Introduction
Breast hyperplasia is a common disease that
generally occurs in women and accounts for more than 90% of all
breast diseases. Breast hyperplasia is characterized by
proliferative pathological changes in the breast acini, epithelial
cells lining the breast ducts, and fibrous connective tissue. The
clinical manifestations of breast hyperplasia are breast pain,
local thickening of the breast tissue, or multiple nodules of
different sizes (1,2). The occurrence of breast hyperplasia can
be associated with an increased risk for breast cancer. The
incidence of breast cancer in patients with severe breast
hyperplasia, atypical breast hyperplasia, and cystic breast
hyperplasia, is significantly higher (3). However, the etiology of breast
hyperplasia is not fully understood. The breast is one of the most
complex endocrine target organs in the human body and represents
the principal target for many hormones. The expression levels of
the estrogen receptor (ER) and the progesterone receptor (PR) in
breast tissue have been associated with the regulation of
self-sensitivity in breast tissue (4). There is a general consensus of opinion
among researchers that the occurrence of breast hyperplasia is
closely related to the disruption of hormones and hormone receptors
across the body (5,6). Currently, the principle treatment for
this disease is drug or surgical treatment. However, because these
lesions generally adopt a diffuse distribution, it is usually
impossible to remove all of the hyperplastic lesions. It is
therefore impossible to cure this disease completely by surgery
alone. It is vital that we develop appropriate drugs for the
treatment of breast hyperplasia.
Melatonin (N-acetyl-5-methoxytryptamine) is an
indoleamine, which is predominantly synthesized by the mammalian
pineal gland. The synthesis and secretion of melatonin is
controlled by light and dark conditions (7). Previous research has shown that
melatonin can regulate the reproductive activities of several
photoperiodic animals (8,9). Early work indicated that the main
function of melatonin was to regulate sleep (10,11).
However, subsequent studies have reported that melatonin has a
multitude of effects against tumors (12) and in the regulation of immunity
(13). With regards to the treatment
of breast cancer, melatonin has been shown to reduce the growth
rate of breast tumor cells and inhibit the invasion and metastasis
of tumor cells. Indeed, such work indicated that melatonin could be
a highly appropriate drug candidate for the treatment of breast
tumors (14).
Phosphatase and tensin homolog (PTEN) is an
effective tumor-suppressor gene located on chromosome 10q23.31
(15); this is the only
tumor-suppressor gene that has been identified to exhibit
phosphatase activity. AKT (also referred to as protein kinase B) is
a serine-threonine kinase and is considered to act as a
proto-oncogene (16). The abnormal
expression of these kinases has been previously linked to
tumorigenesis (15,17). Previous studies have reported that
breast cancer is associated with the abnormal activation of the
PTEN/AKT pathway (14). In the
present study, we investigated the molecular mechanisms underlying
the effects of melatonin on breast hyperplasia. Our research
identified the abnormal activation of PTEN/AKT in the hyperplastic
breast tissue and that the degree of breast hyperplasia was
significantly reduced following treatment with melatonin.
Materials and methods
Animals and the administration of
treatments
Seventy-six virgin female Wistar rats were purchased
from Cavens Lab Animal Co., Ltd. (Changzhou, China), weighing
180–220 g, were fed adaptively for one week and then randomly
divided into two groups: A control group (n=13) and a model group
(n =63). All animals were maintained under 12-h light and 12-h dark
cycles at 22–24°C with free access to food and water. All rats in
the model group, but not the control group, were treated with
estrogen and progesterone to establish the rat model of breast
hyperplasia. In brief, we injected 0.5 mg of estradiol benzoate
into the muscles of the hind legs alternately for 25 days. Then, we
injected 4 mg of progesterone for 5 consecutive days. Rats in the
control group were injected with 0.1 ml of saline into the muscles
of the hind legs for 30 days. After fasting for 12 h on the 30th
day, we selected three rats from the control group and three rats
from the model group. Using these animals, we removed the second
pair of the right mammary glands to prepare pathological sections
for analyses. We then used sections stained with hematoxylin eosin
(H&E) staining to verify the successful creation of the rat
model of breast hyperplasia. During such analysis, we focused on
the acini, ductal epithelium, and the mammary lobules. We observed
obvious acinar and ductal epithelial hyperplasia, an increase in
the diameter and number of mammary acini, an increase in the
average diameter of the mammary lobules, and an increase in the
number of mammary lobules. These results proved that the model had
been successfully established. Rats in which the model had been
successfully established were then randomly divided into a model
group (n=10), a negative control group (n=10), a positive control
group (n=10), a melatonin group (n=10), a PTEN-inhibitor group
(n=10), and an melatonin+PTEN-inhibitor group (n=10). Saline was
used as a solvent.
We administered the PTEN inhibitor bpV (HOpic, EMD
Biosciences) at a dose of 500 µg/kg by local injection every 10
days. The negative control group was administered with normal
saline, while rats in the positive control group were given
tamoxifen. Saline was used as a control. The clinically equivalent
dose was calculated as a dose reference (a factor of 6.25 of the
adult unit body weight). The diameter and height of the second pair
of nipples were measured using a precision Vernier caliper every 10
days. The overall duration of the experiments was 60 days. Prior to
the experiment, the rats were fasted for 12 h and then anesthetized
by intraperitoneal injection of a mixture of ketamine (100 mg/kg)
and xylazine (5 mg/kg). During injection, we closely monitored the
corneal reflex, muscle relaxation, and pain response. Following the
induction of anesthesia, we applied hair removal cream to remove
the fur around the second pair of mammary glands and aseptically
removed the second pair of right mammary gland tissues. Some of
these tissues were immediately stored in a freezer at −80°C to
await western blotting experiments. The rest of the tissues were
fixed with 4% paraformaldehyde for 3 days, embedded in paraffin,
and sectioned for H&E staining. These sections were
subsequently used to investigate histopathological changes in
response to the different treatments. At the end of the experiment
and under continuous anesthesia, the animals were euthanized by
overdose of pentobarbital (125 mg/kg). All of the experiments were
carried out in accordance with the Chinese regulations on the use
and breeding of experimental animals and were approved by the
Animal Experiment Center. All animal experiments complied with the
ARRIVE guidelines and were carried out in accordance with the U.K.
Animals (Scientific Procedures) Act, 1986 and associated
guidelines.
Immunohistochemistry assay
First, we placed the paraffin-embedded tissue
sections into a 60°C incubator for 20 min. These were then dewaxed
twice in xylene for 10 min. Next, the sections were hydrated with a
graded series of ethanol concentrations (from high to low). The
dewaxed and hydrated sections were then treated with citrate buffer
(sodium citrate antigen retrieval solution; 10 mM citric acid, pH
6.0) for 10 min and then washed three times in phosphate-buffered
solution (PBS). The sections were then immersed in 3%
H2O2 at 37°C for 10 min and washed three
times in PBS. The sections were incubated with 5% goat serum at
37°C for 15 min before being incubated overnight with a primary
antibody at 4°C. Two monoclonal antibodies were used: Rabbit
anti-PCNA [dilution 1:500; cat. #13110, Cell Signaling Technology,
Inc. (CST)] and rabbit anti-cleaved-caspase-3 (dilution 1:1,000;
cat. no. 9664, CST). The following morning, the sections were
rinsed three times and then incubated with a goat anti-rabbit IgG
second antibody (dilution 1:1,000; cat. no. ab7090, Abcam) at 37°C
for 15 min. The sections were then washed three times in PBS,
incubated in Strept Avidin Biotin Complex for 15 min at 37°C, and
then stained with 3,3-diaminobenzidine tetrahydrochloride (DAB) at
37°C for 5 min. After staining with DAB, the sections were
re-stained with H&E and mounted with Permount (Thermo Fisher
Scientific, Inc.). Finally, the sections were observed using an
Olympus 1×70 light microscope (at magnifications of ×200 and
×400).
TUNEL assay
Paraffin-embedded tissue sections were placed in a
60°C incubator for 20 min and then dewaxed twice in xylene for 10
min. We then hydrated the sections with ethanol with a gradient
series of ethanol concentrations (from high to low). The dewaxed
and hydrated sections were then incubated with 20 µg/ml of Protease
K for 20 min. TUNEL assay kits were purchased from Abcam and were
used in accordance with the manufacturer's instructions. In brief,
the TUNEL reaction was carried out at 37°C for 1 h. After the
reaction, the sections were washed three times with PBS, incubated
with anti-fluorescein antibody-peroxidase (POD) for 30 min, stained
with DAB for 10 min, washed three times with PBS, and then
re-stained with H&E. Finally, sections were mounted with
Permount. Sections were observed using an Olympus 1×70 light
microscope (at magnifications of ×200 and ×400). Cells showing
brown-stained nuclei were recorded as being positive. According to
the distribution of apoptotic cells, we photographed 7 positive
visual fields from each treatment group under a ×400 objective. We
then counted 200 cells in each visual field using Image-pro Plus
6.0 (Image-pro Plus, Media Cybernetics, Inc., USA). Then, we
calculated the mean proportion of apoptotic cells (in %) as the
apoptotic index (AI) using Equation 1.
Equation 1: AI = the number of apoptotic cells/total
number cells
Western blotting assays
Fresh breast tissues were crushed by grinding in
liquid nitrogen. We then extracted whole cell proteins in RIPA
buffer (CST) and determined protein concentrations using a BCA
protein concentration detection kit (Thermo Fisher Scientific,
Inc.). Protein lysate (20 µg) was mixed with 4X SDS-PAGE loading
buffer and boiled at 99°C for 5 min. The proteins were then
separated by 12% SDS-PAGE electrophoresis alongside a protein
marker. Separated proteins were then transferred, along with the
protein marker, onto a PVDF membrane. After transfer, the PVDF
membrane was incubated with 5% skimmed milk at 37°C for 1 h. The
PDVF membrane was then incubated with several monoclonal antibodies
at 4°C overnight: Rabbit anti-PTEN (dilution 1:1,000; cat. no.
ab267787, Abcam), rabbit anti-p-AKT/AKT (dilution 1:500, cat.
#4060, CST), and rabbit anti-GAPDH (dilution 1:2,000; cat. no.
ab8245, Abcam). After incubation with the primary antibody, the
membrane was rinsed three times in Tris-buffered saline Tween
(TBST) for 10 min. The rinsed membrane was then incubated with a
secondary antibody, goat anti-rabbit IgG (dilution 1:2,000; cat.
no. ab7090, Abcam), at 37°C for 2 h. After incubation, the membrane
was rinsed three times with TBST for 10 min. After rinsing,
positive antibody binding was revealed by an enhanced
chemiluminescence (ECL) immunoblotting photoluminescence kit
(Thermo Fisher Scientific, Inc.). The levels of each protein were
then analyzed by Quantity One software version 4.4.0 (Bio-Rad
Laboratories) and normalized to controls.
Statistical analysis
SPSS version 19.0 statistical software (SPSS Inc.)
was used to analyze all experimental data. Experimental results are
expressed as the mean ± standard deviation (SD). Different groups
were compared by one-way analysis of variance (ANOVA). We also
carried out tests to determine the homogeneity of variance. If
variances were uniform, then we carried out Fisher's least
significant difference (LSD) analysis. However, if the variance was
not uniform, then we used the Games-Howell test. Differences were
considered to be statistically significant if P<0.05.
Results
Changes in nipple diameter, height,
and case characteristics
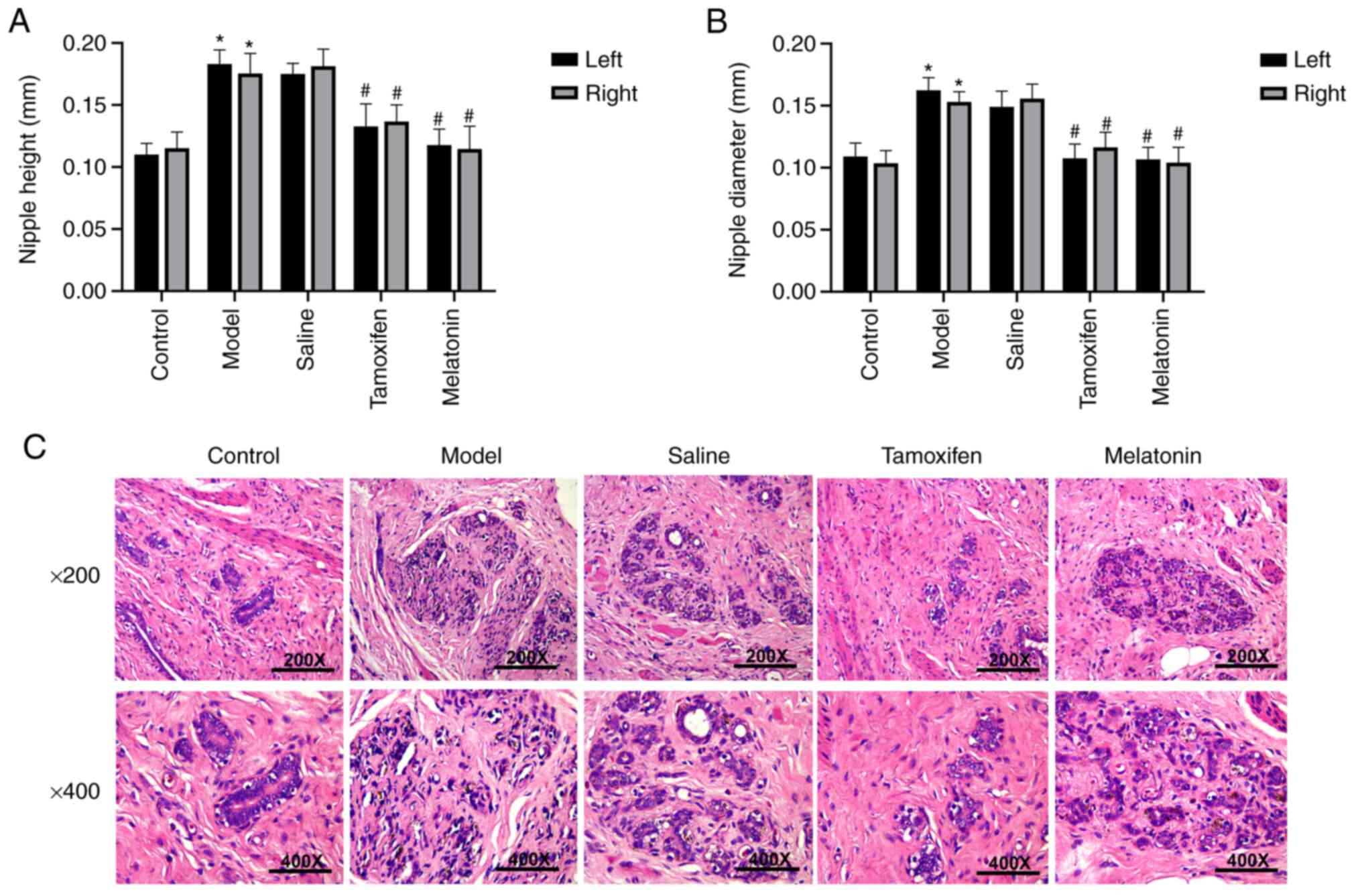
We found that nipple height and diameter were
directly proportional to the severity of breast hyperplasia, and
therefore, represent an intuitive index with which to reflect the
degree of hyperplasia in mammary glands. As shown in Fig. 1A and B, both nipple diameter and
nipple height in the model group were significantly wider and
higher, respectively, than those in the control group (P<0.05).
Compared to the model group, all of the drug treatments led to a
significant reduction in both nipple diameter and height in all
groups, except for the negative control (saline) group (P<0.05).
The therapeutic effect in the melatonin group was similar to that
observed in the tamoxifen group.
Next, we investigated histological changes in each
group by microscopy (Fig. 1C). We
found that in the control group, the breast tissue was in a
quiescent state, the epithelial cells of the mammary duct were
arranged regularly, the ductal lumen and the acinar lumen were not
dilated. In addition, there were only small numbers of lobular
acini, and there was no interstitial hyperplasia. In the model
group, there was significant hyperplasia of the breast acini and
ductal epithelium, and the diameter and number of breast acini and
breast lobules had increased significantly. In addition, we were
able to visualize exfoliated epithelial cells and secretions in the
breast acini and duct. In the melatonin and tamoxifen treatment
groups, the degree of breast tissue hyperplasia was significantly
alleviated, the arrangement of the epithelium cells in the mammary
duct was slightly irregular, the acinar cavity and the ductal lumen
were slightly dilated, a small number of acini were slightly
hyperplastic, and the breast acini and ductal endocrine secretions
were reduced. Our results demonstrated that melatonin can improve
the pathological changes observed in breast tissue.
Melatonin inhibits cell proliferation
in rats with breast hyperplasia
In order to determine the effect of melatonin on
breast cell proliferation, we determined the expression of
proliferating cell nuclear antigen (PCNA) and apoptotic protein
cleaved-caspase-3 by immunohistochemistry. Analysis showed that
PCNA was weakly positive in the ductal and acinar epithelial cells
in the control group (shown as yellow or brown granular staining).
Analysis also showed that there was a significantly lower number of
cells that stained positive for cleaved-caspase-3 in the model
group than that noted in the control group, and also that this
positive staining was distributed wider and was more intense.
Compared with the other treatment groups, we found that the number
of PCNA-positive cells was significantly lower in the melatonin and
tamoxifen groups, and that the number of cells that showed positive
staining for cleaved-caspase-3 was significantly higher than that
in the model group (Fig. 2,
P<0.05). Following TUNEL staining, we re-stained sections with
hematoxylin. Cells showing brown nuclei were considered to be
apoptotic. Analysis showed that there were almost no apoptotic
cells in the control group (Fig. 2);
however, the numbers of apoptotic cells in the melatonin group and
the tamoxifen group were significantly higher.
Melatonin regulates the PTEN/AKT
pathway in rats with breast hyperplasia
In order to investigate whether melatonin regulates
breast hyperplasia via the PTEN/AKT pathway, western blotting was
performed to determine the expression of PTEN, AKT, and
phosphorylated (p-)AKT proteins in each group. As shown in Fig. 3, the expression of PTEN in the control
breast tissue was significantly higher than in the model group, and
the expression of p-AKT/AKT in normal breast tissue was
significantly lower than that in the model group (P<0.05).
Changes were also detected in the expression of PTEN and p-AKT/AKT
in each treatment group. Results showed that the expression of PTEN
was significantly increased in the tamoxifen and melatonin groups
(P<0.05) while the expression of p-AKT/AKT was significantly
decreased (P<0.05). These results suggested that the effect of
melatonin on hyperplasia in the mammary glands of rats is achieved
via the PTEN/AKT pathway.
Melatonin weakens the effects of a
PTEN inhibitor in rats with breast hyperplasia
In order to further confirm that melatonin regulates
cell proliferation in hyperplastic breast tissue via the PTEN/AKT
signaling pathway, our rat model was administered a special PTEN
inhibitor bpV (HOpic). Expression of PTEN was significantly
inhibited and that the expression of p-AKT/AKT had significantly
increased in the PTEN-in group compared with the control group
(P<0.05). In addition, expression of PTEN in the
melatonin+PTEN-in group was significantly higher than that in the
PTEN-in group (P<0.05), while expression of p-AKT/AKT was
significantly inhibited (P<0.05). These data suggested that
melatonin can weaken the activity of the PTEN/AKT signaling pathway
(Fig. 4A). We also measured changes
in nipple diameter and height across the different groups. Results
showed that nipple height and nipple diameter increased
significantly in the PTEN-in group. Furthermore, in the
melatonin+PTEN-in group, we found that nipple diameter and height
was significantly lower than these parameters in the PTEN-in group
(P<0.05), thus, suggesting that breast hyperplasia was
significantly reduced (Fig. 4B and
C). We used H&E staining to investigate the effect of
melatonin on the degree of breast hyperplasia under the
intervention of a PTEN-inhibitor. In the melatonin+PTEN-in group,
we observed that the breast hyperplasia had been significantly
alleviated and was not affected by the PTEN inhibitor (Fig. 4D). These results suggested that
melatonin can weaken the aggravation of breast hyperplasia caused
by the administration of PTEN inhibitor.
The expression of PCNA and cleaved-caspase-3 were
detected by immunohistochemistry in the PTEN-in group and the
melatonin+PTEN-in group. Data showed that the expression of PCNA
was significantly higher in the PTEN-in group while the expression
of cleaved-caspase-3 was significantly lower (P<0.05; Fig. 5). Compared with the PTEN-in group, the
expression of PCNA was significantly lower in the melatonin+PTEN-in
group, while the expression of cleaved-caspase-3 was significantly
higher (P<0.05). TUNEL staining results were consistent with the
expression of cleaved-caspase-3. This suggested that melatonin can
reduce the proliferation of breast tissue cells caused following
administration of the PTEN inhibitor.
Discussion
Breast hyperplasia refers to hyperplasia of the
breast epithelium and fibrous tissue caused by endocrine imbalance
and the structural degeneration of breast ducts and breast lobules
(18,19). Breast hyperplasia is a common benign
disease of the breast and occurs during the normal development and
degeneration of female breasts. Globally, breast hyperplasia is
ranked as the leading cause of female breast disease. If the
disease persists for a long period of time, it can develop into
pre-cancerous lesions or even the development of breast cancer,
thus, representing a serious risk for public health and the safety
of women (20). Over recent years,
there has been a gradual increase in the incidence of breast
hyperplasia; furthermore, the age at onset has become younger. It
is commonly believed that the increases incidence of this condition
is related to changes in social rhythm and life pressure. Moreover,
this condition causes serious adverse effects on the physical and
mental health of women of childbearing age (21). A recent study showed that the
incidence of breast hyperplasia is increasing by 2.7-fold each
year, while the probability of breast cancer in patients with
breast hyperplasia is 1.4- to 1.5-fold higher than that of healthy
women (22). At present, tamoxifen,
toremifene, and other drugs, are predominantly used for the
treatment of breast hyperplasia (23,24).
However, patient compliance for such treatment is poor. This is due
to the requirements for long-term treatment and the associated risk
of a series of side effects (25).
Therefore, there is a critical need to identify new drugs that are
effective for the treatment of breast hyperplasia, but with fewer
side effects and better outcomes.
In the present study, we found that melatonin
performed well in alleviating breast hyperplasia and achieved the
same therapeutic effect as tamoxifen. We established a rat model of
breast hyperplasia induced by estradiol benzoate and progesterone
and then explored the molecular mechanisms underlying the effects
of melatonin in the treatment of breast hyperplasia. By measuring
changes in nipple height and diameter, and by investigating the
pathological changes of breast tissue following H&E staining;
we found that the degree of breast hyperplasia was significantly
reduced after melatonin treatment. Next, we determined the
expression of proliferating cell nuclear antigen (PCNA) and
cleaved-caspase-3 protein, and evaluated proliferation and
apoptosis in breast tissue. PCNA is an essential requirement for
DNA replication. The expression and synthesis of PCNA is closely
related to cell proliferation, and is therefore, an important index
with which to evaluate the state of cell proliferation (26). Our results showed that the expression
of PCNA in the model group was significantly higher (P<0.05).
These data showed that the breast tissue had high rates of
proliferation during the onset of breast hyperplasia. After
treatment, we found that the expression of PCNA decreased, thus,
indicating that melatonin can significantly reduce cell
proliferation in breast hyperplasia. Caspase-3 is the most
important executor of apoptosis in the caspase family, and
represents the final common substrate of both endogenous and
exogenous apoptosis pathways. Activated caspase-3 can cleave
substrate to induce apoptosis, and therefore, represents an
important parameter with which to reflect apoptosis. By measuring
the expression levels or the activity of activated caspase-3, we
can indirectly reflect the level of apoptosis (27). By detecting the expression levels of
cleaved-caspase-3, we found that the expression levels of
cleaved-caspase-3 were lower in the disease model group, thus
suggesting that apoptosis had been inhibited. We also found that
the expression of cleaved-caspase-3 had increased significantly in
the treatment group, thus suggesting that melatonin can promote
apoptosis in hyperplastic breast tissue cells. In the present
study, we also used TUNEL staining and the expression of
cleaved-caspase-3 to provide evidence for apoptosis; these two
techniques yielded similar results. Consequently, our data
suggested that the effects of melatonin in the treatment of breast
hyperplasia are similar to, or even better than, tamoxifen.
Previous studies have found that melatonin has a
significant effect on breast cancer and is a good candidate drug
for the treatment of breast cancer (14). Furthermore, experimental studies have
found that melatonin inhibits cell proliferation and induces
apoptosis by inhibiting the COX-2/PGE2, p300/NF-κB, and PI3K/AKT
signaling pathways, and by activating the APAF-1/caspase-dependent
apoptotic pathways in breast cancer cells (28). We found that the phosphatase and
tensin homolog (PTEN)/protein kinase B (AKT) signaling pathway was
abnormally activated in breast hyperplasia and observed low levels
of PTEN protein in the disease model group; however, the levels of
PTEN protein increased significantly after melatonin treatment.
PTEN is a widely studied tumor-suppressor gene. Many human
cancers are associated with somatic deletion or mutation of the
PTEN gene, including endometrial carcinoma (29), skin (30), breast (31) and prostate cancer (32). The inactivation of PTEN gene protein
products in breast cancer is associated with tumor cell invasion
and metastasis (33). PTEN is a
phosphatase (34), which is the main
negative regulator of class I phosphatidylinositol-3-kinases
(PI3Ks) (35). PTEN can inhibit the
conversion of phosphatidylinositol-4-diphosphate (PIP2) to
phosphatidylinositol-3-triphosphate (PIP3) (36). The reduction or total loss of PTEN
activity is known to contribute to the activation of the PI3K
pathway (37). An increase in the
levels of PIP3 in cancer cells can inhibit apoptosis and affect the
growth and proliferation of tumor cells (38). In our study, the expression levels of
PTEN protein in the model of breast hyperplasia were low. In
contrast, the expression levels of PTEN protein were high in normal
breast tissue. There was a significant difference between the two
groups (P<0.05), thus, indicating that breast hyperplasia is
associated with low levels of PTEN protein.
AKT is a key gene in the PTEN/AKT pathway and acts
as a proto-ncogene that encodes a 58-kDa protein that is
overexpressed in numerous human malignancies (39). The AKT protein contains 480 amino
acids, with catalytic sites at both the N-terminal and C-terminal
that are phosphorylated by PDK and PI3K, respectively (40). Once activated, AKT produces p-Akt,
which can promote tumor progression (41). PTEN exerts its role mainly via the
PI3K/AKT signaling pathway. When PTEN is expressed at high levels,
PIP2 is phosphorylated by PI3K; levels of PIP2 increase in the
nucleus and therefore inhibit the PI3K/AKT pathway. When PTEN is
expressed at low levels, there is a large-scale accumulation of
PIP3. This also leads to a significant reduction in the level of
apoptosis. The reason for this is because AKT is always in a state
of activation, thus inducing a variety of tumors (41–43).
Western blotting data further showed that the expression levels of
PTEN consistently opposed those of p-AKT/AKT. The expression of
p-AKT/AKT was upregulated in the disease model group, but
significantly inhibited in the treatment groups (P<0.05). In the
present study, we applied a special PTEN inhibitor bpV (HOpic) to
inhibit PTEN activity in our rat model. Data showed that the extent
of breast hyperplasia was significantly higher in the PTEN-in
group, as compared with the disease model group. Furthermore, the
height and diameter of the nipple were significantly higher
(P<0.05), the proliferation of breast tissue cells was
significantly increased, and the extent of apoptosis was inhibited.
These data indicated that PTEN had a regulatory effect on the
proliferation of breast tissue cells. In the melatonin and PTEN-in
groups, it was clear that melatonin significantly attenuated the
breast hyperplasia induced by a PTEN inhibitor, thus, suggesting
that melatonin can alleviate breast hyperplasia via PTEN.
In regards to the limitations, in our study, we did
not conduct a detailed study of the therapeutic window and side
effects during melatonin treatment of breast hyperplasia. Thus,
further in-depth studies will be conducted to provide more research
information in order to timely promote the use of melatonin in
clinical treatment.
To conclude, our results indicate that melatonin is
an effective means of treating breast hyperplasia, and that the
PTEN/AKT pathway shows abnormal activation in breast hyperplasia.
Our data also showed that the expression levels of proteins
associated with the PTEN/AKT pathway were related to the degree of
breast hyperplasia. Our data indicate that melatonin can play a
therapeutic role by regulating the PTEN/PI3K/AKT axis. Our data
indicate that melatonin may represent an ideal drug for the
treatment of breast hyperplasia.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant
from funding agencies in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
XZ WA responsible for project development and
manuscript writing. YN conducted the data collection. YH was
responsible for data collection and manuscript writing. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All protocols followed the requirements of the
Animal Experiment Center. All of the experiments were carried out
in accordance with the Chinese regulations on the use and breeding
of experimental animals and were approved by the Animal Experiment
Center. All animal experiments complied with the ARRIVE guidelines
and were carried out in accordance with the U.K. Animals
(Scientific Procedures) Act, 1986 and associated guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
PTEN
|
phosphatase and tensin homolog
|
|
AKT
|
protein kinase B/serine/threonine
protein kinase
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
PI3K
|
class I
phosphatidylinositol-3-kinase
|
|
PIP3
|
phosphatidylinositol-3-triphosphate
|
|
PIP2
|
phosphatidylinositol-4-diphosphate
|
References
|
1
|
Huang J, Tang H, Cao S, He Y, Feng Y, Wang
K and Zheng Q: Molecular targets and associated potential pathways
of danlu capsules in hyperplasia of mammary glands based on systems
pharmacology. Evid Based Complement Alternat Med. 2017:19305982017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer JS: Cell proliferation in normal
human breast ducts, fibroadenomas, and other ductal hyperplasias
measured by nuclear labeling with tritiated thymidine. Effects of
menstrual phase, age, and oral contraceptive hormones. Hum Pathol.
8:67–81. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartmann LC, Degnim AC, Santen RJ, Dupont
WD and Ghosh K: Atypical hyperplasia of the breast-risk assessment
and management options. N Engl J Med. 372:78–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh H, Eliassen AH, Wang M, Smith-Warner
SA, Beck AH, Schnitt SJ, Collins LC, Connolly JL, Montaser-Kouhsari
L, Polyak K and Tamimi RM: Expression of estrogen receptor,
progesterone receptor, and Ki67 in normal breast tissue in relation
to subsequent risk of breast cancer. NPJ Breast Cancer.
2:160322016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee S, Mohsin SK, Mao S, Hilsenbeck SG,
Medina D and Allred DC: Hormones, receptors, and growth in
hyperplastic enlarged lobular units: Early potential precursors of
breast cancer. Breast Cancer Res. 8:R62006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wood CE, Hester JM, Appt SE, Geisinger KR
and Cline JM: Estrogen effects on epithelial proliferation and
benign proliferative lesions in the postmenopausal primate mammary
gland. Lab Invest. 88:938–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wurtman RJ, Axelrod J and Phillips LS:
Melatonin synthesis in the pineal gland: Control by light. Science.
142:1071–1073. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Majidinia M, Reiter RJ, Shakouri SK and
Yousefi B: The role of melatonin, a multitasking molecule, in
retarding the processes of ageing. Ageing Res Rev. 47:198–213.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mura MC, Luridiana S, Pulinas L, Bizzarri
D, Cosso G and Carcangiu V: Melatonin treatment and male
replacement every week on the reproductive performance in Sarda
sheep breed. Theriogenology. 135:80–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arendt J, Bojkowski C, Folkard S, Franey
C, Marks V, Minors D, Waterhouse J, Wever RA, Wildgruber C and
Wright J: Some effects of melatonin and the control of its
secretion in humans. Ciba Found Symp. 117:266–283. 1985.PubMed/NCBI
|
|
11
|
Zhdanova IV, Wurtman RJ, Regan MM, Taylor
JA, Ping SJ and Leclair OU: Melatonin treatment for age-related
insomnia. J Clin Endocrinol Metab. 86:4727–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cutando A, López-Valverde A,
Arias-Santiago S, DE Vicente J and DE Diego RG: Role of melatonin
in cancer treatment. Anticancer Res. 32:2747–2753. 2012.PubMed/NCBI
|
|
13
|
Szczepanik M: Melatonin and its influence
on immune system. J Physiol Pharmacol. 58 (Suppl 6):S115–S124.
2007.
|
|
14
|
Amin N, Shafabakhsh R, Reiter RJ and Asemi
Z: Melatonin is an appropriate candidate for breast cancer
treatment: Based on known molecular mechanisms. J Cell Biochem.
120:12208–12215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mccubrey J, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sainsbury R: Benign disorders and diseases
of the Breast. Breast. 1:113. 1992. View Article : Google Scholar
|
|
19
|
Chen CQ and Dan Y: Research of changes of
endocrine hormones in mammary cancer and hyperplasia of mammary
glands. J Modern Oncol. 6:57–59. 2005.(In Chinese with English
abstract).
|
|
20
|
Hartmann LC, Sellers TA, Frost MH, Lingle
WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS,
Hillman DW, et al: Benign breast disease and the risk of breast
cancer. N Engl J Med. 353:229–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knabben L and Mueller MD: Breast cancer
and pregnancy. Horm Mol Biol Clin Investig. 322017.doi:
10.1515/hmbci-2017-0026.
|
|
22
|
Wang L, Zhao D, Di L, Cheng D, Zhou X,
Yang X and Liu Y: The anti-hyperplasia of mammary gland effect of
Thladiantha dubia root ethanol extract in rats reduced by
estrogen and progestogen. J Ethnopharmacol. 134:136–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li HT, Liu HH, Yang YX, Wang T, Zhou XL,
Yu Y, Li SN, Zheng Y, Zhang P, Wang RL, et al: Therapeutic effects
of a traditional Chinese medicine formula plus tamoxifen vs.
Tamoxifen for the treatment of mammary gland hyperplasia: A
meta-analysis of randomized trials. Front Pharmacol. 9:452018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldstein SR, Suresh S, Ciaccia AV and
Plouffe L Jr: Apharmacological review of selective oestrogen
receptor modulators. Hum Reprod Update. 6:212–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henry NL: Endocrine therapy toxicity:
Management options. Am Soc Clin Oncol Educ Book. e25–e30. 2014.doi:
10.14694/EdBook_AM.2014.34.e25. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muskhelishvili L, Latendresse JR, Kodell
RL and Henderson EB: Evaluation of cell proliferation in rat
tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in
situ hybridization for histone mRNA. J Histochem Cytochem.
51:1681–1688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan WR, Garner DS, Williams SD,
Funckes-Shippy CL, Spath IS and Blomme EA: Comparison of
immunohistochemistry for activated caspase-3 and cleaved
cytokeratin 18 with the TUNEL method for quantification of
apoptosis in histological sections of PC-3 subcutaneous xenografts.
J Pathol. 199:221–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu
L, Liu L, Xie F, Kang T, Huang W and Deng W: Simultaneous
modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin
to inhibit proliferation and induce apoptosis in breast cancer
cells. J Pineal Res. 53:77–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oda K, Stokoe D, Taketani Y and McCormick
F: High frequency of coexistent mutations of PIK3CA and PTEN genes
in endometrial carcinoma. Cancer Res. 65:10669–10673. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ming M and He YY: PTEN: New insights into
its regulation and function in skin cancer. J Invest Dermatol.
129:2109–2112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janaki RM and Vaishnave S: BMI1 and PTEN
are key determinants of breast cancer therapy: A plausible
therapeutic target in breast cancer. Gene. 678:302–311. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwabi-Addo B, Giri D, Schmidt K,
Podsypanina K, Parsons R, Greenberg N and Ittmann M:
Haploinsufficiency of the Pten tumor suppressor gene promotes
prostate cancer progression. Proc Natl Acad Sci USA.
98:11563–11568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giri D and Ittmann M: Inactivation of the
PTEN tumor suppressor gene is associated with increased
angiogenesis in clinically localized prostate carcinoma. Hum
Pathol. 30:419–424. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bonneau D and Longy M: Mutations of the
human PTEN gene. Hum Mutat. 16:109–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guillard S, Clarke PA and Workman P:
Investigating the function of class I phosphatidylinositol 3-kinase
isoforms in glioma cell lines by siRNA-mediated depletion. Cancer
Res. 66:998. 2006.
|
|
36
|
Wei Y, Stec B, Redfield AG, Weerapana E
and Roberts MF: Phospholipid-binding sites of phosphatase and
tensin homolog (PTEN): Exploring the mechanism of
phosphatidylinositol 4,5-bisphosphate activation. J Biol Chem.
290:1592–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goschzik T, Gessi M, Denkhaus D and
Pietsch T: PTEN mutations and activation of the PI3K/Akt/mTOR
signaling pathway in papillary tumors of the pineal region. J
Neuropathol Exp Neurol. 73:747–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leslie NR, Kriplani N, Hermida MA,
Alvarez-Garcia V and Wise HM: The PTEN protein: Cellular
localization and post-translational regulation. Biochem Soc Trans.
44:273–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dennis PA: The PI3K/Akt/mTOR signaling
pathway. Ann Oncol. 192011.PubMed/NCBI
|
|
41
|
Lu XX, Cao LY, Chen X, Xiao J and Chen Q:
PTEN inhibits cell proliferation, promotes cell apoptosis, and
induces cell cycle arrest via downregulating the PI3K/AKT/hTERT
pathway in lung adenocarcinoma A549 cells. Biomed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He L, Fan C, Gillis A, Feng X, Sanatani M,
Hotte S, Kapoor A and Tang D: Co-existence of high levels of the
PTEN protein with enhanced Akt activation in renal cell carcinoma.
Biochim Biophys Acta. 1772:1134–1142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|