Introduction
Osteosarcoma (OS) is a rare tumor type, which mostly
occurs in children and adolescents (1). OS usually occurs at the fixed end of
long bones. Several studies have found that OS occurs mostly in
fast-growing bones (2,3). Chemical substances such as
methylcholanthrene or SV40 virus may promote the generation of OS
(4,5).
Approximately 10–25% of patients with OS have lung metastases, and
lung injury caused by lung metastasis is the main cause of
mortality (6). Osteosarcoma treatment
typically involves chemotherapy and surgery. Some common drug
treatments include doxorubicin, methotrexate, and vincristine.
However, prolonged use of these drugs adversely affects liver
function in patients, and their survival cannot be guaranteed.
Surgical treatment affects the body of patients (7,8–11). Therefore, it is urgent to understand
and explore the pathogenesis of OS and develop effective drugs for
the treatment of OS.
Normal cell proliferation depends on a complete and
effective cell cycle. The regulation of cell proliferation mainly
occurs in the G1 phase cell cycle (12,13). In
malignant cells, cell cycle imbalance is an early step in tumor
development (14). A large number of
signaling pathways and growth factors affect whether the cells
enter the S phase for DNA replication (15,16). Of
these pathways, cyclin D transfers extracellular mitogenic signals
to activate the G1/S phase transition. A variety of
signaling pathways can affect the expression of cyclin D, such as
the classic Ras/Raf/MEK/ERK (MAPK), Rac, NF-κB, Wnt, and Notch
signaling pathways (15). The
Wnt/β-catenin signaling pathway involves the stabilization and
translocation of β-catenin induced by extracellular Wnt. When
β-catenin in the cytoplasm accumulates to a certain level, it
transfers to the nucleus and forms β-catenin-TCF/LEF transcription
complex, which initiates the transcriptional regulation of cyclin D
and c-Myc during the G1-S period of the cell cycle,
causing the cells to enter the S phase for DNA replication
(17). Previous studies have reported
that β-catenin can increase OS cell proliferation and the number of
OS cells in the S phase, and promote the invasion and migration of
OS cells (18–21). Therefore, the Wnt/β-catenin signaling
pathway is a critical pathway in OS.
Most components of the transcriptome that do not
encode proteins have traditionally been considered as
‘transcription junk’. However, with the development of
high-throughput technologies such as next-generation sequencing in
the past decade, researchers have an unprecedented understanding of
non-coding genomes. An lncRNA is a type of non-coding RNA over 200
bp (22). In recent years, studies
have revealed that there are numerous lncRNAs aberrantly expressed
in OS, such as TUG1, UCAI, BCAR4, and HULC, which can be regarded
as prognostic indicators of OS (23–26). Other
lncRNAs, such as DANCR (27) and
FOXC2-AS1 (28), also play important
roles in the progression and metastasis of OS. Long non-coding RNA
(lncRNA) cancer susceptibility candidate 15 (CASC15) is a long
intergenic non-coding RNA (lincRNA) located at chromosome 6p22.3
(29). It has been reported that
lncRNA CASC15 regulated the expression of SOX4 in acute leukemia to
promote its occurrence (30) and
regulated the Wnt/β-catenin signaling pathway through miR-4310 in
colon cancer to promote cancer cell proliferation and metastasis
(31). The latest research has
reported that lncRNA CASC15 is upregulated in OS plasma exosomes.
Knockout of lncRNA CASC15 inhibited the proliferation of OS cells
and the growth of osteosarcoma in xenograft models (32). In addition, lncRNA CASC15
downregulated the expression of E-cadherin and upregulated the
expression of N-cadherin, indicating that lncRNA CASC15 can affect
the metastasis of OS cells (32).
However, it remains unknown whether lncRNA CASC15 affects the
proliferation and metastasis of osteosarcoma by regulating the
Wnt/β-catenin signaling pathway.
Materials and methods
Clinical samples
A total of 30 patients with OS treated at the First
People's Hospital of Shangqiu (Shangqiu, China) were selected as
the observation group, and their OS tissues were obtained as
biological samples, and the paired adjacent normal tissues were
used as controls. The basic information of OS patients is presented
in Table I. The present study was
approved by the Ethics Committee of the First People's Hospital of
Shangqiu, and the patients provided signed informed consent.
 | Table I.Basic information of OS patients. |
Table I.
Basic information of OS patients.
|
| OS (n=30) |
|---|
|
|
|
|---|
|
Characteristics | No. of
patients | % |
|---|
| Sex |
|
|
|
Male | 17 | 56.7 |
|
Female | 13 | 43.3 |
| Tumor size |
|
|
| ≤9
cm | 14 | 46.7 |
| ≥9
cm | 16 | 53.3 |
| Age at
diagnosis |
|
|
|
Mean | – | 22.2 |
| SD | – |
9.2 |
|
Minimum | – | 8 |
|
Maximum | – | 40 |
Cell culture and transfection
The hFOB1.19 cell line, purchased from American type
culture collection (ATCC), was cultured in a 37°C incubator with 5%
CO2. Then, the cells were cultured in high-glucose
DMEM/F12 (1:1) (Cytiva) supplemented with 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.) and 0.3% G418 (Sigma-Aldrich;
Merck KGaA). UMR-106 cells were purchased from ATCC and cultured in
complete α-minimum essential medium (α-MEM) containing 15% FBS. The
cells were placed in an incubator at 37°C and 5% CO2.
Human OS cell line U-2OS (Shanghai Chinese Academy of Sciences Cell
Bank) was cultured in RPMI-1640 medium containing 10% FBS at 37°C
and 5% CO2. Human osteogenic sarcoma cells Saos-2 were
purchased from China Infrastructure of Cell Line Resources. The
cells were cultured with a DMEM containing 10% FBS, 100 U/l
penicillin, and 100 mg/l streptomycin in a culture flask at 5%
CO2 and 37°C.
Lentiviruses were produced using a second-generation
lentiviral system in 293T cells. The recombinant lentiviruses
expressing overexpressed (oe)-CASCS15, short hairpin (sh)-CASCS15,
and their negative controls (oe-NC and sh-NC) were constructed and
packaged by Shanghai GenePharma Co., Ltd. The ratio of lentiviral
plasmid: psPAX2: pMD2.G was 2:2:1. U-2OS cells were infected with a
total of 5 µg lentiviruses expressing oe-CASCS15, sh-CASCS15, and
their negative controls (oe-NC and sh-NC) for 48 h using RNAi-Mate
transfection reagent (MDBio, Inc.). The multiplicity of infection
was 80. Cells were incubated in 5% CO2 at 37°C for 4
days.
Establishment of xenograft model
A total of 15 (female, 4–6 weeks old; weight between
15–20 g; BALb/c; Harlan Sprague Dawley, Inc.) nude mice (n=5 for
each group) were used to establish a xenograft model. All mice were
housed at a 12-h light/dark cycle with a temperature of 25±2°C and
a humidity of 55±5°C. Food and drinking water were provided ad
libitum. All animal experiments were approved by the Animal
Experimentation Ethics Committee of First People's Hospital of
Shangqiu. U-2OS, UMR-106, or Saos-2 cells were inoculated under the
skin of the back of nude mice for 4 weeks. Each mouse was
inoculated with ~2×106 cells. After 16 days, the mice
were euthanized by cervical dislocation following anesthesia with
ketamine (50 mg/kg)/xylazine (5 mg/kg), and tumors were harvested.
Tumor size was measured by calipers and calculated using the
following formula: Volume (mm3) = L (length) × W
(width)2/2.
Metastasis assays
A mouse model of pulmonary metastasis was
established using tail vein injection (33). When the cells reached 80–90%
confluency, U-2OS, Saos-2, UMR-106, and hFOB1.19 cells were
separated with 0.2 mmol/l EDTA in Hanks' Balanced Salt Solution
(HBSS) without Mg+2-Ca+2-NaHC03.
After counting, the cells (2.5×106/ml) were resuspended
in ice-cold HBSS. Female athymic mice (4 weeks old; weight ~15 g;
BALb/c; Harlan Sprague Dawley, Inc.) were injected with 0.2 ml of
the cell suspension through the tail vein. A total of 20 mice was
divided into 4 groups (n=5 per group). At 10 weeks after
inoculation, mice were euthanized by cervical dislocation following
anesthesia with ketamine (50 mg/kg)/xylazine (5 mg/kg). All organs
were examined for metastasis formation macroscopically. Lung
tissues were harvested and fixed in a mixture of Bouin's fixative
and neutral buffered formalin (1:5, v/v). Metastatic nodules in the
lungs were counted using an MZ16F dissecting microscope (Leica).
Mice were housed at a 12-h light/dark cycle with a temperature of
25±2°C and humidity of 55±5°C. Food and drinking water were
provided ad libitum. All animal experiments were approved by
the Animal Experimentation Ethics Committee of First People's
Hospital of Shangqiu.
Cell Counting Kit-8 (CCK-8) assay
U-2OS cells (4×103 cells/well) were
inoculated into a 96-well plate and 8 multiple wells were set up in
each group. Cells were cultured in a cell incubator. After
lentiviral transfection, the cells were cultured for 48 h. The
freshly prepared CCK-8 detection solution (10 µl) was added to each
well and then cultured in the incubator for 4 h at 37°C. Then, the
OD value was detected at 450 nm with a microplate reader. The
experiment was repeated 3 times, and the average value of the
experimental results was considered as the final experimental
results. The growth inhibition rate was calculated according to the
following formula: Cell growth inhibition rate=[(OD control
group-OD experimental group)/OD control group] × 100%.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The Cell-Light EdU DNA Cell Proliferation Kit
(Guangzhou RiboBio Co., Ltd.) was used to detect cell proliferation
by EdU assay. The cells in the logarithmic growth period were
seeded into a 96-well plate with ~4×103−1×105
cells/well, and cells were cultured to the normal growth stage. EdU
medium was added and incubated with cells for 2 h at 37°C. Cells
were washed and incubated with 100 µl of cell immobilization
solution (PBS containing 4% paraformaldehyde) in each well for 30
min at room temperature. Glycine (2 mg/ml) was added and incubated
on a decolorizing shaking bed for 5 min. Then, the glycine solution
was discarded, and cells were washed with PBS for 5 min. Then, 1X
Apollo® dyeing reaction solution (100 µl) was added, and
the reaction continued for 30 min. Subsequently, the cells were
washed with 0.5% Triton X-100 in PBS. After adding 100 µl 1X
Hoechst 33342 reaction solution, the reaction solution was
discarded, and cells were washed with PBS after incubating on a
light-free, room temperature, decolorizing shaking bed for 30 min.
Images were obtained using an FSX100 fluorescence microscope
(Olympus Corporation) at an ×200 magnification. The cell
proliferation rate was evaluated according to the manufacturer's
instructions.
Transwell assay
The cells (1×105 cells/well) were
suspended in serum-free medium and inoculated into the upper
chamber of an 8-µΜ pore Transwell chamber (EMD Millipore). A medium
containing 20% FBS was added to the lower chamber, following
incubation at 37°C for 24 h. The cells were carefully wiped away in
the upper chamber with a cotton swab. Cells that migrated to the
lower chamber were fixed with 4% formaldehyde solution and stained
with 0.1% crystal violet (Solarbio Life Sciences) for 15 min at
37°C. Finally, an inverted microscope (magnification, ×100; Olympus
IX 70; Olympus Corporation) was used to count the number of
migrating cells. The migration activity was quantified by counting
the migrated cells.
Cell scratch assay
A scratch wound assay was also used to evaluate the
cell migration of OS cells. In short, 1×105 U-2OS cells
were seeded in 12-well plates. When the cell density reached 80%
confluence, the cell monolayer was scraped with a sterile pipette
tip. After washing away cell debris with PBS, cells were maintained
in serum-free medium. Images were obtained at 0 and 24 h after the
scratch. Wound width was measured using ImageJ software version
1.8.0 (National Institutes of Health), and migration was expressed
as wound closure fraction.
Cell cycle assay using flow
cytometry
Approximately 2×105 cells were collected
and washed with PBS, fixed in 70% cold ethanol at 4°C for 2 h, and
then filtered through a 70-µm cell strainer (BD Biosciences) to
obtain a single-cell suspension. Subsequently, the cells were
incubated with RNase A at 37°C for 30 min and stained with PI for
30 min at 4°C (Cell Cycle Detection Kit; BD Biosciences). Flow
cytometric analysis was performed on a FACSCalibur flow cytometer
(BD Biosciences), and data were analyzed using CellQuest software
(version 5.1; BD Biosciences).
Subcellular fractionation
U-2OS Cells were processed using Cytoplasmic
Extraction and NE-PER Nuclear kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. For quantification of
β-catenin in indicated fractions, lamin A+C (dilution, 1:200;
product code ab40567; Abcam) and actin (dilution, 1:1,000; cat. no.
sc-8432; Santa Cruz Biotechnology, Inc.) were employed as the
fractionation indicators.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RT-qPCR was performed for gene expression
analysis in hFOB1.19, U-2OS, Saos-2, UMR-106 cells, and OS tissues.
TRIzol reagent (Thermo Fisher Scientific, Inc.) was used to extract
total RNA from these cell lines and grated tissues. Nanodrop
technology (Thermo Fisher Scientific, Inc.) was used to detect the
concentration of RNA, and the ratio of A260/280 was between 1.9 and
2.1. Total RNA was reverse-transcribed using Prime Script™ RT
reagent Kit with gDNA Eraser (Takara Biotechnology Co., Ltd.) to
reverse RNA to cDNA on a PCR instrument (ABI; Thermo Fisher
Scientific, Inc.). The obtained cDNA was mixed with TB Green™
Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.) for RT-qPCR
detection, with a Real-time PCR instrument (Roche
LightCycler® 480; Roche Diagnostics). The PCR thermal
cycling conditions were 95°C for 5 min, and then 45 cycles at 95°C
for 5 sec and 60°C for 30 sec. Primers were designed and
synthesized by TSINGKE Biological Technology Co., Ltd, as presented
in Table SI. β-actin was used as the
internal control. The relative expression was calculated using the
2−∆∆Cq method (34).
Western blotting
RIPA lysis buffer (Bio-Rad Laboratories, Inc.) was
used to lyse the cells and extract soluble proteins. U-2OS cells
were lysed with RIPA lysis buffer. This process lasted 30 min on
ice. The obtained system was centrifuged at 12,000 × g for 10 min
at 4°C. The supernatant was collected in an EP tube, and the
protein concentration was measured with a BCA protein detection kit
(Thermo Fisher Scientific, Inc.). The extracted protein (20 µg) was
subjected to 7.5% SDS-PAGE electrophoresis to separate the protein.
Furthermore, 5% BSA or skimmed milk was prepared in advance, and
the membrane was then placed in 5% BSA or skimmed milk, and it was
blockedfor 2 h on the shaker at room temperature. The incubated
primary antibody was dissolved in 5% BSA or skimmed milk, and the
blots were probed overnight at 4°C with antibodies against Wnt1
(dilution 1:1,000; product code ab15251; Abcam), β-catenin
(dilution 1:1,000; product code ab68183; Abcam), cyclin D1
(dilution 1:1,000; product no. 55506S; Cell Signaling Technology,
Inc.), E-cadherin (dilution 1:50; product code ab1416; Abcam),
N-cadherin (dilution 1:10,000; product code ab76011; Abcam),
vimentin (dilution 1:5,000; product code ab92547; Abcam), actin
(dilution 1:1,000; cat. no. sc-8432; Santa Cruz Biotechnology,
Inc.), and lamin A + C (dilution 1:200; product code ab40567;
Abcam) followed by incubation for 1 h at room temperature with a
horseradish peroxidase-conjugated secondary antibody (cat. no.
sc-2357; Santa Cruz Biotechnology, Inc.; or product code ab205719;
Abcam). Enhanced chemiluminescence (ECL) substrate (Pierce; Thermo
Fisher Scientific, Inc.) was used as the visualization reagent. The
film was soaked in the luminescent liquid for 5 min. Then, the film
was removed and placed in the luminescence imager, and the
luminescent liquid was added dropwise again to collect the
images.
Statistical analysis
All data were analyzed with SPSS (version 22.0; IBM
Corp.), and the quantitative data were expressed as the mean ±
standard deviation (SD). The differences between two groups were
compared using unpaired Student's t-test, and one-way
analysis of variance (ANOVA) followed by post hoc test Tukey-Kramer
was carried out to compare the differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CASC15 is highly expressed in OS cell
lines and clinical samples
RT-qPCR was used to detect the expression of CASC15
in clinical samples of OS from 30 patients and tissues samples with
negative OS biopsy (n=30). The results revealed that the expression
of CASC15 in OS biopsy samples was significantly higher than that
in biopsy samples without cancer (Fig.
1A). To further explore the expression of CASC15 in OS,
different OS cell lines were selected, namely, U-2OS, Saos-2, and
UMR-106 as well as normal cell line hFOB1.19, to detect the
expression of CASC15. The RT-qPCR results demonstrated that the
expression of CASC15 in U-2OS, UMR-106, and Saos-2 was
significantly higher than that in hFOB1.19, and the expression of
CASC15 in U-2OS was significantly lower than that in Saos-2 and
UMR-106 cells (Fig. 1B). U-2OS,
Saos-2, and UMR-106 cells in the logarithmic growth phase were
inoculated subcutaneously into the back of nude mice (n=5) for 4
weeks at 2×106 cells for each mouse, and tumor formation
was observed after 4 weeks. The results revealed that compared with
U-2OS nude mice, the tumors of Saos-2 and UMR-106 nude mice were
significantly enlarged (Fig. 1C and
D). The tail vein injection of U-2OS, Saos-2, UMR-106, and
hFOB1.19 in nude mice (n=5) was used to observe tumor formation in
the lungs of mice. The results indicated that the nude mice
injected with U-2OS had fewer metastatic tumors than the mice
injected with Saos-2 and UMR-106 (Fig.
1E).
 | Figure 1.Expression of CASC15 in OS cell lines
and clinical samples. The OS cell lines used in the present study
were hFOB1.19, U-2OS, Saos-2, UMR-106. (A) qPCR was used to detect
the expression of CASC15 in clinical samples from 30 patients. (B)
qPCR was used to detect the expression of CASC15 in different OS
cell lines and a normal cell line. (C and D) Comparison of
xenograft tumor formation in nude mice with different cell lines.
(E) Nude mice were respectively injected with hFOB1.19, U-2OS,
Saos-2, UMR-106 into the tail vein to observe lung tumor formation.
**P<0.01 and ***P<0.001 vs. OS, hFOB1.19 or U-2OS. CASC15,
cancer susceptibility 15; OS, osteosarcoma; qPCR, quantitative
polymerase chain reaction. |
CASC15 regulates the cell cycle and
promotes the proliferation of OS cells in vitro
Since CASC15 could promote cancer progression and
cancer cell proliferation in leukemia and bowel cancer (30,31,35), and
higher expression of CASC15 had been revealed in OS cell lines,
transplanted tumors, and clinical samples, whether CASC15 could
promote cell proliferation was further studied. Therefore, the
U-2OS cell line with low expression of CASC15 was used for the next
experiments. The results of CCK-8 and Edu assays demonstrated that
compared with the respective control (oe-NC or sh-NC) group, the
cell proliferation of the oe-CASC15 group was increased, while the
cell proliferation of the sh-CASC15 group was decreased (Fig. 2A and B). Flow cytometry was used to
detect the number of cells in the G0/G1, S,
and G2/M phases of the cell cycle. The results indicated
that compared with the control group, S-phase cells were increased
in the oe-CASC15 group, whereas S-phase cells were decreased in the
sh-CASC15 group (Fig. 2C and D).
CASC15 impacts the proliferation and
cell cycle of OS cells by regulating the Wnt/β-catenin pathway
Due to the changes in proliferation and cycle
distribution revealed in the present study, RT-qPCR and western
blotting were then used to detect the expression of Wnt, β-catenin,
and cyclin D, respectively. The transfected U-2OS cells were
grouped into oe-CASC15, sh-CASC15, oe-NC and sh-NC. The RT-qPCR
results revealed that compared with the respective control (oe-NC
or sh-NC) group, the expression of Wnt, β-catenin, and cyclin D was
increased in the oe-CASC15 group, while it was decreased in the
sh-CASC15 group (Fig. 3A). The
western blot results revealed that compared with the control group,
the expression of Wnt, β-catenin, and cyclin D was increased in the
oe-CASC15 group, whereas it was decreased in the sh-CASC15 group
(Fig. 3B). In the rescue experiment,
oe-CASC15, oe-CASC15/Wnt inhibitor, or control were transfected
into U-2OS cells, and then RT-qPCR and western blotting were used
to detect the expression of Wnt, β-catenin, and cyclin D,
respectively. The RT-qPCR and western blot results indicated that
compared with the control group, the expression of Wnt, β-catenin,
and cyclin D was increased in the oe-CASC15 group, whereas it was
decreased in the oe-CASC15/Wnt inhibitor group (Fig. 3C and D). The flow cytometric results
indicated that compared with the control group, S-phase cells were
increased in the oe-CASC15 group, whereas they were decreased in
the oe-CASC15/Wnt inhibitor group (Fig.
3E and F). Furthermore, the results of CCK-8 cell proliferation
demonstrated that the oe-CASC15 group had increased cell
proliferation compared with the control group. The oe-CASC15 + Wnt
inhibitor group inhibited cell proliferation, and the proliferation
was slightly lower than that of the control group (Fig. 3G).
 | Figure 3.CASC15 can activate the Wnt/β-catenin
signaling pathway to regulate the cell cycle and interfere with the
proliferation of osteosarcoma cells. (A and B) U-2OS cells were
transfected with oe-CASC15, sh-CASC15, and their respective
negative controls (oe-NC and sh-NC), and RT-qPCR and western
blotting were used to detect the expression of Wnt, β-catenin, and
cyclin D, respectively. (C and D) U-2OS cells were transfected with
oe-CASC15, oe-CASC15 + Wnt inhibitor, and control, and RT-qPCR and
western blotting were used to detect the expression of Wnt,
β-catenin, and cyclin D, respectively. (E and F) The cell cycle was
detected in U-2OS cells which were transfected with oe-CASC15,
oe-CASC15 + Wnt inhibitor, and control. (G) A Cell Counting Kit-8
assay was performed in U-2OS cells which were transfected with
oe-CASC15, oe-CASC15 + Wnt inhibitor, and control. *P<0.05,
**P<0.01 and ***P<0.001 vs. oe-NC, control or oe-CASC15;
#P<0.05 and ###P<0.001 vs. sh-NC or
control. CASC15, cancer susceptibility 15; oe, overexpressed; NC,
negative control; sh, short hairpin; ns, nonstatistically
significant difference. |
High CASC15 expression promotes the
metastasis of OS cells
In previous studies, it was revealed that cells with
high CASC15 expression were more prone to metastasis, and that
CASC15 expression in metastatic tissues was higher (Fig. 1), while promoting cell proliferation
via the Wnt/β-catenin signaling pathway (Fig. 3). Considering that the Wnt/β-catenin
signaling pathway also promoted the EMT process (36), it was further explored whether the
expression level of CASC15 could affect cell proliferation and
metastasis. U-2OS cells were transfected with lentiviruses
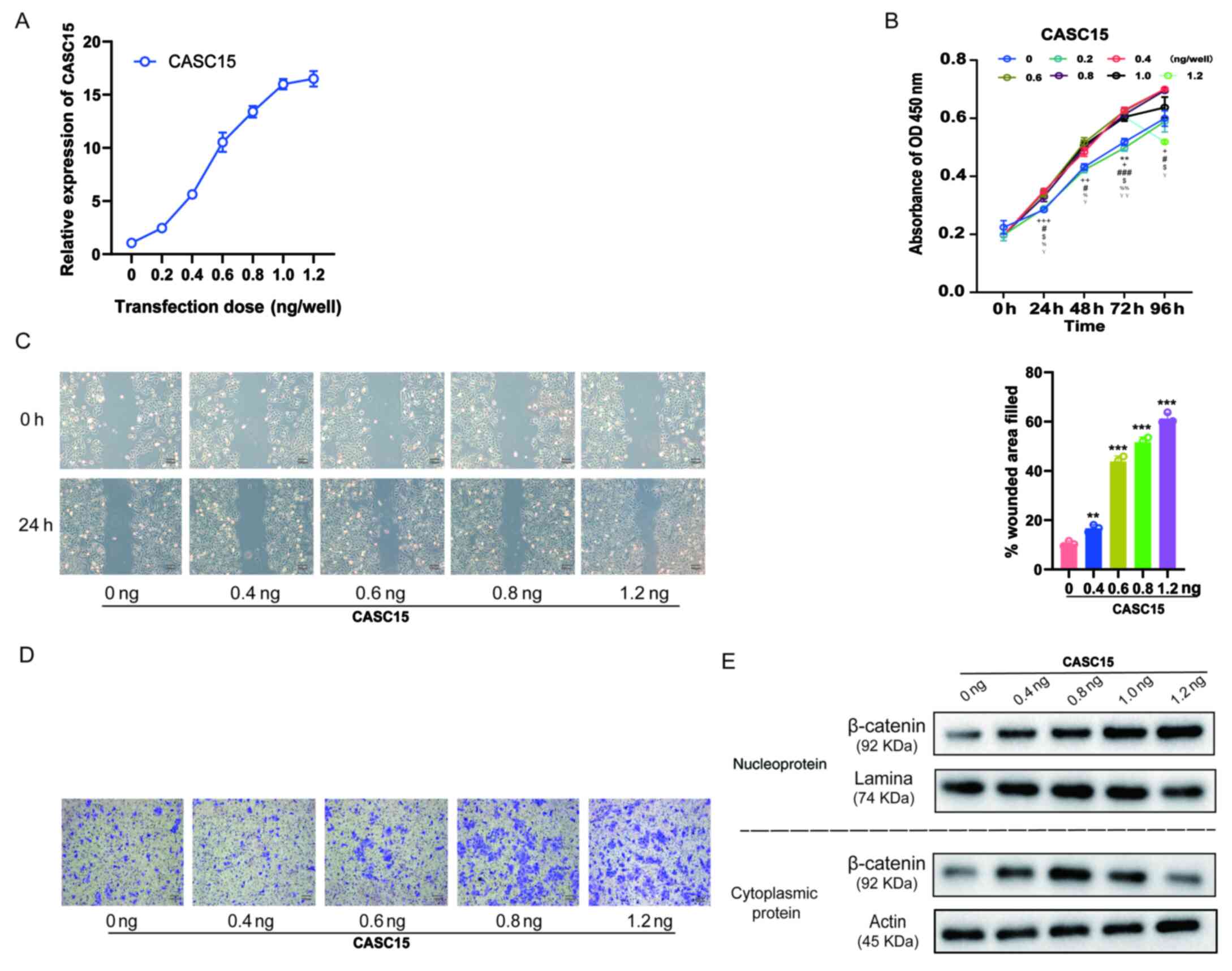
expressing various doses [0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 ng/well
(24 wells)] of oe-CASC15. The RT-qPCR results revealed that with
the increasing transfection dose, the expression of CASC15 was
gradually increased (Fig. 4A).
Furthermore, the results of the CCK-8 assay revealed that with the
increase in the expression of CASC15, cell proliferation quickly
increased, however, when the transfection dose exceeded 0.6
ng/well, the increase of proliferation was insignificant and
slightly decreased (Fig. 4B). The
results of the cell scratch experiments also indicated that with
the increase of CASC15 expression, low-dose transfection did not
improve the healing rate, whereas with high-dose transfection it
was significantly improved (Fig. 4C).
Moreover, the results of the Transwell cell migration experiments
revealed that with the increase of CASC15 expression, low-dose
transfection did not affect cell migration ability, whereas
high-dose transfection significantly increased cell migration
(Fig. 4D). Next, the influence of
various CASC15 expression levels on the cellular location of
β-catenin was also assessed. The western blot results revealed that
with the increased expression of CASC15, low-dose transfection
increased the expression of β-catenin in the cytoplasm, whereas
high-dose transfection increased the expression of β-catenin in the
nucleus and decreased its expression in the cytoplasm (Fig. 4E).
CASC15 promotes metastasis by inducing
EMT of OS cells through activation of the Wnt/β-catenin
pathway
Since in Fig. 4, the
cell proliferation was not obvious whereas cell migration was after
the transfection amount was >0.6 ng/well, a dose of 1 ng/well
was used as the high-expression dose, and 0.6 ng/well was used as
the low-expression dose. Grouping of U-2OS-transfected cells was as
follows: high expression of the CASC15 group, low expression of the
CASC15 group, high expression of CASC15 + Wnt inhibitor group, low
expression of CASC15 + Wnt inhibitor group, and the control group.
Western blotting and RT-qPCR were used to detect the expression
levels of vimentin, E-cadherin, N-cadherin, and cyclin D. The
results revealed that compared with the low-expression CASC15
group, the expression of E-caherin in OS cells was downregulated in
the high-expression CASC15 group, while the expression levels of
N-cadherin, vimentin and cyclin D were upregulated. After
transfection of the Wnt inhibitor, the expression of E-caherin was
upregulated in OS cells, whereas the expression levels of
N-cadherin, vimentin and cyclin D were downregulated (Fig. 5A and B). After nuclear and cytoplasmic
separation, western blotting was used to detect the expression
changes of β-catenin among the aforementioned groups. Western blot
analysis indicated that when CASC15 was in low concentration, the
expression of β-catenin was higher in the cytoplasm than that in
the nucleus, indicating that β-catenin was primarily expressed in
the cytoplasm; when the concentration of CASC15 was 1 ng/well (high
expression), β-catenin was predominantly expressed in the nucleus,
indicating that high expression levels of CAS15 could promote the
entry of β-catenin into the nucleus, thereby causing the occurrence
of EMT in the nucleus (Fig. 5C).
 | Figure 5.CASC15 promotes the
epithelial-mesenchymal transition of osteosarcoma cells by
Wnt/β-catenin pathway. U-2OS cells were transfected with 1 ng/well
of oe-CASC15 as the high expression dose, and 0.6 ng/well as the
low expression dose. The transfection groups were as follows: high
expression, low expression, high expression + Wnt inhibitor, and
low expression + Wnt inhibitor. (A and B) Reverse
transcription-quantitative polymerase chain reaction and western
blotting were used to detect the expression of E-cadherin,
N-cadherin, vimentin, and cyclin D, respectively. (C) After nuclear
and cytoplasmic separation, western blotting was used to detect the
expression changes of β-catenin among the aforementioned groups.
***P<0.01 vs. low expression or control. CASC15, cancer
susceptibility 15. |
Discussion
EMT was first proposed by Elizabeth Hay. In 1980,
she observed that epithelial cells could downregulate epithelial
tissue characteristics to obtain mesenchymal tissue characteristics
(37). EMT reveals that epithelial
cells have a certain degree of plasticity. In the process of EMT,
epithelial cells lose their apical-basal polarity and the
connection mode between cells, the trefoil factor 3 (TFF3)
signaling pathway is involved and altered, and the shape of
epithelial cells presents with slender morphology (38). This increases the ability of
individual cells to develop their ability to infiltrate into
tissues during the period of transformation (39,40). The
EMT process is an important step in the formation of gastrulation
and neural crest (41). However, EMT
occurs again in wound healing, fibrosis, and cancer progression
(42). Cancer invasion and metastasis
are similar to normal embryonic development (43). Recent studies have revealed that the
process of cancer invasion is a recurrence of the EMT process of
embryonic development (44,45). During EMT, the expression of
E-cadherin and cytokeratin that are specifically expressed in
epithelial tissues decrease, whereas the expression of mesenchymal
cytoskeleton proteins such as vimentin increase. When epithelial
cells undergo the EMT process, they usually secrete fibronectin
produced by mesenchymal cells, such as fibroblasts, and
concurrently express fibroblast marker proteins such as N-cadherin
to replace the marker protein E in epithelial tissues. Cancer cells
expressing N-cadherin undergo the EMT process to enhance their
affinity with supporting cells, thereby causing cell infiltration
and metastasis in normal tissues (46). In the present study, it was revealed
that when the expression of CASC15 increased, it could promote the
proliferation of OS cells. However, after it increased to a certain
level, it did not further promote cell proliferation but appeared
to induce cell migration. Therefore, it was considered whether
CASC15 activates the Wnt signaling pathway in order for a large
amount of β-catenin to enter the nucleus to promote EMT. To this
aim, it was first explored whether different doses would affect the
EMT process, and it was revealed that different doses of lncRNA
CASC15 could alter the expression of β-catenin in the nucleus and
cytoplasm. It was revealed that CASC15 activated the Wnt signaling
pathway. It was surmised that the formation of the possible
migration ability was related to the EMT process caused by
β-catenin entering the nucleus. Therefore, the effect of various
doses of CASC15 on the content of β-catenin inside and outside the
nucleus was detected, and it was revealed that CASC15 could lead to
β-catenin entering the nucleus in large quantities via the Wnt
pathway to promote the EMT of OS cells.
The Wnt signaling pathway was first discovered in
the study of Drosophila wingless genes (47). Later, more Wnt family genes were
discovered during embryonic development (48). The discovery of the Wnt1 gene linked
the Wnt signaling pathway to cancer. Nusse and Varmus revealed that
overexpression of the Wnt1 gene could cause mouse mammary tumors
(49). The Wnt signaling pathway is
mainly divided into the classic Wnt signaling pathway, namely the
Wnt/β-catenin signaling pathway and the non-canonical Wnt signaling
pathway (50). The classic Wnt
signaling pathway is mainly composed of a Wnt-secreted protein, a
transmembrane receptor frizzled protein, a loose protein,
β-catenin, glycogen synthesis kinase 3β, and T cell factor/lymph
enhancer factor (51). This pathway
mainly regulates cell behavior through the transcription properties
of DNA-binding proteins of the TCF/LEF family. The key is the
stable β-catenin in the cytoplasm (52). In Wnt signaling, β-catenin is mostly
bound by E-cadherin, which extends from the cell membrane into the
cell. When the Wnt signaling pathway is activated, the Wnt protein
secreted by the cell binds to the Frz and LRP5/6 receptor complex
on the cell surface to activate the Dsh protein in the cytoplasm.
The activated Dsh can inhibit GSK3-β and stimulate β-catenin
activity in the cytoplasm. When β-catenin accumulates to a certain
level, it transfers to the nucleus and competitively binds to the
transcription factor TCF/LEF such as P300 in the nucleus to form a
β-catenin-TCF/LEF transcription complex to regulate target gene
expression (53). The present study
revealed that lncRNA CASC15 triggered the Wnt signaling pathway and
led to the downstream β-catenin in the nucleus. β-catenin plays a
role in the nucleus. When β-catenin begins to appear in the
nucleus, it first regulates the expression of downstream cyclin D
and triggers cell proliferation. When there is excessive β-catenin
in the nucleus, part of it regulates downstream EMT (54,55). In
the present study a competitive binding mechanism of β-catenin with
the lncRNA likely occurred, and this mechanism should be further
explored in future studies. In addition, in-depth research should
be conducted as to how CASC15 regulates the Wnt/β-catenin signaling
pathway and even regulates the expression of β-catenin inside and
outside the nucleus. It is theorized that CASC15 is likely to
inhibit the phosphorylation β-catenin by binding to the β-catenin
protein in OS cells (56,57), leading to the accumulation of
β-catenin and its entering the nucleus to bind to the LEF/TCF
transcription factor family and activate transcription of
downstream target genes (such as cyclin D1).
In conclusion, the present study explored the
function and effect of lncRNA CASC15 on the proliferation,
invasion, and metastasis of OS cells and revealed the preliminary
mechanism of the expression level of lncRNA CASC15 on the
progression and metastasis of OS, thus providing a new treatment
for OS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW proposed and designed the study, collected the
clinical samples, performed the experiments in vivo, and
prepared the manuscript. PZ conducted the experiments in
vitro, and analyzed the data. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Shangqiu, and the
patients provided signed informed consent. All animal experiments
were approved by the Animal Experimentation Ethics Committee of
First People's Hospital of Shangqiu.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, Greenfield EM and Getty PJ: Osteosarcoma. J Am Acad Orthop
Surg. 17:515–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiller AL: Orthopaedic pathology. Semin
Diagn Pathol. 28:1–3. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendoza SM, Konishi T and Miller CW:
Integration of SV40 in human osteosarcoma DNA. Oncogene.
17:2457–2462. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazabraud A: Experimental production of
bone sarcomas in the rabbit by a single local injection of
beryllium. Bull Cancer. 62:49–58. 1975.(In French). PubMed/NCBI
|
|
6
|
Miller BJ, Cram P, Lynch CF and Buckwalter
JA: Risk factors for metastatic disease at presentation with
osteosarcoma: An analysis of the SEER database. J Bone Joint Surg
Am. 95:e892013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabbri A, Motta E, Ferrari S, Longhi C,
Marchi E, Bacci G, Figus E and Marchesini G: High-dose methotrexate
treatment and liver function in patients with osteosarcoma. J
Intern Med. 236:209–214. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin F, Wang Q, Yu W, Tang L, Zheng S, Sun
Y, Shen Z, Yao Y and Dong Y: Clinical analysis of Chinese limb
osteosarcoma patients treated by two combinations of methotrexate,
cisplatin, doxorubicin and ifosfamide. Asia-Pac J Clin Oncol.
7:270–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bacci G and Lari S: Adjuvant and
neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov.
86:253–268. 2001.(Article in English, Italian). PubMed/NCBI
|
|
10
|
Biermann JS and Baker LH: The future of
sarcoma treatment. Semin Oncol. 24:592–597. 1997.PubMed/NCBI
|
|
11
|
La Quaglia MP: Osteosarcoma. Specific
tumor management and results. Chest Surg Clin N Am. 8:77–95.
1998.PubMed/NCBI
|
|
12
|
Yue Z, Guan X, Chao R, Huang C, Li D, Yang
P, Liu S, Hasegawa T, Guo J and Li M: Diallyl disulfide induces
apoptosis and autophagy in human osteosarcoma MG-63 cells through
the PI3K/Akt/mTOR pathway. Molecules. 24:26652019. View Article : Google Scholar
|
|
13
|
Kurowska P, Mlyczyńska E, Dawid M,
Opydo-Chanek M, Dupont J and Rak A: In vitro effects of vaspin on
porcine granulosa cell proliferation, cell cycle progression, and
apoptosis by activation of GRP78 receptor and several kinase
signaling pathways including MAP3/1, AKT, and STAT3. Int J Mol Sci.
20:58162019. View Article : Google Scholar
|
|
14
|
Li B, Zhou P, Xu K, Chen T, Jiao J, Wei H,
Yang X, Xu W, Wan W and Xiao J: Metformin induces cell cycle
arrest, apoptosis and autophagy through ROS/JNK signaling pathway
in human osteosarcoma. Int J Biol Sci. 16:74–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duronio RJ and Xiong Y: Signaling pathways
that control cell proliferation. Cold Spring Harb Perspect Biol.
5:a0089042013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cavallaro G, Cucina A, Coluccia P,
Petramala L, Cotesta D, Polistena A, Zinnamosca L, Letizia C,
Rosato L, Cavallaro A and De Toma G: Role of growth factors on
human parathyroid adenoma cell proliferation. World J Surg.
34:48–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaldis P and Pagano M: Wnt signaling in
mitosis. Dev Cell. 17:749–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Q, Shi SY, Ji HB and Xing SX: LncRNA
BE503655 inhibits osteosarcoma cell proliferation,
invasion/migration via Wnt/β-catenin pathway. Biosci Rep.
39:BSR201822002019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keremu A, Maimaiti X, Aimaiti A, Yushan M,
Alike Y, Yilihamu Y and Yusufu A: NRSN2 promotes osteosarcoma cell
proliferation and growth through PI3K/Akt/MTOR and Wnt/β-catenin
signaling. Am J Cancer Res. 7:565–573. 2017.PubMed/NCBI
|
|
20
|
Zhao H, Hou W, Tao J, Zhao Y, Wan G, Ma C
and Xu H: Upregulation of lncRNA HNF1A-AS1 promotes cell
proliferation and metastasis in osteosarcoma through activation of
the Wnt/β-catenin signaling pathway. Am J Transl Res. 8:3503–3512.
2016.PubMed/NCBI
|
|
21
|
Jin H, Luo S, Wang Y, Liu C, Piao Z, Xu M,
Guan W, Li Q, Zou H, Tan QY, et al: miR-135b stimulates
osteosarcoma recurrence and lung metastasis via Notch and
Wnt/β-catenin signaling. Mol Ther Nucleic Acids. 8:111–122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu X, Duanmu J, Li T and Jiang Q: A
7-lncRNA signature associated with the prognosis of colon
adenocarcinoma. PeerJ. 8:e88772020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Huang Y, Xiang P and Tian W:
LncRNA expression and implication in osteosarcoma: A systematic
review and meta-analysis. Onco Targets Ther. 10:5355–5361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie C, Chen B, Wu B, Guo J and Cao Y:
LncRNA TUG1 promotes cell proliferation and suppresses apoptosis in
osteosarcoma by regulating miR-212-3p/FOXA1 axis. Biomed
Pharmacother. 97:1645–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ju L, Zhou YM and Yang GS: Up-regulation
of long non-coding RNA BCAR4 predicts a poor prognosis in patients
with osteosarcoma, and promotes cell invasion and metastasis. Eur
Rev Med Pharmacol Sci. 20:4445–4451. 2016.PubMed/NCBI
|
|
26
|
Kong D and Wang Y: Knockdown of lncRNA
HULC inhibits proliferation, migration, invasion, and promotes
apoptosis by sponging miR-122 in osteosarcoma. J Cell Biochem.
119:1050–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang N, Wang X, Xie X, Liao Y, Liu N, Liu
J, Miao N, Shen J and Peng T: lncRNA DANCR promotes tumor
progression and cancer stemness features in osteosarcoma by
upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 405:46–55.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang CL, Zhu KP and Ma XL: Antisense
lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by
increasing the expression of FOXC2. Cancer Lett. 396:66–75. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Q, Xiang S, Ma J, Hui P, Wang T, Meng
W, Shi M and Wang Y: Long non-coding RNA CASC15 regulates gastric
cancer cell proliferation, migration and epithelial mesenchymal
transition by targeting CDKN1A and ZEB1. Mol Oncol. 12:799–813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernando TR, Contreras JR, Zampini M,
Rodriguez-Malave NI, Alberti MO, Anguiano J, Tran TM, Palanichamy
JK, Gajeton J, Ung NM, et al: The lncRNA CASC15 regulates SOX4
expression in RUNX1-rearranged acute leukemia. Mol Cancer.
16:1262017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jing N, Huang T, Guo H, Yang J, Li M, Chen
Z and Zhang Y: LncRNA CASC15 promotes colon cancer cell
proliferation and metastasis by regulating the
miR-4310/LGR5/Wnt/β-catenin signaling pathway. Mol Med Rep.
18:2269–2276. 2018.PubMed/NCBI
|
|
32
|
Zhang H, Wang J, Ren T, Huang Y, Yu Y,
Chen C, Huang Q and Guo W: LncRNA CASC15 is upregulated in
osteosarcoma plasma exosomes and CASC15 knockdown inhibits
osteosarcoma progression by regulating miR-338-3p/RAB14 axis. Onco
Targets Ther. 13:12055–12066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hurst DR, Edmonds MD, Scott GK, Benz CC,
Vaidya KS and Welch DR: Breast cancer metastasis suppressor 1
up-regulates miR-146, which suppresses breast cancer metastasis.
Cancer Res. 69:1279–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang B, Xu W, Cai Y, Guo C, Zhou G and
Yuan C: CASC15: A tumor-associated long non-coding RNA. Curr Pharm
Des. 27:127–134. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Cai H, Sun L, Zhan P, Chen M,
Zhang F, Ran Y and Wan J: LGR5, a novel functional glioma stem cell
marker, promotes EMT by activating the Wnt/β-catenin pathway and
predicts poor survival of glioma patients. J Exp Clin Cancer Res.
37:2252018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat (Basel). 154:8–20.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li P, Pan X, Zheng Z, Sun Y, Han Y and
Dong J: LINC00271 inhibits epithelial-mesenchymal transition of
papillary thyroid cancer cells by downregulating trefoil factor 3
expression. Aging Pathobiol Ther. 2:78–85. 2020. View Article : Google Scholar
|
|
39
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li A and Machesky LM: Melanoblasts on the
move: Rac1 sets the pace. Small GTPases. 3:115–119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jayanthi P, Varun BR and Selvaraj J:
Epithelial-mesenchymal transition in oral squamous cell carcinoma:
An insight into molecular mechanisms and clinical implications. J
Oral Maxillofac Pathol. 24:1892020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Qiao B, Zhao T, Hu F, Lam AK and
Tao Q: Sox2 promotes tumor aggressiveness and
epithelial-mesenchymal transition in tongue squamous cell
carcinoma. Int J Mol Med. 42:1418–1426. 2018.PubMed/NCBI
|
|
46
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sharma RP and Chopra VL: Effect of the
Wingless (wg1) mutation on wing and haltere development in
Drosophila melanogaster. Dev Biol. 48:461–465. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yamaguchi TP: Heads or tails: Wnts and
anterior-posterior patterning. Curr Biol. 11:R713–R724. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shetti D, Zhang B, Fan C, Mo C, Lee BH and
Wei K: Low dose of paclitaxel combined with XAV939 attenuates
metastasis, angiogenesis and growth in breast cancer by suppressing
Wnt signaling. Cells. 8:8922019. View Article : Google Scholar
|
|
51
|
Saito-Diaz K, Chen TW, Wang X, Thorne CA,
Wallace HA, Page-McCaw A and Lee E: The way Wnt works: Components
and mechanism. Growth Factors. 31:1–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
de Sousa EM, Vermeulen L, Richel D and
Medema JP: Targeting Wnt signaling in colon cancer stem cells. Clin
Cancer Res. 17:647–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jubb AM, Chalasani S, Frantz GD, Smits R,
Grabsch HI, Kavi V, Maughan NJ, Hillan KJ, Quirke P and Koeppen H:
Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is
upregulated in intestinal neoplasia. Oncogene. 25:3445–3457. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rashid MS, Mazur T, Ji W, Liu ST and
Taylor WR: Analysis of the role of GSK3 in the mitotic checkpoint.
Sci Rep. 8:142592018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiao C, Wu CH and Hu HZ: LncRNA UCA1
promotes epithelial-mesenchymal transition (EMT) of breast cancer
cells via enhancing Wnt/beta-catenin signaling pathway. Euro Rev
Med Pharmacol Sci. 20:2819–2824. 2016.
|
|
56
|
Yue B, Liu C, Sun H, Liu M, Song C, Cui R,
Qiu S and Zhong M: A positive feed-forward loop between
LncRNA-CYTOR and Wnt/β-Catenin signaling promotes metastasis of
colon cancer. Mol Ther. 26:1287–1298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu J, Han Z, Sun Z, Wang Y, Zheng M and
Song C: LncRNA SLCO4A1-AS1 facilitates growth and metastasis of
colorectal cancer through β-catenin-dependent Wnt pathway. J Exp
Clin Cancer Res. 37:2222018. View Article : Google Scholar : PubMed/NCBI
|