Introduction
Genome integrity within normal cells is preserved by
operating surveillance mechanisms including DNA damage checkpoints,
DNA repair systems, and mitotic checkpoints (1). However, defects in genome integrity
cause impaired cellular responses, leading to genome instability.
Genome instability, which is an enabling characteristic for the
acquisition of the hallmarks of cancer, allows normal cells to
undergo genetic alterations including gene mutations, which
contribute to tumor progression (2).
In particular, the elimination of mitotically defective or failed
cells has been regarded as a gateway to avoid genomic instability,
including mitotic catastrophe (3).
Chromosome missegregation resulting from failed mitosis, which has
been extensively characterized as the typical feature of mitotic
catastrophe that is accompanied by mitotic arrest, leads to nuclear
alterations such as micronucleation and multinucleation (4). Furthermore, the disturbance of mitotic
spindle formation induced by the depletion of centrosomal proteins
contributes to the occurrence of mitotic catastrophe because the
mitotic spindle is responsible for perfect chromosome segregation
during mitosis (5). Although the
eventual fate of cells following mitotic catastrophe remains
indefinite, the cells appear to undergo senescence, apoptosis, and
necrosis (3,6,7). Several
microtubule or non-microtubule targeting agents that act as mitotic
catastrophe inducers have been evaluated in preclinical and
clinical trials (8). Natural
compounds have been particularly noted to cause mitotic catastrophe
followed by apoptotic cell death in various types of cancer
(9,10). The induction of mitotic catastrophe in
tumor cells endows therapeutic advantages for the development of
novel anti-cancer agents given the high susceptibility to mitotic
aberrations in aneuploidy or tetraploid tumor cells and their usage
at lower doses before the occurrence of cell death (4). Therefore, inducing mitotic catastrophe
is an attractive strategy for successful therapeutic outcomes.
Active compounds from natural products have
consistently been considered for the identification and development
of potential anti-cancer drug candidates because of their various
pharmacological functions with high safety profiles. Thus, natural
product-derived agents are currently being investigated in clinical
trials to identify valuable agents that can be developed as
anti-cancer drugs (11). The
Juniperus genus, which contains approximately 75 species, is
mainly distributed in large parts of the Northern Hemisphere and
has been used as a traditional medicine for treating several
symptoms, including abdominal spasms, asthma, or diarrhea (12,13). The
biological and pharmacological activities of Juniperus
species seem to be caused by secondary metabolites including five
terpenoids, two flavonoids, and one lignan (13). Among these species, Juniperus
communis has shown a notable ability to induce cell cycle
arrest or apoptotic cell death in various types of cancer cell
lines through regulation of Bcl-2 family proteins, p53 signaling,
or the Akt pathway (14,15). Similarly, the cytotoxic properties of
bioactive compounds derived from Juniperus phoenicea have
been evaluated in several cancer cell lines (16). However, unlike other Juniperus
species, the biological activity of Juniperus squamata (J.
squamata; also referred to as Sabina squamata) against
cancer has not yet been fully studied. Therefore, the aim of this
study was to examine the potential effect of the ethanolic extract
of J. squamata on mitotic catastrophe followed by apoptotic
cell death in human oral cancer cell lines.
Materials and methods
Preparation of plant extracts
The ethanolic extracts were provided by the
International Biological Material Research Center at the Korea
Research Institute of Bioscience and Biotechnology (Daejeon,
Republic of Korea). All extracts were dissolved with dimethyl
sulfoxide (DMSO), aliquoted, and maintained at −20°C. The final
concentration of DMSO did not exceed 0.1%.
Cell cultures
Human oral cancer cell lines HSC-3 and HSC-4 were
obtained from Hokkaido University (Hokkaido), and the HN22 cell
line was provided by Dankook University (Cheonan). The SCC-9 cell
line was supplied by the American Type Culture Collection (ATCC),
and the MC3 cell line was kindly provided by the Fourth Military
Medical University (Xi'an). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM)/F-12 supplemented with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin as an antibiotic
at 37°C with 5% CO2 in a humidified incubator. All
experiments were held after the cells reached approximately 50%
confluency.
Cytotoxicity measurements: Manual cell
counting
Following treatment with EEJS for 24 h, the cells
were washed twice with ice-cold PBS and trypsinized. A
hemocytometer was used to count the number of viable cells. Each
experiment was performed three times, and the results were
expressed as the percentage of surviving cells compared with the
DMSO-treated control group.
Cell counting kit-8 (CCK-8) assay
The cytotoxic effect of EEJS on the human oral
cancer cells was determined via a CCK-8 assay (Dojindo
Laboratories) according to the manufacturer's instructions.
Briefly, cells from all the cell lines were seeded in 96-well
plates and treated and incubated with various doses (2 or 4 µg/ml)
of EEJS for 24 h. CCK-8 solution (10 µl) was added to each well of
the 96-well plates and incubated for 1–2 h at 37°C with 5%
CO2 in a humidified incubator. The absorbance was
measured at 450 nm using a Chameleon microplate reader (Hidex).
Transmission electron microscopy
(TEM)
Following 24 h of treatment with either DMSO or
EEJS, the cells were detached from the cell culture plate using 2X
trypsin, resuspended in DMEM/F-12 media containing 10% FBS, and
centrifuged at 115 × g for 2 min at 4°C. After discarding the
media, the cell pellets were fixed with 2.5% glutaraldehyde in 0.1
M phosphate buffer for at least 24 h at 4°C, post-fixed in 1%
osmium tetroxide, and embedded in Spurr low viscosity resin.
Sections (1 µm thick) were prepared and stained with toluidine blue
O. Ultrathin sections were prepared and stained with uranyl acetate
and lead citrate. TEM was performed by the Electron Microscopy Core
Facility in Seoul National University Hospital Biomedical Research
Institute using JEOL JEM-1400 (JEOL Ltd.). A phase-contrast
microscopy (PCM) was assessed by inverted microscope (CKX53;
Olympus Corporation).
Immunofluorescence staining
The cells were seeded on a 4-well chamber slide.
After the cell confluency reached approximately 50%, the cells were
treated with DMSO or EEJS. After 24 h, the cells were washed three
times with PBS and fixed in 4% paraformaldehyde for 20 min on ice
and then washed three times with 0.1% BSA in PBS. The fixed cells
were permeabilized in buffer containing 0.3% Triton X-100 and 1%
BSA for 1 h at room temperature. The cells were incubated with
α-tubulin (1:300; Santa Cruz Biotechnology, Inc.) overnight at 4°C
in the dark. After being washed with 0.1% BSA in PBS three times,
the cells were further incubated with Alexa Fluor® 488
anti-mouse antibodies (1:400, Jackson ImmunoResearch Inc.). The
nuclei were further stained with DAPI (1:25, 50 µg/ml;
Sigma-Aldrich), and the stained cells were examined by confocal
microscopy (LSM700; Carl Zeiss).
Western blot analysis
Protein was extracted from the EEJS-treated or
untreated cells with RIPA lysis buffer (EMD Millipore) along with
phosphatase inhibitor tablets (Thermo Fisher Scientific, Inc.) and
protease inhibitor cocktails (Roche). The protein concentration of
each sample was quantified using a DC Protein Assay Kit (Bio-Rad
Laboratories). After normalization, the protein lysates containing
approximately 30–50 µg of protein were boiled with 5X protein
sample buffer at 95°C for 5 min and separated by 8, 12, or 15%
SDS-PAGE, after which they were transferred to Immuno-Blot PVDF
membranes. The membranes were blocked with 5% skim milk in
Tris-buffered saline with Tween-20 (TBST) at room temperature for 2
h and incubated with primary antibodies overnight at 4°C, and then
incubated with corresponding horseradish peroxidase
(HRP)-conjugated secondary antibodies for 2 h at RT. The primary
antibodies used to detect phospho-histone H3 (Ser10, cat. no. 9701,
1:1,000), cleaved PARP (cat. no. 9541, 1:1,000), myeloid cell
leukemia-1 (Mcl-1, cat. no. 5453, 1:1,000), and Bcl-xL (cat. no.
2764, 1:1,000) were purchased from Cell Signaling Technology
(Charlottesville). Histone H3 (cat. no. sc-10809, 1:1,000), Bcl-2
(cat. no. sc-7382, 1:1,000), and β-actin (cat. no. sc-47778,
1:3,000) antibodies were obtained from Santa Cruz Biotechnology.
The immunoreactive bands were visualized with ImageQuant™ LAS 500
(GE Healthcare Life Sciences).
Cell cycle analysis
The trypsinized and floating cells were pooled,
washed with PBS, and fixed in 70% ethanol at least overnight at
−20°C. The cells were incubated with propidium iodide solution (20
µg/ml) and RNase A (20 µg/ml) for 15 min at 37°C after PBS washing.
The cell cycle distribution was analyzed with a FACSCalibur Flow
Cytometer (BD Biosciences), and a minimum of 10,000 cells in each
sample was analyzed with BD CellQuest™ Pro software. The
percentages of sub-G1 and G2/M fractions were
quantified by FlowJo software version 9/10 (FlowJo LLC).
Annexin V-FITC/PI double staining
Apoptosis induction was measured using a
FITC-Annexin V Apoptosis Detection Kit (BD Pharmingen) according to
the manufacturer's protocol. Briefly, floating and adherent cells
were collected, washed twice with ice-cold PBS, and pelleted by
centrifugation (180 × g, 5 min, 4°C). Then, the cells were
resuspended in Annexin V binding buffer containing 3 µl of Annexin
V-FITC and 1 µl of PI and incubated for 15 min at room temperature
in the dark. Subsequently, the cells were transferred to a FACS
tube and analyzed by flow cytometry using a FACSCalibur flow
cytometer. The cells showing Annexin V(+)/PI(−) staining indicated
early-stage apoptosis whereas the cells showing Annexin V(+)/PI(+)
staining indicated late-stage apoptosis. The percentage of Annexin
V/PI-stained cells was quantified from a minimum of 10,000 cells by
BD CellQuest™ Pro software. The flow cytometry data were
re-analyzed with FlowJo software version 9/10.
Construction of Mcl-1 overexpression
vector and transient transfection
The Mcl-1 overexpression vector was constructed as
described previously (17). The HSC-3
cells were transfected with either 1 µg of empty pcDNA3.1 or
pcDNA3.1-Mcl-1 using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Statistical analysis
Statistical significance was calculated using a
two-tailed Student's t-test for comparisons between two groups. For
multiple-group comparisons, one-way ANOVA with Tukey's post-hoc
test analyses were applied to determine the significance of
differences between the control and treatment groups. All graphs
are the mean ± SD of three independent experiments. Values of
P<0.05 were considered statistically significant.
Results
EEJS exhibits cytotoxic effects on
human oral cancer cell lines
To identify a novel anti-cancer drug candidate based
on natural products that have a potential chemotherapeutic effect
on human oral cancer cell lines, we screened 49 plant extracts at
doses of 20 µg/ml for 48 h to investigate their cytotoxicity on the
MC3 cell line. The results indicated that the majority of plant
extracts had no significant effect or showed only about 20–30%
inhibitory effect in MC3 cells, whereas the ethanolic extract of
J. squamata (hereafter referred to as EEJS) showed a growth
inhibitory effect of more than 80% (Table S1 and Fig.
S1). We further examined the cytotoxic effect of EEJS on other
human oral cancer cell lines. HSC-3 and HSC-4 cells were treated
with various concentrations (2 and 4 µg/ml) of EEJS for 24 h. As
shown in Fig. 1A, EEJS markedly
decreased the viability of human oral cancer cells in a
dose-dependent manner. Consistently, EEJS significantly suppressed
cell proliferation in HSC-3 and HSC-4 cells according to the CCK-8
assay (Fig. 1B). Similar results were
observed in HN22 and SCC-9 cells, as evidenced by the reduced cell
survival (Fig. S2A). These results
indicate that EEJS exerts a cytotoxic effect on human oral cancer
cell lines.
EEJS provokes mitotic catastrophe in
human oral cancer cell lines
Morphologic changes were observed under a
phase-contrast microscope (PCM) and a transmission electron
microscope (TEM) to examine the effect of EEJS on the occurrence of
mitotic catastrophe. Following treatment with 4 µg/ml EEJS for 24
h, enlarged multinucleated cells with multiple micronuclei were
observed in human oral cancer cells compared with the control group
(Fig. 2 and S2B). Immunofluorescence staining was
performed to confirm whether the enrichment of enlarged
multinucleated cells upon EEJS treatment was accompanied by
disturbances in microtubule formation. As shown in Fig. 3A and S2C, EEJS disrupted the microtubule fibers
and triggered abnormal chromosome segregation. To clarify the
biological function of EEJS, we analyzed the phosphorylation of
histone H3 (Ser10), a specific marker of mitosis.
Western blot analysis revealed that the phosphorylation of histone
H3 at the Ser10 residue was notably increased upon EEJS
treatment in a dose-dependent manner (Fig. 3B and S2D). These data indicate that EEJS
facilitates mitotic catastrophe by disturbing microtubule formation
and chromosome segregation.
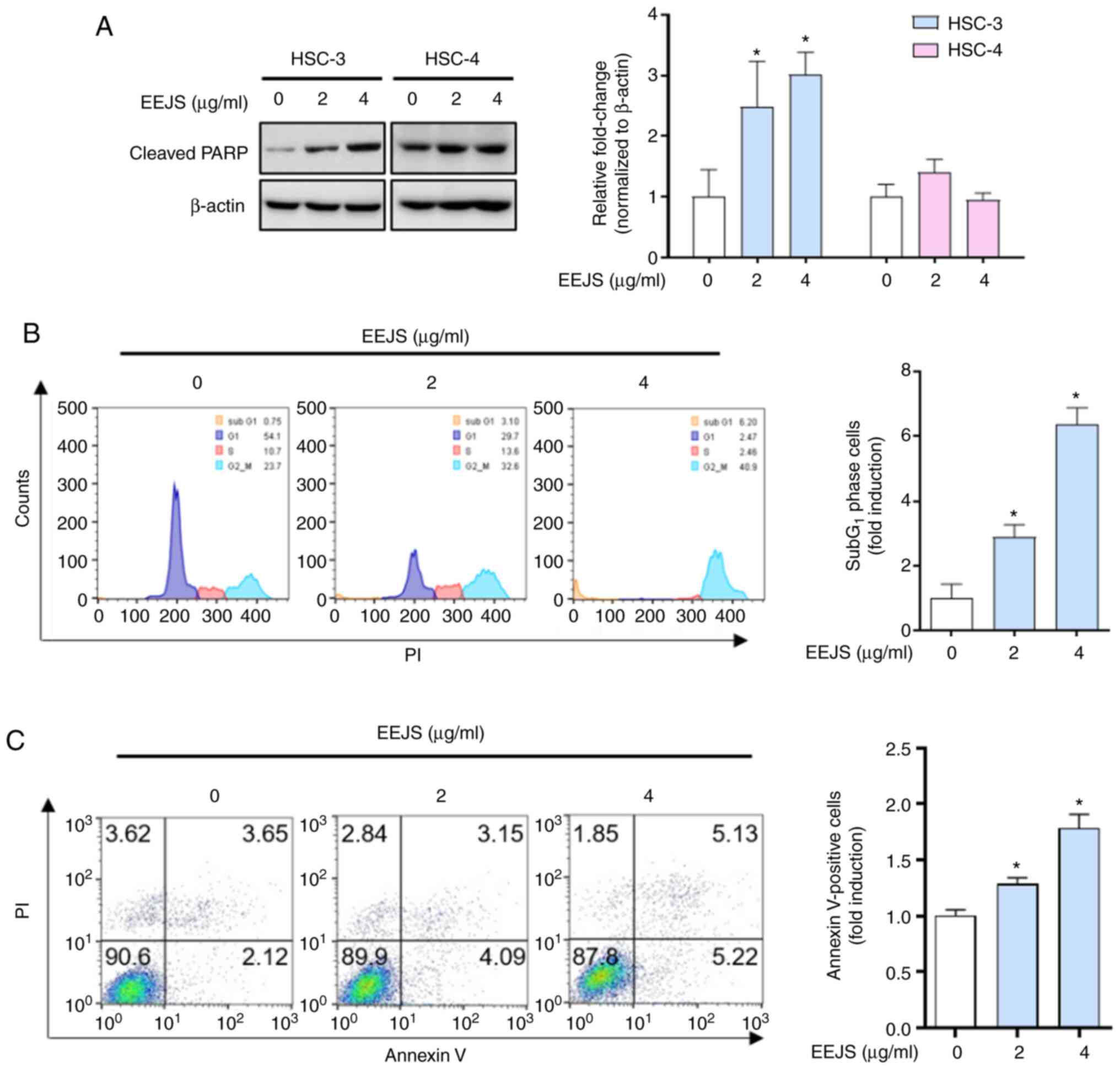
Mitotic catastrophe induced by EEJS
treatment results in apoptotic cell death in human oral cancer
cells
To determine whether the mitotic catastrophe induced
by EEJS treatment was accompanied by apoptotic cell death in human
oral cancer cells, we examined the expression of cleaved PARP, a
hallmark of apoptosis. As shown in Fig.
4A and S2D, EEJS clearly
increased the expression of cleaved PARP in three human oral cancer
cell lines (HSC-3, HN22, and SCC-9); conversely, no sign of an
apoptotic effect from EEJS was observed in the HSC-4 cells. The
flow cytometry analysis results revealed that the percentage of
HSC-3 cells in the sub-G1 phase following EEJS treatment
increased significantly up to 6-fold compared with the vehicle
control group (Fig. 4B).
Consistently, the rate of Annexin V-positive HSC-3 cells was
increased from 5.77% in the vehicle control group to 7.24 or 10.35%
in the EEJS treatment group (Fig.
4C). These data indicate that mitotic catastrophe induced by
EEJS treatment leads to the induction of apoptotic cell death in
human oral cancer cells in a cell context-dependent manner.
Suppression of Mcl-1 following EEJS
treatment determines the susceptibility to apoptotic cell death in
human oral cancer cells
To determine whether EEJS-induced apoptotic cell
death is a consequence of regulating anti-apoptotic Bcl-2 family
proteins, we analyzed Mcl-1, Bcl-xL, and Bcl-2 protein expressions.
As shown in Fig. 5A, EEJS caused a
pronounced reduction of Mcl-1 protein expression in a
dose-dependent manner in HSC-3 cells only; no significant
differences in Bcl-xL or Bcl-2 protein levels were observed in
either cell line. To clarify the biological role of Mcl-1 in
EEJS-induced apoptotic cell death, we overexpressed the Mcl-1
protein in HSC-3 cells. Compared with the control vector
(pcDNA3.1), Mcl-1 overexpression (pcDNA3.1-Mcl-1) partly abolished
the expression of cleaved PARP after EEJS treatment (Fig. 5B). These data indicate that Mcl-1 may
act as a determinant for EEJS-induced apoptotic cell death in human
oral cancer cells.
Discussion
Cancer in the oral cavity and pharynx is the eighth
most prevalent type of neoplasm and was responsible for
approximately 4% of all cancer cases in males in the United States
in 2020 (18). The 5-year survival
rate of patients with oral cavity cancer (hereafter referred to as
oral cancer) is 41.7%, and survival seems to be significantly
correlated with local invasion and distant metastasis (19). Although surgical management has been
considered the standard treatment strategy for oral cancer
patients, adjuvant therapy remains necessary for treating advanced
oral cancer before or after surgery (20). However, the anti-cancer efficacy of
conventional chemotherapy using platinum-based agents is not
sufficient to improve survival in oral cancer patients, which leads
to several therapeutic attempts including targeted therapy and
immunotherapy (21). Natural products
have been proposed as promising anti-cancer drug candidates given
their noteworthy ability to prevent oral cancer belonging to head
and neck cancer (22). Studies on
oral cancer therapy in our laboratory have demonstrated that
natural products function as chemotherapeutic agents by promoting
pro-apoptotic Bcl-2 family proteins and DNA damage responses
(23,24). Thus, a chemotherapeutic approach using
natural products could be a promising therapeutic option for
favorable prognosis in oral cancer patients. In this study, our
data revealed the chemotherapeutic effect of J. squamata on
human oral cancer cells.
Mitotic catastrophe is defined as an oncosuppressive
cell death mechanism for the evasion of genomic instability, which
can precede senescence, apoptotic cell death, or necrotic cell
death (4). It is characterized by
morphological features such as enlarged multinucleated cells that
arise from missegregated chromosomes and microtubule formation
disruption (3). Thus, microtubule
targeting agents have been suggested as effective anti-cancer drugs
in various types of cancer given their obstruction of mitotic
progression. For example, docetaxel treatment of breast cancer
cells primarily leads to mitotic catastrophe, which is considered
the cell death mode (25). The use of
nanocarriers as a drug delivery system for paclitaxel and cetuximab
has resulted in anti-tumor activity in colorectal cancer in
vitro and in vivo by enhancing mitotic catastrophe
followed by apoptotic cell death (26). Notably, several natural products seem
to cause mitotic catastrophe. Bioactive compounds such as gallic
acid, pseudolaric acid B, or thalicthuberine result in arrest at
the G2/M phase of cancer cells by disturbing centrosomal
clustering, microtubule formation, or chromosome segregation, which
contributes to mitotic catastrophe (9,27,28). Chelidonine and glyfoline can
effectively cause the phosphorylation of histone H3
(Ser10), which is an indicator of mitotic catastrophe,
and thereby induce apoptotic cell death (10,29).
Similar to previous studies, we found that EEJS treatment clearly
led to the enrichment of enlarged multinucleated cells,
disturbances of the mitotic spindle, and increased phosphorylation
of histone H3 (Ser10) in human oral cancer cells. These
observations suggest that EEJS is potentially effective against
human oral cancer by facilitating the occurrence of mitotic
catastrophe. Encouraging evidence has shown that mitotic
catastrophe can determine the cell death mode (6). Furthermore, the occurrence of mitotic
catastrophe induced by viriditoxin leads to both apoptotic cell
death and autophagic cell death (30). The current study aimed to investigate
which type of cell death is the endpoint of EEJS-induced mitotic
catastrophe in human oral cancer cells, and our findings indicated
that EEJS treatment generally promoted apoptotic cell death in
three human oral cancer cell lines (HSC-3, HN22, and SCC-9).
Conversely, there was no significant change in the HSC-4 cells,
which suggests that apoptotic cell death from EEJS treatment is
cell context-dependent. Additionally, we observed that the cell
population in G2/M phase was increased upon the EEJS
treatment (Fig. S3), suggesting that
the mitotic catastrophe induced by EEJS treatment may facilitate
G2/M arrest as well as apoptotic cell death in human
oral cancer cells. Thus, our findings provide a possibility that
the mitotic catastrophe induced by EEJS treatment predominantly
causes apoptotic cell death in human oral cancer cells, even if
further studies are necessary to clarify the causality between
mitotic catastrophe and apoptotic cell death.
The Bcl-2 family proteins, which can be divided into
three groups based by their function (e.g., anti-apoptotic,
pro-apoptotic, and BH3-only proteins), orchestrate the balance of
survival and death in cells. Mcl-1 proteins, which are
anti-apoptotic Bcl-2 family proteins, bind and sequester the
pro-apoptotic Bcl-2 family proteins Bax/Bak, resulting in the
disturbance of mitochondria-dependent apoptotic cell death
(31). Mcl-1 is highly expressed in
various types of cancers and is associated with poor prognosis in
cancer patients (31–33). Thus, several Mcl-1 inhibitors have
entered preclinical or clinical trials (32,34).
Natural products have been regarded as one of the most promising
therapeutic strategies given their abilities to regulate Mcl-1
proteins at the transcriptional, post-transcriptional, and
post-translational levels (35). Our
previous findings have demonstrated that bioactive compounds
derived from natural products serve as apoptosis-inducing agents
via modulating the protein stability of Mcl-1 (17,36). Based
on these observations, Mcl-1 is considered a crucial molecular
target for cancer treatment. In this study, we investigated whether
EEJS-induced apoptotic cell death is accompanied by anti-apoptotic
Bcl-2 family proteins and noted that EEJS treatment suppressed
Mcl-1 expression in HSC-3 cells; however, there was no significant
change in Bcl-xL and Bcl-2 expressions. These results caused us to
further investigate the biological role of Mcl-1 in EEJS-induced
apoptotic cell death. As we expected, the ectopic expression of
Mcl-1 almost abrogated the expression of cleaved PARP in HSC-3
cells following EEJS treatment. This highlights the possibility
that the suppression of Mcl-1 upon EEJS treatment plays a decisive
role in the high susceptibility of HSC-3 cells to apoptotic cell
death. Conversely, Mcl-1, as well as Bcl-2 and Bcl-xL, are likely
key regulators during mitosis. Mitotic arrest always precedes
mitotic catastrophe in cells exhibiting defective or failed mitosis
(4), and the sustained CDK1-mediated
phosphorylation of Mcl-1 during mitotic arrest results in cell
death by suppressing Mcl-1 levels (3). However, the mitotic catastrophe induced
by EEJS treatment is not likely a consequence of reduced Mcl-1
expression levels because there was significant enrichment of
mitotic catastrophe in all human oral cancer cell lines regardless
of Mcl-1 expression levels. Therefore, reduced Mcl-1 expression
levels upon EEJS treatment may be a determinant for apoptotic cell
death after mitotic catastrophe.
Based on our observations, the results revealed an
unexpected phenomenon in which EEJS-induced mitotic catastrophe was
insufficient to induce apoptotic cell death in HSC-4 cells only.
Based on this result, we speculate that EEJS may enable HSC-4 cells
to evade apoptotic cell death due to the maintenance of Mcl-1
expression. Although we must continue to elucidate the reasons for
EEJS-induced mitotic catastrophe resulting in the induction of
apoptotic cell death in a cell context-dependent manner, we cannot
exclude the possibility that there are different cell death
mechanisms following EEJS treatment. A previous study showed that
the final endpoints of mitotic catastrophe could be accompanied by
apoptotic cell death or necrotic cell death, depending on the
genetic background of the cells (6).
Thus, we cautiously speculate that EEJS-induced mitotic catastrophe
in HSC-3 and HSC-4 cells is likely to decide their endpoints based
on their different genetic backgrounds. Our results also showed
that the expression level of Mcl-1 was not changed in SCC-9 cells
unlike HN22 cells, even though EEJS clearly induced apoptosis in
both cells (Fig. S2D). This
phenomenon provides the possibility of other molecular targets in
SCC-9 cells treated with EEJS. Thus, we need to further study which
molecular targets could elicit high susceptibility on apoptotic
cell death in SCC-9 cells.
Although we revealed that EEJS acts as a potential
therapeutic agent against human oral cancer, the question remains
as to which main components could elicit the therapeutic effect of
EEJS. Keskes et al reported that the main components of
hexane and methanol extracts from Juniperus phoenicea L.
were α-humulene, pentadecane, cubenene, quercetin 3-O-glucoside,
isoscutellarein 7-O-pentoside, and quercetin 3-O-pentoside
(37). Another group showed that the
ethyl acetate and water extracts from Juniperus communis L.
consist of the eight flavonoids, a α-ionone glycoside, and a
lignin (38). In addition, four
abundant monoterpenoids such as sabinene, elemol, terpinen-4-ol,
and α-pinene were contained in essential oils of Juniperus
squamata var. fargesii (39).
Thus, we modestly speculate that EEJS may contain several
aforementioned components including other species belonging to
Juniperus genus.
In conclusion, the results of the present study
provide evidence that EEJS may function as a promising therapeutic
agent that can induce mitotic catastrophe in human oral cancer
in vitro.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank Enago (www.enago.co.kr) for English language editing.
Funding
This work was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science ICT and Future Planning
[2019R1A2C1085896 and 2020R1C1C1005480]. D.J.H. was funded by the
Health Fellowship Foundation.
Availability of data and materials
All datasets generated or analyzed during this study
are included in the current published article.
Authors' contributions
MJ and JAS conducted most of the experiments and
drafted the manuscript. DJH, CHA, KOH, and YSC were involved in
data acquisition and data interpretation. JSK, HJY, and SDH
performed statistical analysis. JAS and SDC designed the study,
supervised, and wrote the final draft of the paper. All authors
read and approved the final manuscript and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. The authenticity of all the raw data was assessed by
MJ and JAS to ensure its legitimacy.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EEJS
|
ethanolic extract of Juniperus
squamata
|
|
Mcl-1
|
myeloid cell leukemia-1
|
|
PCM
|
phase-contrast microscopy
|
|
TEM
|
transmission electron microscopy
|
References
|
1
|
Yao Y and Dai W: Genomic instability and
cancer. J Carcinog Mutagen. 5:10001652014.PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mc Gee MM: Targeting the mitotic
catastrophe signaling pathway in cancer. Mediators Inflamm.
2015:1462822015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vitale I, Galluzzi L, Castedo M and
Kroemer G: Mitotic catastrophe: A mechanism for avoiding genomic
instability. Nat Rev Mol Cell Biol. 12:385–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimura M, Yoshioka T, Saio M, Banno Y,
Nagaoka H and Okano Y: Mitotic catastrophe and cell death induced
by depletion of centrosomal proteins. Cell Death Dis. 4:e6032013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vakifahmetoglu H, Olsson M and Zhivotovsky
B: Death through a tragedy: Mitotic catastrophe. Cell Death Differ.
15:1153–1162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castedo M, Perfettini JL, Roumier T,
Andreau K, Medema R and Kroemer G: Cell death by mitotic
catastrophe: A molecular definition. Oncogene. 23:2825–2837. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denisenko TV, Sorokina IV, Gogvadze V and
Zhivotovsky B: Mitotic catastrophe and cancer drug resistance: A
link that must to be broken. Drug Resist Updat. 24:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi M, Yao G, Fan S, Cheng W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces mitotic
catastrophe followed by apoptotic cell death in murine fibrosarcoma
L929 cells. Eur J Pharmacol. 683:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YC, Yen WY, Ho HY, Su TL and Yih LH:
Glyfoline induces mitotic catastrophe and apoptosis in cancer
cells. Int J Cancer. 126:1017–1028. 2010.PubMed/NCBI
|
|
11
|
Cragg GM and Pezzuto JM: Natural products
as a vital source for the discovery of cancer chemotherapeutic and
chemopreventive agents. Med Princ Pract. 25 (Suppl 2):S41–S59.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bais S, Gill NS, Rana N and Shandil S: A
phytopharmacological review on a medicinal plant: Juniperus
communis. Int Sch Res Notices. 2014:6347232014.PubMed/NCBI
|
|
13
|
Tavares WR and Seca AML: The current
status of the pharmaceutical potential of Juniperus L.
Metabolites. Medicines (Basel). 5:812018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raasmaja A, Stenius U and Ghalali A: The
Water Extract of Juniperus communis L. Induces cell death
and sensitizes cancer cells to cytostatic drugs through p53 and
PI3K/Akt Pathways. Int J Mol Sci. 20:20542019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CC, Hsiao CY, Lee SC, Huang XF, Chang
KF, Lee MS, Hsieh MC and Tsai NM: Suppression of oral cancer by
induction of cell cycle arrest and apoptosis using Juniperus
communis extract. Biosci Rep. 40:BSR202020832020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al Groshi A, Jasim HA, Evans AR, Ismail
FMD, Dempster NM, Nahar L and Sarker SD: Growth inhibitory activity
of biflavonoids and diterpenoids from the leaves of the Libyan
Juniperus phoenicea against human cancer cells. Phytother
Res. 33:2075–2082. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang IH, Jung W, Kim LH, Shin JA, Cho NP,
Hong SD, Hong KO and Cho SD: Nitidine chloride represses Mcl-1
protein via lysosomal degradation in oral squamous cell carcinoma.
J Oral Pathol Med. 47:823–829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tajmirriahi N, Razavi SM, Shirani S,
Homayooni S and Gasemzadeh G: Evaluation of metastasis and 5-year
survival in oral squamous cell carcinoma patients in Isfahan
(2001–2015). Dent Res J (Isfahan). 16:117–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Omura K: Current status of oral cancer
treatment strategies: Surgical treatments for oral squamous cell
carcinoma. Int J Clin Oncol. 19:423–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szturz P and Vermorken JB: Management of
recurrent and metastatic oral cavity cancer: Raising the bar a step
higher. Oral Oncol. 101:1044922020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crooker K, Aliani R, Ananth M, Arnold L,
Anant S and Thomas SM: A review of promising natural
chemopreventive agents for head and neck cancer. Cancer Prev Res
(Phila). 11:441–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu HJ, Shin JA, Yang IH, Won DH, Ahn CH,
Kwon HJ, Lee JS, Cho NP, Kim EC, Yoon HJ, et al: Apoptosis induced
by caffeic acid phenethyl ester in human oral cancer cell lines:
Involvement of Puma and Bax activation. Arch Oral Biol. 84:94–99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang IH, Shin JA, Lee KE, Kim J, Cho NP
and Cho SD: Oridonin induces apoptosis in human oral cancer cells
via phosphorylation of histone H2AX. Eur J Oral Sci. 125:438–443.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morse DL, Gray H, Payne CM and Gillies RJ:
Docetaxel induces cell death through mitotic catastrophe in human
breast cancer cells. Mol Cancer Ther. 4:1495–1504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin YW, Raj EN, Liao WS, Lin J, Liu KK,
Chen TH, Cheng HC, Wang CC, Li LY, Chen C and Chao JI: Co-delivery
of paclitaxel and cetuximab by nanodiamond enhances mitotic
catastrophe and tumor inhibition. Sci Rep. 7:98142017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan S, Guan X, Grun C, Zhou Z, Schepers U
and Nick P: Gallic acid induces mitotic catastrophe and inhibits
centrosomal clustering in HeLa cells. Toxicol In Vitro. 30:506–513.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levrier C, Rockstroh A, Gabrielli B,
Kavallaris M, Lehman M, Davis RA, Sadowski MC and Nelson CC:
Discovery of thalicthuberine as a novel antimitotic agent from
nature that disrupts microtubule dynamics and induces apoptosis in
prostate cancer cells. Cell Cycle. 17:652–668. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu Z, Zou X, Zhang X, Sheng J, Wang Y,
Wang J, Wang C and Ji Y: Chelidonine induces mitotic slippage and
apoptotic-like death in SGC-7901 human gastric carcinoma cells. Mol
Med Rep. 13:1336–1344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kundu S, Kim TH, Yoon JH, Shin HS, Lee J,
Jung JH and Kim HS: Viriditoxin regulates apoptosis and autophagy
via mitotic catastrophe and microtubule formation in human prostate
cancer cells. Int J Oncol. 45:2331–2340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi R, Lartigue L and Perkins G:
Targeting Mcl-1 and other Bcl-2 family member proteins in cancer
therapy. Pharmacol Ther. 195:13–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiang W, Yang CY and Bai L: MCL-1
inhibition in cancer treatment. Onco Targets Ther. 11:7301–7314.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen Q, Zhan Y, Zheng H, Zang H, Luo J,
Zhang Y, Wang W, Feng J, Lu J, Chen L and Fan S: Elevated
expression of mcl-1 inhibits apoptosis and predicts poor prognosis
in patients with surgically resected non-small cell lung cancer.
Diagn Pathol. 14:1082019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hird AW and Tron AE: Recent advances in
the development of Mcl-1 inhibitors for cancer therapy. Pharmacol
Ther. 198:59–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Muller F, Cerella C, Radogna F, Dicato M
and Diederich M: Effects of natural products on Mcl-1 expression
and function. Curr Med Chem. 22:3447–3461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han JM, Hong KO, Yang IH, Ahn CH, Jin B,
Lee W, Jung YC, Kim KA, Shin JA, Cho SD and Hong SD: Oridonin
induces the apoptosis of mucoepidermoid carcinoma cell lines in a
myeloid cell leukemia-1dependent manner. Int J Oncol. 57:377–385.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Keskes H, Belhadj S, Jlail L, El Feki A,
Damak M, Sayadi S and Allouche N: LC-MS-MS and GC-MS analyses of
biologically active extracts and fractions from Tunisian
Juniperus phoenicea leaves. Pharm Biol. 55:88–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pollio A, Zarrelli A, Romanucci V, Di
Mauro A, Barra F, Pinto G, Crescenzi E, Roscetto E and Palumbo G:
Polyphenolic profile and targeted bioactivity of methanolic
extracts from mediterranean ethnomedicinal plants on human cancer
cell lines. Molecules. 21:3952016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wedge DE, Tabanca N, Sampson BJ, Werle C,
Demirci B, Baser KH, Nan P, Duan J and Liu Z: Antifungal and
insecticidal activity of two Juniperus essential oils. Nat Prod
Commun. 4:123–127. 2009.PubMed/NCBI
|