Introduction
Prostate cancer is one of the major causes of
increased morbidity and mortality worldwide. It is also the most
commonly diagnosed type of cancer among men (1). Currently, the main treatments for
prostate cancer are surgery, radiation and chemotherapy. Despite
improvements in traditional surgical and radiotherapy techniques,
the quality of life of patients with prostate cancer remains poor
following treatment (2). At present,
immunotherapy remains under development and a large number of
future clinical trials are required to evaluate its true
therapeutic potential (3). Currently
androgen blockers are the main therapeutic agents (4). A previous study reported that the
long-term use of the novel androgen receptor antagonists can lead
to the initiation of drug resistance mechanisms (5). Therefore, the development of additional
drug candidates for the treatment of prostate cancer is
particularly important.
Curcumol is a monomer compound isolated from the
Rhizome curcumae, which possesses anti-inflammatory,
anti-oxidation and anti-tumor pharmacological activities (6). Previously, the aromatization of curcumol
derivatives has been reported to exert anti-tumor effects (7). In addition, curcumol has a therapeutic
effect on liver fibrosis (8), liver
cancer (9), bladder cancer (10) and lung adenocarcinoma (11). Some researchers have also observed
that curcumol can not only inhibit the epithelial-to-mesenchymal
transition (EMT) of triple-negative breast cancer (12) but also enhance the drug sensitivity of
cancer cells (13). Thus, it has been
hypothesized that curcumol is a promising anti-tumor drug. However,
the role of curcumol in the treatment of prostate cancer remains to
be elucidated.
Therefore, the present study aimed to screen
curcumol sensitive prostate cancer cells by verifying the
pharmacological activity of curcumol. At the same time, the
internal regulatory pathway of curcumol on prostate cancer was
further investigated via in vitro cell experiments and in
vivo animal experiments. The present findings may provide
reliable scientific information for the treatment of prostate
cancer with curcumol.
Materials and methods
Cell culture and treatment
Prostate cancer cell lines (PC3, DU145, LNCaP) and
normal prostate cells (RWPE-1) were purchased from Shanghai
Zhongqiao Xinzhou Biotechnology Co., Ltd. PC3, DU145, LNCaP and
RWPE-1 cells were cultured in F-12 (Sigma-Aldrich; Merck KGaA), MEM
(Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd.), RPMI-1640
(Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd.) and special
medium (1% keratinocyte growth factor) (Shanghai Zhongqiao Xinzhou
Biotechnology Co., Ltd.) respectively, which were supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution (Beyotime Institute of
Biotechnology), at 37°C with 5% CO2 and saturated
humidity.
In order to screen the cell types and concentration
of curcumol (cat. no. B20342, Shanghai YuanYe Biotechnology Co.,
Ltd.), four types of cells (PC3, DU145, LNCaP and RWPE-1 cells) in
the logarithmic growth phase were selected. Curcumol was dissolved
into the original solution with a concentration of 10 mg/ml using
absolute ethanol and diluted using medium (F-12, MEM, RPMI-1640, or
special medium) (14). The specific
groups were as follows: 0 µg/ml (treated with 0 µg/ml curcumol), 25
µg/ml (treated with 25 µg/ml curcumol), 50 µg/ml (treated with 50
µg/ml curcumol) and 100 µg/ml (treated with 100 µg/ml curcumol).
The cells were treated with these different concentrations of
curcumol for 24 h at 37°C.
Based on curcumol concentration screening results,
it was found that the comparison between different types of cells
had significant statistical significance in the 50 µg/ml group.
Therefore, a concentration of 50 µg/ml was chosen. The following
groups were established: Control group (treated with the same
concentration of absolute ethanol and medium) and a Curcumol group
(treated with 50 µg/ml curcumol). The prepared cells were used in
the subsequent experiments.
Cell transfection and treatment
To investigate the effects of curcumol and miR-9 on
PC3 cells, cells in the logarithmic growth phase were divided into
the following groups: Control group (blank control group), negative
control (NC) group (transfected with NC inhibitor), a miR-9
inhibitor group (transfected with miR-9 inhibitor), Curcumol group
(treated with 50 µg/ml curcumol + NC) and miR-9 inhibitor +
curcumol group (treated with miR-9 inhibitor + 50 µg/ml
curcumol).
For verify the transfection efficiency of miR-9
mimics, cells were divided into the following groups: NC group
(transfected with NC mimics) and miR-9 mimics group (transfected
with miR-9 mimics).
The NC inhibitor, miR-9 inhibitor, NC mimics, and
miR-9 mimics were purchased from HonorGene.
Lipofectamine® 2000 (5 µl; Thermo Fisher Scientific,
Inc.) was used for 5 µl miR-9 inhibitor (NC inhibitor) or miR-9
mimics (NC mimics) transfection into cells at 37°C for 6 h. The
concentration of miR-9 inhibitor, NC inhibitor, miR-9 mimics and NC
mimics was 50 nM. After transfection for 48 h, the curcumol group
and miR-9 inhibitor + curcumol group were treated with 50 µg/ml
curcumol for 24 h at 37°C. The prepared cells were immediately used
in the subsequent experiments. The sequence of has-miR-9 inhibitor
was 5′-UCAUACAGCUAGAUAACCAAAGA-3′. The sequence of NC inhibitor was
5′-CAGUACUUUUGUGUAGUACAA-3′. The sequence of miR-9 mimics was
5′-AUAAAGCUAGAUAACCGAAAGU-3′. The sequence of NC mimics was
5′-UUCUCCGAACGUGUCACGU-3′.
Cell Counting Kit (CCK)-8 assay
Cell Counting Kit-8 (cat. no. NU679; Dojindo
Molecular Technologies, Inc.) was used according to the
manufacturer's instructions. The prepared cells were digested and
counted. Cells were inoculated in 96-well plates at a density of
1×104 cells/well. In total, five duplicate wells were
used. After cells adhered, they were grouped as aforementioned.
Drug-containing medium was removed. The prepared CCK-8 solution
(final concentration 10 µl/well) was added. After incubation at
37°C with 5% CO2 for 4 h, the absorbance was measured at
450 nm using a BioTek plate analyzer (BioTek Instruments,
Inc.).
Wound healing assay
PC3 cells in the logarithmic growth phase were
selected and divided into a Control group (blank control group) and
a Curcumol group (50 µg/ml curcumol group). In total, three
repeated experiments were set in each group. The 6-well plates were
evenly drawn with horizontal lines. Trypsin was utilized to digest
PC3 cells. Following cell counting, ~5×105 cells were
added to each well. After the cells were added onto the plate, a
100 µl pipette tip was used to create a scratch perpendicular to
the horizontal line previously drawn. Sterile PBS was used to wash
the cells and it was repeated three times. Serum-free DMEM was
added. The following time points were selected: 0, 24 and 48 h and
three fields were selected for each time point. The cells were
observed under a light microscope at magnification, ×100. The
culture conditions were 37°C and 5% CO2.
Transwell assay
For the cell invasion assay, Matrigel, Transwell
plates (24-well, 8.0 µm pore membranes) and sterile hypodermic
needles were pre-cooled overnight at 4°C in advance. Each well was
added with 60 µl diluted matrix gel. After incubation at 37°C for
30 min, the supernatant was removed. Then, 500 µl 10% FBS-complete
medium was placed in the lower chamber. Trypsin was utilized to
digest cells. Cells were resuspended to 2×106 cells/ml
on a serum-free medium and 100 µl cells were added to each well.
After incubation at 37°C for 48 h, the upper chamber was removed
and washed with PBS. Then, 4% paraformaldehyde was used for
fixation at room temperature for 20 min and 0.1% crystal violet was
applied for staining at room temperature for 5 min. A light
microscope was used for observation, with three fields of view at a
magnification, ×100. The cavity was decolorized in 500 µl 10%
acetic acid solution. An enzyme plate analyzer was used to
determine the optical density (OD) value at 550 nm, repeated three
times.
For the cell migration assay, 500 µl 10% FBS
complete medium (Gibco; Thermo Fisher Scientific, Inc.) was placed
in the lower layer of the chamber. Each well was added with 100 µl
cells (1×106 cells/ml). After incubation at 37°C for 48
h, the upper chamber was removed and washed with PBS. Methanol and
acetone were prepared to form a stationary solution (equal volume
mix). After being fixed at room temperature for 20 min by mixing
methanol and acetone into a fixed solution, the cells were stained
with 0.1% crystal violet at room temperature for 5 min. A light
microscope was used for observation, with three fields of view at
magnification, ×100. Then, 500 µl 10% acetic acid solution was used
for decolorization. A microplate analyzer was applied to determine
the OD value at 550 nm, repeated three times.
Flow cytometry
The prepared PC3 cells were removed and digested
with trypsin (without EDTA). PBS was used to wash the cells and
then 1–5×105 cells were collected. The following steps
were performed according to the manufacturer's instructions of the
Annexin V-FITC cell apoptosis detection kit (cat. no. KGA108;
Nanjing KeyGen Biotech Co., Ltd.). In total, 500 µl Binding buffer
was added to suspend the cells. Then, 5 µl Annexin V-FITC and 5 µl
PI were added successively and mixed. The reaction was performed at
room temperature in the dark for 10 min. A flow cytometer
(A00-1-1102; Beckman Coulter, Inc.) was used for observational
detection. The apoptotic rate was calculated (the percentage of
early + late apoptotic cells).
Western blotting
Briefly, 200 µl RIPA lysate (cat. no. P0013B;
Beyotime Institute of Biotechnology) was used to extract total
protein from 0.025 g tissues or 2×106 cells. After lysis
of tissue homogenate or cell suspension on ice for 10 min, the
supernatant was obtained by centrifugation with 13,280 × g at 4°C
for 15 min. A BCA Protein Assay Kit (cat. no. P0012S; Beyotime
Institute of Biotechnology) was used to determine protein
concentration according to the manufacturer's instructions. The
mass of protein loaded per lane was ~20 µg. 10% polypropylene gel
was configured. Polyacrylamide gel electrophoresis was performed
with a constant voltage of 75 V at room temperature. The protein
was transferred to the nitrocellulose membrane at a constant
current of 300 mA. Next, 5% skimmed milk was used to block the
membrane at 4°C overnight. The primary antibody was incubated for
90 min at room temperature. The following primary antibodies were
used: PDK1 (cat. no. ab202468; Abcam; dilution 1:2,000), total AKT
(cat. no. ab179463; Abcam; dilution 1:10,000), phosphorylated
(p)-AKT (cat. no. ab131443; Abcam; dilution 1:500), total mTOR
(cat. no. ab32028; Abcam; dilution 1:1,000), p-mTOR (cat. no.
ab109268; Abcam; dilution 1:1,000) and β-actin (cat. no.
66009-1-Ig; ProteinTech Group, Inc.; dilution 1:5,000). The
secondary antibodies HRP goat anti-mouse IgG (cat. no. SA00001-1;
ProteinTech Group, Inc.; dilution 1:5,000) and HRP goat anti-rabbit
IgG (cat. no. SA00001-2; ProteinTech Group, Inc.; dilution 1:6,000)
were incubated at room temperature for 90 min. ECL solution (cat.
no. K-12045-D50; Advansta, Inc.) was used for visualization in a
darkroom. Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc.) was used to analyze the protein expression.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
When the cell density reached 80%, total RNA was
extracted using TRIzol® (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, the RNA
concentration was measured with an ultraviolet spectrophotometer.
The mixture was incubated at 50°C for 50 min, followed by 85°C for
5 min and then cooled on ice. HiFiScript cDNA Synthesis kit (cat.
no. CW2569M; CWBIO) and SYBR-Green PCR Master Mix (cat. no.
CW2601S; CWBIO) were used according to the manufacturer's
protocols. A 30 µl amplification system was used, with three
replicates and 40 cycles. A two-step method was used for
amplification, which included: Extending at 95°C for 15 min,
denaturing at 95°C for 15 sec, and annealing at 60°C for 30 sec.
The 2−∆∆Cq value (15) was
utilized to reflect the sample gene expression levels relative to
the ratio of the control sample. The primer sequences were as
follows: H-U6 forward (F), 5′-CTCGCTTCGGCAGCACA-3′ and reverse (R),
5′-AACGCTTCACGAATTTGCGT-3′, product length 125 bp; hsa-miR-9,
5′-CATTATTACTTTTGGTACGCG-3′; H-actin F, 5′-ACCCTGAAGTACCCCATCGAG-3′
and R, 5′-AGCACAGCCTGGATAGCAAC-3′, product length 224 bp; and
H-PDK1 F, 5′-CTTTCTGTCCCCACCGCACA-3′ and R,
5′-GCAGAAGCCTCCAGAAACTCACA-3′, product length 236 bp. These
experiments were repeated three times.
Dual luciferase reporter gene
assay
The potential binding sites between miR-9 and PDK1
were predicted using TargetScan online software (http://www.targetscan.org/vert_72/). After the
cells were transfected with NC mimics and miR-9 mimics, the
expression of miR-9 was measured for transfection efficiency. Cells
were divided into the wild-type (wt)-PDK1 + NC mimics group
(wt-PDK1 + NC), the wt-PDK1 + miR-9 mimics group (wt-PDK1 + miR-9
mimics), the mutant (Mut)-PDK1 + NC mimics group (Mut-PDK1 + NC)
and the Mut-PDK1 + miR-9 mimics group (Mut-PDK1 + miR-9 mimics).
pHG-MirTarget-WT PDK1-3U, pHG-MirTarget-mut PDK1-3U, miR-9 mimics,
NC mimics and 293A cells were purchased from HonorGene. According
to the instructions of the Dual-Luciferase Reporter assay system
(cat. no. E1910; Promega Corporation), 1XPLB lysis buffer, LAR II
and Stop&Glo buffer were prepared. After transfection for 48 h,
the luciferase activity was determined with a GloMax20/20
chemiluminescence detector (Promega Corporation).
Animal model
A total of 30 BALB/C male nude mice (age, 6 weeks;
weight, 13~15 g) were purchased from the SLAC Laboratory. Mice were
housed with conditions of 22–24°C, 12 h light/dark cycle and 40–60%
relative humidity. The feeding conditions of mice were as described
in the previous literature (16).
After adaptive feeding for 1 week, the mice were randomly divided
into five groups (6 mice in each group): A Control group, an NC
group, a miR-9 inhibitor group, a Curcumol group and a miR-9
inhibitor + curcumol group. PC3 cells were transfected with NC
inhibitor and miR-9 inhibitor as aforementioned. The mice were
subcutaneously injected with 5×106 transfected cells.
When tumor growth reached 100 mm3, the curcumol
administration group received curcumol (20 mg/kg) peritoneal
injection every 2 days (13,17). The tumor was measured twice a week.
The tumor volume was calculated as length × width2 ×
0.5. When the maximum tumor volume reached 2,000 mm3,
the mice were sacrificed via an intraperitoneal injection of
pentobarbital sodium (150 mg/kg). The tumor was removed to prepare
for further experiments.
All of the experiments were conducted in accordance
with the recommendation and approval of the Animal Welfare
Committee of Hunan University of Chinese Medicine (approval no.
2019-0019).
Immunohistochemistry
The tissues were sectioned at 3 µm and fixed with 4%
paraformaldehyde at room temperature for 24 h. The tumor tissue was
dewaxed.
After EDTA buffer was boiled, the slices were
immersed in a boiling water bath for 22 min. Endogenous peroxidase
was inactivated with 1% periodate acid at room temperature for 10
min. Diluted Ki67 (cat. no. ab15580; Rabbit; Abcam) with dilution
1:100 was added and it was incubated overnight at 4°C. The
ready-to-use type of secondary antibody (cat. no. PV-9001; OriGene
Technologies, Inc.) was added and incubated at 37°C for 30 min.
After DAB color development at room temperature for ~10 sec, the
nuclei were stained at room temperature for 1 min. The sections
were sealed using neutral resin and observed under a light
microscope (BA210; Motic; magnification, ×400).
Statistical analysis
SPSS 25.0 software (IBM Corp.) and GraphPad 8.0
(GraphPad Software, Inc.) were used for statistical analysis and
the measurement data are presented as the mean ± standard
deviation. Unpaired t-test, one-way ANOVA and two-way ANOVA
analysis were used. Tukey's multiple and Sidak's multiple
comparisons tests were employed. P<0.05 was considered to
indicate a statistically significant difference.
Results
Curcumol cytotoxicity assay and
screening of sensitive cells
To test the cytotoxicity of curcumol and drug
sensitivity of prostate cancer cells, PC3, DU145, LNCaP and RWPE-1
cells were treated with different concentrations of curcumol (0,
25, 50 and 100 µg/ml). The CCK-8 results demonstrated that curcumol
had no significant effect on the proliferation of RWPE-1 cells.
Compared with DU145 and LNCaP cells, PC3 cells were the most
sensitive to curcumol (Fig. 1). In
the 50 µg/ml group, the comparison between different types of cells
had significant statistical significance. Thus, PC3 cells and 50
µg/ml curcumol were selected for subsequent experiments.
Curcumol inhibits the development of
PC3 cells and regulates the PDK1/AKT/mTOR signaling pathway
Next, the effect of curcumol on PC3 cells was
examined. The wound healing assay results revealed that the
migration of PC3 cells in the curcumol group was significantly
lower compared with the control group (Fig. 2A). Transwell (Fig. 2B) and flow cytometry (Fig. 2C) assays were conducted to detect the
invasion and apoptosis of PC3 cells. The results indicated that
curcumol inhibited the invasive activity of PC3 cells and increased
their apoptosis rate. Next, the PDK1/AKT/mTOR signaling pathway was
examined at the protein expression level. The results demonstrated
that curcumol could decrease the expression level of PDK1. In
addition, curcumol inhibited the phosphorylation levels of AKT and
mTOR (Fig. 2D).
miR-9 targets PDK1
The previous study has reported that curcumol can
regulate the expression level of miR-9 (17). The RT-qPCR results suggested that
curcumol could upregulate the expression level of miR-9 (Fig. 3A). Following treatment with curcumol,
the expression of miR-9 was significantly different in the PC3
cells (Fig. 3C). Following treatment
with 50 µg/ml curcumol, the expression of miR-9 was upregulated in
three prostate cancer cell lines and the difference of miR-9
expression was most obvious in PC3 cells. PC3 cells were treated
with different concentrations of curcumol (0, 25, 50 and 100
µg/ml). The expressions of miR-9 increased with the increase of
curcumol concentration (Fig. 3B).
This suggested that curcumol could promote the expression of miR-9.
No significant difference was observed in the level of miR-9
between the 50 µg/ml group and 100 µg/ml groups. Therefore, 50
µg/ml curcumol was used in the subsequent experiments. After the
cells were transfected with NC mimics and miR-9 mimics, the
expression of miR-9 was measured for transfection efficiency
(Fig. 3D). Based on the TargetScan
online software prediction, it was identified that miR-9 might have
a direct targeting relationship with PDK1 (Fig. 3E). Therefore, a luciferase reporter
assay was utilized to verify the relationship between miR-9 and
PDK1. The results revealed that miR-9 could directly target PDK1
(Fig. 3F).
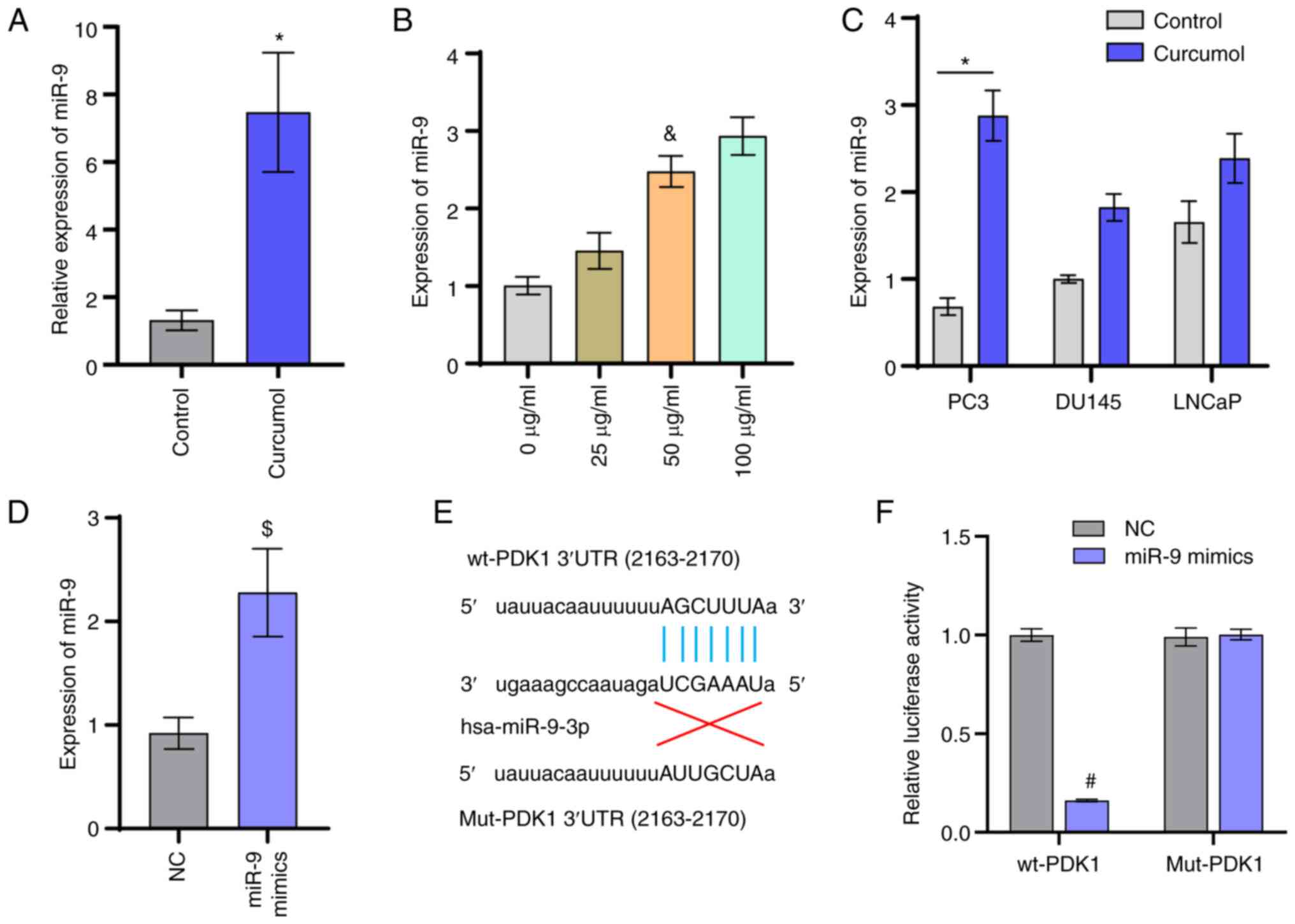 | Figure 3.miR-9 targets PDK1. (A) Gene
expression level of miR-9. (B) Following treatment with different
concentrations of curcumol (0, 25, 50, and 100 µg/ml) for 24 h, the
expression of miR-9 was determined. (C) Expression of miR-9 in
prostate cancer cell lines (PC3, DU145, and LNCaP). (D) Following
transfection with NC mimics and miR-9 mimics, the expression of
miR-9 was determined. (E) Diagram of PDK1 and miR-9 interactions.
(F) Luciferase reporter assay examining miR-9 and PDK1. t-test,
one-way ANOVA and two-way ANOVA analysis were used. *P<0.05 vs.
Control group; &P<0.05 vs. 0 µg/ml;
$P<0.05 vs. NC group; #P<0.05 vs. wt +
NC group. miR, microRNA; PDK1, pyruvate dehydrogenase kinase 1; NC,
negative control; wt, wild-type; Mut, mutant. |
Curcumol regulates the PDK1/AKT/mTOR
signaling pathway and affects the activity of PC3 cells via
miR-9
To determine whether the regulation of the
PDK1/AKT/mTOR signaling pathway by curcumol was mediated by
regulating the expression of miR-9, PC3 cells were transfected with
miR-9 inhibitor and treated with curcumol. The successful
transfection of miR-9 was verified via RT-qPCR (Fig. 4A). It was found that the expression
level of PDK1 in the miR-9 inhibitor group was significantly higher
compared with that in the control and NC groups. Compared with the
miR-9 inhibitor group, the expression level of PDK1 in the miR-9
inhibitor + Curcumol group was significantly decreased (Fig. 4B). This suggested that curcumol could
affect the expression of PDK1 via miR-9. The results at the protein
level also indicated that curcumol could regulate the PDK1/AKT/mTOR
signaling pathway via miR-9 (Fig.
4C). In addition, the experimental results of CCK-8 (Fig. 5A), Transwell (Fig. 5B and C) and flow cytometry (Fig. 5D) assays further indicated that
curcumol could affect the activity of PC3 cells via miR-9.
Curcumol inhibits tumor growth in
mice
In order to evaluate the effect of curcumol on tumor
formation in nude mice, relevant animal models were constructed and
treated with curcumol. Tumor volume was measured at different time
points in vivo (Fig. 6A). The
results demonstrated that curcumol significantly inhibited tumor
growth. Compared with the curcumol group, the tumor volume of the
miR-9 inhibitor + curcumol group was significantly increased. It
was hypothesized that curcumol inhibited tumor growth via miR-9.
Following removal, the volume and mass of the tumor were also
consistent with this hypothesis (Fig. 6B
and C).
Curcumol inhibits the development of
prostate cancer and the activation of the PDK1/AKT/mTOR signaling
pathway via miR-9
To further verify the effect of miR-9 on curcumol in
prostate cancer, a series of experimental tests were performed on
the removed tumor bodies. RT-qPCR was used to verify the expression
level of miR-9 in each group (Fig.
7A). Examination of the gene and protein expression levels
revealed that the miR-9 inhibitor could alleviate the inhibition of
curcumol on PDK1, p-AKT and p-mTOR expression (Fig. 7B and C). Thus, in vivo, it was
hypothesized that the miR-9 inhibitor might reduce the inhibitory
effect of curcumol on the PDK1/AKT/mTOR signaling pathway. Ki67
expression (Fig. 7D) indicated the
inhibition of curcumol on prostate cancer. Combined with the above
experimental results, miR-9 could mediate curcumol to inhibit the
development of prostate cancer cells and the PDK1/AKT/mTOR pathway,
at least in part.
Discussion
The present study demonstrated that curcumol had no
cytotoxicity or side effects on normal prostate cells. The
inhibitory effect on prostate cancer cells was clear. PC3, LNCaP
and DU145 cells demonstrated different drug sensitivities to
curcumol. In a previous study examining the sensitivity of prostate
cancer cells to docetaxel, it was reported that rapamycin could
resist docetaxel-induced apoptosis and that rapamycin can induce
autophagy in PC3 cells but not in DU145 cells (18). Pickard et al (19) also demonstrated that docetaxel
produced significant toxicity in PC3 cells, but no toxicity was
observed in LNCaP cells. The studies above reveal that PC3 cell
lines are more sensitive to drug toxicity compared with LNCaP and
DU145 cell lines. This was consistent with the findings of the
present study that PC3 cells were more sensitive to curcumol than
LNCaP and DU145 cell lines. Combined with the expression of miR-9
in different cell lines, it is reasonable to hypothesize that the
sensitivity of these three prostate cancer cell lines (PC3, LNCaP
and DU145 cell lines) to curcumol is different, which may be
mediated by miR-9. However, the specific mechanism by which
curcumol regulates miR-9 remains to be elucidated.
Curcumol, a naturally extracted drug, has been used
in the study of a variety of types of cancer. In bladder cancer
cells, curcumol can affect cell proliferation and apoptosis by
targeting enhancer of zeste homolog 2 and regulating the
mitochondrial apoptosis pathway (10). Curcumol can affect cell proliferation
by increasing reactive oxygen species, decreasing mitochondrial
membrane potential and downregulating isocitrate dehydrogenase 1 in
gastric adenocarcinoma (20). In
hepatocellular carcinoma cells, curcumol inhibits the expression of
programmed cell death-ligand 1 through hypoxia-inducible factor-1α
and signal transducer and activator of transcription 3 signaling
pathways, restoring the tumor-killing ability of cytotoxic T cells
(9). All these suggested that
curcumol has a certain anti-tumor activity. The regulatory pathway
of curcumol may be different in different cell types. In addition,
curcumol may be a developmental neurotoxic substance that may
affect the neurites' growth via the neural cell adhesion
molecule/focal adhesion kinase signaling pathway (21). Wang et al (22) also reported that curcumol had a
certain inhibitory effect on CD4+ T cells. Collectively,
these results suggest that curcumol may affect the body's
anti-tumor immune regulation in vivo and hinder its
anti-tumor activity. Notably, a previous study has shown that
curcumol can induce cancer cell cycle arrest by inhibiting the
insulin-like growth factor 1 receptor/PI3K/AKT pathway (23). Curcumol inhibits the development of
melanoma (17) and colorectal cancer
(14) through PI3K/AKT pathway. All
these studies suggest that curcumol may affect tumor development
through the PI3K/AKT pathway, at least in part. The results of the
present study also demonstrated that curcumol inhibited the
development of prostate cancer and regulated the PDK1/AKT/mTOR
signaling pathway. Thus, the PDK1/AKT/mTOR signaling pathway might
be a possible pathway for curcumol to affect the development of
prostate cancer.
The present study further investigated the internal
regulatory pathways of curcumol inhibition of PC3 cell activity.
The results indicated that curcumol could significantly upregulate
the expression level of miR-9. It has been revealed that miR-9 was
differentially expressed in young and elderly patients with
prostate cancer (24). Thus, miR-9
may be involved in the development of prostate cancer. A previous
study has shown that miR-9 can promote the development of multiple
myeloma by regulating the tripartite motif-containing protein
56/NF-κB pathway (25). miR-9 can
promote cell migration and invasion of synovial osteosarcoma by
directly targeting E-cadherin (26).
miR-9 can induce angiogenesis by targeting sphingosine-1-phosphate
receptor 1 (27). All these results
suggest that miR-9 may be an oncogene. By contrast, the current
study found that in PC3 cells, curcumol could regulate the
downstream signaling pathway by upregulating the expression of
miR-9, thus affecting the proliferation, migration, invasion and
apoptosis of PC3 cells. Some studies have shown that miR-9 can
inhibit cancer. Wang et al (28) also reported that miR-9 could inhibit
colorectal cancer cell invasion and EMT by targeting forkhead box
P2. miR-9 could inhibit the proliferation and invasion of
pancreatic cancer cells by targeting glutamate oxaloacetate
transaminase 1 (29). miR-9 in
exosomes can inhibit angiogenesis in nasopharyngeal carcinoma by
targeting Midkine (30). These
findings support the conclusion of the current study that miR-9
could also act as a tumor suppressor gene to alleviate tumor
progression. Therefore, it was concluded that the internal
regulatory mechanism of miR-9 is different in different
environments and that its role will also be different.
The in vivo and in vitro results
demonstrated that miR-9 at least partly mediated the inhibitory
effect of curcumol on prostate cancer. Following prediction
analysis using the TargetScan website and luciferase reporter
assays, it was identified that miR-9 could directly target PDK1.
The present study found that the expression level of PDK1 in PC3
cells was significantly altered after curcumol treatment. In
glioblastoma, it has been reported that activation of the
PDK1/c-Jun pathway can induce EMT and promote the proliferation and
invasion of tumor cells (31). In a
related study of hypopharyngeal cancer, PDK1 has been shown to
induce EMT and promote cancer cell invasion (32). In ovarian cancer, PDK1 can regulate
tumor-mesothelial adhesion and angiogenesis, as well as promoting
cancer cell invasion (33). However,
reduced PDK1 expression can inhibit the proliferation, migration
and EMT of renal cancer cells (34).
These findings were similar to the results of the current study,
that the expression level of PDK1 in cancer cells was higher
compared with that in the curcumol treatment group. By contrast,
PDK1 acts as an independent driver of the PI3K/AKT/mTOR signaling
pathway (35). This further confirms
the hypothesis of the present study, that curcumol regulated the
PDK1/AKT/mTOR signaling pathway via miR-9 and affected the
progression of prostate cancer.
Considering the limited experimental conditions,
there remain several deficiencies in the current study. Due to
limited time and funding availability, it did not include the
pharmacokinetics of non-injected curcumol in humans. At present,
the specific mechanism of the resistance of different prostate
cancer cell lines to curcumol is unable to be fully determined due
to a shortage of funds. The mechanism of miR-9 upregulation caused
by curcumol therapy has not been fully elucidated due to
limitations of the experimental conditions. The question of whether
inhibition of the PDK1/AKT/mTOR signaling pathway is the cause of
inhibition of curcumol-mediated prostate cancer cell proliferation
has not yet been completely determined. These unanswered questions
will be the focus of future research. Future studies will further
examine the mechanism of action and drug resistance of curcumol in
prostate cancer cells. The regulatory mechanism between curcumol
and miR-9 will also be further studied, as will the role of the
PDK1/AKT/mTOR signaling pathway in the influence of curcumol on the
progression of prostate cancer cells. The pharmacokinetics and
bioavailability data of curcumol in the human body also will be
further collect.
In conclusion, the present study demonstrated that
curcumol could upregulate the expression level of miR-9 and inhibit
the activity of PC3 cells. It was identified that miR-9 could
directly target PDK1 and regulate the PDK1/AKT/mTOR signaling
pathway. Therefore, curcumol regulated the PDK1/AKT/mTOR signaling
pathway and inhibited the development of prostate cancer through
miR-9. These findings provided a reliable scientific basis for the
treatment of prostate cancer with curcumol.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation (grant no. 81774324), and the Postgraduate
Innovation Project of Hunan Province (grant no. CX2018B463).
Availability of data and materials
The analyzed datasets generated during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WS and WX conceived and designed the experiments. JD
and XY performed the experiments. LL analyzed the data. LL and YW
confirm the authenticity of all the raw data. YW and QH drafted the
manuscript and revised it critically for important intellectual
content. WS, WX, and QH agreed to be accountable for all aspects of
the present study in ensuring that questions related to the
accuracy or integrity of any part of the present study are
appropriately investigated and resolved. All authors approved the
final manuscript.
Ethics approval and consent to
participate
All of experiments were conducted in accordance with
the recommendation and approval of Animal Welfare Committee of
Hunan University of Chinese Medicine (approval number:
2019-0019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sebesta EM and Anderson CB: The surgical
management of prostate cancer. Semin Oncol. 44:347–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cordes LM, Gulley JL and Madan RA: The
evolving role of immunotherapy in prostate cancer. Curr Opin Oncol.
28:232–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gourdin T: Optimization of therapies for
men with advanced prostate cancer: A review of recent developments
with a look toward the future. Curr Opin Oncol. 31:188–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu H, You L, Li Y, Zhao Z, Shi G, Chen Z,
Wang Z, Li X, Du S, Ye W, et al: Loss of a negative feedback loop
between IRF8 and AR promotes prostate cancer growth and
enzalutamide resistance. Cancer Res. 80:2927–2939. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei W, Rasul A, Sadiqa A, Sarfraz I,
Hussain G, Nageen B, Liu X, Watanabe N, Selamoglu Z, Ali M, et al:
Curcumol: From plant roots to cancer roots. Int J Biol Sci.
15:1600–1609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YQ, Li GZ, Dong Y, Ma X, Dong HJ, Wu QQ
and Zhao WJ: Orobanone analogues from acid-promoted aromatization
rearrangement of curcumol inhibit hypoxia-inducible factor-1
(HIF-1) in cell-based reporter assays. Bioorg Chem. 85:357–363.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li G, Lin J, Peng Y, Qin K, Wen L, Zhao T
and Feng Q: Curcumol may reverse early and advanced liver
fibrogenesis through downregulating the uPA/uPAR pathway. Phytother
Res. 34:1421–1435. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuo HX, Jin Y, Wang Z, Li MY, Zhang ZH,
Wang JY, Xing Y, Ri MH, Jin CH, Xu GH, et al: Curcumol inhibits the
expression of programmed cell death-ligand 1 through crosstalk
between hypoxia-inducible factor-1α and STAT3 (T705) signaling
pathways in hepatic cancer. J Ethnopharmacol. 257:1128352020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou L, Wei E, Zhou B, Bi G, Gao L, Zhang
T, Huang J, Wei Y and Ge B: Anti-proliferative benefit of curcumol
on human bladder cancer cells via inactivating EZH2 effector.
Biomed Pharmacother. 104:798–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Wang Z and Chen T: Curcumol
induces apoptosis via caspases-independent mitochondrial pathway in
human lung adenocarcinoma ASTC-a-1 cells. Med Oncol. 28:307–314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Dong Y and Liu H: Curcumol
enhances the anti-tumor effects of metformin via suppressing
epithelial-mesenchymal transition in triple-negative breast cancer.
Ann Transl Med. 8:9462020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng C, Fan D, Xu Y, Li X, Yuan J, Yang Q,
Zhou X, Lu J, Zhang C, Han J, et al: Curcumol enhances the
sensitivity of doxorubicin in triple-negative breast cancer via
regulating the miR-181b-2-3p-ABCC3 axis. Biochem Pharmacol.
174:1137952020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Wang J, Tao Y, Li X, Qin J, Bai Z,
Chi B, Yan W and Chen X: Curcumol inhibits colorectal cancer
proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt
pathways. Life Sci. 221:354–361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C, Zhang JJ, Zhang XP, Xiao R and Li
PG: Sporamin suppresses growth of xenografted colorectal carcinoma
in athymic BALB/c mice by inhibiting liver β-catenin and vascular
endothelial growth factor expression. World J Gastroenterol.
25:3196–3206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ning N, Liu S, Liu X, Tian Z, Jiang Y, Yu
N, Tan B, Feng H, Feng X and Zou L: Curcumol inhibits the
proliferation and metastasis of melanoma via the
miR-152-3p/PI3K/AKT and ERK/NF-κB signaling pathways. J Cancer.
11:1679–1692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cristofani R, Montagnani Marelli M,
Cicardi ME, Fontana F, Marzagalli M, Limonta P, Poletti A and
Moretti RM: Dual role of autophagy on docetaxel-sensitivity in
prostate cancer cells. Cell Death Dis. 9:8892018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pickard RD, Spencer BH, McFarland AJ,
Bernaitis N, Davey AK, Perkins AV, Chess-Williams R, McDermott CM,
Forbes A, Christie D and Anoopkumar-Dukie S: Paradoxical effects of
the autophagy inhibitor 3-methyladenine on docetaxel-induced
toxicity in PC-3 and LNCaP prostate cancer cells. Naunyn
Schmiedebergs Arch Pharmacol. 388:793–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zang S, Tang Q, Dong F, Liu H, Li L, Guo
F, Pan X, Lin H, Zeng W, Cai Z, et al: Curcumol inhibits the
proliferation of gastric adenocarcinoma MGC-803 cells via
downregulation of IDH1. Oncol Rep. 38:3583–3591. 2017.PubMed/NCBI
|
|
21
|
Yu C, Sun X and Niu Y: An investigation of
the developmental neurotoxic potential of curcumol in PC12 cells.
Toxicol Mech Methods. 26:635–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Wang Y, Jiang X, Wang Z, Zhong B
and Fang Y: The molecular mechanism of curcumol on inducing cell
growth arrest and apoptosis in Jurkat cells, a model of
CD4+ T cells. Int Immunopharmacol. 21:375–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Liu H, Wang J, Qin J, Bai Z, Chi B,
Yan W and Chen X: Curcumol induces cell cycle arrest and apoptosis
by inhibiting IGF-1R/PI3K/Akt signaling pathway in human
nasopharyngeal carcinoma CNE-2 cells. Phytother Res. 32:2214–2225.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valera VA, Parra-Medina R, Walter BA,
Pinto P and Merino MJ: microRNA expression profiling in young
prostate cancer patients. J Cancer. 11:4106–4114. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang G, Liu X, Zhao X, Zhao J, Hao J, Ren
J and Chen Y: miR-9 promotes multiple myeloma progression by
regulating TRIM56/NF-κB pathway. Cell Biol Int. 43:1223–1233. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu XZ, Li XA, Luo Y, Liu JF, Wu HW and
Huang G: miR-9 promotes synovial sarcoma cell migration and
invasion by directly targeting CDH1. Int J Biochem Cell Biol.
112:61–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao X, Xie L and Zeng Y: miR-9 promotes
angiogenesis via targeting on sphingosine-1-phosphate receptor 1.
Front Cell Dev Biol. 8:7552020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang WX, Yu HL and Liu X: miR-9-5p
suppresses cell metastasis and epithelial-mesenchymal transition
through targeting FOXP2 and predicts prognosis of colorectal
carcinoma. Eur Rev Med Pharmacol Sci. 23:6467–6477. 2019.PubMed/NCBI
|
|
29
|
Wang J, Wang B, Ren H and Chen W: miR-9-5p
inhibits pancreatic cancer cell proliferation, invasion and
glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun.
509:241–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu J, Liu QH, Wang F, Tan JJ, Deng YQ,
Peng XH, Liu X, Zhang B, Xu X and Li XP: Exosomal miR-9 inhibits
angiogenesis by targeting MDK and regulating PDK/AKT pathway in
nasopharyngeal carcinoma. J Exp Clin Cancer Res. 37:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo D, Xu X, Li J, Chen C, Chen W, Wang F,
Xie Y and Li F: The PDK1/c-Jun pathway activated by TGF-β induces
EMT and promotes proliferation and invasion in human glioblastoma.
Int J Oncol. 53:2067–2080. 2018.PubMed/NCBI
|
|
32
|
Jing P, Zhou S, Xu P, Cui P, Liu X, Liu X,
Liu X, Wang H and Xu W: PDK1 promotes metastasis by inducing
epithelial-mesenchymal transition in hypopharyngeal carcinoma via
the Notch1 signaling pathway. Exp Cell Res. 386:1117462020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siu MK, Jiang YX, Wang JJ, Leung TH, Ngu
SF, Cheung AN, Ngan HY and Chan KK: PDK1 promotes ovarian cancer
metastasis by modulating tumor-mesothelial adhesion, invasion, and
angiogenesis via α5β1 integrin and JNK/IL-8 signaling. Oncogenesis.
9:242020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou WM, Wu GL, Huang J, Li JG, Hao C, He
QM, Chen XD, Wang GX and Tu XH: Low expression of PDK1 inhibits
renal cell carcinoma cell proliferation, migration, invasion and
epithelial mesenchymal transition through inhibition of the
PI3K-PDK1-Akt pathway. Cell Signal. 56:1–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bamodu OA, Chang HL, Ong JR, Lee WH, Yeh
CT and Tsai JT: Elevated PDK1 expression drives PI3K/AKT/MTOR
signaling promotes radiation-resistant and dedifferentiated
phenotype of hepatocellular carcinoma. Cells. 9:7462020. View Article : Google Scholar : PubMed/NCBI
|





















