Introduction
Irradiation with specific wavelengths of light using
light-emitting diodes (LEDs) has several different effects on
living cells and organisms. In the field of anticancer treatment,
photodynamic therapy (PDT) and photothermal therapy (PTT), which
combines LED light with photosensitizers, have been developed. PDT
has gained attention as a novel minimally invasive alternative
therapy to chemoradiotherapy and it is being used for cancers such
as skin cancer, prostate cancer, lung cancer, brain tumors, and
esophageal cancer in clinical settings (1). There are also some reports on the
effects of PDT and PTT on colorectal cancer in vitro and
in vivo (2–4); however, blue LED light itself has
been demonstrated to be effective in killing certain types of
cells. Recent studies suggest that irradiation with blue visible
light inhibits tumor growth in various cancer cell lines (5–8). The
phototoxic and antiproliferative effects of blue light have been
reported to cause apoptosis (7),
autophagy (5), and cell cycle
arrest (8). Blue LED irradiation
also causes reactive oxygen species (ROS) production and DNA damage
in colorectal cancer cells (9);
however, the mechanism of photoreception in ‘nonvisual’ tumor cells
is unknown. We previously reported that blue LED light irradiation
at 465 nm (30 mW/cm2, 30 min) inhibits the proliferation
of colon cancer cells through the autophagy pathway (10). Furthermore, we found that the
photoreceptor opsin 3 (Opn3) promotes the cytostatic effect of blue
LED light (10).
Opsin is a G protein-coupled receptor (GPCR)
expressed in photoreceptors, such as retinal rods and cone cells.
It is activated by light and is involved in neurotransmission via G
proteins. In recent years, it has been revealed that Opn3 is widely
expressed not only in the retina but also in organs unrelated to
vision, such as the brain, respiratory epithelium, liver, kidney,
and heart, and it is thus referred to as a nonvisual opsin
(11,12). Opn3 functions as a receptor of blue
light and regulates GPCR signaling (13). Opn3 has an absorption maximum at
460–470 nm and activates the Gi/o subtypes of
G proteins, which inhibit adenylate cyclase and decrease cyclic
adenosine monophosphate (cAMP) (13,14).
Previous reports have shown that opsin is associated with
phototherapy as a photoreceptor for malignant melanocytes (15), and we reported that Opn3 is
associated with phototherapy as a photoreceptor in colon cancer
cells (10). However, the role of
photoreceptors in colorectal cancer in vivo and the effects
of blue LED on the tumor microenvironment are unknown.
In recent years, it has become evident that not only
tumor cells but also the tumor microenvironment, including
fibroblasts and immune cells, are extremely important in cancer
treatment. Fibroblasts are also activated by tumors to generate
cancer-associated fibroblasts (CAFs) and secrete various cytokines,
chemokines, growth factors and extracellular vesicles, and play an
important role in crosstalk in the tumor microenvironment and
increased tumor malignancy (16,17).
It has been reported that blue light significantly
decreases fibroblast-induced α-SMA protein expression in
fibroblastic diseases associated with elevated α-smooth muscle
actin (α-SMA) expression (18),
and it has been suggested that blue LED irradiation is a useful
tool for not only fibroblastic diseases but also the tumor
microenvironment.
The aim of this study was to investigate the impact
of blue LED light on colon cancer and the tumor microenvironment
and to examine the contribution of photoreceptors in
vivo.
Materials and methods
Cell culture
The human colon cancer cell lines HCT-116 and HT-29
(KAC Co., Ltd.; cat nos. EC91091005-F0 and EC91072201-F0) and human
intestinal fibroblasts (ScienCell Research Laboratories, Inc.; cat.
no. 2920) were used for in vitro and in vivo
experiments. The cells were tested for mycoplasma and confirmed to
be negative, and the cell lines were maintained and cultured in
accordance with international guidelines for proper cell culture
practices. The cells were cultured in RPMI-1640 medium (Wako Pure
Chemical Industries, Ltd.) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin
(Sigma-Adrich; Merck KGaA) in a 37°C humidified incubator
containing 5% CO2.
Animals
Sixteen 4 weeks old female nude mice (BALB/c) were
purchased from Charles River Japan, Inc. (Kanagawa, Japan). The
average weight of the mice used was 18.2±0.6 g. During the
experiment, the animals were maintained behind barriers under
controlled conditions and provided with water and a standard
laboratory diet for at least 7 days before use. The animals had
free access to tap water and food before and after surgery. Two
weeks after irradiation, the mice were sacrificed by cervical
dislocation and the tumors were removed for tissue processing.
The research was conducted in compliance with the
Division for Animal Research Resources, Institute of Health
Biosciences, University of Tokushima, Japan. The experiments and
procedures were approved by the Animal Care and Use Committee of
the University of Tokushima, Japan. All animal experiments were
performed in accordance with the U.K. Animals (Scientific
Procedures) Act, 1986 and associated guidelines (https://www.legislation.gov.uk/ukpga/1986/14/contents),
EU Directive 2010/63/EU for animal experiments (https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063),
and the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications No. 8023, revised 1978)
(https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf).
Orthotopic model
HCT-116 cells were harvested with 1 mM
ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS),
washed three times with PBS, and resuspended at a density of
1×107 cells/ml in PBS containing 500 mg/ml of Matrigel
(Becton Dickinson Labware). Animals were anesthetized with ether
and then placed in a supine position. A 7-mm incision was made in
the anterior rectal wall to prevent colonic obstruction caused by
rectal tumor progression. Suspended HCT-116 cells
(1×106) were slowly injected into the submucosa of the
posterior rectal wall using a 29-gauge needle. Long and short
diameters of the primary tumor mass were measured every week after
tumor implantation. Tumor volume was calculated using the following
formula: Tumor volume (mm3) =1/2×(long diameter)x(short
diameter)2.
LED irradiation
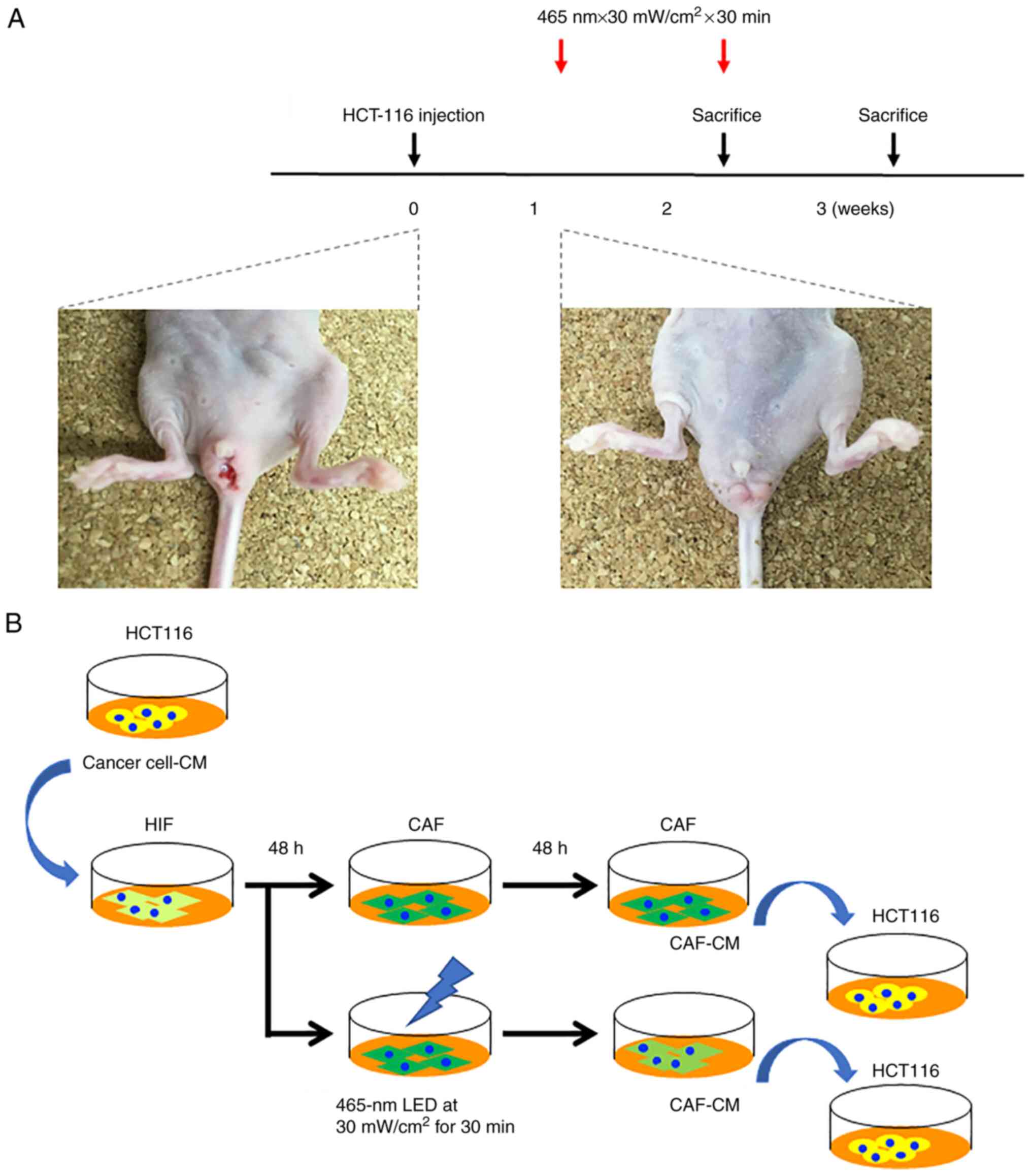
Sixteen mice developed rectal cancer at 7 days after
cell implantation and were randomized into four experimental groups
comprising four animals each and irradiated as in the following
settings in accordance with our previous reports (10,19):
i) LED irradiation (465 nm, 30 mW/cm2, 30 min, once)
group sacrificed 2 weeks after cell implantation; ii) LED
irradiation (465 nm, 30 mW/cm2, 30 min, 1 day/week)
group sacrificed 3 weeks after cell implantation; iii) control
group sacrificed 2 weeks after cell implantation; iv) control group
sacrificed 3 weeks after cell implantation (Fig. 1A). The blue LED light was
irradiated onto the tumor from the surface of the skin without any
surgical procedure.
Immunohistochemistry
The tissues resected from the orthotopic model or
cultured cells were fixed in 10% formalin (Wako Pure Chemical
Industries, Ltd.; cat. no. 066-03847) for 24 h at room temperature,
paraffin-embedded, and sliced to 4-µm thickness. Next, 3% BSA
(MACS® BSA Stock Solution; MACS Miltenyi Biotec; cat.
no. 130-091-376) was used for blocking for 1 h at room temperature,
and the anti-β-arrestin 1 antibody (15361-1-AP; Proteintech;
dilution 1:100), anti-Opn3 antibody (SAB2700986; Sigma-Adrich;
Merck KGaA; dilution 1:250), anti-TGF-β antibody (SAB4502954;
Sigma-Adrich; Merck KGaA; dilution 1:200) and anti-α-SMA antibody
(ab7817; Abcam K.K.; dilution 1:100) were used for immunostaining.
Secondary antibodies were Alexa Flour® 594 goat
anti-mouse lgG (A11005; Abcam K.K.; dilution 1:500) or Alexa
Flour® 488 goat anti-rabbit lgG (A11008; Abcam K.K.
dilution 1:500) for 1 h at room temperature. A KEYENCE BZ-710
fluorescence microscope (Keyence, Osaka, Japan) with ×20 or ×40
magnification was used for imaging.
RT-PCR analysis
Total RNA was isolated using the RNeasy Mini Kit
(Qiagen GmbH) and was reverse transcribed using a high-capacity
cDNA reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The 7500 real-time PCR system, TaqMan gene
expression assays on demand, and TaqMan universal master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to
perform quantitative real-time PCR. The following TaqMan primers
(identification number) were used: LC-3 (Hs01076567_g1), beclin-1
(Hs01061917_g1), α-SMA (Hs00426835_g1), IL-6 (Hs00985639_m1), and
PD-L1 (Hs00204257_m1). GAPDH (4326317E) was used as an internal
control for mRNA expression (all from Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermal cycling conditions were as
follows: 2 min at 50°C, 10 min at 95°C, and then 40 cycles of 15
sec at 95°C followed by 1 min at 60°C. Amplification data were
analyzed using the Prism 7500 Sequence Detection System ver. 1.3.1
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The Step One
Plus Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to perform RT-qPCR. The relative
abundance of target transcripts was evaluated and normalized to the
expression of GAPDH as an internal control using the
2−ΔΔCq method (20).
Preparation of the conditioned medium
(CM)
HCT-116 cells were cultured in a 10-cm dish up to
80% confluency. The cells were washed with PBS and then incubated
in FBS-free medium. After 48 h of incubation, the medium was
collected, centrifuged (750 × g, 10 min), and passed through a
0.2-µm filter to obtain cancer cell-CM. The CM was used without
additional FBS. CAF-derived cancer cell-CM was prepared by adding
cancer cell-CM to human intestinal fibroblasts for 48 h. The CAFs
of the irradiation group were treated with blue LED light (465 nm,
30 mW/cm2, 30 min). Next, the medium was changed once
and the supernatant was collected. These supernatants were used as
CAF-CM (Fig. 1B).
Migration and scratch assays
Transwell inserts (Corning, Inc.) with an 8-µm pore
size were used for the migration assays. Cancer cells
(2.0×104) were seeded into the upper chamber. After cell
attachment, the medium in the upper chambers was discarded and
fresh medium containing 1% FBS was added. Each CM containing 10%
FBS was added to the lower chamber. After incubation at 37°C for 24
h, the cells that migrated to the bottom of the Transwell membrane
were fixed with 4% paraformaldehyde for 15 min, stained with 0.2%
crystal violet solution (Wako Pure Chemical Industries, Ltd.) for
20 min at room temperature, and examined under a phase contrast
microscope (BX43; Olympus Corp.). The stained cells were counted in
three random fields per membrane (×100 magnification).
For the scratch assays, cancer cells were seeded at
a density of 2.0×104 cells/well in 6-well plates. Once
the cells attained confluency, a plastic pipette tip was scraped
across the center of the well to produce a 1-mm wide wound area.
The medium was discarded and then replenished with fresh DMEM
medium containing 1% FBS followed by the addition of corresponding
CM at a ratio of 1:1; the final FBS concentration was 0.5%, and the
cancer cells were cultured for a further 24 h at 37°C. Images of
the wound areas were captured using a phase-contrast microscope
(magnification, ×40; DP22-CU; Olympus Corp.) at 0 and 24 h after
scratching. The wound healing rates were calculated using ImageJ
v1.46r software (National Institutes of Health) and using the
following equation: Wound healing rate (%)=[area (0 h)-area (24
h)]/area (0 h) ×100.
Statistical analysis
All statistical analyses were performed using Stat
View version 5.0 software (SAS Institute). The Mann-Whitney U-test
and Wilcoxon signed-rank test were used for statistical
comparisons. Subsequently, the multiple comparisons were assessed
by Dunnett or Tukey's test. P-values of <0.05 were
considered statistically significant.
Results
Opn3 expression in colon cancer
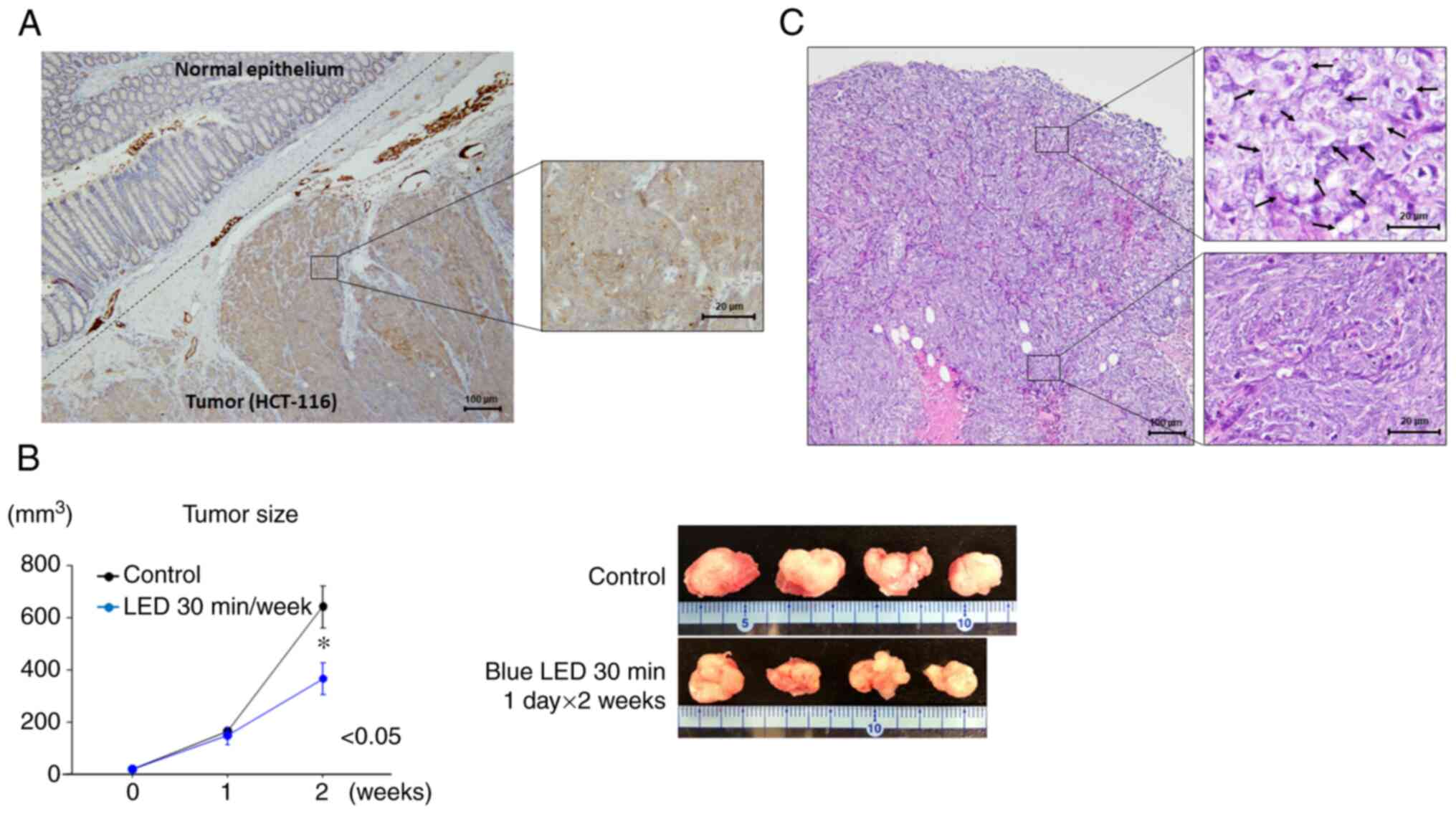
Opn3 expression was observed in colon cancer cells
by immunohistochemistry. No Opn3 expression was detected in normal
intestinal epithelial cells, whereas diffuse Opn3 expression was
observed in the cytoplasm of tumor cells (Fig. 2A).
Effects of blue LED irradiation on
colon cancer
Two weeks after the start of irradiation, the growth
of colon tumors was suppressed by blue LED irradiation (P=0.048)
(Fig. 2B). The largest tumor
diameter and volume were 15 mm and 750 mm3,
respectively. Regarding morphological changes, the tumor cells were
found to be swollen near the tumor surface following LED
irradiation (Fig. 2C). In the
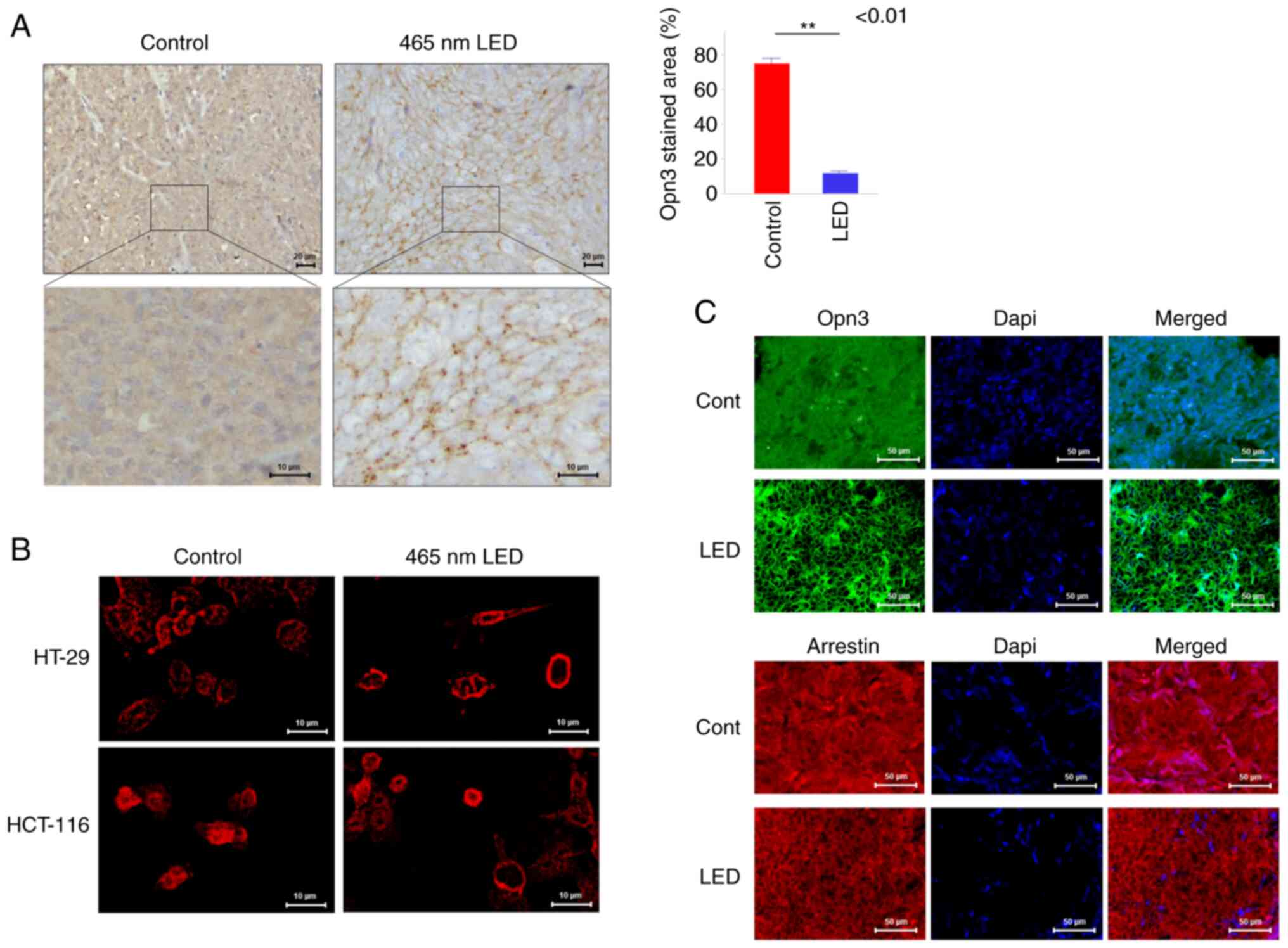
control group, Opn3 expression was diffuse throughout the
cytoplasm, whereas in the irradiation group, Opn3 expression was
observed at the cell membrane (Fig.
3A). Using immunostaining, the localization of Opn3 was
evaluated by the stained area relative to the cytoplasm, and the
stained area was significantly decreased in the irradiated group
(P<0.01). When this phenomenon was examined in vitro by
immunostaining, Opn3 expression was diffuse throughout the
cytoplasm in both colon cancer cell lines in the control group and
localized to the cell membrane in the LED group (Fig. 3B). Arrestin was also expressed in
the cytoplasm of the control group, and it was observed on the cell
membrane of the LED group in fluorescent immunostaining of the
orthotopic models (Fig. 3C). In
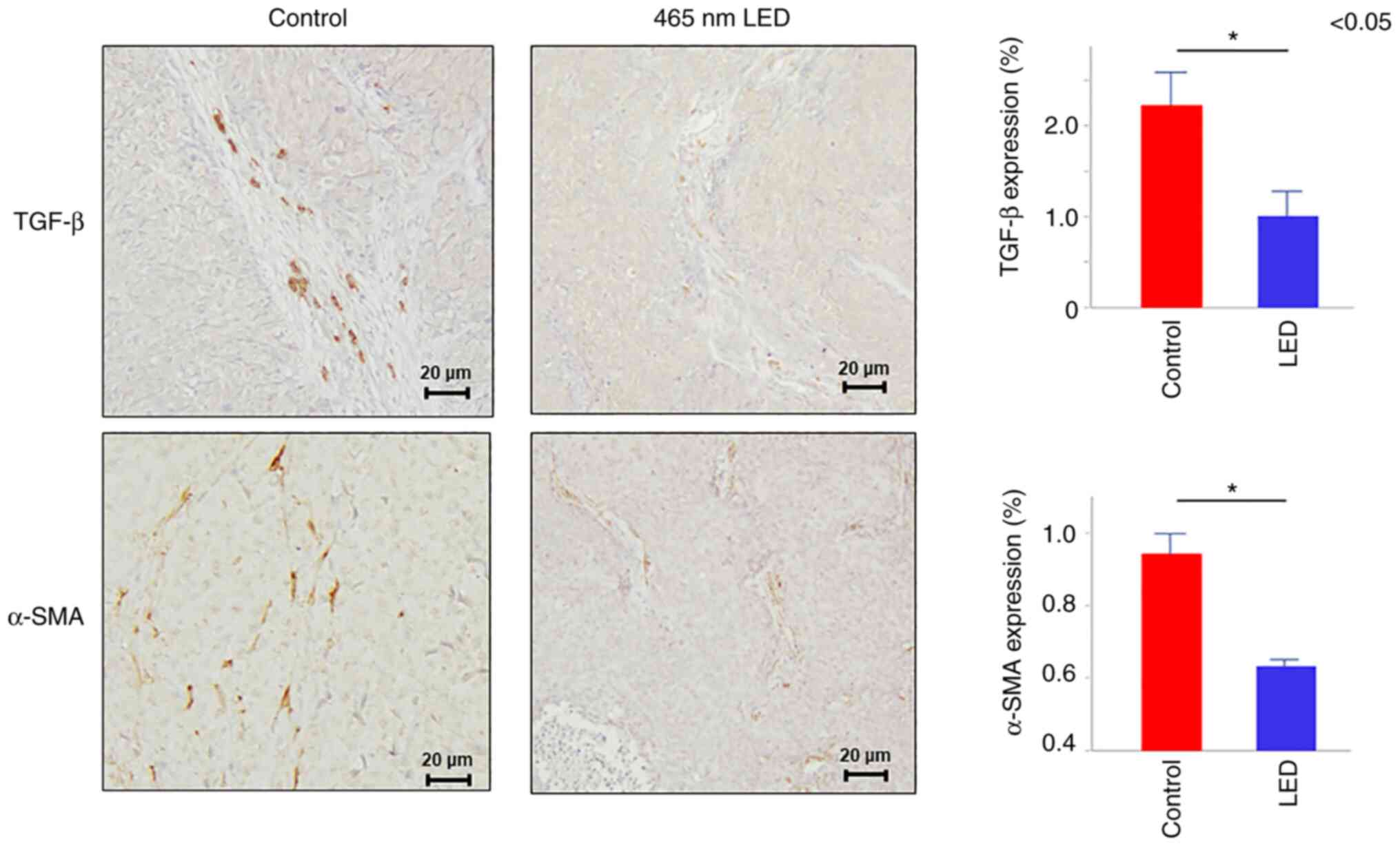
addition, immunostaining was performed and the results revealed
that transforming growth factor (TGF)-β and α-SMA expression levels
in the fibroblasts were attenuated in the LED group (TGF-β:
P=0.035, α-SMA: P=0.022) (Fig.
4).
Blue LED irradiation induces the
upregulation of autophagy-related factors
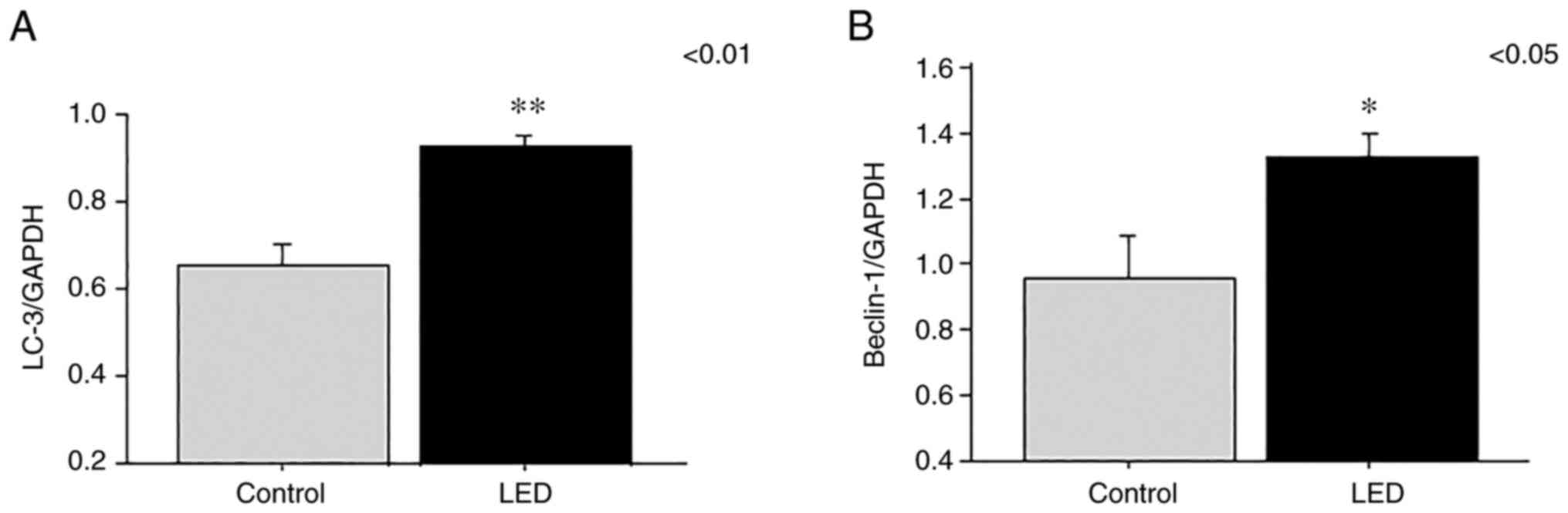
The expression levels of the LC-3 and beclin-1 genes
were analyzed by RT-PCR at 1 week following LED irradiation. RNA
levels of LC-3 (P=0.0025) and beclin-1 (P=0.031) were significantly
elevated in the LED group compared with those in the control group
(Fig. 5A and B).
Blue LED irradiation suppresses the
CAF activation in vitro
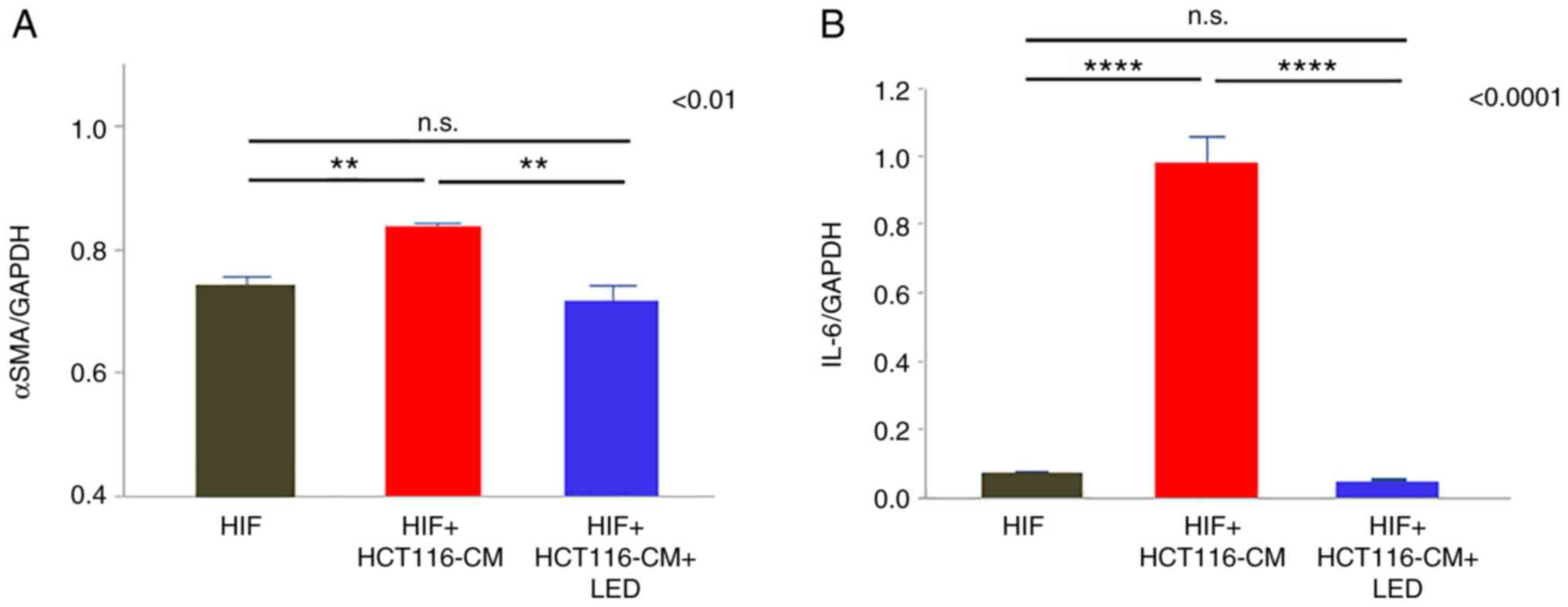
α-SMA and IL-6 expression in the CAFs was evaluated
by PCR. α-SMA expression was attenuated by blue LED irradiation
(P=0.0036) (Fig. 6A) and IL-6
expression was significantly decreased in the LED group
(P<0.0001) (Fig. 6B).
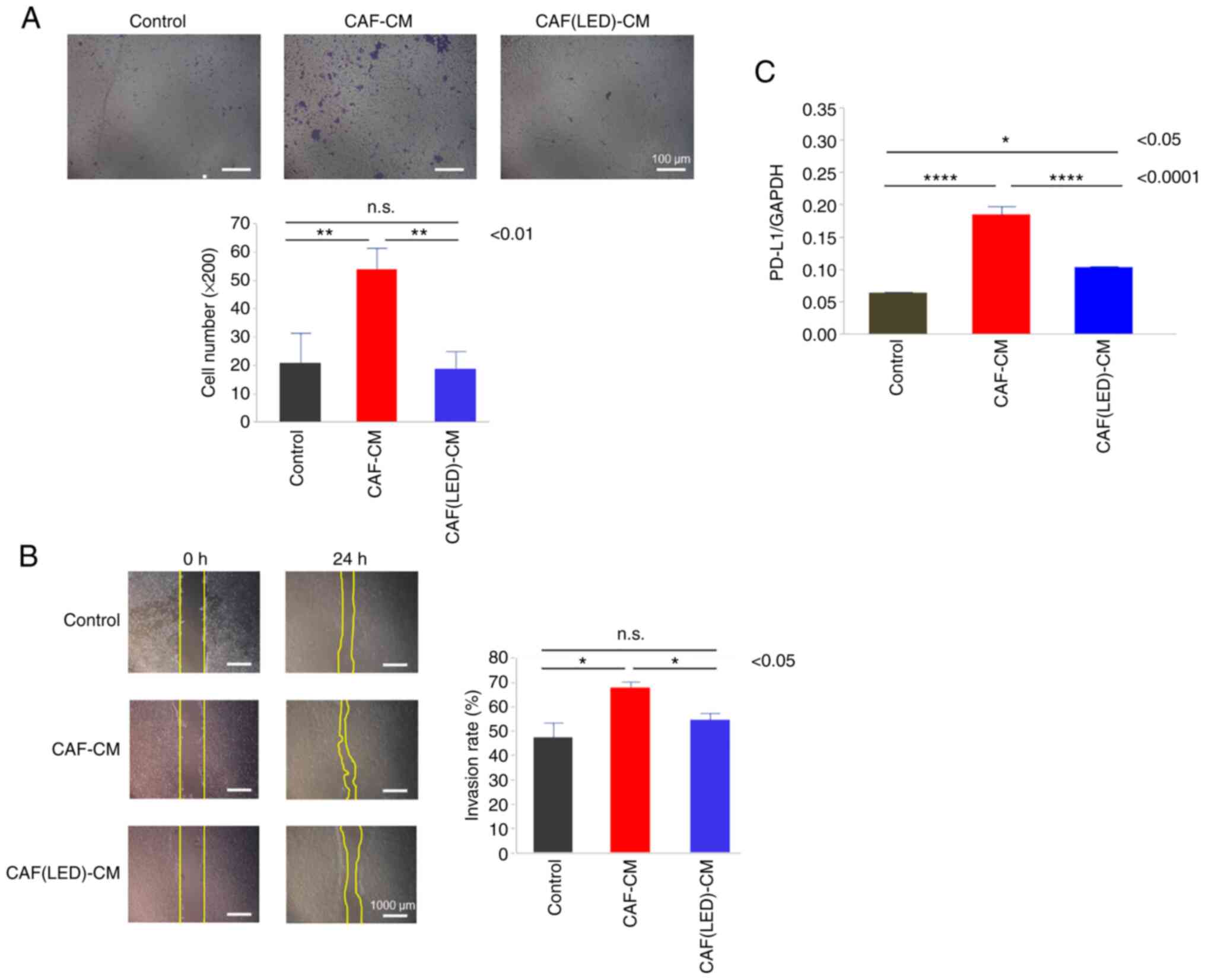
Furthermore, migration (Fig. 7A)
and scratch assays (Fig. 7B) for
HCT-116 cells cultured in CAF-CM were performed. CAF-CM promoted
the migration and invasion by HCT-116 cells. In contrast, compared
with CAF-CM, CAF-CM derived from LED-irradiated CAF did not promote
the migration or invasion of HCT-116 cells (P=0.0053 and P=0.019,
respectively).
Finally, the expression of PD-L1 in HCT-116 cells
was evaluated by PCR. PD-L1 expression was increased in the HCT-116
cells cultured in CAF-CM compared to the control group
(P<0.0001); however, the relative increase in PD-L1 expression
was lower in the CAFs irradiated with blue LED light than that in
the CAFs without LED irradiation (P<0.0001) (Fig. 7C).
Discussion
It has been reported that radiation therapy,
photodynamic therapy (PDT), and phototherapy induce autophagy in
colon cancer cells (21).
Recently, we reported that blue light-emitting diode (LED) light
irradiation at 465 nm (30 mW/cm2, 30 min) induced
autophagy via opsin 3 (Opn3) and coupled G protein in colon cancer
cells (10). In the present study,
irradiation for 30 min with LED light at 465 nm suppressed tumor
growth in human colon cancer, and autophagy was determined to be
the mechanism underlying growth suppression in vivo.
Furthermore, Opn3 was diffusely expressed throughout the cytoplasm
of colon cancer cells in the control group but strongly expressed
in the cell membrane of the LED group. Opn3 is known as a blue
light receptor that is widely expressed in nonvisual cells
(22). It is predicted that Opn3
must be expressed in the cell membrane to function as a
photoreceptor, and the translocation of opsins from the cytoplasm
to the cell membrane in melanocytes has been reported (15). These results suggest that
irradiation with blue LED light induces Opn3 translocation from the
cytoplasm to the cell membrane where Opn3 acts as a photoreceptor.
Cell membrane-associated Opn3 may then promote autophagy in colon
cancer cells. It is known that arrestin inactivates opsin expressed
in the cell membrane of photoreceptors by drawing it into the
cytoplasm (23). In the present
study, arrestin was also diffusely expressed in the cytoplasm of
the controls and localized to the cell membrane following blue LED
irradiation. These results suggest that arrestin is also involved
in the migration of Opn3 into colorectal cancer cells.
Previous reports have shown that autophagy may
induce caspase-independent cell death and inhibit tumor growth
(24). In addition, autophagy was
negatively regulated by cAMP signaling (25,26).
Irradiated Opn3 activates the Gi/o subtype of the G proteins which
inhibits cAMP activation. Based on these findings, blue LED
irradiation may induce autophagy and inhibit the growth of colon
cancer cells via the Opn3 photoreceptor pathway. In addition, we
previously found that Opn3 protein was highly expressed in human
colon cancer cells and no expression of Opn3 was observed in human
normal colonic mucosa (10). Also
in the present in vivo experiments, Opn3 was not expressed
in the normal colonic mucosa, and since the tumor-suppressive
effect of blue LED was canceled when Opn3 was knocked down in a
previous study, it is considered that there is no Opn3-mediated
cytotoxicity to the normal mucosa. These findings indicate that
blue light irradiation can be used clinically for the treatment of
colon cancer.
In recent years, it has been determined that the
tumor microenvironment, including not only tumor cells but also
fibroblasts and immune cells, is extremely important to the
efficacy of anticancer therapy. Fibroblasts are also activated by
cancer cells and become cancer-associated fibroblasts (CAFs).
Transforming growth factor (TGF)-β and Interleukin (IL)-6 are
markers of CAFs (27), and CAFs
contribute to the malignant phenotype. In a previous study, we also
reported that IL-6 in the tumor microenvironment increases
malignancy (28,29). In the present study, immunostaining
for TGF-β and α-smooth muscle actin (α-SMA) revealed that their
expression in fibroblasts in the tumor microenvironment was
attenuated in the LED group, which suggests the use of phototherapy
to target the tumor microenvironment, including CAFs. We found that
LED irradiation on CAFs not only reduced the migration and invasion
ability of colorectal cancer cells, but also had no cell
proliferation-promoting effect in the conditioned medium (CM) of
LED-irradiated CAFs (data not shown).
An in vitro study also showed the effects of
LEDs that suppress CAF activation and IL-6 expression. It has been
reported that IL-6 increases programmed cell death 1-ligand (PD-L1)
expression via STAT3 (30).
Therefore, we considered the possibility that blue light suppresses
the activation of CAFs as well as tumor cells, further reducing
tumor malignancy. Consequently, LED irradiation of CAFs indirectly
reduced tumor malignancy and suppressed PD-L1 expression in the
HCT-116 cells. PD-L1 expression in the colon cancer cells was also
reduced by LED irradiation on CAFs in vitro. It has been
reported that the expression of TGF-β in the tumor microenvironment
correlates with PD-L1 expression in cancer cells and is closely
related to tumor immunity (31).
Thus, blue LED may exert antitumor effects by influencing tumor
immunity.
The major limitation of this study is that the
significance of Opn3 expression in tumors and fibroblasts has not
been fully investigated because we have not conducted experiments
with tumors without Opn3 or Opn3-knockout mice. In addition, since
we were unable to collect the amount of samples necessary for the
evaluation of protein expression in this in vivo experiment,
it is necessary to conduct the experiment again to examine protein
expression levels such as LC-3, Beclin1, PDL-1, aSMA and IL-6. In
our previous in vitro studies, daily irradiation for 5 min
and once-irradiation for 30 min were used (10,19),
and similar tumor suppressive effects were observed. Therefore,
weekly irradiation for 30 min was used in vivo this time. In
the future, it will be necessary to study the effects of
short-duration divided irradiation and multiple 30-min irradiation
sessions.
The mechanism by which blue light suppresses CAFs is
unclear, and there are no reports of photoreceptor-mediated
effects, thus the role of photoreceptors in CAFs is an important
issue for future investigation. Although further studies are
warranted to elucidate the mechanism of action of blue LED
irradiation on tumors and the microenvironment, including Opn3
expression, translocation and photoimmunotherapeutic effects, this
study suggests that blue LED irradiation may have an inhibitory
effect on colorectal cancer and the tumor microenvironment.
Acknowledgements
Not applicable.
Funding
Funding: No funding has been received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
TY, SO and MS conceived and designed the study. TY,
SO, HK and YW performed the experiments and collected the data. TY,
SO, MN and CT analyzed and interpreted the data. TY and SO wrote
the paper. TT, TN, and MN reviewed the data and information and
edited the manuscript. KY and MS revised the paper critically for
important intellectual content. KY, TY and TT confirmed the
authenticity of all the raw data. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The research was conducted in compliance with the
Division for Animal Research Resources, Institute of Health
Biosciences, University of Tokushima, Japan. The experiments and
procedures were approved by the Animal Care and Use Committee of
the University of Tokushima, Japan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Glossary
Abbreviations
Abbreviations:
|
LED
|
light-emitting diode
|
|
CAFs
|
cancer-associated fibroblasts
|
|
CM
|
conditioned medium
|
|
HIFs
|
human intestinal fibroblasts
|
|
Opn3
|
opsin 3
|
|
cAMP
|
cyclic adenosine monophosphate
|
|
GPCR
|
G protein-coupled receptors
|
|
PDT
|
photodynamic therapy
|
|
PTT
|
photothermal therapy
|
|
PD-L1
|
programmed cell death 1-ligand
|
|
α-SMA
|
α-smooth muscle actin
|
|
TGF
|
transforming growth factor
|
References
|
1
|
Gunaydin G, Gedik ME and Ayan S:
Photodynamic therapy for the treatment and diagnosis of cancer-a
review of the current clinical status. Front Chem. 9:6863032021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hatakeyama T, Murayama Y, Komatsu S,
Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H,
Okamoto K, et al: Efficacy of 5-aminolevulinic acid-mediated
photodynamic therapy using light-emitting diodes in human colon
cancer cells. Oncol Rep. 29:911–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodgkinson N, Kruger CA and Abrahamse H:
Targeted photodynamic therapy as potential treatment modality for
the eradication of colon cancer and colon cancer stem cells. Tumor
Biology. 39:10104283177346912017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan J, Wang C, Jiang X, Wei Y, Wang Q, Cui
K, Xu X, Wang F and Zhang L: Application of phototherapeutic-based
nanoparticles in colorectal cancer. Int J Biol Sci. 17:1361–1381.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh PS, Hwang H, Jeong HS, Kwon J, Kim HS,
Kim M, Lim S, Sohn MH and Jeong HJ: Blue light emitting diode
induces apoptosis in lymphoid cells by stimulating autophagy. Int J
Biochem Cell Biol. 70:13–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
del Olmo-Aguado S, Núñez-Álvarez C and
Osborne NN: Blue light action on mitochondria leads to cell death
by necroptosis. Neurochem Res. 41:2324–2335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh PS, Na KS, Hwang H, Jeong HS, Lim S,
Sohn MH and Jeong HJ: Effect of blue light emitting diodes on
melanoma cells: Involvement of apoptotic signaling. J Photochem
Photobiol B. 142:197–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sparsa A, Faucher K, Sol V, Durox H,
Boulinguez S, Doffoel-Hantz V, Calliste CA, Cook-Moreau J, Krausz
P, Sturtz FG, et al: Blue light is phototoxic for B16F10 murine
melanoma and bovine endothelial cell lines by direct cytocidal
effect. Anticancer Res. 30:143–147. 2010.PubMed/NCBI
|
|
9
|
Yan G, Zhang L, Feng C, Gong R,
Idiiatullina E, Huang Q, He M, Guo S, Yang F, Li Y, et al: Blue
light emitting diodes irradiation causes cell death in colorectal
cancer by inducing ROS production and DNA damage. Int J Biochem
Cell Biol. 103:81–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimoto T, Morine Y, Takasu C, Feng R,
Ikemoto T, Yoshikawa K, Iwahashi S, Saito Y, Kashihara H, Akutagawa
M, et al: Blue light-emitting diodes induce autophagy in colon
cancer cells by Opsin 3. Ann Gastroenterol Surg. 2:154–161. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koyanagi M and Terakita A: Diversity of
animal opsin-based pigments and their optogenetic potential.
Biochim Biophys Acta. 1837:710–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiao J, Hong S, Zhang J, Ma L, Sun Y,
Zhang D, Shen B and Zhu C: Opsin3 sensitizes hepatocellular
carcinoma cells to 5-fluorouracil treatment by regulating the
apoptotic pathway. Cancer Lett. 320:96–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koyanagi M, Takada E, Nagata T, Tsukamoto
H and Terakita A: Homologs of vertebrate Opn3 potentially serve as
a light sensor in nonphotoreceptive tissue. Proc Natl Acad Sci USA.
110:4998–5003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terakita A and Nagata T: Functional
properties of opsins and their contribution to light-sensing
physiology. Zoolog Sci. 31:653–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Assis L, Moraes M, da Silveira
Cruz-Machado S and Castrucci AM: The effect of white light on
normal and malignant murine melanocytes: A link between opsins,
clock genes, and melanogenesis. Biochim Biophys Acta.
1863:1119–1133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berzaghi R, Islam A, Hellevik T and
Martinez-Zubiaurre I: Secretion rates and protein composition of
extracellular vesicles released by cancer-associated fibroblasts
after radiation. J Radiat Res. 62:401–413. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ham IH, Lee D and Hur H: Cancer-associated
fibroblast-induced resistance to chemotherapy and radiotherapy in
gastrointestinal cancers. Cancers (Basel). 13:11722021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krassovka J, Borgschulze A, Sahlender B,
Lögters T, Windolf J and Grotheer V: Blue light irradiation and its
beneficial effect on Dupuytren's fibroblasts. PLoS One.
14:e02098332019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsumoto N, Yoshikawa K, Shimada M,
Kurita N, Sato H, Iwata T, Higashijima J, Chikakiyo M, Nishi M,
Kashihara H, et al: Effect of light irradiation by light emitting
diode on colon cancer cells. Anticancer Res. 34:4709–4716.
2014.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang B and Liu L: Autophagy is a double
edged sword in the therapy of colorectal cancer. Oncol Lett.
21:3782021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakai K, Yamashita T, Imamoto Y and
Shichida Y: Diversity of active states in TMT opsins. PLoS One.
10:e01412382015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luttrell LM and Lefkowitz RJ: The role of
β-arrestins in the termination and transduction of
G-protein-coupled receptor signals. J Cell Sci. 115:455–465. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JY and Juhnn YS: cAMP signaling
increases histone deacetylase 8 expression by inhibiting
JNK-dependent degradation via autophagy and the proteasome system
in H1299 lung cancer cells. Biochem Biophys Res Commun.
470:336–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang R, Zeh H, Lotze M and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia C, Wang G, Wang T, Fu B, Zhang Y,
Huang L, Deng Y, Chen G, Wu X, Chen J, et al: Cancer-associated
fibroblasts induce epithelial-mesenchymal transition via the
transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular
carcinoma. Int J Biol Sci. 16:2542–2558. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nab-paclitaxel interrupts
cancer-stromal interaction through C-X-C motif chemokine
10-mediated interleukin-6 downregulation in vitro. Cancer Sci.
109:2509–2519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iwahasi S, Rui F, Morine Y, Yamada S,
Saito Y, Ikemoto T, Imura S and Shimada M: Hepatic stellate cells
contribute to the tumor malignancy of hepatocellular carcinoma
through the IL-6 pathway. Anticancer Res. 40:743–749. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao
Y, Chen Y and Jiang R: IL-6 promotes PD-L1 expression in monocytes
and macrophages by decreasing protein tyrosine phosphatase receptor
type O expression in human hepatocellular carcinoma. J Immunother
Cancer. 8:e0002852020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shima T, Shimoda M, Shigenobu T, Ohtsuka
T, Nishimura T, Emoto K, Hayashi Y, Iwasaki T, Abe T, Asamura H and
Kanai Y: Infiltration of tumor-associated macrophages is involved
in tumor programmed death-ligand 1 expression in early lung
adenocarcinoma. Cancer Sci. 111:727–738. 2020. View Article : Google Scholar : PubMed/NCBI
|