Introduction
Bladder cancer (BC) is an important malignancy in
the urinary system, whose incidence ranks 11th among all malignant
tumors, 7th among malignant tumors in male patients, and 10th among
malignant tumors in female patients (1). Based on histopathology, BC is divided
into urothelial carcinoma, squamous carcinoma, adenocarcinoma, and
undifferentiated carcinoma, of which urothelial carcinoma is a
major type, accounting for more than 90% of all malignant bladder
tumors (2). In addition, according to
the BC invasion and infiltration of the muscular layer, BC may be
stratified into muscle invasive bladder cancer (MIBC) and
non-muscle invasive bladder cancer (NMIBC), accounting for
approximately 30 and 70%, respectively (3). MIBC has a higher degree of malignancy
and a much higher probability of tumor recurrence and metastasis
than NMIBC. High-grade MIBC is the major cause of mortality in BC
patients (4). Mechanisms underlying
the occurrence and development of BC remain unclear. Biological
behaviors of BC cells are characterized by multicentricity,
invasion, and susceptibility to recurrence. Some patients present
with early stage NMIBC, progress to MIBC, and develop drug
resistance post-treatment, with a high recurrence rate (5). Therefore, an in-depth study to clarify
the biological process of BC and molecular regulatory network at
various stages as early as possible can help patients diagnose
tumors early, improve treatment effects as well as disease
prognosis.
Non-coding RNAs (ncRNAs) are RNA transcripts, and
the vast majority of ncRNAs are not translated into proteins
(6). In spite of the lack of protein
translation function, ncRNAs are vital participants in cellular
activities. Based on different lengths, structures, and functions,
ncRNAs are mainly divided into microRNA, rRNA, tRNA, lncRNA, and
circular RNA, of which lncRNA is a transcript with more than 200 nt
and no open reading frame (7).
Although lncRNAs do not code proteins, they are expressed in many
differentiated tissues or specific cancers. lncRNAs are regarded as
noise produced during the transcription process and have no
influence on biological effects. In addition, lncRNAs are
considered irreplaceable in vital activities such as the regulation
of the stability of telomeres, the control of nuclear structure and
transcription, X inactivation, nucleosome generation, the
regulation of mRNA splicing and maturity, and microRNA activity
(8). Through epigenetic regulation of
coding gene expression involved in cellular activities, lncRNAs
regulate vital bioprocesses including cell autophagy, cell cycle,
differentiation, proliferation, migration, invasion, and apoptosis
(9). Therefore, lncRNAs are expected
to become diagnostic markers and therapeutic targets for tumors.
For example, lncRNA highly upregulated in liver cancer promotes the
progression of liver cancer by binding to microRNA-186 to increase
the expression of high mobility group AT-hook 2 (10), lncRNA LINC00958 inhibits the
occurrence of pancreatic cancer by sponging microRNA-330-5p to
downregulate the expression of paired box 8 (11), and lncRNA small nucleolar RNA host
gene 1 (SNHG1) promotes the development of non-small cell lung
cancer by spongy bonding to miR-145-5p to upregulate the expression
of metadherin (12).
LINC00649 is an lncRNA abnormally highly expressed
in BC through database screening; however, its biological roles and
potential molecular mechanisms in BC remain unknown. This study
investigated the expression of LINC00649 in BC tissues, adjacent
tissues, normal cell lines, and BC cell lines, and analyzed the
association of the expression with clinicopathological
characteristics of BC patients. In addition, in vitro cell
experiments were conducted to test the effects of LINC00649 on the
proliferation, migration, and invasion of BC cells. Furthermore,
bioinformatics and relevant experiments were used to explore the
possible LINC00649 molecular mechanisms of action, thus providing a
new direction and molecular target for the targeted therapy of
BC.
Materials and methods
GEPIA database
A total of 404 BC tissues and 28 normal (control)
tissues were obtained from the TCGA database to analyze mRNA
expression profiles. GEPIA is a new interactive website for TCGA-
and GTEx-based analysis of RNA sequence data (http://gepiacancer-pku.cn/index.html).
LINC00649 expression in BC tissues and control tissues was
analyzed, and the GEPIA database was used to calculate the
correlation between LINC00649 expression and disease-free survival
(DFS) and overall survival (OS) of BC patients.
Study subjects and sample
collection
Approval for the study was obtained from the ethics
committee of The First People's Hospital of Wenling (Zhejiang,
China). The patients volunteered to participate in this study and
signed a written informed consent form. All experiments were
performed in accordance with the Declaration of Helsinki.
A total of 60 BC tissues and 60 adjacent tissues
were collected from The First People's Hospital of Wenling. All
tissue specimens were confirmed via pathological diagnosis by an
experienced pathologist. Samples were frozen in liquid nitrogen
(−196°C) immediately post-dissection and stored in a −80°C freezer.
Age >55 years was considered the cut-off point as indicated in
previous studies (13,14). Pathological classification and tumor
staging were performed according to the cancer staging criteria of
the Union for International Cancer Control. None of the patients
had preoperative radiotherapy, chemotherapy history, or other tumor
history and met the clinical and pathological data integrity. The
patient sex, age, tumor staging, lymphatic metastasis, or distant
metastasis was listed in Table I.
 | Table I.Correlation between LINC00649
expression and clinicopathological factors. |
Table I.
Correlation between LINC00649
expression and clinicopathological factors.
|
|
| Expression of
LINC00649 |
|
|---|
|
|
|
|
|
|---|
| Factors | Cases | High | Low | P-value |
|---|
| Overall | 60 | 45 | 15 |
|
| Sex |
|
|
| 0.9236 |
|
Male | 43 | 32 | 11 |
|
|
Female | 17 | 13 | 4 |
|
| Age |
|
|
| 0.5223 |
|
≤55 | 10 | 7 | 3 |
|
|
>55 | 50 | 38 | 12 |
|
| Stage |
|
|
|
|
| Primary tumor |
|
|
| 0.0056 |
| NMI
(Ta+Tis+TI) | 30 | 17 | 13 |
|
| MI
(T2+T3+T4) | 30 | 28 | 2 |
|
| Lymph node
metastasis |
|
|
| 0.6325 |
| Absent
(N0) | 20 | 15 | 5 |
|
| Present
(N1+N2+N3) | 40 | 30 | 10 |
|
| Distant
metastasis |
|
|
| 0.05869 |
| Absent
(N0) | 54 | 40 | 14 |
|
| Present
(M1) | 6 | 5 | 1 |
|
| Grade |
|
|
| 0.0969 |
| Low
grade | 18 | 12 | 6 |
|
| High
grade | 42 | 33 | 9 |
|
Cell culture
Normal control cell line SV-HUC-1 and human bladder
cancer cell lines (5637, SW780, T24, and UM-UC-3) were purchased
from the American Type Culture Collection (ATCC, Rockville). Cells
were cultured in RPMI-1640 (Gibco) culture medium with 10% fetal
bovine serum (FBS; Invitrogen) and placed in an incubator
containing 5% CO2 at 37°C, followed by passage culturing
at 80–90% of cell density.
Cell transfection
For LINC00649 overexpression, LINC00649
overexpression plasmids were constructed into the pSicoR lentiviral
vector, and 293T cells were used for virus production. Lentiviruses
were used to infect BC cells, and purinomycin was used for two
weeks to produce LINC00649-overexpressed cell lines. Transfection
efficiency was measured using RT-qPCR, and cells were collected for
subsequent experiments. Then, 3,000 cells were seeded into a 6-well
plate, culture medium was added, and transfection was performed at
70–80% of cell density at 37°C for 48 h, referencing the IFU of
Lipofectamine 3000 (Invitrogen). GenePharma (Shanghai) designed and
synthesized LINC00649 siRNA, miR-15a-5p mimics, HMGA1
overexpression plasmids, and corresponding negative
control-transfected cells. The mixed solution was added to a cell
culture plate or culture flask containing culture solution and
shaken gently. After 24 h of culturing in a CO2
incubator at 37°C, subsequent experiments were conducted.
RNA extraction and real-time
quantitative PCR
BC tissues and cells required for experiments were
collected, and 1 ml TRIzol (Invitrogen) was added for cell lysis.
Subsequently, chloroform (250 µl) was added and mixed for 30 sec,
and centrifuged at 1,500 × g at 4°C for 15 min. The resultant
aqueous phase was then drawn and added to isometric pre-cooling
isopropanol. After centrifugation, precipitates were gently purged
with 75% ethanol and dissolved in 30 µl DEPC water. The
quantification of total RNA was performed with a NanoDrop 2000
(Thermo Fisher Scientific, Inc.) device and placed in a −80°C
refrigerator for future use. Total RNA (1,000 ng) was reverse
transcribed to cDNA using the PrimeScript RT reagent kit.
Quantitative PCR of miRNA was performed according to
the IFU (Takara; code no. RR036A) of the miScript SYBR-Green PCR
Kit. cDNA (500 ng) was used to conduct RT-qPCR using the ABI7900
device according to the IFU of SYBR®-Green Master Mix
(Takara). GAPDH and U6 were used as an internal
reference, respectively. Analysis of relative gene expression data
using real-time quantitative PCR and the 2(-Delta Delta C(T))
method. The primer sequences used were as follows: LINC00649
forward: TCCCCACGTAAGGAGGGTAG, reverse: CAGCAACAGGCCTTGTCAAC;
miR-15a-5p forward: CACAGAGTGTCGGAGGTGATTC, reverse:
CCTGTAGTACGGGTATGTTGAGC; HMGA1 forward: TTTACAGAAGGAGCCCAGCG,
reverse: AGGGAAGGGGGTACACAACT;GAPDH forward: GGAGCGAGATCCCTCCAAAAT,
reverse: GGCTGTTGTCATACTTCTCATGG; U6 forward:
CGCTTCGGCAGCACATATACTAAAATTGGAAC, reverse:
GCTTCACGAATTTGCGTGTCATCCTTGC.
Cell proliferation experiment
EdU experiment: EdU kit (RiboBio, China) was used
for the detection of cell proliferation. Then, 3,000 cells were
seeded into 96-well plates at cells/well, and then 50 mM EdU
solution was added to the culture medium. After 24 h, the cells
were fixed in 4% formaldehyde and infiltrated using Triton X-100.
Treated cells were incubated with the EDU reaction mixture and
counterstained with DAPI. The staining results were observed under
a fluorescence microscope and recorded according to the
manufacturer. Images of five randomly selected fields of view were
captured to calculate the number of EdU fusion cells.
For clone formation the transfected BC cells were
digested using trypsin, re-suspended, and counted. The same number
of BC cells was then calculated and plated into a 3.5-cm well.
After 14 days of culturing using complete medium, crystal violet
was used to stain visible colonies. Finally, stained colonies were
photographed, counted, and compared among different groups.
Transwell experiment
Transwell experiments were performed using 24-well
plates (Corning) with a membrane filter chamber at an aperture of 8
µm. For the invasion experiment, 100 µg Matrigel was precoated in
the upper chamber. BC cells were seeded into the upper chamber at
4×104 cells/well using 100 µl FBS-free RPMI-1640 culture
medium, while 500 µl RPMI-1640 culture medium with 10% FBS was
added into the lower chamber. After 24 h of culturing in a cell
incubator containing 5% CO2 at 37°C, cells in the upper
chamber were removed using a cotton swab. Cells were fixed in
methanol for 15 min, stained with crystal violet for 20 min,
photographed in five random fields of view under a microscope
(×200), and counted. For the migration experiment, steps were
identical to those of the invasion experiment, except that no
Matrigel was required.
Nucleoplasm isolation experiment
Cells were transferred to a 1.5-ml EP tube, with
cytoplasmic lysate (RLA) added, incubated on ice for 20 min, and
centrifuged at 1,500 × g at 4°C for 15 min to obtain cytoplasmic
protein as the supernatant. After three repeated runs of washing
and precipitation using RLA, the cells were incubated with nuclear
lysate (RIPA), incubated on ice for 20 min, mixed for 30 sec every
5 min, and centrifuged at 11,000 × g at 4°C for 15 min to obtain
nucleoproteins as the supernatant.
Luciferase assay
Bioinformatics website was used to predict the
binding sites of miR-15a-5p to LINC00649 or HMGA1. The LINC00473
sequence was analyzed and predicted on the website (http://starbase.sysu.edu.cn/). Subsequently,
3×104 treated BC cells were seeded into 24-well plates.
After 48 h of co-transfection with the corresponding plasmids
(GenePharma Shanghai) and miR-15a-5p mimics or negative control
according to the manufacturer, a Promega kit (Promega) was used to
measure luciferase activity as per the IFU and recorded. The
experiments were performed in triplicate, with the mean taken for
statistical analysis.
Western blot analysis
The treated BC cells were collected. For protein
extraction, the cells were added to the cell lysis solution
(Beyotime) of protease inhibitor PMSF, placed on ice, centrifuged
at 1,500 × g at 4°C for 15 min to collect the supernatant, and
protein concentration was measured according to the BCA kit
(Beyotime) manufacturer's instructions. After adding SDS-PAGE
protein loading buffer, the cells were heated at 100°C to make
albuminous degeneration. After loading, the transfer membrane was
performed. The corresponding size of the PVDF membrane was cut as
per the molecular weight for antigen blocking in 5% skim milk
powder blocking buffer. Then the membrane was incubated with
primary antibodies targeting HMGA1 (cat. no. ab202070; Abcam,
Cambridge) or GAPDH (cat. no. ab128915; Abcam) overnight at 4°C.
Next, the membranes were washed three times using TBS in Tween-20
and incubated with an HRP-linked goat antirabbit secondary antibody
(cat. no. ab205718; Abcam) at room temperature for 2 h. The ECL
Western Blotting Substrate Kit (cat. no. ab65623; Abcam) was used
to detect protein signals.
Statistical analysis
Each experiment was performed in triplicate.
Experimental data were reported as mean ± standard deviation and
analyzed with GraphPad Prism 6 (GraphPad Software Inc.). If the
data conformed to normal distribution, Student's t-test was used to
compare the mean of measurement data between the two groups, and
analysis of variance (ANOVA) was used to compare the difference
between multiple groups with Tukey honestly significant difference
(HSD) post hoc test. The correlation between LINC00649 and
miR-15a-5p expression was assessed using the Pearson's correlation
analysis. Kaplan-Meier analysis with log-rank tests were used for
overall survival analysis. P-value <0.05 was considered
statistically significant.
Results
LINC00649 is highly expressed in
BC
TCGA database analysis showed significantly higher
expression of LINC00649 in BC tissues relative to control tissues
(Fig. 1A). Further analysis revealed
that LINC00649 was strongly associated with the OS of BC patients
(HR=0.74, P=0.047) (Fig. 1B), but not
significantly correlated with DFS (HR=0.61, P=0.6) (Fig. 1C). Subsequently, LINC00649 expressions
in 60 BC tissues and control tissues were verified, which were
consistent with the database results. LINC00649 expression was
considerably increased in BC tissues (P<0.05) (Fig. 1D), and highly expressed LINC00649
indicated poor prognosis of BC patients (P<0.05)
(Fig. 1E). The results were different
from those of the TCGA database analysis. Further verification by
RT-qPCR revealed significant upregulation of LINC00649 expression
in the BC cell lines (P<0.05) (Fig. 1F). Moreover, the analysis of clinical
data revealed an association between LINC00649 and muscle invasion
of BC (P=0.0056), while no notable association with patient sex,
age, tumor staging, lymphatic metastasis, or distant metastasis was
observed (Table I). These results
showed that LINC00649 may be important in BC.
LINC00649 overexpression promotes the
proliferation, migration, and invasion of BC cells
To explore the biological functions of LINC00649,
lentivirus stable transfection was used to construct T24 and
UM-UC-3 cell lines with highly expressed LINC00649, and RT-qPCR was
performed to analyze LINC00649 expression, revealing that
LV-LINC00649 enhanced LINC00649 expression (P<0.05)
(Fig. 2A). Subsequently, EdU and
clone formation experiments were used to assess the influence of
LINC00649 on cell proliferation. As shown in Fig. 2B and C, highly expressed LINC00649 in
T24 and UM-UC-3 cells promoted cell proliferation and clone
formation. Transwell experiment revealed that the migration and
invasion of T24 and UM-UC-3 cells in the LV-LINC00649 group were
significantly enhanced relative to the LV-NC group
(P<0.05) (Fig. 2D and E).
These results indicated that LINC00649 overexpression notably
enhanced the proliferation, migration, and invasion of BC
cells.
Inhibition of LINC00649 suppresses the
proliferation, migration, and invasion of BC cells
LINC00649 expressions in T24 and UM-UC-3 were
inhibited by siRNA. In addition, for transfection efficiency,
RT-qPCR revealed that LINC00649 siRNA remarkably suppressed
LINC00649 expression (P<0.05) (Fig. 3A). si-LINC00649#1 was selected for
subsequent experiments owing to its higher interference efficiency.
From the EdU experiment, si-LINC00649 notably inhibited the
proliferation of T24 and UM-UC-3 cells (P<0.05) (Fig. 3B). Based on the results of the clone
formation experiment, the inhibition of LINC00649 in T24 and
UM-UC-3 cells suppressed cell colony formation. Furthermore,
Transwell experiments indicated that the migration and invasion of
T24 and UM-UC-3 cells in the si-LINC00649 group were notably
weakened relative to the si-NC group (P<0.05) (Fig. 3D-E). These results suggest that the
downregulation of LINC00649 significantly suppresses the
proliferation, migration, and invasion of BC cells.
LINC00649 binds to miR-15a-5p
To investigate the possible molecular mechanisms of
LINC00649 in BC, subcellular localization was performed via
nucleoplasm isolation experiments. As shown in Fig. 4A, LINC00649 was mainly located in the
cytoplasm of T24 and UM-UC-3 cells, suggesting that LINC00649 may
participate in the regulation at the post-transcriptional level
(P<0.05). Subsequently, the bioinformatics website was
used to predict miRNAs that may be bound to LINC00649. The binding
analysis found that miR-15a-5p, miR-15b-5p, miR-195-5p, miR-424-5p,
and miR-6838-5p may be potential binding targets of LINC00649
(P<0.05) (Fig. 4B).
LINC00649 was overexpressed in T24 and UM-UC-3 cells. In RT-qPCR,
LINC00649 significantly suppressed the expression of miR-15a-5p,
miR-15b-5p, and miR-424-5p, exerting the highest inhibition against
miR-15a-5p; thus, miR-15a-5p was selected for subsequent studies
(P<0.05) (Fig. 4C). Through
predicted binding sites, LINC00649 wild-type plasmids (LINC00649
WT) and LINC00649 mutant plasmids (LINC00649 MUT) were designed and
constructed (P<0.05) (Fig.
4D). For transfection efficiency, RT-qPCR revealed that
miR-15a-5p mimics significantly promoted miR-15a-5p expression
(P<0.05) (Fig. S1A). Their
binding relationship was detected via a dual luciferase reporter
gene experiment, which revealed that post-transfection with
miR-15a-5p mimics in T24 and UM-UC-3 cells, luciferase activity was
significantly decreased in LINC00649-WT 3′UTR group and was not
notably changed in LINC00649-MUT 3′UTR group compared with the
control group, indicating that miR-15a-5p bound to LINC00649
(P<0.05) (Fig. 4E).
miR-15a-5p expression in BC and control tissues were analyzed by
RT-qPCR, which revealed markedly low expression of miR-15a-5p in BC
tissues (P<0.05) (Fig. 4F).
Additionally, LINC00649 and miR-15a-5p expression levels were
analyzed in BC tissues, and a negative correlation was observed
between them (R=−0.5945, P<0.001) (Fig. 4G). These results showed that LINC00649
may bind to miR-15a-5p and suppress its expression in BC.
miR-15a-5p targeted HMGA1
Bioinformatics websites (microT, TargetScan, PicTar,
miRanda, RNA22, and miRmap) were used to predict target genes that
could bind to miR-15a-5p and found that HMGA1 was a potential
target (P<0.05) (Fig. 5A).
The analysis of the TCGA database revealed a significantly high
expression of HMGA1 in BC tissues (P<0.05) (Fig. 5B). Using predicted binding sites,
HMGA1 wild-type plasmids (HMGA1 WT) and HMGA1 mutant plasmids
(HMGA1 MUT) were designed and constructed (P<0.05)
(Fig. 5C). As shown by reporter gene
results, following transfection of T24 and UM-UC-3 cells with
miR-15a-5p mimics, luciferase was reduced in the HMGA1-WT 3′UTR
group and was not notably different in the HMGA1-MUT 3′UTR group
(P<0.05) (Fig. 5D),
indicating that miR-15a-5p could bind to HMGA1. After miR-15a-5p
and LINC00649 were highly expressed in both T24 and UM-UC-3 cells,
RT-qPCR and western blot analysis were employed to examine HMGA1
expression and indicated that post miR-15a-5p overexpression, mRNA
and protein expressions of HMGA1 were reduced, whereas LINC00649
overexpression partially reversed the inhibitory effect of
miR-15a-5p on HMGA1 (P<0.05) (Fig. 5E and F). This indicated that LINC00649
enhanced HMGA1 expression by binding to miR-15a-5, thus exerting
its role in BC.
miR-15a-5p suppressed the
proliferation, migration, and invasion of BC cells by inhibiting
HMGA1
For transfection efficiency, RT-qPCR and western
blot analysis revealed that HMGA1 OE markedly promoted HMGA1 mRNA
and protein expressions (P<0.05) (Fig. S1B and C). After high expression of
miR-15a-5p and HMGA1 in both T24 and UM-UC-3 cells, RT-qPCR was
employed to detect HMGA1 expression and indicated that miR-15a-5p
overexpression repressed HMGA1 expression, whereas HMGA1
overexpression showed a partial reversal effect on the inhibitory
effect of miR-15a-5p (P<0.05) (Fig. 6A). Subsequently, the effects of
miR-15a-5p and HMGA1 on cell proliferation, migration, and invasion
were assessed in vitro. Results indicated that post
miR-15a-5p overexpression in T24 and UM-UC-3 cells, cell
proliferation, and colony formation were both markedly weakened,
whereas HMGA1 overexpression reversed this inhibitory effect
(P<0.05) (Fig. 6B and C).
As such, miR-15a-5p overexpression in T24 and UM-UC-3 cells
repressed cell migration and invasion, which was reversed by HMGA1
expression (P<0.05) (Fig. 6D
and E). These results suggest that miR-15a-5p inhibits the
proliferation, migration, and invasion of BC cells by inhibiting
HMGA1.
LINC00649 promotes the proliferation,
migration, and invasion of BC cells by elevating HMGA1
expression
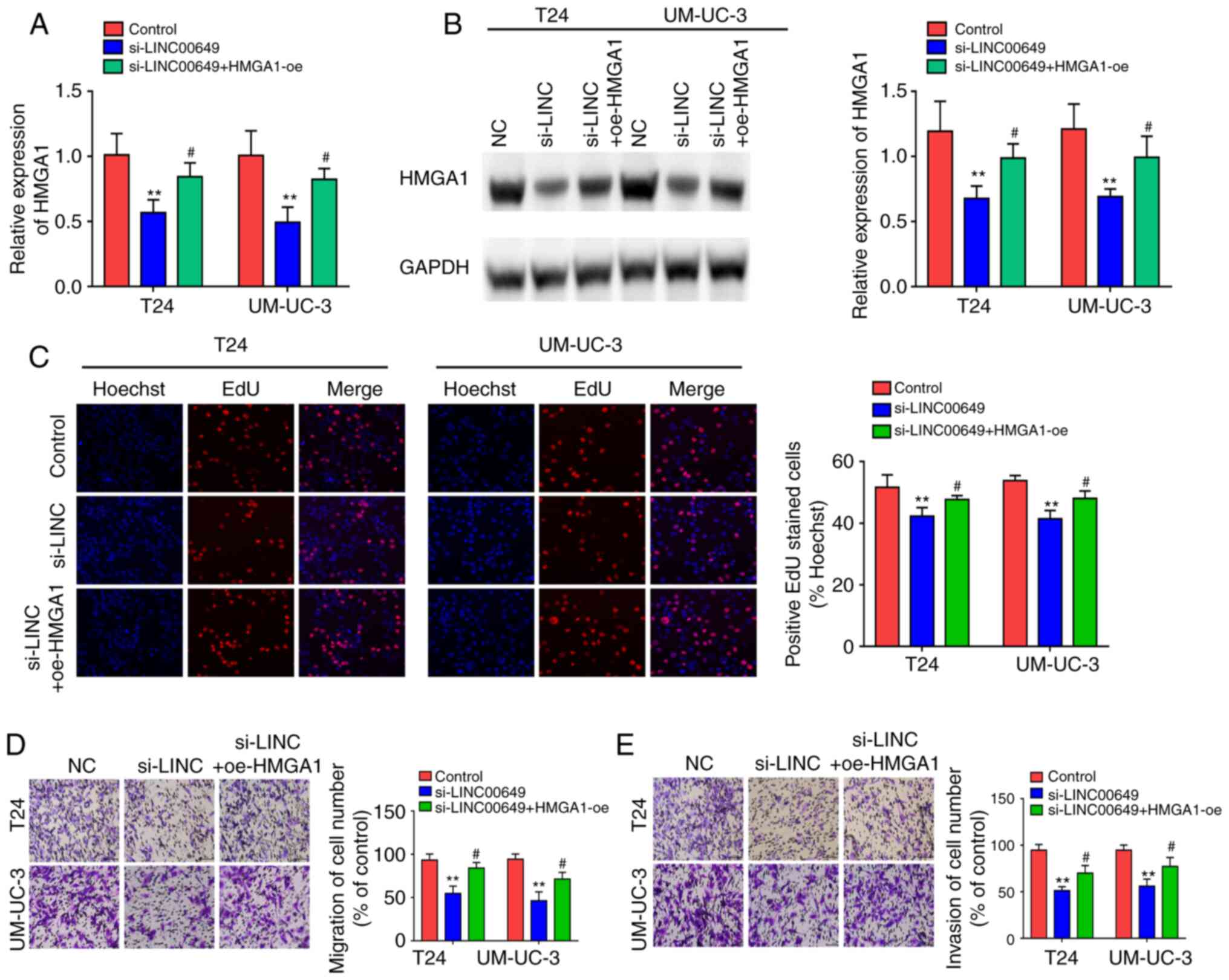
After co-transfection of LINC00649 siRNA and HMGA1
OE in both T24 and UM-UC-3 cells, RT-qPCR and western blotting were
employed to examine HMGA1 expression and indicated that LINC00649
siRNA repressed HMGA1 expression, whereas HMGA1 overexpression
showed a partial reversal effect on the inhibitory effect of
si-LINC00649 (P<0.05) (Fig. 7A
and B). Subsequently, the effects of LINC00649 and HMGA1 on
cell proliferation, migration, and invasion were assessed in
vitro. Results indicated that LINC00649 downregulation in T24
and UM-UC-3 cells markedly weakened cell proliferation, whereas
HMGA1 overexpression reversed this inhibitory effect
(P<0.05) (Fig. 7C). As
such, LINC00649 downregulation in T24 and UM-UC-3 cells repressed
cell migration and invasion, which was reversed by HMGA1 expression
(P<0.05) (Fig. 7D and E).
These results suggest that LINC00649 could promote the
proliferation, migration, and invasion of BC cells by elevating
HMGA1 expression.
Knockdown of LINC00649 inhibited the
EMT of BC cells
We determined whether the LINC00649/miR-15a-5p/HMGA1
regulatory axis regulated EMT in BC cells. The expressions of EMT
markers were determined using RT-qPCR and western blot analysis.
Knockdown of LINC00649 increased E-cadherin expression and
decreased expression of N-cadherin and vimentin in BC cells, while
the overexpression of HMGA1 promoted EMT. In addition, miR-15a-5p
mimics increased E-cadherin expression and decreased N-cadherin and
vimentin expressions in BC cells, while miR-15a-5p inhibitor showed
an opposite effect (P<0.05) (Fig. 8A and B). These results indicated that
the LINC00649/miR-15a-5p/HMGA1 regulatory axis promoted the EMT of
BC cells.
Discussion
BC is the most prevalent malignant tumor in the
urinary system, and its incidence is increasing worldwide (15). The occurrence and development of BC is
a complex process involving multiple genes that are diversely
regulated and may be associated with uncontrolled proto-oncogene
activation, inactivation or inhibition of cancer suppressor genes,
damage of self-repairing systems of DNA, extracellular matrix
formation, and living habits of individual patients; however,
specific mechanisms of action involved remain unclear (16). BC is currently mainly treated
surgically. BC, especially MIBC, is characterized by a high
recurrence rate, albeit with continuous improvement of surgical
methods and techniques combined with comprehensive treatments such
as radiotherapy, chemotherapy, and targeted drugs (17). Therefore, the exploration of molecular
markers and new effective therapeutic targets for the early
diagnosis of BC has become a hot spot in malignant bladder tumor
research.
It has been suggested that lncRNAs may affect the
occurrence and progression of multiple diseases, including
malignant tumors, through the regulation of cell cycle, immune
surveillance, cell differentiation, cell apoptosis, and other links
(18–20). Although studies of BC-related lncRNAs
started late, their progress has been rapid. lncRNA SNHG16 enhances
the malignant development of BC by regulating the
miR-98/STAT3/Wnt/β-catenin pathway axis (21), and lncRNA GAS5 contributes to the
apoptosis of BC cells by inhibiting EZH2 transcription (13). However, further research on BC-related
lncRNAs is required. Further in-depth studies on the search for
highly sensitive and highly specific tumor markers, the influence
of lncRNA on biological behaviors of BC cells, as well as specific
mechanisms of action should also be considered.
Abnormally highly expressed lncRNA-LINC00649 in BC
tissues was found through database analysis. LINC00649 was observed
to be significantly highly expressed in BC tissues via RT-qPCR in
60 BC tissues and adjacent tissues. Results revealed that high
expression of LINC00649 predicted poor prognosis of BC patients.
Notably, we identified that the opposite also occurred between TCGA
database and our results. Therefore, the study of more samples is
required to verify the association between LINC00649 expression and
BC patient OS. The in vitro cell experiments revealed that
LINC00649 contributed to the proliferation, migration, and invasion
of BC cells, indicating that it may act as a tumor promoter gene
involved in the malignant progression of BC.
lncRNAs are characterized by sequence conservation
and functional conservation, specific spatial secondary structure,
strong tissue and cell expression specificity, and relative
certainty of chromosomal localization, suggesting the vital
biological functions of these genes that are widely expressed in
human tissues and cells without protein-coding ability (22). Recent findings have shown that lncRNAs
participate in and constitute important and complex gene expression
regulatory networks in humans and may regulate gene expression via
interaction with protein or DNA specificity. lncRNAs may regulate
gene expression at multiple levels, such as transcriptional,
post-transcriptional, and epigenetic regulation (23).
During a nucleoplasm isolation experiment, LINC00649
was mainly located in the BC cytoplasm, suggesting that it exerted
crucial regulatory roles at the post-transcriptional level. The
LINC00473 sequence was analyzed and predicted on the website
(http://starbase.sysu.edu.cn/). We
observed that LINC00473 had multiple miRNA binding sites and may
act on multiple miRNAs, suggesting that it plays a role by binding
to miRNA. miR-15a-5p is a potential target miRNA with a higher
score and better correlation compared to other miRNAs. Further
analysis showed that LINC00649 binds to miR-15a-5p and inhibits its
expression, miR-15a-5p expression was notably downregulated in BC
tissues, and LINC00649 expression was negatively correlated with
miR-15a-5p expression. This indicated that LINC00649 promoted
cancer by binding to miR-15a-5p.
Prediction using Bioinformatics website identified
HMGA1 as a possible target of miR-15a-5p. HMGA1 has been identified
in the human cervical cancer cell line HeLa with highly malignant
proliferation. It is highly expressed in multiple
bio-embryogeneses, but barely or only weakly expressed in most
mature tissues. Additionally, it participates in many basic
cellular processes, including cell cycle regulation, embryogenesis,
tumor transformation, cell differentiation, apoptosis, metabolism,
and DNA repair. Evidence indicates that HMGA1 is highly expressed
in multiple human cancers such as cervical (24), breast (25), colorectal (26), and thyroid (27) cancer. The present study found that
miR-15a-5p targeted HMGA1, and LINC00649 contributed to HMGA1
expression by binding to miR-15a-5p. Overexpression of miR-15a-5p
repressed the proliferation, migration, and invasion of BC cells,
which was partially reversed by simultaneous HMGA1
overexpression.
In conclusion, findings of the present showed that
LINC00649 is abnormally highly expressed in BC, and that LINC00649
contributes to HMGA1 expression by binding to miR-15a-5p to enhance
the proliferation, migration, and invasion of BC, thus enhancing
its malignant progression. However, there were some limitations to
the study. First, the role of LINC00649 in the regulation of HMGA1
expression was not directly assessed. Second, although it was
demonstrated that LINC00649 is involved in the progression of BC by
binding to miR-15a-5p to regulate HMGA1 expression, LINC00649 had
multiple binding sites for miRNA. Thus, further studies are needed
to determine whether LINC00649 participates in disease progression
by binding to multiple miRNAs to regulate the expression of
multiple tumor promoter genes. Finally, the biological effects and
molecular mechanisms of LINC00649 were not verified in
vivo.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data included in this study are available upon
request by contact with the corresponding author.
Authors' contributions
XC and SC designed this study. XC conducted the
experiments, and SC served as scientific advisor. XC and SC
participated in writing or technical editing of the manuscript.
Both authors have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
Approval for the study was obtained from the ethics
committee of The First People's Hospital of Wenling (Zhejiang,
China). The patients volunteered to participate in this study and
signed a written informed consent form. All experiments were
performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no known
competing financial interests or personal relationships that could
have appeared to influence the work reported in this paper.
References
|
1
|
Afonso J, Santos LL, Longatto-Filho A and
Baltazar F: Competitive glucose metabolism as a target to boost
bladder cancer immunotherapy. Nat Rev Urol. 17:77–106. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alifrangis C, McGovern U, Freeman A,
Powles T and Linch M: Molecular and histopathology directed therapy
for advanced bladder cancer. Nat Rev Urol. 16:465–483. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng MV, Gschwend JE, Shore N, Grossfeld
GD, Mostafid H and Black PC: Emerging immunotherapy options for
bacillus calmette-guerin unresponsive nonmuscle invasive bladder
cancer. J Urol. 202:1111–1119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soria F, D'Andrea D, Pohar K, Shariat SF
and Lotan Y: Diagnostic, prognostic and surveillance urinary
markers in nonmuscle invasive bladder cancer: Any role in clinical
practice? Curr Opin Urol. 28:577–583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dobruch J, Daneshmand S, Fisch M, Lotan Y,
Noon AP, Resnick MJ, Shariat SF, Zlotta AR and Boorjian SA: Gender
and bladder cancer: A collaborative review of etiology, biology,
and outcomes. Eur Urol. 69:300–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo X and Hua Y: CCAT1: An oncogenic long
noncoding RNA in human cancers. J Cancer Res Clin Oncol.
143:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Hamblin MH and Yin KJ: The long
noncoding RNA Malat1: Its physiological and pathophysiological
functions. RNA Biol. 14:1705–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen LL: Linking long noncoding RNA
localization and function. Trends Biochem Sci. 41:761–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sallam T, Sandhu J and Tontonoz P: Long
noncoding RNA discovery in cardiovascular disease: Decoding form to
function. Circ Res. 122:155–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Chen F, Zhao M, Yang Z, Li J,
Zhang S, Zhang W, Ye L and Zhang X: The long noncoding RNA HULC
promotes liver cancer by increasing the expression of the HMGA2
oncogene via sequestration of the microRNA-186. J Biol Chem.
292:15395–15407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Qiu
FN, Yan ML, Tian YF, Peng CH, Shen BY, Chen YL and Wang YD:
Silencing of long noncoding RNA LINC00958 prevents tumor initiation
of pancreatic cancer by acting as a sponge of microRNA-330-5p to
down-regulate PAX8. Cancer Lett. 446:49–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Q, Shan S, Li Y, Zhu D, Jin W and Ren
T: Long noncoding RNA SNHG1 promotes non-small cell lung cancer
progression by up-regulating MTDH via sponging miR-145-5p. FASEB J.
32:3957–3967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Guo C, Wang L, Luo G, Huang C, Li
Y, Liu D, Zeng F, Jiang G and Xiao X: Long noncoding RNA GAS5
promotes bladder cancer cells apoptosis through inhibiting EZH2
transcription. Cell Death Dis. 9:2382018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Xie D and Zhang H: Long noncoding
RNA neuroblastoma-associated transcript 1 gene inhibits malignant
cellular phenotypes of bladder cancer through miR-21/SOCS6 axis.
Cell Death Dis. 9:10422018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soukup V, Capoun O, Cohen D, Hernandez V,
Babjuk M, Burger M, Compérat E, Gontero P, Lam T, MacLennan S, et
al: Prognostic Performance and Reproducibility of the 1973 and
2004/2016 World Health organization grading classification systems
in non-muscle-invasive bladder cancer: A european association of
urology non-muscle invasive bladder cancer guidelines panel
systematic review. Eur Urol. 72:801–813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lobo N, Mount C, Omar K, Nair R,
Thurairaja R and Khan MS: Landmarks in the treatment of
muscle-invasive bladder cancer. Nat Rev Urol. 14:565–574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin C and Yang L: Long noncoding RNA in
cancer: Wiring signaling circuitry. Trends Cell Biol. 28:287–301.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen M, Li J, Zhuang C and Cai Z:
Increased lncRNA ABHD11-AS1 represses the malignant phenotypes of
bladder cancer. Oncotarget. 8:28176–28186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Zhuang C, Liu Y, Li J, Dai F, Xia
M, Zhan Y, Lin J, Chen Z, He A, et al: Tetracycline-inducible shRNA
targeting antisense long non-coding RNA HIF1A-AS2 represses the
malignant phenotypes of bladder cancer. Cancer Lett. 376:155–164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng F, Chen A, Huang J, Xia Q, Chen Y and
Jin X: Long noncoding RNA SNHG16 contributes to the development of
bladder cancer via regulating miR-98/STAT3/Wnt/β-catenin pathway
axis. J Cell Biochem. 119:9408–9418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jandura A and Krause HM: The New RNA
World: Growing Evidence for Long Noncoding RNA Functionality.
Trends Genet. 33:665–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu F, Wang T, Wu Z, Feng Y, Wang W, Zhou
S, Ma X and Wang S: HMGA1 exacerbates tumor growth through
regulating the cell cycle and accelerates migration/invasion via
targeting miR-221/222 in cervical cancer. Cell Death Dis.
9:5942018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zanin R, Pegoraro S, Ros G, Ciani Y,
Piazza S, Bossi F, Bulla R, Zennaro C, Tonon F, Lazarevic D, et al:
HMGA1 promotes breast cancer angiogenesis supporting the stability,
nuclear localization and transcriptional activity of FOXM1. J Exp
Clin Cancer Res. 38:3132019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei F, Zhang T, Deng SC, Wei JC, Yang P,
Wang Q, Chen ZP, Li WL, Chen HC, Hu H and Cao J: PD-L1 promotes
colorectal cancer stem cell expansion by activating HMGA1-dependent
signaling pathways. Cancer Lett. 450:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong J, Liu C, Zhang QH, Chen L, Shen YY,
Chen YJ, Zeng X, Zu XY and Cao RX: TGF-β1 induces HMGA1 expression:
The role of HMGA1 in thyroid cancer proliferation and invasion. Int
J Oncol. 50:1567–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|