/II feasibility and
efficacy study. Radiother Oncol. 112:284–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ajani JA, Winter K, Okawara GS, Donohue
JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD,
Willett C and Rich TA: Phase II trial of preoperative
chemoradiation in patients with localized gastric adenocarcinoma
(RTOG 9904): Quality of combined modality therapy and pathologic
response. J Clin Oncol. 24:3953–3958. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ajani JA, Mansfield PF, Janjan N, Morris
J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS and
Gunderson LL: Multi-institutional trial of preoperative
chemoradiotherapy in patients with potentially resectable gastric
carcinoma. J Clin Oncol. 22:2774–2780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Newton AD, Datta J, Loaiza-Bonilla A,
Karakousis GC and Roses RE: Neoadjuvant therapy for gastric cancer:
Current evidence and future directions. J Gastrointest Oncol.
6:534–543. 2015.PubMed/NCBI
|
|
37
|
Smalley SR, Benedetti JK, Haller DG,
Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson
JA, Jessup JM, et al: Updated analysis of SWOG-directed intergroup
study 0116: A phase III trial of adjuvant radiochemotherapy versus
observation after curative gastric cancer resection. J Clin Oncol.
30:2327–2333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SH, Sohn TS, Lee J, Lim DH, Hong ME,
Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, et al: Phase III trial to
compare adjuvant chemotherapy with capecitabine and cisplatin
versus concurrent chemoradiotherapy in gastric cancer: Final report
of the adjuvant chemoradiotherapy in stomach tumors trial,
including survival and subset analyses. J Clin Oncol. 33:3130–3136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), ; Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J,
et al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: Meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Van de Steene J, Soete G and Storme G:
Adjuvant radiotherapy for breast cancer significantly improves
overall survival: The missing link. Radiother Oncol. 55:263–272.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ragaz J, Olivotto IA, Spinelli JJ,
Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CM, Weir L,
Gelmon K, et al: Locoregional radiation therapy in patients with
high-risk breast cancer receiving adjuvant chemotherapy: 20-year
results of the British Columbia randomized trial. J Natl Cancer
Inst. 97:116–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J,
Luo Y, Xing P, Lan B, Li M, et al: Phase I study and biomarker
analysis of pyrotinib, a novel irreversible Pan-ErbB receptor
tyrosine kinase inhibitor, in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Clin Oncol.
35:3105–3112. 2017. View Article : Google Scholar : PubMed/NCBI
|