Introduction
RNA helicases are enzymes that unwind nucleic acids
in an energy-dependent manner via hydrolysis of nucleotide
triphosphate (1); these enzymes are
implicated in processes involving RNA, including transcription,
splicing, RNA export, ribosome biogenesis, mRNA translation and RNA
decay (2–5). Moreover, novel functions of RNA
helicases include their activity as transcriptional coregulators
and regulators of post-translational modifications, and their
ability to modulate cellular signaling pathways such as
Wnt-ß-catenin, NF-κB and MAPK (6–9).
Deregulation of these RNA helicase functions may contribute to
tumor development and progression (10–12).
Dysregulation of RNA helicase expression levels, its mutation
status in tumors and its role in the regulation of molecules have
been implicated in cancer (13).
High-throughput sequencing technology, with its rapidly decreasing
costs and increasing applications, allows researchers to
investigate the biological function of RNA helicases in cancer,
which may be the key to ‘unwind’ cancer in the future.
DEAD-box helicase 41 (DDX41) is a highly conserved
protein considered essential for cell growth and viability
(14), which is mutated in primary
tumor and relapse samples from patients with acute myeloid leukemia
(AML) (15). Polprasert et
al (16) identified somatic
DDX41 mutations in myeloid neoplasms that resulted in loss of tumor
suppressor function due to altered pre-mRNA splicing and RNA
processing. RNA-sequencing (seq) analysis on peripheral bold
mononuclear cells of DDX41 mutation carriers with hematological
malignancy (HM) revealed altered expression levels of genes
involved in hemoglobin complex and innate immunity (17). The majority of germline mutations
are frameshift mutations, suggesting loss of function, with DDX41
serving as a tumor suppressor, which may impact initiation,
maintenance or progression of tumorigenesis (18). The identification of DDX41 mutations
has improved understanding of the potential mechanisms underlying
HM; however, the expression and mechanism by which DDX41
contributes to tumorigenesis is still unknown.
As an upstream DNA sensor of stimulator of
interferon gene (STING), DDX41 may serve dual roles in various
types of cancer. The preventive role of DDX41 in HM is associated
with inhibition of proliferation and promotion of apoptosis and
DDX41 negative regulates p21 at the translational level (19); in addition, DDX41 silencing promotes
apoptosis of HCT116 cells in a p21-dependent manner (19). These studies suggest that the role
of DDX41 may be tumor type-dependent. However, the precise function
of DDX41, and how it influences the formation and development of
tumors remains poorly understood.
DDX41 is also associated with the immune response.
Recent studies revealed that DDX41 primarily senses the viral
DNA/RNA hybrid and is required for the anti-retroviral innate
immune response to murine leukemia and HIV in primary mouse
macrophages and dendritic cells (DCs) (20,21).
Zhang et al (22) identified
DDX41 as an intracellular DNA sensor in myeloid DCs; silencing of
DDX41 was shown to block the activation of
STING-TANK-binding kinase 1 signaling and the transcription factors
NF-κB and interferon regulatory factor 3, resulting in decreased
production of type I interferon. As a pattern-recognition receptor,
DDX41 also recognizes bacterial di-GMP and di-AMP to activate the
immune response via the STING-dependent signaling pathway (23). Intravenous administration of
c-di-GMP into mice efficiently activates DDX41-STING signaling,
production of interferon and activation of natural killer (NK)
cells, and also facilitates antigen-specific cytotoxic T cell
activity, resulting in a significant antitumor effect in a lung
metastasis mouse model using malignant melanoma cells (24,25).
Thus, DDX41 may trigger the production of molecules involved in the
immune response.
Given the involvement of helicases in RNA splicing
(26), Polprasert et al
(16) expressed epitope-tagged
versions of wild-type (WT) and R525H-mutated DDX41 in 293 cells.
Epitope pull-down coupled with peptide sequencing analysis revealed
that spliceosome proteins constitute a DDX41-associated group,
which includes pre-mRNA processing factor 8 and splicing factor 3B
subunit 1 (16). Furthermore, DDX41
mutation in the DEAD domain (such as R525H) alters this
interaction, especially for major components in the U2 and U5
spliceosomes (16). In addition,
DDX41 has been reported to be involved in pre-RNA splicing and
DDX41 silencing may be associated with increased exon skipping
(16). DDX41 is specifically
recruited to the catalytically active C complex, which performs the
second step of splicing, in which the 5′ and 3′ exons are ligated
and an intronic lariat is released (27,28).
These findings indicated that DDX41 may lead to the impairment of
RNA processing and pre-mRNA splicing, which may partially
contribute to tumorigenesis.
The present study aimed to investigate the function
of DDX41 in HeLa cells and cervical cancer. DDX41 was cloned and
overexpressed in HeLa cells. RNA-sequencing (RNA-seq) analysis of
the effect of DDX41 on the transcriptome and alternative splicing
of the overexpressed and normal control cell samples was performed.
These results may improve understanding of the biological role of
DDX41 in cancer.
Materials and methods
DDX41 cloning and plasmid
construction
Primer pairs used for Hot Fusion were designed using
CE Design v1.04 (Vazyme Biotech Co., Ltd.) with gene-specific
sequences and oligonucleotides encoding 3× FLAG tag were added
before the TAG termination codon, along with a portion of the
vector pIRES-hrGFP-1a sequence, each with a 17–30 bp overlap. The
following primers were used: Forward,
5′-agcccgggcggatccgaattcATGGAGGAGTCGGAACCCG-3′; and reverse,
5′-gtcatccttgtagtcctcgagGAAGTCCATGGAGCTGTGGGC-3′. Non-capitalized
letters indicate sequence for homologous recombination during
cloning, whereas capitalized letters indicate sequence paired with
DDX41 gene. Vector pIRES-hrGFP-1a was digested by
EcoRI and XhoI (New England BioLabs, Inc.) at 37°C
for 2–3 h. The enzyme-digested vector was then run on a 1.0%
agarose gel and purified using a QIAquick Gel Extraction kit (cat.
no. 28704; Qiagen, Inc.). Total RNA from HeLa cells was isolated
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). Then, 1 µg total RNA was transcribed into cDNA using a
PrimeScript™ RT Reagent kit (Takara Bio, Inc.), according to the
manufacturer's protocol. The thermocycling condition were as
follows; 98°C for 30 sec; 28 cycles of 98°C for 10 sec and 60°C for
30 sec; final extension at 72°C for 1 min and holding at 4°C.
Linearized vector digested by EcoRI and XhoI (New
England BioLabs, Inc.) and the PCR insert (1,866 bp) were added to
a PCR microtube and cloned using the ClonExpress® II One
Step Cloning kit (Vazyme Biotech Co., Ltd.), according to the
manufacturer's protocol. Plasmids were introduced into
Escherichia coli by chemical transformation. Cells
(5×106) were plated onto LB plates containing ampicillin
and incubated overnight at 37°C. Colonies were screened using a
TaKaRa Taq™ HS PCR kit (Takara Bio, Inc.) according to the
manufacturer's protocol, with M13 primers (forward,
5′-TGTAAAACGACGGCCAGT-3′ and reverse, 5′-CAGGAAACAGCTATGACC-3′).
The PCR insert was verified by Sanger sequencing.
Cell culture and transfection
The human HeLa cell line was purchased from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences, Shanghai, China. Cells were seeded into a petri dish (100
mm) at a density of 1×105 cells/ml and cultured at 37°C
with 5% CO2 in DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences), penicillin (100 U/ml) and streptomycin
(100 g/ml). Transfection of 1×105 HeLa cells with 0.5 µg
DDX41 overexpression or empty plasmid was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Transfected cells were harvested after 48 h for subsequent
experimentation.
Assessment of DDX41
overexpression
GAPDH was used as an internal control. cDNA
synthesis was performed according to standard procedure (29) and reverse transcription-quantitative
(RT-q)PCR was performed on the Bio-Rad S1000 system with Bestar
SYBR Green RT-PCR Master Mix kit according to the manufacturer's
instructions. (DBI Bioscience; Shanghai Xinghan Biotechnology Co.,
Ltd.). The concentration of each transcript was then normalized to
the GAPDH mRNA levels using the 2−ΔΔCq method
(30). Comparisons were performed
by one-way ANOVA using GraphPad Prism 8 software (GraphPad
Software, Inc.).
Western blot analysis
In brief, for preparation of total cell lysates,
normal and DDX41-overexpressing HeLa cells were lysed in
RIPA buffer containing 50.0 mM Tris-HCl (pH, 7.4), 150.0 mM NaCl,
1.0% deoxycholate, 1.0% Triton X-100, 1.0 mM EDTA and 0.1% SDS. The
samples were centrifuged (10,000 × g; 5 min; 4°C). The protein
level was determined by Pierce™ BCA protein assay kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Then, 50 µg protein was incubated for 10 min in
boiling water with 1X SDS sample buffer, separated by SDS-PAGE on a
10% gel, then transferred to a PVDF membrane (EMD Millipore, Inc.).
The membranes were blocked with 5% skimmed milk [in buffer
containing 10 mM Tris (pH, 8.0), 150 mM NaCl and 0.05% Tween-20]
for 1 h. DDX41 was detected using monoclonal Flag antibody
(1:2,000; cat. no. F7425; Sigma-Aldrich; Merck KGaA) with actin as
a control (1:2,000; cat. no. AC038; ABclonal Biotech Co., Ltd.)
diluted in TBST (0.05% Tween-20) at 4°C overnight. The blots were
incubated with horseradish peroxidase-conjugated secondary antibody
(goat anti-rabbit IgG; 1:10,000; cat. no. ab7090; Abcam) for 1 h at
room temperature. Bands were detected using enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.).
MTT assay
MTT assay was used to measure cell proliferation.
Briefly, cells (1×104) were seeded in 96-well culture
plates containing 200 µl cell growth medium. Upon reaching 70%
confluence, the HeLa cells were transfected with DDX41
overexpression vector or control vector using Lipofectamine 2000
according to the manufacturer's protocol. The cells were then
incubated at 37°C for 48 h. Subsequently, 0.025 ml MTT solution (5
mg/ml) was added to each well and cells were incubated for 4 h.
Following centrifugation (5,000 × g; 5 min; room temperature), the
supernatant was removed from each well. The colored formazan
crystals produced from MTT in each well were dissolved in 0.15 ml
DMSO and the optical density values were measured at 490 nm.
Flow cytometric analysis of cell
apoptosis
HeLa cells (5×104) were seeded in 24-well
culture plates. At 70% confluence, the cells were transfected with
the DDX41 overexpression or control vector using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The cells were then
incubated at 37°C for 48 h and viable cells were harvested and
washed twice with PBS. The cell pellets were resuspended in 400 µl
ice-cold 1X binding buffer at ~1×106 cells/ml, then
incubated with 10 µl FITC-conjugated Annexin V/7-AAD (Beijing 4A
Biotech Co., Ltd.) for 10 min in the dark at room temperature.
Samples were analyzed within 1 h of staining using a A00-1-1102
CytoFLEX flow cytometer (Beckman Coulter, Inc.) and Flow-Jo
software (v8.8.6; Tree Star, Inc.).
Library preparation and
sequencing
Total RNA was extracted from HeLa cells using
TRIzol. The RNA was further purified with two phenol-chloroform
treatments and then treated with RQ1 DNase (Promega Corporation) at
37°C for 10 min to remove DNA. The quality and quantity of the
purified RNA were determined by measuring the absorbance at 260/280
nm using Smartspec Plus (Bio-Rad Laboratories, Inc.). The integrity
of RNA was further verified by 1.5% agarose gel electrophoresis.
For each sample, 1 µg total RNA was used for RNA-seq library
preparation using the VAHTS Stranded mRNA-seq Library Prep kit
(cat. no. NR612-01; Vazyme Biotech Co., Ltd.). Polyadenylated mRNA
was purified and fragmented, and then converted into double
stranded cDNA. Following end repair and A tailing, DNA was ligated
to VAHTS RNA Adapters (Vazyme Biotech Co., Ltd.). Purified ligation
products corresponding to 200–500 bp were digested with heat-labile
uracil-DNA glycosylase and the single stranded cDNA was amplified,
purified, quantified and stored at −80°C before sequencing. Next,
the 3M library was sequenced on an Illumina HiSeq X Ten platform in
a 2 × 150 bp paired-end mode using HiSeq X Ten reagent kit v2.5
(cat. no. FC-501-2501; Illumina, Inc.). The fastq files were
produced using the CASAVA pipeline v1.8.2 (Illumina, Inc.).
Preliminary quality control analysis of the fastq files was
performed using FASTQC software v 1.11.4 (fastqc.software.informer.com/).
Data processing and alignment
Raw reads containing >2-N bases were first
discarded. Adaptors and low-quality bases were then trimmed from
the raw sequencing reads using the FASTX-Toolkit (Version 0.0.13;
hannonlab.cshl.edu/fastx_toolkit/). Short reads
(<16 nucleotides in length) were also excluded. Clean reads were
aligned to the GRCh38 genome by Tophat2 2.0.0 (ccb.jhu.edu/software/tophat/index.shtml) allowing four
mismatches. Uniquely mapped reads were used to calculate the read
number and fragments per kilobase of transcript per million
fragments mapped for each gene.
Differentially expressed genes (DEGs)
analysis
The R Bioconductor package edgeR 3.12 (31) (bioconductor.org/packages/release/bioc/html/edgeR.html)
was utilized to screen out DEGs. A false discovery rate (FDR)
<0.05 and fold change >1.5 or <0.7 were set as the cut-off
criteria for identifying DEGs. In order to predict the gene
function and calculate the functional category distribution
frequency, Gene Ontology (GO) and enriched Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathways were identified using the KOBAS
2.0 server (kobas.cbi.pku.edu.cn/) (32). Hypergeometric tests and the
Benjamini-Hochberg FDR controlling procedure were used to define
the enrichment of each pathway (corrected P-value <0.05).
Alternative splicing analysis
The alternative splicing events (ASEs) and regulated
ASEs (RASEs) in the samples were defined and quantified using the
ABLas pipeline, as described previously (33). In brief, detection of eight types of
ASE was based on the splice junction reads. The eight types of ASE
included cassette exon (CassetteExon), exon skipping (ES), mutually
exclusive ES (MXE), alternative 5′ splice site (A5SS), alternative
3′ splice site (A3SS), MXE combined with an alternative 5′ promoter
(5pMXE) and MXE combined with an alternative polyadenylation site
(3pMXE). After detecting the ASEs in each RNA-seq sample, the
paired t-test was chosen to calculate the P-value, with the
alternative reads and model reads of samples as input data. The
changed ratio of alternatively spliced reads and constitutively
spliced reads between compared samples was defined as the RASE
ratio. P<0.05 and RASE ratio >0.2 were set as the threshold
for RASE detection.
RT-qPCR validation of DEGs and
ASEs
In order to confirm the validity of the RNA-seq
data, RT-qPCR was performed for selected DEGs and normalized to the
reference gene GAPDH. The primer sequences are presented in
Table SI. The same RNA samples for
RNA-seq were used for qPCR. The PCR conditions consisted of
denaturation at 95°C for 10 min, followed by 40 cycles of
denaturing at 95°C for 15 sec and annealing and extension at 60°C
for 1 min. A total of three independent repeats was performed for
each sample. RT-qPCR assay was also used to analyze ASEs in HeLa
cells. PCR primer pairs (Table SI)
were designed to amplify the long and short splicing isoforms in
the same reaction. In order to detect one of the alternative
isoforms, one primer was designed for the alternative exon, and an
opposing primer was designed for the constitutive exon; to detect
the other alternative isoforms, a boundary-spanning primer was
designed for the sequence encompassing the exon-exon junction, with
the opposing primer in a constitutive exon. The changed ratio of AS
events in RNA-seq was calculated using the formula: AS1 junction
reads/AS1 junction reads + AS2 junction reads. The altered ratio of
AS events in RT-qPCR was calculated using the formula: AS1
transcripts level/AS2 transcripts level.
Downloading data
The RNA-seq data of cervical cancer samples were
downloaded from The Cancer Genome Atlas (TCGA) database (xenabrowser.net/datapages/) to analyze the
expression levels of DDX41-regulated genes. Tumor suppressor genes
were downloaded from TSGene 2.0 database (bioinfo.mc.vanderbilt.edu/TSGene/). The oncogenes were
downloaded from an oncogene database (ongene.bioinfominzhao.org/index.html).
Gene expression profiling interactive
analysis (GEPIA)
GEPIA was used to analyze RNA-seq expression data of
9,736 tumors and 8,587 normal samples from TCGA and Genotype-Tissue
Expression (GTEx) projects, using a standard processing pipeline
(gepia.cancer-pku.cn/). GEPIA provides customizable functions, such
as tumor or normal differential expression analysis, profiling
according to cancer types or pathological stages, patient survival
analysis, similar gene detection, correlation analysis and
dimensionality reduction analysis.
Immunofluorescence assay
HeLa cells were placed on glass slides using a
cytospin (Shandong Junteng Medical Technology Co., Ltd.). Cells
were fixed in 4% paraformaldehyde and permeabilized in 1% Triton
X-100/PBS at room temperature for 15 min. Cells were incubated with
primary antibody for DDX41 (1:100; cat. no. A6576; ABclonal) for 1
h at room temperature in a humidified chamber, washed with 0.1%
Tween-20/PBS and incubated with species-appropriate FITV-conjugated
secondary antibody (1:500; cat. no. AS011; ABclonal) for 1 h at
room temperature. Slides were mounted with 100 ng/ml 4,6
diamidino-2-phenylindole Vectashield (Vector Laboratories, Inc.;
Maravai Life Sciences) to stain nuclei at room temperature for 15
min and cells were visualized using an Olympus AX70 epifluorescence
microscope (Olympus Corporation) with triple color filters and a
20× air objective. Numeric aperture varied automatically according
to the light emitted. Images were captured with a Zeiss Axiocam
camera (Zeiss GmbH).
Statistical analysis
Data are presented as the mean ± SD of ≥3
independent repeats. The statistical difference between groups was
analyzed using one-way ANOVA with SPSS software (version 17.0;
SPSS, Inc.). Patient survival analysis was conducted using the
Kaplan-Meier method and analyzed using the log-rank test. The
heatmap analysis compared the data obtained from DEG of RNA-seq
(34) and sashimi plot
representation of RNA-seq data was used in the Integrative Genomics
Viewer (35). P<0.05 was
considered to indicate a statistically significant difference.
Results
DDX41 inhibits proliferation and
promotes apoptosis of HeLa cells
Previous studies have reported that in AML cell line
xenografts in a mouse model, DDX41 knockdown accelerates
tumor growth compared with control cells (16,48).
In order to investigate the potential biological function of DDX41
in cervical cancer, DDX41-3×Flag plasmids was stably
overexpressed in HeLa cells and compared with empty
vector-transfected cells. The results showed significantly
increased expression levels of DDX41, as assessed by western
blotting and RT-qPCR (Fig. 1A). MTT
assay was performed to determine cell proliferation; overexpression
of DDX41 suppressed HeLa cell proliferation (Fig. 1B). In order to investigate the
function of DDX41 in cell apoptosis, flow cytometry was performed.
The results revealed that DDX41 overexpression significantly
induced apoptosis of HeLa cells (Fig.
1C). By immunostaining with DDX41 antibody, DDX41 protein was
detected both in nucleus and cytoplasm (Fig. S2B). Therefore, DDX41 may serve as a
tumor suppressor in HeLa cells.
RNA-seq analysis of the impact of
DDX41 overexpression on the HeLa cell transcriptome
In order to analyze the gene expression profile of
DDX41, RNA-seq was performed for 83±4 million total raw
reads per sample (Table SII).
Filtered reads were aligned to the human GRCH38 genome by Tophat2
(36) and expression patterns of
28,914 known annotated genes were detected and characterized
(Table SIII). edgeR (31) and (cutoff, fold change ≥1.5 or ≤0.7;
P<0.01) was used to identify the DEGs between the
DDX41-overexpressing cells and controls. An M-versus-A plot
was constructed to display the DEGs that were associated with
DDX41 overexpression and identified 504 up- and 455
downregulated genes (Fig. 2A;
Table SIV). Heatmap analysis of
the expression patterns of all DEGs in each sample was performed;
the results showed high consistency for DDX41-mediated
transcription in all datasets (Fig.
2B).
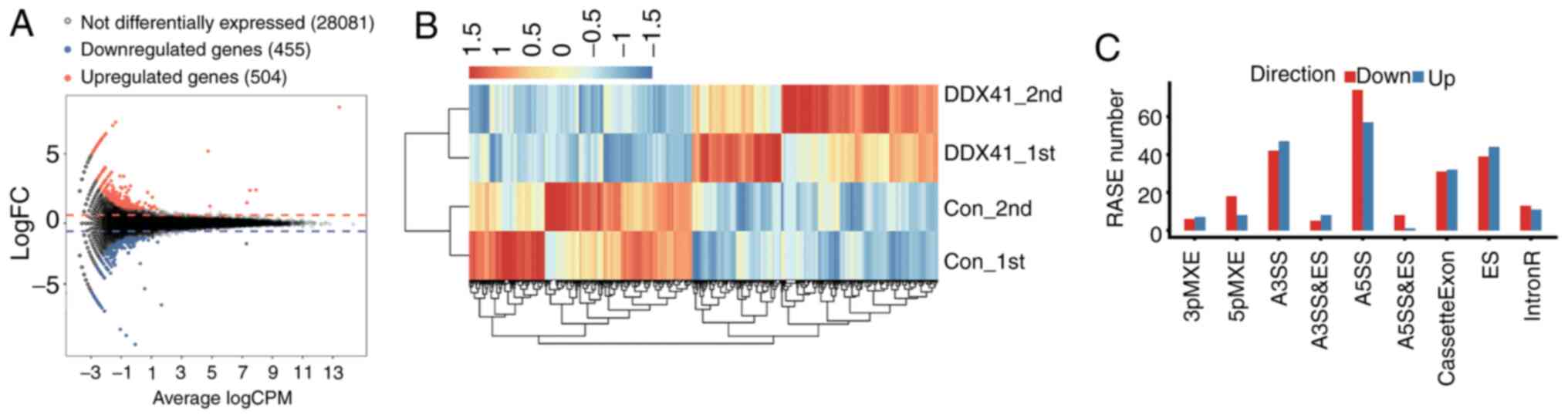 | Figure 2.Differential gene expression and ASEs
in response to DDX41 overexpression. (A) Detection of
DDX41-regulated genes on the M-versus-A plot. Red, upregulated
(FC≥1.5, P-value<0.01); blue, downregulated (FC≤0.7,
P-value<0.01). (B) Hierarchical clustering of the expression
levels of 959 DEGs regulated by DDX41 overexpression in HeLa
cells expressing either the control or DDX41 plasmid. (C)
Classification of different alternative spicing types regulated by
DDX41 protein. FC, fold change; RASE, regulated alternative
splicing events. 3pMXE, mutually exclusive 3′ untranslated regions;
5pMXE, mutually exclusive 5′ untranslated regions; A3SS,
alternative 3′ splice site; A5SS, alternative 5′ splice site; ES,
exon skipping; IntronR, intron retention. |
The ABLas software tool (under permission) was used
to analyze global changes in DDX41-mediated ASEs in HeLa cells. A
total of 20,532 annotated ASEs were detected when comparing these
uniquely mapped reads to the reference genome, as well as 60,654
novel ASEs (Table SV). A stringent
cutoff of P≤0.05 was set to identify high-confidence RASEs and 707
RASEs were identified (Tables SVI
and SVII). DDX41-regulated ASEs
included 24 known intron retention events, 63 CassetteExons, 83 ES,
89 A3SS and 131 A5SS. The other event types included 11 3pMXE, 26
5pMXE, 13 A3SS + ES, 9 A5SS + ES and 18 MXE (Fig. 2C). These results suggested that
DDX41 primarily regulated A5SS, A3SS and ES events in HeLa
cells.
DDX41 regulates transcription and
alternative splicing of certain tumorigenesis-associated genes
In order to test the hypothesis that DDX41 may
regulate tumorigenesis-associated gene transcription, a gene
dataset was constructed by downloading tumor suppressor genes from
TSGene 2.0 (bioinfo.mc.vanderbilt.edu/TSGene/) (37) and oncogenes from an oncogene
database (ongene.bioinfominzhao.org/index.html). The
tumorigenesis-associated gene dataset was overlapped with
DDX41-regulated genes (RNA-seq-identified DEGs). Heatmap analysis
of the expression patterns of the overlapped genes was performed
and showed a high consistency for DDX41-regulated gene expression
in each sample (Fig. 3A). Certain
oncogenes were downregulated by DDX41, such as LCN2, TP63, KIT,
MYB, CDK5R2 and TNFRSF1B, whereas others were
upregulated, including PANO1, GLI1, HOMER2 and RND3
(Fig. 3A). In order to assess the
association between DDX41-regulated expression levels of oncogenes
and tumor suppressors, expression data from CESC and control
samples were downloaded (xenabrowser.net/datapages/) and analyzed. Expression
levels of five oncogenes, TP63, LCN2, MYB, CSF2 and
CYP24A1, were upregulated (Fig.
3B), whereas the expression levels of tumor suppressors
GLI1 and PANO1 were downregulated in cancer samples
(Fig. 3C).
In order to identify the cellular function of
DDX41-regulated alternatively spliced genes, high-confidence
regulated alternatively spliced genes were analyzed by performing
GO and KEGG enrichment analysis. DDX41-regulated genes with
alternative splicing were enriched in a number of
tumorigenesis-associated pathways, including ‘EGFR signaling’,
‘FGFR signaling’, ‘MAPK signaling’ and ‘prostate cancer’ (Fig. 4A). DDX41-regulated
tumorigenesis-associated genes included FGFR1 and
FGFR4 (Fig. 4B).
DDX41 selectively regulates
transcription of immunity-associated genes in HeLa cells
In order to assess the functional role of DDX41, all
959 DEGs were subjected to GO and KEGG annotation to identify their
potential biological roles. Based on the cut-off criteria, the
results showed that upregulated DEGs were enriched in six GO terms
and downregulated DEGs were enriched in 30 GO terms (Tables SVIII and SIX). In the GO analysis, the upregulated
genes were enriched in ‘regulation of transcription’ (Fig. S1A), while the downregulated genes
were enriched in ‘cell adhesion’, ‘extracellular matrix
organization’ and ‘angiogenesis’ (Fig.
5A). KEGG analysis showed that upregulated genes were
associated with ‘antigen processing and presentation’,
‘endocytosis’ and ‘MAPK signaling pathway’ (Fig. 5B; Table
SX). Moreover, the downregulated genes were enriched in ‘cell
adhesion molecules’, ‘cytokine-cytokine receptor interaction’ and
‘B cell receptor signaling pathway’ (Fig. S1B; Table SXI. DDX41-downregulated
immune-associated genes included CD22, CD177, CSF2, C4A, SELPLG,
TNFRSF1B, HLA-DOB, HLA-DMA, GH1 and PDGFC.
In order to investigate the DDX41-regulated
transcription of immune-associated genes, heatmap analysis of these
genes was performed, demonstrating a high consistency for
DDX41-regulated transcription in both replicates (Fig. S1C). Expression levels of these
genes were confirmed via qPCR analysis, showing a significant
change in expression levels of CD22, CD177, SCF2, IL12A and
TNFRSF1B, in agreement with results of RNA-seq analysis
(Fig. 5C). HeLa is a
well-characterized HPV+ cervical cancer cell line, so
activated expression of immune response genes by DDX41
overexpression may have resulted from an effort to reactivate
expression of the HPV genome (38,39).
In order to assess this possibility, expression levels of HPV genes
E6, E7 and L1 were evaluated via qPCR with the primer pairs shown
in Table I, in ref. 40. None of
these HPV genes showed significantly increased expression upon
DDX41 overexpression (Fig. 5D).
From these results, it was concluded that DDX41 regulated
immune-associated gene expression.
Antigen processing genes upregulated
by DDX41 in HeLa cells are associated with survival rate in
patients with cervical cancer
Previous studies have reported that antigen
processing presents antigen-derived peptides into MHC-I and MHC-II
molecules to be recognized by T cell receptors expressed by
CD8+ and CD4+ T cells, respectively (41–43).
The top KEGG pathway in analysis of DDX41 overexpression was
‘antigen processing and presentation’, with significant
upregulation of HSPA1A, HSPA1B, HSPA6, HLA-DMB and
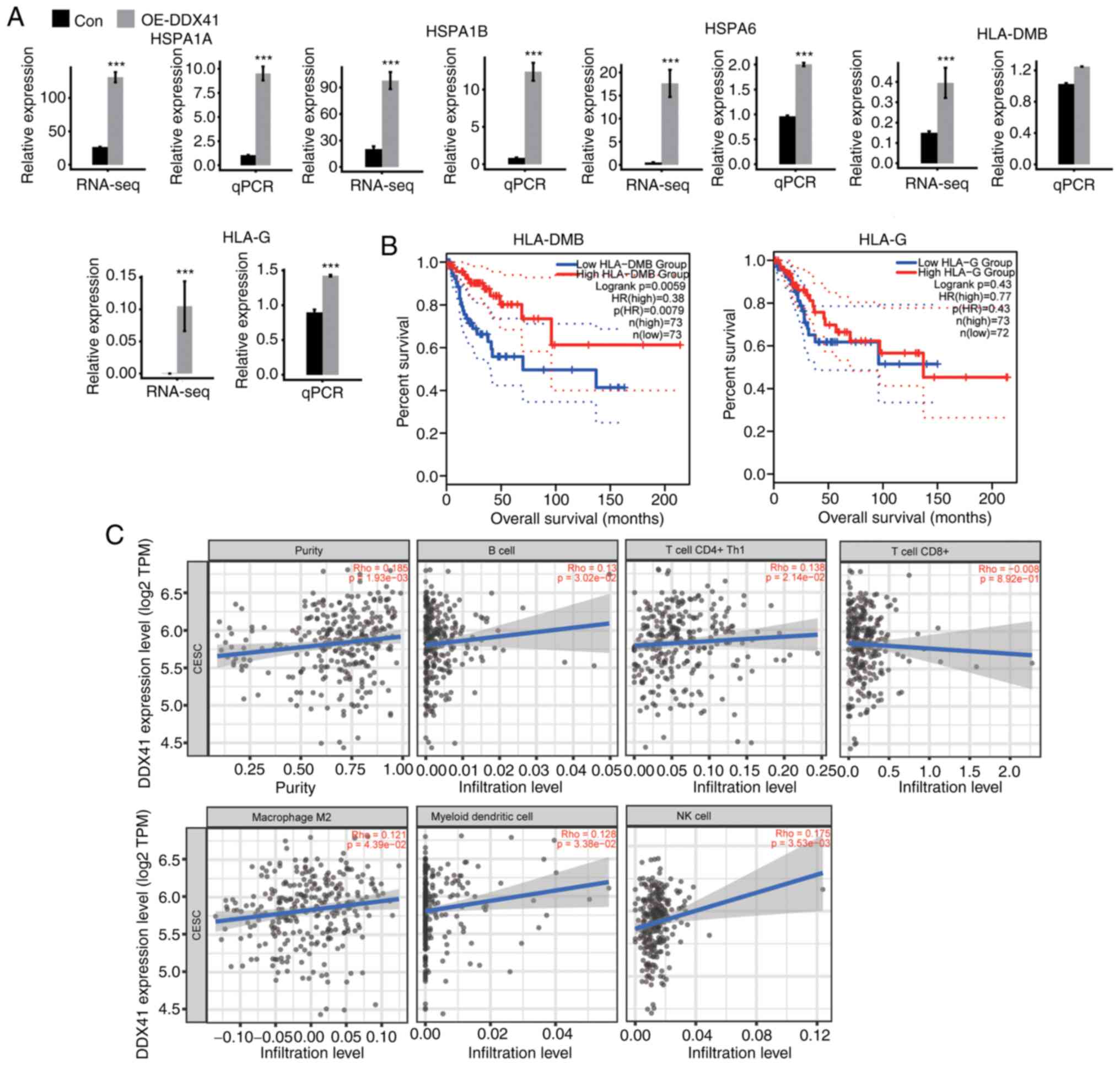
HLA-G detected (Fig. 6A).
Expression levels of these genes were confirmed via RT-qPCR, and
the results were consistent with the RNA-seq analysis (Fig. 6A).
DDX41 regulation of the expression of
immune-associated genes in HeLa cells may reflect an important role
of DDX41 in cervical cancer. To test the hypothesis, the
association between the expression levels of DDX41-regulated genes
and the survival rates of patients with CESC was analyzed in the
GEPIA databases. Expression levels of HLA-DMB were positively
associated with survival rate of patients with CESC in the whole
disease course of >200 months (Fig.
6B). The expression levels of HLA-G, HLA-1A and HLA-1B were
positively (though not significantly) associated with survival rate
in either the early or late disease stage, while those of HSPA6
were negatively associated with survival rate (Figs. 6B and S2A).
Infiltration of tumor immune cell populations in
solid tumors can be estimated from the transcriptomic changes in
the bulk tumor samples (44).
TIMER2.0 (timer.cistrome.org/) is a
user-friendly web interface for dynamic exploring and visualizing
the interaction between tumor-infiltrating immune cells and
important tumoral transcriptome changes using deconvolution-based
approaches (45). The TIMER2.0
database was next searched to estimate the correlation between
DDX41 mRNA expression and immune cell infiltration in CESC.
As illustrated in the scatter plot (Fig. 6C), expression of DDX41 was
positively correlated with the population of B, CD4+ Th1
and NK cells, macrophage M2 and myeloid DCs. The correlation
between DDX41 expression and CD8+ T cells was not
significant. Collectively, the association between DDX41 expression
levels and tumor immune cell populations supported the hypothesis
that DDX41-regulated expression of immune-associated and antigen
presentation genes evident in HeLa may regulate immune cell
infiltration and cancer immunity in patients with CESC.
DDX41 regulates the alternative
splicing of certain immune-response genes
Genes regulated by DDX41-mediated alternative
splicing were enriched in the ‘innate immune response’ GO pathway
(MAP4K2, PGLYRP1, NFATC3, TNRC6B, RAF1, MAPKAP1, TANK, NLRX1,
PLA2G6, NOD1, ZC3HAV1, PML, ATG9A, ERBB3, NR4A1, FGFR4, MLST8,
FGFR1, LRRFIP1, CFD, UBA52, GRB2, PRKACB, CHID1, AGO3 and
ATF2; Table SXII).
Enriched KEGG pathways included those involved in ‘B cell receptor
signaling pathway’ (GRB2, IFITM1, PPP3CC, RAF1 and
NFATC3; Table SXIII). In
order to validate the ASEs identified in the RNA-seq data, 10
potential ASEs were analyzed by RT-qPCR. Out of 11 tested events,
four ASEs validated by RT-qPCR showed a significant change, which
was in agreement with the RNA-seq results. The four validated
splicing events were located in the genes IFITM1, CFD, NLRX1
and NFATC3 (Figs. 7 and
S3).
Discussion
The present study demonstrated that DDX41 acted as a
tumor suppressor by inhibiting cell proliferation and promoting
apoptosis in HeLa cells. To the best of our knowledge, the present
study is the first to profile changes in the HeLa cell
transcriptome following DDX41 overexpression. A total of 959
DEGs were identified; GO/KEGG analysis of these DEGs revealed
significant enrichment in ‘cell adhesion’, ‘angiogenesis’, ‘MAPK
signaling pathway’ and ‘immune response’. Genes regulated by
DDX41-mediated alternative splicing were highly enriched in ‘EGFR
signaling pathway’ and ‘FGFR signaling pathway’. Identification and
characterization of DDX41-regulated genes in cancer cells provided
mechanistic insights into the link between tumorigenesis and cancer
immunity, which may aid the development of novel therapeutic
strategies against cervical and endocervical cancer (46,47).
A number of previously published works have
indicated the diverse tumor cell type-specific roles of DDX41 in
regulating tumorigenesis. For example, knockdown (KD) of DDX41 by
short hairpin RNA promoted the proliferation of K562 cells, whereas
DDX41 overexpression in U937 cells inhibited growth
(16,48). Compared with that of WT DDX41
or DDX41-knockdown 293 cells, overexpression of
DDX41R525H mutant increased cell soft agar colony
formation (48). Similarly,
DDX41 knockdown in CD34+ hematopoietic progenitor
cells significantly enhanced colony formation compared with
controls (16). An in vivo
xenograft experiment of K562 cells in which DDX41 was
knocked down showed accelerated tumor growth (16). Taken together, these studies support
DDX41 as a tumor suppressor gene in multiple cell lines. The
present results revealed that DDX41 inhibited proliferation and
promoted apoptosis of HeLa cells and expression of DDX41 was
associated with immune infiltration in CESC; this extends knowledge
of the tumor suppressor function of DDX41 in cancer.
In the present study, DDX41 downregulated the
expression levels of oncogenes LCN2, TP63, KIT, MYB and
CDK5R2. Moreover, certain tumor suppressor genes were
upregulated by DDX41, such as PANO1, GLI1 and HOMER2;
expression of these genes was also deregulated and associated with
survival rate in patients with CESC. p63 protein is a homologue of
transcription factor p53, a tumor suppressor which serves key
functions in tumorigenesis and oncogenesis (49,50).
p63 protein is a useful immunohistochemical marker of
differentiation of squamous neoplasms within the cervix and is a
marker of premalignant lesions of the cervix used to predict
malignant potential (51–54). The oncogenic roles of transcription
factor MYB have been widely studied in different types of cancer
including leukemia, colon and breast cancer and adenoid cystic
carcinoma (55–57). Overexpression of MYB has been
reported to be associated with poor prognosis in leukemogenesis
(58), colorectal (59) and breast cancer (60) and Burkitt lymphoma (61). Therefore, DDX41 may function in
tumorigenesis by regulating the expression levels of certain
oncogenes and tumor suppressors.
In the present study, DDX41 regulated alternative
splicing pre-mRNAs from genes enriched in cancer progression
pathways, including ‘EGFR signaling’, ‘FGFR signaling’, ‘MAPK
signaling’ and ‘prostate cancer’ pathways. Tumorigenesis-associated
genes were FGFR1, FGFR4, NR4A1, CSK, MLST8, IGF1R and
RRAF1. Alternative inclusion of one of two unique exons
results in three versions of Ig-like domain III in FGFR 1–3
(62–64), but FGFR4 is not alternatively
spliced in this region (65). Two
FGFR1 IIIc splice variants (FGFR1a and FGFR1β)
have been shown to be expressed at similar levels in normal
urothelial cells, but FGFR1β is more highly expressed in
tumor cells and results in increased proliferation (66). The FGFR IIIb isoform is
primarily expressed in epithelial tissue, whereas the IIIc isoform
is primarily expressed in mesenchymal tissue (67). Therefore, the FGF/FGFR axis may
serve an important role in cancer progression (68,69).
The present finding of DDX41-regulated alternative splicing of
FGFR1 and FGFR4 suggested that DDX41 may regulate
tumor progression via the FGF/FGFR axis.
GO/KEGG analysis revealed that DDX41 regulated the
expression of genes enriched in immune-associated pathways
including ‘leukocyte migration’, ‘cytokine-cytokine receptor
interaction’, ‘antigen processing and presentation’ and ‘autoimmune
disease’. This finding suggested a potential function of DDX41 in
regulating cancer immunity. Among these pathways, the mRNA
expression levels of CD22, CD177 and CSF2 were significantly
downregulated and IL-12A was upregulated by DDX41. It has been
reported that administration of IL-12 promotes the development of T
helper type 1 CD4+ T cells, inducing rapid onset of
insulin-dependent diabetes mellitus in non-obese diabetic mice
(70). The interaction of CD22 with
α2,6-linked sialic acid ligands has been proposed to regulate B
lymphocyte function and migration (71). DDX41 selectively upregulated antigen
processing and presentation genes including HSPA1A, HSPA1B
and HSPA6. When these HSPs are released into the
extracellular space, they act as a source of antigens due to their
ability to chaperone peptides and induce DCs to cross-present
antigens to T cells, and also stimulate the innate immune system
independently of peptides (72).
Castelli et al (73)
reported that HSP70 purified from human melanoma activated T cells
recognizing melanoma differentiation antigens by gaining access to
the class I HLA presentation pathway. Noessner et al
(74) reported that tumor-derived
HSP70-peptide complexes have the immunogenic potential to instruct
cross-presentation of antigenic peptide for T cell recognition by
DCs. It has been suggested that combinatorial approaches
encompassing DCs and tumor-associated antigens, such as HSP70, may
be useful options for cancer therapies (75,76).
The unique immune properties of HSP70 have enabled development of
innovative prophylactic and therapeutic vaccines, particularly
those for cervical and endocervical cancer (77).
DDX41 is an RNA helicase, which also acts as a
dsDNA sensor in the cytoplasm, thus defending against pathogen
invasion and activating the type 1 IFN response (22). Bruton's tyrosine kinase has been
shown to phosphorylate Tyr414 of DDX41, which is critical for DDX41
recognition of DNA and binding to STING (78). In a zebrafish model, DDX41
contributed to STING-STAT6-mediated chemokine production via its
DEADc domain (79). STING signaling
is crucial for activation of transcription of type 1 IFN production
genes, and therefore protects cells against pathogen invasion and
the development of cancer by promoting antitumor immune response
(80–82). The present results revealed that
DDX41 regulated the expression of alternative splicing of hundreds
of genes. Cytoplasmic and nuclear localization of DDX41 was also
demonstrated. DDX41 may mediate gene regulation indirectly by
affecting the function of its protein partners and/or directly by
its RNA and DNA binding activity.
In summary, the present findings suggested that
DDX41 selectively regulated the alternative splicing of numerous
important genes involved in tumorigenesis, as well as expression of
genes involved in the immune response in HeLa cells. The expression
levels of DDX41-regulated cancer-associated genes were deregulated
in cervical cancer samples and the expression of DDX41-regulated
antigen processing and presentation genes was associated with
survival rate of patients with CESC. These findings identified a
DDX41-regulatory network connecting transcription and alternative
splicing that predicted the biological functions of DDX41 in
suppressing tumor cell growth, as well as regulating
cross-presentation of antigens and other antitumor immune
responses. Further study of DDX41 may lead to novel vaccine
strategies in the treatment of cancer.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Professor. Yi Zhang
(ABLife, Inc., Wuhan, China) for helpful discussions.
Funding
The present study was supported by the Health
Commission of Hubei Province Scientific Research Project (grant no.
WJ2019M118) and ABLife (grant no. ABL-7702157).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the National Center for
Biotechnology Information Gene Expression Omnibus repository
(series accession no. GSE122986).
Authors' contributions
KQ, YZ and XLY designed and supervised the
experiments. KQ, XLY, DNJ, YC, PZ, JZ, and HHX performed the
experiments. YQX, YXW and YZ analyzed the data. KQ, YQX and YZ
drafted the manuscript. All authors reviewed, read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jankowsky E: RNA helicases. 511. 1st
edition. Academic Press; 2012
|
|
2
|
Tanner NK and Linder P: DExD/H Box RNA
helicases: From generic motors to specific dissociation functions.
Mol Cell. 8:251–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourgeois CF, Mortreux F and Auboeuf D:
The multiple functions of RNA helicases as drivers and regulators
of gene expression. Nat Rev Mol Cell Biol. 17:426–438. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rocak S and Linder P: DEAD-Box proteins:
The driving forces behind RNA metabolism. Nat Rev Mol Cell Biol.
5:232–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jankowsky E: RNA helicases at work:
Binding and rearranging. Trends Biochem Sci. 36:19–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuller-Pace FV: DExD/H box RNA helicases:
Multifunctional proteins with important roles in transcriptional
regulation. Nucleic Acids Res. 34:4206–4215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cruciat CM, Dolde C, de Groot RE, Ohkawara
B, Reinhard C, Korswagen HC and Niehrs C: RNA helicase DDX3 is a
regulatory subunit of casein kinase 1 in wnt-β-catenin signaling.
Science. 339:1436–1441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bleichert F and Baserga SJ: The long
unwinding road of RNA helicases. Mol Cell. 27:339–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mosallanejad K, Sekine Y,
Ishikura-Kinoshita S, Kumagai K, Nagano T, Matsuzawa A, Takeda K,
Naguro I and Ichijo H: The DEAH-Box RNA helicase DHX15 activates
NF-κB and MAPK signaling downstream of MAVS during antiviral
responses. Sci Signal. 7:ra402014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuller-Pace FV: DEAD box RNA helicase
functions in cancer. RNA Biol. 10:121–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Francis R and Jerry P: Perturbations of
RNA helicases in cancer. Wiley Interdiscip Rev RNA. 4:333–349.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdelhaleem M: Do human RNA helicases have
a role in cancer? Biochim Biophys Acta. 1704:37–46. 2004.PubMed/NCBI
|
|
13
|
Cai W, Chen ZX, Rane G, Singh SS, Choo Z,
Wang C, Yuan Y, Tan TZ, Arfuso F, Yap CT, et al: Wanted DEAD/H or
alive: Helicases winding up in cancers. J Natl Cancer Inst.
25:1092017.
|
|
14
|
Chlon TM, Stepanchick E, Choi K, Zheng Y,
Hueneman K, Davis A and Starczynowski DT: The inherited MDS gene
DDX41 is required for ribosome biogenesis and cell viability.
Blood. 134 (Suppl 1):S7732019. View Article : Google Scholar
|
|
15
|
Ding L, Ley TJ, Larson DE, Miller CA,
Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan
MD, et al: Clonal evolution in relapsed acute myeloid leukaemia
revealed by whole-genome sequencing. Nature. 481:506–510. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polprasert C, Schulze I, Sekeres MA,
Makishima H, Przychodzen B, Hosono N, Singh J, Padgett RA, Gu X,
Phillips JG, et al: Inherited and somatic defects in DDX41 in
myeloid neoplasms. Cancer Cell. 27:658–670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venugopal P, Cheah JJC, Eshraghi L,
Shahrin NH, Homan C, Feng J, Schreiber AW, Fine M, Phillips K,
Poplawski N, et al: An integrative genomic approach to examine
mutations and biological pathways associated with hematological
malignancy development in DDX41 mutated families. Blood. 134 (Suppl
1):S26862019. View Article : Google Scholar
|
|
18
|
Cheah JJC, Hahn CN, Hiwase DK, Scott HS
and Brown AL: Myeloid neoplasms with germline DDX41 mutation. Int J
Hematol. 106:163–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peters D, Radine C, Reese A, Budach W,
Sohn D and Jänicke RU: The DEAD-box RNA helicase DDX41 is a novel
repressor of p21 WAF1/CIP1 mRNA translation. J Biol
Chem. 292:8331–8341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stavrou S, Aguilera AN, Blouch K and Ross
SR: DDX41 recognizes RNA/DNA retroviral reverse transcripts and is
critical for in vivo control of murine leukemia virus infection.
mBio. 9:e00923–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duan Y, Zeng J, Fan S, Liao Y, Feng M,
Wang L, Zhang Y and Li Q: Herpes simplex virus type 1-encoded
miR-H2-3p manipulates cytosolic DNA-stimulated antiviral innate
immune response by targeting DDX41. Viruses. 15:7562019. View Article : Google Scholar
|
|
22
|
Zhang Z, Yuan B, Bao M, Lu N, Kim T and
Liu YJ: The helicase DDX41 senses intracellular DNA mediated by the
adaptor STING in dendritic cells. Nat Immunol. 12:959–965. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parvatiyar K, Zhang Z, Teles RM, Ouyang S,
Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, et al: The
helicase DDX41 recognizes the bacterial secondary messengers cyclic
di-GMP and cyclic di-AMP to activate a type I interferon immune
response. Nat Immunol. 13:1155–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura T, Miyabe H, Hyodo M, Sato Y,
Hayakawa Y and Harashima H: Liposomes loaded with a STING pathway
ligand, cyclic di-GMP, enhance cancer immunotherapy against
metastatic melanoma. J Control Release. 216:149–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyabe H, Hyodo M, Nakamura T, Sato Y,
Hayakawa Y and Harashima H: A new adjuvant delivery system ‘cyclic
di-GMP/YSK05 liposome’ for cancer immunotherapy. J Control Release.
184:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cordin O and Beggs JD: RNA helicases in
splicing. RNA Biol. 10:83–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jurica MS, Licklider LJ, Gygi SR,
Grigorieff N and Moore MJ: Purification and characterization of
native spliceosomes suitable for three-dimensional structural
analysis. RNA. 8:426–439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bessonov S, Anokhina M, Will CL, Urlaub H
and Lührmann R: Isolation of an active step I spliceosome and
composition of its RNP core. Nature. 452:846–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nam DK, Lee S, Zhou G, Cao X, Wang C,
Clark T, Chen J, Rowley JD and Wang SM: Oligo(dT) primer generates
a high frequency of truncated cDNAs through internal poly(A)
priming during reverse transcription. Proc Natl Acad Sci USA.
99:6152–6156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robinson MD, McCarthy DJ and Smyth GK:
EdgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li CY and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. 39((Web Server Issue)): W316–W322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia H, Chen D, Wu Q, Wu G, Zhou Y, Zhang Y
and Zhang L: CELF1 preferentially binds to exon-intron boundary and
regulates alternative splicing in HeLa cells. Biochim Biophys Acta
Gene Regul Mech. 1860:911–921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pryke A, Mostaghim S and Nazemi A: Heatmap
visualization of population based multi objective algorithms.
Evolutionary multi-criterion optimization. Obayashi S, Deb K,
Poloni C, Hiroyasu T and Murata T: Springer Berlin Heidelberg;
Berlin, Heidelberg: pp. 361–375. 2007, View Article : Google Scholar
|
|
35
|
Katz Y, Wang ET, Silterra J, Schwartz S,
Wong B, Thorvaldsdóttir H, Robinson JT, Mesirov JP, Airoldi EM and
Burge CB: Quantitative visualization of alternative exon expression
from RNA-seq data. Bioinformatics. 31:2400–2402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao M, Kim P, Mitra R, Zhao J and Zhao Z:
TSGene 2.0: An updated literature-based knowledgebase for tumor
suppressor genes. Nucleic Acids Res. 44(D1): D1023–D1031. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adey A, Burton JN, Kitzman JO, Hiatt JB,
Lewis AP, Martin BK, Qiu R, Lee C and Shendure J: The
haplotype-resolved genome and epigenome of the aneuploid HeLa
cancer cell line. Nature. 500:207–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Landry JJ, Pyl PT, Rausch T, Zichner T,
Tekkedil MM, Stütz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, et al:
The genomic and transcriptomic landscape of a HeLa cell line. G3
(Bethesda). 7:1213–1224. 2013. View Article : Google Scholar
|
|
40
|
Li Y, Qi H, Li X, Hou X, Lu X and Xiao X:
A novel dithiocarbamate derivative induces cell apoptosis through
p53-dependent intrinsic pathway and suppresses the expression of
the E6 oncogene of human papillomavirus 18 in HeLa cells.
Apoptosis. 20:787–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jensen PE: Recent advances in antigen
processing and presentation. Nat Immunol. 8:1041–1048. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murata S, Takahama Y, Kasahara M and
Tanaka K: The immunoproteasome and thymoproteasome: Functions,
evolution and human disease. Nat Immunol. 19:923–931. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rock KL, Reits E and Neefjes J: Present
yourself! by MHC class I and MHC class II molecules. Trends
Immunol. 37:724–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Becht E, Giraldo NA, Lacroix L, Buttard B,
Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman
C, Fridman WH and de Reyniès A: Estimating the population abundance
of tissue-infiltrating immune and stromal cell populations using
gene expression. Genome Biol. 17:2182016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48((W1)): W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Flood BA, Higgs EF, Li S, Luke JJ and
Gajewski TF: STING pathway agonism as a cancer therapeutic. Immunol
Rev. 290:24–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoneyama-Hirozane M, Kondo M, Matsumoto
SI, Morikawa-Oki A, Morishita D, Nakanishi A, Kawamoto T and
Nakayama M: High-throughput screening to identify inhibitors of
DEAD box helicase DDX41. SLAS Discov. 22:1084–1092. 2017.PubMed/NCBI
|
|
48
|
Polprasert C, Schulze I, Sekeres MA,
Makishima H, Przychodzen BP, Hosono N, Singh J, Padgett RA, Gu X,
Jankowsky E, et al: DDX41 is a tumor suppressor gene associated
with inherited and acquired mutations. Blood. 124:1252014.
View Article : Google Scholar
|
|
49
|
Nekulova M, Holcakova J, Coates P,
Vojtesek BJC and Letters MB: The role of P63 in cancer, stem cells
and cancer stem cells. Cell Mol Biol Lett. 16:296–327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Graziano V and De Laurenzi V: Role of p63
in cancer development. Biochim Biophys Acta. 1816:57–66.
2011.PubMed/NCBI
|
|
51
|
Wang TY, Chen BF, Yang YC, Chen H, Wang Y,
Cviko A, Quade BJ, Sun D, Yang A, McKeon FD and Crum CP: Histologic
and immunophenotypic classification of cervical carcinomas by
expression of the p53 homologue p63: A study of 250 cases. Hum
Pathol. 32:479–486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
McCluggage WG: Immunohistochemistry as a
diagnostic aid in cervical pathology. Pathology. 39:97–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Houghton O and McCluggage WG: The
expression and diagnostic utility of p63 in the female genital
tract. Adv Anat Pathol. 16:316–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saritha VN, Veena VS, Jagathnath KM,
Somanathan T and Sujathan K: Significance of DNA replication
licensing proteins (MCM2, MCM5 and CDC6), p16 and p63 as markers of
premalignant lesions of the uterine cervix: Its usefulness to
predict malignant potential. Asian Pac J Cancer Prev. 27:141–148.
2018.
|
|
55
|
Pattabiraman D and Gonda T: Role and
potential for therapeutic targeting of MYB in leukemia. Leukemia.
27:269–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ramsay RG and Gonda TJ: MYB function in
normal and cancer cells. Nat Rev Cancer. 8:523–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Drier Y, Cotton MJ, Williamson KE,
Gillespie SM, Ryan RJ, Kluk MJ, Carey CD, Rodig SJ, Sholl LM,
Afrogheh AH, et al: An oncogenic MYB feedback loop drives alternate
cell fates in adenoid cystic carcinoma. Nat Genet. 48:265–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sarvaiya PJ, Schwartz JR, Hernandez CP,
Rodriguez PC and Vedeckis WV: Role of c-myb in the survival of pre
B-cell acute lymphoblastic leukemia and leukemogenesis. Am J
Hematol. 87:969–976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Biroccio A, Benassi B, D'Agnano I,
D'Angelo C, Buglioni S, Mottolese M, Ricciotti A, Citro G,
Cosimelli M, Ramsay RG, et al: C-Myb and bcl-x overexpression
predicts poor prognosis in colorectal cancer: Clinical and
experimental findings. Am J Pathol. 158:1289–1299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Knopfová L, Biglieri E, Volodko N, Masařík
M, Hermanová M, Garzón JF, Dúcka M, Kučírková T, Souček K, Šmarda
J, et al: Transcription factor c-myb inhibits breast cancer lung
metastasis by suppression of tumor cell seeding. Oncogene.
37:1020–1030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma M, Zhao R, Yang X, Zhao L, Liu L, Zhang
C, Wang X and Shan B: Low expression of Mda-7/IL-24 and high
expression of C-myb in tumour tissues are predictors of poor
prognosis for burkitt lymphoma patients. Hematology. 23:448–455.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Werner S, Duan DS, de Vries C, Peters KG,
Johnson DE and Williams LT: Differential splicing in the
extracellular region of fibroblast growth factor receptor 1
generates receptor variants with different ligand-binding
specificities. Mol Cell Biol. 12:82–88. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Johnson DE, Lu J, Chen H, Werner S and
Williams LT: The human fibroblast growth factor receptor genes: A
common structural arrangement underlies the mechanisms for
generating receptor forms that differ in their third immunoglobulin
domain. Mol Cell Biol. 11:4627–4634. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chellaiah AT, McEwen DG, Werner S, Xu J
and Ornitz M: Fibroblast growth factor receptor (FGFR) 3.
Alternative splicing in immunoglobulin-like domain III creates a
receptor highly specific for acidic FGF/FGF-1. J Biol Chem.
269:11620–11627. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vainikka S, Partanen J, Bellosta P,
Coulier F, Birnbaum D, Basilico C, Jaye M and Alitalo K: Fibroblast
growth factor receptor-4 shows novel features in genomic structure,
ligand binding and signal transduction. EMBO J. 12:4273–4280. 1992.
View Article : Google Scholar
|
|
66
|
Tomlinson DC and Knowles MA: Altered
splicing of FGFR1 is associated with high tumor grade and stage and
leads to increased sensitivity to FGF1 in bladder cancer. Am J
Pathol. 177:2379–2386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang S, Hao Y, Yuan Y, Liu R and Chen Q:
Role of fibroblast growth factor receptor 4 in cancer. Cancer Sci.
109:3024–3031. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Touat M, Ileana E, Postel-Vinay S, André F
and Soria JC: Targeting FGFR signaling in cancer. Clin Cancer Res.
21:2684–2694. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Babina IS and Turner NC: Advances and
challenges in targeting FGFR signalling in cancer. Nat Rev Cancer.
17:318–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Trembleau S, Penna G, Bosi E, Mortara A,
Gately MK and Adorini L: Interleukin 12 administration induces T
helper type 1 cells and accelerates autoimmune diabetes in NOD
mice. J Exp Med. 181:817–821. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Poe JC, Fujimoto Y, Hasegawa M, Haas KM,
Miller AS, Sanford IG, Bock CB, Fujimoto M and Tedder TF: CD22
regulates B lymphocyte function in vivo through both
ligand-dependent and ligand-independent mechanisms. Nat Immunol.
5:1078–1087. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
72
|
Milani V, Noessner E, Ghose S, Kuppner M,
Ahrens B, Scharner A, Gastpar R and Issels RD: Heat shock protein
70: Role in antigen presentation and immune stimulation. Int J
Hyperthermia. 18:563–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Castelli C, Ciupitu AM, Rini F, Rivoltini
L, Mazzocchi A, Kiessling R and Parmiani G: Human heat shock
protein 70 peptide complexes specifically activate antimelanoma T
cells. Cancer Res. 61:222–227. 2001.PubMed/NCBI
|
|
74
|
Noessner E, Gastpar R, Milani V, Brandl A,
Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK
and Issels RD: Tumor-Derived heat shock protein 70 peptide
complexes are cross-presented by human dendritic cells. J Immunol.
169:5424–5432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Banchereau J and Palucka AK: Dendritic
cells as therapeutic vaccines against cancer. Nat Rev Immunol.
5:296–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Noessner E: Thermal stress-related
modulation of tumor cell physiology and immune responses. Cancer
Immunol Immunother. 55:289–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Albakova Z, Armeev GA, Kanevskiy LM,
Kovalenko EI and Sapozhnikov AM: HSP70 multi-functionality in
cancer. Cells. 9:5872020. View Article : Google Scholar
|
|
78
|
Lee KG, Susana SY, Kui L, Chih-Cheng Voon
D, Mauduit M, Bist P, Bi X, Pereira NA, Liu C, Sukumaran B, et al:
Bruton's tyrosine kinase phosphorylates DDX41 and activates its
binding of dsDNA and STING to initiate type 1 interferon response.
Cell Rep. 10:1055–1065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ma JX, Li JY, Fan DD, Feng W, Lin AF,
Xiang LX and Shao JZ: Identification of DEAD-box RNA helicase DDX41
as a trafficking protein that involves in multiple innate immune
signaling pathways in a zebrafish model. Front Immunol. 9:13272018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Woo SR, Corrales L and Gajewski TF: The
STING pathway and the T cell-inflamed tumor microenvironment.
Trends Immunol. 36:250–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Barber GN: STING-Dependent cytosolic DNA
sensing pathways. Trends Immunol. 35:88–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Barber GN: STING: Infection, inflammation
and cancer. Nat Rev Immunol. 15:760–770. 2015. View Article : Google Scholar : PubMed/NCBI
|