Introduction
Breast cancer is the most frequently diagnosed
neoplasm and the second leading cause of cancer-related mortality
among women worldwide (1,2); four molecular features are used for
breast cancer subtypes based on the expression of estrogen receptor
(ER), progesterone receptor, human epidermal growth factor receptor
(HER)2 and Ki-67 (3). ER-positive
breast cancer is the most common clinical subtype, constituting
almost 70% of all breast cancer cases (4). Endocrine therapy to block ER activity
is the mainstay therapy for ER-positive breast cancer (3,5).
Tamoxifen is the most commonly used endocrine treatment for
ER-positive breast cancer, particularly for pre-menopausal patients
(6). It decreases
estrogen-responsive gene transcription by competitively inhibiting
the binding of estrogen to ER, thereby suppressing the
proliferation of ER-positive breast cancer (7). Treatment with tamoxifen reportedly
decreases the risk of recurrence at 5 years by 47% and mortality at
15 years by 34% in patients with early ER-positive breast cancer
and prolongs the survival of patients with metastatic breast cancer
for ~8 months (8,9). However, ~40% of patients with
ER-positive breast cancer develop tamoxifen resistance, leading to
metastasis, recurrence and even mortality (10–12).
Therefore, tamoxifen resistance plays a main role in the mortality
rate of patients with ER-positive breast cancer. Various mechanisms
have been proposed to combat this resistance; for example, the
modification or loss of ER expression, the upregulation of
oncogenic signaling pathways and epigenetic alterations (11,13,14).
Nevertheless, the crucial question regarding the definition of
therapeutic targets to overcome tamoxifen resistance in ER-positive
breast cancer remains unanswered. Therefore, it is important to
identify therapeutic targets for overcoming or reversing tamoxifen
resistance in ER-positive breast cancer.
In recent years, the importance of the
epithelial-mesenchymal transition (EMT) process in the gain of
aggressive characteristics in cancers has been recognized (15–17).
EMT is a complex process characterized by epithelial cells that
lose cell-cell junctions and acquire mesenchymal properties
(18). It is characterized by the
downregulated expression of epithelial markers, including
E-cadherin, and the upregulated expression of mesenchymal markers,
including N-cadherin and vimentin, and EMT-inducing transcription
factors, including snail family transcriptional repressor 1
(Snai1), twist family BHLH transcription factor 1 (Twist) and snail
family transcriptional repressor 2 (Slug) (19). Several studies have demonstrated
that EMT is associated with the gain of migratory and invasive
properties, and an increased tolerance to chemotherapy, being also
a prominent hallmark of cancer progression (20,21).
In addition, the decreased expression of E-cadherin, and the
increased expression of N-cadherin and vimentin have been
associated with a poor survival in breast, melanoma and prostate
cancer (22–24). Furthermore, the EMT phenotype has
been identified in a number of cancer cells, including
erlotinib-resistant lung cancer cells, doxorubicin-resistant
gastric cancer cells and tamoxifen-resistant breast cancer cells
(25–27). Therefore, therapeutic strategies
based on reversing EMT may provide a novel approach with which to
overcome acquired tamoxifen resistance in ER-positive breast
cancer.
Tamoxifen-resistant breast cancer cells have been
reported to exhibit an EMT phenotype and an EMT gene expression
pattern (28,29). Transcription factors, including
Snail, Slug and Twist have been reported to mediate EMT by
regulating the expression of E-cadherin, N-cadherin and vimentin
(30,31). In addition, the dysregulation of
EMT-inducing transcription factors exhibits clinical relevance in
patients with tamoxifen-resistant breast cancer (32,33).
Several growth factor receptors, including fibroblast growth factor
1 receptor (FGFR1), insulin-like growth factor 1 receptor (IGF1R)
and epidermal growth factor receptor (EGFR), which are involved in
the EMT process, are also highly expressed in ER-positive breast
cancer cells, supporting the link between EMT and insensitivity to
endocrine therapy (34,35). However, the central molecules
inducing the EMT process during the development of tamoxifen
resistance remain largely unknown.
In the present study, a tamoxifen-resistant MCF-7
(MCF-7/TR) breast cancer cell line was established. MCF-7/TR cells
underwent EMT and exhibited an enhanced cell motility and invasive
behavior. In addition, Snail and Twist silencing
reversed the EMT phenotype and decreased the tamoxifen resistance,
migration and invasion of MCF-7/TR cells. Of note, gefitinib, a
known inhibitor of EGFR, reversed EMT and decreased the tamoxifen
resistance, migration and invasion of MCF-7/TR cells via the
downregulation of Snail and Twist. The findings of
the present study indicate that EGFR may be a promising therapeutic
target for tamoxifen-resistant breast cancer treatment. Moreover,
it is suggested that gefitinib may serve as a potent novel
therapeutic strategy for breast cancer patients, who have developed
tamoxifen resistance.
Materials and methods
Reagents
Tamoxifen (MilliporeSigma) and gefitinib (Funakoshi
Co., Ltd.) were first dissolved in dimethyl sulfoxide (DMSO;
FUJIFILM Wako Pure Chemical Corporation) up to a concentration of
50 mM (stock solution) and stored at −20°C. Stealth small
interfering RNA (siRNA) targeting Snail (HSS143995;
5-CCTCGCTGCCAATGCTCATCTGGGA-3′) and Twist (HSS144372;
5-TGGCGGCCAGGTACATCGACTTCCT-3′) were purchased from Thermo Fisher
Scientific, Inc. Antibodies against phosphorylated (p)-EGFR (cat.
no. 2235; dilution 1:1,000) and EGFR (cat. no. 4267; dilution
1:1,000) were obtained from Cell Signaling Technology, Inc.
Antibodies against β-actin (cat. no. A2228; dilution 1:3,000) were
purchased from MilliporeSigma. Anti-rabbit secondary antibody (cat.
no. 7074; dilution 1:5,000) and anti-mouse secondary antibody (cat.
no. 7076; dilution 1:5,000) were obtained from Cell Signaling
Technology, Inc.
Cells and cell culture
The tamoxifen-sensitive human breast cancer cell
line, MCF-7 (cat. no. JCRB0134), was obtained from the Health
Science Research Resources Bank. The MCF-7/TR cell line was
established from the MCF-7 cells, following continuous exposure to
tamoxifen along with a gradual increase in the concentration from 1
to 25 µM over a period of 6 months. The MCF-7/TR cells were
maintained in 25 µM tamoxifen. These cells were cultured in
RPMI-1640 (MilliporeSigma) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 2 mM L-glutamine (FUJIFILM Wako Pure
Chemical Corporation), 25 mM HEPES (FUJIFILM Wako Pure Chemical
Corporation), 100 µg/ml penicillin/streptomycin (Gibco; Thermo
Fisher Scientific, Inc.), at 37°C in a CO2 incubator
(Sanyo Co., Ltd.) with 95% air and 5% CO2.
Cell viability assay
Cell viability assay was performed using trypan blue
staining. The MCF-7 and MCF-7/TR cells were plated in 96-well
plates in RPMI-1640 medium, containing 10% FBS at a concentration
of 2×103 cells per well. Subsequently, tamoxifen (0.1,
0.5, 1.5, 10, 25, 50, 100, 250 and 500 µM), Snail siRNA (10 nM),
Twist siRNA (10 nM), or gefitinib (1, 5, 10, and 25 µM) were added
to the wells. All cells were stained with 0.4% trypan blue
(FUJIFILM Wako Pure Chemical Corporation) for 3 min at room
temperature, and counted at a magnification of ×100 under a light
microscope (Olympus CK2; Olympus Corporation) at 3 days. IC50
values were calculated using GraphPad Prism 9.0 (GraphPad Prism
software, Inc.).
Transwell invasion and migration
assays
For Transwell invasion assay, the Cell Culture
Inserts (8.0 µm pore size; Becton, Dickinson and Company) were
coated with 20 µl Matrigel (Corning, Inc.) for 30 min at 37°C.
Subsequently, MCF-7 (5×104 cells) and MCF-7/TR
(5×104 cells) cells previously transfected (as described
below) with Snail siRNA (10 nM), Twist siRNA (10 nM), or gefitinib
(5 µM) were plated in the upper chamber, and the lower chamber was
supplemented with medium containing 10% FBS (Gibco; Thermo Fischer
Scientific, Inc.). Following a 24-h incubation, all cells on the
upper chamber surface were removed using a wet cotton swab, and
those attached on the lower side of the membrane were fixed with
95% ethanol for 10 min at room temperature and stained hematoxylin
(MilliporeSigma) for 5 min at room temperature. The cells passing
through the Cell Culture Insert were counted at a magnification of
×200 under a light microscope (Olympus BX50; Olympus Corporation)
in five randomly selected fields. Transwell migration assay was
performed similarly to the Transwell invasion assay, without using
Matrigel.
Reverse transcription-quantitative PCR
(RT-qPCR)
The MCF-7/TR cells were cultured with Snail siRNA
(10 nM), Twist siRNA (10 nM), or gefitinib (5 µM). Total RNA
extraction from the MCF-7 and MCF-7/TR cells was performed using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Reverse transcription reactions were performed using the
PrimeScript RT reagent kit (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol under the following
thermocycling conditions: 37°C for 15 min, followed by 85°C for 5
sec. qPCR was performed using TB Green Premix Ex Taq (Takara Bio,
Inc.) and an ABI Prism 7000 detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Primers for RT-qPCR were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The
following primer sequences were used: E-cadherin forward,
5-GAACGCATTGCCACATACAC-3 and reverse, 5-GAATTCGGGCTTGTTGTCAT-3;
N-cadherin forward, 5-CTCCTATGAGTGGAACAGGAACG-3 and reverse,
5-TTGGATCAATGTCATATTCAAGTGCTGTA-3; vimentin forward,
5-AGATGGCCCTTGACATTGAG-3 and reverse, 5-CCAGAGGGAGTGAATCCAGA-3;
Snail forward, 5-GCGAGCTGCAGGACTCTAAT-3 and reverse,
5-GGACAGAGTCCCAGATGAGC-3; Slug forward, 5-CGTTTTTCCAGACCCTGGTT-3
and reverse, 5-CTGCAGATGAGCCCTCAGA-3; Twist forward,
5-CGCCCCGCTCTTCTCCTCT-3 and reverse, 5-GACTGTCCATTTTCTCCTTCTCTG-3;
GAPDH was used as an internal control and the following primer
sequences were used: GAPDH forward, 5-ACTTTGTCAAGCTCATTT-3 and
reverse, 5-TGCAGCGAACTTTATTG-3. Relative mRNA expression was
calculated by the 2−ΔΔCq method (36).
RNA interference/transfection
The MCF-7/TR cells were transfected with 10 nM Snail
siRNA, 10 nM Twist siRNA and 10 nM Stealth™ RNAi Negative Control
(Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine
3000® (Invitrogen; Thermo Fisher Scientific, Inc.).
Lipofectamine 3000 and siRNAs were diluted in RPMI-1640 medium,
respectively, and were incubated for 5 min at room temperature. The
diluted Lipofectamine 3000 and siRNAd were then mixed at a ratio of
1:1, and subsequently they were incubated for 15 min at room
temperature. Subsequently, the complexes were added to the cells
followed by incubation for 48 h at 37°C in a 5% CO2.
Following transfection, the cells were treated according to the
subsequent experimental protocol requirements.
Receptor tyrosine kinase (RTK)
analysis
RTK analyses were conducted using the 7-Plex RTK
Mitogenesis Phosphoprotein Magnetic Bead kit (cat. no. 48-671MAG;
Merck Life Science UK, Ltd.) according the manufacturer's protocol.
Briefly, the MCF-7 and MCF-7/TR cells were collected and lysed
using lysis buffer [20 mM Tris-HCl pH 8.0 (FUJIFILM Wako Pure
Chemical Corporation), 150 mM NaCl (FUJIFILM Wako Pure Chemical
Corporation), 2 mM ethylenediaminetetraacetic acid (EDTA; FUJIFILM
Wako Pure Chemical Corporation), 100 mM NaF, 1% NP40 (both from
FUJIFILM Wako Pure Chemical Corporation), 1 µg/ml leupeptin
(MilliporeSigma), 1 µg/ml antipain (MilliporeSigma) and 1 mM
phenylmethylsulfonyl fluoride (PMSF) (MilliporeSigma)]. The samples
were mixed with 7-Plex RTK Mitogenesis magnetic beads and incubated
overnight at 4°C. Subsequently, the samples were washed and mixed
Biotin-Labeled Detection Antibody (dilution 1:20; cat. no.
48-671MAG; Merck Life Science UK, Ltd.). RTK expression was
measured using the Luminex® 200 instrument (Luminex
Corporation).
Western blot analysis
The MCF-7 and MCF-7/TR cells were cultured with
gefitinib (5 µM). Subsequently, the MCF-7 and MCF-7/TR cells were
collected and lysed with lysis buffer [20 mM Tris-HCl (pH 7.5), 10
mM NaCl, 1 mM EDTA, 0.5% NP-40, 1 µM pepstatin, 1 µM leupeptin, 2
mM sodium orthovanadate, 1 µM calpain inhibitor, phosphatase
inhibitor cocktail I/II and 1 mM phenylmethylsulfonyl fluoride
(PMSF)]. Protein samples were quantified using the BCA Protein
assay kit (Thermo Fischer Scientific, Inc.). The extracts (40 µg)
were separated using 10% sodium dodecyl sulfate (FUJIFILM Wako Pure
Chemical Corporation)-polyacrylamide gel electrophoresis
(SDS-PAGE), followed by a transfer to polyvinylidene fluoride
(PVDF) membranes (Cytiva). The membranes were blocked with 5% skim
milk for 30 min at room temperature and incubated with the primary
antibodies (as indicated above in the ‘Reagents’ paragraph)
overnight at 4°C. The membranes were then incubated with secondary
antibodies (as indicated above in the ‘Reagents’ paragraph)
for 2 h at room temperature. The immunoreactive bands were
visualized using Luminata Forte Western HRP substrate (Merck Life
Science UK, Ltd.). β-actin was used as the loading control. The
bands were analyzed using Densitograph software CS Analyzer ver 3.0
(Atto Corporation).
Statistical analysis
GraphPad Prism 9.0 (GraphPad Prism software, Inc.)
was used for analysis. All data are expressed as the mean ±
standard deviation (SD). Data comparisons between two groups were
performed using an unpaired Student's t-test. Comparisons among
multiple groups were performed using analysis of variance (ANOVA)
followed by Dunnett's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
MCF-7/TR cells exhibit an enhanced
motility and invasive behavior
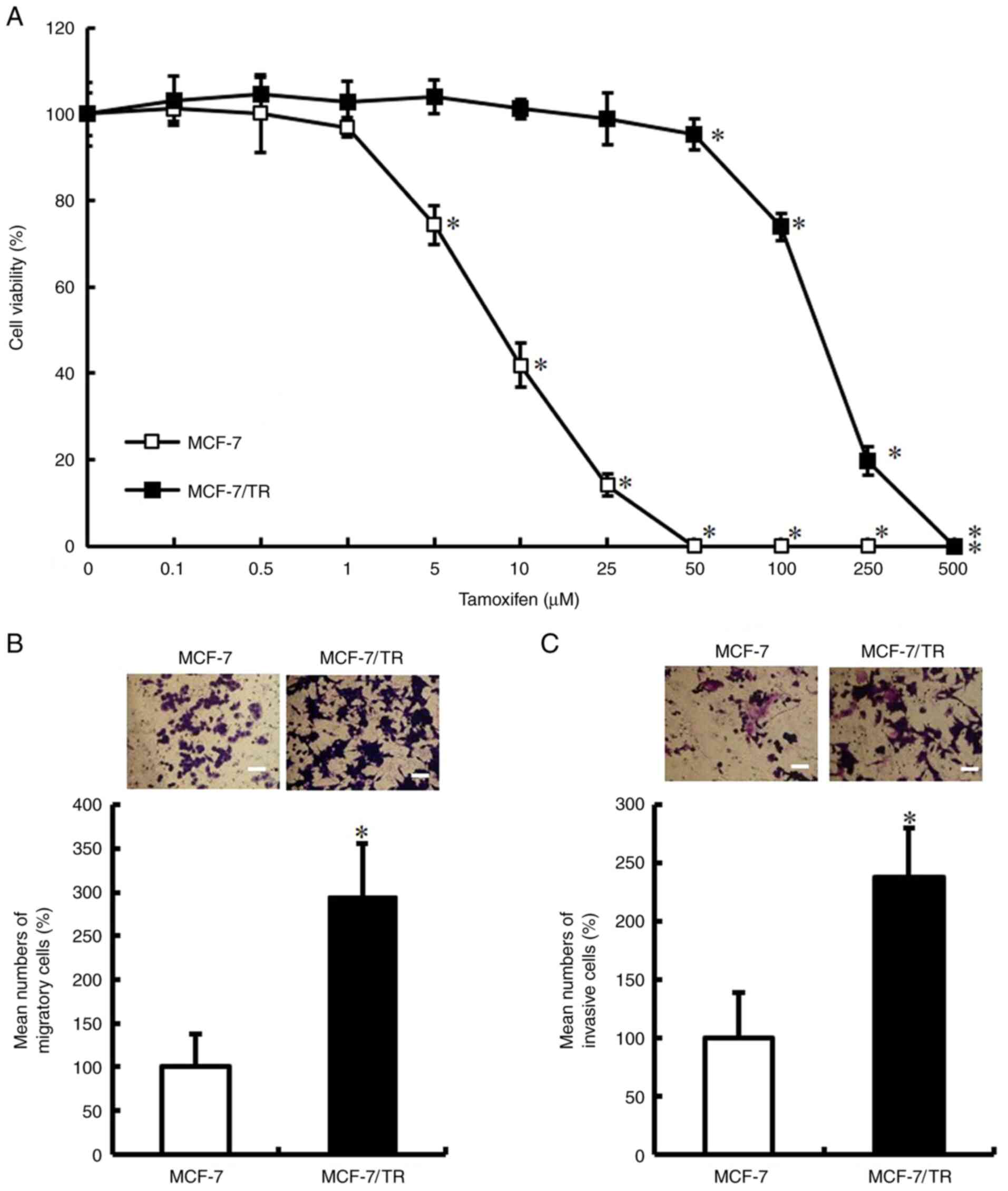
To confirm whether MCF-7/TR cells acquired a
tamoxifen-resistant phenotype, parental MCF-7 and MCF-7/TR cells
were treated with various concentrations of tamoxifen for 72 h.
Tamoxifen decreased the viability of the MCF-7 cells; however, it
exerted a limited effect on the viability of MCF-7/TR cells
(Fig. 1A). The IC50 value was 8.0
µM for the parental MCF-7 cells and 107.2 µM for the MCF-7/TR
cells. Subsequently, it was examined whether the acquisition of a
tamoxifen-resistant phenotype enhances cell motility and invasive
behavior. It was observed that the MCF-7/TR cells exhibited a
significantly increased migratory and invasive ability in
comparison with the MCF-7 cells (Fig.
1B and C). These results indicated that the MCF-7/TR cells
exhibit an enhanced motility and invasive behavior.
MCF-7/TR cells acquire the EMT
phenotype
To determine whether the MCF-7/TR cells acquired the
EMT phenotype, morphological changes in the MCF-7/TR cells were
examined. The MCF-7/TR cells exhibited a spindle shape,
intercellular spaces and scattering, whereas the MCF-7 cells
exhibited firmly packed cobblestone-like clusters (Fig. S1A). Moreover, E-cadherin
expression was downregulated, and N-cadherin and
vimentin expression was upregulated in the MCF-7/TR cells,
but not in the MCF-7 cells (Fig.
2). These results indicated that the MCF-7/TR cells acquired
the EMT phenotype.
Silencing of Snail and Twist reverses
the EMT phenotype in MCF-7/TR cells
Snail, Slug and Twist are three well-documented EMT
regulatory transcription factors. Therefore, the present study
examined their expression levels in MCF-7 and MCF-7/TR cells.
Snail and Twist expression levels were upregulated in
the MCF-7/TR cells compared with the MCF-7 cells, while Slug
expression was not significantly unaltered (Fig. 2). Furthermore, it was investigated
whether Snail and Twist silencing reversed the EMT phenotype in
MCF-7/TR cells. Transfection with Snail and Twist siRNA induced
morphological changes, resulting in EMT in MCF-7/TR cells (Fig. S1B). In addition, Snail and
Twist silencing resulted in E-cadherin upregulation,
and N-cadherin and vimentin downregulation (Fig. 3). These results indicated that the
silencing of Snail and Twist may reverse the EMT phenotype in
MCF-7/TR cells.
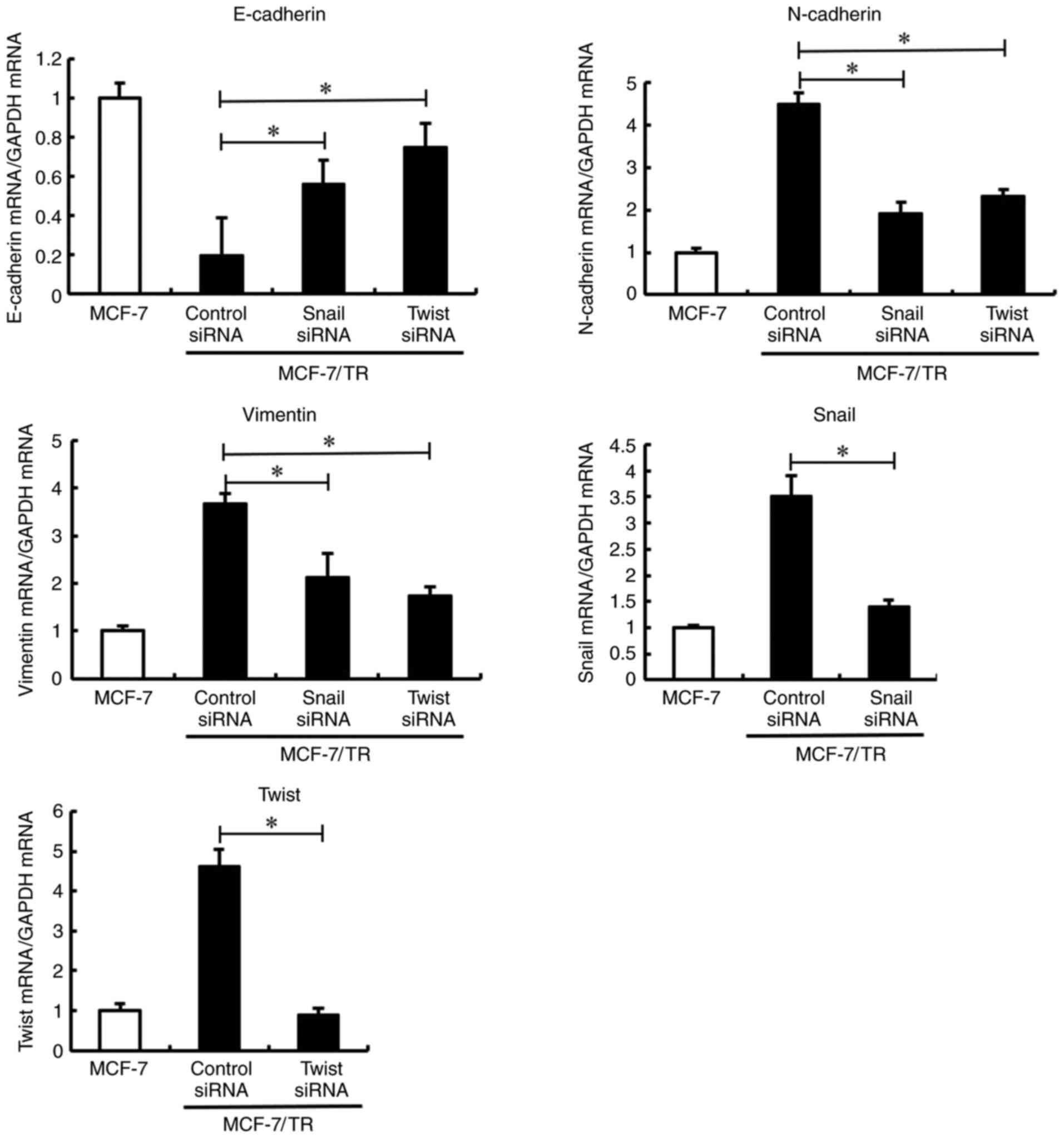 | Figure 3.Snail and Twist inhibition reverses
the epithelial-mesenchymal transition phenotype in MCF-7/TR cells.
MCF-7/TR cells were transfected with Snail siRNA (10 nM), Twist
siRNA (10 nM), or Stealth™ RNAi Negative Control (siRNA control)
for 3 days. E-cadherin, N-cadherin, vimentin, Snail, and
Twist mRNA expression levels were measured using reverse
transcription-quantitative PCR. Data are presented as the mean ± SD
of three independent experiments. *P<0.05 as compared to the
siRNA control. The F values are 43.6 (E-cadherin), 79.52
(N-cadherin), 41.13 (Vimentin), 46.48 (Snail) and 158.5 (Twist).
Snai1, snail family transcriptional repressor 1; Twist, twist
family BHLH transcription factor 1; Slug, snail family
transcriptional repressor 2; MCF-7/TR, tamoxifen-resistant MCF-7
cells. |
Silencing of Snail and Twist decreases
the tamoxifen resistance, migration and invasion of MCF-7/TR
cells
The present study then examined whether the
inhibition of Snail and Twist impaired tamoxifen resistance, and
decreased the migration and invasion of MCF-7/TR cells. It was
revealed that transfection with Snail and Twist siRNA impaired the
tamoxifen resistance of MCF-7/TR cells (Fig. 4A). In addition, Snail and Twist
siRNA inhibited cell migration and invasion (Fig. 4B and C). These results indicated
that the silencing of Snail and Twist may decrease tamoxifen
resistance, migration, and invasion in MCF-7/TR cells.
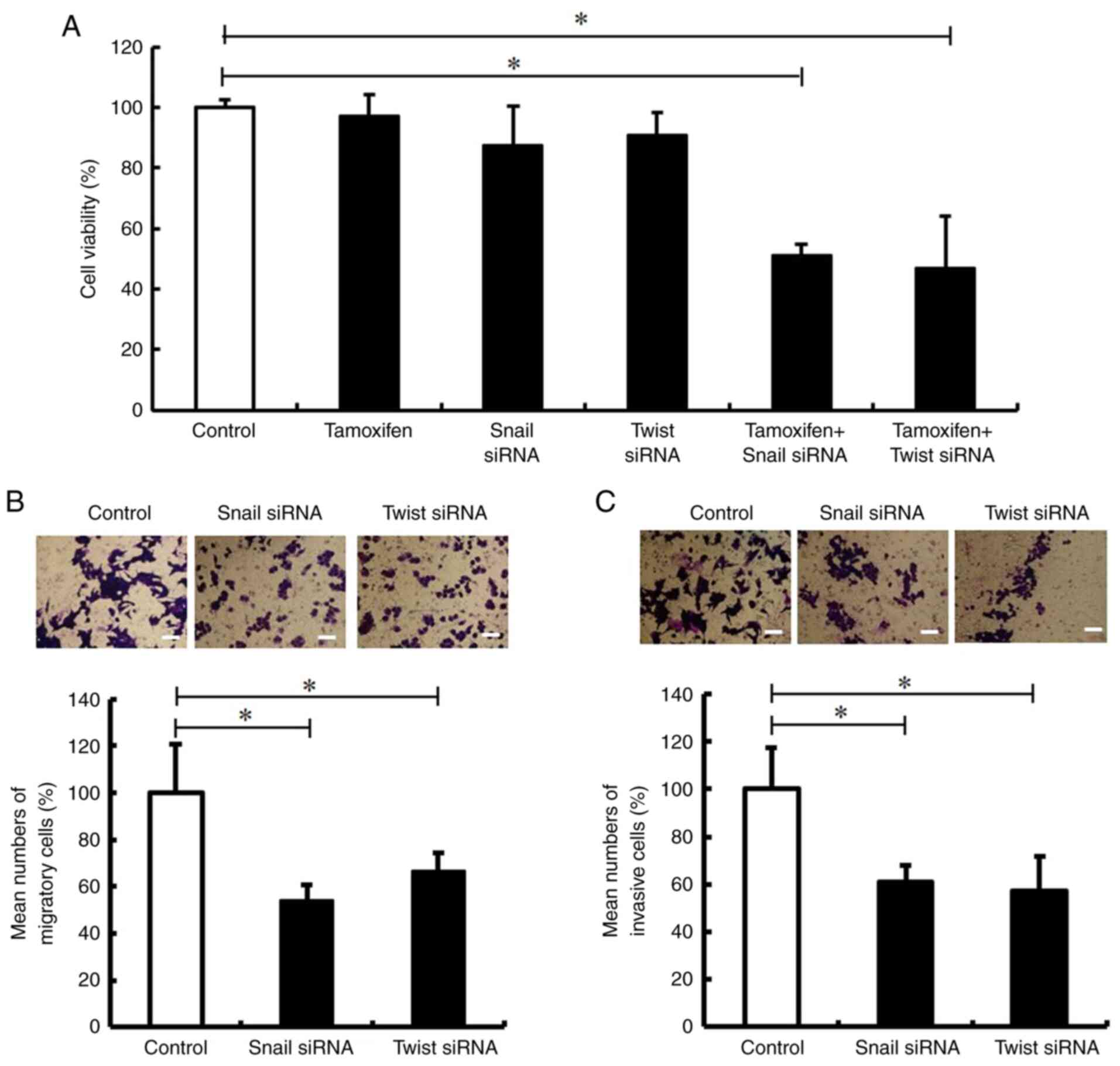 | Figure 4.Snail and Twist inhibition decreases
the tamoxifen resistance, migration and invasion of MCF-7/TR cells.
MCF-7/TR cells were transfected with Snail siRNA (10 nM), Twist
siRNA (10 nM), or Stealth™ RNAi Negative Control (Control) for 3
days. (A) MCF-7/TR cells were treated with 25 µM tamoxifen. The
cells were stained with trypan blue, and the number of stained
cells was counted on day 3. The results are presented as the mean ±
SD of three independent experiments. *P<0.05 compared to the
control. The F value is 25.29. (B and C) Cell migration was
analyzed using Transwell culture inserts, whereas cell invasion was
analyzed using Transwell culture inserts coated with Matrigel.
Representative images of invasion assay of MCF-7/TR cells are
presented on the top panels. Magnification, ×20. Scale bar, 50 µm.
The results are presented as the mean ± SD of three independent
experiments. *P<0.05 as compared to the control. The F value is
27.22 (cell migration) and 12.32 (cell invasion). Snai1, snail
family transcriptional repressor 1; Twist, twist family BHLH
transcription factor 1; MCF-7/TR, tamoxifen-resistant MCF-7
cells. |
Inhibition of EGFR reverses the EMT
phenotype in MCF-7/TR cells by downregulating Snail and Twist
expression
The molecular mechanisms underlying the increased
expression levels of Snail and Twist in the MCF-7/TR cells have not
yet been fully elucidated. Recent research has reported that
several RTKs, including EGFR, IGF1R and fibroblast growth factor 1
receptor, which are involved in the EMT process, are highly
expressed in tamoxifen-resistant breast cancer, supporting the link
between EMT and insensitivity to endocrine therapy (37). Therefore, the present study
examined RTK expression in MCF-7 and MCF-7/TR cells using
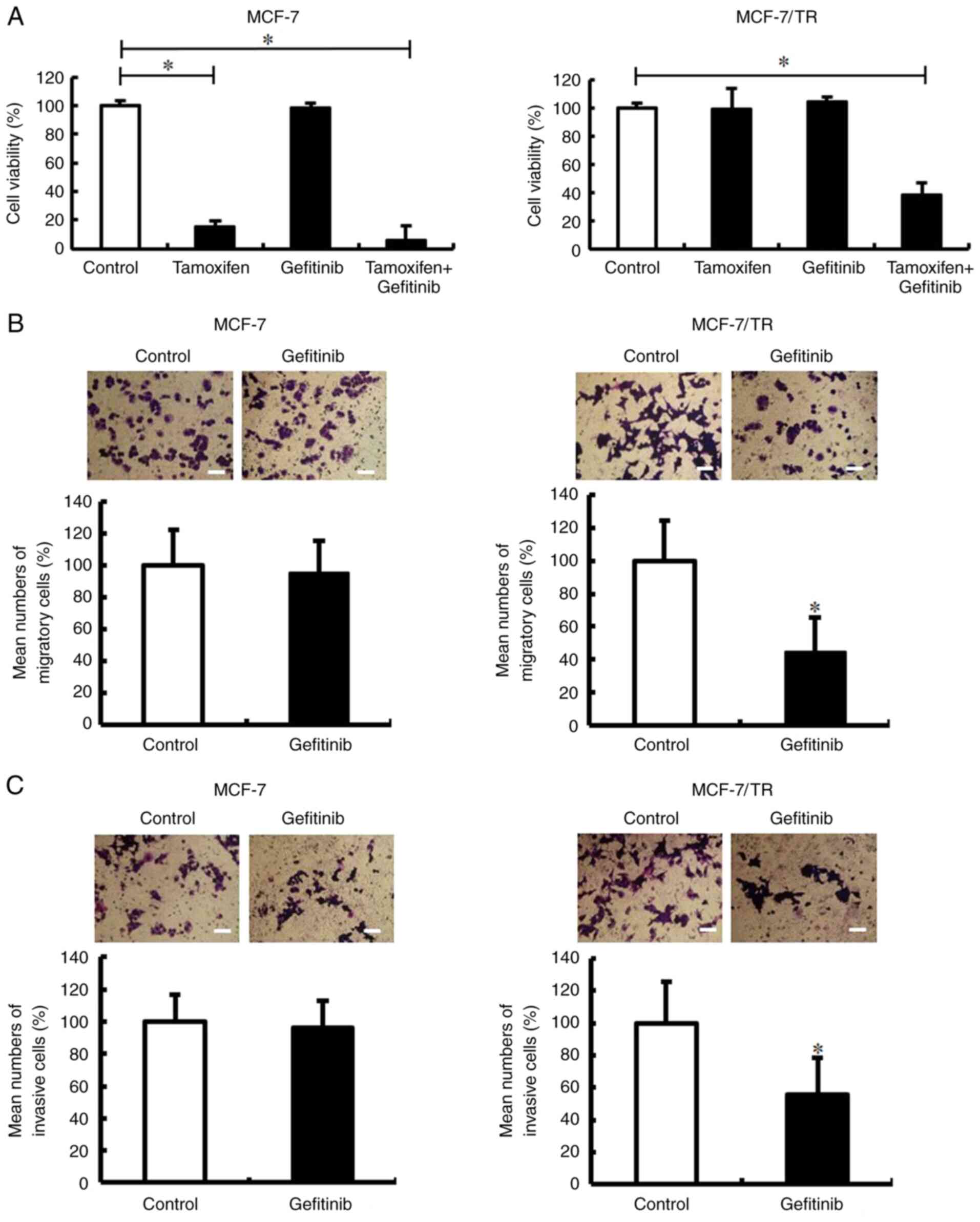
Luminex® 200. It was revealed that EGFR expression was
higher in the MCF-7/TR cells in comparison with the MCF-7 cells
(Fig. 5). However, no changes in
the expression of c-Met, IGF1R, insulin receptor (IR), HER3 and
HER4 proteins were observed between the MCF-7 and MCF-7/TR cells.
It was then examined whether EGFR inhibition reversed the EMT
phenotype through Snail and Twist inhibition. Firstly, the effect
of the EGFR inhibitor, gefitinib, on the viability of MCF-7 and
MCF-7/TR cells was examined using trypan blue exclusion assay. The
MCF-7 cells treated with 1, 5 and 10 µM gefitinib, and the MCF-7/TR
cells treated with 1 and 5 µM gefitinib did not exhibited an
inhibition of cell viability (Fig.
6A). However, the MCF-7 cells treated with 25 µM gefitinib, and
the MCF-7/TR cells treated with 10 and 25 µM gefitinib exhibited a
decrease in cell viability compared to the untreated cells. In
addition, the expression of EGFR in the gefitinib-treated MCF-7 and
MCF-7/TR cells was examined using western blot analysis. It was
revealed that gefitinib suppressed the expression of p-EGFR
(Figs. 6B and S2). These results revealed that 5 µM
gefitinib did not inhibit cell viability, whereas at a
concentration >10 µM, it inhibited the viability of the MCF-7/TR
cells. Therefore, the MCF-7/TR cells were treated with gefitinib at
5 µM in subsequent experiments. It was thus demonstrated that
gefitinib may reverse the EMT phenotype through the inhibition of
Snail and Twist (Figs.
7 and S1A). These results
suggested that EGFR inhibition reversed the EMT phenotype in
MCF-7/TR cells via the downregulation of Snail and Twist.
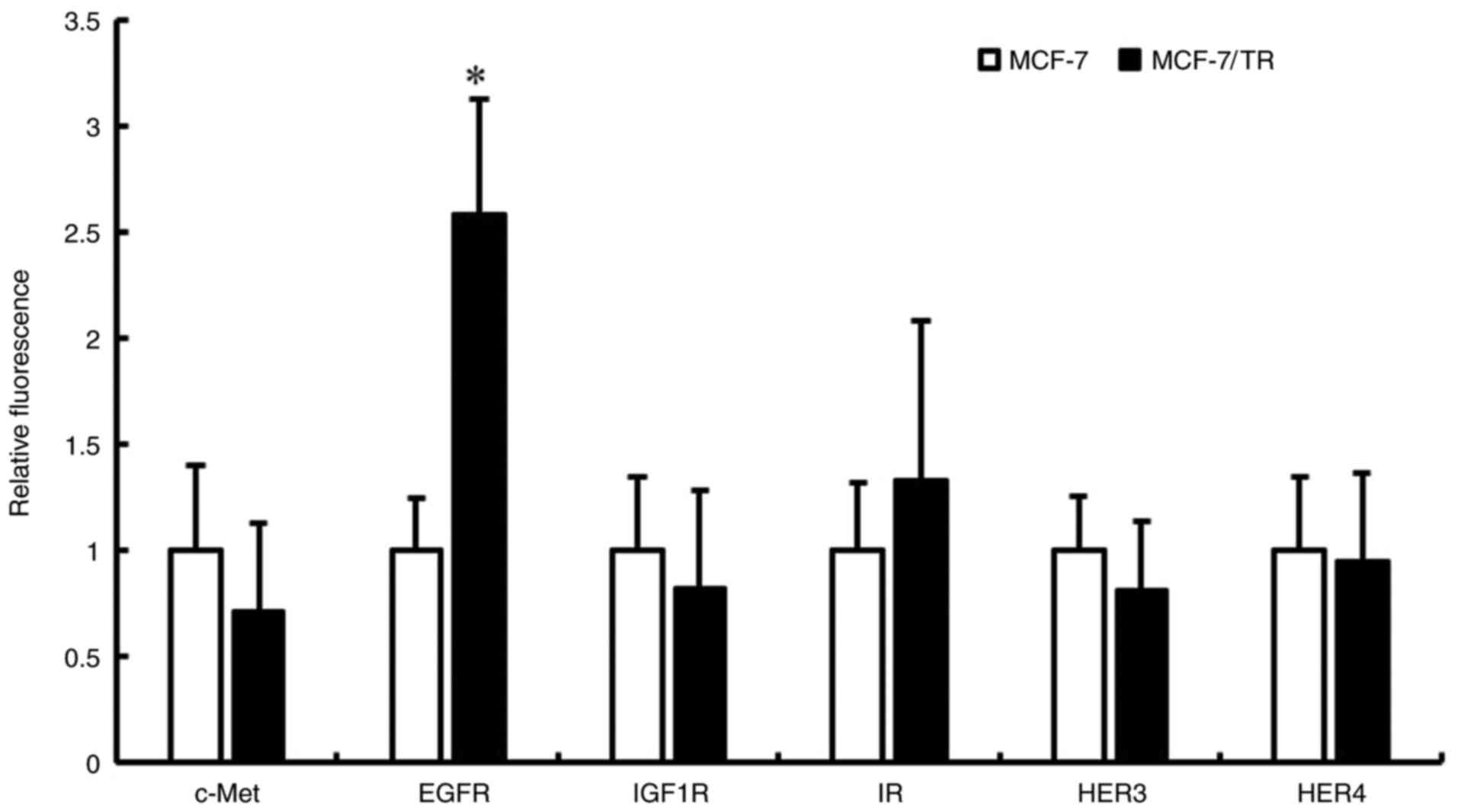 | Figure 5.MCF-7/TR cells exhibit an increased
EGFR phosphorylation in comparison with MCF-7 cells. c-Met, EGFR,
IGF-1R, IR, HER3 and HER4 protein phosphorylation levels were
measured using Luminex® 200. Data are presented as the
mean ± SD of three independent experiments. *P<0.05 as compared
to MCF-7 cells. MCF-7/TR, tamoxifen-resistant MCF-7 cells; c-Met,
tyrosine-protein kinase Met; HER, human epidermal growth factor
receptor; EGFR, epidermal growth factor receptor; IGF1R,
insulin-like growth factor 1 receptor; IR, insulin receptor. |
 | Figure 7.EGFR inhibition reverses the EMT
phenotype in MCF-7/TR cells via Snail and Twist downregulation.
MCF-7/TR cells were treated with gefitinib (5 µM) for 3 days.
E-cadherin, N-cadherin, vimentin, Snail, and Twist
levels were measured using reverse transcription-quantitative PCR.
Data are presented as the mean ± SD of three independent
experiments. *P<0.05 as compared to MCF-7/TR cells. The F values
are 236.4 (E-cadherin), 483.8 (N-cadherin), 49.86 (Vimentin), 49.87
(Snail), and 220.3 (Twist). EGFR, epidermal growth factor receptor;
EMT, epithelial-mesenchymal transition; MCF-7/TR,
tamoxifen-resistant MCF-7 cells; Snai1, snail family
transcriptional repressor 1; Twist, twist family BHLH transcription
factor 1; Slug, snail family transcriptional repressor 2. |
Inhibition of EGFR decreases the
tamoxifen resistance, migration and invasion of MCF-7/TR cells
The present study then examined whether gefitinib
decreases the tamoxifen resistance, migration and invasion of
MCF-7/TR cells. Gefitinib treatment was found to decrease the
tamoxifen resistance of MCF-7/TR cells (Fig. 8A). The combination of tamoxifen and
gefitinib slightly reduced the viability of the MCF-7 cells
compared to the tamoxifen-treated MCF-7 cells. In addition,
Transwell invasion and migration assays revealed that gefitinib
treatment inhibited the migration and invasion of MCF-7/TR cells
(Fig. 8B and C). However, no
changes were observed in the migration and invasion of the
gefitinib-treated MCF-7 cells. These results indicated that
gefitinib may successfully decrease the tamoxifen resistance,
migration and invasion of MCF-7/TR cells.
Discussion
Tamoxifen has been used in the treatment of both
pre- and post-menopausal patients with ER-positive breast cancer
for >40 years. However, ~40% of ER-positive breast cancer
patients develop resistance to tamoxifen (10). Numerous studies have been conducted
to identify the underlying mechanisms of tamoxifen resistance in
various research and clinical settings (11,14,38,39).
EMT has been reported to contribute to drug resistance, an
increased motility and cancer metastasis in a variety of cancer
types, including breast, pancreatic, and colorectal cancers
(40). Furthermore,
tamoxifen-resistant breast cancer cells undergo EMT morphological
changes, which alters their growth rate and increases aggressive
behavior (41,42). Additionally, restoring E-cadherin
expression or reversing EMT in resistant cancer cells has been
reported to enhance cancer cell susceptibility to chemotherapy and
radiotherapy (43). Taken
together, therapeutic strategies that reverse EMT may be a novel
approach which may be used to overcome acquired tamoxifen
resistance in breast cancer. However, crucial questions concerning
the central molecules controlling the EMT process during the
development of tamoxifen resistance remain unanswered.
In the present study, a tamoxifen-resistant breast
cancer cell line, MCF-7/TR, was established, that exhibited an
enhanced cell motility and invasive behavior. In addition, an
increased expression of the mesenchymal protein, vimentin, and a
decreased expression of the epithelial marker, E-cadherin, were
revealed, as well as morphological changes consistent with EMT. It
was also demonstrated that Snail and Twist silencing may reverse
the EMT phenotype, and decrease the tamoxifen resistance, migration
and invasion of MCF-7/TR cells. Increased Snail expression levels
may induce an EMT phenotype, and increased migration and invasion
in various physiological and pathological settings (44–46).
The expression of Twist has also been found to be associated with
various aggressive cancer types, including breast, gastric and
bladder cancer (47–51). Previous studies have reported that
Snail and Twist may function by inducing epigenetic silencing at
the E-cadherin promoter in the form of hypermethylation and histone
deacetylation (40,44,45–54).
Twist overexpression has been reported to increase the expression
of protease-activated receptor 1 (PAR1), and promote the EMT,
migration and invasion of ER-positive breast cancer cells (55). The results of the present study
suggested that Snail and Twist may be important targets for
overcoming tamoxifen resistance, and controlling cancer migration
and invasion.
Tamoxifen-resistant breast cancer is unresponsive to
the majority of targeted clinical therapies; thus, there is an
urgent need for alternative therapies. Therapeutic strategies based
on the reversal of EMT may be a novel approach for overcoming
acquired tamoxifen resistance in breast cancer. The RTK signaling
pathway has been demonstrated to contribute to EMT and tumor cell
invasion (56). The activation of
RTK and its downstream signaling effectors, including MAPK or PI3K,
is crucial for an increased rate of cell proliferation in
epithelial cells (57). In the
present study, it was demonstrated that EGFR expression was
increased in MCF-7/TR cells in comparison with MCF-7 cells.
Notably, the EGFR inhibitor, gefitinib, reversed the EMT phenotype
through the inhibition of Snail and Twist. In addition, gefitinib
decreased the tamoxifen resistance, migration and invasion of
MCF-7/TR cells. EGFR is an important transmembrane protein that is
involved in normal epithelial development, as well as in tumor cell
proliferation, migration and metastasis. It has been reported to be
overexpressed in breast cancer, particularly in more aggressive
breast tumor phenotypes associated with poor disease prognosis
(58–60). Furthermore, EGFR activation has
been reported to induce EMT in cancer cells via the upregulation of
Snail and Twist (61,62). The findings of the present study
indicated that EGFR activation was an independent biomarker in
tamoxifen-resistant breast cancer, and a potential novel
therapeutic target that may contribute to reversing EMT and
re-sensitizing breast cancer cells to tamoxifen treatment.
Increased knowledge of the signaling factors and
pathways inducing tamoxifen resistance could not only aid in the
discovery of novel drug targets in ER-positive breast cancer, but
also in expanding further the use of presently available
medications. Although a number of studies have revealed that
tamoxifen resistance promotes EMT-like behavior, the underlying
molecular mechanisms and the participating cellular signaling
pathways have not yet been studied in detail (63–65).
In the present study, it was indicated that EGFR is a promising
therapeutic target for tamoxifen-resistant breast cancer. In
addition, the EGFR inhibitor, gefitinib, decreased tamoxifen
resistance, migration and invasion through the inhibition of
Snail and Twist. Gefitinib has been approved by the
FDA for the treatment of metastatic non-small cell lung cancer.
Moreover, gefitinib is well-tolerated and has been shown to be
effective in treating acquired tamoxifen-resistance in breast
cancer patients in a phase II study (66). Therefore, gefitinib may serve as a
potent novel therapeutic strategy for breast cancer patients, who
have developed tamoxifen resistance. In addition, repurposing
gefitinib may be a more effective and inexpensive approach than
traditional drug development.
The present study has a few limitations, however.
The present study clarified that the EGFR inhibitor, gefitinib,
reversed the EMT phenotype through the inhibition of Snail and
Twist. Consistent with these findings, Hiscox et al
(41) reported that the inhibition
of EGFR may alter the EMT-like phenotype in tamoxifen-resistant
breast cancer cells. However, previous studies concerning the
association between EGFR and EMT in tamoxifen resistance have been
contradictory. Jiang et al (67) reported that the inhibition of the
EGFR pathway, which successfully restored the tamoxifen sensitivity
of Snail-expressing breast cancer cells, could not reverse their
mesenchymal phenotype. In the present study, tamoxifen-resistant
MCF-7 cells were used, established from the MCF-7 cells, following
a continuous exposure to tamoxifen and a gradual increase in the
tamoxifen concentration. By contrast, Jiang et al (67) used stable Snail-overexpressing
breast cancer cells (MCF-7 and T47D). Therefore, these inconsistent
results may be attributed to the methods of tamoxifen-resistant
breast cancer cell establishment. The association between EGFR and
EMT warrants further investigations using tamoxifen-resistant
breast cancer cell studies. In addition, the EGFR inhibition
efficacy in MCF/TR cells should be validated in vivo.
In conclusion, the present study demonstrated that
tamoxifen-resistant breast cancer cells may undergo EMT, and
exhibit an enhanced cell motility and invasive behavior. Snail and
Twist silencing reversed the EMT phenotype, and decreased tamoxifen
resistance, migration and invasion. More importantly, the EGFR
inhibitor, gefitinib, may be capable of reversing the EMT phenotype
through the inhibition of Snail and Twist, and enhancing tamoxifen
susceptibility in breast cancer cells. Taken together, the results
of the present study suggest that EGFR may be a promising
therapeutic target in tamoxifen-resistant breast cancer, and
gefitinib may have potential clinical treatment applications.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported in part by a Grant-in-Aid for Young
Scientists from the Japan Society for the Promotion of Science
(JSPS) (grant no. 20K16343).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT wrote the manuscript, and performed the cell
viability assay, Transwell and migration assays, RNA
interference/transfection assays, western blot analysis and the
statistical analyses. MTs performed the cell viability assay,
RT-qPCR and RNA interference/transfection assays. TM, AK, MJ and TO
performed the Transwell and migration assays, RT-qPCR and western
blot analysis. MTa contributed to the statistical analyses. SN
conceptualized and coordinated the present study. All authors have
read and approved the final manuscript. SN and TT confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
EGFR
|
epidermal growth factor receptor
|
|
Snai1
|
snail family transcriptional repressor
1
|
|
Twist
|
twist family BHLH transcription factor
1
|
|
Slug
|
snail family transcriptional repressor
2
|
|
MCF-7/TR cells
|
tamoxifen-resistant MCF-7 cells
|
References
|
1
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Viedma-Rodríguez R, Baiza-Gutman L,
Salamanca-Gómez F, Diaz-Zaragoza M, Martínez-Hernández G, Ruiz
Esparza-Garrido R, Velázquez-Flores MA and Arenas-Aranda D:
Mechanisms associated with resistance to tamoxifen in estrogen
receptor-positive breast cancer (review). Oncol Rep. 32:3–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaufmann M, Jonat W, Hilfrich J, Eidtmann
H, Gademann G, Zuna I and von Minckwitz G: Improved overall
survival in postmenopausal women with early breast cancer after
anastrozole initiated after treatment with tamoxifen compared with
continued tamoxifen: The ARNO 95 study. J Clin Oncol. 25:2664–2670.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clemons M, Danson S and Howell A:
Tamoxifen (‘Nolvadex’): A review. Cancer Treat Rev. 28:165–180.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Musgrove EA and Sutherland RL: Biological
determinants of endocrine resistance in breast cancer. Nat Rev
Cancer. 9:631–643. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies C, Godwin J, Gray R, Clarke M,
Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al:
Relevance of breast cancer hormone receptors and other factors to
the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of
randomised trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Liu S, Luo H, Chen C, Zhang X, He L
and Tu G: GPR30-mediated HMGB1 upregulation in CAFs induces
autophagy and tamoxifen resistance in ERalpha-positive breast
cancer cells. Aging (Albany NY). 13:16178–16197. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Gong X and Zhang Y: Network-based
approach to identify prognosis-related genes in tamoxifen-treated
patients with estrogen receptor-positive breast cancer. Biosci Rep.
41:BSR202030202021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan W, Chang J and Fu P: Endocrine therapy
resistance in breast cancer: Current status, possible mechanisms
and overcoming strategies. Future Med Chem. 7:1511–1519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dittmer J: Nuclear mechanisms involved in
endocrine resistance. Front Oncol. 11:7365972021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arpino G, De Angelis C, Giuliano M,
Giordano A, Falato C, De Laurentiis M and De Placido S: Molecular
mechanism and clinical implications of endocrine therapy resistance
in breast cancer. Oncology. 77 (Suppl 1):23–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsubaki M, Komai M, Fujimoto S, Itoh T,
Imano M, Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, et
al: Activation of NF-κB by the RANKL/RANK system up-regulates snail
and twist expressions and induces epithelial-to-mesenchymal
transition in mammary tumor cell lines. J Exp Clin Cancer Res.
32:622013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saitoh M: Involvement of partial EMT in
cancer progression. J Biochem. 164:257–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derynck R and Weinberg RA: EMT and cancer:
More than meets the eye. Dev Cell. 49:313–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ribatti D, Tamma R and Annese T:
Epithelial-mesenchymal transition in cancer: A historical overview.
Transl Oncol. 13:1007732020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Z, Shan Z and Shaikh ZA:
Epithelial-mesenchymal transition in breast epithelial cells
treated with cadmium and the role of snail. Toxicol Appl Pharmacol.
344:46–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baranwal S and Alahari SK: Molecular
mechanisms controlling E-cadherin expression in breast cancer.
Biochem Biophys Res Commun. 384:6–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mariotti A, Perotti A, Sessa C and Rüegg
C: N-cadherin as a therapeutic target in cancer. Expert Opin
Investig Drugs. 16:451–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li
J and Zhang Q: Resveratrol reverses Doxorubicin resistance by
inhibiting epithelial-mesenchymal transition (EMT) through
modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin
Cancer Res. 36:192017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Gun M and Hong XY: Induced
tamoxifen resistance is mediated by increased methylation of
e-cadherin in estrogen receptor-expressing breast cancer cells. Sci
Rep. 9:141402019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PLoS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Işeri OD, Kars MD, Arpaci F, Atalay C, Pak
I and Gündüz U: Drug resistant MCF-7 cells exhibit
epithelial-mesenchymal transition gene expression pattern. Biomed
Pharmacother. 65:40–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MR, Choi HK, Cho KB, Kim HS and Kang
KW: Involvement of Pin1 induction in epithelial-mesenchymal
transition of tamoxifen-resistant breast cancer cells. Cancer Sci.
100:1834–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Nes JG, de Kruijf EM, Putter H,
Faratian D, Munro A, Campbell F, Smit VT, Liefers GJ, Kuppen PJ,
van de Velde CJ and Bartlett JM: Co-expression of SNAIL and TWIST
determines prognosis in estrogen receptor-positive early breast
cancer patients. Breast Cancer Res Treat. 133:49–59. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The e-cadherin and
n-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells.
8:11182019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gooding AJ and Schiemann WP:
Epithelial-mesenchymal transition programs and cancer stem cell
phenotypes: Mediators of breast cancer therapy resistance. Mol
Cancer Res. 18:1257–1270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luqmani YA and Alam-Eldin N: Overcoming
resistance to endocrine therapy in breast cancer: New approaches to
a nagging problem. Med Princ Pract. 25 (Suppl 2):28–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alves CL, Elias D, Lyng MB, Bak M and
Ditzel HJ: SNAI2 upregulation is associated with an aggressive
phenotype in fulvestrant-resistant breast cancer cells and is an
indicator of poor response to endocrine therapy in estrogen
receptor-positive metastatic breast cancer. Breast Cancer Res.
20:602018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Butti R, Das S, Gunasekaran VP, Yadav AS,
Kumar D and Kundu GC: Receptor tyrosine kinases (RTKs) in breast
cancer: Signaling, therapeutic implications and challenges. Mol
Cancer. 17:342018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Gu J, Neuwald AF, Hilakivi-Clarke
L, Clarke R and Xuan J: Identifying intracellular signaling modules
and exploring pathways associated with breast cancer recurrence.
Sci Rep. 11:3852021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao J, Deng K, Huang J, Zeng R and Zuo J:
Progress in the understanding of the mechanism of tamoxifen
resistance in breast cancer. Front Pharmacol. 11:5929122020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hiscox S, Jiang WG, Obermeier K, Taylor K,
Morgan L, Burmi R, Barrow D and Nicholson RI: Tamoxifen resistance
in MCF7 cells promotes EMT-like behaviour and involves modulation
of beta-catenin phosphorylation. Int J Cancer. 118:290–301. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Q, Cheng Y, Wang Y, Fan Y, Li C,
Zhang Y, Wang Y, Dong Q, Ma Y, Teng YE, et al: Tamoxifen reverses
epithelial-mesenchymal transition by demethylating miR-200c in
triple-negative breast cancer cells. BMC Cancer. 17:4922017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:9652016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peinado H, Marin F, Cubillo E, Stark HJ,
Fusenig N, Nieto MA and Cano A: Snail and E47 repressors of
E-cadherin induce distinct invasive and angiogenic properties in
vivo. J Cell Sci. 117:2827–2839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Blanco MJ, Moreno-Bueno G, Sarrio D,
Locascio A, Cano A, Palacios J and Nieto MA: Correlation of Snail
expression with histological grade and lymph node status in breast
carcinomas. Oncogene. 21:3241–3246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee YM, Park T, Schulz RA and Kim Y:
Twist-mediated activation of the NK-4 homeobox gene in the visceral
mesoderm of Drosophila requires two distinct clusters of E-box
regulatory elements. J Biol Chem. 272:17531–17541. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng MY, Wang K, Song HT, Yu HW, Qin Y,
Shi QT and Geng JS: Metastasis-induction and apoptosis-protection
by TWIST in gastric cancer cells. Clin Exp Metastasis.
26:1013–1023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Z, Xie D, Li X, Wong YC, Xin D, Guan
XY, Chua CW, Leung SC, Na Y and Wang X: Significance of TWIST
expression and its association with E-cadherin in bladder cancer.
Hum Pathol. 38:598–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu Y, Xu Y, Liao L, Zhou N, Theissen SM,
Liao XH, Nguyen H, Ludwig T, Qin L, Martinez JD, et al: Inducible
knockout of Twist1 in young and adult mice prolongs hair growth
cycle and has mild effects on general health, supporting Twist1 as
a preferential cancer target. Am J Pathol. 183:1281–1292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alexander NR, Tran NL, Rekapally H,
Summers CE, Glackin C and Heimark RL: N-cadherin gene expression in
prostate carcinoma is modulated by integrin-dependent nuclear
translocation of Twist1. Cancer Res. 66:3365–3369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Liu J, Ying X, Lin PC and Zhou BP:
Twist-mediated epithelial-mesenchymal transition promotes breast
tumor cell invasion via inhibition of hippo pathway. Sci Rep.
6:246062016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Casaletto JB and McClatchey AI: Spatial
regulation of receptor tyrosine kinases in development and cancer.
Nat Rev Cancer. 12:387–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gschwind A, Fischer OM and Ullrich A: The
discovery of receptor tyrosine kinases: Targets for cancer therapy.
Nat Rev Cancer. 4:361–370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ueno NT and Zhang D: Targeting EGFR in
triple negative breast cancer. J Cancer. 2:324–328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Normanno N, De Luca A, Maiello MR, Mancino
M, D'Antonio A, Macaluso M, Caponigro F and Giordano A: Epidermal
growth factor receptor (EGFR) tyrosine kinase inhibitors in breast
cancer: Current status and future development. Front Biosci.
10:2611–2617. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
60
|
Drury SC, Detre S, Leary A, Salter J,
Reis-Filho J, Barbashina V, Marchio C, Lopez-Knowles E, Ghazoui Z,
Habben K, et al: Changes in breast cancer biomarkers in the
IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen
treatment. Endocr Relat Cancer. 18:565–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei
Y, Abbruzzese JL, Hortobagyi GN and Hung MC: Epidermal growth
factor receptor cooperates with signal transducer and activator of
transcription 3 to induce epithelial-mesenchymal transition in
cancer cells via up-regulation of TWIST gene expression. Cancer
Res. 67:9066–9076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu H, Zhang HW, Sun XF, Guo XH, He YN,
Cui SD and Fan QX: Tamoxifen-resistant breast cancer cells possess
cancer stem-like cell properties. Chin Med J (Engl). 126:3030–3034.
2013.PubMed/NCBI
|
|
64
|
Johansson HJ, Sanchez BC, Forshed J, Stål
O, Fohlin H, Lewensohn R, Hall P, Bergh J, Lehtiö J and Linderholm
BK: Proteomics profiling identify CAPS as a potential predictive
marker of tamoxifen resistance in estrogen receptor positive breast
cancer. Clin Proteomics. 12:82015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sakunrangsit N, Kalpongnukul N, Pisitkun T
and Ketchart W: Plumbagin enhances tamoxifen sensitivity and
inhibits tumor invasion in endocrine resistant breast cancer
through EMT regulation. Phytother Res. 30:1968–1977. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gutteridge E, Agrawal A, Nicholson R,
Cheung KL, Robertson J and Gee J: The effects of gefitinib in
tamoxifen-resistant and hormone-insensitive breast cancer: A phase
II study. Int J Cancer. 126:1806–1816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang Y, Zhao X, Xiao Q, Liu Q, Ding K, Yu
F, Zhang R, Zhu T and Ge G: Snail and slug mediate tamoxifen
resistance in breast cancer cells through activation of EGFR-ERK
independent of epithelial-mesenchymal transition. J Mol Cell Biol.
6:352–354. 2014. View Article : Google Scholar : PubMed/NCBI
|