Introduction
Cholangiocarcinoma (CCA) is a term used to describe
a cluster of highly heterogeneous malignant tumors that arise in
any part of the biliary tree (1). CCA
is a highly malignant, relatively rare disease, yet the incidence
of CCA is increasing worldwide (2).
At present, the main treatment strategies of CCA include surgical
resection, liver transplantation, systemic chemotherapy and local
treatment (3). In addition, CCA often
remains concealed, is highly heterogeneous, highly invasive and has
a high tolerance to chemotherapy. These factors have a detrimental
impact on the effectiveness of existing treatments which leads to
high rates of mortality (4). Thus,
the complexity of the tumor requires further investigation to allow
the development of innovative therapies to improve patient
outcomes. Currently, candidate molecular targets for precision
medicine are being identified and evaluated in clinical trials
(5). The molecular mechanisms
underlying CCA require in depth investigation to identify key small
molecular targets and associated signaling pathways that may serve
as novel therapeutic approaches (5,6). The
minichromosome maintenance (MCM) protein family was initially
discovered in the budding yeast Saccharomyces cerevisiae and
received extensive attention (7).
Initial study has revealed that the MCM family plays a key role in
DNA replication (8). In previous
studies, it has been reported that MCM2-7 are overexpressed in
esophageal squamous cell carcinoma, cervical, gastric and breast
cancer as well as other cancers (9–12).
Minichromosome maintenance 8 homologous recombination repair factor
(MCM8) and the related MCM9 were recently discovered as members of
the MCM family. MCM8 has significant homology with MCM7, and is a
helicase involved in the elongation step of DNA replication
(13). The findings of a recent study
indicated that MCM2-7, MCM8 and MCM10 are overexpressed in
hepatocellular carcinoma (HCC) (14).
However, the specific molecular mechanism underlying the role of
MCM8 in CCA has yet to be elucidated.
In the present study, the clinical significance and
biological function of MCM8 in CCA progression were investigated.
The expression of MCM8 in CCA and the effects of MCM8 knockdown on
the proliferation, apoptosis and migration of CCA cells were
examined. It was also investigated whether the effects of MCM8
knockdown were mediated via regulation of apoptosis-related protein
expression. The aim was to elucidate whether MCM8 knockdown exerts
an inhibitory effect on CCA and determine its therapeutic potential
as a molecular target.
Materials and methods
Tissue collection and
immunohistochemical (IHC) staining
A total of 122 human CCA and paired paracancerous
tissue samples were purchased from Shanghai Outdo Biotech Co., Ltd.
(cat. no. JS W-11-01; http://www.superchip.com.cn). All tissues were from
patients with CCA who underwent surgery, and none of the patients
received any chemotherapy or radiation before surgery. IHC staining
was performed as previously described in the literature (15). The experimental protocols and the
human tissues used were approved (approval no. 20200305) by the
Ethics Committee of Clinical Research of The First Affiliated
Hospital of Chengdu Medical College (Chengdu, China).
Formalin-fixed and paraffin-embedded tissues (4 µm) were
deparaffinized in xylene and rehydrated in ethanol. Subsequently,
the tissues were incubated with MCM8 primary antibody (1:100; cat.
no. PA5-41325; Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C
for 4 h and an HRP-conjugated secondary antibody (1:400; cat. no.
ab6721; Abcam) at room temperature for 1 h. Following incubation,
the tissues were stained with 3,3′-diaminobenzidine solution and
hematoxylin at room temperature for 10 min. IHC scores were
determined by staining percentage and staining intensity as
previously described by Haonon et al (15). In addition, the median IHC score was
used to verify the expression levels of MCM8 in CCA.
Cell culture
The human CCA cell lines HCCC-9810, RBE and HuCCT1
were obtained from the Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and cultured at 37°C in RPMI-1640
medium supplemented with 10% FBS, 100 mg/ml streptomycin and 100
IU/ml penicillin (all from Gibco; Thermo Fisher Scientific, Inc.)
in a humidified atmosphere of 5% CO2.
Lentivirus production and cell
transduction
A total of three short hairpin (sh)RNAs specifically
targeting MCM8 (shMCM8) were designed for MCM8 knockdown and the
sequences were as follows: RNAi-1: 5′-TGGCAATACATCAGGTGTTAA-3′;
RNAi-2: 5′-CTGGAATTGTCAAAGTCTCAA-3′; and RNAi-3:
5′-AGGCAGCTGGAATCTTTGATT-3′. The negative control was a scrambled
short interfering (si)RNA (shCtrl:
5′-CCGGAGGGATTGACTTAGAGCAAATCTCGAGATTTGCTCTAAGTCAATCCCTTTTTTG-3′).
Lentivirus vectors BR-V108 were purchased from Yi Berry Biological
Medicine Technology Co., LTD (http://www.bioscienceres.com). and labeled with green
fluorescent protein (GFP) and resistance tag (puromycin, 400 ng/ml)
for cell screening. Human CCA cell lines HCCC-9810 and RBE were
cultured in 6-well plates (2×105 cells/well; Corning,
Inc.) at 37°C for 24 h for second-generation lentiviral
transduction. Recombinant lentivirus particles (1×107
TU/ml) contained in shMCM8 or shCtrl sequences were used to
transduce CCA cell lines HCCC-9810 and RBE (5×105
cells/ml) using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in RPMI-1640 medium with 10% FBS at a
multiplicity of infection of 10 for 40 min at 37°C. A total of 72 h
after transduction, the expression of GFP in the cells was observed
under a fluorescence microscope (magnification, ×200; Olympus
Corporation), and the cells were cultured for 24 h for subsequent
experiments.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
RNA was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) from target cells. Total RNA was
reverse transcribed into cDNA using the M-MLV Reverse Transcriptase
kit (Promega Corporation), according to the manufacturer's
protocol. qPCR was subsequently performed as follows: Initial
denaturation at 95°C for 15 min; 40 of cycles of denaturation,
annealing and elongation at 55°C for 15 min; and a final extension
at 85°C for 2 min using SYBR-Green Master Mix Kit (Vazyme Biotech
Co., Ltd.). The relative mRNA expression of MCM8 was quantified
with cycle threshold (Ct) values and normalized using the
2−∆∆Cq method (16). The
primer sequences used were as follows: MCM8 upstream,
5′-ATGGCTTTTCTTTGTGCTGC-3′ and downstream,
5′-CCAGTCCATCGTAACTGTGAGA-3′; GAPDH (internal control) upstream,
5′-TGACTTCAACAGCGACACCCA-3′ and downstream,
5′-CACCCTGTTGCTGTAGCCAAA-3′.
Western blotting
Total protein obtained using RIPA lysis buffer
(Beyotime, Jiangsu, China) from HCCC-9810 and RBE cells and
quantified using a BCA Protein Assay Kit (Pierce; Thermo Fisher
Scientific, Inc.) and 20 µg/lane protein was separated by SDS-PAGE
on a 10% gel [30% acrylamide/bis-acrylamide; 1.5 mol/l Tris (pH
8.8); 10% SDS; 10% ammonium persulfate and
tetramethylethylenediamine]. The separated proteins were
transferred onto PVDF membranes and blocked using TBST solution
containing 5% skimmed milk and 0.5 ml Tween-20 at 4°C for 1 h.
Immunostaining was performed using target primary antibodies:
Anti-MCM8 (1:1,000; cat. no. PA5-41325; Invitrogen), anti-Akt,
(1:1,000; product no. 4685; CST), anti-p-Akt (1:1,000; cat. no.
BS-5193R; Bioss), anti-MAPK9 (1:1,000; product code ab76125;
Abcam), anti-PIK3CA (1:1,000; product code ab40776; Abcam), GAPDH
(1:3,000; cat. no. AP0063; Bioworld) at 4°C for 4 h and
corresponding secondary antibody goat anti-rabbit (1:3,000; cat.
no. A0208; Beyotime Institute of Biotechnology) at room temperature
for 2 h. Protein bands were visualized using the Amersham ECL + TM
Western Blot system (Cytiva) and signal intensities were analyzed
using ImageJ software version 1.8.0.112 (National Institutes of
Health).
MTT assay
After 5 days of transduction, HCCC-9810 and RBE
cells were inoculated overnight on 6-well plates at 2,000
cells/well. A total of 20 µl of 5 mg/ml MTT solution (cat. no.
JT343; Genview) was added to each well for 4 h. Subsequently, 100
ml/well DMSO was added and OD values at 490 nm were obtained at 24,
48, 72, 96 and 120 h using a microplate reader. The cell viability
was calculated as a ratio of the OD of treated cells to the OD of
control cells.
Cell colony formation assay
After 5 days of transduction, HCCC-9810 and RBE
cells were inoculated on 6-well plates at a density of 1,000
cells/well for 8 days. The number of cells in a single clone was
greater than 50, and the size between 0.3–1.0 mm. Subsequently, the
cells were fixed with 1 ml 4% paraformaldehyde at room temperature
for 30–60 min and stained with 500 µl Giemsa for 10–20 min at room
temperature. After washing the cells several times with double
distilled water, the cells were captured and counted with a camera
(Sony Group Corporation).
Cell apoptosis detection by flow
cytometry
After 5 days of transduction, HCCC-9810 and RBE
cells were inoculated on 6-well plates at a seeding density of
1,000 cells/well for 10 days. The cells were washed with PBS,
centrifuged (4°C, 12,000 × g, 1 min) and resuspended. Subsequently,
500 µl of diluted 1X Annexin V binding buffer (cat. no. 88-8007-74;
eBioscience; Thermo Fisher Scientific, Inc.) was added and cells
were stained with 10 µl Annexin V-APC for 10–15 min at room
temperature in the dark. Subsequently, 5 µl of 50 µg/ml PI was used
for staining the cells at room temperature for 20 min and 1X
binding buffer was added to assess cell apoptosis using the Guava
easyCyte 12HT Benchtop Flow Cytometer and GuavaSuite 19.0 Software
(Java; both from Luminex Corporation).
Human apoptosis antibody array
Prepared target cell protein was used to investigate
the expression levels of apoptosis-related proteins using the human
apoptosis antibody array (cat. no. ab134001; Abcam). After the
membrane was blocked with array buffer at room temperature for 1 h,
prepared HuCCT1 cell protein was incubated with the membrane at 4°C
overnight. The array was subsequently washed, and incubated with
detection antibodies (1:100; cat. no. ab134001; Abcam) and
streptavidin-HRP (cat. no. ab134001; Abcam) at room temperature for
1 h. Protein expression was visualized using the Amersham ECL + TM
Western Blot system (Cytiva) and signal intensities were analyzed
using ImageJ software version 1.8.0.112.
Wound-healing assay
After 5 days of transduction, HCCC-9810 and RBE
cells were inoculated on 6-well plates at a seeding density of
5×104 cells/well. When the cells had reached a
confluence of >90%, the culture medium was replaced with fresh
medium. The cell layer was scratched using the scratch instrument
(cat. no. VP408FH; V&P Scientific, Inc.) and the cells were
cultured in serum-free medium. The shadow area in the center of the
6-well plate was used as a reference and the scratch was in the
center of the image. According to the preliminary experiments,
images were captured at 0, 4, 8, 24 and 48 h using a fluorescence
microscope (magnification, ×200; Olympus Corporation) and the cell
migration rate of each group was calculated.
Transwell assay
A total of five days after transduction, HCCC-9810
and RBE cells were seeded on a 24-well plate (5×104
cells/well) for 24 h. The chamber in the Transwell transfer test
kit (Corning, Inc.) was composed of 24-well tissue culture plates
and 12-well cell culture inserts. A total of 100 µl of the cell
suspension (5×105 cells) was loaded into the upper
chamber of the Transwell and the lower chamber of the Transwell was
filled with 500 µl culture medium containing 30% FBS for incubation
at 37°C for 48 h. The insert contained a polycarbonate membrane
with a pore size of 8 µm, and the cells migrated and adhered to the
bottom of the polycarbonate membrane. The cells were fixed with 4%
paraformaldehyde for 30 min and stained with 0.1% crystal violet
solution at room temperature for 20 min. The non-migrating cells
were removed and migrated cells were stained. Following washing
with PBS, 5 fields of view per well were selected randomly under a
fluorescence microscope (magnification, ×200; Olympus Corporation),
and images were captured for enumeration of the cells.
Mouse xenograft model
Mouse experiments were approved by the Ethics
Committee of Chengdu Medical College (approval no. 20190213),
following the Guidelines and Procedures for Animal Care and
Protection (http://www.lascn.net/Item/7281.aspx). A total of
4-week-old female BALB/c nude mice (Shanghai Lingchang
Biotechnology Co., Ltd.) with a body weight of 15–25 g were used to
construct the pathogen-free xenograft model. Mice were kept in a
non-toxic metal cage with a 12-h light/dark cycle, a constant
temperature of 20–22°C, humidity at 30–70%, ventilation every 10–20
times/h and food (cat. no. 000521; Shanghai ZeYa; http://www.shzeya.com) and water intake at any time.
At 5 days after transduction, 0.2 ml of shMCM8 and shCtrl HuCCT1
suspended with PBS with a cell concentration of 4×106
cells/ml were injected into the left back of each mouse. The weight
and volume of tumors was monitored twice a week, starting at 10
days following injection (calculated by π/6 × L × W2;
where L was the long diameter and W was the short diameter). At 46
days after injection, mice were anesthetized using 0.7%
pentobarbital sodium at a dose of 40 mg/kg. Subsequently,
fluorescence was observed under the imaging system (emission
wavelength 510 nm; IVIS Spectrum; Perkin Elmer). Mice were
sacrificed by intraperitoneal injection of sodium pentobarbital at
a dose of 100 mg/kg according to the American Veterinary Medical
Association guidelines (http://www.lascn.net/Item/91107.aspx). The tumors were
removed, weighed and images were captured and tumor slides were
subjected to Ki67 expression detection using the IHC staining
method. Tumor specimens of shMCM8 and shCtrl (4 µm) were fixed with
formalin for 30 min, dewaxed with xylene for 15 min and rehydrated
with 100% alcohol for 10 min. PBS-H2O2 with
0.1% Tween-20 were added and the tissues were washed three times
with PBS, 5 min each time. Citric acid buffer was added for antigen
retrieval, with heating at 120°C for 20 min. The tumor tissues were
incubated with the anti-Ki67 (1:200; cat. no. ab16667, Abcam) at
4°C overnight and secondary antibody incubation with HRP goat
anti-rabbit IgG (1:400; cat. no. ab6721; Abcam) at room temperature
for 2 h. Images were collected with a microscope (magnification,
×200; Olympus Corporation).
Statistical analysis
Experimental data were presented as the mean ±
standard deviation (n=3) and statistical analyses were performed
using SPSS 19.0 (IBM Corp.). The relationship between MCM8
expression and tumor characteristics in patients with CCA was
analyzed using chi-square test or Fisher's exact test. Data
produced using RT-qPCR was analyzed using 2−∆∆Cq method.
The results of the MTT OD 490-nm value were obtained and
wound-healing as well as Transwell assays were investigated using
the paired two-tailed Student's t-test or one-way ANOVA followed by
Bonferroni's post hoc test analysis. The relationship between MCM8
and clinicopathological features of CCA were analyzed using
Mann-Whitney U and Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Upregulation of MCM8 in CCA
The results of IHC staining revealed a lower signal
intensity of MCM8 in paracancerous tissues when compared with CCA
tissues, which indicated that MCM8 expression was significantly
higher in CCA tissues compared with paracancerous tissues (Table I and Fig.
1A). Moreover, the relationship between MCM8 expression and
tumor characteristics in patients with CCA was analyzed using
chi-square test or Fisher's exact test. The results indicated that
there was no significant association between the expression level
of MCM8 and the pathological stage of CCA (Table II). In addition, Pearson's
correlation analysis indicated that the higher the expression level
of MCM8, the higher the pathological stage of CCA (Table III). Thus, investigating the
expression levels of MCM8 may be clinically relevant in predicting
the deterioration of CCA. Furthermore, CCA cell models with MCM8
knockdown were constructed to clarify the effects of MCM8 in CCA
cells. The results of the RT-qPCR analysis revealed that the
knockdown efficiency of MCM8 in the shMCM8-3 group was the highest
compared with other groups (Fig.
S1). In addition, a ratio of more than 80% of GFP was observed,
indicating that the lentiviruses shCtrl and shMCM8 had successfully
infected the CCA cells (Fig. 1B). The
results of RT-qPCR demonstrated that the MCM8 expression was
decreased by 79.3 and 90.7% in HCCC-9810 and RBE cells compared
with the shCtrl group, respectively (Fig.
1C). The protein expression level of MCM8 was also decreased in
HCCC-9810 and RBE cells infected with lentivirus shMCM8 (Fig. 1D). Consequently, the low
shMCM8-mediated expression levels of MCM8 in HCCC-9810 and RBE
cells were used for further functional analysis.
 | Table I.Expression patterns in
cholangiocarcinoma tissues and para-carcinoma tissues revealed by
immunohistochemical analysis. |
Table I.
Expression patterns in
cholangiocarcinoma tissues and para-carcinoma tissues revealed by
immunohistochemical analysis.
|
| Tumor tissue | Para-carcinoma
tissue |
|
|---|
|
|
|
|
|
|---|
| MCM8
expression | Cases | Percentage (%) | Cases | Percentage (%) | P-value |
|---|
| Low | 47 | 52.8 | 23 | 74.2 |
4.098×10−6 |
| High | 42 | 47.2 | 8 | 25.8 |
|
 | Table II.Relationship between MCM8 expression
and tumor characteristics in patients with cholangiocarcinoma. |
Table II.
Relationship between MCM8 expression
and tumor characteristics in patients with cholangiocarcinoma.
|
|
| MCM8
expression |
|
|---|
|
|
|
|
|
|---|
| Features of the
patients | No. of patients
89 | Low 47 | High 42 | P-value |
|---|
| Age (years) |
|
|
| 0.930 |
|
≤57 | 46 | 25 | 21 |
|
|
>57 | 43 | 22 | 21 |
|
| Sex |
|
|
| 0.736 |
|
Male | 46 | 23 | 23 |
|
|
Female | 43 | 24 | 19 |
|
| Grade |
|
|
| 0.443 |
| I | 5 | 4 | 1 |
|
| II | 66 | 35 | 31 |
|
|
III | 18 | 8 | 10 |
|
| N |
|
|
| 0.698 |
| N0 | 58 | 32 | 26 |
|
| N1 | 31 | 15 | 16 |
|
| T |
|
|
| 0.338 |
| T1 | 7 | 4 | 3 |
|
| T2 | 61 | 34 | 27 |
|
| T3 | 17 | 8 | 9 |
|
| T4 | 3 | 0 | 3 |
|
| Tumor size |
|
|
| 0.639 |
| ≤3
cm | 50 | 28 | 22 |
|
| >3
cm | 39 | 19 | 20 |
|
| Stage |
|
|
| 0.078 |
| 1 | 8 | 4 | 4 |
|
| 2 | 40 | 27 | 13 |
|
| 3 | 27 | 11 | 16 |
|
| 4 | 14 | 5 | 9 |
|
| Lymphoid positive
number |
|
|
| 0.659 |
| ≤0 | 58 | 32 | 26 |
|
|
>0 | 30 | 15 | 15 |
|
 | Table III.Pearson correlation analysis of MCM8
expression and tumor characteristics in patients with
cholangiocarcinoma. |
Table III.
Pearson correlation analysis of MCM8
expression and tumor characteristics in patients with
cholangiocarcinoma.
|
| Expression
correlation between MCM8 and stage |
|
|---|
|
|
|
|
|---|
| Number of
samples | Pearson correlation
coefficient | P-value |
|---|
| 89 | 0.218 | 0.040 |
Reduced MCM8 expression suppresses
HCCC-9810 and RBE cell proliferation in vitro
As revealed in Fig.
2A, the effect of shCtrl and shMCM8 on the OD 490-nm values of
HCCC-9810 and RBE cells was investigated. The results of the
present study demonstrated that the level of cell proliferation was
weakened in the shMCM8 group compared with the shCtrl group.
Additionally, the colony formation assay in HCCC-9810 and RBE cells
indicated that the shMCM8 group yielded smaller and fewer colonies
compared with the control group (Fig.
2B). In conclusion, CCA cells with reduced MCM8 expression
exhibited impaired proliferation.
Reduced MCM8 expression increases
HCCC-9810 and RBE cell apoptosis in vitro
The level of cell apoptosis was assessed using flow
cytometry. The results indicated that the decrease of MCM8
expression greatly enhanced the apoptotic levels of HCCC-9810 and
RBE cells (Fig. 3A). Furthermore,
expression levels of a number of apoptotic factors associated with
apoptotic signaling pathways were analyzed in cells transfected
with shCtrl and shMCM8. As revealed in the Fig. 3B, the expression levels of HSP27,
IGF-1sR, survivin, sTNF-R1, sTNF-R2, TNF-α, TNF-β and XIAP were
significantly downregulated in HCCC-9810 cells. The results of the
present study revealed that MCM8 knockdown enhanced the apoptosis
of CCA cells and led to the abnormal downregulation of
apoptosis-related proteins.
Reduced MCM8 expression suppresses
HCCC-9810 and RBE cell migration in vitro
The effect of MCM8 knockdown on the migration
ability of CCA cells was preliminarily evaluated using
wound-healing and Transwell assays. Compared with the shCtrl group,
the decreased expression of MCM8 resulted in a 16.9% reduction in
the migration rate of HCCC-9810 cells within 24 h. The migration
rate of RBE cells at 0–48 h decreased by 26.7% (Fig. 4A). Additionally, the results of
Transwell assay further indicated that the migration ability of
HCCC-9810 and RBE cells in the MCM8-knockdown group was inhibited
(Fig. 4B). In conclusion, knockdown
of MCM8 inhibited the migration of CCA cells. To further
investigate the effect of MCM8 on protein expression in downstream
pathways, the expression levels of a number of proteins in
well-established signaling pathways were detected in HCCC-9810
cells. The results of western blot analysis indicated that the
expression level of MAPK9 was upregulated, while the expression
levels of phosphorylated Akt, G1/S-specific cyclin D1 (CCND1) and
PIK3 catalytic subunit alpha (CA) were downregulated in the shMCM8
group (Fig. 4C). Mechanistically, the
knockdown of MCM8 led to abnormalities in the expression levels of
proteins in well-established downstream pathways, such as PI3K/Akt,
CCND1 and MAPK.
Reduced MCM8 expression impairs tumor
generation in vivo
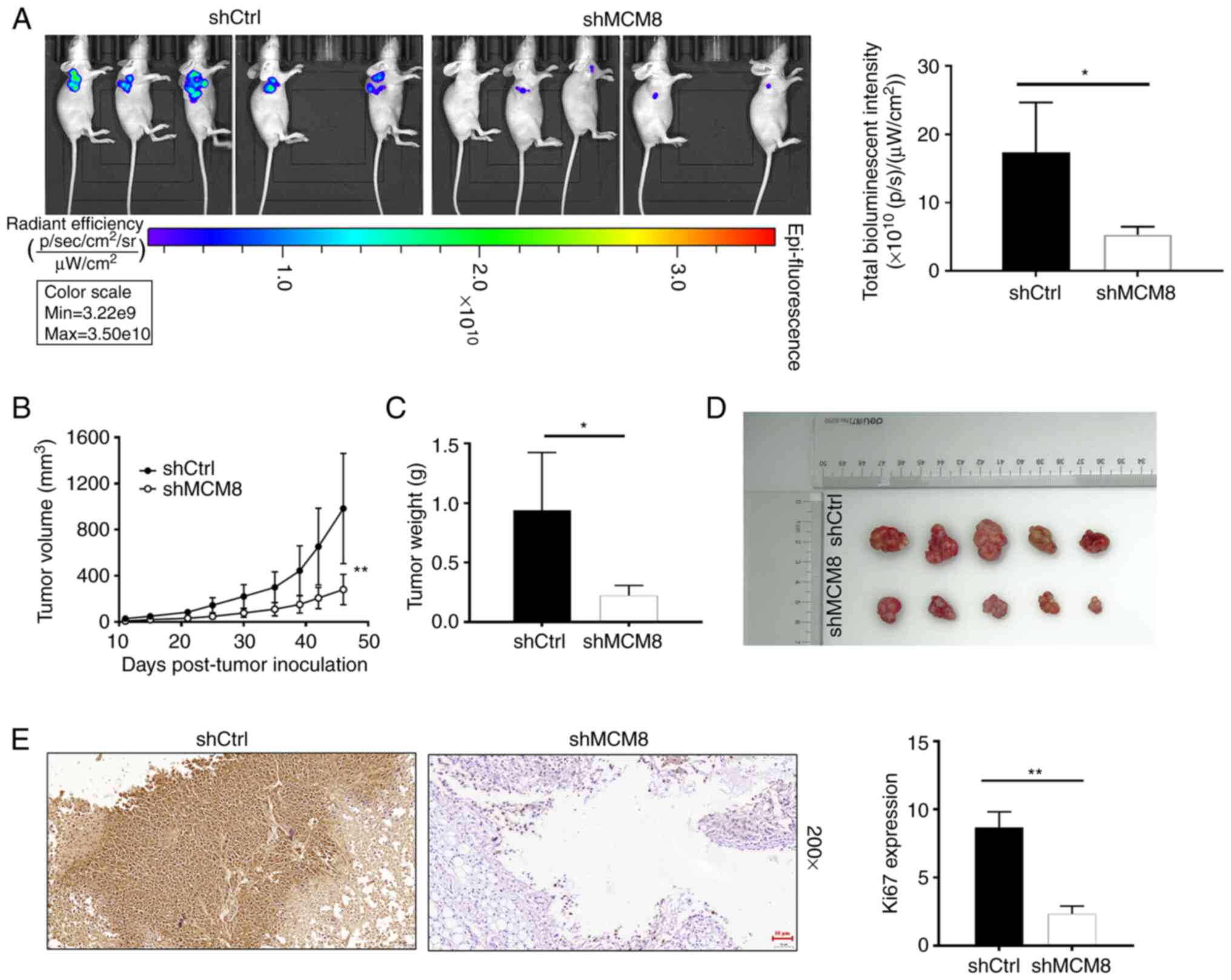
shMCM8 and shCtrl-mediated HuCCT1 cells were
subcutaneously injected into nude mice to determine the effect of
MCM8 knockdown in mice. Both groups of mice were anesthetized and
images were subsequently captured using an imaging system. The
results demonstrated that the intensity of bioluminescence in the
shMCM8 group was significantly weaker compared with shCtrl group
(Fig. 5A). The results indicated that
tumor burden was reduced in mice with low MCM8 expression (Fig. 5B and C). Monitoring of the
experimental mice for more than a month revealed that the largest
tumor weighed 1.33 g, with a volume of 1,399.91 mm3. In
addition, mice with MCM8 knockdown developed tumors that were
smaller in size. A maximum tumor diameter of 17.23 mm was observed
in the shCtrl group and only 11.59 mm in the shMCM8 group (Fig. 5D). The IHC staining displayed in
Fig. 5E further demonstrated that the
expression of Ki67 was lower in the shMCM8 group compared with the
control group. These results indicated that downregulation of MCM8
suppressed tumor formation in vivo.
Discussion
MCM8 is involved in the initiation and elongation
during DNA replication (13,17,18). The
high growth stimulation of carcinogenesis leads to a high level of
pressure on cancer cells to proliferate, compared with
non-cancerous cells (19,20). Furthermore, MCM8 plays an essential
role in the repair process of replication stress. Thus, it was
hypothesized that MCM8 may be associated with tumor progression. In
addition, the results of a previous study revealed that the
upregulation of MCM8 led to increased aggressiveness in a variety
of human tumors (21). For example,
elevated expression of MCM8 resulted in increased aggressiveness in
prostate cancer (21). MCM8 is also
considered as an independent prognostic factor in patients with
pancreatic cancer (22). However, the
physiological functions and potential molecular mechanisms
underlying MCM8 in CCA are yet to be fully elucidated.
In the present study, the overexpression of MCM8 in
CCA was preliminarily identified using IHC staining. The biological
function of MCM8 was investigated using a lentivirus-mediated CCA
cell model. The loss-of-function assays revealed that the reduced
expression of MCM8 resulted in reduced proliferation, induction of
apoptosis and suppressed migration of CCA cells in vitro.
Furthermore, decreased expression of MCM8 led to the decreased
ability of tumor formation in mice. Thus, knockdown of MCM8
inhibited the malignant behavior of CCA, indicating that MCM8
exerted a role in the promotion of CCA.
Mechanistically, MCM8 knockdown contributed to the
abnormal downregulation of apoptosis-related proteins such as
survivin, XIAP, HSP27, IGF-1sR, sTNF-R1, sTNF-R2, TNF-α and TNF-β.
Furthermore, MCM8 knockdown induced apoptosis in CCA cells, which
required a number of pro-apoptotic and anti-apoptotic factors.
However, the exact mechanism underlying MCM8 regulation of CCA cell
apoptosis is yet to be fully elucidated, and requires further
discussion. In addition, Abnormalities in intracellular signaling
pathways affected cell proliferation, survival, migration or
invasion. Previous studies have demonstrated that PI3K/Akt,
CCND1/CDK6 and MAPK signaling pathways serve key functions in CCA
cells (23–26). For instance, Wang et al
revealed that the PI3K/Akt signaling pathway played a critical role
in mediating epithelial-mesenchymal transition and metastasis of
CCA (27). Additionally, knockdown of
CCND1 expression inhibited the proliferation and migration of CCA
cells (28). Proline-rich homeodomain
protein transcription factor is an oncogenic driver of CCA and is
sensitive to CDK4/6 inhibitors (29).
Zhang et al indicated that an abnormal p38/MAPK signaling
pathway affected the proliferation of CCA cells (30). In conclusion, the PI3K/Akt, CCND1/CDK6
and MAPK signaling pathways played an important role in the
progression of CCA cells. The present study demonstrated that the
decrease of MCM8 expression inhibited the phosphorylation of Akt,
downregulated the expression levels of CCND1, PIK3CA, and
upregulated the expression of MAPK9. Therefore, it was hypothesized
that MCM8 is involved in the progression of CCA cells through
regulation of the PI3K/Akt, CCND1 and MAPK9 signaling pathways.
The present study, however, still has certain
limitations. For instance, the clinical sample size that was used
was limited. In addition, the present study only initially screened
the changes of certain apoptosis-related proteins, and the exact
mechanism of MCM8 affecting CCA apoptosis still requires a large
number of studies for more in-depth insights.
The findings of the present study indicated that the
high expression of MCM8 in CCA has the potential to be clinically
significant in predicting disease progression. In addition,
knockdown of MCM8 decreased proliferation and migration, and
promoted apoptosis of CCA cells. In summary, MCM8 played a key role
in the malignant progression of CCA, indicating that inhibition of
MCM8 may prove to be of value as a molecular targeted therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Institutional
Research Funding from The First Affiliated Hospital of Chengdu
Medical College (grant no. CYFY-GQ20).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HDu designed the present study. HDe, LC, PY, JL and
BL completed all in vitro experiments operation. YY, QW and
XJ performed the animal assays. Data collection and statistical
analysis were performed by HW. JH wrote the manuscript and it was
reviewed by HDu. JH and HDu confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols and the human tissues
used were approved (approval no. 20200305) by the Ethics Committee
of Clinical Research of The First Affiliated Hospital of Chengdu
Medical College (Chengdu, China). Mouse experiments were approved
(approval no. 20190213) by the Ethics Committee of Chengdu Medical
College, following the Guidelines and Procedures for Animal Care
and Protection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan AS and Dageforde LA:
Cholangiocarcinoma. Surg Clin North Am. 99:315–335. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckholz AP and Brown RS Jr:
Cholangiocarcinoma: Diagnosis and management. Clin Liver Dis.
24:421–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dondossola D, Ghidini M, Grossi F, Rossi G
and Foschi D: Practical review for diagnosis and clinical
management of perihilar cholangiocarcinoma. World J Gastroenterol.
26:3542–3561. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banales JM, Marin JJ, Lamarca A, Rodrigues
PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB,
Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Labib PL, Goodchild G and Pereira SP:
Molecular pathogenesis of cholangiocarcinoma. BMC Cancer.
19:1852019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang T, Chen G, Liu C, Zu L, Wang Q, Wang
Y, Lv J, An Y, Dong L, Cheng H, et al: A phase I Study comparing
the pharmacokinetics, safety, and immunogenicity of proposed
biosimilar GB242 and reference infliximab in healthy subjects.
BioDrugs. 33:93–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhai Y, Li N, Jiang H, Huang X, Gao N and
Tye BK: Unique roles of the non-identical MCM subunits in DNA
replication licensing. Mol Cell. 67:168–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong X, Chen X, Guan X, Zhang H, Ma Y,
Zhang S, Wang E, Zhang L and Han Y: Overexpression of G9a and MCM7
in oesophageal squamous cell carcinoma is associated with poor
prognosis. Histopathology. 66:192–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das M, Prasad SB, Yadav SS, Govardhan HB,
Pandey LK, Singh S, Pradhan S and Narayan G: Over expression of
minichromosome maintenance genes is clinically correlated to
cervical carcinogenesis. PLoS One. 8:e696072013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giaginis C, Giagini A, Tsourouflis G,
Gatzidou E, Agapitos E, Kouraklis G and Theocharis S: MCM-2 and
MCM-5 expression in gastric adenocarcinoma: Clinical significance
and comparison with Ki-67 proliferative marker. Dig Dis Sci.
56:777–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cobanoglu U, Mungan S, Gundogdu C, Ersoz
S, Ozoran Y and Aydin F: The expression of MCM-2 in invasive breast
carcinoma: A stereologic approach. Bratisl Lek Listy. 111:45–49.
2010.PubMed/NCBI
|
|
13
|
Gozuacik D, Chami M, Lagorce D, Faivre J,
Murakami Y, Poch O, Biermann E, Knippers R, Bréchot C and
Paterlini-Bréchot P: Identification and functional characterization
of a new member of the human Mcm protein family: hMcm8. Nucleic
Acids Res. 31:570–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Li J, Chen J, Shan Q, Dai H, Xie H,
Zhou L, Xu X and Zheng S: MCM family in HCC: MCM6 indicates adverse
tumor features and poor outcomes and promotes S/G2 cell cycle
progression. BMC Cancer. 18:2002018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haonon O, Rucksaken R, Pinlaor P,
Pairojkul C, Chamgramol Y, Intuyod K, Onsurathum S, Khuntikeo N and
Pinlaor S: Upregulation of 14-3-3 eta in chronic liver fluke
infection is a potential diagnostic marker of cholangiocarcinoma.
Proteomics Clin Appl. 10:248–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maiorano D, Cuvier O, Danis E and Méchali
M: MCM8 is an MCM2-7-related protein that functions as a DNA
helicase during replication elongation and not initiation. Cell.
120:315–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volkening M and Hoffmann I: Involvement of
human MCM8 in prereplication complex assembly by recruiting hcdc6
to chromatin. Mol Cell Biol. 25:1560–1568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotsantis P, Silva LM, Irmscher S, Jones
RM, Folkes L, Gromak N and Petermann E: Increased global
transcription activity as a mechanism of replication stress in
cancer. Nat Commun. 7:130872016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hills SA and Diffley JF: DNA replication
and oncogene-induced replicative stress. Curr Biol. 24:R435–R444.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He DM, Ren BG, Liu S, Tan LZ, Cieply K,
Tseng G, Yu YP and Luo JH: Oncogenic activity of amplified
miniature chromosome maintenance 8 in human malignancies. Oncogene.
36:3629–3639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng YP, Zhu Y, Yin LD, Zhang JJ, Guo S,
Fu Y, Miao Y and Wei JS: The expression and prognostic roles of
MCMs in pancreatic cancer. PLoS One. 11:e01641502016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song X, Liu X, Wang H, Wang J, Qiao Y,
Cigliano A, Utpatel K, Ribback S, Pilo MG, Serra M, et al: Combined
CDK4/6 and Pan-mTOR inhibition is synergistic against intrahepatic
cholangiocarcinoma. Clin Cancer Res. 25:403–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samukawa E, Fujihara S, Oura K, Iwama H,
Yamana Y, Tadokoro T, Chiyo T, Kobayashi K, Morishita A, Nakahara
M, et al: Angiotensin receptor blocker telmisartan inhibits cell
proliferation and tumor growth of cholangiocarcinoma through cell
cycle arrest. Int J Oncol. 51:1674–1684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Ji G, Han S, Shao Z, Lu Z, Huo L,
Zhang J, Yang R, Feng Q, Shen H, et al: Tip60 Suppresses
cholangiocarcinoma proliferation and metastasis via PI3k-AKT. Cell
Physiol Biochem. 50:612–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng R, Zhang PF, Zhang C, Huang XY, Ding
YB, Deng B, Bai DS and Xu YP: Elevated TRIM44 promotes intrahepatic
cholangiocarcinoma progression by inducing cell EMT via MAPK
signaling. Cancer Med. 7:796–808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Liang Y, Yang G, Lan Y, Han J,
Wang J, Yin D, Song R, Zheng T, Zhang S, et al: Tetraspanin 1
promotes epithelial-to-mesenchymal transition and metastasis of
cholangiocarcinoma via PI3K/AKT signaling. J Exp Clin Cancer Res.
37:3002018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang W, Wang Y, Li W, Shi L and Geng Z:
MicroRNA-551b-3p inhibits tumour growth of human cholangiocarcinoma
by targeting Cyclin D1. J Cell Mol Med. 23:4945–4954. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitchen P, Lee KY, Clark D, Lau N,
Lertsuwan J, Sawasdichai A, Satayavivad J, Oltean S, Afford S,
Gaston K and Jayaraman PS: A runaway PRH/HHEX-notch3-positive
feedback loop drives cholangiocarcinoma and determines response to
CDK4/6 inhibition. Cancer Res. 80:757–770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang MX, Gan W, Jing CY, Zheng SS, Yi Y,
Zhang J, Xu X, Lin JJ, Zhang BH and Qiu SJ: S100A11 promotes cell
proliferation via P38/MAPK signaling pathway in intrahepatic
cholangiocarcinoma. Mol Carcinog. 58:19–30. 2019. View Article : Google Scholar : PubMed/NCBI
|