Introduction
Prostate cancer (PCa) is a genitourinary malignancy
that threatens the health of men worldwide. In Europe and the
United States, the incidence (19%) of PCa ranks first among male
malignant tumours, and its mortality (9%) ranks second (1,2).
Currently, the incidence of PCa in China has surpassed that of
bladder cancer to rank first among male genitourinary tumours. For
PCa treatment, surgery and chemotherapy are currently used.
Surgical treatment is expensive and causes a certain degree of
inconvenience to patients. Moreover, the therapeutic effects of
existing targeted drugs are unsatisfactory. To improve the
therapeutic effect of targeted drugs, it is particularly important
to explore the aetiology of PCa development. Only by clarifying the
pathogenesis of PCa can we find specific molecular markers for use
as predictors and therapeutic targets.
The cell division cycle-associated 5 (CDCA5) gene
encodes the CDCA5 protein, which is a major regulator of sister
chromatid cohesion and segregation (3). CDCA5 maintains sister chromatid
cohesion by stabilizing the cohesive complex, and it ensures
accurate cell chromosome separation during meiosis and mitosis; it
also plays an essential role in DNA repair (4). Moreover, CDCA5 regulates the activity
of cell cycle-associated proteins and transcription factors,
thereby promoting proliferation and participating in apoptosis in
cancer cells (5). Studies have
reported that high expression of the CDCA5 gene is closely related
to the incidence of bladder (6),
lung (7), liver (8,9) and
colorectal cancer (10). Notably,
researchers have concluded through bioinformatics analysis that
CDCA5 is closely related to the development of PCa (11–13).
This is a strong indicator that CDCA5 may be an important protein
in the development of PCa. However, the specific mechanism of
action of CDCA5 in PCa development has not been reported.
The MAPK cascade signalling pathway is composed of
serine and threonine kinases. This signalling pathway transfers
extracellular molecules, such as hormones and tumour-promoting and
differentiation molecules, into cells to regulate cell
proliferation and differentiation (14). Extracellular signal-regulated kinase
(ERK) is a member of the MAPK signalling pathway. Studies have
found that aberrant Ras/Raf/MEK/ERK activation leads to
tumourigenesis (15). Increased
levels of ERK phosphorylation have been revealed to be associated
with poor prognosis and the progression of multiple cancers,
including PCa (16,17). Researchers have already revealed the
relationship between CDCA5 and ERK in colorectal (10) and liver cancer (18). Based on this, we further explored
whether CDCA5 exerts its function through the ERK signalling
pathway in PCa.
In the present study, the expression level of CDCA5
in PCa and the effect of CDCA5 on PCa progression via
bioinformatics and molecular and cell biology assays were explored.
Through existing literature support and experimental verification,
it was further elucidated that CDCA5 functions through the ERK
signalling pathway to promote PCa progression. The purpose of the
present study was to confirm that CDCA5 has become a potential
molecular target for PCa diagnosis and treatment.
Materials and methods
Ethical approval for the study
protocol
The present study was approved by the Medical Ethics
Committee of The Second Hospital of Tianjin Medical University,
Tianjin, China (nos. KY2019K107 and YN2019Y99). For the collection
of clinical patient samples, written informed consent was obtained
from all patients. For in vivo experiments, all experiments
passed the approval of the Ethics Committee of the Second Hospital
of Tianjin Medical University. All studies were conducted in
accordance with the Declaration of Helsinki. The Ethics approval
no. KY2019K107 was used for human samples and the Ethics approval
no. YN2019Y99 was used for animal experiments.
Human samples
Prostate tissue specimens were surgically removed
from prostate cancer patients, with complete clinicopathological
data, and cancer tissues and paracancerous tissue from each patient
were collected. These patients were treated at the Second Hospital
of Tianjin Medical University. The collection period was from
September 2017 to February 2019. During this period, all prostate
cancer patients treated in Ward B of the Department Urology of the
Second Hospital of Tianjin Medical University were included in the
present study. All included patients signed the informed consent
form. A total of 106 patients were included, thus, a total of 106
pairs of prostate tissue and paracancerous tissue from patients
were collected. Among them, 96 pairs of tissues were formalin-fixed
paraffin-embedded (FFPE) for immunohistochemical (IHC) analysis,
and 10 pairs of tissues were fresh tissue for reverse
transcription-quantitative (RT-q)PCR. The clinicopathological data
of all patients are presented in Table
SI.
Antibodies
The antibodies used in the present study are listed
in Table I.
 | Table I.Antibodies used in the present
study. |
Table I.
Antibodies used in the present
study.
| ID | Product code or
cat. no., supplier | Instructions |
|---|
| CDCA5 | ab192237,
Abcam | 1:1,000 dilution
for western blotting |
|
|
| 1:100 dilution for
IHC |
| Ki67 | ab16667, Abcam | 1:1,000 dilution
for western blotting |
|
|
| 1:200 dilution for
IHC |
| Cleaved
caspase-3 | ab2302, Abcam | 1:500 dilution for
western blotting |
|
|
| 1:100 dilution for
IHC |
| Pro caspase-3 | ab32150, Abcam | 1:1,000 dilution
for western blotting |
|
|
| 1:200 dilution for
IHC |
| ERK | ab54230,
Abcam, | 1:2,000 dilution
for western blotting |
|
|
| 1:100 dilution for
IHC |
| p-ERK | ab50011, Abcam | 1:1,000 dilution
for western blotting |
|
|
| 1:100 dilution for
IHC |
| CDK1 | ab131450,
Abcam | 1:100 dilution for
western blotting |
| Cyclin B1 | ab72, Abcam | 1:1,000 dilution
for western blotting |
| GAPDH | KM9002, Tianjin
Sungene Biotech Co, Ltd. | 1:5,000 dilution
for western blotting |
Bioinformatics analysis
Gene Expression Profiling Interactive Analysis
(GEPIA) (19) is an online data
processing tool at http://gepia.cancer-pku.cn/index.html. The GEPIA
database is based on data from the UCSC Xena project. It can
analyze and process The Cancer Genome Atlas (TCGA) database
(20). In the present study, GEPIA
was used to determine the difference in CDCA5 mRNA between PCa
tissue and normal prostate tissue in TCGA database. In addition,
GEPIA was also used to explore the relationship between CDCA5
expression level and survival of PCa patients in TCGA database.
Cell culture and In vitro
transfection
The cell lines RWPE-1, LNCaP, 22RV1, C4-2 and PC-3
were purchased from the ATCC. All the cell lines were cultured in
RPMI-1640 medium containing 10% foetal bovine serum (both from
Gibco; Thermo Fisher Scientific, Inc.) at 37°C under a volume
fraction of 5% CO2. Transfection was performed using
cells in the logarithmic growth phase. The cells were seeded in
6-well plates at a density of 5×105 cells/well one day
prior to transfection. The following day, transfection was
performed at a cell density of 60–70%, and the specific procedure
was carried out with Lipofectamine 3000 according to the
manufacturers instructions (Invitrogen; Thermo Fisher Scientific,
Inc.). Transfection was carried out for 30 min at 37°C. The
following short hairpin (sh)RNA plasmids were used: CDCA5 human
shRNA plasmid (cat. no. TL305489), and HuSH shRNA RFP cloning
vector (cat. no. TR30014; both from OriGene Technologies, Inc.). In
a 6-well plate, 2.5 µg plasmid was added to each well. The time
interval between transfection and subsequent experimentation was 24
h. In the present study, the aforementioned shRNA plasmids were
used to transfect PC-3 and C4-2 cell lines.
RT-qPCR
Total RNA from tissue was extracted using TRIzol and
reverse-transcribed into cDNA using an RT kit (both Sigma-Aldrich;
Merck KGaA) according to the manufacturers instructions. TaqMan
(Invitrogen; Thermo Fisher Scientific, Inc.) was used according to
the manufacturers instructions for RT-qPCR. Using the cDNA as a
template, RT-qPCR was performed to detect the expression of CDCA5.
Using GAPDH as an internal reference, relative quantitative
analysis was performed using the 2−ΔΔCq method (21). The primer sequences were as follows:
CDCA5 forward, 5′-GACGCCAGAGACTTGGAAATG-3′ and reverse,
5′-GGACCTCGGTGAGTTTGGAG-3′; GAPDH forward,
5′-GATTCCACCCATGGCAAATT-3′ and reverse, 5′-TCTCGCTCCTGGAAGATGGT-3′.
The reaction conditions for RT-qPCR were 95°C for 3 min, 95°C for 5
sec, and 60°C for 30 sec. The aforementioned reaction conditions
were carried out for 40 cycles, and the assays were repeated 3
times.
IHC staining
For IHC staining, 4% paraformaldehyde was used to
fix the tissue at 25°C for 24 h. Paraffin was used for embedding,
and the thickness of sections was 0.4 mm. The specimen slices were
routinely dewaxed with water, antigen retrieval was performed, and
the sections were washed with PBS. Next, use blocking reagent to
block endogenous peroxidase activity, and the sections were washed
again with PBS. Primary antibodies were added dropwise and
incubated at 4°C for 18 h. The sections were washed again with PBS,
and secondary antibodies (general purpose secondary antibody;
dilution 1:10; PV-6000; ZSGB-BIO; OriGene Technologies, Inc.) were
added dropwise and incubated at 37°C for 1 h. The sections were
washed again with PBS; then, DAB colouring solution was added
dropwise for colour development, and the colour development was
immediately stopped. After washing with tap water, the nuclei were
counterstained with haematoxylin (25°C, 15 sec). Finally, the
samples were dehydrated, cleared, sealed and observed under a light
microscope.
Western blotting
Protein lysis buffer (Thermo Fisher Scientific,
Inc.) was used to extract total cell protein. After using the
bicinchoninic acid (BCA) method to determine the protein
concentration, loading buffer was used to prepare the protein
samples (30 µg per lane) for SDS-PAGE (the percentage of the gel
was 10%) electrophoresis. After electrophoresis, the protein was
transferred to a PVDF (Thermo Fisher Scientific, Inc.) membrane at
a constant flow rate of 250 mA for 130 min. Next, the membrane was
sealed with 5% skimmed milk at room temperature for 1 h. Then, the
membrane was washed with TBST solution, the PVDF membrane was
incubated with primary antibody and incubated overnight at 4°C.
Subsequently, the membrane was washed 3 times with TBST for 5 min
each time. Then, secondary antibodies [(mouse IgG (H+L)
cross-adsorbed secondary antibody; cat. no. G-21040; 1:10,000
dilution); or (rabbit IgG (H+L) secondary antibody cat. no. 31460,
1:10,000 dilution); both from Thermo Fisher Scientific, Inc.] was
added and incubated for 1 h at room temperature. The PVDF membrane
was washed 3 times with TBST again for 5 min each time. Finally, a
visualisation reagent (Pierce™ ECL Western Blotting Substrate;
Thermo Fisher Scientific, Inc.) was dropped on the PVDF membrane
for exposure.
Flow cytometry
Culture growth was terminated when the total number
of cells reached 1×106. The cells were digested with
trypsin, centrifuged at 157 × g at 4°C for 3 min, and the
supernatant was discarded. The cells were fixed with 70% ice-cold
alcohol and allowed to stand at 4°C overnight. The cells were
washed twice with ice-cold PBS, centrifuged at 157 × g at 4°C for 3
min, stained with a fluorescent solution (PE Annexin V Apoptosis;
cat. no. 559763; BD Pharmingen™; BD Biosciences) at 25°C for 3 min,
and then gently mixed. After incubation for 30 min at room
temperature, the cells were analyzed and mapped using flow
cytometer (Cytomics FC 500 MCL; Beckman Coulter, Inc.).
MTT assay
Cells were seeded on 96-well plates at 2,000 cells
per well, each well contained 150 µl of culture medium, and the
cells were cultured in a conventional cell culture method for 6
days. Then, 20 µl of MTT (Sigma-Aldrich; Merck KGaA) solution was
added to each well. After incubation at 37°C for 4 h, the culture
supernatant in the wells was discarded. Then, 150 µl of DMSO
(Sigma-Aldrich; Merck KGaA) was added to each well and shaken for
10 min to allow the crystals to fully dissolve. A microplate reader
(FC; Thermo Fisher Scientific, Inc.) was used for detection. The
light absorption value at 570 nm was detected, and the cell growth
curve was plotted with time as the abscissa and absorbance as the
ordinate.
Colony formation assay
Cells in the logarithmic growth phase were
collected, and 1×103 cells/well were seeded in a 6-well
plate. Three replicate wells were used for each group. When
macroscopic colonies appeared after 7 days of culture, the culture
solution was discarded. After washing with PBS, the cells were
fixed in a 4% methanol solution at 25°C for 15 min and stained with
10% haematoxylin at 25°C for 15 min. The cells were then observed
under an optical microscope (magnification ×40), and the number of
colonies was counted. The minimum number of cells per colony was
50.
Transwell invasion assay
Matrigel (BD Biosciences) was melted at 4°C
overnight. On ice, a pre-cooled pipette tip was used to draw 100 µl
Matrigel into the pre-cooled 300 µl serum-free medium and was well
mixed. Then, 25 µl of the aforementioned-diluted Matrigel was
obtained and added to a Transwell plate chamber (BD FALCON; product
no. 353097; pore size of the inserts, 8 µm; Corning, Inc.), and
placed at 37°C for 30 min to polymerize the Matrigel into a gel. A
single cell suspension was prepared with a serum-free medium using
a conventional method (22), and
the concentration of the cell suspension was 5×105
cells/ml. Then, 100 µl of cell suspension was added to the chamber
of the Transwell culture plate and 200 µl of serum-free medium.
Subsequently, 500 µl of medium containing 10% FBS was added to the
lower chamber of the Transwell culture plate, and cultured in
RPMI-1640 medium containing 10% fetal bovine serum (both from
Gibco; Thermo Fisher Scientific, Inc.) at 37°C under a volume
fraction of 5% CO2. The Matrigel gel and the cells on
the upper surface of the Transwell culture plate were then wiped
off. The cells were fixed with 10% methanol at 25°C for 30 min.
Crystal violet dye at a concentration of 0.1% was used to stain the
cells at 37°C for 1 min. The excess crystal violet dye was rinsed
with PBS, and the cells were observed and counted under a light
microscope (magnification, ×200).
Cell cycle analysis
The cells (1×106/ml) to be assessed were
fixed with 70% ethanol at 4°C for 8 h, and stained with propidium
iodide (PI; 0.5 mg/ml; Thermo Fisher Scientific, Inc.) at 37°C for
30 min. A FACSCaliber flow cytometer (BD Biosciences) was used to
analyse the number of cells in different cell-cycle stages and
calculate the corresponding percentages.
In vivo experiments
The animal studies were approved by The Second
Hospital of Tianjin Medical University, Tianjin, China. Male nude
mice (6 weeks old; n=6; 16–20 g per mouse) were purchased from
Beijing HFK Bioscience Co., Ltd. There were no more than 5 mice in
each cage. The temperature in the cage was maintained at 26–28°C,
and the relative humidity at 40–60%. A high-efficiency filter was
used to ensure that constant ventilation. The experimental mice
were fed with sterilized feed and water ad libitum. Subcutaneous
tumour growth assays were performed with shCON-PC-3 and
shCDCA5-PC-3 stable cell lines (1×106 shCON-PC-3 cells
were injected into 3 mice and 1×106 shCDCA5-PC-3 cells
were injected into the other 3 mice). After two weeks, the 6
injected mice developed tumours. The tumour size was measured every
7 days with a Vernier calliper, and the volume change of the tumour
was calculated. When the length of the largest tumour among all
tumours reached 20 mm, the experiment required termination to avoid
tumor rupture. The experimental mice were euthanized by inhaling
excessive CO2. The air displacement rate of
CO2 in %/min was 15%/min. Animal experiments were
performed from September 5, 2019 to November 17, 2019. The tumours
were harvested under standard, institutionally approved processes.
The mass of each tumour was measured with an electronic balance.
Tumour samples were paraffin-fixed and processed for IHC
analysis.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
statistical software (IBM Corp.). Each experiment in the present
study was repeated independently 3 times. All results are expressed
as the mean ± standard deviation (SD). Comparisons between groups
were performed using one-way analysis of variance (ANOVA). CDCA5
mRNA expression levels in different cell lines were compared using
ANOVA followed by Dunnetts post hoc test. An independent sample
unpaired Students t-test was used for direct comparisons between
two groups. Pearson χ2 test was used to analyse the
relationship between CDCA5 protein expression level and
clinicopathological characteristics. Differences were considered
statistically significant at P<0.05.
Results
CDCA5 expression is higher in PCa
tissues than in normal prostate tissues
In the present study, GEPIA was used to determine
the difference in CDCA5 mRNA between PCa tissue and normal prostate
tissue in TCGA database. The results revealed that the mRNA level
of CDCA5 was significantly increased in PCa tissues (P<0.05,
num(T)=492; num(N)=52; Fig. 1A). To
verify the aforementioned results, PCa tissues and prostate
paracancerous tissues were collected concurrently from 10 patients
with tumours at the Second Hospital of Tianjin Medical University.
Total RNA was extracted from the tissues, and the CDCA5 mRNA
expression level was examined. The RT-qPCR results revealed that
CDCA5 mRNA expression was significantly higher in the 10 pairs of
PCa tissues than in paracancerous tissues (Fig. 1B). To further explore the protein
expression of CDCA5 in PCa tissues, relevant paraffin sections were
obtained from the Second Hospital of Tianjin Medical University.
The pathologists confirmed that the normal prostate tissue acinus
was small and round, the basal cells were flat and in a continuous
line at the base of the gland, and the nucleus was arranged neatly.
IHC results revealed that the protein level of CDCA5 was
significantly higher in PCa tissues than in paracancerous tissues
(Fig. 1C) (n=96). In vitro,
it was confirmed that CDCA5 mRNA and protein expression levels were
higher in several PCa cell lines (LNCAP, 22RV1, C4-2 and PC-3) than
in human normal prostate epithelial cells (RWPE-1) (Fig. 1D and E).
 | Figure 1.CDCA5 expression is higher in PCa
tissues than in adjacent tissues. (A) GEPIA was used to assess the
differences in CDCA5 mRNA levels in PCa tissues and normal prostate
tissues in TCGA database [(num(T)=492, num(N)=52, *P<0.05]. (B)
RT-qPCR was used to detect the CDCA5 mRNA expression level in PCa
and adjacent surgical specimens from tumour patients (n=10 pairs)
(***P<0.001). (C) Representative IHC images of CDCA5 in PCa and
paracancerous tissues (magnification, ×200 and ×400). The histogram
revealed the statistical results of the histochemical positive rate
(right panel) (n=96). (D) RT-qPCR was used to detect the mRNA
expression levels of CDCA5 in RWPE-1, LNCaP, 22RV1, C4-2 and PC-3
cells. GAPDH was used as an internal control (n=3; **P<0.01 and
***P<0.001). (E) Western blotting was used to detect the protein
expression levels of CDCA5 in RWPE-1, LNCaP, 22RV1, C4-2 and PC-3
cells. GAPDH was used as an internal control. CDCA5, cell division
cycle-associated 5; PCa, prostate cancer; GEPIA, Gene Expression
Profiling Interactive Analysis; TCGA, The Cancer Genome Atlas;
RT-qPCR, reverse transcription-quantitative PCR. |
CDCA5 expression affects the prognosis
of PCa patients
The CDCA5 staining and clinicopathological features
of the aforementioned clinical specimens were comprehensively
analysed. Table II revealed that
patients with high CDCA5 expression had a high initial PSA.
Additionally, seminal vesicle invasion and lymph node metastasis
were more likely to occur.
 | Table II.Clinicopathologic variables and CDCA5
expression in PCa patients (n=96). |
Table II.
Clinicopathologic variables and CDCA5
expression in PCa patients (n=96).
|
|
| CDCA5
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Group | n=96 | High | Low |
P-valuea |
|---|
| Age (years) | <65 | 46 | 32 | 14 | 0.56 |
|
| ≥65 | 50 | 32 | 18 |
|
| Preoperative
PSA | ≤10 | 25 | 12 | 13 | 0.02b |
|
| >10 | 71 | 52 | 19 |
|
| Clinical stage | T1 | 30 | 16 | 14 | 0.06 |
|
| T2-3 | 66 | 48 | 18 |
|
| Gleason score | <7 | 35 | 20 | 15 | 0.13 |
|
| ≥7 | 61 | 44 | 17 |
|
| Seminal vesicle
invasion | Absence | 36 | 19 | 17 | 0.03b |
|
| Presence | 60 | 45 | 15 |
|
| Lymph node
metastasis | Absence | 40 | 22 | 18 | 0.04b |
|
| Presence | 56 | 42 | 14 |
|
GEPIA was used to explore the relationship between
CDCA5 expression level and survival in PCa patients. All the
relevant data were obtained from TCGA database. CDCA5 mRNA
expression was classified as low (n=246) or high (n=246) based on
the median CDCA5 mRNA expression level as a cut-off point. The
analysis results revealed that both the overall survival (P=0.025)
and disease-free survival (P=0.00048) were significantly different
between the high and low CDCA5 expression groups (Fig. 2A and B). Thus, it could be inferred
that the expression of CDCA5 may be used as a predictor of
prognosis in PCa patients.
CDCA5 knockdown inhibits PCa cell
proliferation and invasion in vitro
In vitro experiments further explored the
effect of CDCA5 on PCa cell function. The protein expression levels
of CDCA5 were examined in the PCa cell lines LNCaP, 22RV1, C4-2 and
PC-3. Among them, the CDCA5 protein expression level was the
highest in C4-2 and PC-3 cells (Fig.
1E). Therefore, C4-2 and PC-3 cells were selected for our
experimental research. Next, two stable cell lines with CDCA5
knockdown were established. Through proliferation-related
functional protein markers, it was determined that when CDCA5 was
knocked down, Ki67 expression levels decreased in C4-2 and PC-3
cells and cleaved caspase-3 expression was increased (Fig. 3A). Moreover, flow cytometric results
revealed that the apoptotic rate (early apoptosis + late apoptosis)
of shCDCA5-C4-2 and shCDCA5-PC-3 cells was significantly higher
than the rate of apoptotic C4-2 and PC-3 control cells respectively
(Fig. 3B). An MTT assay revealed
that the proliferative capacity of C4-2 and PC-3 cells was
significantly decreased after CDCA5 was knocked down (Fig. 3C). Moreover, colony formation assays
revealed that the cloning ability of C4-2 and PC-3 cells was
significantly decreased after CDCA5 was knocked down (Fig. 3D). The statistical results for the
number of colonies revealed significant differences between the two
groups (Fig. 3E). The experimental
results revealed that when the CDCA5 of prostate cancer cells PC-3
or C4-2 was knocked down, their invasion ability was significantly
reduced (Fig. 3F). The statistical
results for the number of colonies revealed significant differences
between the two groups (Fig. 3G).
These data indicated that after knocking down CDCA5, the
proliferation and invasion abilities of PCa cells were
decreased.
 | Figure 3.Knockdown of CDCA5 inhibits PCa cell
proliferation and invasion in vitro. (A) Western blotting
was used to detect the expression levels of CDCA5, Ki67 and
cleaved/pro caspase-3 after CDCA5 knockdown in C4-2 and PC-3 cells.
GAPDH was used as an internal control. (B) Flow cytometry was used
to detect apoptosis in C4-2 and PC-3 cells after CDCA5 knockdown.
The apoptotic rate (early apoptosis + late apoptosis) of C4-2 and
PC-3 after knockdown CDCA5 was counted and plotted on a graph
(**P<0.01 and ***P<0.001). The UL region indicates cell
necrosis, the LL region indicates cell survival, the LR region
indicates early cell apoptosis, and the UR region indicates late
cell apoptosis. The LR region + UR region indicate total apoptosis.
(C) An MTT assay was used to detect C4-2 and PC-3 cell growth after
CDCA5 was knocked down. The absorbance value at a wavelength of 570
nm was detected (*P<0.05). (D) A colony formation assay was used
to detect C4-2 and PC-3 cell growth after CDCA5 knockdown. (E) The
number of colonies from D was counted and plotted (***P<0.001).
(F) Transwell invasion assays detected the invasiveness of the
prostate cancer cells after knockdown of CDCA5. (G) The number of
invaded cells in F was counted and plotted on a graph
(***P<0.001). CDCA5, cell division cycle-associated 5; PCa,
prostate cancer; sh, short hairpin; CON, control; UL, upper left;
LL, lower left; LR, lower right; UR, upper right. |
CDCA5 regulates the prostate cancer
cell division cycle
The proliferation of eukaryotic cells is mainly
mediated by the cell cycle. The G2/M process is one of the main
checkpoints and is tightly regulated by the cyclin B1/CDK1 protein
complex (10). CDCA5 is required
for the stable binding of adhesion and chromatin in the G2/M phase
and plays a vital role in cell cycle regulation. Researchers have
found that CDCA5 can affect the cell division cycle in several
tumours (23). Thus, the effects
CDCA5 on the prostate cancer cell division cycle were explored.
Cell cycle analysis revealed a decreased percentage of G0/G1 phase
cells and an increased percentage of G2/M phase cells for both the
C4-2 and PC-3 cell lines (Fig. 4A and
B). These phenomena indicated that after CDCA5 knockdown in
PC-3 and C4-2 cells, the G2/M process was blocked. The expression
of the G2/M-related proteins CDK1 and cyclin B1 was decreased by
CDCA5 knockdown in C4-2 and PC-3 cells (Fig. 4C and D).
CDCA5 knockdown inhibits ERK
signalling pathway activation
Researchers have reported that activation of the ERK
signalling pathway plays a key role in PCa development, and
activation of this pathway is dependent on ERK phosphorylation
(24). We also obtained similar
conclusions in our experiment. The phosphorylation level of ERK in
C4-2 and PC-3 cells was significantly decreased after knocking down
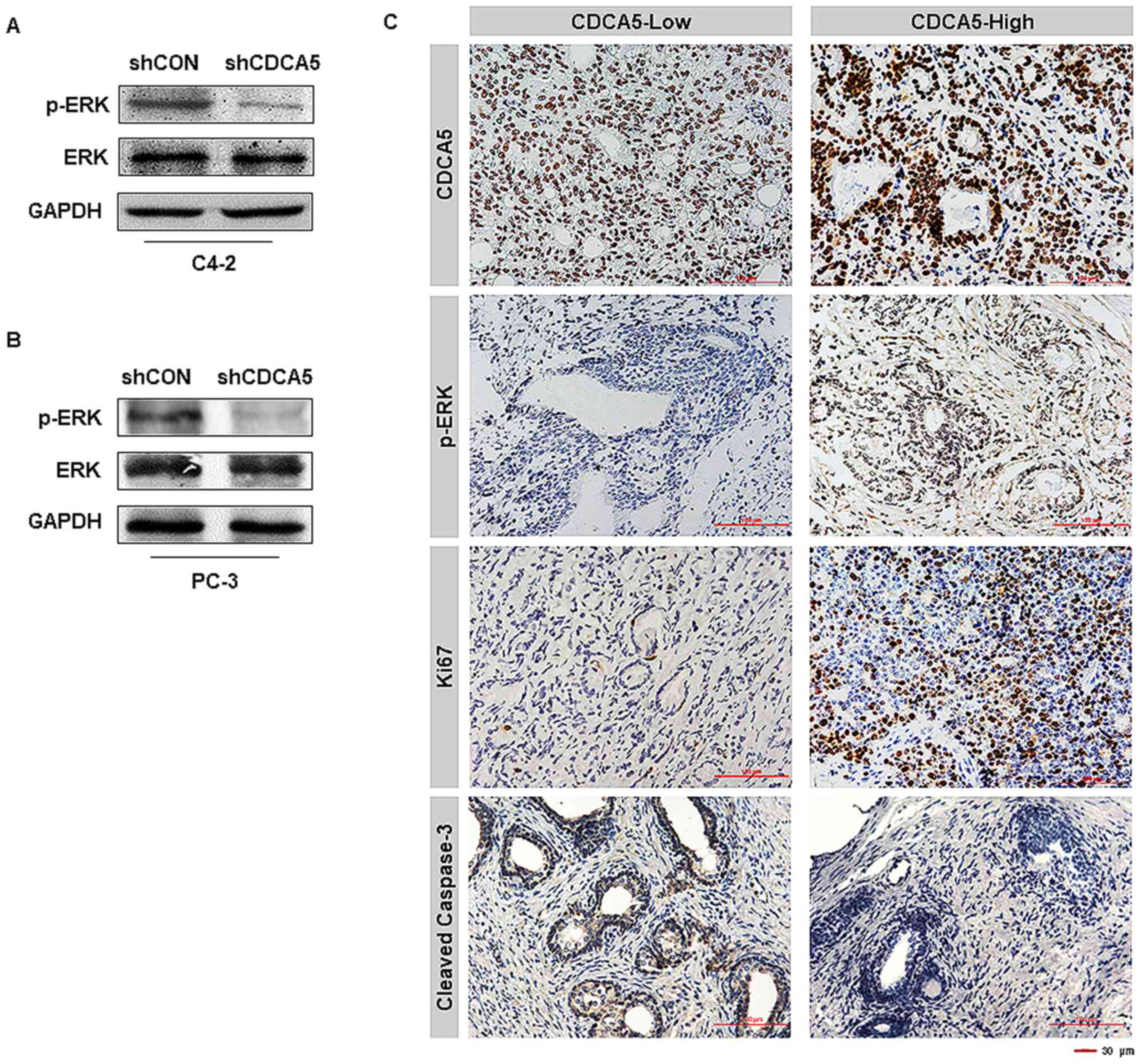
CDCA5 (Fig. 5A and B). IHC analysis
of the 96 clinical specimens aforementioned (CDCA5-low, n=32;
CDCA5-high, n=64) was conducted. Notably, it was revealed that PCa
patients with high levels of CDCA5 also had high ERK
phosphorylation levels and Ki67 expression levels (Fig. 5C). In contrast, the expression level
of caspase-3 in prostate cancer patients with high expression of
CDCA5 was low (Fig. 5C). This
revealed that highly expressed CDCA5 could promote the
proliferation of prostate cancer cells and inhibit their apoptosis.
In summary, it was inferred that CDCA5 may participate in the
activation of the ERK signalling pathway in prostate cancer.
Knockdown of CDCA5 suppresses prostate
tumour growth in vivo
To investigate the effect of CDCA5 expression on
prostate tumour growth, a mouse xenograft model was established.
Because the expression of CDCA5 protein in the PC-3 cell line was
higher than that of C4-2 (Fig. 1E),
PC-3 was selected for in vivo experiments. Another reason
for this selection was that PC-3 cells were more capable of forming
tumours in vivo. The six nude mice were equally divided into
two groups and were injected with shCON-PC-3 and shCDCA5-PC-3 cells
subcutaneously. Tumour formation was visible to the naked eye in
the nude mice two weeks later. Starting from the second week, the
sizes of the tumours were measured once a week and the tumour
growth curve was plotted. The tumours were removed at the fifth
week (Fig. 6A), and the weight of
the tumour was measured. It was revealed that the tumour growth
rate and weight were greater in the shCON-PC-3 group than in the
shCDCA5-PC-3 group (Fig. 6B and C).
This indicated that the growth of PC-3 cells was inhibited after
knocking down CDCA5 in vivo. Immunohistochemical staining on
the tumours was performed, and the results revealed that CDCA5
knockdown significantly decreased the phosphorylation of ERK
(Fig. 6D). Similar to previous
results, when CDCA5 was knocked down, the Ki67 expression level
decreased, and the cleaved caspase-3 level was increased in the
tumours (Fig. 6D). Proteins were
extracted from the aforementioned tumours and the relevant protein
markers were detected by western blotting. The experimental results
obtained were consistent with the IHC results (Fig. 6E). Thus, after knocking down CDCA5,
the proliferation ability of PCa cells was decreased, and apoptosis
was more likely to occur.
 | Figure 6.CDCA5 knockdown inhibits prostate
tumour growth in vivo. (A and B) Volumes of PC-3
subcutaneous xenograft tumours were compared between the shCDCA5
and shCON groups (n=6, *P<0.05). (C) Tumour weights were
compared between the shCDCA5 and shCON groups (n=6, *P<0.05).
(D) Representative images of the protein expression levels of
CDCA5, p-ERK, Ki67 and cleaved caspase-3 in xenograft tumours
(n=6). (E) Western blot analysis of CDCA5, ERK, p-ERK, Ki67 and
cleaved/pro caspase-3 expression levels in the tumours from the
shCDCA5 and shCON groups. GAPDH was used as an internal control.
CDCA5, cell division cycle-associated 5; sh, short hairpin; CON,
control; p-, phosphorylated; ERK, extracellular signal-regulated
kinase. |
Discussion
Malfunctions in cell division can lead to cancer
(25). Disturbance of cell cycle
regulation is an important biological feature of malignant tumours
and can lead to reduced apoptosis, unlimited proliferation, and
metastasis in malignant cells. Cell cycle disruption is one of the
most important causes of malignant tumours (26). Numerous cell cycle-related genes are
dysregulated in cancer, and these genes may be potential targets
for drug therapy (27,28). CDCA5 belongs to a family of cell
division cycle-associated proteins whose primary role is to promote
sister chromatid binding and to ensure accurate sister chromatid
segregation to maintain genomic integrity (3). CDCA5 regulates the activity of cell
cycle-associated proteins and transcription factors, promotes the
proliferation of cancer cells, and participates in the apoptosis of
cancer cells (5).
Researchers have revealed that CDCA5 was highly
expressed in endometrial cancer tissues (12), consistent with the findings of high
CDCA5 expression in liver (8) and
gastric cancer (29) tissues.
Nguyen et al also revealed that CDCA5 was upregulated in
lung cancer tissues (7), and poor
prognosis in non-small cell lung cancer patients was associated
with CDCA5 overexpression. In a study of urothelial carcinoma,
CDCA5 was revealed to play a crucial role in DNA repair, and CDCA5
overexpression was a predictor of poor prognosis (6). After searching the current literature,
it was revealed that there is little research on the correlation
between CDCA5 and prostate cancer pathogenesis. In the present
study, the upregulation of CDCA5 expression in prostate cancer was
confirmed using both clinical data and cell lines. The results of
the chi-square test indicated that high CDCA5 expression was
positively associated with the malignant degree of prostate
cancer.
CDCA5 is required for G2/M phase chromatid
condensation and stable binding and plays an important role in cell
cycle regulation (30–32). G2/M progression, as one of the main
checkpoints of the cell division cycle, is regulated by the protein
complex of cyclin B1/CDK1 (9,10,33).
It has been reported that inhibiting the expression of the CDCA5
gene can affect the cell division cycle of liver cancer cells and
colorectal cancer cells, resulting in a decrease in viability
(10,18). shRNA was used to knock down CDCA5 in
PCa cells and it was determined that their proliferative capacity
was significantly reduced, and G2/M progression in the cell cycle
was effectively blocked. These results are consistent with previous
research results (9,10).
The association of CDCA5 with tumour-associated
signalling pathways was further explored. It has been revealed that
ERK acts as a protein-regulating kinase involved in the regulation
of a variety of cellular processes, including proliferation,
differentiation, inflammation, transcriptional regulation and
development (34–38). Activation of the ERK signalling
pathway has been revealed to be closely associated with the
development of multiple tumours, including prostate cancer
(24). Because ERK is primarily
activated via phosphorylation (39,40),
the level of ERK phosphorylation in PCa cells after CDCA5 knockdown
was examined in vivo and in vitro. Notably, once
CDCA5 was suppressed, the phosphorylation level of ERK was
significantly reduced, and the activity of this signalling pathway
was diminished. In addition, it was revealed that patients with
prostate cancer with high CDCA5 levels also had high levels of ERK
phosphorylation. This again confirms that CDCA5 promotes prostate
cancer progression through the ERK signalling pathway. In addition
to the ERK signalling pathway, researchers revealed that CDCA5
could also inhibit the apoptosis of liver cancer cells through the
AKT signaling pathway (41); CDCA5
could regulate cyclin E1 to affect the progression of gastric
cancer (29); CDCA5 promoted
bladder cancer cell proliferation by upregulating two key
cell-cycle factors, cell division cycle protein 2 (CDC2) and cyclin
B1, and by activating the PI3K/AKT/mTOR pathway (42). These signalling pathways may become
the direction of follow-up progress in this research.
In conclusion, it was demonstrated that CDCA5 was
highly expressed in PCa tissues and that its upregulated expression
was significantly associated with poor outcomes. In vitro
and in vivo studies revealed that CDCA5 knockdown induced
G2/M phase arrest and inhibited PCa cell growth. In addition,
targeted silencing of CDCA5 expression by PCa reduced the activity
of the ERK signalling pathway, thereby inhibiting disease
progression. In summary, CDCA5 is anticipated to be an important
indicator for the early diagnosis and prognosis of prostate cancer.
We will further explore its role in the development and progression
of prostate cancer, thus providing more insight and new targets for
the clinical diagnosis and treatment of prostate cancer. The
present study also has some limitations. For example, the number of
specimens was not large enough; the specific mechanism of CDCA5 and
the ERK signalling pathway was not explored; and the upstream and
downstream proteins of CDCA5 have not been explored in depth. In
follow-up research, these shortcomings will be addressed.
In conclusion, CDCA5 was upregulated and affected
the prognosis in patients with PCa. Decreased expression of CDCA5
inhibited PCa cell proliferation by inhibiting the ERK signalling
pathway. Consequently, CDCA5 may be a potential therapeutic target
for PCa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present stuy was supported by the National
Natural Science Foundation of China (grant nos. 81872100 and
81772756) and the Natural Science Foundation of Tianjin (grant nos.
17JCZDJC35300, 17JCYBJC26000 and 18JCZDJC34800).
Availability of data and materials
The data used or analysed during the current study
are available from the corresponding author upon reasonable
request.
Authors contributions
TS, ZS and YN designed the experiments. JJ and YaL
performed the molecular biology experiments. JJ and YiL performed
the animal experiments and participated in the sequence alignment.
TS performed the statistical analysis. TS, YaL and JJ analysed the
data and wrote the manuscript. All the authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of The Second Hospital of Tianjin Medical University,
Tianjin, China (nos. KY2019K107 and YN2019Y99). For the collection
of clinical patient samples, written informed consent was obtained
from all patients. All studies were conducted in accordance with
the Declaration of Helsinki. The Ethics approval no. KY2019K107 was
used for human samples and the Ethics approval no. YN2019Y99 was
used for animal experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang N and Pati D: Sororin is a master
regulator of sister chromatid cohesion and separation. Cell Cycle.
11:2073–2083. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang N and Pati D: Handcuff for sisters:
A new model for sister chromatid cohesion. Cell Cycle. 8:399–402.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang N, Panigrahi AK, Mao Q and Pati D:
Interaction of Sororin protein with polo-like kinase 1 mediates
resolution of chromosomal arm cohesion. J Biol Chem.
286:41826–41837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeh CR, Hsu I, Song W, Chang H, Miyamoto
H, Xiao GQ, Li L and Yeh S: Fibroblast ERalpha promotes bladder
cancer invasion via increasing the CCL1 and IL-6 signals in the
tumor microenvironment. Am J Cancer Res. 5:1146–1157.
2015.PubMed/NCBI
|
|
7
|
Nguyen MH, Koinuma J, Ueda K, Ito T,
Tsuchiya E, Nakamura Y and Daigo Y: Phosphorylation and activation
of cell division cycle associated 5 by mitogen-activated protein
kinase play a crucial role in human lung carcinogenesis. Cancer
Res. 70:5337–in vivo5347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Chen J, Zhao L, Song W, Xuan Z,
Chen J, Li Z, Song G, Hong L, Song P, et al: CDCA5, Transcribed by
E2F1, promotes oncogenesis by enhancing cell proliferation and
inhibiting apoptosis via the AKT pathway in hepatocellular
carcinoma. J Cancer. 10:1846–1854. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen Z, Yu X, Zheng Y, Lai X, Li J, Hong
Y, Zhang H, Chen C, Su Z and Guo R: CDCA5 regulates proliferation
in hepatocellular carcinoma and has potential as a negative
prognostic marker. OncoTargets Ther. 11:891–901. 2018. View Article : Google Scholar
|
|
10
|
Shen A, Liu L, Chen H, Qi F, Huang Y, Lin
J, Sferra TJ, Sankararaman S, Wei L, Chu J, et al: Cell division
cycle associated 5 promotes colorectal cancer progression by
activating the ERK signaling pathway. Oncogenesis. 8:192019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Tang H, Thayanithy V, Subramanian
S, Oberg AL, Cunningham JM, Cerhan JR, Steer CJ and Thibodeau SN:
Gene networks and microRNAs implicated in aggressive prostate
cancer. Cancer Res. 69:9490–9497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HY, Jin N, Han YP and Jin XF: Pathway
crosstalk analysis in prostate cancer based on protein-protein
network data. Neoplasma. 64:22–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tolkach Y, Merseburger A, Herrmann T,
Kuczyk M, Serth J and Imkamp F: Signatures of adverse pathological
features, androgen insensitivity and metastatic potential in
prostate cancer. Anticancer Res. 35:5443–5451. 2015.PubMed/NCBI
|
|
14
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolden A, Bernard L, Jones D, Akinyeke T
and Stewart LV: The PPAR gamma agonist troglitazone regulates Erk
1/2 phosphorylation via a PPARgamma-independent, MEK-dependent
pathway in human prostate cancer cells. PPAR Res.
929052:20122012.
|
|
17
|
Caraglia M, Marra M, Leonetti C, Meo G,
D'Alessandro AM, Baldi A, Santini D, Tonini G, Bertieri R, Zupi G,
et al: R115777 (Zarnestra)/Zoledronic acid (Zometa) cooperation on
inhibition of prostate cancer proliferation is paralleled by
Erk/Akt inactivation and reduced Bcl-2 and bad phosphorylation. J
Cell Physiol. 211:533–543. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Xia C, Pu M, Dai B, Yang X, Shang
R, Yang Z, Zhang R, Tao K and Dou K: Silencing of CDCA5 inhibits
cancer progression and serves as a prognostic biomarker for
hepatocellular carcinoma. Oncol Rep. 40:1875–1884. 2018.PubMed/NCBI
|
|
19
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper LA, Demicco EG, Saltz JH, Powell
RT, Rao A and Lazar AJ: PanCancer insights from The Cancer Genome
Atlas: The pathologist's perspective. J Pathol. 244:512–524. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Methods Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen T, Li Y, Zhu S, Yu J, Zhang B, Chen
X, Zhang Z, Ma Y, Niu Y and Shang Z: YAP1 plays a key role of the
conversion of normal fibroblasts into cancer-associated fibroblasts
that contribute to prostate cancer progression. J Exp Clin Cancer
Res. 39:362020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai L, Ren Y and Cui T: Overexpression of
CDCA5, KIF4A, TPX2, and FOXM1 coregulated cell cycle
and promoted hepatocellular carcinoma development. J Comput Biol.
27:965–974. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu B, Wang N, Wang X, Tong N, Shao N, Tao
J, Li P, Niu X, Feng N, Zhang L, et al: MiR-146a suppresses tumor
growth and progression by targeting EGFR pathway and in a
p-ERK-dependent manner in castration-resistant prostate cancer.
Prostate. 72:1171–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lopez-Lazaro M: The stem cell division
theory of cancer. Crit Rev Oncol Hematol. 123:95–113. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boeynaems S, Tompa P and Van Den Bosch L:
Phasing in on the cell cycle. Cell Div. 13:12018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YC, Chang KC, Lin BW, Lee JC, Lai CH,
Lin LJ, Yen Y, Lin CS, Yang SJ, Lin PC, et al: The EGF/hnRNP Q1
axis is involved in tumorigenesis via the regulation of cell
cycle-related genes. Exp Mol Med. 50:1–14. 2018. View Article : Google Scholar
|
|
28
|
Lu J, Lin JX, Zhang PY, Sun YQ, Li P, Xie
JW, Wang JB, Chen QY, Cao LL, Lin Y, et al: CDK5 suppresses the
metastasis of gastric cancer cells by interacting with and
regulating PP2A. Oncol Rep. 41:779–788. 2019.PubMed/NCBI
|
|
29
|
Zhang Z, Shen M and Zhou G: Upregulation
of CDCA5 promotes gastric cancer malignant progression via
influencing cyclin E1. Biochem Biophys Res Commun. 496:482–489.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmitz J, Watrin E, Lenart P, Mechtler K
and Peters JM: Sororin is required for stable binding of cohesin to
chromatin and for sister chromatid cohesion in interphase. Curr
Biol. 17:630–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishiyama T, Ladurner R, Schmitz J, Kreidl
E, Schleiffer A, Bhaskara V, Bando M, Shirahige K, Hyman AA,
Mechtler K, et al: Sororin mediates sister chromatid cohesion by
antagonizing Wapl. Cell. 143:737–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rankin S, Ayad NG and Kirschner MW:
Sororin, a substrate of the anaphase-promoting complex, is required
for sister chromatid cohesion in vertebrates. Mol Cell. 18:185–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng W, Cai D, Zhang B, Lou G and Zou X:
Combination of HDAC inhibitor TSA and silibinin induces cell cycle
arrest and apoptosis by targeting survivin and cyclinB1/Cdk1 in
pancreatic cancer cells. Biomed Pharmacother. 74:257–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li P, Jia YF, Ma XL, et al: DEC2
suppresses tumor proliferation and metastasis by regulating
ERK/NF-kappaB pathway in gastric cancer. Am J Cancer Res.
6:1741–1757. 2016.PubMed/NCBI
|
|
35
|
Zhang L, Mao Y, Mao Q, Fan W, Xu L, Chen
Y, Xu L and Wang J: FLOT1 promotes tumor development, induces
epithelial-mesenchymal transition, and modulates the cell cycle by
regulating the Erk/Akt signaling pathway in lung adenocarcinoma.
Thorac Cancer. 10:909–917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li ZH, Li L, Kang LP and Wang Y:
MicroRNA-92a promotes tumor growth and suppresses immune function
through activation of MAPK/ERK signaling pathway by inhibiting PTEN
in mice bearing U14 cervical cancer. Cancer Med. 7:3118–3131. 2018.
View Article : Google Scholar
|
|
37
|
Wang S, Huang X, Li Y, Lao H, Zhang Y,
Dong H, Xu W, Li JL and Li M: RN181 suppresses hepatocellular
carcinoma growth by inhibition of the ERK/MAPK pathway. Hepatology.
53:1932–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B,
Yang J, Pan J, Hu S, Zhang C, et al: Tumor-derived lactate induces
M2 macrophage polarization via the activation of the ERK/STAT3
signaling pathway in breast cancer. Cell Cycle. 17:428–438. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumari G and Mahalingam S: Extracellular
signal-regulated kinase 2 (ERK-2) mediated phosphorylation
regulates nucleo-cytoplasmic shuttling and cell growth control of
Ras-associated tumor suppressor protein, RASSF2. Exp Cell Res.
315:2775–2790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Zhang GL, Sun X, Cao KX, Shang
YW, Gong MX, Ma C, Nan N, Li JP, Yu MW, et al: Gubenyiliu II
inhibits breast tumor growth and metastasis associated with
decreased heparanase expression and phosphorylation of ERK and AKT
pathways. Molecules. 22:7872017.doi: 10.3390/molecules22050787.
View Article : Google Scholar
|
|
41
|
Tian Y, Wu J, Chagas C, Du Y, Lyu H, He Y,
Qi S, Peng Y and Hu J: CDCA5 overexpression is an indicator of poor
prognosis in patients with hepatocellular carcinoma (HCC). BMC
Cancer. 18:11872018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu G, Xu Z, Chen X, Pan H, Wang Y and Jin
B: CDCA5 functions as a tumor promoter in bladder cancer by
dysregulating mitochondria-mediated apoptosis, cell cycle
regulation and PI3k/AKT/mTOR pathway activation. J Cancer.
11:2408–2420. 2020. View Article : Google Scholar : PubMed/NCBI
|