Introduction
Colorectal cancer (CRC) is a common malignancy of
the digestive system, with incidence and mortality rates that rank
third and second among all cancers worldwide, respectively
(1). Surgical resection and
chemoradiotherapy are the primary methods of CRC treatment.
However, despite advancements in these modalities, the therapeutic
effect on CRC is unsatisfactory, as the overall survival rate of
patients remains at ~65% (2).
Increasing evidence has verified the presence of cancer stem cells
(CSCs) in tumor tissues, which are the origin of cancers and
essential for metastasis, recurrence and drug resistance (3,4). CSCs have
the potential for high tumorigenicity, self-renewal and unlimited
proliferation, and exhibit the characteristics of normal cancer
cells (5–7). Conventional chemoradiotherapy is
effective against common cancer cells, but ineffective against
CSCs; thus, targeting CSCs has the potential to eradicate cancer at
the developmental stage (8–10). Although major advances have been made
in the molecular characterization of CSCs, the molecular regulation
of their tumor-initiation capacity is poorly understood. Therefore,
it is imperative to further investigate the molecular mechanisms of
CSCs to identify novel targets for CRC treatment.
Octamer-binding transcription factor 4-B1 (OCT4B1)
is involved in regulating the self-renewal of colorectal cancer
stem cells (CRCSCs), as was indicated in our previous study, in
which four groups of cells with different OCT4B1 expression levels
(different CSCs self-renewal ability) were established (11). This was later confirmed using an
Affymetrix microarray, where the expression trend of heterogeneous
nuclear ribonucleoprotein AB (hnRNPAB) was found to mirror that of
OCT4B1, while opposing that of miR-8063. Furthermore,
bioinformatics analysis indicated that miR-8063 and hnRNPAB may
share common binding sites (11).
hnRNPAB belongs to the family of heterogeneous
nuclear ribonucleoproteins (hnRNPs), a class of RNA-binding
proteins that are closely associated with the biological functions
of mRNAs. As such, hnRNPs are involved in nucleic acid metabolism,
including RNA splicing, maintenance of telomerase activity, cell
signal transduction, and regulation of transcription and
translation (12–14). hnRNPAB (also known as hnRNPA/B) is
divided into four subgroups: hnRNPA1, hnRNPA2/B1 (also known as
hnRNPA2 or hnRNPB1), hnRNPA3 and hnRNPA0 (12,15). While
hnRNPA1 and hnRNPA2/B1 have been widely studied, studies on hnRNPA0
and hnRNPA3 are limited. Notably, hnRNPAB and its subgroups are
closely associated with malignant biological behaviors such as
proliferation and apoptosis reduction, as well as poor patient
prognosis in various cancer types (16–21). Our
previous study confirmed the upregulation of hnRNPAB expression in
CRC tissues compared with adjacent tissues, which was closely
associated with poor prognosis (21).
hnRNPAB and its subgroups were also found to regulate the
epithelial-mesenchymal transition (EMT) of cancer cells, thus
promoting metastasis (22–24). Increasing evidence also indicates that
cancer cells can acquire CSC characteristics through EMT (25–27).
Therefore, hnRNPAB may be involved in the regulation of CRCSCs.
Emerging evidence has indicated that hnRNPAB and its
subgroups can regulate the expression of Wnt/β-catenin signaling
pathway proteins. Stockley et al (28) discovered that the colony-formation
ability of prostate cancer cells was decreased following
hnRNPA2/B1-knockdown, while the proliferative ability was improved
following overexpression of hnRNPA2/B1. These effects are related
to the enhanced expression of β-catenin mRNA by hnRNPA2/B1, hence
increasing the synthesis of β-catenin protein. Meng et al
(29) revealed that hnRNPA1 promoted
the differentiation of mesenchymal stem cells into cartilage cells,
which is associated with enhanced expression of Wnt3a, Wnt5a and
β-catenin. The Wnt/β-catenin signaling pathway is involved in the
regulation of various CSC types, which has been confirmed by
several studies (30–33). Therefore, hnRNPAB may regulate CRCSCs
through the Wnt/β-catenin signaling pathway.
MicroRNAs (miRNAs/miRs) are a class of small
non-coding single-stranded RNAs comprising ~18–25 nucleotides.
miRNAs regulate post-transcriptional gene expression by binding to
the 3′ untranslated region (3′-UTR) of specific mRNAs, resulting in
translational repression or mRNA degradation (34,35).
Previous studies have suggested that miRNAs also play an important
role in maintaining the self-renewal and drug resistance of CSCs
(36–41).
In view of our previous studies and the associated
literature, we hypothesize that miR-8063 may bind to hnRNPAB and
regulate its expression, promoting Wnt/β-catenin signaling pathway
activation to regulate the self-renewal of CRCSCs. Therefore, the
purpose of the present study was to determine whether miR-8063 and
hnRNPAB are involved in the regulation of CRCSC self-renewal, as
well as the underlying molecular mechanisms involved.
Materials and methods
Cell lines and 3D microsphere
culture
Human colorectal cancer cell lines (SW480 and HT29)
and 293T cells were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
L-15 or DMEM medium (Hyclone; Cytiva) containing 10% fetal bovine
serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.). On reaching
80% confluency, SW480 and HT29 cells in the logarithmic growth
phase were seeded into low-adhesion 12-well plates
(1×105 cells/well) and maintained in stem cell culture
medium: 25 µl B27 (1:50; Gibco; Thermo Fisher Scientific, Inc.), 20
µl EGF (20 ng/ml; Invitrogen; Thermo Fisher Scientific, Inc.), 15
µl basic fibroblast growth factor (bFGF; 10 ng/ml; Invitrogen;
Thermo Fisher Scientific, Inc.) and 940 µl L-15 medium. The stem
cell culture medium was replenished every 72 h, and the cells were
cultured continuously for 14 days; the resulting SW480-3D and
HT29-3D cell microspheres were maintained in stem cell culture
medium and harvested for subsequent experimentation as
required.
Patients and tissue samples
A total of 118 primary CRC and paired-adjacent
tissue samples were collected from patients with CRC at the
Affiliated Hospital of Zunyi Medical University (Zunyi, China), who
underwent radical resection in a blinded manner between January
2015 and December 2015. The patient data are presented in Table I. CRC diagnosis was based on
histopathological features. No patient underwent radiotherapy or
chemotherapy preoperatively. Following surgery, the tissue samples
were immediately stored at −80°C for reverse
transcription-quantitative (RT-q)PCR analysis. A 5-year follow-up
survey of the patients was performed to determine their survival
status. The present study was reviewed and approved by the Ethics
Review Committee of the Affiliated Hospital of Zunyi Medical
University (approval no. [2015] 1-040), and written informed
consent was obtained from all patients.
 | Table I.Association between miR-8063
expression and the clinicopathological characteristics of patients
with colorectal cancer. |
Table I.
Association between miR-8063
expression and the clinicopathological characteristics of patients
with colorectal cancer.
|
| microRNA-8063
expression |
|---|
|
|
|
|---|
| Clinicopathological
characteristics | Category | cases (n=118) | Low (n=52) | High (n=66) |
χ2-value | P-value |
|---|
| Age, years | <60 | 42 | 20 | 22 | 0.334 | 0.564 |
|
| ≥60 | 76 | 32 | 44 |
|
|
| Sex | Male | 65 | 28 | 37 | 0.058 | 0.810 |
|
| Female | 53 | 24 | 29 |
|
|
| Tumor site | Rectum | 89 | 41 | 48 | 0.587 | 0.443 |
|
| Colon | 29 | 11 | 18 |
|
|
| Tumor
infiltration | T1+T2 | 33 | 21 | 12 | 7.117 | 0.008 |
|
| T3+T4 | 85 | 31 | 54 |
|
|
| Vascular
invasion | Negative | 48 | 28 | 20 | 6.681 | 0.010 |
|
| Positive | 70 | 24 | 46 |
|
|
| Differentiation
status | High +
moderate | 34 | 18 | 16 | 1.526 | 0.217 |
|
| poor | 84 | 34 | 50 |
|
|
| Lymph node
metastasis | Negative | 43 | 25 | 18 | 5.435 | 0.020 |
|
| Positive | 75 | 27 | 48 |
|
|
| TNM stage | I+II | 44 | 27 | 17 | 8.515 | 0.004 |
|
| III+IV | 74 | 25 | 49 |
|
|
RT-qPCR
Total RNA from tissues and cells was extracted using
TRIzol® reagent according to the manufacturer's
instructions (Takara Biotechnology Co., Ltd.), and reverse
transcribed into cDNA using the Prime Script RT kit (Takara
Biotechnology Co., Ltd.). Subsequent qPCR detection was performed
using the 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with a 20 µl reaction mixture comprising
10 µl qPCR SYBR Green Mix (Takara Biotechnology Co., Ltd.), 0.8 µl
each of the forward and reverse primers, 2 µl cDNA and diethyl
pyrocarbonate-treated H2O up to the final volume. The
qPCR conditions were: Initial denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec, annealing
at 60°C for 30 sec and melting curve analysis. Each test set
included three duplicate wells, and the experiment was repeated
three times. Relative gene expression was normalized to that of
GAPDH or U6, and calculated using the 2−ΔΔCq method
(42). The primers were designed and
synthesized by General Biosystems, Inc., the sequence of which are
as follows: miR-8063 forward, 5′-TGCGGTCAAAATCAGGAGTCGGGG-3′ and
reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′; U6 forward,
5′-GCTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGTG-3′;
hnRNPAB forward, 5′-AAGAAGTCTATCAGCAGCAGCAGTATG-3′ and reverse,
5′-CTCCACCTCCACCACCACCTC-3′; and GAPDH forward,
5′-ATGACATCAAGAAGGTGGTGAAGCAGG-3′ and Reverse,
5′-GCGTCAAAGGTGGAGGAGTGGG-3′.
Western blotting
Total cellular protein was extracted, and its
concentration determined, using the Protein Extraction kit and the
BCA protein concentration kit (both Beyotime Institute of
Biotechnology), respectively. Protein samples (40 µg) were
separated using 10% SDS-PAGE and transferred to a PVDF membrane.
After blocking with 5% skim milk for 1 h at room temperature, the
membrane was incubated overnight at 4°C with anti-hnRNPAB (cat. no.
14813-1-AP; 1:2,000), anti-Wnt3a (cat. no. 26744-1-AP; 1:2,000),
anti-Wnt5a (cat. no. 55184-1-AP; 1:2,000), anti-β-catenin (cat. no.
17565-1-AP; 1:2,000) and anti-β-tubulin (cat. no. 10094-1-AP;
1:5,000) (all ProteinTech Group, Inc.). The following day, the
membrane was washed three times with PBS containing 0.1% Tween-20,
and incubated with a horseradish peroxidase-conjugated antibody
solution (cat. no. SA00001-2; 1:10,000; ProteinTech Group, Inc.)
for 1 h at room temperature. Protein bands were visualized using
Super ECL plus super-sensitive luminescent solution (Bio-Rad
Laboratories, Inc.) and exposed using X-ray film. Quantity One
software v4.6.6 (Bio-Rad Laboratories, Inc.) was used to quantify
band intensities.
Dual-luciferase reporter assay
The target gene of hnRNPAB (miR-8063) was predicted
using TargetScan (http://www.targetscan.org/vert_71/) bioinformatics
software. The wild-type plasmid pmirGLO/hnRNPAB-3′UTR and the
mutant plasmid pmirGLO/hnRNPAB-3′UTR-mut were constructed by
General Biosystems, Inc. 293T cells (1×105/well) were
seeded into 24-well plates 24 h before transfection. Cells were
co-transfected with pmirGLO/hnRNPAB-3′UTR or
pmirGLO/hnRNPAB-3′UTR-mut and miR-8063 mimics using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After transfection for 48 h, the dual-luciferase
reporter assay kit (Beyotime Institute of Biotechnology) was used
according to the manufacturer's instructions, and the relative
light unit (RLU) value was determined using the FLx800 fluorescence
analyzer (BioTek Instruments, Inc.). The firefly RLU value was
normalized to that of Renilla luciferase.
Colony formation assay
A soft agar colony-formation assay was used to
evaluate the CSC characteristics of HT29-3D microspheres, and the
self-renewal ability of CRCSCs (SW480CSCs and HT29CSCs) after
miR-8063 overexpression. First, two different concentrations of
soft agarose (0.6 and 1.2%) were prepared. The 3D microspheres and
corresponding parent cells were digested into a single cell
suspension using Accutase enzyme (PAN-Biotech) and 0.25% trypsin
(Hyclone; Cytiva), respectively. The digested cells were then
resuspended in a medium containing 10% FBS (1×103
cells/ml), and mixed with 1.2% agarose at a 1:1 ratio. Then, 3 ml
of the mixture was added to each 6-cm-diameter glass dishes, and
left to solidify to form the lower agar layer. Medium and 0.7%
agarose were then mixed at a 1:1 ratio, and 0.5 ml cell suspension
was added to the mix, which was then added atop the previously
prepared 1.2% agarose to form the upper agar layer. Finally, the
culture dishes were incubated at 37°C for 2 weeks. The number of
colonies in each plate was counted using a light microscope.
For parent cells (SW480 and HT29), a plate
colony-formation assay was used to detect CSC characteristics after
hnRNPAB overexpression. Cells were seeded into 6-well plates
(1×103/well) in 1 ml medium containing 10% FBS, and
cultured at 37°C for 2 weeks. Next, 1 ml 4% paraformaldehyde was
added as a fixative at room temperature for 15 min, and 1 ml Giemsa
dye was then added for 15 min at room temperature. The number of
colonies was manually counted using a light microscope (>50
cells are considered a clone). Colony-formation rate=number of
clones/number of seeded cells ×100%.
In vivo tumorigenicity
A total of 12 male specific pathogen-free-grade
NOD/SCID mice (weight, 20–25 g; age, 6 weeks) and 44 male BALB/C
nude mice (weight, 15–20 g; age, 6 weeks) were used in the
experiment. All mice were housed at 25°C, 50% humidity and in
specific-pathogen-free conditions with a 12/12-h light/dark cycle.
Sterile food and water were provided daily. NOD/SCID mice and nude
mice were purchased from the Animal Experimental Center, Institute
of Radiology Medicine, Chinese Academy of Medical Sciences
(Tianjin, China). NOD/SCID mice were used to evaluate the
tumorigenicity of HT29-3D microspheres in vivo. HT29-3D
microspheres and HT29 cells were harvested and counted. Then,
1×107 cells were collected and resuspended in 1 ml
medium; ~100 µl suspension (containing 1×106 cells) was
subcutaneously injected into the left armpits of NOD/SCID mice (six
mice in each group). Similarly, 5×106 SW480 and HT29
cells (overexpressing hnRNPAB), SW480CSCs and HT29CSCs cells
(overexpressing the miR-8063), and the corresponding NC cells, were
subcutaneously injected into the left armpits of the nude mice.
When the tumors had reached a maximum diameter of 15 mm, the mice
were anesthetized with an intraperitoneal injection of 1%
Pentobarbital (50 mg/kg), and then sacrificed by cervical
dislocation. Tumor volume was calculated using the following
formula: Tumor volume = ½ (length × width2) (43).
Flow cytometric analysis
The expression of CSC markers was detected using
flow cytometry. Cells in the logarithmic growth phase were
homogenized into a single cell suspension. Then, 1×106
cells were resuspended in 100 ml PBS containing 5% bovine serum
albumin and 10 µl fluorophore-conjugated primary anti-CD44-PE (cat.
no. PE-6506), anti-CD133-PE (cat. no. PE-62403) and the
corresponding negative control antibodies (cat. no. PE-48642) (all
ProteinTech Group, Inc.). The cells were mixed and incubated for 10
min in the dark at 4°C. After centrifugation (500 × g, 5 min at
room temperature), the cells were washed three times with PBS and
resuspended in 200 µl PBS each. Finally, NovoExpress software 1.2.4
(Novocyte; ACEA Biosciences, Inc.) was used to detect the
percentage of fluorescence-positive cells.
Cell infection
The lentiviruses overexpressing hnRNPAB
(hnRNPAB-GFP-PURO), miR-8063-mimic-GFP-PURO and
miR-8063-inhibitor-GFP-PURO were purchased from Hanbio
Biotechnology Co., Ltd. The miR-8063-inhibitor-GFP-PURO lentiviral
vector sequence was as follows: 5′-UUCGGGGCUGAGACUAAAACU-3′. Cells
(1×105) were seeded into 12-well plates 24 h before
infection. The virus was used to infect the cells according to the
optimal MOI value obtained in the pre-experiment: Lentivirus
hnRNPAB-GFP-PURO (MOI of 30 for both SW480 and HT29),
miR-8063-mimic-GFP-PURO (MOI of 20 for SW480CSCs, and 30 for
HT29CSCs), and miR-8063-inhibitor GFP-PURO (MOI 20 for both SW480
and HT29). Then, 8 mg/ml Polybrene (Hanheng Biological Technology
Co., Ltd.) and enhanced infection solution (Shanghai GeneChem Co.,
Ltd.) were added at 37°C for 12 h, and then replaced with fresh
medium. After a further 72 h, the cells were collected, and the
effect of gene overexpression or silencing was determined by
RT-qPCR or western blotting.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Multigroup comparisons were conducted by one-way ANOVA
followed by Tukey's post hoc test. The χ2 test was used
to determine the association between miR-8063 expression and
patient clinical characteristics. The Kaplan-Meier method was used
to assess the association between overall survival rate and the
expression level of miR-8063. P<0.05 was considered to indicate
a statistically significant difference.
Results
SW480-3D and HT29-3D microspheres
exhibit CSC properties
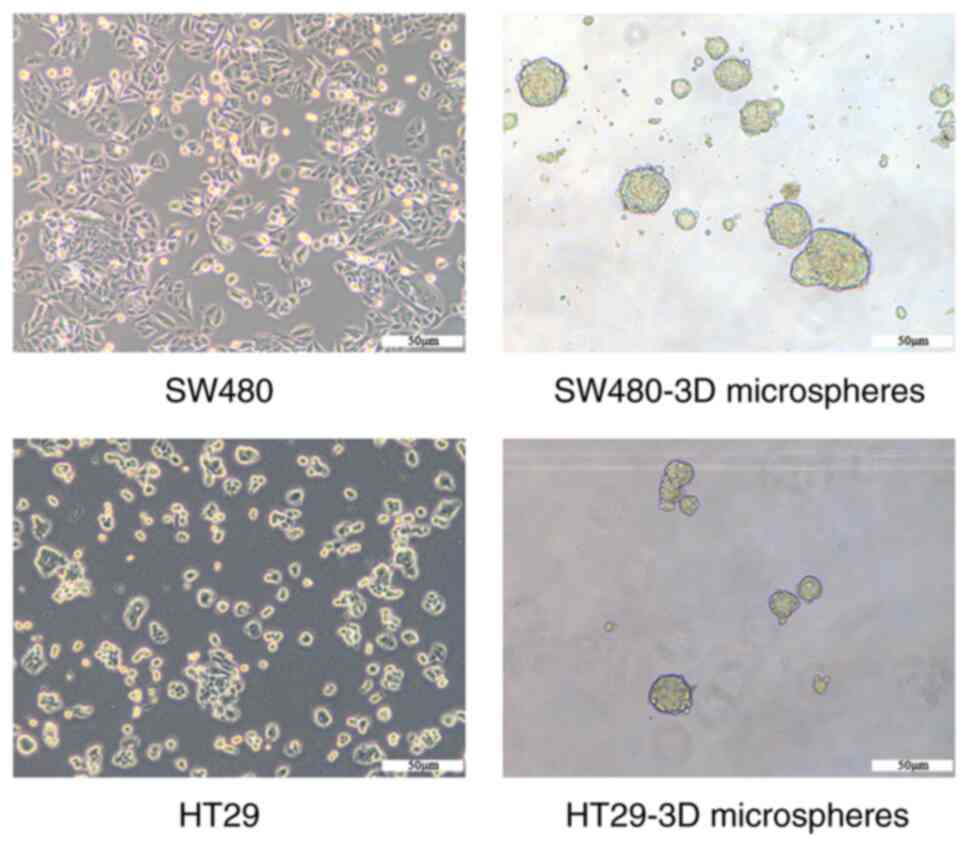
SW480-3D and HT29-3D microspheres were generated
using a suspension culture of human CRC cells (SW480 and HT29;
Fig. 1). A colony formation assay was
performed to verify the self-renewal properties of the HT29-3D
microspheres, and the results indicated that the colony-formation
rates of HT29-3D microspheres and HT29 cells (45.67±9%) were
significantly higher than those of the parent cells (16.98±5%)
(Fig. 2A and B) (P<0.01). Next,
the tumorigenicity of HT29-3D microspheres was detected using an
in vivo tumorigenicity assay in NOD/SCID mice. The tumor
volume of the mice inoculated with HT29-3D microspheres was
significantly larger than that of the HT29 cell group, suggesting
that HT29-3D microspheres had greater tumorigenicity than the
parent cells (Fig. 2C and D)
(P<0.01). Finally, the results of flow cytometry showed that the
positive expression rates of CD44 in HT29-3D microspheres and HT29
cells were 37.14±1.62 and 9.37±2.28%, respectively. The
corresponding positive expression rates of CD133 were 38.40±1.74
and 3.64±0.95%, respectively. These findings demonstrate
significantly higher expression of CD44 and CD133 in HT29-3D
microspheres compared with the parent cells (Fig. 2E and F) (P<0.01). The results
suggest that HT29 microspheres exhibit CSC characteristics.
SW480-3D microspheres with CSC characteristics were identified and
named SW480CSCs in our previous study (11). Similarly, HT29-3D microspheres were
termed HT29CSCs.
miR-8063 expression is downregulated,
while hnRNPAB expression is upregulated in SW480CSCs and
HT29CSCs
RT-qPCR detection revealed that the relative
expression levels of miR-8063 in SW480 and SW480CSCs were 1.03±0.04
and 0.45±0.06, respectively. miR-8063 expression in HT29 and
HT29CSCs were 0.99±0.11 and 0.32±0.12, respectively (Fig. 3A) (P<0.01). Furthermore, relative
hnRNPAB mRNA expression in SW480CSCs and HT29CSCs, compared with
the parental cells, was 2.6- and 3.2-fold, respectively, as
detected by RT-qPCR. (Fig. 3B).
Moreover, the expression of hnRNPAB protein in SW480, SW480CSCs,
HT29 and HT29CSCs were 0.74±0.04, 1.04±0.06, 0.68±0.03 and
0.98±0.07, respectively (Fig. 3C and
D). These results indicate that compared with the parent cells,
the expression level of miR-8063 in SW480CSCs and HT29CSCs was
significantly decreased, while hnRNPAB expression was significantly
increased (P<0.01).
miR-8063 expression is downregulated
in human CRC tissues, which is associated with poor overall
survival in patients with CRC
The patient population comprised 65 men and 53 women
(mean age, 58 years; age range, 30–82 years). Compared with the
adjacent tissues, miR-8063 expression in CRC tissues was
significantly downregulated (Fig. 4A;
P<0.001). Next, the expression levels were classified as low and
high according to the median value (0.62), and association between
miR-8063 expression and patient clinicopathological characteristics
was determined. As shown in Table I,
low miR-8063 expression was significantly associated with advanced
TNM stage (P=0.004), tumor infiltration (P=0.008), vascular
invasion (P=0.010) and lymph node metastasis (P=0.020). No
association was found between miR-8063 expression and age, sex,
differentiation and tumor site. These data indicated that low
expression of miR-8063 was closely associated with the malignant
behaviors of CRC. The relationship between miR-8063 expression and
the overall survival rate of patients was estimated using the
Kaplan-Meier method; the overall survival rate of CRC patients with
high miR-8063 expression was lower than those with low miR-8063
expression, suggesting an association between high expression and
poorer prognosis (Fig. 4B;
P=0.023).
hnRNPAB is a direct target gene of
miR-8063
To verify whether there is a direct relationship
between miR-8063 and hnRNPAB, bioinformatics software (TargetScan
Human) was used to predict common binding sites between the two
molecules. The results showed that the hnRNPAB gene possesses a
potential miR-8063 binding sequence in its 3′UTR region (Fig. 5A). Furthermore, the dual-luciferase
reporter assay revealed that compared with the other groups, the
relative RLU value of the pmirGLO/hnRNPAB-3′UTR+miR-8063 mimics
group was significantly lower (P<0.01), confirming that hnRNPAB
is the direct target gene of miR-8063 (Fig. 5B and C) (P<0.01).
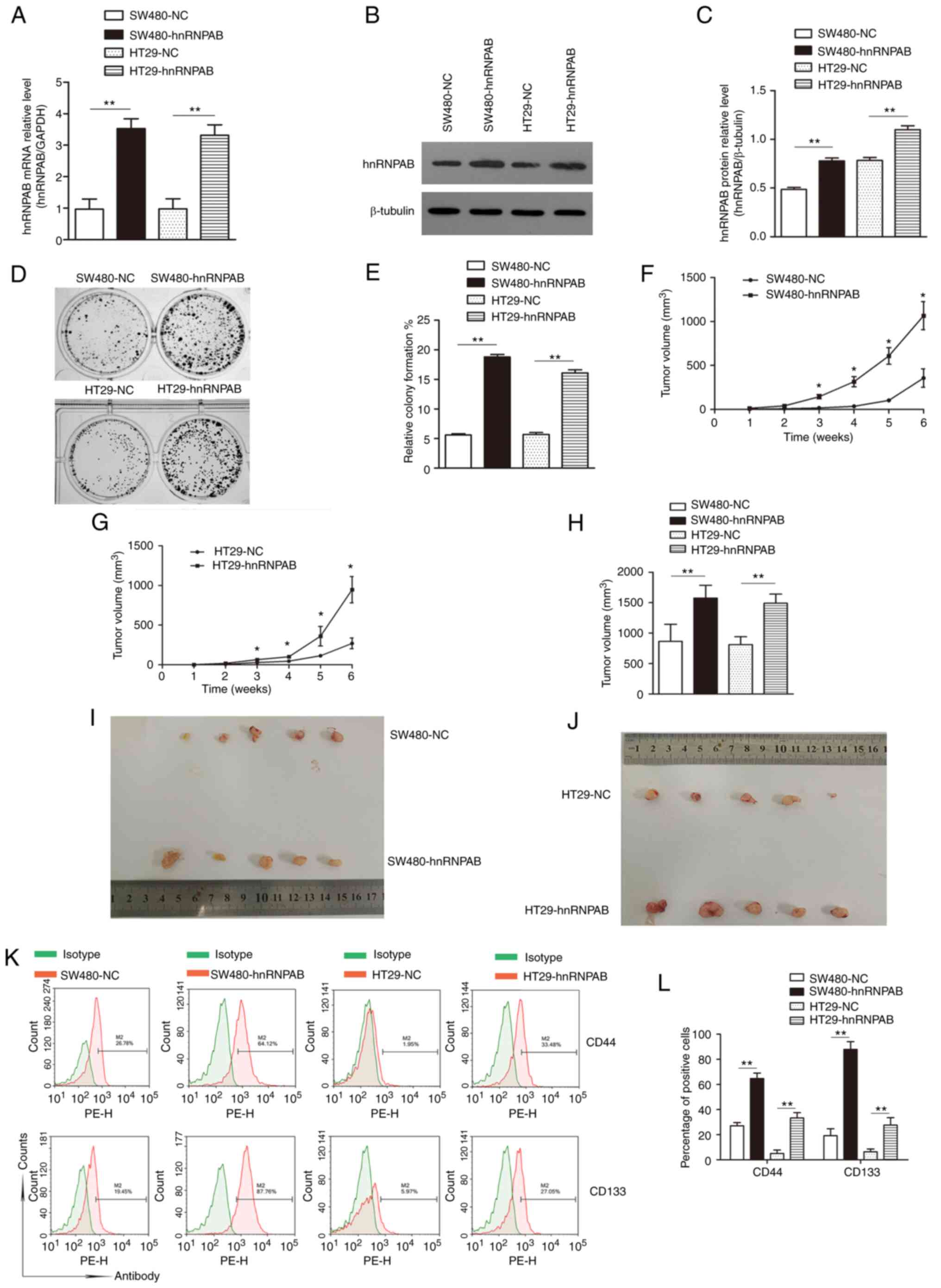
Overexpression of hnRNPAB promotes the
acquisition of stemness in SW480 and HT29 cells
To investigate the effect of hnRNPAB on the stemness
of CRC cells, a lentiviral vector overexpressing hnRNPAB
(hnRNPAB-GFP-PURO) and the corresponding NC (NC-GFP-PURO) were
transfected into SW480 and HT29 cells, and the experimental groups
(SW480-hnRNPAB and HT29-hnRNPAB) and the NC group (SW480-NC and
HT29-NC) were established. First, overexpression efficiency was
tested. The hnRNPAB mRNA expression in the SW480-hnRNPAB and
HT29-hnRNPAB groups was 3.5-fold and 3.3-fold, respectively,
compared with that of the NC groups (Fig.
6A). The expression of hnRNPAB protein also showed a similar
trend (Fig. 6B and C) (P<0.01).
These results indicated that the hnRNPAB overexpression model was
successfully constructed.
Furthermore, the colony formation rates of the
SW480-hnRNPAB, SW480-NC, HT29-hnRNPAB and HT29-NC groups were
18.8±0.42, 5.6±0.21, 16.1±0.53 and 5.7±0.31%, respectively,
indicating a significant increase in the colony-formation ability
of SW480 and HT29 cells following hnRNPAB overexpression (Fig. 6D and E) (P<0.01).
The results of tumor formation in nude mice showed
that all groups formed tumors, though the SW480-hnRNPAB and
HT29-hnRNPAB groups exhibited increased tumor volumes compared with
the NC groups (Fig. 6F and G).
Similarly, the SW480-hnRNPAB and HT29-hnRNPAB groups possessed a
significantly greater tumor volume than the NC-treated mice
(Fig. 6H-J). These findings suggested
that the tumorigenicity of CRC cells was significantly increased
following hnRNPAB overexpression.
In addition, the proportions of CD44+
cells in the SW480-NC, SW480-hnRNPAB, HT29-NC and HT29-hnRNPAB
groups were determined by flow cytometry, and were 27.05±2.57,
64.68±4.40, 5.08±2.72 and 33.27±5.27%, respectively. The
corresponding proportions of CD133+ cells were
19.19±5.49, 87.88±6.18, 6.37±2.26 and 27.62±5.89% respectively
(Fig. 6K and L) (P<0.01). These
results suggested that the positive expression rates of CD44 and
CD133 in the SW480-hnRNPAB and HT29-hnRNPAB groups were
significantly greater than those of the corresponding NC
groups.
Overexpression of hnRNPAB activates
the Wnt/β-catenin signaling pathway
The relative protein expression levels of Wnt3a in
the SW480-NC, SW480-hnRNPAB, HT29-NC and HT29-hnRNPAB groups were
0.27±0.03, 0.87±0.04, 0.52±0.04 and 0.91±0.03, respectively; and
those of Wnt5a were 0.37±0.04, 0.92±0.05, 0.28±0.05 and 1.01±0.06,
respectively. The corresponding protein expression levels of
β-catenin in the four groups were 0.61±0.06, 0.96±0.06, 0.59±0.04
and 0.99±0.15, respectively. Thus, compared with the NC groups, the
expression of Wnt/β-catenin pathway proteins in the experimental
groups was significantly increased (Fig.
7A and B) (P<0.01).
Overexpression of miR-8063 inhibits
self-renewal of SW480CSCs and HT29CSCs
SW480CSCs and HT29CSCs (obtained from SW480 and HT29
cells by suspension culture) were transfected with a lentiviral
vector overexpressing miR-8063 and the corresponding NC. The
experimental groups (SW480CSCs-miR-8063 and HT29CSCs-miR-8063) and
NC groups (SW480CSCs-NC and HT29CSCs-NC) were established. Compared
with the NC group, the SW480CSCs-miR-8063 and HT29CSCs-miR-8063
groups exhibited 2.7-fold and 3.3-fold relative expression of
miR-8063, respectively, as detected by RT-qPCR (Fig. 8A; P<0.01).
The colony formation rates of the
SW480CSCs-miR-8063, SW480CSCs-NC, HT29CSCs-miR-8063 and HT29CSCS-NC
groups were 17.43±4.30, 42.59±3.48, 39.52±7.28, and 74.93±8.52%,
respectively. Compared with those in the NC groups, the colony
formation rates in the experimental groups were significantly
decreased (Fig. 8B and C)
(P<0.01). These results showed that following miR-8063
overexpression, the colony-formation ability of SW480CSCs and
HT29CSCs was significantly decreased.
Following subcutaneous inoculation, the subcutaneous
tumor growth of nude mice was observed weekly, and a growth curve
was plotted (Fig. 8D and E). While
all the groups formed tumors, the tumor volumes in the
SW480CSCs-miR-8063 and HT29CSCs-miR-8063 groups were significantly
lower than those of the corresponding NC-treated mice (Fig. 8F and G), suggesting that the
tumorigenic ability of SW480CSCs and HT29CSCs in nude mice was
significantly reduced by the overexpression of miR-8063.
The positive expression rates of CD44 in the
SW480CSCs-miR-8063, SW480CSCs-NC, HT29CSCs-miR-8063 and HT29CSCs-NC
groups were 3.35±1.87, 24.86±3.27, 6.42±1.3 and 35.06±2.40%,
respectively. The corresponding positive expression rates of CD133
were 31.33±2.61, 87.91±4.99, 10.47.±2.52 and 33.07±3.86%,
respectively (Fig. 8H and I)
(P<0.01). These results indicate that the expression of
CD44+ and CD133+ in the SW480CSCS-miR-8063
and the HT29CSCS-miR-8063 groups was significantly lower than that
in the NC groups.
Overexpression of miR-8063 inhibits
the expression of hnRNPAB, thus inhibiting the Wnt/β-catenin
signaling pathway
The mRNA expression levels of hnRNPAB in the
SW480CSCS-NC, SW480CSCS-miR-8063, HT29CSCS-NC and HT29CSCS-miR-8063
groups were 1.03±0.11, 0.68±0.04, 0.96±0.05 and 0.53±0.02,
respectively (Fig. 9A; P<0.01).
The same trend was observed for protein expression of hnRNPAB
(Fig. 9B and C; P<0.01). These
results indicated that overexpression of miR-8063 inhibited the
hnRNPAB expression.
Next, the relative protein expression of Wnt3a in
the SW480CSCS-NC, SW480CSCS-miR-8063, HT29CSCS-NC and
HT29CSCS-miR-8063 groups was 0.68±0.09, 0.47±0.04, 1.10±0.13 and
0.65±0.047, respectively. Concerning Wnt5a, the relative protein
expression in the four groups was 0.49±0.03, 0.29±0.06, 0.85±0.15
and 0.52±0.06, respectively. The corresponding relative expression
of the β-catenin protein was 0.68±0.04, 0.46±0.08, 0.71±0.05 and
0.58±0.03, respectively. Compared with the corresponding NC group,
the expression levels of the three proteins in the
SW480CSCs-miR-8063 and HT29CSCs-miR-8063 groups were significantly
decreased (Fig. 9B and C; P<0.01).
These results suggest that the overexpression of the miR-8063 gene
inhibited the Wnt/β-catenin signaling pathway.
Silencing miR-8063 promotes the
expression of hnRNPAB and activates the Wnt/β-catenin signaling
pathway
miR-8063 was silenced in SW480 and HT29 cells using
the lentiviral-mediated RNA interference (RNAi) technique, and
experimental groups (SW480-sh-miR-8063 and HT29-sh-miR-8063) and NC
groups (SW480-sh-NC and HT29-sh-NC) transfected with NC virus were
established. Compared with the NC groups, the relative expression
of miR-8063 in the experimental groups was significantly decreased
(Fig. 10A; P<0.01). The results
of RT-qPCR showed that compared with the NC group, the relative
expression of hnRNPAB mRNA in the SW480-sh-miR-8063 and
HT29-sh-miR-8063 groups was 2.9-fold and 5.7-fold, respectively
(Fig. 10B; P<0.01). Similarly,
the expression of hnRNPAB protein (detected by western blotting)
was also significantly upregulated compared with that of the NC
groups (Fig. 10C and D). These
results indicated that hnRNPAB expression was upregulated following
miR-8063 silencing. Next, we examined whether the upregulation of
hnRNPAB expression induced by silencing miR-8063 was accompanied by
the activation of the Wnt/β-catenin signaling pathway. Compared
with the NC groups, the relative expression levels of Wnt3a, Wnt5a
and β-catenin in the SW480CSCs-miR-8063 and HT29CSCs-miR-8063
groups were significantly increased (Fig. 10C and D) (P<0.01). These findings
suggest that miR-8063 silencing promoted the expression of hnRNPAB,
resulting in the activation of the Wnt/β-catenin signaling
pathway.
 | Figure 10.Expression of hnRNPAB, Wnt3a, Wnt5a
and β-catenin after silencing miR-8063 in SW480 and HT29 cells. (A)
Relative expression level of miR-8063 in SW480-sh-miR-8063 and
HT29-sh-miR-8063 groups was detected using RT-qPCR, compared with
those of the NC groups. (B) hnRNPAB mRNA expression in
SW480-sh-miR-8063 and HT29-sh-miR-8063 groups was detected by
RT-qPCR, compared with the NC groups. (C and D) Protein expression
of hnRNPAB, Wnt3a, Wnt5a and β-catenin in SW480-sh-miR-8063 and
HT29-sh-miR-8063 groups was detected by western blotting, compared
with the NC groups. **P<0.01. hnRNPAB, heterogeneous nuclear
ribonucleoprotein AB; miR, microRNA; NC, negative control; CSC,
cancer stem cells; RT-q, reverse transcription-quantitative. |
Discussion
According to the CSC theory, these cells are the
root cause of cancer recurrence, metastasis and drug resistance.
Therefore, direct targeting of CSCs is predicted to reverse
chemotherapeutic resistance and prevent cancer recurrence and
metastasis, thus potentially eradicating the associated cancers.
Therefore, the regulatory mechanism and related therapeutic targets
of CSCs are the focus of current cancer research (44). Due to the small proportion of CSCs in
cancer tissue, the enrichment of these cells for further research
is a challenge. The enrichment of CSCs using chemotherapeutics
(45) and suspension culture
(11) has been described in our
previous study. In addition, various studies have indicated that
CSCs can be enriched by flow cytometry (46) or magnetic activated cell sorting
(47) for specific CSC markers.
Due to morphological similarities, it is difficult
to distinguish CSCs from tumor cells; hence, the identification of
CSCs is largely based on their self-renewal ability, tumorigenicity
after transplantation into immunodeficient mice, and the expression
level of specific cell surface markers (48,49). In
vivo and in vitro experiments can be performed for CSC
identification and characterization. For in vivo
experiments, CSCs can form tumors when inoculated into
immunodeficient mice, while general cancer cells cannot, or possess
weak tumorigenic ability. By contrast, in vitro experiments
encompass the use of soft agar colony formation to verify the
self-renewal ability of CSCs; CSCs can form colonies on soft agar
medium, but general cancer cells do not, or have reduced ability to
form colonies. In addition, CSCs possess stemness, and the
expression of specific cell surface markers is significantly
upregulated. Following in-depth research, CD44 and CD133 are
recognized markers of colorectal cancer stem cells (50,51).
Furthermore, the SW480-3D microspheres obtained by suspension
culture of SW480 cells (in serum-free medium containing growth
factors such as EGF and bFGF) were confirmed to exhibit CSC
characteristics in our previous study (11). In the present study, a soft agar
colony formation experiment, flow cytometric detection of CSC
markers CD44 and CD133, and NOD/SCID mouse tumorigenesis studies
were used to determine whether HT29-3D microspheres exhibited CSC
characteristics. Compared with parental cells, HT29-3D microspheres
exhibited significantly enhanced colony formation ability (Fig. 2A and B), NOD/SCID mice had
significantly enhanced tumorigenic ability (Fig. 2C and D), and CD44 and CD133 had
significantly upregulated positive expression rates (Fig. 2E and F). The results also showed that
HT29-3D microspheres exhibited CSC characteristics.
Next, the expression levels of miR-8063 and hnRNPAB
in SW480CSCs and HT29CSCs were detected to verify our previous
microarray screening results. Compared with the parent cells, the
expression of miR-8063 in SW480CSCs and HT29CSCs was significantly
decreased (Fig. 3A), while hnRNPAB
expression was significantly upregulated (Fig. 3B-D). It was also confirmed that the
expression trend of hnRNPAB and OCT4B1 in CRCSCs was consistent,
while hnRNPAB and miR-8063 showed the opposite expression
trend.
hnRNPAB is highly expressed in CRC and is closely
associated with cancer stage and poor prognosis, as indicated in
our previous study (21). To verify
miR-8063 expression in CRC tissues, and its relationship with
prognosis, the expression level of miR-8063 was determined in CRC
tissues by RT-qPCR. The results showed that compared with the
adjacent tissues, miR-8063 expression in CRC tissues was
significantly downregulated (Fig.
4A), which was associated with advanced TNM stage, tumor
infiltration, vascular invasion and lymph node metastasis (Table I). Furthermore, patients with low
miR-8063 expression had a worse prognosis (Fig. 4B). These results indicate that
miR-8063 may be a tumor suppressor gene that negatively regulates
the progression of CRC.
Using bioinformatics software, the existence of
binding sites between miR-8063 and hnRNPAB was predicted (Fig. 5A), and through dual-luciferase
experiments, hnRNPAB was further confirmed as a downstream gene of
miR-8063 (Fig. 5B and C). Then, SW480
and HT29 cells were infected with a lentiviral vector
overexpressing hnRNPAB. Consequently, the changes in CSC
characteristics were assessed by plate colony formation assays,
nude mouse tumor formation assays and flow cytometry. Compared with
the NC groups, the overexpression of hnRNPAB significantly
increased colony formation ability (Fig.
6D and E), tumorigenic ability in nude mice (Fig. 6H-J), and the positive expression rates
of CSC markers, CD44 and CD133 (Fig. 6K
and L). These results suggest that overexpression of hnRNPAB
promotes the acquisition of CSC characteristics in SW480 and HT29
cells. Moreover, the Wnt/β-catenin signaling pathway has been shown
to be involved in cellular proliferation and differentiation,
regulation of tissue homeostasis, and maintenance of the
self-renewal of CSCs (30–33). The expression of the Wnt/β-catenin
signaling pathway proteins can be regulated by hnRNPAB and its
subtypes (28,29). Therefore, the expression levels of
Wnt3a, Wnt5a and β-catenin protein, involved in the Wnt/β-catenin
signaling pathway, were detected using western blotting, and the
expression levels of the three proteins were significantly
increased (Fig. 7A and B). This
suggests that the overexpression of the hnRNPAB gene activates the
Wnt/β-catenin signaling pathway. These results indicate that
hnRNPAB overexpression promotes the acquisition of stemness in
SW480 and HT29 cells by activating the Wnt/β-catenin signaling
pathway.
Subsequently, the effect of miR-8063 on the function
of CRCSCs was further investigated by overexpressing miR-8063 in
SW480CSCs and HT29CSCs. Following miR-8063 overexpression, the cell
colony formation ability was significantly weaker than that of the
control cells (Fig. 8B and C), and
tumorigenicity in nude mice was significantly reduced (Fig. 8F and G). In addition, the positive
expression rates of CD44 and CD133 were significantly downregulated
(Fig. 8H and I). These results
indicate that miR-8063 is important in regulating the self-renewal
of CRCSCs.
To investigate the molecular mechanism underlying
the regulation of CRCSCs using miR-8063, the expression level of
hnRNPAB, which has a direct binding site for miR-8063, was
detected. The results showed that overexpression of miR-8063
significantly inhibited hnRNPAB expression (Fig. 9A and B). Moreover, western blot
analysis revealed that the expression levels of key proteins in the
Wnt/β-catenin signaling pathway (Wnt3a, Wnt5a and β-catenin)
decreased with the downregulation of hnRNPAB (Fig. 9B and C). The expression level of
hnRNPAB in SW480 and HT29 cells was detected after miR-8063
silencing using a lentiviral-mediated RNAi technique. The mRNA and
protein expression of hnRNPAB were significantly upregulated after
miR-8063 silencing (Fig. 10B-D). The
expression of Wnt3a, Wnt5a and β-catenin were also increased
following the upregulation of hnRNPAB (Fig. 10C and D). These results indicated
that hnRNPAB expression was upregulated, and that the Wnt/β-catenin
signaling pathway was activated, after silencing the miR-8063
gene.
In conclusion, the present study confirmed that as a
tumor suppressor, miR-8063 is involved in regulating the
self-renewal of CRCSCs, and its molecular mechanism is through the
loss of miR-8063 expression, which weakens its inhibition on
hnRNPAB; this leads to the activation of the Wnt/β-catenin
signaling pathway to promote the self-renewal of CRCSCs. These
results provide new insights into the molecular targeted therapy of
CRC, and highlight miR-8063 and hnRNPAB as potential therapeutic
targets.
Acknowledgements
The authors would like to thank the Department of
Immunology, Zunyi Medical University for providing the experimental
platform.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560404), the
Science and Technology Fund Foundation of Guizhou (grant no.
[2017]5733-053), the Science and Technology Fund Foundation of
Zunyi City (grant no. [2019]69) and the Fund Foundation of Guizhou
Health Committee (grant no. gzwjkj2019-1-122).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQC, TY, HJ and KMW conceived and designed the
study. ZQC, TY, HJ and LW performed the experiments and data
analysis. TY and HJ performed the cytological experiments. YYY,
RMF, SQL, TZ, ZYW and KMW participated in the discussion and
interpretation of data. TY and KMW confirm the authenticity of all
the raw data. TY wrote the paper. KMW supervised all experimental
work. All authors have read and approved the final manuscript.
Ethis approval and consent to
participate
Human primary CRC tissues were obtained from
patients admitted to the Department of Gastrointestinal Surgery,
Affiliated Hospital of Zunyi Medical University. The present study
was reviewed and approved by the Ethics Review Committee of the
Affiliated Hospital of Zunyi Medical University (approval no.
[2015] 1-040) and was conducted according to the recognized ethical
guidelines (Declaration of Helsinki, CIOMS). All patients included
in the study provided written informed consent. All animal
experiments complied with Animal Research: Reporting In Vivo
Experiment Guidelines, and were approved by the Animal Experiment
Ethics Committee of Zunyi Medical University (approval no. [2015]
2-030).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Das PK, Pillai S, Rakib MA, Khanam JA,
Gopalan V, Lam AKY and Islam F: Plasticity of cancer stem cell:
Origin and role in disease progression and therapy resistance. Stem
Cell Rev Rep. 16:397–412. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuşoğlu A and Avcı ÇB: Cancer stem cells:
A brief review of the current status. Gene. 681:80–85. 2019.
View Article : Google Scholar
|
|
5
|
Yadav AK and Desai NS: Cancer stem cells:
Acquisition, characteristics, therapeutic implications, targeting
strategies and future prospects. Stem Cell Rev Rep. 15:331–355.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bakhshinyan D, Adile AA, Qazi MA, Singh M,
Kameda-Smith MM, Yelle N, Chokshi C, Venugopal C and Singh SK:
Introduction to cancer stem cells: Past, present, and future.
Methods Mol Biol. 1692:1–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SH, Reed-Newman T, Anant S and
Ramasamy TS: Regulatory role of quiescence in the biological
function of cancer stem cells. Stem Cell Rev Rep. 16:1185–1207.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Najafi M, Mortezaee K and Majidpoor J:
Cancer stem cell (CSC) resistance drivers. Life Sci.
234:1167812019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steinbichler TB, Dudas J, Skvortsov S,
Ganswindt U, Riechelmann H and Skvortsova II: Therapy resistance
mediated by cancer stem cells. Semin Cancer Biol. 53:156–167. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou JM, Hu SQ, Jiang H, Chen YL, Feng JH,
Chen ZQ and Wen KM: OCT4B1 promoted EMT and regulated the
self-renewal of CSCs in CRC: Effects associated with the balance of
miR-8064/PLK1. Mol Ther Oncolytics. 15:7–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geuens T, Bouhy D and Timmerman V: The
hnRNP family: Insights into their role in health and disease. Hum
Genet. 135:851–867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang TH, Chen CC, Hsiao YC, Lin YH, Pi WC,
Huang PR, Wang TCV and Chen CY: Heterogeneous nuclear
ribonucleoproteins A1 and A2 function in telomerase-dependent
maintenance of telomeres. Cancers (Basel). 11:3342019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weighardt F, Biamonti G and Riva S: The
roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA
metabolism. Bioessays. 18:747–756. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han SP, Tang YH and Smith R: Functional
diversity of the hnRNPs: Past, present and perspectives. Biochem J.
430:379–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xuan Y, Wang J, Ban L, Lu JJ, Yi C, Li Z,
Yu W, Li M, Xu T, Yang W, et al: hnRNPA2/B1 activates
cyclooxygenase-2 and promotes tumor growth in human lung cancers.
Mol Oncol. 10:610–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Y, Sun Z, Deng J, Hu B, Yan W, Wei H
and Jiang J: Splicing factor hnRNPA2B1 contributes to tumorigenic
potential of breast cancer cells through STAT3 and ERK1/2 signaling
pathway. Tumour Biol. 39:10104283176943182017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi X, Ran L, Liu Y, Zhong SH, Zhou PP,
Liao MX and Fang W: Knockdown of hnRNP A2/B1 inhibits cell
proliferation, invasion and cell cycle triggering apoptosis in
cervical cancer via PI3K/AKT signaling pathway. Oncol Rep.
39:939–950. 2018.PubMed/NCBI
|
|
19
|
Ma Y, Yang L and Li R: HnRNPA2/B1 is a
novel prognostic biomarker for breast cancer patients. Genet Test
Mol Biomarkers. 24:701–707. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin D, Kong C and Chen M: Effect of
hnRNPA2/B1 on the proliferation and apoptosis of glioma U251 cells
via the regulation of AKT and STAT3 pathways. Biosci Rep.
40:BSR201903182020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou JM, Jiang H, Yuan T, Zhou GX, Li XB
and Wen KM: High hnRNP AB expression is associated with poor
prognosis in patients with colorectal cancer. Oncol Lett.
18:6459–6468. 2019.PubMed/NCBI
|
|
22
|
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q,
Zhao YM, Shi YH, Gao Q, Wu WZ, Qiu SJ, et al: HNRNPAB induces
epithelial-mesenchymal transition and promotes metastasis of
hepatocellular carcinoma by transcriptionally activating SNAIL.
Cancer Res. 74:2750–2762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tauler J, Zudaire E, Liu H, Shih J and
Mulshine JL: hnRNP A2/B1 modulates epithelial-mesenchymal
transition in lung cancer cell lines. Cancer Res. 70:7137–7147.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai S, Zhang J, Huang S, Lou B, Fang B, Ye
T, Huang X, Chen B and Zhou M: HNRNPA2B1 regulates the
epithelial-mesenchymal transition in pancreatic cancer cells
through the ERK/snail signalling pathway. Cancer Cell Int.
17:122017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai Z, Cao Y, Luo Y, Hu H and Ling H:
Signalling mechanism(s) of epithelial-mesenchymal transition and
cancer stem cells in tumour therapeutic resistance. Clin Chim Acta.
483:156–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishiyama M, Tsunedomi R, Yoshimura K,
Hashimoto N, Matsukuma S, Ogihara H, Kanekiyo S, Iida M, Sakamoto
K, Suzuki N, et al: Metastatic ability and the
epithelial-mesenchymal transition in induced cancer stem-like
hepatoma cells. Cancer Sci. 109:1101–1109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stockley J, Villasevil ME, Nixon C, Ahmad
I, Leung HY and Rajan P: The RNA-binding protein hnRNPA2 regulates
β-catenin protein expression and is overexpressed in prostate
cancer. RNA Biol. 11:755–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meng X, Cui J, Wang Y, Zhang X, Li D, Hai
Y and Du H: Heterogeneous nuclear ribonucleoprotein A1 interacts
with microRNA-34a to promote chondrogenic differentiation of
mesenchymal stem cells. Am J Transl Res. 9:1774–1782.
2017.PubMed/NCBI
|
|
30
|
Jiang S, Song C, Gu X, Wang M, Miao D, Lv
J and Liu Y: Ubiquitin-specific peptidase 22 contributes to
colorectal cancer stemness and chemoresistance via Wnt/β-catenin
pathway. Cell Physiol Biochem. 46:1412–1422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng X, Xu X, Chen D, Zhao F and Wang W:
Therapeutic potential of targeting the Wnt/β-catenin signaling
pathway in colorectal cancer. Biomed Pharmacother. 110:473–481.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin-Orozco E, Sanchez-Fernandez A,
Ortiz-Parra I and Ayala-San Nicolas M: WNT signaling in tumors: The
way to evade drugs and immunity. Front Immunol. 10:28542019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang T, Guo C, Xia T, Zhang R, Zen K, Pan
Y and Jin L: LncCCAT1 promotes breast cancer stem cell function
through activating WNT/β-catenin signaling. Theranostics.
9:7384–7402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao Q, Chen Y and Zhou X: The roles of
microRNAs in epigenetic regulation. Curr Opin Chem Biol. 51:11–17.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu XM, Fu Q, Du Y, Yang YX and Cho WC:
MicroRNA as regulators of cancer stem cells and chemoresistance in
colorectal cancer. Curr Cancer Drug Targets. 16:738–754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo JC, Yang YJ, Zhang JQ, Guo M, Xiang L,
Yu SF, Ping H and Zhuo L: MicroRNA-448 inhibits stemness
maintenance and self-renewal of hepatocellular carcinoma stem cells
through the MAGEA6-mediated AMPK signaling pathway. J Cell Physiol.
234:23461–23474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang S, Miao D, Wang M, Lv J, Wang Y and
Tong J: miR-30-5p suppresses cell chemoresistance and stemness in
colorectal cancer through USP22/Wnt/β-catenin signaling axis. J
Cell Mol Med. 23:630–640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mukohyama J, Isobe T, Hu Q, Hayashi T,
Watanabe T, Maeda M, Yanagi H, Qian X, Yamashita K, Minami H, et
al: miR-221 targets QKI to enhance the tumorigenic capacity of
human colorectal cancer stem cells. Cancer Res. 79:5151–5158. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng CW, Liao WL, Chen PM, Yu JC, Shiau
HP, Hsieh YH, Lee HJ, Cheng YC, Wu PE and Shen CY: miR-139
modulates cancer stem cell function of human breast cancer through
targeting CXCR4. Cancers (Basel). 13:25822021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ni H, Qin H, Sun C, Liu Y, Ruan G, Guo Q,
Xi T, Xing Y and Zheng L: miR-375 reduces the stemness of gastric
cancer cells through triggering ferroptosis. Stem Cell Res Ther.
12:3252021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
44
|
Gupta R, Bhatt LK, Johnston TP and
Prabhavalkar KS: Colon cancer stem cells: Potential target for the
treatment of colorectal cancer. Cancer Biol Ther. 20:1068–1082.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wen K, Fu Z, Wu X, Feng J, Chen W and Qian
J: Oct-4 is required for an antiapoptotic behavior of
chemoresistant colorectal cancer cells enriched for cancer stem
cells: Effects associated with STAT3/Survivin. Cancer Lett.
333:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin QY, Wang JQ, Wu LL, Zheng WE and Chen
PR: miR-638 represses the stem cell characteristics of breast
cancer cells by targeting E2F2. Breast Cancer. 27:147–158. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Erdogan S and Turkekul K: Neferine
inhibits proliferation and migration of human prostate cancer stem
cells through p38 MAPK/JNK activation. J Food Biochem.
44:e132532020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abbaszadegan MR, Bagheri V, Razavi MS,
Momtazi AA, Sahebkar A and Gholamin M: Isolation, identification,
and characterization of cancer stem cells: A review. J Cell
Physiol. 232:2008–2018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bhutia SK, Naik PP, Praharaj PP, Panigrahi
DP, Bhol CS, Mahapatra KK, Saha S and Patra S: Identification and
characterization of stem cells in oral cancer. Methods Mol Biol.
2002:129–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zahran AM, Rayan A, Fakhry H, Attia AM,
Ashmawy AM, Soliman A, Elkady A and Hetta HF: Pretreatment
detection of circulating and tissue CD133(+) CD44(+) cancer stem
cells as a prognostic factor affecting the outcomes in Egyptian
patients with colorectal cancer. Cancer Manag Res. 11:1237–1248.
2019. View Article : Google Scholar : PubMed/NCBI
|