Introduction
Based on literary data, breast cancer comprises 30%
of all diagnosed carcinomas and is the second leading cause of
cancer-related mortality among women (1). A total of 208,8849 new cases of breast
cancer and 626,679 mortalities due to breast cancer occurred
worldwide in 2018 (2), and it has
been reported that there will be 279,100 new breast cancer cases,
as well as 42,690 mortalities due to breast cancer in 2020 in the
United States (3). Despite great
progress in improving the survival rates of patients with breast
cancer in recent years, breast cancer, particularly
‘triple-negative’ breast cancer (TNBC), remains a threat to the
health of women. TNBC, which is characterized by absent or low
expression of estrogen receptor (ER), progesterone receptor (PR) or
human epidermal growth factor receptor 2 (HER2), exhibits intensive
invasion, high metastasis and poor prognosis (4). Compared with patients without TNBC,
patients with advanced TNBC experience an aggressive clinical
outcome with a poor prognosis and high metastasis to distant organs
including the lung, liver, brain and lymphatic nodes (5). Distant metastasis, which has become
the leading obstacle to breast cancer therapy, accounts for the
majority of breast cancer-related deaths (6). However, there are currently few
effective methods to treat the metastasis and recurrence of breast
cancer, and thus it is urgent to develop novel therapeutic agents
for breast cancer.
It is well known that various signaling pathways are
involved in breast cancer progression and metastasis, including
NF-κB (6,7). NF-κB, which was defined as a
DNA-binding protein in 1986, is widely involved in various human
disorders including inflammatory diseases, viral infection and
metabolic disorders, as well as cell proliferation and oxidative
stress (9–12). With regard to breast cancer,
dysregulated activation of NF-κB is involved in the regulation of
cell proliferation, differentiation, apoptosis, angiogenesis and
metastasis (13–16). Epithelial-mesenchymal transition
(EMT), which is critical to primary metastasis of breast cancer, is
regulated by the NF-κB signaling pathway (17). Moreover, previous studies have
revealed that blockade of abnormally activated NF-κB using
inhibitors could induce apoptosis and suppress metastasis in breast
cancer cells (18–20). Thus, inhibition of NF-κB offers a
potential strategy to the therapy of breast cancer. While increased
effort has been made in the discovery of potent NF-κB inhibitors
and numerous inhibitors targeting NF-κB have been reported, to
date, few NF-κB inhibitor drugs have been approved by the Food and
Drug Administration. Therefore, it is urgent to develop novel NF-κB
inhibitors for the treatment of breast cancer.
Chlorogenic acid (CGA), a polyphenol compound that
is abundant in the human diet, such as coffee, possesses multiple
biological activities, including anticarcinogenic, antibacterial,
anticancer and antioxidant effects (21–24).
CGA has been reported to be non-toxic and safe in animals and
humans (25). Accumulating evidence
has revealed that CGA suppresses migration and invasion, and
induces apoptosis in numerous cancer cell lines including colon,
breast and lung (25, 26). For instance, Feng et al (27) demonstrated that CGA inhibited lung
cancer cell proliferation by suppressing the NF-κB and MAPK
signaling pathways. Furthermore, Kang et al (28) revealed that CGA derived from coffee
impaired colorectal cancer cell metastasis by suppressing NF-κB,
MEK and T-LAK cell-originated protein kinase. Additional studies
indicated that CGA could attenuate LPS-induced acute kidney injury,
protect cardiomyocytes and ameliorate lead-induced renal damage via
suppressing the NF-κB signaling pathway (29–31).
Considering the important role of NF-κB in breast cancer, it was
hypothesized that CGA, a potent NF-κB signaling inhibitor, may be a
potential drug for clinical therapy of breast cancer.
The present study aimed to investigate the role of
CGA in proliferation, apoptosis, migration and invasion in breast
cancer cell lines. Moreover, two mouse models of breast cancer were
established to further evaluate the therapeutic activity of CGA. It
was hypothesized that CGA could slow tumor growth and suppress
pulmonary metastasis by impairing the NF-κB signaling pathway.
Materials and methods
Materials and reagents
CGA (Fig. 1A)
(purity, >98%, as measured by high-performance liquid
chromatograph analysis; purchased from J&K Scientific, Ltd.)
was made into a stock solution (40 mM) by dissolving in DMSO, and
was stored at −20°C for further use. The medium containing 0.1%
DMSO served as the control.
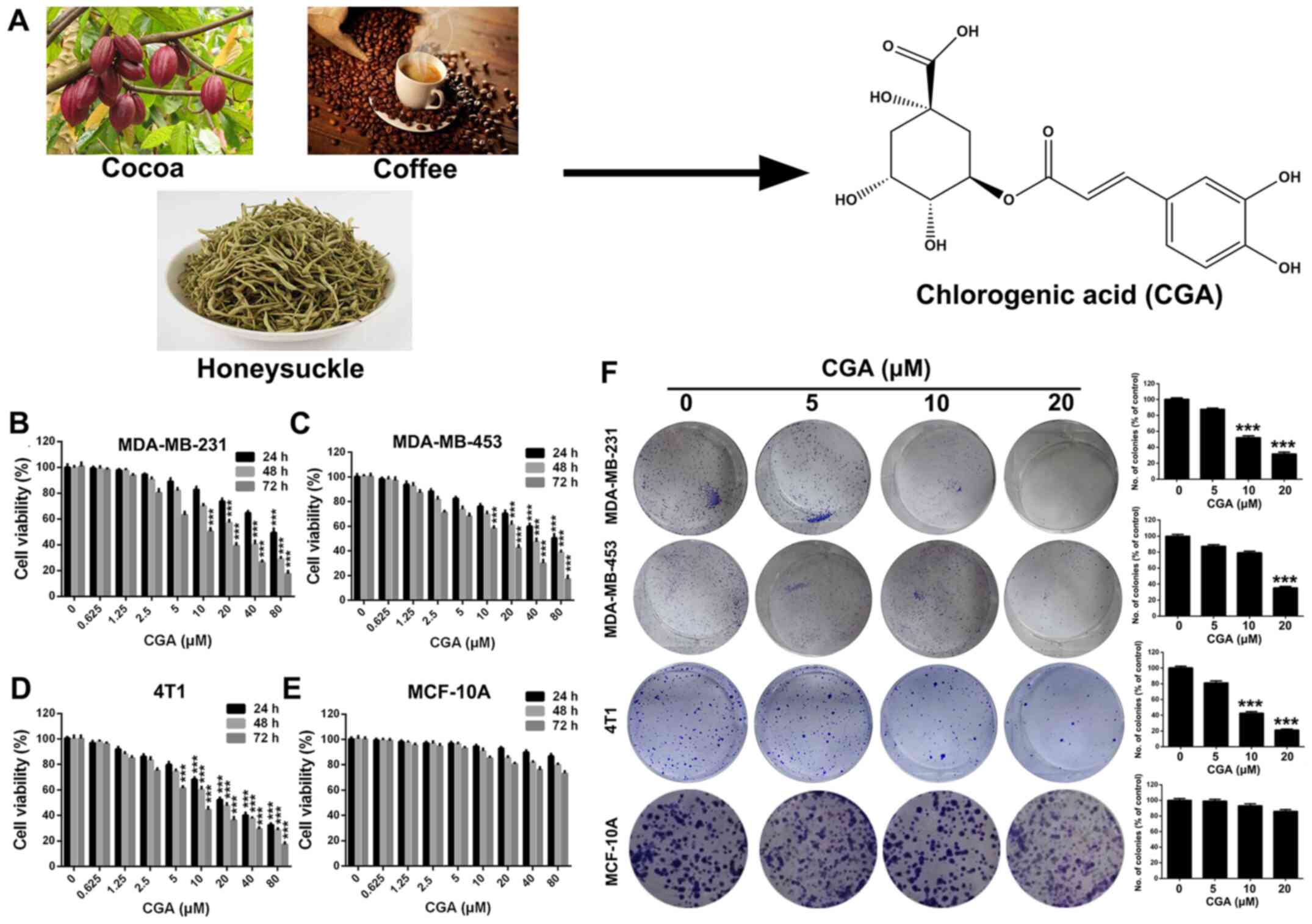 | Figure 1.CGA suppresses the viability of
breast cancer cell lines. (A) Natural sources and chemical
structure of CGA. (B-D) Viabilities of breast cancer cells line of
MDA-MB-231, MDA-MB-453 and 4T1 were measured by MTT assay after
treatment with various concentrations of CGA (0, 0.625, 1.25, 2.5,
5, 10, 20, 40 and 80 µM) for various time-points (24, 48 and 72 h).
(E) Viability of human mammary epithelial cells MCF-10A was
measured by MTT assay after treatment with various concentrations
of CGA for various time-points. (F) Abilities of colony formation
of MDA-MB-231, MDA-MB-453, 4T1 and MCF-10A cells were measured
after treatment with various concentrations of CGA (0, 5, 10 and 20
µM). Bars represent the means ± SD of at least three independent
experiments. ***P<0.001 in comparison with the control group.
CGA, chlorogenic acid. |
MTT and Hoechst 33258 were purchased from
Sigma-Aldrich (Merck KGaA), while the Annexin V-FITC apoptosis
detection kit was obtained from 4A Biotech Co., Ltd. The mouse
monoclonal antibody of Ki-67 (dilution: 1:500, cat. no. SAB5300423)
was purchased from EMD Millipore. Primary antibodies against
cleaved caspase-3 (product no. 9661; dilution 1:1,000), NF-κB p65
(product no. 8242; dilution 1:1,000), phosphorylated (p)-IκBα
(product no. 2859; dilution 1:1,000), N-cadherin (product no.
13116; dilution 1:1,000), E-cadherin (product no. 14472; dilution
1:1,000) and β-actin (product no. 3700; dilution 1:1,000) used for
western blotting were obtained from Cell Signaling Technology,
Inc.
Cell lines and cell culture
The human breast cancer cell lines MDA-MB-231 and
MDA-MB-453, human mammary epithelial cells MCF-10A and the murine
breast cancer cell line 4T1 were purchased from the American Type
Culture Collection. All cells were cultured in DMEM or RPMI-1640
media (Gibco; Thermo Fisher Scientific, Inc.) containing 10%
heat-inactivated FBS (Hyclone; Cytiva) and 1% antibiotics
(penicillin and streptomycin) in 5% CO2 at 37°C.
Cell viability
Cells were seeded into 96-well plates at a density
of 2-6×103 cells/well and cultured for 24 h. Then, cells
were treated with various concentrations (0, 0.625, 1.25, 2.5, 5,
10, 20, 40 and 80 µM) of CGA for 24, 48 and 72 h. Subsequently, 20
µl MTT solution (5 mg/ml) was added to each well and incubated for
another 2-4 h at 37°C. The culture medium was replaced with 150 µl
DMSO, which is used to dissolve formazan secreted by living cells.
A Spectra MAX M5 microplate spectrophotometer (Molecular Devices,
LLC) was used to measure absorbance (570 nm) of each well. The data
are obtained from ≥3 independent experiments.
Colony formation assay
Briefly, cells were seeded at a determined number
(300-500 cells/well) in 6-well plates and exposed to various
concentrations (0, 5, 10 and 20 µM) of CGA for ~12 days. The medium
was replaced with fresh culture medium with or without CGA every 3
days. Cells were washed gently with cold PBS, fixed with methanol
for 20 min and stained with crystal violet solution (0.5%, m/v) for
25 min at room temperature. Finally, the colonies (number of cells,
>50) were imaged and counted using a florescence microscope
(magnification, ×10; Olympus Corporation).
Morphological analysis via Hoechst
staining
Cells (1×105 cells/well) were seeded into
6-well plates and incubated with various concentrations (0, 5, 10
and 20 µM) of CGA for 24 h, followed by staining with Hoechst 33258
dye solution for 10 min at 25°C based on the manufacturer's
instructions. Then, the nuclear morphologies of treated cells were
observed and imaged with a fluorescence microscope (magnification,
×100; Olympus Corporation).
Cell apoptosis
Cells (1×105 cells/well) were seeded into
6-well plates and incubated with various concentrations (0, 5, 10
and 20 µM) of CGA for 24 h. Then, cells were harvested, washed with
cold PBS three times and incubated with Annexin V/PI dual labeling
kit (4A Biotech Co., Ltd.) according to the manufacturer's
instructions. Finally, apoptotic cells were detected via flow
cytometry (BD Biosciences). The data was analyzed by FlowJo 7.6
software (Tree Star, Inc.).
Wound-healing assay
Cells (1×105 cells/well) were seeded into
6-well plates and were scraped with a sterile 100-µl pipette tip
when cell confluence reached 80-90%. Then, the cultured medium was
replaced with fresh medium (0.5% FBS) containing determined
concentrations (0, 5, 10 and 20 µM) of CGA. After treatment with
CGA for 24 h, the cells were washed with cold PBS and imaged using
a microscope (Olympus Corporation).
Body chamber invasion assay
Briefly, 1×105 4T1 or 5×106
MDA-MB-231 cells, which were suspended in 100 µl FBS-free culture
medium, were added in the upper chamber that was precoated with
Martrigel. The lower chamber was filled with 600 µl cultured medium
that contained 10% FBS. Various concentrations (0, 5, 10 and 20 µM)
of CGA were added in the upper chamber medium. After 48 h, invasive
cells located on the downside of the filter were washed with cold
PBS, fixed in methanol for 15 min and incubated with crystal violet
(0.5%, m/v) for 20 min at room temperature. Finally, invasive cells
located on the membrane were imaged and counted with a microscope
(Olympus Corporation).
Western blot analysis
Cells with various treatments (0, 5, 10 and 20 µM of
CGA) were harvested, washed with cold PBS for three times and lysed
with RIPA buffer (Beyotime Institute of Biotechnology).
Concentration of protein was determined by BCA method and the OD
value of protein was measured by Spectra MAX M5 microplate
spectrophotometer (Molecular Devices, LLC). Then, equal amounts of
proteins (30-50 µg) of each sample were separated via SDS-PAGE
(10%, w/v), and the separated proteins on the gel were transferred
onto PVDF membranes. Subsequently, membranes containing targeted
proteins were blocked with non-fat milk for 1 h at 37°C and treated
with specific primary antibodies overnight at 4°C. After incubation
with the corresponding horseradish peroxidase-conjugated secondary
antibodies (cat. no. BA1056; dilution 1:20,000; Wuhan Boster
Biological Technology, Ltd.) for 1 h at 37°C, protein bands were
visualized with an enhanced chemiluminescence kit (EMD
Millipore).
Immunofluorescence analysis
Cells were cultured on circular glasses in 24-well
plates and underwent different treatment regimes. Cells were fixed
with 4% paraformaldehyde at 25°C for 10 min, treated with
permeabilization solution (1% Triton X-100) for 15 min, washed with
PBS three times and blocked with BSA (5%, m/v; Biological
Technology Co., Ltd.) at room temperature for 1 h. After being
treated with corresponding primary antibodies (NF-κB p65; product
no. 8242; dilution 1:500; Cell Signaling Technology, Inc.)
overnight at 4°C, cells were washed gently with cold PBS three
times and incubated with FITC-conjugated goat anti-rabbit IgG
secondary antibody (product no. A0562; 1:500; Beyotime Institute of
Biotechnology) at room temperature for 1 h. Cells were stained with
DAPI (dilution 1:10,000) at 25°C for 10 min and imaged via Laser
Scanning Confocal Microscopy (Leica Microsystems GmbH).
In vivo antitumor assessment
Animal experiments in the present study were
approved by the Ethics Committee of Chengdu University of
Traditional Chinese. Mice were kept in a specific-pathogen-free
(SPF) condition facility with an air-conditioned room at 25±2°C
with a relative humidity of 40-70%, and a 12-h light/dark cycle.
Twenty-seven female BALB/c mice (age, 6-8 weeks; weight, 18-20 g)
were purchased from Beijing HFK Bioscience Co., Ltd. 4T1 cells
(1×106 cells per mouse) suspended in culture medium with
no FBS and antibodies were subcutaneously injected into the right
flank of every BALB/c mice. When the tumor volume reached ~100
mm3, tumor-bearing mice were randomly divided into three
groups (control group, 20 and 40 mg/kg; n=9), and intraperitoneally
administered different doses of CGA every 2 days. The tumor volume
was calculated according to the following formula: V = 0.5 ×
LW2, where L represents the length of the tumors and L
represents the width of the tumors. At the termination of the
experiment (18 days after CGA treatment), three mice from each
group were euthanized via cervical dislocation, while the remaining
tumor-bearing mice were maintained for an additional time period to
record the survival rate. Tumors isolated from mice in the various
treated groups were imaged, weighed and fixed with paraformaldehyde
(4%, w/v) for 24 h at 25°C for further immunohistochemistry
evaluation. For immunohistochemistry, the frozen (−20°C) 4-µm-thick
sections were incubated with primary rabbit anti-mouse antibodies
Ki-67 (product no. 9449; dilution 1:500), cleaved caspase-3
(product no. 9661; dilution 1:500), PD-L1 (product no. 13684;
dilution 1:200), N-cadherin (product no. 13116; dilution 1:500) and
p-IκBα (product no. 2859; dilution 1:200) at 25°C for 1 h, blocked
with goat serum (10% in PBS; Beyotime Institute of Biotechnology)
at 25°C for 15 min, and subsequently treated with biotinylated goat
anti-rabbit immunoglobulin secondary antibody (dilution 1:1,000;
cat. no. ab6721; Abcam) at 37°C for 30 min. Finally, sections were
incubated with streptavidin-peroxidase and DAB solution (Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C for 20 min to
visualize the biotinylated goat anti-rabbit immunoglobulin. Organs
(heart, liver, spleen, lung and kidney) were isolated from mice in
different groups and were fixed with paraformaldehyde (4%, w/v) for
24 h at 25°C for further histopathological analysis. Sections
(4-µm-thick) were stained with hematoxylin (1%) and eosin (1%) at
25°C for 2-5 min. Finally, the sections were visualized by light
microscope.
Anti-pulmonary metastasis
evaluation
For anti-pulmonary metastasis evaluation, 4T1 cells
(5×105 cells per mouse) suspended in culture medium with
no FBS and antibodies were intravenously injected into mice. Then,
2 days after inoculation, mice were randomly divided into three
groups (control group, 20 and 40 mg/kg; n=3), and intraperitoneally
administered different doses of CGA every 2 days. After treatment
with various doses of CGA for 14 days, the mice from each group
were euthanized via cervical dislocation. Lung tissues were
isolated, weighted and imaged. Metastatic nodules (>3 and <3
mm3) on lung tissues from each group were counted.
Finally, lung tissues were fixed with paraformaldehyde (4%, w/v)
for 24 h at 25°C for histopathological analysis. Sections
(4-μm-thick) were stained with hematoxylin (1%) and eosin (1%) at
25°C for 2-5 min. Finally, sections were visualized by light
microscope.
To investigate the effect of CGA on antitumor
immunity, single-cell suspensions of spleens from various treated
groups were prepared and stained with various antibodies CD3 (cat.
no. 100236), CD4 (cat. no. 100406) and CD8 (cat. no. 100707; all
from BioLegend, Inc.) at 25°C for 30 min to analyze CD4+
T cells and CD8+ T cells via flow cytometric analysis
(FCM).
Statistical analysis
Data are presented as the mean ± SEM of three
independent experiments. GraphPad Prism 5 (GraphPad Software, Inc.)
was used to analyze data in the present study. One-way ANOVA
followed by Dunnett's post hoc test or Tukey's post hoc test were
used for multi-group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
CGA inhibits the proliferation of
breast cancer cell
In order to evaluate the cytotoxicity of CGA in
breast cancer, MDA-MB-231, MDA-MB-453 and 4T1 cells were treated
with various concentrations of CGA and cell viability was measured
using an MTT assay. The viabilities of MDA-MB-231, MDA-MB-453 and
4T1 cells were significantly impaired by treatment with CGA
(Fig. 1B-D). Additionally, CGA
exhibited a notably dose- and time-dependent toxic effect on breast
cancer cells (Fig. 1E). However,
CGA had almost no influence on viability of human mammary
epithelial cells (MCF-10A), suggesting that CGA could selectively
cause toxicity in breast cancer cells.
A colony formation assay was conducted to further
investigate the anti-proliferation effect of CGA on breast cancer.
The colony formation ability of MDA-MB-231, MDA-MB-453 and 4T1
cells was significantly inhibited after treatment with various
concentrations of CGA (Fig. 2F).
Furthermore, the colony formation ability of MCF-10A cells was
unaffected by treatment of CGA, which was consistent with the
results of the MTT assay.
CGA induces breast cancer cells
apoptosis
To examine whether the cytotoxicity of CGA in breast
cancer resulted from cell apoptosis, Hoechst 33258 staining was
conducted to detect the apoptotic induction ability of CGA.
Treatment with CGA significantly changed the morphologies of
MDA-MB-231 and 4T1 cells (Fig. 2A).
Additionally, bright blue fluorescent condensed nuclei and nuclear
fragmentations were observed in MDA-MB-231 and 4T1 cells after
various treatments with CGA.
To further confirm the apoptotic induction ability
of CGA, an Annexin V/PI dual staining assay was performed to detect
the apoptotic rate in breast cancer cells. Compared with the
control group (1.27±0.98%), the apoptotic rate in MDA-MB-231 cells
increased from 4.74±0.95 to 15.10±1.26% (P<0.001) when the
concentration of CGA was increased from 5 to 20 µM (Fig. 2B). Similarly, treatment with 20 µM
CGA induced significant apoptosis (22.65±1.79%; P<0.001) in 4T1
cells in comparison with the control group (11.00±1.25%).
CGA suppresses the migration and
invasion in breast cancer via the NF-κB/EMT signaling pathway
Migration and invasion of tumor cells are key
processes for the successful metastasis from primary tumor sites to
distant organs (32). Therefore, a
wound-healing assay was performed to evaluate the anti-migratory
effect of CGA in breast cancer cells. The migratory abilities of
MDA-MB-231 and 4T1 cells were significantly (P<0.001) suppressed
by treatment with CGA, in comparison with the control group
(Fig. 3A). Moreover, a Transwell
invasion assay was conducted to assess the anti-invasive ability of
CGA in breast cancer cells. Compared with the control group, CGA
significantly (P<0.001) inhibited the invasive ability in both
MDA-MB-231 and 4T1 cells (Fig. 3B),
thereby demonstrating the anti-migration and anti-invasion effects
of CGA in breast cancer cells.
To investigate the intrinsic anti-migration and
anti-invasion mechanism of CGA in breast cancer, the expression
levels of NF-κB signaling pathway-related proteins were detected
after treatment with various concentrations of CGA via western
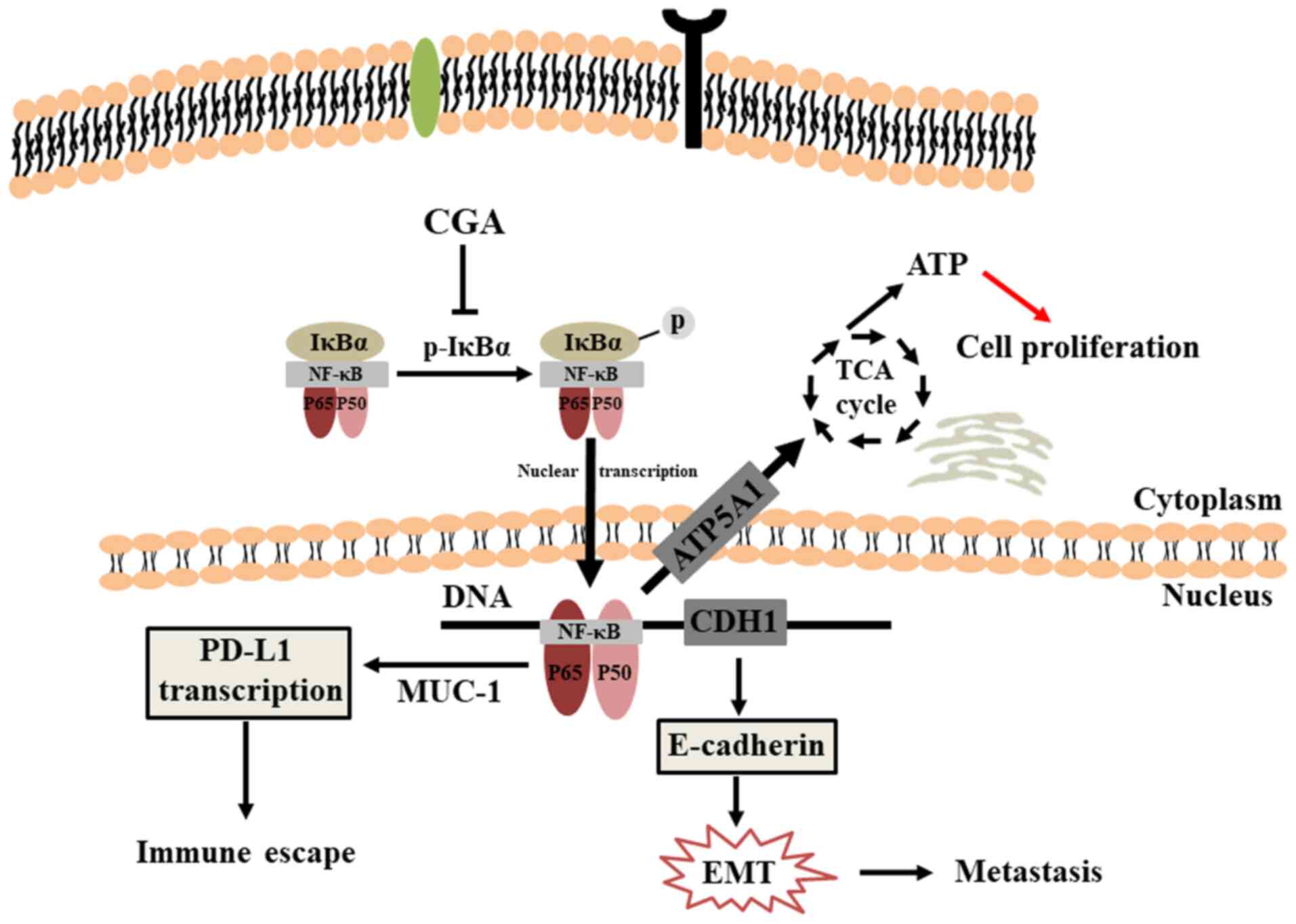
blotting. The expression levels of NF-κB p65 and p-IκBα in both
MDA-MB-231 and 4T1 cells were significantly downregulated after
various treatments with of CGA (Fig.
4A), indicating that the NF-κB signaling pathway participated
the in anti-migration and anti-invasion effects of CGA in breast
cancer cells. Additionally, the expression of N-cadherin was
downregulated, while the expression of E-cadherin in both
MDA-MB-231 and 4T1 cells was upregulated, suggesting an impairment
of the EMT process in breast cancer.
The present study also investigated the
translocation of NF-κB p65 after treatment with CGA in MDA-MB-231
cells. NF-κB p65 was located in both the cytoplasm and nucleus,
suggesting constitutive activation of NF-κB in MDA-MB-231 cells
(Fig. 4B). After treatment with
TNF-α, NF-κB p65 protein was markedly transported into nuclei of
MDA-MB-231 cells. CGA could significantly attenuate the nuclear
translocation of NF-κB p65 protein that was triggered by treatment
with TNF-α. Collectively, CGA significantly inhibited breast cancer
cell migration and invasion by impairing the NF-κB/EMT signaling
pathway.
CGA exerts an antitumor effect in a
tumor mouse model
To investigate the antitumor effect of CGA in vivo,
a xenograft tumor mouse model of 4T1 cells was established to
assess the tumor inhibitory effect of CGA. After treatment with 20
or 40 mg/kg of CGA, the tumor growth rate was significantly slowed
in comparison with the control group (Fig. 5A). At the end point of the animal
experiment, tumors of each group were isolated. Treatment with CGA
significantly (P<0.001) diminished the volume and weight of
tumors in comparison with the control group (Fig. 5B and C). Moreover, compared with the
control group, the survival rate of tumor-bearing mice was
significantly prolonged after treatment with various concentrations
of CGA (Fig. 5D).
Immunohistochemistry was conducted to further
evaluate the antitumor mechanism of CGA. The number of
proliferative cells, which were defined as Ki67-positive, in the
tumor sections of the CGA-treated groups were significantly lower
compared with that of the control group, and the expression level
of cleaved caspase-3 in the CGA-treated tumor sections was
upregulated (Fig. 5E). In addition,
CGA could downregulate the expression level of PD-L1 in the tumor
sections, implying improvement in the tumor immunosuppressive
microenvironment. Furthermore, compared with the control group, the
expression of p-IκBα and N-cadherin, which are important in the
NF-κB and EMT signaling pathways respectively, were both
significantly downregulated in tumor sections after treatment with
CGA. The major organs of various treated groups exhibited no
pathological changes (Fig. 5F),
which demonstrated that CGA had no organ toxicity. Collectively, it
was indicated that CGA suppressed breast cancer proliferation and
induced tumor cell apoptosis via impairing the NF-κB signaling
pathway.
CGA suppresses the pulmonary
metastasis of breast cancer by enhancing antitumor immunity
To further confirm whether CGA, which exhibited
efficient anti-migration and anti-invasion abilities in breast
cancer cells, had an anti-metastasis effect in vivo, a lung
metastatic model of 4T1 cells was used to assess anti-pulmonary
metastasis potency of CGA. As presented in Fig. 6A, pulmonary metastasis was
significantly inhibited in the CGA-treated groups. The weight of
lung tissues isolated from the CGA-treated groups was lower
compared with that of the control group (Fig. 6B). Furthermore, the number of
metastatic nodules (>3 and <3 mm) of the CGA-treated groups
was decreased compared with that of the control group (Fig. 6C). Histopathological analysis of
sections of isolated lung tissues identified that lung tissues of
CGA treated groups had fewer metastatic nodules (Fig. 6D).
It has been reported that CGA could activate CD4 T
lymphocytes by suppressing Toll-like receptor (TLR) 4 signal
molecules, including TLR4, p-IRAK1, p-IκB and p-p38 (33). Thus, the present study used flow
cytometry to investigate whether CGA could enhance antitumor
immunity in the pulmonary metastasis mouse model of 4T1 cells. As
demonstrated in Fig. 6E, the
proportion of CD4+ and CD8+ T cells in
spleens was significantly upregulated after treatment with CGA in
comparison with the control groups. Collectively, the results
demonstrated that CGA exhibited efficient anti-pulmonary metastasis
effects in breast cancer by enhancing antitumor immunity.
Discussion
Breast cancer is the most common type of diagnosed
cancer among women worldwide and demonstrates considerable
metastatic potential, multi-drug resistance and high mortality
(34). With developments in medical
technology, early diagnosis of breast cancer in early stage and
treatment with chemoradiation therapy on its own or in combination
with surgery can efficiently suppress cancer progression. However,
patients with breast cancer, especially advanced TNBC, experience
an aggressive clinical outcome with a poor prognosis and high
metastasis, as well as an unsatisfactory overall survival rate
(4). Previous studies have revealed
that abnormal activation of NF-κB is closely associated with the
malignance of breast cancer, and blockade of the aberrantly
activated NF-κB could trigger apoptosis and suppress metastasis in
breast cancer cells (18–20). Thus, targeting NF-κB may be a
potential method for therapy of breast cancer. In the present
study, CGA, a potent natural inhibitor derived from cocoa and
coffee, was assessed for its antitumor efficacy in breast cancer
in vitro and in vivo.
The present results firstly demonstrated that CGA
exhibited potent cytotoxicity in breast cancer cells in a
concentration- and time-dependent manner, while significantly
inhibited the breast cancer cell colony formation ability.
Furthermore, CGA could not significantly impair the viability or
inhibit colony formation in human mammary epithelial cells
(MCF-10A), indicating that CGA could selectively impair viability
and inhibit proliferation in breast cancer cells. Next, it was
investigated whether the anti-proliferation effect of CGA on breast
cancer cells was caused by apoptotic induction. Results of Hoechst
and Annexin V/PI dual staining demonstrated that CGA significantly
induced condensed nuclei and nuclear fragmentations, as well as
triggered apoptosis in breast cancer cells. However, the proportion
of apoptosis was low. The cell viability and colony formation
results indicated that CGA significantly suppressed these abilities
of the breast cancer cells. There may be other antitumor mechanisms
of CGA in cancer therapy. For example, Huang et al (35) reported that CGA effectively treated
cancer types via the induction of cancer cell differentiation,
while Yamagata et al (36)
demonstrated that CGA regulated stem cell marker-related gene
expression in A549 human lung cancer cells. Moreover, CGA was
identified to decrease the abundance of HIF-1α and sphingosine
kinase-1 in hypoxia-induced prostate cancer cells, thus exhibiting
antitumor activity (37).
Therefore, other antitumor mechanisms of CGA should be investigated
in future studies.
Metastasis of early stage cancer is a huge challenge
to breast cancer therapy and is a major cause of breast cancer
mortality (6). Additionally, the
migration and invasion of cancer cells are key steps in the primary
metastasis of various cancer types (38). Therefore, it is crucial to suppress
the migration and invasion of cancer cells during the treatment of
cancer metastasis. The present results demonstrated that CGA could
significantly inhibit the migration and invasion of breast cancer
cells in a dose-dependent manner. Previous studies have reported
that excessive activation of NF-κB serves a key role in cancer
metastasis (16,39). EMT is important for movements of
cells during embryogenesis. Tumor cells can reactivate EMT
programs, which increases their aggressiveness. In addition to
motility, EMT is implicated in enhanced properties of stem cell and
multi-drug resistance, thereby promoting recurrence, distant
metastasis and resistance (40). It
has been revealed that microRNA (miR)-1224-5p inhibited metastasis
and EMT in colorectal cancer by targeting the SP1-mediated NF-κB
signaling pathways (41). In
addition, curcumol could inhibit the proliferation and metastasis
of melanoma via the miR-152-3p/PI3K/AKT and ERK/NF-κB signaling
pathways (42). In the present
study, mechanism analysis via western blotting demonstrated that
CGA significantly downregulated the expression levels of
NF-κB-associated proteins and EMT process-associated proteins,
indicating that CGA impaired NF-κB, and then inhibited the EMT
signaling pathway, thereby suppressing breast cancer cell migration
and invasion. In addition, evidence has demonstrated that various
signaling pathway/proteins are involved in the EMT progression of
numerous cancers. Pallasch and Schumacher revealed that TGF-β, a
known driver of malignancy is also an inducer of EMT in various
cancers (43). It is reported that
diphenyl urea derivative could serve as an inhibitor on human lung
cancer cell migration by disrupting EMT via Wnt/β-catenin and
PI3K/Akt signaling (44). Research
from Du et al demonstrated that chronic stress promotes
EMT-mediated metastasis through activation of the STAT3 signaling
pathway by miR-337-3p in breast cancer (45). The EMT signaling pathway, as an axis
center, has been revealed to largely participate in the regulation
of cancer metastasis (46).
The antitumor effect of CGA was evaluated in a
subcutaneous tumor mouse model of 4T1 cells. In vivo results
demonstrated that CGA significantly slowed tumor growth and
diminished tumor weight by inhibiting tumor cell proliferation and
suppressing the NF-κB signaling pathway. Moreover, the survival
rate of tumor-bearing mice was significantly improved after
treatment with CGA. In the pulmonary metastasis model of 4T1,
treatment with CGA significantly decreased metastatic nodules in
lung tissues. Tumor immune escape, which is featured as suppressive
antitumor immunity, is responsible for tumor malignancy and distant
metastasis (47). Based on the
efficient anti-metastasis efficacy of CGA, the present study
investigated the effect of CGA on antitumor immunity. The results
indicated that treatment with CGA significantly upregulated the
proportion of CD4+ and CD8+ T cells in the
spleens of tumor-bearing mice, indicating that CGA could improve
antitumor immunity. The possible reason why CGA enhances antitumor
immunity may be that CGA could regulate the tumor immune
microenvironment via the repolarization of macrophages from the M2
to the M1 phenotype (48). Further
studies should be performed to investigate the regulatory effect
and mechanism of CGA on the tumor immune microenvironment.
In conclusion, the present study provides important
information regarding the antitumor activity of CGA in breast
cancer (Fig. 7). The results
demonstrated that CGA could significantly inhibit viability and
induce apoptosis in breast cancer cells. Additionally, CGA
suppressed the migration and invasion of breast cancer cells by
impairing the NF-κB/EMT signaling pathway. Furthermore, CGA
significantly slowed tumor growth, prolonged the survival rate and
inhibited pulmonary metastasis by increasing the proportion of
CD4+ and CD8+ T cells in spleens, thus
improving antitumor immunity.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chinese
Postdoctoral Science Foundation Program (grant no. 2019M653833XB),
the Foundation of Science and Technology Department of Sichuan
Province (grant no. 2020YJ0147), the Foundation of ‘Apricot Grove
Scholar’ of Chengdu University of Traditional Chinese Medicine
(grant no. 2019yky09), the Postdoctoral Science Foundation of
Chengdu University of Traditional Chinese Medicine (grant no.
030054080), and the Foundation of Sichuan Academy of Chinese
Medical Science (grant no. A-2019N-16).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QZ, JZ and LS designed the research and were
responsible for the project conception. AZ, SZ and XL performed
experiments and acquired the data. LS, AZ, SW and CL were
responsible for statistical analyses and interpretation of the
data. LS drafted the manuscript, along with SZ and CL. LS revised
the manuscript, along with JZ and SW. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
This article does not contain any studies with human
participants performed by any of the authors. All the animal
experiments in the present study were performed according to the
National Institutes of Health guidelines and were approved by the
Institutional Animal Care and Treatment Committee of Chengdu
University of Traditional Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Waks AG and Winer EP: Breast cancer
treatment: A Review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Couch FJ, Hart SN, Sharma P, Toland AE,
Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, et
al: Inherited mutations in 17 breast cancer susceptibility genes
among a large triple-negative breast cancer cohort unselected for
family history of breast cancer. J Clin Oncol. 33:304–311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Claessens AK, Erdkamp FL, Lopez-Yurda M,
Bouma JM, Rademaker-Lakhai JM, Honkoop AH, de Graaf H, Tjan-Heijnen
VC and Bos ME: Secondary analyses of the randomized phase III
Stop&Go study: Efficacy of second-line intermittent versus
continuous chemotherapy in HER2-negative advanced breast cancer.
Acta Oncol. 59:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bale R, Putzer D and Schullian P: Local
treatment of breast cancer liver metastasis. Cancers (Basel).
11:112019. View Article : Google Scholar
|
|
7
|
Song L, Chen X, Mi L, Liu C, Zhu S, Yang
T, Luo X, Zhang Q, Lu H and Liang X: Icariin-induced inhibition of
SIRT6/NF-kappaB triggers redox mediated apoptosis and enhances
anti-tumor immunity in triple-negative breast cancer. Cancer Sci.
11:4242–4256. 2020. View Article : Google Scholar
|
|
8
|
Zeng A, Liang X, Zhu S, Liu C, Luo X,
Zhang Q and Song L: Baicalin, a potent inhibitor of NF-kappaB
signaling pathway, enhances chemosensitivity of breast cancer cells
to docetaxel and inhibits tumor growth and metastasis both in vitro
and in vivo. Front Pharmacol. 11:8792020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A, Takada Y, Boriek AM and Aggarwal
BB: Nuclear factor-kappaB: Its role in health and disease. J Mol
Med (Berl). 82:434–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong ET and Tergaonkar V: Roles of
NF-kappaB in health and disease: Mechanisms and therapeutic
potential. Clin Sci (Lond). 116:451–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gyrd-Hansen M and Meier P: IAPs: From
caspase inhibitors to modulators of NF-kappaB, inflammation and
cancer. Nat Rev Cancer. 10:561–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Y, Luo JL and Karin M: IkappaB kinase
alpha kinase activity is required for self-renewal of
ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl
Acad Sci USA. 104:15852–15857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang S, Pettaway CA, Uehara H, Bucana CD
and Fidler IJ: Blockade of NF-kappaB activity in human prostate
cancer cells is associated with suppression of angiogenesis,
invasion, and metastasis. Oncogene. 20:4188–4197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poligone B and Baldwin AS: Positive and
negative regulation of NF-kappaB by COX-2: Roles of different
prostaglandins. J Biol Chem. 276:38658–38664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad A, Biersack B, Li Y, Kong D, Bao B,
Schobert R, Padhye S and Sarkar F: Targeted regulation of
PI3K/Akt/mTOR/NF-kappaB signaling by indole compounds and their
derivatives: Mechanistic details and biological implications for
cancer therapy. Anticancer Agents Med Chem. 13:1002–1013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhen X, Choi HS, Kim JH, Kim SL, Ren Liu
R, Yun BS and Lee DS: Machilin D, a lignin derived from Saururus
chinensis, suppresses breast cancer stem cells and inhibits
NF-kappaB signaling. Biomolecules. 10:2452020. View Article : Google Scholar
|
|
19
|
Senthil Kumar KJ, Gokila Vani M, Hsieh HW,
Lin CC, Liao JW, Chueh PJ and Wang SY: MicroRNA-708 activation by
glucocorticoid receptor agonists regulate breast cancer
tumorigenesis and metastasis via downregulation of NF-kappaB
signaling. Carcinogenesis. 40:335–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orlova Z, Pruefer F, Castro-Oropeza R,
Ordaz-Ramos A, Zampedri C, Maldonado V, Vazquez-Santillan K and
Melendez-Zajgla J: IKKepsilon regulates the breast cancer stem cell
phenotype. Biochim Biophys Acta Mol Cell Res. 1866:598–611. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng S, Cao J, Feng Q, Peng J and Hu Y:
Roles of chlorogenic Acid on regulating glucose and lipids
metabolism: A review. Evid Based Complement Alternat Med.
801457:20132013.
|
|
22
|
dos Santos MD, Almeida MC, Lopes NP and de
Souza GE: Evaluation of the anti-inflammatory, analgesic and
antipyretic activities of the natural polyphenol chlorogenic acid.
Biol Pharm Bull. 29:2236–2240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kono Y, Kobayashi K, Tagawa S, Adachi K,
Ueda A, Sawa Y and Shibata H: Antioxidant activity of polyphenolics
in diets. Rate constants of reactions of chlorogenic acid and
caffeic acid with reactive species of oxygen and nitrogen. Biochim
Biophys Acta. 1335:335–342. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neuwirthova J, Gal B, Smilek P and
Urbankova P: Coffee in cancer chemoprevention. Klin Onkol.
30:106–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo C, Xu X, Wei X, Feng W, Huang H, Liu
H, Xu R, Lin J, Han L and Zhang D: Natural medicines for the
treatment of fatigue: Bioactive components, pharmacology, and
mechanisms. Pharmacol Res. 148:1044092019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buldak RJ, Hejmo T, Osowski M, Bułdak L,
Kukla M, Polaniak R and Birkner E: The impact of coffee and its
selected bioactive compounds on the development and progression of
colorectal cancer in vivo and in vitro. Molecules. 23:33092018.
View Article : Google Scholar
|
|
27
|
Feng R, Lu Y, Bowman LL, Qian Y,
Castranova V and Ding M: Inhibition of activator protein-1,
NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme
activity by chlorogenic acid. J Biol Chem. 280:27888–27895. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang NJ, Lee KW, Kim BH, Bode AM, Lee HJ,
Heo YS, Boardman L, Limburg P, Lee HJ and Dong Z: Coffee phenolic
phytochemicals suppress colon cancer metastasis by targeting MEK
and TOPK. Carcinogenesis. 32:921–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang T, Chen S, Chen L, Zhang L, Meng F,
Sha S, Ai C and Tai J: Chlorogenic acid ameliorates lead-induced
renal damage in mice. Biol Trace Elem Res. 189:109–117. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian L, Su CP, Wang Q, Wu FJ, Bai R, Zhang
HM, Liu JY, Lu WJ, Wang W, Lan F, et al: Chlorogenic acid: A potent
molecule that protects cardiomyocytes from TNF-alpha-induced injury
via inhibiting NF-kappaB and JNK signals. J Cell Mol Med.
23:4666–4678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arfian N, Wahyudi DA, Zulfatina IB, Citta
AN, Anggorowati N, Multazam A, Romi MM and Sari DC: Chlorogenic
acid attenuates kidney ischemic/reperfusion injury via reducing
inflammation, tubular injury, and myofibroblast formation. BioMed
Res Int. 5423703:20192019.
|
|
32
|
Hamidi H and Ivaska J: Every step of the
way: Integrins in cancer progression and metastasis. Nat Rev
Cancer. 18:533–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan Y, Gong X, Zhang L, Jiang R, Yang J,
Wang B and Wan J: Chlorogenic acid ameliorated concanavalin
A-induced hepatitis by suppression of Toll-like receptor 4
signaling in mice. Int Immunopharmacol. 44:97–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peart O: Metastatic breast cancer. Radiol
Technol. 88:519M–539M. 2017.PubMed/NCBI
|
|
35
|
Huang S, Wang LL, Xue NN, Li C, Guo HH,
Ren TK, Zhan Y, Li WB, Zhang J, Chen XG, et al: Chlorogenic acid
effectively treats cancers through induction of cancer cell
differentiation. Theranostics. 9:6745–6763. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamagata K, Izawa Y, Onodera D and Tagami
M: Chlorogenic acid regulates apoptosis and stem cell
marker-related gene expression in A549 human lung cancer cells. Mol
Cell Biochem. 441:9–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee MS, Lee SO, Kim KR and Lee HJ:
Sphingosine kinase-1 involves the inhibitory action of HIF-1alpha
by chlorogenic acid in hypoxic DU145 cells. Int J Mol Sci.
18:3252017. View Article : Google Scholar
|
|
38
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bollrath J and Greten FR: IKK/NF-kappaB
and STAT3 pathways: Central signalling hubs in
inflammation-mediated tumour promotion and metastasis. EMBO Rep.
10:1314–1319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aiello NM and Kang Y: Context-dependent
EMT programs in cancer metastasis. J Exp Med. 216:1016–1026. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Peng W, Yang P, Chen R, Gu Q, Qian
W, Ji D, Wang Q, Zhang Z, Tang J, et al: MicroRNA-1224-5p inhibits
metastasis and epithelial-mesenchymal transition in colorectal
cancer by targeting SP1-mediated NF-kappaB signaling pathways.
Front Oncol. 10:2942020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ning N, Liu S, Liu X, Tian Z, Jiang Y, Yu
N, Tan B, Feng H, Feng X and Zou L: Curcumol inhibits the
proliferation and metastasis of melanoma via the
miR-152-3p/PI3K/AKT and ERK/NF-kappaB signaling pathways. J Cancer.
11:1679–1692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pallasch FB and Schumacher U: Angiotensin
inhibition, TGF-beta and EMT in cancer. Cancers (Basel).
12:27852020. View Article : Google Scholar
|
|
44
|
Dai B, Fan M, Yu R, Su Q, Wang B, Yang T,
Liu F and Zhang Y: Novel diphenyl urea derivative serves as an
inhibitor on human lung cancer cell migration by disrupting EMT via
Wnt/beta-catenin and PI3K/Akt signaling. Toxicol In Vitro.
69:1050002020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du P, Zeng H, Xiao Y, Zhao Y, Zheng B,
Deng Y, Liu J, Huang B, Zhang X, Yang K, et al: Chronic stress
promotes EMT-mediated metastasis through activation of STAT3
signaling pathway by miR-337-3p in breast cancer. Cell Death Dis.
11:7612020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pastushenko I and Blanpain C: EMT
Transition States during Tumor Progression and Metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mohme M, Maire CL, Schliffke S, Joosse SA,
Alawi M, Matschk J, Schüller U, Dierlamm J, Martens T, Pantel K, et
al: Molecular profiling of an osseous metastasis in glioblastoma
during checkpoint inhibition: Potential mechanisms of immune
escape. Acta Neuropathol Commun. 8:282020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xue N, Zhou Q, Ji M, Jin J, Lai F, Chen J,
Zhang M, Jia J, Yang H, Zhang J, et al: Chlorogenic acid inhibits
glioblastoma growth through repolarizating macrophage from M2 to M1
phenotype. Sci Rep. 7:390112017. View Article : Google Scholar : PubMed/NCBI
|