Introduction
Oral cancer affects various parts of the oral
cavity, such as the tongue, gingiva, floor of the mouth and buccal
mucosa, and is the sixth most common malignant neoplasm worldwide
(1). Oral squamous cell carcinoma
(OSCC) is the most frequently occurring malignancy in the oral
cavity (1). Although significant
advances in the development of comprehensive and multimodality
therapies for OSCC have been achieved over the past few decades,
the long-term survival rates have remained relatively unchanged,
particularly in patients with advanced lesions (2). Locoregional relapse and cervical lymph
node metastasis are the most prevalent and significant factors that
affect the prognosis of patients with OSCC (2). Although the initiation and progression
of OSCC are closely associated with the activation of aberrant
oncogenes, inactivation of tumor suppressors and other epigenetic
abnormalities, the molecular carcinogenesis of OSCC has not yet
been elucidated in detail, and this has hindered the development of
potent and sensitive biomarkers, and therapeutic strategies
(3). Therefore, the underlying
molecular mechanisms and novel therapeutic targets need to be
identified in order to improve the prognosis of patients with OSCC.
Tumor biomarkers that accurately reflect tumor cell characteristics
or could be used as treatment targets have been the focus of
continuing research. Two-dimensional fluorescence difference gel
electrophoresis (2D-DIGE) has been employed for protein separation
(4), and proteomic approaches have
contributed to the identification of biomarkers in various types of
cancer (5,6). However, they have not yet been
extensively applied to oral cancer (7,8). In
the present study, the protein expression profiles in normal
epidermal keratinocytes and OSCC cell lines were examined using
2D-DIGE and liquid chromatography tandem-mass spectrometry
(LC-MS/MS). A common upstream search was performed for proteins
with expression abnormalities in a proteomic analysis of OSCC
cells. The results obtained identified heat shock protein 90
(HSP90) as a target that regulated the functional maintenance and
stability of numerous client proteins, which serve important roles
in OSCC cell proliferation and survival.
The molecular chaperone HSP90 is involved in
regulating the maturation and functional stability of an extensive
array of cellular client proteins, an activity that is often
exploited by cancer cells to confer an aberrant proliferative,
survival and/or metastatic potential (9,10). The
HSP90 machinery functions as a biochemical buffer for a number of
oncogenic signaling proteins that have been causally implicated in
various human tumors; mutant oncoproteins have been previously
demonstrated to rely on the chaperone (11,12).
The functional inhibition of HSP90 results in the simultaneous
degradation of hundreds of client proteins, thereby providing a
mechanism to concomitantly disrupt multiple oncogenic signaling
cascades through a single molecular target (13). The pharmacological blockade of HSP90
has emerged as an innovative approach for the development of novel
antineoplastic agents (13).
Therefore, the potential of HSP90 as a candidate for molecular
targeted therapy was investigated in OSCC cells treated with HSP90
inhibitors in the present study. In addition, a functional analysis
of the HSP90 protein was conducted using in vitro assays,
and the relationships between the expression levels of this protein
and clinicopathological factors, as well as the prognosis of
patients, were assessed using immunohistochemistry (IHC).
Materials and methods
Cells
The OSCC KON, OSC-20, HSC-3, HSC-4, SAS and Ca9-22
cell lines were obtained from the Japanese Collection of Research
Bioresources Cell Bank. The spontaneously transformed immortal
keratinocyte cell line, HaCaT (cat. no. 300493), was obtained from
CLS Cell Lines Service GmbH. All cell lines were maintained at 37°C
in a humidified atmosphere of 5% CO2/95% air. FBS was
purchased from Sigma-Aldrich; Merck KGaA. The KON cells were
cultured in DMEM (Sigma-Aldrich; Merck KGaA) supplemented with 10%
FBS and 50 U/ml penicillin and streptomycin (Sigma-Aldrich; Merck
KGaA). The OSC-20 and SAS cells were cultured in DMEM/F-12 medium
(Sigma-Aldrich; Merck KGaA) with 10% FBS and 50 U/ml penicillin and
streptomycin. The HSC-3, HSC-4 and Ca9-22 cells were cultured in
minimum essential medium (Sigma-Aldrich; Merck KGaA) supplemented
with 10% FBS and 50 U/ml penicillin and streptomycin. The HaCaT
cells, which were used as controls in the present study, were
cultured in DMEM supplemented with 10% FBS and 50 U/ml penicillin
and streptomycin. The culture medium was changed twice a week for
all cells. It has been reported that the Ca9-22 cell line is
contaminated with MSK-922 cells (14). Therefore, short tandem repeat (STR)
analysis was performed and it was confirmed that the cell line used
in the present study was not contaminated. STR analysis was
performed by a third party (Promega Corporation) using the
PowerPlex 16 kit (Promega Corporation) which analyses 16
independent genetic sites specific for human DNA that include the
13 CODIS loci, plus PENTA E, PENTA D and amelogenin (15).
2D-DIGE and image analysis
2D-DIGE was performed as described previously
(16,17). In brief, a common internal control
sample was created by mixing a small portion from all protein
samples used in the present study, and this was then labeled using
a Cy3 fluorescent dye (CyDye DIGE Fluor saturation dye; GE
Healthcare Bio-Sciences). Individual samples were labeled with Cy5
fluorescent dye (CyDye DIGE Fluor saturation dye; GE Healthcare
Bio-Sciences). The protein samples were mixed together and
separated using 2D-DIGE based on their isoelectric points and
molecular weights. First-dimension separation was performed with an
Immobiline DryStrip Gel (IPG; length, 24 cm; pH 3.0-10.0; GE
Healthcare Bio-Sciences) and the Multiphor Electrophoresis System
(GE Healthcare Bio-Sciences). The second-dimension separation was
performed using a homemade gradient gel with GiantGelRunner
(separation distance, 36 cm; Everseiko Corporation). The gels were
scanned using a laser scanner (Typhoon Trio; GE Healthcare
Bio-Sciences) at the appropriate wavelengths for Cy3 or Cy5 (Cy3:
Excitation 532 nm, fluorescence 580 nm; Cy5: Excitation 633 nm,
fluorescence 670 nm). The gel images were analyzed automatically
using the DeCyder-BVA (biologic variation analysis) software
(version 7.0; GE Healthcare Bio-Sciences). The statistical
significance of each expression level was calculated using
Student's t-test on the logged ratios.
Mass spectrometry analysis
Proteins (100 µg/lane) separated by 12% SDS-PAGE
were visualized using SYPRO Ruby staining (Molecular Probes; Thermo
Fisher Scientific, Inc.) at room temperature for 3 h. The peptide
samples were excised from the gels and digested by trypsin using
the In Gel Digest Kit (EMD Millipore) as described previously
(18). Following the extraction of
the peptides, the proteolytic peptide mixture was evaporated to ~5
ml, and 35 ml of 2% acetonitrile and 0.1% trifluoroacetic acid were
added to the mixture, which was then subjected to an autosampler
(HTC PAL; CTC Analytics AG) for nanoscale capillary LC-MS/MS
analysis. A capillary LC system (Magic 2002; Bruker-Michrom, Inc.)
coupled to an in-line nanoelectrospray mass spectrometer (LCQ
Advantage; Thermo Fisher Scientific, Inc.) with a silica-coated
glass capillary (PicoTip; New Objective, Inc.) was used. The
analysis conditions were as follows: Ionization mode used, positive
mode; column temperature, room temperature; flow rate, 2.5 µl/min.
The samples were loaded in 5% acetonitrile with 0.1% formic acid.
The gradient consisted of 6.4% acetonitrile for 5 min followed by
6.4 to 76.8% acetonitrile for 45 min. The spectra were collected as
MS and MS/MS scans. The MS scan defined the ion composition at an
m/z range of 450-2,000, and the MS/MS scan acquired the mass
spectrum of the parental ion upon collision-induced dissociation.
The collision-induced dissociation spectra acquired were then
analyzed by direct inspection using the Mascot software program
(version 2.2.04; Matrix Science, Inc.) as described previously
(19,20).
Hierarchical clustering and molecular
network analyses
Hierarchical clustering analysis of protein
expression data was performed using the Multi Experiment View
cluster software version 4.9.0 (21,22). A
molecular network analysis was performed using the
KeyMolnet® software version 5.8 (23), which encompasses the majority of
relationships among human genes and proteins, molecules, diseases,
pathways and drugs. This information is manually collected,
carefully curated and regularly updated by expert biologists. The
database is categorized into core contents, which are collected
from selected review articles with the highest reliability and
secondary contents, which are extracted from abstracts in the
PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Human Protein
Reference databases (https://www.hprd.org/). KeyMolnet® provides
information on the corresponding molecules as a node on networks by
importing microarray data, such as protein IDs and fold changes in
individual probes. The ‘common upstream’ search algorithm aids in
the extraction of the most relevant molecular network comprising
genes that are coordinately regulated by putative common upstream
transcription factors (23,24).
HSP90 inhibitors
In the in vitro experiments,
17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; AdooQ
Bioscience) and ganetespib (KareBay Biochem, Inc.) were dissolved
in DMSO to a stock concentration of 1 mM and the same final
concentrations (5, 10 and 15 µM) were used, as previously described
(25,26). The stock solutions were stored at
−20°C. The inhibitors were diluted in culture medium prior to each
in vitro experiment, and 0.01% DMSO in culture medium was
used as the vehicle control.
Cell proliferation assay
KON cells that strongly expressed the HSP90 protein
were plated in 96-well plates (density, 5×103
cells/well) in sextuplicate and incubated at 37°C in a humidified
5% CO2 atmosphere. Following an overnight attachment
period, the cells were exposed to 17-DMAG (5, 10 and 15 µM),
ganetespib (5, 10 and 15 µM) or 0.01% DMSO (as control) at 37°C.
The number of viable cells was counted after 24, 48 and 72 h using
the RealTime-Glo MT Cell Viability Assay (Promega Corporation) and
a GloMax 96 Microplate Luminometer (Promega Corporation) (27). All assays were performed with five
technical replicates and each assay was repeated three times.
Cell invasion assay
The in vitro invasion assay was performed
using the CultreCoat 96-well Basement Membrane Extract-Coated
Invasion Assay Kit (Trevigen, Inc.) (28). The KON cells (1×105)
suspended in serum-free culture medium were seeded on the upper
surface of each insert chamber in triplicate. The lower part was
filled with culture medium containing 10% FBS. The cells were
exposed to 17-DMAG (5, 10 and 15 µM), ganetespib (5, 10 and 15 µM)
or 0.01% DMSO at 37°C for 48 h. Following incubation, the cells
that had migrated to the other side of the membrane were detected
using cell dissociation solution/calcein AM. The fluorescence was
read using a 480/520 nm filter set.
Gap closure assay
KON cells were seeded on culture inserts
(3.0×105 cells/insert; cat. no. 80206; Ibidi GmbH) in
triplicate. When the cells reached confluency, the inserts were
removed and a gap was created. After washing with PBS to remove the
cell debris, the cells were exposed to 17-DMAG (5, 10 and 15 µM),
ganetespib (5, 10 and 15 µM) or 0.01% DMSO, and incubated at 37°C
in a 5% CO2 humidified incubator to allow for gap
closure. The FBS concentration used in this experiment was 3%
(29). The closure of the gap was
imaged under a BZ-X710 microscope (light microscope; magnification,
×40; Keyence Corporation) immediately after adding fresh culture
medium and at the indicated time points (6, 12, 18, 24 and 30 h
later) (27,30). The area of the gap at each time
point was calculated using the MRI Wound healing tool (http://dev.mri.cnrs.fr/projects/imagej-macros/wiki/Wound_Healing_Tool)
in ImageJ software (ver. 1.50; National Institutes of Health).
Western blot analysis
Western blotting was performed as previously
described (27,30). The KON cells were seeded on culture
plates (10×10 mm2). At 60-70% confluency, they were
treated with 17-DMAG (5, 10 and 15 µM), ganetespib (5, 10 and 15
µM) or 0.01% DMSO for 48 h. Proteins were extracted using the
Mammalian Protein Extraction Reagent (Thermo Fisher Scientific,
Inc.) containing a protease inhibitor mixture (FUJIFILM Wako Pure
Chemical Corporation), Phosphatase Inhibitor Cocktail 2
(Sigma-Aldrich; Merck KGaA) and Phosphatase Inhibitor Cocktail 3
(Sigma-Aldrich; Merck KGaA) at a ratio of 1:100. The protein
samples were fractionated by SDS-PAGE and blotted onto
polyvinylidene difluoride membranes (Merck KGaA). The membranes
were blocked with 5% PhosphoBLOCKE powder (Cell Biolabs, Inc.) in
Tris-buffered saline and 1% Tween-20, and probed using the
following antibodies: EGF receptor rabbit monoclonal antibody
(dilution, 1:1,000; cat. no. 4267; Cell Signaling Technology,
Inc.), phospho-EGF receptor rabbit monoclonal antibody (dilution,
1:1,000; cat. no. 3777; Cell Signaling Technology, Inc.), MEK1/2
rabbit monoclonal antibody (dilution, 1:1,000; cat. no. 8727; Cell
Signaling Technology, Inc.), phospho-MEK1/2 rabbit monoclonal
antibody (dilution, 1:1,000; cat. no. 9154; Cell Signaling
Technology, Inc.), p44/42 MAPK rabbit polyclonal antibody
(dilution, 1:1,000; cat. no. 9102; Cell Signaling Technology,
Inc.), phospho-p44/42 MAPK rabbit monoclonal antibody (dilution,
1:2,000; cat. no. 4370; Cell Signaling Technology, Inc.), heat
shock protein 70 (HSP70) rabbit polyclonal antibody (dilution,
1:1,000; cat. no. 4872; Cell Signaling Technology, Inc.), HSP90
rabbit monoclonal antibody (dilution, 1:1,000; cat. no. 4877; Cell
Signaling Technology, Inc.) and β-actin mouse monoclonal antibody
(dilution, 1:2,500; cat. no. ab6276; Abcam). Following overnight
incubation with the primary antibodies at 4°C, signals were
detected using the relevant horseradish peroxidase-conjugated
anti-mouse or anti-rabbit IgG antibodies (GE Healthcare) and the
SuperSignal™ West Dura Extended Duration Substrate (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
levels of total and phosphorylated proteins were analyzed using the
same protein sample. The experiments were performed in
triplicate.
Tissue samples
Tumor tissues and patient-matched normal oral
tissues (near the resection margin) were obtained at the time of
surgical resection from Tokyo Dental College Chiba Hospital (Chiba,
Japan) according to a protocol approved by the Institutional Review
Board of Tokyo Dental College (approval no. 709). The inclusion
criteria were: i) Having an OSCC of the tongue, ii) available
formalin-fixed paraffin-embedded tumor samples; and iii) existence
of essential clinical records corresponding to the tumor. The
exclusion criteria were: i) Lack of essential survival data; ii)
lack of sufficient quantity of tumor tissue in the paraffin block;
and iii) microinvasive carcinomas or carcinoma in situ. Written
informed consent was obtained from all patients involved. The
subjects included 37 men and 21 women ranging in age between 30 and
86 years, with a mean age of 66 years, who underwent surgical
excision between January 2009 and March 2014. The resected tissues
were divided into two parts: One was frozen immediately and stored
at −80°C for further analyses and the other was fixed in 10%
buffered formaldehyde solution for pathological diagnosis. Fixation
was performed at room temperature for 24 h. The histopathological
diagnosis of each tissue was performed according to the
International Histological Classification of Tumors (31) at the Department of Pathology, Tokyo
Dental College. Clinicopathological staging was conducted according
to the TNM Classification of the International Union against Cancer
(32).
IHC
IHC staining was performed as previously described
(17,27). Paraffin-embedded specimens
(4-µm-thick) were subjected to IHC staining. Briefly, following
deparaffinization and hydration, the slides were treated with 0.3%
H2O2 for 30 min to block endogenous
peroxidase. Subsequently, the sections were blocked at room
temperature for 2 h with 1.5% blocking serum (Santa Cruz
Biotechnology, Inc.) in PBS and treated with the anti-HSP90 rabbit
monoclonal antibody at a dilution of 1:1,000. The same primary
antibodies were used for IHC and western blot analysis. Sections
were incubated with the primary antibody in a moist chamber at room
temperature for 30 min. Following incubation, the sections were
washed three times in PBS and treated with Envision reagent (Dako;
Agilent Technologies, Inc.), followed by color development in
3,3′-diaminobenzidine tetrahydrochloride (Dako; Agilent
Technologies, Inc.). The slides were then lightly counterstained
with hematoxylin and mounted. Duplicate sections immunostained
without exposure to the primary antibodies served as negative
controls. A scoring method was used to quantitate the protein
expression levels of HSP90, with the mean percentage of positive
tumor cells being assessed in at least five random fields
(magnification, ×400) in each section. The intensity of the HSP90
immunoreaction was scored as follows: 1+, weak; 2+, moderate; and
3+, intense. The percentage of positive tumor cells and staining
intensity were multiplied to produce an HSP90-IHC staining score
for each case (27,30). The IHC scores of the tumor tissues
were compared with those of the healthy surrounding normal tissues
obtained from the same patient. Cancer tissues with higher IHC
scores than those of the adjacent normal tissues were designated as
HSP90 high expression cases. The cases were scored by two
independent specialists who were blinded to the clinical status of
the patients.
Statistical analysis
The in vitro assay results were evaluated
using Student's t-test and ANOVA with Bonferroni's correction
applied. Data are presented as the mean ± SD. All assays were
repeated three times. Significant differences between HSP90-IHC
scores and clinicopathological features were assessed using the
Mann-Whitney U test and Fisher's exact test. The overall survival
rate was calculated by Kaplan-Meier analysis, and the log-rank test
was used for comparisons between groups. Statistical analyses were
performed using EZR, ver. 1.42 (Saitama Medical Center, Jichi
Medical University, Saitama, Japan), which is a graphical user
interface for R (The R Foundation) and a modified version of R
Commander that adds statistical functions frequently used in
biostatistics (27,33). All P-values were two-sided, and
P≤0.05 was considered to indicate a statistically significant
difference.
Results
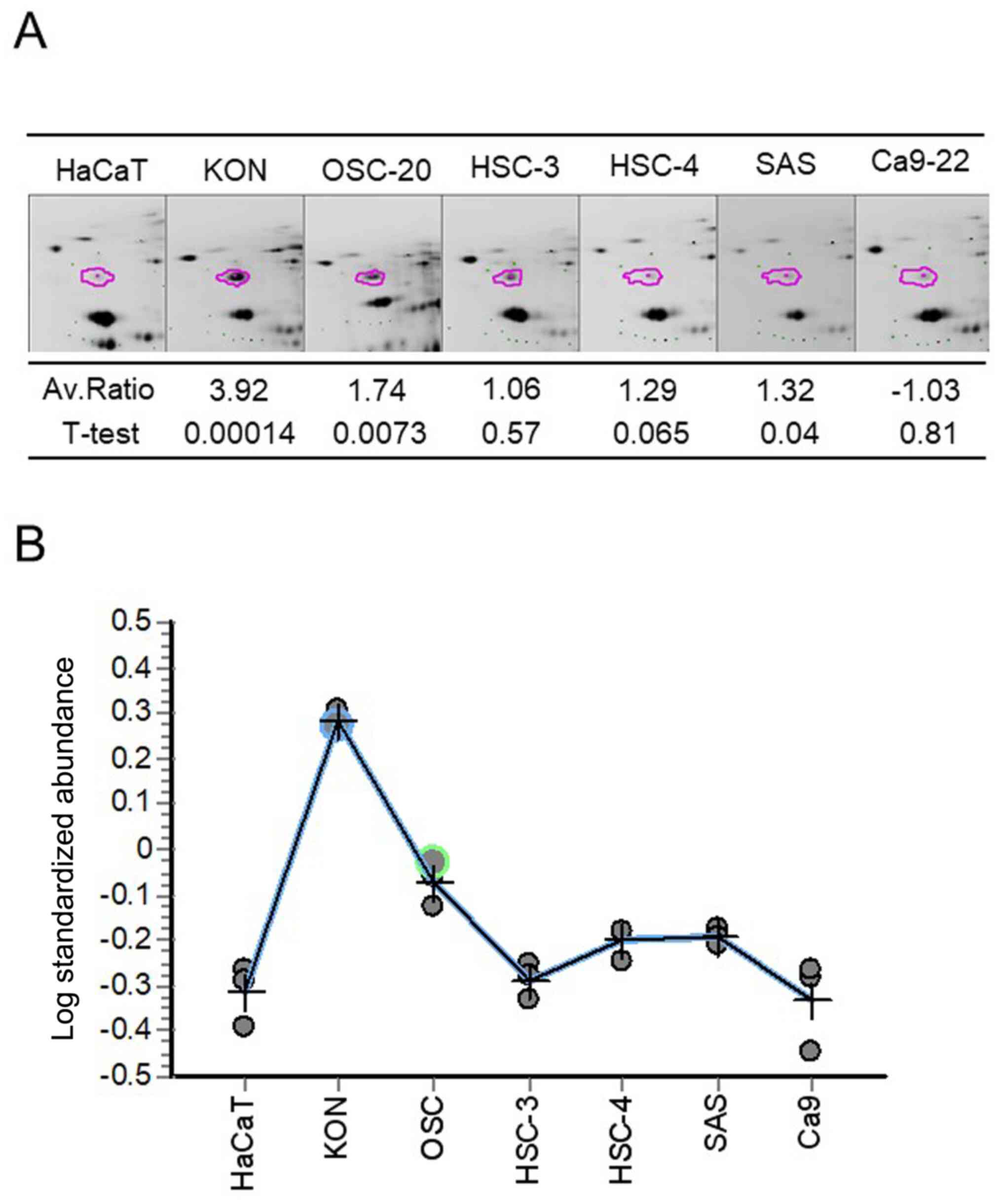
Proteomic profiling by 2D-DIGE
OSCC-derived cell lines and HaCaT cells were
subjected to protein extraction. From each proteome, ~3,000 protein
spots were successfully identified (Fig. 1A). The expression levels of 49
proteins were significantly increased and those of 77 proteins were
significantly decreased in the OSCC cells compared with in the
HaCaT cells. LC-MS/MS identified 92 types of proteins, except those
with post-translational modifications (Fig. 1B).
Hierarchical clustering and partition
analyses of samples
Fig. 1B shows the
results obtained in the clustering analysis. Samples with high
expression levels are shown in green, and those with low expression
levels in red. The columns indicate the samples, whereas the rows
indicate the proteins. The dendrograms represent the distances
between the clusters. To identify relationships between the
molecular network of the OSCC cells and the canonical pathway, an
‘interrelation’ network search was performed using the
KeyMolnet® software. A common upstream search was
performed using this software for proteins with expression
abnormalities that were identified in the proteomic analysis of
OSCC cells. In the extracted molecular network, the five pathways
with the highest scores were the ‘HSP90 signaling pathway’ (score,
47.28), ‘transcriptional regulation by HIF’ (score, 25.15),
‘Annexin signaling pathway’ (score, 14.32), ‘PI3K signaling
pathway’ (score, 12.65) and ‘Spliceosome assembly’ (score, 12.13;
Table I). The results obtained
revealed HSP90 as a target which regulates the functional
maintenance and stability of numerous client proteins that serve
roles in OSCC cell proliferation and survival. A typical network
including HSP90 (Table I; rank 1)
is shown in Fig. 1C. HSP90 was
listed as a candidate protein that frequently exhibited high
expression levels in OSCC cell lines (Fig. 2A and B).
 | Table I.List of top 5 pathways contributing
to the common upstream network identified by KeyMolnet. |
Table I.
List of top 5 pathways contributing
to the common upstream network identified by KeyMolnet.
| Rank | Name | Score | Score(p) | Score(v) |
|---|
| 1 | HSP90 signaling
pathway | 47.28 |
5.853×10−15 | 0.121 |
| 2 | Transcriptional
regulation by HIF | 25.15 |
2.683×10−8 | 0.053 |
| 3 | Annexin signaling
pathway | 14.32 |
4.870×10−5 | 0.030 |
| 4 | PI3K signaling
pathway | 12.65 |
1.550×10−4 | 0.038 |
| 5 | Spliceosome
assembly | 12.13 |
2.230×10−4 | 0.023 |
17-DMAG and ganetespib decrease cell
viability and invasion in OSCC-derived cell lines in vitro
The HSP90 inhibitors 17-DMAG and ganetespib were
selected for in vitro validation as potential therapeutic
target molecules for OSCC. HSP90 was more highly expressed in KON
cells than in the other OSCC-derived cell lines. Therefore, this
cell line was examined in subsequent assays. The viability of cells
treated with increasing concentrations of 17-DMAG or ganetespib for
24, 48 and 72 h was assessed using the MT Cell Viability Assay. As
shown in Fig. 3A, the viability of
KON cells treated with 17-DMAG or ganetespib was significantly
reduced within 24 h of treatment (P<0.05). To clarify the
effects of 17-DMAG or ganetespib on the invasive ability of the
cells, the KON cell lines were treated with 17-DMAG (5, 10 and 15
µM), ganetespib (5, 10 and 15 µM) or 0.01% DMSO for 48 h. The
results obtained revealed significantly lower invasion rates in
treated cells compared with the untreated cells (Fig. 3B). The gap closure assay
investigated gap closure in KON cells 30 h after the DMSO
treatment. Additional cells were observed in the vicinity of the
gap. By contrast, gap closure was slower in HSP90 inhibitor-treated
KON cells (Fig. 3C and D). This
difference in gap closure was statistically significant at 18, 24
and 30 h (P<0.05).
 | Figure 3.In vitro effects of 17-DMAG
and ganetespib on cell proliferation and migration in OSCC-derived
cell lines. (A) KON cells were treated with increasing
concentrations of 17-DMAG and ganetespib. Cell viability was
assessed after 24, 48 and 72 h of treatment. Data are presented as
the mean ± SD of at least three independent experiments in
quintuplicate. *P<0.05. (B) Cell invasion was evaluated in KON
cells treated with 17-DMAG or ganetespib (5, 10 and 15 µM) for 48
h. DMSO-treated KON cells were used as the control. Data are
presented as the mean ± SD. *P<0.05 (t-test and ANOVA with
Bonferroni's correction). (C) Gap closure assay after treatment
with the HSP90 inhibitor. After uniform gaps were created in
confluent cultures of 17-DMAG-, ganetespib- and DMSO-treated KON
cells, the extent of closure was visually monitored. The gap was
completely sealed after 30 h in the DMSO-treated control cells, but
remained open in the HSP90 inhibitor-treated cells. *P<0.05 vs.
0 µM (t-test and ANOVA with Bonferroni's correction). (D) Widths of
the gaps were measured at predefined positions and specific time
points. Magnification, ×40. The migration of HSP90
inhibitor-treated cells was decreased compared with that of the
control cells at 18, 24 and 30 h after the creation of the gaps.
17-DMAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin;
HSP90, heat shock protein 90; OSCC, oral squamous cell
carcinoma. |
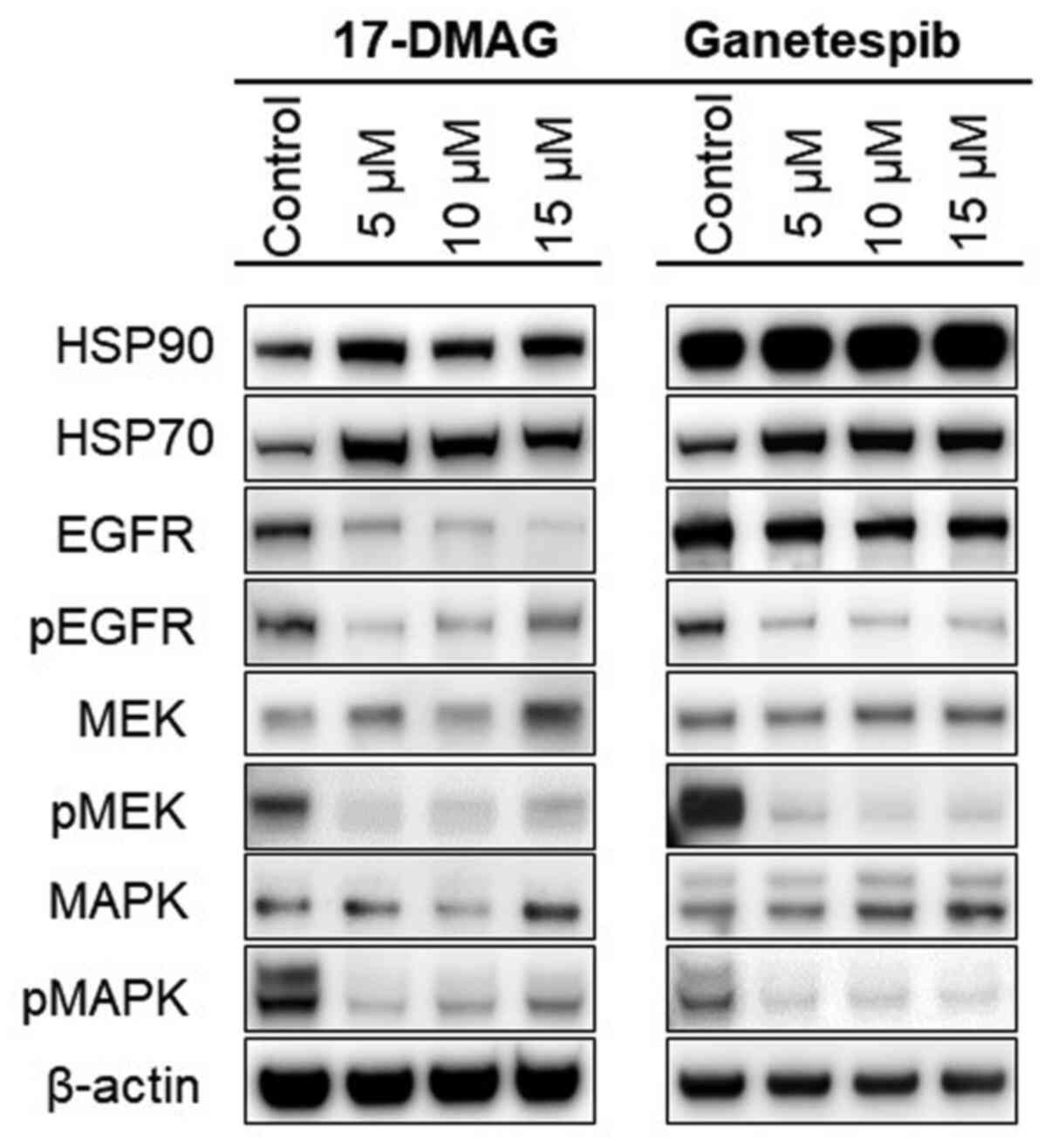
17-DMAG and ganetespib alter the in
vitro expression levels of HSP90-related proteins in OSCC
cells
In an attempt to clarify the in vitro effects
of 17-DMAG and ganetespib in the OSCC-derived cell lines, the
expression levels of HSP90 and its related proteins were assessed
by western blot analysis. The cells were exposed to 17-DMAG (5, 10
and 15 µM), ganetespib (5, 10 and 15 µM) or DMSO for 48 h, lysed
and then subjected to western blotting using commercial antibodies.
As shown in Fig. 4, the expression
levels of HSP90, HSP70, MEK and MAPK were increased in the HSP90
inhibitor-treated KON cells, whereas those of the HSP90 target
proteins EGFR, phospho-EGFR, phospho-MEK and phospho-MAPK were
decreased in the 17-DMAG- and ganetespib-treated cells.
HSP90 protein expression in normal
oral tissues and primary OSCCs
Twenty-six out of the 58 OSCC samples subjected to
IHC staining exhibited increased expression levels of HSP90. By
contrast, normal tissues exhibited weak cytoplasmic immunoreactions
for HSP90. Fig. 5A-C shows
representative results for HSP90 protein expression in normal oral
tissue and primary OSCCs. A significant association between high
HSP90 expression and regional lymph node metastasis was noted
(Table II). The protein expression
levels of HSP90 in normal oral tissue and primary OSCC samples are
shown in Fig. 5D. The HSP90-IHC
scores ranged between 2.89 and 165.98 (mean, 75.66), 31.37 and
258.77 (mean, 104.39), 31.37 and 165.45 (mean, 79.53), and 63.81
and 258.77 (mean, 146.54) in the normal tissues, OSCC samples, and
the node-negative (pN-) and node-positive (pN+) OSCC samples,
respectively. Significant differences in HSP90-IHC scores between
the normal oral tissues and OSCC samples were observed (P=0.005;
Fig. 5D). Furthermore, the
expression levels of HSP90 were significantly higher (P=0.015) in
the pN+ OSCC samples compared with the pN−
OSCC samples. The results of the Kaplan-Meier analysis revealed
that, based on the overall survival rates of 58 patients, high
expression levels of HSP90 were not associated with poor outcomes
(P=0.606; Fig. 5E).
 | Table II.Association between the expression
levels of HSP90 and clinical classification in patients with oral
squamous cell carcinoma (n=58). |
Table II.
Association between the expression
levels of HSP90 and clinical classification in patients with oral
squamous cell carcinoma (n=58).
|
|
| HSP90
expression |
|---|
|
|
|
|
|---|
| Clinical
classification | No. of patients
(n=58) | Low, n (%) | High, n (%) | P-value |
|---|
| Age at surgery,
years |
|
<60 | 22 | 14 (64) | 8
(36) | 0.67 |
| ≥60,
<70 | 18 | 9
(50) | 9
(50) |
|
|
≥70 | 18 | 9
(50) | 9
(50) |
|
| Sex |
|
Male | 37 | 20 (54) | 17 (46) | 1 |
|
Female | 21 | 12 (57) | 9
(43) |
|
| T-primary
tumor |
| T1 | 16 | 8
(50) | 8
(50) | 0.897 |
| T2 | 20 | 11 (55) | 9
(45) |
|
| T3 | 6 | 3
(50) | 3
(50) |
|
| T4 | 16 | 10 (63) | 6
(37) |
|
| N-regional lymph
node |
|
pN(+) | 21 | 7
(33) | 14 (67) | 0.015 |
|
pN(−) | 37 | 25 (68) | 12 (32) |
|
| Stage |
| I | 16 | 8
(50) | 8
(50) | 0.709 |
| II | 17 | 8
(47) | 9
(53) |
|
|
III | 9 | 6
(67) | 3
(33) |
|
| IV | 16 | 10 (63) | 6
(37) |
|
| I,
II | 33 | 16 (48) | 17 (52) | 0.292 |
| III,
IV | 25 | 16 (64) | 9
(36) |
|
| Histological
type |
| Well
differentiated | 46 | 25 (54) | 21 (46) | 0.444 |
|
Moderately differentiated | 7 | 3
(43) | 4
(57) |
|
| Poorly
differentiated | 5 | 4
(80) | 1
(20) |
|
Discussion
Proteome analysis, which is the study of protein
complements in a cell, has the potential to accurately identify
proteins that can be used as novel targets during therapeutic
interventions and biomarkers for early cancer detection (34,35).
The establishment of effective cancer therapeutics based on the
inhibition of a protein that regulates numerous signaling pathways
and shows abnormalities in cancer cells is an attractive approach
for cancer therapy (36). A common
upstream search was performed using the protein nucleic acid
database for proteins with expression abnormalities that were
identified following a proteomic analysis of OSCC cells. HSP90 was
identified as a potentially novel molecular marker and target,
which regulates the functional maintenance and stability of
numerous client proteins that are involved in the proliferation and
survival of OSCC cells. Previous studies of proteomic analysis of
OSCC tissues also reported abnormal protein expression levels of
HSP90 (37,38). HSP90 has emerged as a novel
therapeutic target that simultaneously regulates numerous oncogenic
client proteins, which are the pathological hallmarks of malignancy
(39). HSP90 functions as a
chaperone protein in the stabilization and conformational
maturation of a large number of oncoproteins (11). HSP90 has been implicated in the
pathogenesis, poor prognosis and resistance to therapy of various
types of cancer in humans (40,41).
Furthermore, HSP90 target proteins are involved in most aspects of
the oncogenic process, such as immortality, survival,
anti-apoptosis, genomic instability, neoangiogenesis and metastasis
(42,43). In a previous study, blocking of
ATP-binding sites on the HSP90-partner complex resulted in the
dephosphorylation and/or proteasomal degradation of these target
proteins, thus demonstrating a potent antitumor activity (44). Among the various HSP90 inhibitors
currently available, geldanamycin (GA), a benzoquinone ansamycin
compound, belongs to the originally identified benzoquinone class
of compounds. GA derivatives, such as the less toxic
17-allylamino-17-demethoxygeldanamycin (17-AAG) and 17-DMAG, have
been developed. 17-DMAG is water-soluble and, therefore, orally
available (45,46). Furthermore, it has numerous
advantages over 17-AAG, such as less hepatotoxicity, high potency,
less extensive metabolism and a longer plasma half-time (45,46). A
phase I trial of 17-DMAG validated its clinical usefulness in
various types of cancer, including acute myeloid leukemia (partial
response), castration-refractory prostate cancer (complete
response), melanoma (partial response), renal cancer (stable
disease) and chondrosarcoma (stable disease) (47). However, to the best of our
knowledge, this was the first study to examine its effect in oral
cancer.
Ganetespib (previously referred to as STA-9090), a
second-generation HSP90 inhibitor, is cytotoxic in vitro and
exhibits antitumor activity against a wide range of cancer types
with promising safety profiles in vivo (48,49).
Previous clinical trials have reported the promising effects of
this agent against human breast and lung cancer (50,51). A
few studies on HSP90 suppression using 17-AAG in OSCC have been
published (52,53). However, to the best of our
knowledge, the anticancer effects of 17-DMAG and ganetespib against
OSCC have not yet been examined in detail.
Similar to previous findings obtained in other tumor
cells treated with HSP90 inhibitors (25,51),
the present results demonstrated that cell viability and migration
were reduced in OSCC cells treated with 17-DMAG and ganetespib.
These effects were associated with markedly decreased expression
levels of target proteins of HSP90, including EGFR, phospho-EGFR,
phospho-MEK and phospho-MAPK. Furthermore, in concordance with the
findings of other studies (54,55), a
marked increase in the expression levels of HSP90 and HSP70
following 17-DMAG and ganetespib treatment was observed in the
present study, probably through the activation of the heat shock
transcription factor 1 (HSF1), which is a master stress-inducible
regulator in the cytoplasm and the nucleus (56,57),
or due to the disruption of the nuclear HSP90/multichaperone
complexes that inhibit the activation of DNA-bound HSF1 (58). The majority of the HSP90 inhibitors
activate HSF1 and induce survival factors, such as HSP70,
indicating that HSF1 is one of the client proteins of HSP90.
Normally, HSF1 binds to the chaperone complex, which consists of
HSP90 and remains in an inactive state. In other words, HSP90
negatively regulates HSF1 activity. Therefore, when the function of
HSP90 is suppressed by an inhibitor, HSF1 is released and activated
leading to the induction of several HSPs, thus making the cells
stress-resistant. This offsets the cytocidal effect of HSP90
inhibitors (56–58). Additionally, the induction of HSP70
is an indicator of the presence of HSP90 inhibitors (56–58).
The induction of HSP70 by an HSP90 inhibitor may enhance the
antitumor effects when combined with other inhibitors, such as an
HSP70 inhibitor, or radiation. Musha et al (59) previously examined the effects of
17-AAG in combination with X-rays or carbon ion beams on OSCC cells
and reported that it exerted synergistic effects on cell lethality
with X-rays, but not with carbon ion beams. Further studies are
required to investigate the synergistic effects of 17-DMAG or
ganetespib with X-rays or carbon ion beams.
In the present study, the HSP90 expression status
based on IHC scores in the clinical tissue samples obtained from
primary OSCC and corresponding normal oral tissues revealed high
frequencies of HSP90-high cases. Furthermore, associations were
noted between the HSP90 expression status and clinicopathological
features. Although most primary OSCC samples with HSP90-high
expression presented with regional lymph node metastasis, tumors
without metastatic lesions had low HSP90 expression levels
(P=0.015). These results are consistent with previous findings
reported by Chang et al (60), which revealed high HSP90 protein
expression in 36 OSCC cases, and an association between HSP90-high
expression cases and lymph node metastasis. However, unlike the
findings of a previous study, which reported a lower survival rate
in patients with high HSP90 expression levels (60,61),
no such relationship was observed in the present study. The study
by Chang et al (60)
comprised only 36 cases, whereas the cases reported by Ono et
al (61) had an extremely poor
5-year survival rate compared with those in the present study.
These differences may have influenced the results of the present
study.
One of the limitations of the present study was the
small sample size, which may have affected the results. On the
other hand, the in vitro analysis verified the effect of
HSP90 inhibitors on only one KON cell line that expressed HSP90
protein at the highest level. Analyzing the effects of HSP90
inhibitors on a number of other types of OSCC cell lines is a
future challenge. Nonetheless, to the best of our knowledge, the
present study was the first to demonstrate the effects of 17-DMAG
and ganetespib in OSCC. OSCC cells treated with 17-DMAG and
ganetespib exhibited reduced cell viability and migration, which
were associated with markedly decreased expression levels of the
HSP90 target proteins EGFR, phospho-EGFR, phospho-MEK and
phospho-MAPK. Therefore, the present results indicate the potential
of HSP90 as a novel target for the early detection, prevention and
treatment of oral cancer metastasis. It was speculated that the
suppression of HSP90 may prevent metastasis associated with oral
carcinogenesis, while the upregulated expression levels of HSP90 in
primary OSCC may promote invasiveness, motility and metastasis.
Further studies with a large sample size will contribute to the
development of strategies for the diagnosis, prevention and
treatment of this neoplasm.
Acknowledgements
Not applicable.
Funding
The present study was supported by JSPS KAKENHI
(grant no. 16K11701 and 19K10366).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NS, TO and TS were involved in the conception and
design of the present study. NS and TO performed experiments,
analyzed data and drafted the manuscript. KH, KO, KW and SS
interpreted the data and assisted in manuscript preparation. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Tokyo Dental College (approval no. 709), and
written informed consent was obtained from all patients
involved.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prestwich R, Dyker K and Sen M: Improving
the therapeutic ratio in head and neck cancer. Lancet Oncol.
11:512–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, et al: The mutational landscape of head and neck
squamous cell carcinoma. Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Srinivas PR, Verma M, Zhao Y and
Srivastava S: Proteomics for cancer biomarker discovery. Clin Chem.
48:1160–1169. 2002.PubMed/NCBI
|
|
5
|
Dabbous MK, Jefferson MM, Haney L and
Thomas EL: Biomarkers of metastatic potential in cultured
adenocarcinoma clones. Clin Exp Metastasis. 28:101–111. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poli G, Ceni E, Armignacco R, Ercolino T,
Canu L, Baroni G, Nesi G, Galli A, Mannelli M and Luconi M: 2D-DIGE
proteomic analysis identifies new potential therapeutic targets for
adrenocortical carcinoma. Oncotarget. 6:5695–5706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merkley MA, Weinberger PM, Jackson LL,
Podolsky RH, Lee JR and Dynan WS: 2D-DIGE proteomic
characterization of head and neck squamous cell carcinoma.
Otolaryngol Head Neck Surg. 141:626–632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bijian K, Mlynarek AM, Balys RL, Jie S, Xu
Y, Hier MP, Black MJ, Di Falco MR, LaBoissiere S and Alaoui-Jamali
MA: Serum proteomic approach for the identification of serum
biomarkers contributed by oral squamous cell carcinoma and host
tissue microenvironment. J Proteome Res. 8:2173–2185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solit DB and Rosen N: Hsp90: A novel
target for cancer therapy. Curr Top Med Chem. 6:1205–1214. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trepel J, Mollapour M, Giaccone G and
Neckers L: Targeting the dynamic HSP90 complex in cancer. Nat Rev
Cancer. 10:537–549. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taldone T, Gozman A, Maharaj R and Chiosis
G: Targeting Hsp90: Small-molecule inhibitors and their clinical
development. Curr Opin Pharmacol. 8:370–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neckers L and Workman P: Hsp90 molecular
chaperone inhibitors: Are we there yet? Clin Cancer Res. 18:64–76.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao M, Sano D, Pickering CR, Jasser SA,
Henderson YC, Clayman GL, Sturgis EM, Ow TJ, Lotan R, Carey TE, et
al: Assembly and initial characterization of a panel of 85
genomically validated cell lines from diverse head and neck tumor
sites. Clin Cancer Res. 17:7248–7264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu M, Selvaraj SK, Liang-Chu MM, Aghajani
S, Busse M, Yuan J, Lee G, Peale F, Klijn C, Bourgon R, et al: A
resource for cell line authentication, annotation and quality
control. Nature. 520:307–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koike H, Uzawa K, Nakashima D, Shimada K,
Kato Y, Higo M, Kouzu Y, Endo Y, Kasamatsu A and Tanzawa H:
Identification of differentially expressed proteins in oral
squamous cell carcinoma using a global proteomic approach. Int J
Oncol. 27:59–67. 2005.PubMed/NCBI
|
|
17
|
Onda T, Uzawa K, Nakashima D, Saito K,
Iwadate Y, Seki N, Shibahara T and Tanzawa H: Lin-7C/VELI3/MALS-3:
An essential component in metastasis of human squamous cell
carcinoma. Cancer Res. 67:9643–9648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishikawa H, Ooka S, Sato K, Arima K,
Okamoto J, Klevit RE, Fukuda M and Ohta T: Mass spectrometric and
mutational analyses reveal Lys-6-linked polyubiquitin chains
catalyzed by BRCA1-BARD1 ubiquitin ligase. J Biol Chem.
279:3916–3924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dyrlund TF, Poulsen ET, Scavenius C,
Sanggaard KW and Enghild JJ: MS Data Miner: A web-based software
tool to analyze, compare, and share mass spectrometry protein
identifications. Proteomics. 12:2792–2796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao P, Ren Y, Tam JP and Sze SK:
Correction of errors in tandem mass spectrum extraction enhances
phosphopeptide identification. J Proteome Res. 12:5548–5557. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav R and Srivastava P: Clustering,
Pathway Enrichment, and Protein-Protein Interaction Analysis of
Gene Expression in Neurodevelopmental Disorders. Adv Pharmacol Sci.
2018:36321592018.PubMed/NCBI
|
|
22
|
Lu J, Tao YF, Li ZH, Cao L, Hu SY, Wang
NN, Du XJ, Sun LC, Zhao WL, Xiao PF, et al: Analyzing the gene
expression profile of anaplastic histology Wilms' tumor with
real-time polymerase chain reaction arrays. Cancer Cell Int.
15:442015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato H, Ishida S, Toda K, Matsuda R,
Hayashi Y, Shigetaka M, Fukuda M, Wakamatsu Y and Itai A: New
approaches to mechanism analysis for drug discovery using DNA
microarray data combined with KeyMolnet. Curr Drug Discov Technol.
2:89–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satoh J, Tabunoki H and Arima K: Molecular
network analysis suggests aberrant CREB-mediated gene regulation in
the Alzheimer disease hippocampus. Dis Markers. 27:239–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber H, Valbuena JR, Barbhuiya MA, Stein
S, Kunkel H, García P, Bizama C, Riquelme I, Espinoza JA, Kurtz SE,
et al: Small molecule inhibitor screening identifified HSP90
inhibitor 17-AAG as potential therapeutic agent for gallbladder
cancer. Oncotarget. 8:26169–26184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klameth L, Rath B and Hamilton G: In vitro
Cytotoxic Activities of the Oral Platinum(IV) Prodrug Oxoplatin and
HSP90 Inhibitor Ganetespib against a Panel of Gastric Cancer Cell
Lines. J Cancer. 8:1733–1743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sekikawa S, Onda T, Miura N, Nomura T,
Takano N, Shibahara T and Honda K: Underexpression of
α-1-microglobulin/bikunin precursor predicts a poor prognosis in
oral squamous cell carcinoma. Int J Oncol. 53:2605–2614.
2018.PubMed/NCBI
|
|
28
|
Islam F, Gopalan V, Law S, Tang JC and Lam
AK: FAM134B promotes esophageal squamous cell carcinoma in vitro
and its correlations with clinicopathologic features. Hum Pathol.
87:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tricarico PM, Zupin L, Ottaviani G, Pacor
S, Jean-Louis F, Boniotto M and Crovella S: Photobiomodulation
therapy promotes in vitro wound healing in nicastrin KO HaCaT
cells. J Biophotonics. 11:e2018001742018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogane S, Onda T, Takano N, Yajima T,
Uchiyama T and Shibahara T: Spleen tyrosine kinase as a novel
candidate tumor suppressor gene for human oral squamous cell
carcinoma. Int J Cancer. 124:2651–2657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pindborg JJ, Reichart PA, Smith CJ and
Waal IV: Histological typing of cancer and precancer of the oral
mucosa. International Histological Classification of Tumours. 2nd
edition. World Health Organization; Geneva: pp. 1–83. 1997
|
|
32
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Singapore: pp. 22–58. 2009
|
|
33
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herrmann PC, Liotta LA and Petricoin EF
III: Cancer proteomics: The state of the art. Dis Markers.
17:49–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johann DJ Jr, McGuigan MD, Patel AR, Tomov
S, Ross S, Conrads TP, Veenstra TD, Fishman DA, Whiteley GR,
Petricoin EF III, et al: Clinical proteomics and biomarker
discovery. Ann N Y Acad Sci. 1022:295–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang T, Hamza A, Cao X, Wang B, Yu S,
Zhan CG and Sun D: A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37
complex against pancreatic cancer cells. Mol Cancer Ther.
7:162–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thiel UJ, Feltens R, Adryan B, Gieringer
R, Brochhausen C, Schuon R, Fillies T, Grus F, Mann WJ and Brieger
J: Analysis of differentially expressed proteins in oral squamous
cell carcinoma by MALDI-TOF MS. J Oral Pathol Med. 40:369–379.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chanthammachat P, Promwikorn W,
Pruegsanusak K, Roytrakul S, Srisomsap C, Chokchaichamnankit D,
Svasti J, Boonyaphiphat P, Singkhamanan K and Thongsuksai P:
Comparative proteomic analysis of oral squamous cell carcinoma and
adjacent non-tumour tissue from Thailand. Arch Oral Biol.
58:1677–1685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Workman P, Burrows F, Neckers L and Rosen
N: Drugging the cancer chaperone HSP90: Combinatorial therapeutic
exploitation of oncogene addiction and tumor stress. Ann N Y Acad
Sci. 1113:202–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCarthy MM, Pick E, Kluger Y,
Gould-Rothberg B, Lazova R, Camp RL, Rimm DL and Kluger HM: HSP90
as a marker of progression in melanoma. Ann Oncol. 19:590–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin P, Yi Y, Lu M, Wang M, Yang Y, Lu Y,
Song S, Zheng Z, Deng X and Zhang L: Heat shock protein 90
inhibitor mycoepoxydiene modulates kinase signaling in cervical
cancer cells and inhibits in-vivo tumor growth. Anticancer Drugs.
26:25–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaplan KB and Li R: A prescription for
‘stress’ - the role of Hsp90 in genome stability and cellular
adaptation. Trends Cell Biol. 22:576–583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Azoitei N, Diepold K, Brunner C, Rouhi A,
Genze F, Becher A, Kestler H, van Lint J, Chiosis G, Koren J III,
et al: HSP90 supports tumor growth and angiogenesis through PRKD2
protein stabilization. Cancer Res. 74:7125–7136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kamal A and Burrows FJ: Hsp90 inhibitors
as selective anticancer drugs. Discov Med. 4:277–280.
2004.PubMed/NCBI
|
|
45
|
Jhaveri K, Miller K, Rosen L, Schneider B,
Chap L, Hannah A, Zhong Z, Ma W, Hudis C and Modi S: A phase I
dose-escalation trial of trastuzumab and alvespimycin hydrochloride
(KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin
Cancer Res. 18:5090–5098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eiseman JL, Lan J, Lagattuta TF, Hamburger
DR, Joseph E, Covey JM and Egorin MJ: Pharmacokinetics and
pharmacodynamics of 17-demethoxy
17-[[(2-dimethylamino)ethyl]amino]geldanamycin (17DMAG, NSC 707545)
in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer
xenografts. Cancer Chemother Pharmacol. 55:21–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pacey S, Wilson RH, Walton M, Eatock MM,
Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U,
Roels B, et al: A phase I study of the heat shock protein 90
inhibitor alvespimycin (17-DMAG) given intravenously to patients
with advanced solid tumors. Clin Cancer Res. 17:1561–1570. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu H, Xiao F, Serebriiskii IG, O'Brien
SW, Maglaty MA, Astsaturov I, Litwin S, Martin LP, Proia DA,
Golemis EA, et al: Network analysis identifies an HSP90-central hub
susceptible in ovarian cancer. Clin Cancer Res. 19:5053–5067. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Proia DA, Zhang C, Sequeira M, Jimenez JP,
He S, Spector N, Shapiro GI, Tolaney S, Nagai M, Acquaviva J, et
al: Preclinical activity profile and therapeutic efficacy of the
HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin
Cancer Res. 20:413–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Socinski MA, Goldman J, El-Hariry I,
Koczywas M, Vukovic V, Horn L, Paschold E, Salgia R, West H,
Sequist LV, et al: A multicenter phase II study of ganetespib
monotherapy in patients with genotypically defined advanced
non-small cell lung cancer. Clin Cancer Res. 19:3068–3077. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Powers MV, Valenti M, Miranda S, Maloney
A, Eccles SA, Thomas G, Clarke PA and Workman P: Mode of cell death
induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent
on the expression of pro-apoptotic BAX. Oncotarget. 4:1963–1975.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hadley KE and Hendricks DT: Use of NQO1
status as a selective biomarker for oesophageal squamous cell
carcinomas with greater sensitivity to 17-AAG. BMC Cancer.
14:3342014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shintani S, Zhang T, Aslam A, Sebastian K,
Yoshimura T and Hamakawa H: P53-dependent radiosensitizing effects
of Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin on human
oral squamous cell carcinoma cell lines. Int J Oncol. 29:1111–1117.
2006.PubMed/NCBI
|
|
54
|
Lin SF, Lin JD, Hsueh C, Chou TC, Yeh CN,
Chen MH and Wong RJ: Efficacy of an HSP90 inhibitor, ganetespib, in
preclinical thyroid cancer models. Oncotarget. 8:41294–41304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ghadban T, Jessen A, Reeh M, Dibbern JL,
Mahner S, Mueller V, Wellner UF, Güngör C, Izbicki JR and Vashist
YK: In vitro study comparing the efficacy of the water-soluble
HSP90 inhibitors, 17-AEPGA and 17-DMAG, with that of the non
water-soluble HSP90 inhibitor, 17-AAG, in breast cancer cell lines.
Int J Mol Med. 38:1296–1302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Westerheide SD and Morimoto RI: Heat shock
response modulators as therapeutic tools for diseases of protein
conformation. J Biol Chem. 280:33097–33100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bagatell R, Paine-Murrieta GD, Taylor CW,
Pulcini EJ, Akinaga S, Benjamin IJ and Whitesell L: Induction of a
heat shock factor 1-dependent stress response alters the cytotoxic
activity of hsp90-binding agents. Clin Cancer Res. 6:3312–3318.
2000.PubMed/NCBI
|
|
58
|
Guo Y, Guettouche T, Fenna M, Boellmann F,
Pratt WB, Toft DO, Smith DF and Voellmy R: Evidence for a mechanism
of repression of heat shock factor 1 transcriptional activity by a
multichaperone complex. J Biol Chem. 276:45791–45799. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Musha A, Yoshida Y, Takahashi T, Ando K,
Funayama T, Kobayashi Y, Negishi A, Yokoo S and Nakano T:
Synergistic effect of heat shock protein 90 inhibitor,
17-allylamino-17-demethoxygeldanamycin and X-rays, but not
carbon-ion beams, on lethality in human oral squamous cell
carcinoma cells. J Radiat Res (Tokyo). 53:545–550. 2012. View Article : Google Scholar
|
|
60
|
Chang WC, Tsai PT, Lin CK, Shieh YS and
Chen YW: Expression pattern of heat shock protein 90 in patients
with oral squamous cell carcinoma in northern Taiwan. Br J Oral
Maxillofac Surg. 55:281–286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ono K, Eguchi T, Sogawa C, Calderwood SK,
Futagawa J, Kasai T, Seno M, Okamoto K, Sasaki A and Kozaki KI:
HSP-enriched properties of extracellular vesicles involve survival
of metastatic oral cancer cells. J Cell Biochem. 119:7350–7362.
2018. View Article : Google Scholar : PubMed/NCBI
|