Introduction
Non-small cell lung cancer (NSCLC), including
adenocarcinoma, squamous cell carcinoma and large-cell carcinoma,
is the most common subtype of lung cancer (1,2).
Currently, the standard treatment options for patients with
advanced NSCLC include chemotherapy, targeted therapy and
immunotherapy, with traditional chemotherapeutics such as cisplatin
and paclitaxel (PTX), epidermal growth factor receptor tyrosine
kinase inhibitors (TKIs) and immune checkpoint inhibitors (3). However, tumor metastasis and drug
resistance limit the efficacy of these drugs (4). Thus, considerable efforts are focused
on identifying new targets and developing novel therapeutic
methods.
Mitochondrial outer membrane-anchored monoamine
oxidase (MAO) catalyzes the oxidative deamination of various
monoamines, including dopamine, 5-hydroxytryptamine,
norepinephrine, phenethylamine and tyrosine (5). MAOA, a subtype of MAO, is mainly
distributed in catecholaminergic neurons (6). MAOA inhibitors are primarily used for
treatment of nervous disorders, depression and anxiety (7). However, it was recently demonstrated
that overexpression of MAOA contributed to prostate cancer (PCa)
progression and the degree of malignancy (8,9).
Multiple studies have investigated the mechanism through which MAOA
promotes PCa progression and demonstrated that MAOA functionally
induces epithelial-to-mesenchymal transition (EMT) through
activation of a vascular endothelial growth factor
(VEGF)A/neuropilin-1 signaling pathway and the protein kinase B
(AKT)/FOXO1 pathway, stabilizing hypoxia-inducible factor-1α
(HIF-1α) by generating reactive oxygen species (9).
Overexpression of MAOA was also observed in Hodgkin
lymphoma and brain glioma (10).
Furthermore, MAOA inhibitors have shown promising inhibitory
effects on several types of tumors, such as PCa, classical Hodgkin
lymphoma and resistant gliomas (11–13).
The role of MAOA in NSCLC has also been reported, with MAOA
expression found to be significantly increased in cancerous tissue,
and possibly associated with the expression of EMT-promoting genes
(N-cadherin, Slug and TWIST) (14).
These results indicate that MAOA may play a pivotal role in
promoting the progression of NSCLC by mediating EMT. Thus, MAOA
inhibitors may hold considerable promise as treatment for
NSCLC.
Near-infrared heptamethine carbocyanine dyes have
been used for optical therapy and imaging due to their excellent
fluorescence properties, and can generate fluorescence emissions in
the range of 700–1,000 nm (15,16).
Of particular relevance is that some heptamethine carbocyanine dyes
preferentially accumulate in tumor tissues (17–19).
The selective accumulation of these dyes in neoplastic rather than
normal tissues is induced by organic anion transporting
polypeptides (OATPs), a hypoxic tumor environment and an increased
mitochondrial membrane potential in cancer cells (20–22).
Therefore, heptamethine carbocyanine dyes may serve as functional
selective transporters of therapeutic drugs to tumors. In our
previous study, the non-selective small molecule MAOA inhibitor
isoniazid was used to target MAOA, and was coupled with
heptamethine carbocyanine dyes via amide bonds to design and
synthesize tumor-targeting MAOA inhibitor-heptamethine carbocyanine
dye conjugates (23). Amongst these
conjugates, compound G10 demonstrated potent cytotoxicity against
PC-3 cells. The MAOA inhibitory activity of G10 was consistent with
the cell viability assay results. The aim of the present study was
to investigate the effects of G10 against NSCLC in vitro and
in vivo and elucidate the underlying antitumor molecular
mechanisms.
Materials and methods
Materials
4-Methylphenylhydrazine hydrochloride,
4-methoxyphenylhydrazine hydrochloride and other chemical
intermediates were purchased from Meryer Chemical Technology Co.,
Ltd.; 3-methyl-2-butanone and isoniazid were purchased from Aladdin
Reagent Company. Other reagents were all synthesized in the
laboratory. Primary antibodies against hypoxia-inducible factor-1α
(HIF-1α, cat. no. 3716, dilution 1:1,000), matrix metallopeptidase
2 (MMP2, cat. no. 87809, dilution 1:1,000), vascular endothelial
growth factor (VEGF, cat. no. 2463, dilution 1:1,000),
phosphorylated AKT (p-AKT, cat. no. 4060, dilution 1:2,000), AKT
(cat. no. 9272, dilution 1:1,000), p21 (cat. no. 2947, dilution
1:1,000) and β-actin (cat. no. 4970, dilution 1:2,000), were
obtained from Cell Signaling Technology, Inc.
Synthesis of G10
2,3,3,5-Tetramethyl-3H-indole (intermediate
compound 1A; 5 g; 28.9 mmol) and ethyl 4-bromobutyrate (28 g; 144
mmol) were added to 200 ml CH3CN and the reaction
proceeded under reflux for 40 h. After purification with silica
column chromatography, 4 g of a yellow oily liquid was obtained.
Subsequently, the obtained intermediate was dissolved in 20 ml 5
mol/l HCl and heated to reflux for 14 h to obtain 3 g of a
colorless liquid (intermediate compound 2A), with a yield of 81%.
5-Methoxy-2,3,3-trimethyl-3H-indole (5 g; 26.5 mmol) and
1-bromobutane (17.9 g; 132 mmol) were suspended in 200 ml
CH3CN, and the mixture was refluxed for 40 h. Colorless
liquid (intermediate compound 2B; 3.5 g; yield, 41%) was obtained
by purification with a silica gel column. Next,
1-phenylamino-5-phenylimino-1,3-pentadiene hydrochloride (1 g; 3.5
mmol), 2B (1.14 g; 3.5 mmol) together with 1 ml Et3N
were added to 30 ml acetic anhydride at 50°C for 1 h. After cooling
to an ambient temperature, the reaction mixture was poured into 500
ml Et2O and filtered to obtain 1.5 g of a brownish
precipitate (intermediate compound 3; yield, 83%). Subsequently,
intermediate 3 (1 g; 1.92 mmol) and intermediate 2A (649 mg; 1.92
mmol) were added to 30 ml pyridine and heated to 50°C with stirring
for 1 h. The mixture was evaporated under reduced pressure and
acidified to obtain intermediate compound 4 (300 mg; yield, 24%)
that was purified with a silica gel column. Finally, PyBOP (241 mg;
0.46 mmol), N,N-diisopropylethylamine (DIPEA; 59 mg; 0.46 mmol) and
intermediate 4 (300 mg; 0.46 mmol) were added to 100 ml
dichloromethane of ice-salt bath, and isoniazid (63 mg; 0.46 mmol)
was added. The resulting mixture was stirred for an additional 24 h
at room temperature, filtered, and the solvent was removed by
reduced pressure distillation and purified by silica gel to obtain
G10 (90 mg; yield, 26%).
Cell lines and culture
A549, NCI-H460 and PC9 human lung adenocarcinoma
cell lines were purchased from American Type Culture Collection.
Cells were cultured in RPMI-1640 medium supplemented with 10% FBS
(both from Gibco; Thermo Fisher Scientific, Inc.), and maintained
at 37°C in a humidified incubator with 5% CO2. The
chemoresistant cells were obtained after treatment with cisplatin,
PTX and erlotinib (MedChemExpress) at concentrations between 0.5
and 5 µM for 3 months.
MAOA enzymatic activity assay
MAOA enzymatic activity was measured in A549,
A549/CDDP, A549/PTX, H460, H460/CDDP, H460/PTX, PC9 and PC9/Er
cells as described previously (24). Briefly, cells were plated in culture
medium supplemented with 10% FBS in a 10-mm dish for 24 h. Through
extracting the reaction products, MAOA activity was measured using
a Cell MAOA assay kit (Shanghai Chengong Biotechnology) according
to the manufacturer's protocol, and was based on substrate
p-tyramine levels following treatment with the MAOB inhibitor
pargyline.
Cell viability assay
An MTT assay was used to determine the effects of
G10 on cell viability in vitro. Briefly, cells
(1×105 cells/ml) were seeded into 96-well culture plates
and incubated overnight. Subsequently, the cells were treated with
a range of concentrations (0.1–100 µM) of G10 for 72 h. MTT
solution (10 µl, 2.5 mg/ml) was added to each well, and the plates
were incubated for another 4 h at 37°C. After centrifugation (1,000
× g for 10 min at room temperature), the medium was replaced with
100 µl DMSO. The optical density of these samples was measured
using a Spectra Max Paradigm Reader (Molecular Devices, LLC) at 570
nm.
Flow cytometry analysis
Apoptosis analysis was performed using an Annexin
V-FITC apoptosis detection kit (BioVision, Inc.). Cells
(1×106) were treated with G10 for 48 h [RNA interference
cells were transfected with a specific MAOA siRNA or scramble siRNA
(Santa Cruz Biotechnology, Inc.) for 24 h]. Subsequently, cells
were collected by centrifugation (1,000 × g at 20°C for 5 min) and
resuspended in 500 µl binding buffer. Annexin V and PI were added
to the cells. After incubation at room temperature for 5 mins in
the dark, cells were analyzed using fluorescence activated cell
sorting using a flow cytometer (Becton, Dickinson and Company). For
autophagy analysis, the cells were treated with G10 for 48 h and
incubated with MDC dye, followed by flow cytometry.
Microarray analysis and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA from untreated and G10-treated H460/PTX
cells was isolated using an RNeasy Mini kit (Qiagen, Inc.)
according to the manufacturer's protocol. Changes in gene
expression were detected using an Affymetrix GeneChip PrimeView
Human Gene Expression Arrays. In order to confirm the changes in
specific gene expression, RT-qPCR was used. The sequences of the
primers are listed in Table
SI.
Transwell assays
Transwell chambers (Corning, Inc.) with
6.5-mm-diameter polycarbonate filters (pore size, 8 µm) were used
to determine the effect of G10 on A549/PTX and H460/PTX cell
migration and invasion in vitro (25). Briefly, the upper chambers of the
insert were coated with Matrigel and the lower chambers were filled
with fresh M200 medium (1% FBS) containing 10 ng/ml VEGF. After
trypsinization, the cells were resuspended at a concentration of
1×106 cells/ml in M200 containing 1% FBS and treated
with different concentrations of G10 for 30 min at room temperature
before seeding. A total of 100 µl of the cell suspension per well
was loaded into the upper wells, and the chamber was incubated at
37°C for 12 h. The cells were fixed and stained with Calcein-AM at
room temperature for 30 min. Cells on the upper surface of the
filter were removed by wiping with a cotton swab, and chemotaxis
was quantified using the high-content drug screening system
ImageXpressR Micro (Molecular Devices, LLC) by counting the cells
that had migrated to the lower side of the filter.
Western blot analysis
A total of 1×107 H460/PTX cells treated
with G10 were collected. Western blotting was performed as
previously described (26).
Briefly, total proteins (50 µg) were extracted from the cells
(determined by BCA method), resolved using 8–15% SDS-PAGE and
transferred to PVDF membranes. The membranes were blocked with 5%
non-fat dry milk at room temperature for three times (10 min/per
times). Primary and secondary antibodies (Thermo Fisher Scientific,
Inc.; cat. nos. 31430 and 31460, dilution 1:5,000) were then used
to visualize the resolved proteins. Finally, an enhanced
chemiluminescence system (ECL Plus; Amersham; Cytiva) was used to
develop the blots.
In vivo antitumor efficacy
studies
To evaluate the antitumor activity of G10 in
vivo, a total of 20 7–8-week old male BALB/c nude mice (weight,
18–22 g) were anesthetized with 3% sodium pentobarbital (50 mg/kg,
i.p.) and injected subcutaneously at the right flank with viable
(confirmed using trypan blue staining) H460/PTX cells
(5×106/100 µl PBS per mouse). When the mean subcutaneous
size of the tumor reached 100 mm3, the mice were
randomly divided into four groups treated with different
concentrations of G10 and a control group (4–6 mice per group). The
G10 single treatment group and PTX single treatment group were
administered G10 [19 mg/kg/2 days, intraperitoneally (i.p.); n=5]
or PTX (3 mg/kg/2 days, i.p.; n=5), respectively. The combination
group was treated with both G10 (19 mg/kg/2 days; i.p.; n=5) and
PTX (3 mg/kg/2 days; i.p.; n=5). Tumor size was measured with a
caliper every 3 days using the following formula: Tumor
volume=shortest diameter2 × longest diameter/2 (maximum
tumor volume, 930 mm3). Body weight was also recorded
every 3 days. After 21 days, the mice were sacrificed with cervical
dislocation, and lack of breathing and heartbeat for 3 min were
observed to confirm death. The study protocol complied with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the Shenyang Pharmaceutical University and all animal
experiments were approved by the Committee on the Ethics of Animal
Experiments of the Shenyang Pharmaceutical University.
Statistical analysis
Differences between experimental groups were
evaluated by one-way ANOVA followed by Turkey's post hoc test using
the SPSS v.11.5 for Windows (SPSS, Inc.). P<0.05 (two-tailed
test) was considered to indicate statistically significant
differences.
Results
Chemical synthesis of G10
The target compound (G10) was synthesized as shown
in Fig. 1 (23). First, intermediates 2A and 2B were
synthesized by SN2 nucleophilic substitution of 1A and
1B, with ethyl 4-bromobutyrate and 4-bromobutane as alkylating
agents, respectively. Subsequently, the asymmetric heptamethine
cyanine dye intermediate 4 was obtained by two-step aldol reactions
of 2A and 2B reacting with
1-phenylamino-5-phenylimino-1,3-pentadiene hydrochloride in acetic
anhydride, with pyridine as a catalyst. Finally, target compound
G10 was obtained by condensation of compound 4 with isoniazid in
the presence of DIPEA and PyBOP in anhydrous dichloromethane.
 | Figure 1.Synthesis of G10. (A) Ethyl
4-bromobutyrate, CH3CN, 75°C, 40 h; 5 mol/l HCl, 100°C,
16 h; (B) 4-bromobutane, CH3CN, 75°C, 40 h; (C)
1-phenylamino-5-phenylimino-1,3-pentadiene hydrochloride,
Et3N, acetic anhydride, 50°C, 1 h; (D) pyridine, 70°C, 1
h; and (E) isoniazid, PyBOP, DIPEA, anhydrous DCM, room
temperature. |
G10 significantly inhibits the
proliferation and viability of PTX-resistant NSCLC cells exhibiting
enhanced MAOA activity
In order to investigate the association between MAOA
and drug resistance in lung cancer, the MAOA activity was examined
in NSCLC cells resistant to chemotherapy (cisplatin and PTX),
erlotinib-resistant NSCLC cells and their parental cells. As shown
in Fig. 2A, the activity of MAOA
was increased in chemotherapy-resistant NSCLC cells, but not in the
TKI-resistant NSCLCs. In particular, PTX-resistant NSCLCs exhibited
notably increased MAOA activity compared with the parental cells.
Subsequently, cell viability was measured following treatment with
the novel MAOA inhibitor G10. As shown in Fig. 2B, G10 exerted potent inhibitory
effects on H460/PTX cells, and was more effective against the
PTX-resistant cells compared with the parental cells (H460); in
addition, the cytotoxicity of G10 was negatively correlated with
MAOA activity, suggesting that inhibition of MAOA could reverse PTX
resistance of NSCLC. To further confirm that G10 induced
PTX-resistant cell death, cell apoptosis was measured by flow
cytometry (Fig. 2C). The results
demonstrated that G10 induced cell apoptosis at relatively higher
concentration (10 µM). Of note, when MAOA gene expression was
knocked down, the apoptosis induced by G10 was abrogated
(supplementary Fig. S1),
indicating that cell apoptosis induced by G10 was dependent on MAOA
activity.
 | Figure 2.Relative MAOA activity and the
ability of G10 to reduce cell viability in lung cancer cells lines.
(A) Relative MAOA activity was determined using an MAOA enzymatic
activity assay. (B) The effect of G10 (0.1, 1, 10 or 100 µM) on the
viability of A549, A549/CDDP, A549/PTX, H460, H460/CDDP, H460/PTX,
PC9 and PC9/Er cells was measured using an MTT assay. The
IC50 values are shown. (C) Cell apoptosis was assessed
by flow cytometry analysis in A549/PTX cells. Annexin V-positive
cells were considered as apoptotic. *P<0.05, **P<0.01,
***P<0.001 vs. respective parental cell line. All experiments
were repeated ≥2 times. MAOA, monoamine oxidase A; PTX, paclitaxel;
CDDP, cisplatin; Er, erlotinib. |
G10 suppresses expression of
extracellular matrix (ECM)-related genes
Acquisition of invasive ability by epithelial cells
is required for metastasis (27).
It has been previously demonstrated that MAOA promotes the
proliferation, invasion and metastasis of PCa cells through
inducing EMT and stabilizing HIF-1α (9). Therefore, the effect of G10 on the
expression of ECM-related genes, changes in which are closely
associated with the migration, invasion and drug resistance of
tumor cells, were examined. Using Gene Ontology (GO) cellular
component (CC) analysis, the changes in the gene expression profile
of H460/PTX cells, found to be relatively sensitive to G10 in the
cell viability assay, following treatment with G10 were evaluated
(Fig. 3A). The differentially
expressed genes are involved in the intrinsic component of the
plasma membrane, ECM, proteinaceous ECM, nuclear pores, endoplasmic
reticulum lumen, anchored component of the membrane, cell surface,
voltage-gated calcium channel complexes, external side of the
plasma membrane and keratin filaments. Among these genes, the
distinct changes in the expression of genes associated with the ECM
that are closely associated with tumor cell migration, invasion and
drug resistance were further explored (28–30).
As shown in Fig. 3B, heatmap
analysis was used to further analyze these genes, and revealed that
changes in the expression levels of several genes associated with
tumor invasion and migration. As MMP2, MMP14 and COL6A have been
shown to be involved in the pathophysiological processes underlying
migration and invasion of several types of cancer cells (31–33),
the mRNA levels of MMP2, MMP14 and COL6A in H460/PTX cells
following G10 administration was further explored. Treatment with
G10 reduced the expression of MMP2, MMP14 and COL6A in H460/PTX
cells (Fig. 3C). Of note, in
addition to ECM-related genes, GO CC analysis suggested that the
expression of cell surface-related genes was also significantly
altered following treatment with G10. This may be explained by the
fact that cell surface proteins may transduce signals from the ECM
into the cells, which in turn regulate a range of cellular
functions, such as survival, proliferation, migration and
differentiation (34). Taken
together, these results suggest that G10 may suppress tumor
invasion and migration by affecting the expression of genes
associated with the ECM.
G10 suppresses A549/PTX and H460/PTX
cell migration and invasion in vitro
To determine whether G10 affects tumor cell
migration and invasion, Transwell assays were performed. First, to
avoid the direct cytotoxic effects of G10, Transwell assays were
performed in cells treated with non-cytotoxic concentrations of G10
(cell viability >80%), which was determined using an MTT assay
(data not shown). As shown in Fig.
4A, the number of migrating cells in both the A549/PTX and
H460/PTX cell lines was decreased significantly following treatment
with non-cytotoxic doses of G10. Similarly, G10 suppressed the
invasive ability of A549/PTX and H460/PTX cells in a
concentration-dependent manner (Fig.
4B). Taken together, these data suggest that G10 can inhibit
the migration and invasion of PTX-resistant cells, consistent with
the results of the GO CC analysis.
G10 reduces the expression of HIF-1α
and the phosphorylation of AKT, increases the expression of p21 and
induces autophagy
Upregulation of MAOA can activate HIF-1α and AKT
signaling (9). The transcription
factor HIF-1α mediates hypoxia by activating target genes
associated with the mechanisms involved in tumor progression, such
as increased tumor glycolysis, angiogenesis and metastasis
(35). Activated HIF-1α regulates
the expression of multiple genes in response to hypoxia, including
VEGF (a key regulator of angiogenesis) and MMP2, which is an
important enzyme promoting migration and invasion. Additionally,
MMPs and VEGF are both involved in the metastatic process through
modifying ECM components of neoplastic and stromal cells (36). HIF-1α, MMP2 and VEGF all participate
in tumor invasion, migration and metastasis. The role of the
MAOA-dependent HIF-1α/MMP2 and VEGF pathways on the inhibitory
effects of G10 on cell invasion was examined using H460/PTX cells.
As shown in Fig. 5A and B, G10
suppressed the expression levels of HIF-1α in a
concentration-dependent manner and inhibited the expression of its
target genes MMP2 and VEGF. Furthermore, the effects of G10 on the
EMT biomarker E-cadherin were also assessed. The data revealed that
G10 upregulated the expression of E-cadherin. These results suggest
that G10 exerts its inhibitory effects on tumor cell migration and
invasion through downregulating HIF-1α/MMP2 and VEGF under hypoxic
conditions.
 | Figure 5.Effects of G10 on the expression
levels of invasion-, migration- and proliferation-related proteins
and on the autophagy of PTX-resistant cell lines. (A-D) Protein
expression levels of HIF-1α, MMP2, VEGF, E-cadherin, p-AKT, AKT and
p21 were assessed using western blot analyses of H460/PTX cells
following treatment with G10 (0, 0.5 or 1 µM) for 48 h under
hypoxic conditions. Densitometry analysis of western blot data was
performed using the Quantity One system. β-actin expression was
used as the loading control. (E) MDC content was measured by flow
cytometry and was used to evaluate cell autophagy in A549/PTX
cells. Data are presented as the mean ± the standard error of the
mean, and experiments were repeated twice. *P<0.05 vs. control.
PTX, paclitaxel; HIF, hypoxia-inducible factor; MMP, matrix
metallopeptidase; VEGF, vascular endothelial growth factor; AKT,
protein kinase B; MDC, monodansylcadaverine dye. |
AKT, a serine/threonine kinase, requires
phosphorylation to be activated to regulate cancer cell
proliferation (37). G10 was shown
to suppress AKT phosphorylation, thereby inhibiting AKT signaling
(Fig. 5C and D). When AKT signaling
is activated, the expression of p21, a tumor suppressor gene that
is associated with tumor proliferation, is reduced. The results
also confirmed that G10 treatment increased the expression of p21
(Fig. 5C and D), suggesting that
inhibiting AKT signaling resulted in p21 activation. The mechanism
underlying the suppressive effect of G10 on tumor progression may
involve the regulation of the p-AKT/p21 signaling pathway. Due to
the involvement of the AKT pathway in autophagy, cell autophagy was
assessed using flow cytometry and was found to be induced by G10 in
a concentration-dependent manner (Fig.
5E), suggesting that autophagy may also be involved in the
antitumor effects of G10.
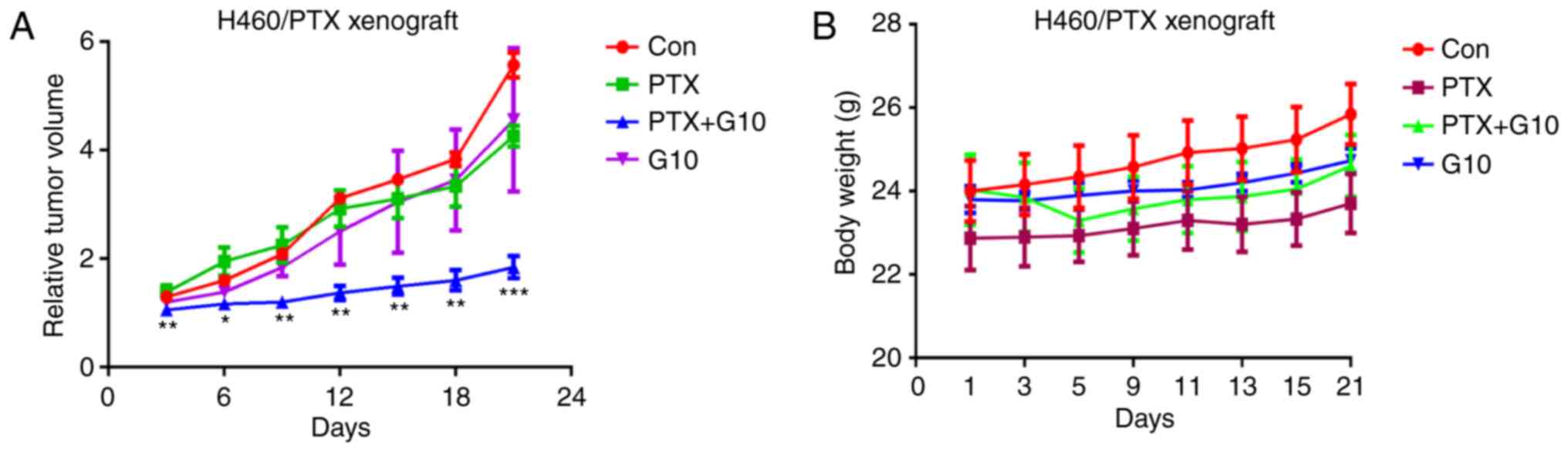
G10 combined with PTX reduces the
growth rate of H460/PTX xenografts in vivo
As G10 exerted prominent antitumor effects in
vitro, further investigation of the efficacy of G10 on the
growth of H460/PTX xenograft models was assessed. In view of the
obvious action of G10 on H460/PTX cells, they were selected to
establish xenograft models. The results revealed that treatment
with PTX exerted no significant effect on tumor growth (Fig. 6A), confirming that H460/PTX cells
were PTX-resistant. In addition, treatment with G10 alone was
ineffective in inhibiting tumor progression. However, combined
treatment with G10 and PTX significantly inhibited the growth of
the H460/PTX xenografts within a 3-week treatment period (Fig. 6A). During this combined treatment,
there were no notable differences in mouse body weight and visceral
index (organ weight vs. body weight) between the control and
treatment groups (Fig. 6B),
suggesting that there were no gross toxic effects induced by the
combination of G10 and PTX.
Discussion
Although MAOA appears to be a promising target for
cancer therapy, the role of MAOA in NSCLC progression requires
further elucidation. MAOA activity was assessed in a range of NSCLC
cell lines, and PTX-resistant NSCLC cells were shown to exhibit
increased MAOA activity. Therefore, the effects of G10, an
isoniazide conjugate of a heptamethine dye targeting tumors and
irreversible MAOA inhibitor, which was synthesized and described in
our previous study, on NSCLC were assessed in vitro and
in vivo. Despite being a notable structural transformation
of isoniazide, the isoniazide moiety may still be the critical
structure underlying the MAOA inhibitory activity of G10, based on
our previous molecular docking analysis, showing that the larger
cyanine dye could not enter into the active hydrophobic cavity of
MAOA. More importantly, recent studies verified that cyanine dyes
may serve as the vectors for targeting and delivering cargo to
mitochondria in cancer cells (38,39).
Nödling et al combined the mitochondria-targeting cyanine
dyes Cy3 and Cy5 with ciprofloxacin (Cip) or the carboplatin
derivative CPT, and evaluated the mitochondria-targeting ability
and the cytotoxicity of the conjugates in human cervical carcinoma
HeLa cells. The results revealed that the conjugates successfully
accumulated in the mitochondria of HeLa cells and were more
cytotoxic compared with their parent drug (increase in toxicity
from 100-fold to 1,000-fold), indicating that the conjugation of
drugs and cyanine dyes did not affect the uptake mechanism, but
rather altered the subcellular localization of the conjugates
(38). However, in the present
study, the application of heptamethine dye greatly improved the
antiproliferative activity of isoniazid against PTX-resistant NSCLC
cells. This likely contributes to the mitochondria-targeting
ability of heptamethine dyes and inhibition of the mitochondrial
outer membrane enzyme MAOA. Choi et al also summarized the
application of tumor-targeting heptamethine cyanine dyes in cancer
treatment. These drug-dye conjugates were used in several cancers,
such as brain cancer, Burkitt's lymphoma, prostate cancer and
breast cancer (39). However, to
the best of our knowledge, there has been no research on NSCLC
treatment by heptamethine cyanine dye-drug conjugates to date.
Hence, our findings may broaden the application of heptamethine
dye-drug conjugates. Of note, the findings of the present study
suggest that MAOA may be involved in the proliferation, migration
and invasion of PTX-resistant NSCLC cells, and inhibition of MAOA
may reverse PTX resistance of NSCLC. The conjugate G10 exerted a
notable inhibitory effect on the migration and invasion of A549/PTX
and H460/PTX cells. Additionally, G10 combined with PTX reduced the
growth rate of H460/PTX xenografts, whereas treatment with G10 or
PTX alone exerted no notable inhibitory effect. In conclusion, G10
may serve as an alternative treatment for PTX-resistant NSCLC.
However, the mechanisms underlying the exact role of MAOA in
PTX-resistant NSCLC require elucidation through further
experimentation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of materials and data
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XGY contributed to the conception and design of the
study, analyzed and interpreted the research data, and drafted the
manuscript. YYL was a major contributor to writing the manuscript
and conducted the cell viability assay. DXZ, WC and HL performed
the other activity tests. XYL and YXL were involved in the analysis
and interpretation of the data. DW contributed to the conception
and design of the study, critically revised the manuscript and
provided final approval of the version to be published. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study protocol complied with the recommendations
of the Guide for the Care and Use of Laboratory Animals of the
Shenyang Pharmaceutical University and all animal experiments were
approved by the Committee on the Ethics of Animal Experiments of
the Shenyang Pharmaceutical University.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Leighl NB, Wu YL and Zhong WZ:
Emerging therapies for non-small cell lung cancer. J Hematol Oncol.
12:452019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terlizzi M, Colarusso C, Pinto A and
Sorrentino R: Drug resistance in non-small cell lung cancer
(NSCLC): Impact of genetic and non-genetic alterations on
therapeutic regimen and responsiveness. Pharmacol Ther.
202:140–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shih JC, Chen K and Ridd MJ: Monoamine
oxidase: From genes to behavior. Annu Rev Neurosci. 22:197–217.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brunner HG, Nelen M, Breakefield XO,
Ropers HH and Van Oost BA: Abnormal behavior associated with a
point mutation in the structural gene for monoamine oxidase A.
Science. 262:578–580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz TL: A neuroscientific update on
monoamine oxidase and its inhibitor. CNS Spectr. 18 (Suppl
1):S25–S33. 2013. View Article : Google Scholar
|
|
8
|
Lin YC, Chang YT, Campbell M, Lin TP, Pan
CC, Lee HC, Shih JC and Chang PC: MAOA-a novel decision maker of
apoptosis and autophagy in hormone refractory neuroendocrine
prostate cancer cells. Sci Rep. 7:463382017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li
Y, Chen YT, Yin F, Liao CP, et al: Monoamine oxidase A mediates
prostate tumorigenesis and cancer metastasis. J Clin Invest.
124:2891–2908. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li PC, Siddiqi IN, Mottok A, Loo EY, Wu
CH, Cozen W, Steidl C and Shih JC: Monoamine oxidase A is highly
expressed in classical Hodgkin lymphoma. J Pathol. 243:220–229.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shih JC: Monoamine oxidase isoenzymes:
Genes, functions and targets for behavior and cancer therapy. J
Neural Transm (Vienna). 125:1553–1566. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kushal S, Wang W, Vaikari VP, Kota R, Chen
K, Yeh TS, Jhaveri N, Groshen SL, Olenyuk BZ, Chen TC, et al:
Monoamine oxidase A (MAO A) inhibitors decrease glioma progression.
Oncotarget. 7:13842–13853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu JB, Lin TP, Gallagher JD, Kushal S,
Chung LW, Zhau HE, Olenyuk BZ and Shih JC: Monoamine oxidase A
inhibitor-near-infrared dye conjugate reduces prostate tumor
growth. J Am Chem Soc. 137:2366–2374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu F, Hu L, Ma Y, Huang B, Xiu Z, Zhang
P, Zhou K and Tang X: Increased expression of monoamine oxidase A
is associated with epithelial to mesenchymal transition and
clinicopathological features in non-small cell lung cancer. Oncol
Lett. 15:3245–3251. 2018.PubMed/NCBI
|
|
15
|
Yang X, Shao C, Wang R, Chu CY, Hu P,
Master V, Osunkoya AO, Kim HL, Zhai HE and Chung LWK: Optical
imaging of kidney cancer with novel near infrared heptamethine
carbocyanine fluorescent dyes. J Urol. 189:702–710. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Shi C, Tong R, Qian W, Zhau HE,
Wang R, Zhu G, Cheng J, Yang VW, Cheng T, et al: Near IR
heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res.
16:2833–2844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Yan F, Wang F, Qin W, Wu G, Yang X,
Shao C, Chung LW and Yuan J: IR-780 dye for near-infrared
fluorescence imaging in prostate cancer. Med Sci Monit. 21:511–517.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Liu T, Zhang E, Luo S, Tan X and
Shi C: Preferential accumulation of the near infrared heptamethine
dye IR-780 in the mitochondria of drug-resistant lung cancer cells.
Biomaterials. 35:4116–4124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Long S, Li M, Gao F, Du J, Fan J
and Peng X: Bromo-pentamethine as mitochondria-targeted
photosensitizers for cancer cell apoptosis with high efficiency.
Dyes Pigments. 149:633–638. 2018. View Article : Google Scholar
|
|
20
|
Shi C, Wu JB and Pan D: Review on
near-infrared heptamethine cyanine dyes as theranostic agents for
tumor imaging, targeting, and photodynamic therapy. J Biomed Opt.
21:509012016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang E, Luo S, Tan X and Shi C:
Mechanistic study of IR-780 dye as a potential tumor targeting and
drug delivery agent. Biomaterials. 35:771–778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Usama SM, Lin CM and Burgess K: On the
mechanisms of uptake of tumor-seeking cyanine dyes. Bioconjug Chem.
29:3886–3895. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang XG, Mou YH, Wang YJ, Wang J, Li YY,
Kong RH, Ding M, Wang D and Guo C: Design, synthesis, and
evaluation of monoamine oxidase A inhibitors-indocyanine dyes
conjugates as targeted antitumor agents. Molecules. 24:14002019.
View Article : Google Scholar
|
|
24
|
Wu JB, Chen K, Li Y, Lau YF and Shih JC:
Regulation of monoamine oxidase A by the SRY gene on the Y
chromosome. FASEB J. 23:4029–4038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Chen G, Lu X, Wang S, Han S, Li Y,
Ping G, Jiang X, Li H, Yang J and Wu C: Novel chalcone derivatives
as hypoxia-inducible factor (HIF)-1 inhibitor: Synthesis,
anti-invasive and anti-angiogenic properties. Eur J Med Chem.
89:88–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang LH, Jiang XR, Yang JY, Bao XF, Chen
JL, Liu X, Chen GL and Wu CF: SYP-5, a novel HIF-1 inhibitor,
suppresses tumor cells invasion and angiogenesis. Eur J Pharmacol.
791:560–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kai F, Drain AP and Weaver VM: The
extracellular matrix modulates the metastatic journey. Dev Cell.
49:332–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brown Y, Hua S and Tanwar PS:
Extracellular matrix-mediated regulation of cancer stem cells and
chemoresistance. Int J Biochem Cell Biol. 109:90–104. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eble JA and Niland S: The extracellular
matrix in tumor progression and metastasis. Clin Exp Metastasis.
36:171–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43 (Suppl):S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiu KH, Chang YH, Wu YS, Lee SH and Liao
PC: Quantitative secretome analysis reveals that COL6A1 is a
metastasis-associated protein using stacking gel-aided purification
combined with iTRAQ labeling. J Proteome Res. 10:1110–1125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou T, Tong C, Kazobinka G, Zhang W, Huang
X, Huang Y and Zhang Y: Expression of COL6A1 predicts prognosis in
cervical cancer patients. Am J Transl Res. 8:2838–2844.
2016.PubMed/NCBI
|
|
34
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gonzalez-Avila G, Sommer B, Mendoza-Posada
DA, Ramos C, Garcia-Hernandez AA and Falfan-Valencia R: Matrix
metalloproteinases participation in the metastatic process and
their diagnostic and therapeutic applications in cancer. Crit Rev
Oncol Hematol. 137:57–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hafner C, Landthaler M and Vogt T:
Activation of the PI3K/AKT signalling pathway in non-melanoma skin
cancer is not mediated by oncogenic PIK3CA and AKT1 hotspot
mutations. Exp Dermatol. 19:e222–e227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nödling AR, Mills EM, Li X, Cardella D,
Sayers EJ, Wu SH, Jones AT, Luk LYP and Tsai YH: Cyanine dye
mediated mitochondrial targeting enhances the anti-cancer activity
of small-molecule cargoes. Chem Commun (Camb). 56:4672–4675. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi PJ, Park TIH, Cooper E, Dragunow M,
Denny WA and Jose J: Heptamethine cyanine dye mediated drug
delivery: Hype or hope. Bioconjug Chem. 31:1724–1739. 2020.
View Article : Google Scholar : PubMed/NCBI
|