Introduction
As the most common cause of cancer-associated
mortality worldwide, lung cancer accounted for ~18.4% of all
cancer-associated mortality in 185 countries in 2018 (1). Small cell lung cancer (SCLC) and
non-SCLC (NSCLC) are the two primary types of lung cancer. NSCLC
accounts for ~85% of all histological subtypes of lung cancer
(2). Despite improvements in surgical
resection, chemotherapy, targeted therapy and radiotherapy, the
prognosis of NSCLC remains unfavorable, since it presents with
locally advanced or metastatic disease in ~70% of patients at time
of diagnosis (2). Therefore, it is
urgent to identify new molecules and clarify their functions in
NSCLC.
PCNA clamp associated factor (KIAA0101) is located
on human chromosome 15q22.31 and contains five exons. KIAA0101 has
been found to serve an oncogenic role in cancer (3–6). Fan et
al (7) reported that KIAA0101 is
a key promoter of renal cell carcinoma malignancy induced by
erythropoietin. Lv et al (4)
found that KIAA0101 knockdown suppresses cell growth, colony
formation and G1/S phase transition in breast cancer
cells. To the best of our knowledge, there is limited research on
KIAA0101 and NSCLC. By analyzing 357 NSCLC tissue microarrays via
immunohistochemical staining, Kato et al (8) found that KIAA0101 is highly expressed in
33.9% (121 of 357) of cases, and that overexpression of KIAA0101
predicted poor prognosis in patients with NSCLC. However, the
detailed mechanism of KIAA0101 in NSCLC remains largely
unknown.
Previously, circular (circ)RNAs have attracted
increasing attention in non-coding RNA research (9,10).
circRNAs are a group of stable, covalently closed RNAs transcripts
with gene-regulatory potential (11).
circRNAs serve pivotal regulatory roles, and can be valuable
diagnostic and prognostic biomarkers in NSCLC (12). The function of circRNA transcriptional
adaptor 2A (TADA2A; circTADA2A; also named hsa_circ_0043278 in
circBase) in cancer remains controversial. circTADA2A serves as a
tumor suppressor in several types of malignancy, including breast
and colorectal cancer (13,14). Wu et al (15) reported that circTADA2A competes with
cyclic AMP-responsive element-binding protein 3 (CREB3) via similar
microRNA (miRNA/miR)-203a-3p response elements and promotes cancer
progression and metastasis in osteosarcoma. However, the role of
circTADA2A in NSCLC remains largely unknown.
The present study investigated the expression and
function of circTADA2A and its potential oncogenic role in
NSCLC.
Materials and methods
Patients and tissue samples
A total of 60 NSCLC (38 males and 22 females; age,
49–83 years; mean age, 61.7 years) and paired paratumor tissue
specimens (2 cm from NSCLC tissue) were collected from patients
following surgical resection between January 2015 and April 2018 at
Liaoning Cancer Hospital and Institute (Shenyang, China). All
patients provided written informed consent, and the study was
performed in accordance with the Declaration of Helsinki. Approval
of the study was granted by the Institute Research Medical Ethics
Committee of Liaoning Cancer Hospital & Institute. All patients
were histologically diagnosed by two pathologists, and classified
as stage I, II, III or IV, using the Tumor-Node-Metastasis (TNM)
staging system (version 8) (16).
Cell culture
The human NSCLC (A549, H460, H1299 and H1975) and
normal bronchial epithelial cell line 16HBE were purchased from the
Institute of Biochemistry and Cell Biology of the Chinese Academy
of Sciences. The 16HBE cells were cultured in Airway Epithelial
Cell Basal Medium (American Type Culture Collection); NSCLC cell
lines were cultured in Dulbecco's modified Eagle medium (Gibco;
Thermo Fisher Scientific, Inc.). All media were supplemented with
10% FBS (Thermo Fisher Scientific, Inc.). All cells were incubated
at a constant temperature of 37°C in a humidified atmosphere
containing 5% CO2.
Bioinformatics and GEO database
analysis
CircPrimer (17) was
used to compare the nucleotide sequences of circTADA2A and TADA2A
mRNA. The clinical value of miR-632-targeted genes in The Cancer
Genome Atlas was analyzed using online software UALCAN (18). Data on differentially expressed
circRNAs (ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101586), miRNAs
(ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19945 and
ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74190) and mRNAs in NSCLC
were downloaded from the GEO database
(ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19188 and
ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30219). Differentially
expressed circRNAs, miRNAs and mRNAs were analyzed using the online
software GEO2R (19).
RNase R treatment
In order to detect the stability of circTADA2A,
RNase R assay was performed as previously described (15). A total of 5 U/µg RNase R (Epicentre;
Illumina, Inc.) was added to 2 mg total RNA (isolated from A549 or
H1299 cells) and incubated for 30 min at 37°C. Controls were
untreated. Subsequently, RNeasy MinElute Cleaning kit (Qiagen,
Inc.) was applied to purify the RNAs according to the
manufacturer's instructions. Next, reverse
transcription-quantitative (RT-q)PCR was performed to detect
circRNAs or mRNAs.
Actinomycin D assay
Actinomycin D assay was performed as previously
reported (20). Briefly, 2 µg/ml
actinomycin D (Sigma-Aldrich; Merck KGaA) was added to A549 and
H1299 cells and incubated at 37°C for 4, 8, 12 and 24 h. Next,
total RNA from A549 or H1299 cells was extracted, and the levels of
circTADA2A and linear TADA2A mRNA were measured by RT-qPCR.
RNA extraction and RT-qPCR
RNA extraction and RT-qPCR were performed as
previously described (21). Total RNA
from CRC tissue and cells was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. To qualify the level of circTADA2A,
RNase R (3 U/1,000 ng; Epicentre; Illumina, Inc.) was added to
digest linear RNAs during RNA extraction and incubated for 10 min
at 37°C. A total of 500 ng RNA was reverse transcribed into cDNA
using a Goscript™ Reverse Transcription system (Promega
Corporation) according to the manufacturer's instructions.
Stem-loop RT was performed for miR-638 using the TaqMan MicroRNA
detection kit (Thermo Fisher Scientific, Inc.). Then, qPCR was
performed using SYBR Premix Ex Taq II (Takara Bio, Inc.). β-actin
and U6 were employed as endogenous controls for mRNA and miR-637,
respectively. PCR amplification conditions were as follows: 2 min
at 95°C for one cycle, followed by denaturation for 15 sec at 95°C
and extension for 60 sec at 60°C for 38 cycles. The relative
expression levels of the genes were determined using the
2−ΔΔCq method (16). The
primer sequences are listed in Table
I.
 | Table I.Primer and oligonucleotide
sequences. |
Table I.
Primer and oligonucleotide
sequences.
| A, Primers |
|---|
|
|---|
| Name | Sequence,
5′→3′ |
|---|
| circTADA2A | F:
TGTGCACCAAGACCAAGGAG |
|
| R:
AGGAAAATCTGAAGTAGTGA |
| circNOL10 | F:
TGCTTCTGACGGCCAATGAA |
|
| R:
AACAGGCCAACTCTGTTTCGA |
| TADA2A | F:
CCCAAAAATATATGTGAAGGCCA |
|
| R:
AAGCAGTTTTGCAAAGGAAACAG |
| KIAA0101 | F:
TCAGTTCGTTCTCTCCTCTCC |
|
| R:
TAGTGGCAGAGGTGGAAGAAC |
| β-actin | F:
CTTCTACAATGAGCTGCGTG |
|
| R:
TCATGAGGTAGTCAGTCAGG |
| miR-638 | F: ATCCAGTGCGTG
TCGTG |
|
| R:
TGCTAGGGATCGCGGGCGGGTG |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
|
| B,
Oligonucleotides |
|
| Name | Sequence,
5′→3′ |
|
| sicircTADA2A-1 | CCAUUUCACUGCAGGAUGU
dTdT |
| sicircTADA2A-2 | CACUGCAGGAUGUAGCCAA
dTdT |
| siSCR |
UUCUCCGAACGUGUCACGUTT |
| miR-638 |
AGGGAUCGCGGGCGGGUGGGCGGC |
| mimics | CU |
| miR-638 |
AGGCCGCCCACCCGCCCGCGAUCC |
| inhibitor | CU |
| miR-NC |
CAGUACUUUUGUGUAGUACAA |
Western blot analysis
Western blotting was performed as previously
reported (21). Total protein from
A549 and H1299 cells was extracted using radio immunoprecipitation
assay lysis buffer (Sigma-Aldrich; Merck KGaA) and quantified by
BCA kit (Santa Cruz Biotechnology, Inc.). Total proteins (20 µg)
were separated by 10% SDS-PAGE and transferred onto PVDF membranes
(EMD Millipore), which was blocked with 5% BSA (Sigma-Aldrich;
Merck KGaA) for 1 h at room temperature. Primary anti bodies
against KIAA0101 (1:1,000; cat.no. ab226255; Abcam) and GAPDH
(1:500; cat.no. ab8245; Abcam) were then added to the membranes and
incubated at 4°C overnight. Next, a secondary antibody [horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG; 1:2,000; cat. no.
ab205719; Abcam] was added to the membranes and incubated at 24°C
for 1 h. Signals of the targeted proteins were detected using an
ECL Western Blotting Substrate kit (cat. no. ab65623; Abcam) and
bands were analyzed with Image J software version 2 (National
Institutes of Health).
Plasmids and oligonucleotide
transfection
Specific small interfering (si)RNAs targeting
circTADA2A and KIAA0101 and scramble control siRNAs (siSCRs) were
purchased from Shanghai GenePharma Co., Ltd. miR-638 mimics and
miR-negative control (NC), as well as miR-638 inhibitor (inh) and
inh-NC were purchased from Shanghai GenePharma Co., Ltd. circTADA2A
overexpression plasmids containing miR-638 binding sequences
(oecircTADA2A) were also synthesized by Shanghai GenePharma Co.,
Ltd. When cells reached 80–90% confluence, 5 nm miR-638
mimics/inhibitors and 50 nM siRNAs were transfected into A549 and
H1299 cells using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions at room temperature. Subsequent experiments were
performed 24 h after transfection. Sequences of siRNAs, miR-638
mimics, miR-NC, miR-638 inhibitor and inh-NC are listed in Table I.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was performed as previously described
(20). In brief, 2×103
A549 and H1299 cells were seeded into 96-well plates, and 200 µl
culture medium was added to each well. The cells were then
incubated at 37°C with 5% CO2. Following incubation for
1–5 days, each well was supplemented with 20 µl CCK-8 solution
(Dojindo Molecular Technologies, Inc.). Following 2 h incubation, a
microplate reader (Bio-Rad Laboratories, Inc.) was used to measure
the absorbance at 450 nm. The assays were repeated three times.
Transwell chamber assay
Transwell assay was conducted as previously reported
(22). For invasion assays, upper
chambers (Corning, Inc.) were pre-coated with Matrigel (1:8; BD
Biosciences) for 1 h at room temperature. A549 and H1299 cells
(5×104) were incubated in the upper chambers. Serum-free
DMEM was added to the upper chamber; medium in the lower chamber
was filled with DMEM supplemented with 10% FBS and the cells were
incubated at 37°C for 12 h. After 12 h, non-migratory cells were
removed and while migratory or invasive A549 and H1299 cells were
fixed with 4% paraformaldehyde at room temperature for 30 min,
stained with crystal violet at 25°C for 1 min and counted using an
inverted microscope (Olympus Corporation).
RNA fluorescence in situ hybridization
(FISH)
The assay was performed as previously described
(20). Specific probes targeting
circTADA2A or miR-638 were synthesized by Shanghai GenePharma Co.,
Ltd. The assay was conducted using a FISH kit (Shanghai GenePharma
Co., Ltd.) according to the manufacturer's instructions.
Cy3-labeled circTADA2A and Dig-labeled locked nucleic acid miR-638
probes (Shanghai GenePharma Co., Ltd.) were measured using the
aforementioned FISH kit, followed by visualization with a confocal
microscope (Carl Zeiss AG).
Immunohistochemistry (IHC)
IHC assay was performed using a specific HRP/DAB
(ABC) Detection IHC kit (Abcam) as previously described (23). NSCLC tissues were sliced following 4%
paraformaldehyde fixation for 4 h at room temperature, 10% EDTA
(pH, 7.3) decalcification, dehydration and paraffin embedding.
Then, the tissue sections (4-µm thick) were incubated with
anti-KIAA0101 antibody (1:500; cat.no. ab226255; Abcam) at 4°C
overnight. The tissue sections were incubated with secondary
antibodies (1:1,000; cat. no. E043201; Dako; Agilent Technologies,
Inc.) at 37°C for 30 min. Subsequently, the tissue sections were
incubated with streptavidin HRP for another 30 min at room
temperature (Labeled Streptavidin Biotin kit; cat. no. K060911-8;
Dako; Agilent Technologies, Inc) at room temperature and stained
with 0.05% 3,3-diaminobenzidine for 60 sec at room temperature.
Finally, the slides were counterstained with 10% hematoxylin for 3
min at room temperature, dehydrated in a graded ethanol series
(absolute ethyl alcohol for 3 min, 95% ethanol for 3 min and 85%
ethanol for 3 min), then observed under a light microscope.
Dual luciferase reporter assay
Reporter plasmids containing wild-type (wt) or
mutant (mut) circTADA2A sequences (luc-circTADA2A-wt and
luc-circTADA2A-mut), as well as reporter plasmids containing wt or
mut KIAA0101 sequences (luc-KIAA0101-wt and luc-KIAA0101-mut) were
synthesized by Shanghai GenePharma Co., Ltd. The procedures were
performed as previously described (15). When A549 and H1299 cells reached 70%
confluence, reporter plasmids and miR-638 mimics or miR-NC were
co-transfected into A549 and H1299 cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, luciferase activity was measured and
compared with Renilla luciferase activity using the
Dual-Luciferase Reporter Assay system (Promega Corporation)
according to the manufacturer's protocol.
RNA immunoprecipitation (RIP)
assay
RIP assay was performed as previously reported
(24) using a Magna RIP RNA-Binding
Protein Immunoprecipitation kit (EMD Millipore). Argonaute (Ago)2
plasmid or vector were transfected into A549 and H1299 cells.
Subsequently, RIP Lysis Buffer combined with a protease inhibitor
cocktail and RNase inhibitors was used to lyse A549 and H1299
cells. Cell lysates (200 µl) were incubated with 5 µg antibody
against Ago2 (EMD Millipore) or rabbit IgG-coated beads at 4°C
overnight with rotation. RNeasy MinElute Cleanup kit (Qiagen, Inc.)
was used to extract the immunoprecipitated RNA and extracted RNAs
were reverse transcribed and subjected to qPCR to detect the
abundance of circTADA2A.
RNA pull-down assay
The targeted binding effect between circTADA2A and
miR-638 was verified by RNA pull-down assay as previously reported
(25). Biotinylated miR-638 probe was
purchased from Shanghai GenePharma Co., Ltd. A549 and H1299 cells
(1×106) were harvested, lysed by 200 µl
Pierce® IP lysis buffer (Thermo Fisher Scientific, Inc.)
and sonicated. The pull-down assay was performed using a Pierce™
Magnetic RNA-Protein Pull-Down kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. In brief, 50 µl C-1
magnetic beads (Thermo Fisher Scientific, Inc.) were co-incubated
with 50 pmol biotinylated miR-638 or NC probes at 25°C for 30 min
to generate probe-coated beads. The beads were harvested by a
magnetic stand (Thermo Fisher Scientific, Inc.), then incubated
with cell lysate at 4°C for 60 min. The beads were harvested using
a magnetic separation stand (Thermo Fisher Scientific, Inc.). The
pulled down RNA complexes were eluted and extracted using a RNeasy
Mini kit (Qiagen, Inc.), Pulled down circTADA2A and circular
nucleolar protein 10 (NOL10) were determined by RT-qPCR, as
aforementioned.
Statistical analysis
All data are presented as the mean ± SD (n=3).
Statistical analysis was performed with GraphPad Prism v5.0
(GraphPad Software, Inc.) and SPSS 19.0 statistical software (IBM
Corp.). Survival analysis was performed by Kaplan-Meier analysis
using log-rank test. The clinical value of circTADA2A was measured
by receiver operating characteristic (ROC) curve analysis. The
association between circTADA2A and miR-638 was analyzed by Spearman
correlation analysis. For comparison of two groups, χ2
or paired Student's t test were performed. For the comparison of
multiple groups, one-way ANOVA followed by post hoc Dunnett's or
Tukey's multiple comparisons test were performed, as appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
circTADA2A is upregulated in patients
with NSCLC
By analyzing the profile of circRNA expression in
five lung adenocarcinoma and paired normal lung tissue samples from
a genome-wide study (GSE101586), it was found that 22 upregulated
circRNAs and 17 downregulated circRNAs were significantly
differentially expressed in lung cancer compared with normal tissue
(cut-off criteria: P<0.05 and |logFC|≥1.2; Fig. 1A; Table
SI). circTADA2A (probe ID: ASCRP002384; circBase ID:
hsa_circ_0043278) was selected for further study since it was the
most significantly upregulated circRNA (Fig. 1B; Table
SI). The expression of circTADA2A was determined in 60 NSCLC
tissue specimens and cell lines. circTADA2A was upregulated in
NSCLC tissue specimens and cell lines (Fig. 1C and D). Additionally, it was
uncovered that the changes of circTADA2A in A549 and H1299 were
more significantly than in H460 and H1975 cells. A549 and H1299
were selected for use in subsequent experiments. In comparison with
its parental gene, circTADA2A was found to form a covalently closed
continuous loop, and comprised exons 5 and 6 of the TADA2A gene,
using the online software CircPrimer (17) (Fig. 1E).
RNase R and actinomycin D assays were performed to detect the
stability of circTADA2A. circTADA2A was more stable than its
parental TADA2A mRNA following treatment with RNase R and
actinomycin D (Fig. 1F-I).
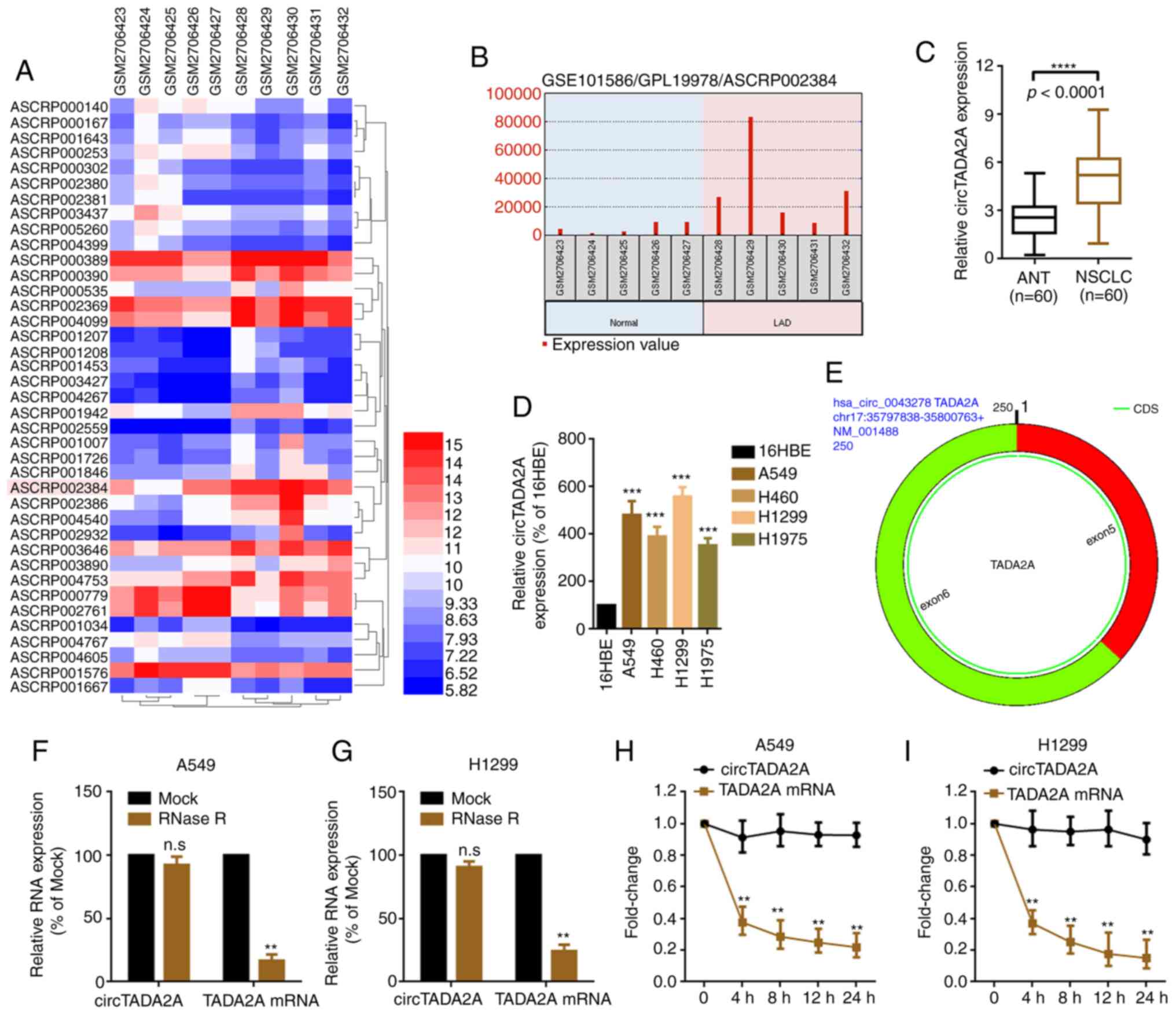 | Figure 1.circTADA2A is upregulated in patients
with NSCLC. (A) Differentially expressed circRNAs in five LAD and
paired normal lung tissue samples, as illustrated by a heatmap
(P<0.05 and |logFC|≥1.2). (B) circTADA2A was highly expressed in
LAD compared with paired normal lung tissue. Probe ID ASCRP002384
represents circTADA2A. Expression of circTADA2A in (C) NSCLC and
ANT and (D) cell lines was determined by RT-qPCR (n=38).
***P<0.001 vs. 16HBE; ****P<0.0001. (E) circTADA2A derives
from exons 5 and 6 of linear TADA2A, as verified by the online
software CircPrimer version 1.2. A total of 5 U/µg RNase R was
added to (F) A549 and (G) H1299 cells, and the mRNA expression of
circTADA2A and TADA2A was evaluated by RT-qPCR. A total of 2 mg/ml
actinomycin D was added to (H) A549 and (I) H1299 cells, and the
mRNA expression of circTADA2A and TADA2A was evaluated by RT-qPCR.
**P<0.01 vs. Mock. All data are shown as the mean ± SD (n=3).
circTADA2A, circular RNA transcription adaptor 2A; circRNA,
circular RNA; NSCLC, non-small cell lung cancer; RT-q, reverse
transcription-quantitative; ANT, adjacent non-tumor tissue; FC,
fold change; LAD, lung adenocarcinoma. |
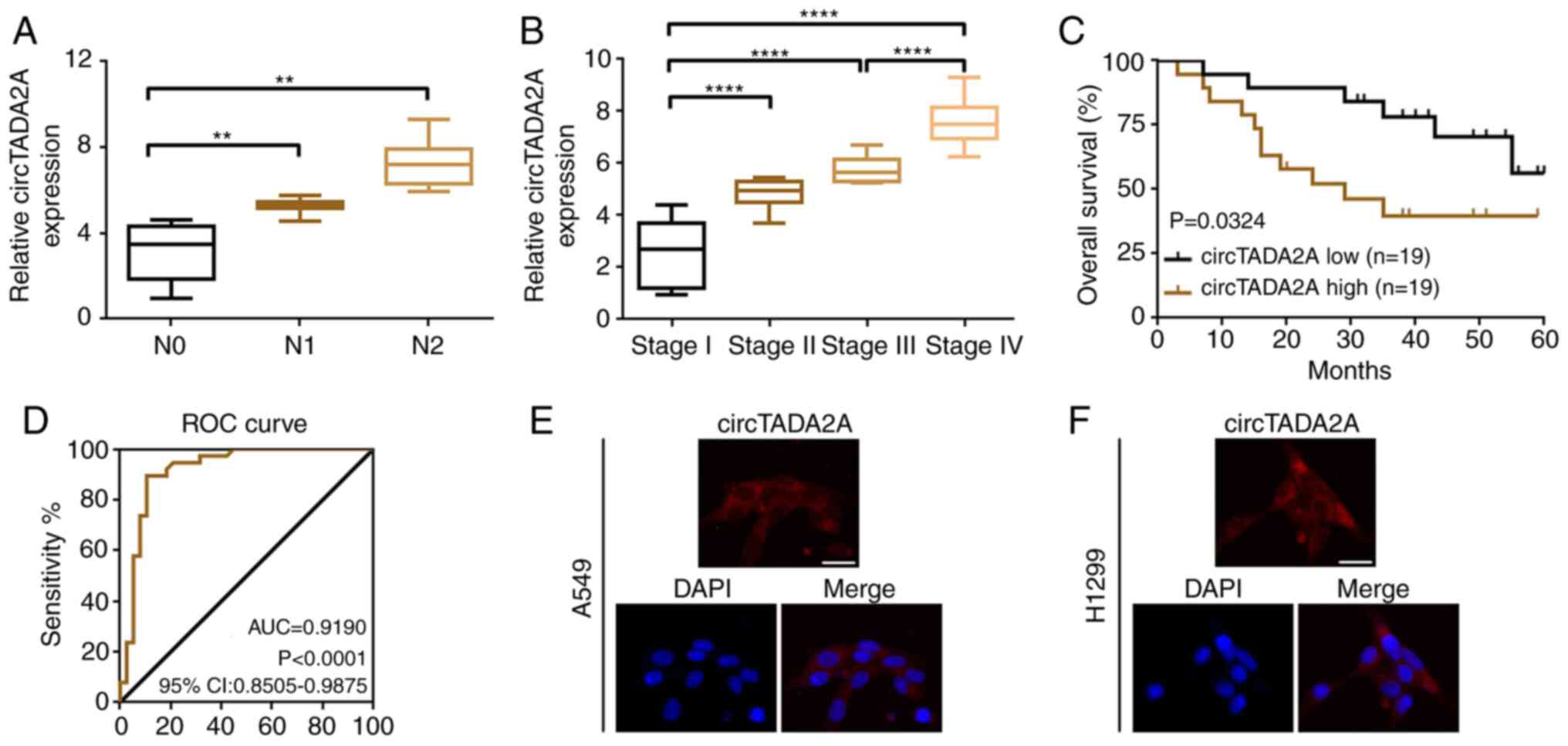
Upregulated circTADA2A predicts
aggressive clinicopathological characteristics and poor prognosis
in patients with NSCLC
In order to determine the clinical role of
circTADA2A, the association between circTADA2A expression levels
and clinicopathological characteristics of patients with NSCLC was
analyzed. Higher levels of circTADA2A were more frequently observed
in patients with lymph node metastasis and advanced stage disease
(Fig. 2A and B). Furthermore, high
expression of circTADA2A was associated with clinicopathological
characteristics of patients with NSCLC, particularly TNM stage and
lymph node metastasis. (Table II).
Kaplan-Meier analysis indicated that patients with NSCLC who had a
higher circTADA2A level had poorer overall survival (Fig. 2C). circTADA2A may be a valuable
biomarker in NSCLC according to the results of ROC curve analysis
(area under the curve=0.8606; Fig.
2D).
 | Table II.Association between circTADA2A and
clinicopathological characteristics of patients with non-small cell
lung cancer. |
Table II.
Association between circTADA2A and
clinicopathological characteristics of patients with non-small cell
lung cancer.
|
|
| circTADA2A
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=60) | High | Low | P-value |
|---|
| Age, years |
|
|
| 0.791 |
|
≤60 | 23 | 12 | 11 |
|
|
>60 | 37 | 18 | 19 |
|
| Sex |
|
|
| 0.284 |
|
Male | 38 | 21 | 17 |
|
|
Female | 22 | 9 | 13 |
|
| Smoking
history |
|
|
| 0.787 |
|
Yes | 39 | 19 | 20 |
|
| No | 21 | 11 | 10 |
|
| Pathology |
|
|
| 0.301 |
|
Adenocarcinoma | 28 | 16 | 12 |
|
|
Squamous carcinoma | 32 | 14 | 18 |
|
| TNM stage |
|
|
| 0.038 |
| I +
II | 28 | 10 | 18 |
|
| III +
IV | 32 | 20 | 12 |
|
| Lymph node
metastasis |
|
|
| 0.001 |
|
Yes | 38 | 25 | 13 |
|
| No | 22 | 5 | 17 |
|
| Tumor size, cm |
|
|
| 0.020 |
| ≤3 | 29 | 10 | 19 |
|
|
>3 | 31 | 20 | 11 |
|
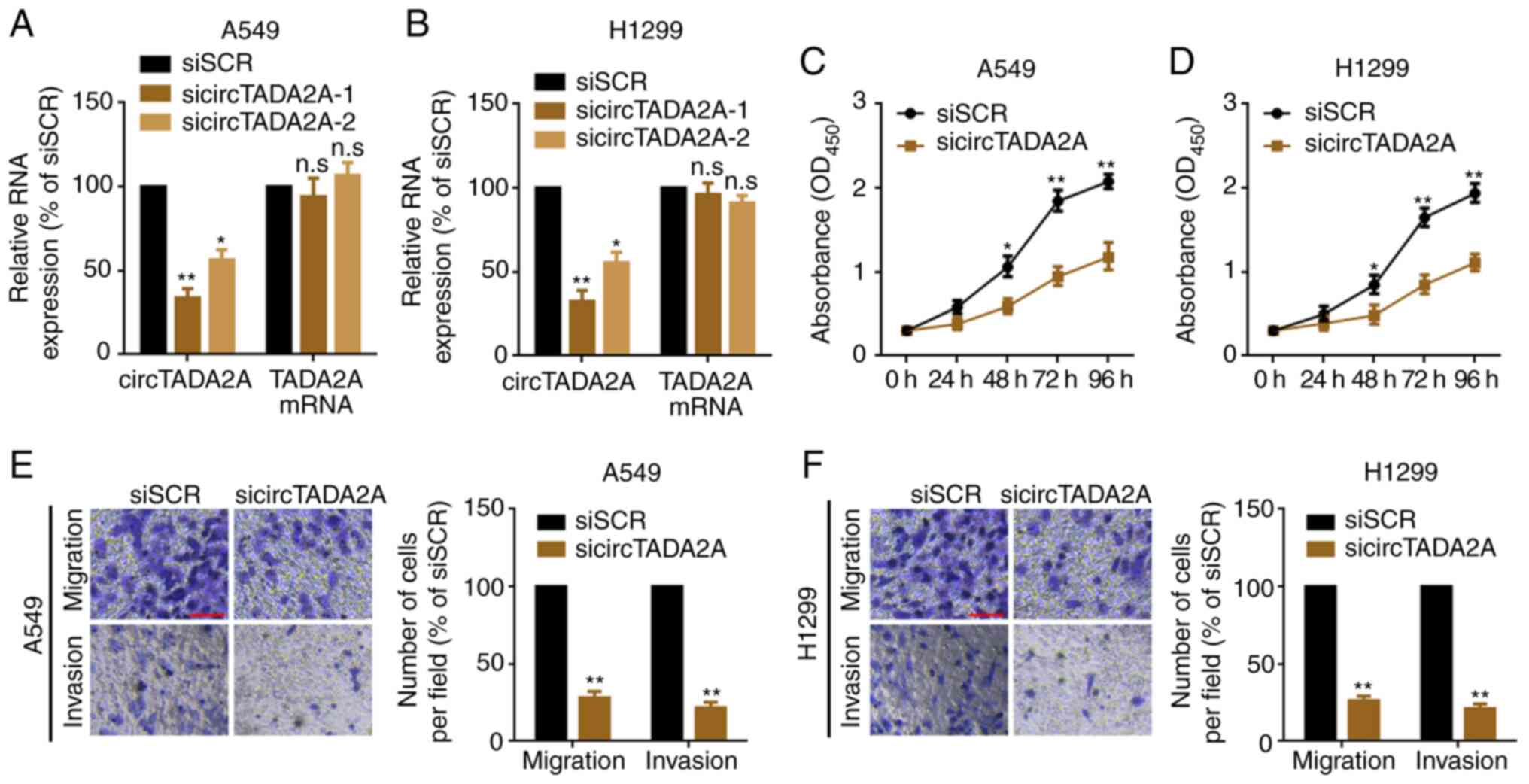
circTADA2A suppression inhibits
proliferation and migration of A549 and H1299 cells
In order to evaluate the role of circTADA2A on
proliferation and migration of NSCLC cells, siRNAs targeting the
back-splice sites were used to knock down expression of circTADA2A
in A549 and H1299 cells. circTADA2A was significantly knocked down,
while TADA2A mRNA was not changed following transfection with
circTADA2A siRNAs (Fig. 3A and B).
sicircTADA2A-1 was selected as the silencing tool for subsequent
experiments as it exhibited the greatest efficacy. Next, the
proliferation, migration and invasion of A549 and H1299 cells were
detected following knockdown of circTADA2A. The results indicated a
significant delay in the proliferation, migration and invasion of
A549 and H1299 following knockdown of circTADA2A compared with
cells transfected with siSCR (Fig.
3C-F).
circTADA2A serves as a sponge for
miR-638
circRNAs have been reported to exert their functions
by acting as miRNAs sponges in various cancer types (26). It was hypothesized that circTADA2A
functions via a similar mechanism in NSCLC. circTADA2A was
primarily located in the cytoplasm of A549 and H1299 cells
(Fig. 2E and F). RIP assay showed
that circTADA2A was significantly enriched in Ago2 protein
(Fig. 4A). Online prediction software
Circular RNA Interactome (27), was
used to predict potential miRNAs that may interact with circTADA2A.
A total of 8 potential miRNAs that could bind to circTADA2A were
found (Table SII). Among them, only
miR-638 was expressed at low levels in NSCLC tissue according to
genome-wide studies using GSE74190 and GSE19945 datasets (Fig. 4B-D, and Tables SIII and SIV). Furthermore, miR-638 was expressed at
low levels in NSCLC tissue and cells (Fig. 4E and F). Additionally, an inverse
correlation between circTADA2A and miR-638 was observed (Fig. 4G). RNA FISH assay indicated that
circTADA2A and miR-638 co-localized in the cytoplasm (Fig. 4H). A luciferase reporter assay was
conducted to confirm the targeted binding effect between circTADA2A
and miR-638. Compared with that of miR-NC, miR-638 mimic
significantly decreased luciferase reporter activity (Fig. 4I and J). When the predictive target
sites for miR-638 were mutated, the change in luciferase activity
was insignificant. Ago RISC catalytic component 2 (AGO2) is a key
RNA binding protein for the ceRNA theory of RNA-RNA interactions
among miRNAs, long non-coding RNAs and circRNAs (28–30). In
order to determine the mechanism of circTADA2A, RIP assay was
performed, using IgG as a control. The enriched circTADA2A and
circNOL10 [a circRNA reported not to bind to Ago2 (31)] were analyzed. The results indicated
that, compared with the NC probe, circTADA2A but not circNOL10 was
pulled down by the miR-638 probe (Fig. 4K
and L). Lastly, it was observed that circTADA2A was not
affected by up- or downregulation of miR-638. Similarly, the level
of miR-638 was not affected either by up- or downregulation of
circTADA2A (Fig. 4M and N).
 | Figure 4.circTADA2A serves as a sponge for
miR-638. (A) RNA immunoprecipitation experiments were performed
using antibody against Ago2 on extracts from A549 and H1299 cells,
and circTADA2A was enriched in the Ago2 group. **P<0.01. (B)
Schematic illustration exhibiting overlap of target miRNAs of
circTADA2A predicted by Circular RNA Interactome, as well as the
miRNAs that were expressed at low levels in genome-wide studies
GSE74190 and GSE19945. The expression of miR-638 in NSCLC was lower
than that in normal lung tissue according to genome-wide studies
(C) GSE74190 and (D) GSE19945. Expression of miR-638 in (E) NSCLC
and paired adjacent non-tumor tissue and (F) cell lines was
determined by RT-qPCR. **P<0.01, ***P<0.001 vs. 16HBE. (G) An
inverse correlation between circTADA2A and miR-638 was demonstrated
by Spearman correlation analysis. (H) Subcellular localization of
circTADA2A and miR-638, as revealed by RNA-fluorescence in
situ hybridization assay. The targeted binding effect between
miR-638 and circTADA2A was confirmed by luciferase assay in (I)
A549 and (J) H1299 cells. **P<0.01 vs. miR-NC. Cell lysates from
(K) A549 and (L) H1299 cells were incubated with biotin-labeled
miR-638 or NC probe, and the enriched circTADA2A and circNOL10 were
detected by RT-qPCR. **P<0.01 vs. NC. The relative expression of
circTADA2A and miR-638 in (M) A549 and (N) H1299 cells was measured
by RT-qPCR. **P<0.01, ***P<0.001, ****P<0.0001. All data
are shown as the mean ± SD (n=3). circTADA2A, circular RNA
transcription adaptor 2A; RT-q, reverse transcription-quantitative;
miR, microRNA; NSCLC, non-small cell lung cancer; Ago2, argonaute
2; NC, negative control; NOL10, nucleolar protein 10; wt,
wild-type; mut, mutant; inh, inhibitor; SCR, scramble; oe,
overexpression; n.s., not significant. |
miR-638 suppresses cell proliferation
and migration by targeting KIAA0101 in A549 and H1299 cells
In order to identify the downstream genes of
miR-638, the online prediction results of TargetScan (32) and upregulated genes according to two
genome-wide studies, GSE19188 and GSE30219 (cut-off criteria:
P<0.05 and logFC value ≥2), were combined. It was found that
four mRNAs, matrix metalloproteinase 1, BUB1 mitotic checkpoint
serine/threonine kinase, KIAA0101 and steroid 5 α-reductase 1,
overlapped (Fig. 5A; Table SV,Table
VI,Table SVII). KIAA0101 has
been reported to be implicated in NSCLC proliferation and
metastasis (8). Among the
aforementioned four genes, KIAA0101 was more closely correlated
with shorter OS rate in lung cancer according to Kaplan-Meier
analysis using the online software UALCAN (18) (Fig.
S1). Therefore, KIAA0101 was selected for subsequent
experiments. KIAA0101 was upregulated in NSCLC according to the
results of IHC staining and genome-wide studies using GSE19188 and
GSE30219 datasets (Fig. 5B-D).
Furthermore, up- and downregulation of miR-638 inversely regulated
KIAA0101 protein expression (Fig. 5E and
F). Functionally, upregulation of miR-638 suppressed A549 and
H1299 cell proliferation and metastasis. When the level of KIAA0101
was elevated (following co-transfection of miR-638 mimic and
specific KIAA0101 overexpression plasmids), the suppressive effect
of miR-638 was abolished (Fig. 5G-J).
Lastly, it was verified that KIAA0101 was a direct target of
miR-638 via six base pair binding sites (positions 1,473-1,479;
Fig. 5K-M).
 | Figure 5.miR-638 suppresses proliferation and
migration by targeting KIAA0101 in A549 and H1299 cells. (A)
Schematic illustration exhibiting overlapping target mRNAs of
miR-638 predicted by TargetScan and the mRNAs that were highly
expressed in genome-wide studies GSE19188 and GSE30219. KIAA0101
expression in NSCLC tissues was higher than that in healthy and NTL
tissue according to the results of genome-wide studies (B) GSE19188
and (C) GSE30219. ****P<0.01. (D) KIAA0101 expression in three
NSCLC and paired paratumor tissue samples was evaluated by
immunohistochemistry. KIAA0101 protein expression in (E) A549 and
(F) H1299 cells following different miR-638 treatments was
evaluated by western blot assay. **P<0.01. Proliferation of (G)
A549 and (I) H1299 cells were determined by Cell Counting Kit-8
assay. Migration and invasion of (H) A549 and (J) H1299 cells were
measured by Transwell assay. Magnification, ×20; scale bar, 200 µm.
*P<0.05. (K) Diagram of the constructed luciferase reporter
plasmids. A luciferase assay was conducted to verify the targeted
binding effect between miR-638 and KIAA0101 in (L) A549 and (M)
H1299 cells. All data are presented as mean ± SD (n=3). **P<0.01
vs. miR-NC. miR, microRNA; NSCLC, non-small cell lung cancer;
KIAA0101, PCNA clamp associated factor; NTL, non-tumor lung; NC,
negative control; BUB1, BUB1 mitotic checkpoint serine/threonine
kinase; SRD5A1, steroid 5 α-reductase 1; inh, inhibitor; OD,
optical density; wt, wild-type; mut, mutant; n.s., not
significant. |
circTADA2A promotes cell proliferation
and migration partially via the miR-638/KIAA0101 pathway in A549
and H1299 cells
The present study investigated whether the oncogenic
role of circTADA2A in A549 and H1299 cell proliferation and
migration was achieved, at least partially, via the
miR-638/KIAA0101 signaling pathway. The protein expression of
KIAA0101 following overexpression or knockdown of circTADA2A was
analyzed by western blotting. Overexpression of circTADA2A
positively regulated KIAA0101 protein expression and vice versa
(Fig. 6A and B). circTADA2A sponged
miR-638, and KIAA0101 was a downstream target of miR-638. The
present study investigated whether circTADA2A regulated KIAA0101
via miR-638 sponging. circTADA2A promoted KIAA0101 protein
expression; this effect was abolished by miR-638 mimics (Fig. S2A and B). CCK-8 assay demonstrated
that overexpression of circTADA2A promoted the proliferation of
A549 and H1299 cells; this effect was abrogated by miR-638 mimics
(upregulation of miR-638; Fig. 6C and
D). Similarly, overexpression of circTADA2A promoted migration
and invasion of A549 and H1299 cells but this effect was attenuated
by miR-638 mimics (Fig. 6E and F).
Overall, the present study indicated that circTADA2A promoted cell
proliferation and migration, at least partially, via the
miR-638/KIAA0101 pathway in A549 and H1299 cells.
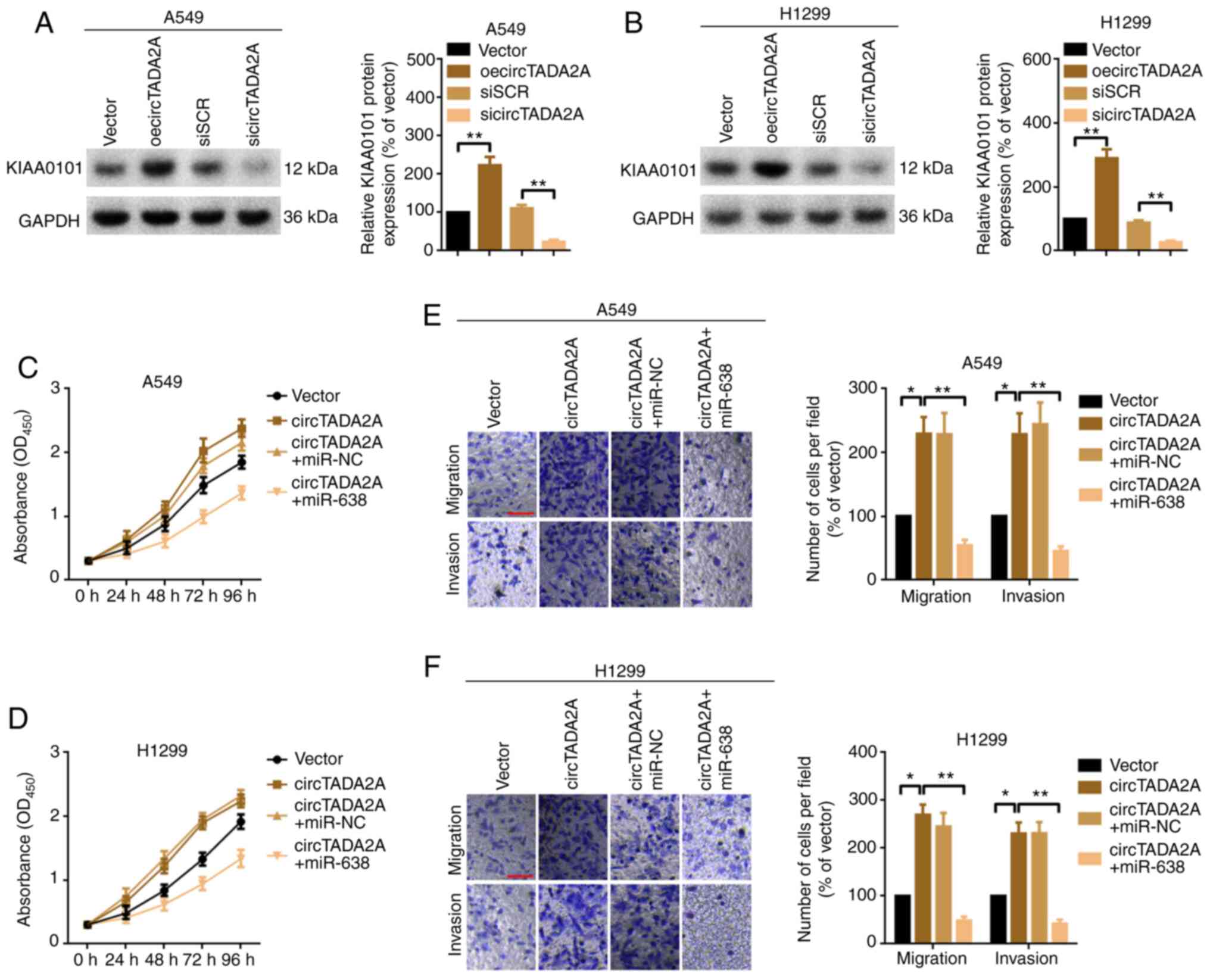 | Figure 6.circTADA2A promotes cell
proliferation and migration partially via the miR-638/KIAA0101
pathway in A549 and H1299 cells. KIAA0101 protein expression in (A)
A549 and (B) H1299 cells following treatment with circTADA2A was
evaluated by western blotting. Proliferation of (C) A549 and (D)
H1299 cells, as determined by Cell Counting Kit-8 assay. Migration
and invasion of (E) A549 and (F) H1299 cells were measured by
Transwell assay. Magnification, ×20; scale bar, 200 µm. All data
are presented as the mean ± SD (n=3). *P<0.05, **P<0.01.
circTADA2A, circular RNA transcription adaptor 2A; KIAA0101, PCNA
clamp associated factor; oe, overexpression; miR, microRNA; si,
small interfering; SCR, scramble; NC, negative control. |
Discussion
Increasing evidence indicates that non-coding RNAs
are involved in various human diseases, including cancer (33). As a novel type of non-coding RNAs,
circRNAs, which are abundant in the human transcriptome, exhibit
diverse biological functions associated with their specific
structural features (34–37). circTADA2A (circBase ID
hsa_circ_0043278) is derived from exons 5 and 6 of the TADA2A gene
(15). The role of circTADA2A in
cancer is not well known, and its function remains controversial.
Wu et al (15) reported that
circTADA2A is upregulated in osteosarcoma tissue specimens and cell
lines and serves as a tumor promoter and increases malignant tumor
behavior via miR-203a-3p/CREB3 signaling in osteosarcoma. In
another study, Xu et al (14)
found that circTADA2A is consistently and significantly decreased
in a large cohort of patients with breast cancer, and its
downregulation is associated with poor patient survival for
triple-negative breast cancer. In the present study, the
differentially expressed circRNAs in NSCLC were analyzed according
to the GEO dataset GSE101586. It was found that circTADA2A was
upregulated in five NSCLC tissue samples compared with paired
paratumor tissue. RT-qPCR verification revealed that circTADA2A was
upregulated in 38 NSCLC tissue samples and four NSCLC cell lines.
CCK-8 and Transwell assays verified that circTADA2A promoted NSCLC
cell proliferation and migration.
circRNAs function via multiple mechanisms, including
serving as sponges of miRNAs and proteins, protein translation and
derivation of pseudogenes (38).
Since the majority of circRNAs are located in the cytoplasm and
function as competitive endogenous (ce)RNAs for miRNAs, circRNA
research has attracted attention (39,40). Here,
RNA-FISH assay demonstrated that circTADA2A and miR-638 were
primarily located in the cytoplasm. miR-638, which is located at
human chromosome 19p13.2, is a well-known tumor-suppressor miRNA
that is implicated in multiple types of malignant tumor including
hepatocellular carcinoma, breast, gastric, cervical and colon
cancer, as well as glioma (41–46). Xia
et al (47) reported that
miR-638 is expressed at low levels in NSCLC, and downregulation of
miR-638 promotes proliferation, invasion and epithelial-mesenchymal
transition via SRY-box transcription factor 2 regulation in NSCLC.
In the present study, miR-638 was downregulated in NSCLC according
to analysis of the GEO datasets GSE74190 and GSE19945 and by
RT-qPCR. Furthermore, the expression of miR-638 was negatively
correlated with circTADA2A. Since the majority of circRNAs interact
with miRNAs by serving as ceRNAs (48), the present findings indicate a
biological basis for interaction between circTADA2A and miR-638, as
both were localized in the cytoplasm. RNA pull-down and luciferase
reporter assay confirmed that miR-638 targeted circTADA2A
directly.
KIAA0101 interacts with several miRNAs, and is
involved in cell cycle progression, cell proliferation, migration,
metastasis and chemoresistance in multiple types of cancer
(4,49–51). In
the present study, bioinformatics analysis and IHC detection
revealed that KIAA0101 was highly expressed in NSCLC. CCK-8 and
Transwell assays indicated that KIAA0101 was involved in
miR-638-mediated cell proliferation and migration. Luciferase
reporter assay found that KIAA0101 was a downstream target of
miR-638. The ceRNA hypothesis proposes that mRNAs, transcribed
pseudogenes and other RNA transcripts communicate via miRNA
response elements (52). The present
study indicated that circTADA2A communicated with KIAA0101 and
regulated KIAA0101-mediated proliferation and migration of A549 and
H1299 cells via miR-638.
In summary, the present study provides evidence that
circTADA2A promoted proliferation and migration via regulation of
the miR-638/KIAA0101 axis in NSCLC cells. circTADA2A and its
downstream miR-638/KIAA0101 signaling pathway may be novel
therapeutic targets for the molecular treatment of NSCLC.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Natural Science Foundation of Liaoning Province (grant no.
20180550318), Youth Talent Support Program of Liaoning Province
(grant no. XLYC1907011), Key R&D Program of Liaoning Province
(grant no. 2018225014) and Technological Innovation Fund of
Shenyang Technology Division (grant nos. RC190008 and
19-112-4-023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and YW conceived the study. YZ, HY, YL and LY
performed the experiments. XL and YZ confirmed the authenticity of
all the raw data. LZ and JC analyzed the data. YW wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institute
Research Medical Ethics Committee of Liaoning Cancer Hospital &
Institute. All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lei H, Wang K, Jiang T, Lu J, Dong X, Wang
F, Li Q and Zhao L: KIAA0101 and UbcH10 interact to regulate
non-small cell lung cancer cell proliferation by disrupting the
function of the spindle assembly checkpoint. BMC Cancer.
20:9572020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv W, Su B, Li Y, Geng C and Chen N:
KIAA0101 inhibition suppresses cell proliferation and cell cycle
progression by promoting the interaction between p53 and Sp1 in
breast cancer. Biochem Biophys Res Commun. 503:600–606. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain M, Zhang L, Patterson EE and Kebebew
E: KIAA0101 is overexpressed, and promotes growth and invasion in
adrenal cancer. PLoS One. 6:e268662011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tantiwetrueangdet A, Panvichian R,
Sornmayura P, Leelaudomlipi S and Macoska JA: PCNA-associated
factor (KIAA0101/PCLAF) overexpression and gene copy number
alterations in hepatocellular carcinoma tissues. BMC Cancer.
21:2952021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan S and Li X, Tie L, Pan Y and Li X:
KIAA0101 is associated with human renal cell carcinoma
proliferation and migration induced by erythropoietin. Oncotarget.
7:13520–13537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato T, Daigo Y, Aragaki M, Ishikawa K,
Sato M and Kaji M: Overexpression of KIAA0101 predicts poor
prognosis in primary lung cancer patients. Lung Cancer. 75:110–118.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patop IL, Wüst S and Kadener S: Past,
present, and future of circRNAs. EMBO J. 38:e1008362019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rybak-Wolf A, Stottmeister C, Glazar P,
Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss
R, et al: Circular RNAs in the mammalian brain are highly abundant,
conserved, and dynamically expressed. Mol Cell. 58:870–885. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Zhang L, Meng G, Wang Q, Lv X and
Zhang J: Circular RNAs: Pivotal molecular regulators and novel
diagnostic and prognostic biomarkers in non-small cell lung cancer.
J Cancer Res Clin Oncol. 145:2875–2889. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Yao H, Wang S, Li G and Gu X:
CircTADA2A suppresses the progression of colorectal cancer via
miR-374a-3p/KLF14 axis. J Exp Clin Cancer Res. 39:1602020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu JZ, Shao CC, Wang XJ, Zhao X, Chen JQ,
Ouyang YX, Feng J, Zhang F, Huang WH, Ying Q, et al: circTADA2As
suppress breast cancer progression and metastasis via targeting
miR-203a-3p/SOCS3 axis. Cell Death Dis. 10:1752019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y, Xie Z, Chen J, Ni W, Ma Y, Huang K,
Wang G, Wang J, Ma J, Shen S and Fan S: Circular RNA circTADA2A
promotes osteosarcoma progression and metastasis by sponging
miR-203a-3p and regulating CREB3 expression. Mol Cancer. 18:732019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong S, Wang J, Zhang Q, Xu H and Feng J:
CircPrimer: A software for annotating circRNAs and determining the
specificity of circRNA primers. BMC Bioinformatics. 19:2922018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T,
Sun H, Pan Y, He B and Wang S: CircHIPK3 promotes colorectal cancer
growth and metastasis by sponging miR-7. Cell Death Dis. 9:4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Lu Z, Wang N, Feng J, Zhang J,
Luan L, Zhao W and Zeng X: Long noncoding RNA DANCR promotes
colorectal cancer proliferation and metastasis via miR-577
sponging. Exp Mol Med. 50:1–17. 2018. View Article : Google Scholar
|
|
23
|
Wang Y, Yang T, Liu Y, Zhao W, Zhang Z, Lu
M and Zhang W: Decrease of miR-195 promotes chondrocytes
proliferation and maintenance of chondrogenic phenotype via
targeting FGF-18 pathway. Int J Mol Sci. 18:9752017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Zeng X, Wang N, Zhao W, Zhang X,
Teng S, Zhang Y and Lu Z: Long noncoding RNA DANCR, working as a
competitive endogenous RNA, promotes ROCK1-mediated proliferation
and metastasis via decoying of miR-335-5p and miR-1972 in
osteosarcoma. Mol Cancer. 17:892018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng Z, Yu C, Cui S, Wang H, Jin H, Wang
C, Li B, Qin M, Yang C, He J, et al: circTP63 functions as a ceRNA
to promote lung squamous cell carcinoma progression by upregulating
FOXM1. Nat Commun. 10:32002019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu S, Liu Z, Yang X, Zhou J, Yu H, Zhang R
and Li H: The emerging functions and roles of circular RNAs in
cancer. Cancer Lett. 414:301–309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma
JZ, Sun SH, Yang F and Zhou WP: Circular RNA cSMARCA5 inhibits
growth and metastasis in hepatocellular carcinoma. J Hepatol.
68:1214–1227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai H, Liu X, Zheng J, Xue Y, Ma J, Li Z,
Xi Z, Li Z, Bao M and Liu Y: Long non-coding RNA taurine
upregulated 1 enhances tumor-induced angiogenesis through
inhibiting microRNA-299 in human glioblastoma. Oncogene.
36:318–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song YX, Sun JX, Zhao JH, Yang YC, Shi JX,
Wu ZH, Chen XW, Gao P, Miao ZF and Wang ZN: Non-coding RNAs
participate in the regulatory network of CLDN4 via ceRNA mediated
miRNA evasion. Nat Commun. 8:2892017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nan A, Chen L, Zhang N, Jia Y, Li X, Zhou
H, Ling Y, Wang Z, Yang C, Liu S and Jiang Y: Circular RNA
circNOL10 inhibits lung cancer development by promoting
SCLM1-mediated transcriptional regulation of the humanin
polypeptide family. Adv Sci (Weinh). 6:18006542019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK and Hansen TB: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salzman J: Circular RNA Expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang
Y, Li X, Wu Z, Yang D, Zhou Y, et al: Circular RNAs in Cancer:
Emerging functions in hallmarks, stemness, resistance and roles as
potential biomarkers. Mol Cancer. 18:902019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui C, Yang J, Li X, Liu D, Fu L and Wang
X: Functions and mechanisms of circular RNAs in cancer radiotherapy
and chemotherapy resistance. Mol Cancer. 19:582020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Yang L and Chen LL: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Q, Li F, He AT and Yang BB: Circular
RNAs: Expression, localization, and therapeutic potentials. Mol
Ther. 29:1683–1702. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong
F, Ren D, Ye X, Li C, Wang Y, et al: Circular RNAs function as
ceRNAs to regulate and control human cancer progression. Mol
Cancer. 17:792018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng J, Chen Y, Zhao P, Li N, Lu J, Li J,
Liu Z, Lv Y and Huang C: Dysregulation of miR-638 in hepatocellular
carcinoma and its clinical significance. Oncol Lett. 13:3859–3865.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li M, Wang J and Liu H: Downregulation of
miR-638 promotes progression of breast cancer and is associated
with prognosis of breast cancer patients. Onco Targets Ther.
11:6871–6877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen Y, Chen H, Gao L, Zhang W, He J, Yang
X, Qin L, Xue X and Guo Z: MiR-638 acts as a tumor suppressor gene
in gastric cancer. Oncotarget. 8:108170–108180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei H, Zhang JJ and Tang QL: MiR-638
inhibits cervical cancer metastasis through Wnt/β-catenin signaling
pathway and correlates with prognosis of cervical cancer patients.
Eur Rev Med Pharmacol Sci. 21:5587–5593. 2017.PubMed/NCBI
|
|
45
|
Yan S, Dang G, Zhang X, Jin C, Qin L, Wang
Y, Shi M, Huang H and Duan Q: Downregulation of circulating
exosomal miR-638 predicts poor prognosis in colon cancer patients.
Oncotarget. 8:72220–72226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng DH, Wang X, Lu LN, Chen DL, Chen JM,
Lin FM and Xu XB: MiR-638 serves as a tumor suppressor by targeting
HOXA9 in glioma. Eur Rev Med Pharmacol Sci. 22:7798–7806.
2018.PubMed/NCBI
|
|
47
|
Xia Y, Wu Y, Liu B, Wang P and Chen Y:
Downregulation of miR-638 promotes invasion and proliferation by
regulating SOX2 and induces EMT in NSCLC. FEBS Lett. 588:2238–2245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu CY, Li TC, Wu YY, Yeh CH, Chiang W,
Chuang CY and Kuo HC: The circular RNA circBIRC6 participates in
the molecular circuitry controlling human pluripotency. Nat Commun.
8:11492017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen H, Xia B, Liu T, Lin M and Lou G:
KIAA0101, a target gene of miR-429, enhances migration and
chemoresistance of epithelial ovarian cancer cells. Cancer Cell
Int. 16:742016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jain N, Roy J, Das B and Mallick B:
miR-197-5p inhibits sarcomagenesis and induces cellular senescence
via repression of KIAA0101. Mol Carcinog. 58:1376–1388. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Samantarrai D and Mallick B: miR-429
inhibits metastasis by targeting KIAA0101 in soft tissue sarcoma.
Exp Cell Res. 357:33–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|