Introduction
Breast cancer is a heterogeneous disease categorized
into several subtypes according to the expression of estrogen
receptor (ER) and human epidermal growth factor receptor 2 (HER2).
ER-positive (ER+) breast cancer is present in
approximately 70% of all breast cancer patients (1,2).
Tamoxifen is the most effective primary drug compared with other
selective estrogen receptor modulators. However, approximately 20%
of patients administered tamoxifen experience tumor recurrence, and
those with advanced breast cancer who respond to tamoxifen at the
beginning of treatment also eventually experience disease
progression (1,3,4). To
date, well-known theories explaining the mechanisms of resistance
to endocrine therapy include loss of ER expression, mutations in
genes encoding ER, overactivation of growth factor signaling
pathways, and difficulty in regulation of cell cycle signaling
pathways (3,5,6).
Among these, mutations in proteins involved in the phosphoinositide
3-kinase (PI3K)/AKT/mTOR pathway are frequently observed in
ER+ breast cancer and have been studied extensively as
one of the mediators of resistance to endocrine therapy. Several
clinical trials targeting this pathway have also been conducted
(7,8). However, the mechanism underlying
endocrine resistance is complex, and recent studies have shown that
characteristics of cancer stem cell-like properties and
Wnt/β-catenin signaling are associated with drug resistance and
poor prognosis (9–13). Transcription factors that regulate
cell proliferation and survival, such as signal transducer and
activator of transcription 3 (STAT3), have also been reported to be
associated with the development of stem cell-like properties in
breast cancer (14).
Wnt/β-catenin signaling plays an important role in
cell growth, proliferation, and differentiation and is associated
with the pathogenesis of various types of cancer (15–20).
In breast cancer, activation of β-catenin signaling is associated
with poor outcomes in basal-like or triple-negative breast cancer
subtypes lacking ER expression (21,22).
Moreover, the number of breast cancer stem cells, the ability to
initiate tumors, and metastasis have been demonstrated to be
related to the Wnt/β-catenin signaling pathway (23). In a previous study by the authors,
it was reported that β-catenin is associated with endocrine
resistance in breast cancer and that inhibition of β-catenin can
overcome endocrine resistance (5).
ICG-001, a β-catenin small-molecule inhibitor, selectively binds to
the CREB-binding protein (CBP) in the nucleus and is proposed to
prevent β-catenin from performing CBP-dependent transcription of
genes in the canonical Wnt/β-catenin signaling pathway that are
related to stem cell-like properties (24). The inhibitory effects of ICG-001
have recently been demonstrated in several carcinomas associated
with Wnt/β-catenin signaling, including pancreatic ductal
adenocarcinoma (25), gastric
cancer (19), and head and neck
cancer (26). However, to the best
of our knowledge, studies examining the effects of ICG-001 on
endocrine-resistant breast cancer have been scarce, and its
mechanism underlying the effects has not been established.
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors
were introduced as primary drugs to inhibit the cell cycle pathway,
overcome endocrine resistance, and increase the effectiveness of
endocrine therapy. Moreover, several treatment combinations are
being studied to increase the potency of CDK4/6 inhibitors in an
endocrine-resistant setting; these include the combination of two
different CDK4/6 inhibitors and a CDK4/6 inhibitor with fulvestrant
(27–29). Considering the role of β-catenin in
endocrine resistance and the acquisition of cancer stem cell-like
properties, in the present study, the effects of a combination of
palbociclib (a CDK4/6 inhibitor) and ICG-001 (a β-catenin small
molecule inhibitor), as well as of ICG-001 on MCF-7 and an
endocrine-resistant cell line, were explored with the aim of
developing novel therapeutic strategies.
Materials and methods
Cell lines and culture
MCF-7 (KCBL no. 30022), an ER+ human
breast cancer cell line, was obtained from the Korean Cell Line
Bank. These cells were cultured in phenol-red-free RPMI-1640 medium
containing 10% fetal bovine serum and antibiotics (1%
penicillin/streptomycin; all Invitrogen; Thermo Fisher Scientific,
Inc.). The medium was changed twice a week during culture.
Tamoxifen-resistant MCF-7 (TamR) cells were prepared via sequential
exposure of MCF-7 cells to increasing concentrations (from 0.05 to
3 µM) of 4-hydroxy-tamoxifen at 37°C over a period of 9 months.
Although the cell growth rate was not quantitated, TamR cells
generally tended to show slow growth when exposed to the drug.
Cell viability and mammosphere
assay
Cell viability was determined at different doses of
the drugs using the Cell Counting Kit-8 (CCK-8; Enzo Life Sciences,
Farmingdale, Inc.). Briefly, MCF-7 and TamR cells were seeded in a
96-well plate at a density of 5×103 cells/well. The
concentrations of palbociclib (0.1, 1, 10, 25, 50 and 100 µM; also
known as PD-0332991; Sigma-Aldrich; Merck KGaA), ICG-001 (0, 12.5,
25, 50 and 100 µM; Selleck Chemicals) and S3I-201 (3, 6.25, 12.5,
50 and 100 µM; cat. no. S1155; Selleck Chemicals) were added to the
wells and the plate was incubated for 24 h at 37°C. The optical
density at 450 nm was measured using a spectrophotometer (VersaMax;
Molecular Devices, LLC). The mammosphere assay was performed using
the MammoCult™ Human Media kit (cat. no. 05620; STEMCELL
Technologies, Inc.) according to the manufacturer's protocol. Cells
were seeded at 3.5×104 cells/2 ml culture medium in
6-well ultra-low attachment plates. Following incubation for 7 days
in a 37°C CO2 incubator, spheres with a diameter of 60
µm or more were counted.
Western blot analysis
Protein extracts were prepared by lysing the cells
in RIPA buffer [25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1%
sodium deoxycholate, and 0.1% SDS] containing a protease and
phosphatase inhibitor cocktail (Thermo Fisher Scientific, Inc.).
The protein concentration was determined using the Bradford assay.
Total protein lysates (40 µg) were loaded into each lane,
size-fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and transferred onto polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked in
Tris-buffered saline (TBS)-0.1% Tween-20 containing 5% skim milk
for 1 h at room temperature, and then incubated with primary
antibodies against ERα (D8H8; rabbit mAb; product no. 8644;
dilution 1:1,000), epidermal growth factor (EGFR; C74B9; rabbit
mAb; product no. 2646; dilution 1:1,000), HER2 (29D8; rabbit mAb;
product no. 2165; dilution 1:1,000), Nanog (D73G4; rabbit mAb;
product no. 4903; dilution 1:1,000), Sox2 (D6D9; rabbit mAb;
product no. 3579; dilution 1:1,000), Oct4 (product no. 2750;
dilution 1:1,000), ALDH1 (D4R9V; rabbit mAb; product no. 12035;
dilution 1:1,000), phosphorylated (p)-STAT3 (D3A7; rabbit mAb;
product no. 9145; dilution 1:1,000), STAT3 (124H6; mouse mAb;
product no. 9139; dilution 1:1,000), mTOR (7C10; rabbit mAb;
product no. 2983; dilution 1:1,000), p-mTOR (D9C2; rabbit mAb;
product no. 5536; dilution 1:1,000), NOTCH1 (D1E11; rabbit mAb;
product no. 3608; dilution 1:1,000), β-catenin (D10A8; rabbit mAb;
product no. 8480; dilution 1:1,000), active β-catenin (D13A1;
rabbit mAb; product no. 8814; dilution 1:1,000), E-cadherin (24E10;
rabbit mAb; product no. 3195; dilution 1:1,000), N-cadherin
(product no. 4061), α-tubulin (11H10; rabbit mAb; product no. 2125;
dilution 1:1,000), or GAPDH (D16H11; rabbit mAb; product no. 5174;
dilution 1:1,000) (all the antibodies were procured from Cell
Signaling Technology, Inc.) overnight at 4°C. Following washing
with TBS-0.1% Tween-20 three times, the membranes were incubated
for 1 h at room temperature with goat anti-rabbit (product no.
7074; dilution 1:1,000) or horse anti-mouse IgG HRP-conjugated
secondary antibodies (product no. 7076; dilution 1:1,000; both from
Cell Signaling Technology, Inc.). Western blot images were
visualized with enhanced chemiluminescence (ECL) reagents (Thermo
Fisher Scientific, Inc.) and recorded using a LAS-4000 Mini camera
(Fujifilm). Band intensities were quantified using the ImageJ
software (version 1.53k; http://imagej.nih.gov/ij/). Protein phosphorylation
data were further expressed as the p-STAT3/STAT3 or p-mTOR/mTOR
ratio.
Luciferase assay
Luciferase reporter assays were performed using the
luciferase assay system (Promega Corporation). MCF-7 and TamR cells
were plated in 24-well plates, a day before transfection, at a
density of 4×104 cells/well. The cells were
co-transfected with the pGL4.49[luc2P/TCF-LEF RE/Hygro] and
pRL-TK constructs (Promega Corporation) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The pRL-TK vector
provides constitutive expression of Renilla luciferase as a
control reporter vector. The cells were incubated for 24 h after
transfection, and then treated with palbociclib (25 µM) or ICG-001
(50 µM) or S3I-201 (100 µM) for 24 h at 37°C in an incubator. The
cells were subsequently processed using the DUAL-Glo Luciferase
Reporter Assay System (Promega Corporation), and luciferase
activity was measured using a luminometer (Veritas Microplate
Luminometer; Turner BioSystems, Inc.). The ratio of firefly to
Renilla luciferase activity was representative of the
transcriptional activity of β-catenin.
Flow cytometric analysis of the cell
cycle
Cells (1×106/ml; 30–40%) were treated
with palbociclib (25 µM) and ICG-001 (50 µM) for 24 h for cell
cycle analysis. The cells were detached with TrypLE (Invitrogen
Life Technologies; Thermo Fisher Scientific, Inc.) and pelleted by
centrifugation at 500 × g for 5 min at 4°C. The cell pellets were
fixed with 66% ethanol, overnight at −20°C, followed by washing
with phosphate-buffered saline [PBS; 0.137 M sodium chloride, 2.7
mM potassium chloride, 4.3 mM sodium phosphate (dibasic,
anhydrous), 1.4 mM potassium phosphate (monobasic, anhydrous)], and
stained with a propidium iodide solution for 30 min at room
temperature. Cell cycle analysis was carried out using the
Propidium Iodide Flow Cytometry Kit (Abcam) and performed on a
NovoCyte Flow Cytometer (ACEA Biosciences, Inc.; Agilent
Technologies, Inc.). The data were analyzed using NoveExpress
software (version 1.2.5; ACEA Biosciences, Inc.; Agilent
Technologies, Inc.).
Immunofluorescence
Cells (1×106/ml; 30–40%) were rinsed with
PBS, fixed for 15 min at room temperature with 4% paraformaldehyde,
permeabilized with 0.1% Triton X-100 in PBS (PBS-0.1% Tween-20),
and blocked with 1% BSA in PBS-Tween-20 for 10 min at room
temperature. The slides with fixed cells were incubated with
primary antibodies against β-catenin (D10A8; rabbit mAb; product
no. 8480) (Cell Signaling Technology, Inc.) at 4°C overnight.
Fluorescence-labeled secondary antibody, goat anti-rabbit IgG (H+L)
cross-adsorbed secondary antibody, Alexa Fluor™ 488 (cat. no.
A11008; Invitrogen; Thermo Fisher Scientific, Inc.) was applied for
1 h at room temperature. The cell nuclei were counterstained with
10 µl DAPI (stock solution 1:1, DAPI:glycerol). All images were
recorded using an LSM5 microscope (Carl Zeiss AG).
siRNA transfection
Cells (1×106/ml; 30–40%) were transfected
with small interfering (si)RNAs targeting STAT3 (siSTAT3) and a
non-targeting siRNA (both from Bioneer Corporation). AccuTarget™
Negative control siRNA (cat. no. SN-1002) was used as the negative
control (siNC). The siSTAT3 sequences were as follows:
5′-UGUAGGAAACUUUUUGCUG-3′ (sense) and 5′-CAGCAAAAAGUUUCCUACA-3′
(antisense). Transfection experiments were performed using the
jetPRIME reagent (Polyplus-transfection). Briefly, cells in the
exponential growth phase were seeded in a 6-well plate, grown for
24 h, transfected with 200 pmol of the siRNA for 10 min at room
temperature, and subsequently cultured for 3 days.
Statistical analysis
The statistical significance of differences between
the groups was determined using unpaired Student's t-test and
one-way ANOVA. The post hoc test employed the Tukey HSD method
after one-way ANOVA. Data were expressed as the mean ± standard
deviation (SD). All data were analyzed for statistical significance
using SPSS (version 25; IBM Corp.) and GraphPad Prism 7.0 for
Windows (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
TamR cells have enhanced self-renewal
properties of stem-like cells
The characteristics of TamR and MCF-7 cells were
compared by determining the expression of selected proteins using
western blot analysis. TamR cells exhibited reduced expression of
ERα and increased expression of EGFR and HER2 compared with MCF-7
cells (Fig. 1A). In addition, the
expression of Nanog, Sox2, and ALDH1 was upregulated in TamR cells,
indicating the characteristics of stem-like cells, and p-STAT3,
which is associated with cell signaling related to stem-like cell
characteristics, was also overexpressed compared with that in MCF-7
cells (Fig. 1B). The self-renewal
properties of TamR cells were visualized using the mammosphere
assay (Fig. 1C and D). The number
of microspheres in TamR cells (average 569 spheres/well) was
significantly increased (more than twice) compared with that in
MCF-7 cells (average 273 spheres/well). Increased active β-catenin
levels are shown in Fig. 2A and B.
Western blot analysis revealed no significant difference in
β-catenin levels between MCF-7 and TamR cells; however, the
upregulation of active β-catenin was considered significant because
β-catenin enters the nucleus and is activated. In addition, the
results of the luciferase assay, which reflects the levels of only
active β-catenin, revealed a significant increase in the levels of
active β-catenin in TamR cells, consistent with the results of
western blot analysis. These findings suggest that an increase in
active β-catenin levels is associated with the acquisition of
Wnt/β-catenin signals, which are known to be related to drug
resistance. The localization of β-catenin in TamR cells was
determined using immunofluorescence (Fig. 2C). Compared with that in the MCF-7
control group, the nuclear localization of β-catenin was high in
TamR cells. These findings are in agreement with those of a
previous study showing that β-catenin accumulates in the nucleus
due to activation of the Wnt/β-catenin signaling pathway, leading
to downstream signaling (30).
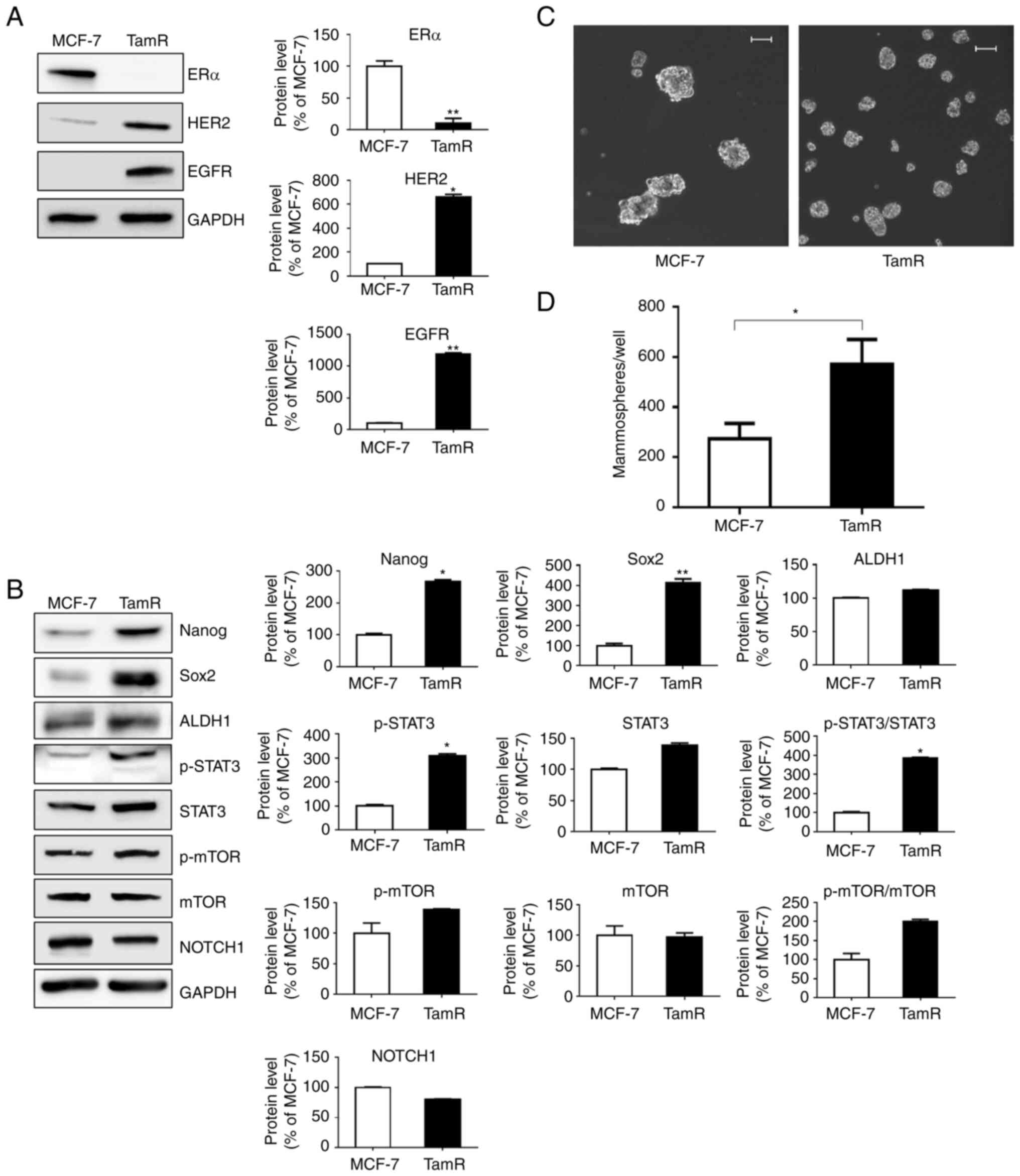 | Figure 1.TamR cells have enhanced self-renewal
properties of stem-like cells. (A) TamR cells exhibited decreased
expression of ERα and increased expression of HER2 and EGFR.
*P<0.05 and **P<0.005 vs. MCF-7 cells. (B) TamR cells
exhibited overexpression of the markers of stem-like properties and
cell signaling pathways compared with that in control MCF-7 cells.
Conversely, among the markers related to cell signaling pathways,
p-mTOR, mTOR, and NOTCH1 exhibited no difference between MCF-7 and
TamR cells. Representative western blots are presented along with
the densitometric analysis of the protein bands. (C) TamR cells
exhibited a greater number of microspheres compared with that in
MCF-7 cells. (D) The number of microspheres, with a diameter >60
µm was counted. Scale bars, 100 µm. Data are expressed as the mean
± SD of values from three independent experiments under the same
conditions. The statistical hypothesis was examined using an
unpaired Student's t-test. *P<0.05 and **P<0.005 vs. MCF-7
cells. TamR, tamoxifen-resistant MCF-7; ER, estrogen receptor;
HER2, human epidermal growth factor receptor 2; EGFR, epidermal
growth factor; p-, phosphorylated. |
Combination therapy with palbociclib
and ICG-001 results in additive inhibition of growth in TamR
cells
To evaluate the combinatorial effect of palbociclib
and ICG-001 on TamR cells, a cell viability assay was performed.
First, the appropriate therapeutic dose of both drugs as the
concentration of drug resulting in 50% inhibition of the viability
(the inhibitory concentration 50 or IC50 value) of naïve
MCF-7 cells, was determined. The IC50 value of
palbociclib was 25 µM, whereas that of ICG-001 was 50 µM (Fig. 3A and B). For palbociclib, no
difference was observed between MCF-7 and TamR cells, and for
ICG-001, TamR cells required relatively higher therapeutic
concentrations than MCF-7 cells. Next, the combination index (CI)
values were determined to identify the synergistic effect of
palbociclib and ICG-001. CI values <0.8, 0.8-1.2, and >1.2
were defined as synergism, additive, and antagonism, respectively
(31). The CI values of MCF-7 and
TamR cells exposed to 25 µM palbociclib and 50 µM ICG-001 were
1.1±0.04 and 1.1±0.02, respectively, representing additive effects
(Fig. 3C). Likewise, the viability
of TamR cells decreased by 29.8 and 57.5% upon treatment with
palbociclib and ICG-001, respectively, and by 71% when treated
concurrently with palbociclib and ICG-001.
Next, cell cycle analysis of MCF-7 and TamR cells
treated with palbociclib and ICG-001 alone or in combination was
performed (Figs. 3D and S1). Both the drugs suppressed the cell
cycle progression in the two cell lines. Compared with
vehicle-treated TamR cells, those treated with palbociclib and
ICG-001 exhibited increased G0/G1 arrest. The percentage of cells
exhibiting G0/G1 arrest was further increased in the combination
treatment compared with that in the treatment with the two drugs
individually.
Combination therapy with palbociclib
and ICG-001 inhibits stem cell-like properties
A mammosphere assay was conducted to visually
confirm the effects of the two drugs (Fig. 4A and B). Treatment with palbociclib
or ICG-001 significantly suppressed microsphere formation in TamR
cells compared with that in the vehicle-treated cells. The
suppression was greater in the combination treatment than in the
treatment with the two drugs individually. However, there was no
significant difference in P-values among the three treatment groups
(palbociclib vs. ICG-001, P=0.431; palbociclib vs. combination,
P=0.900; ICG-001 vs. combination, P=0.225).
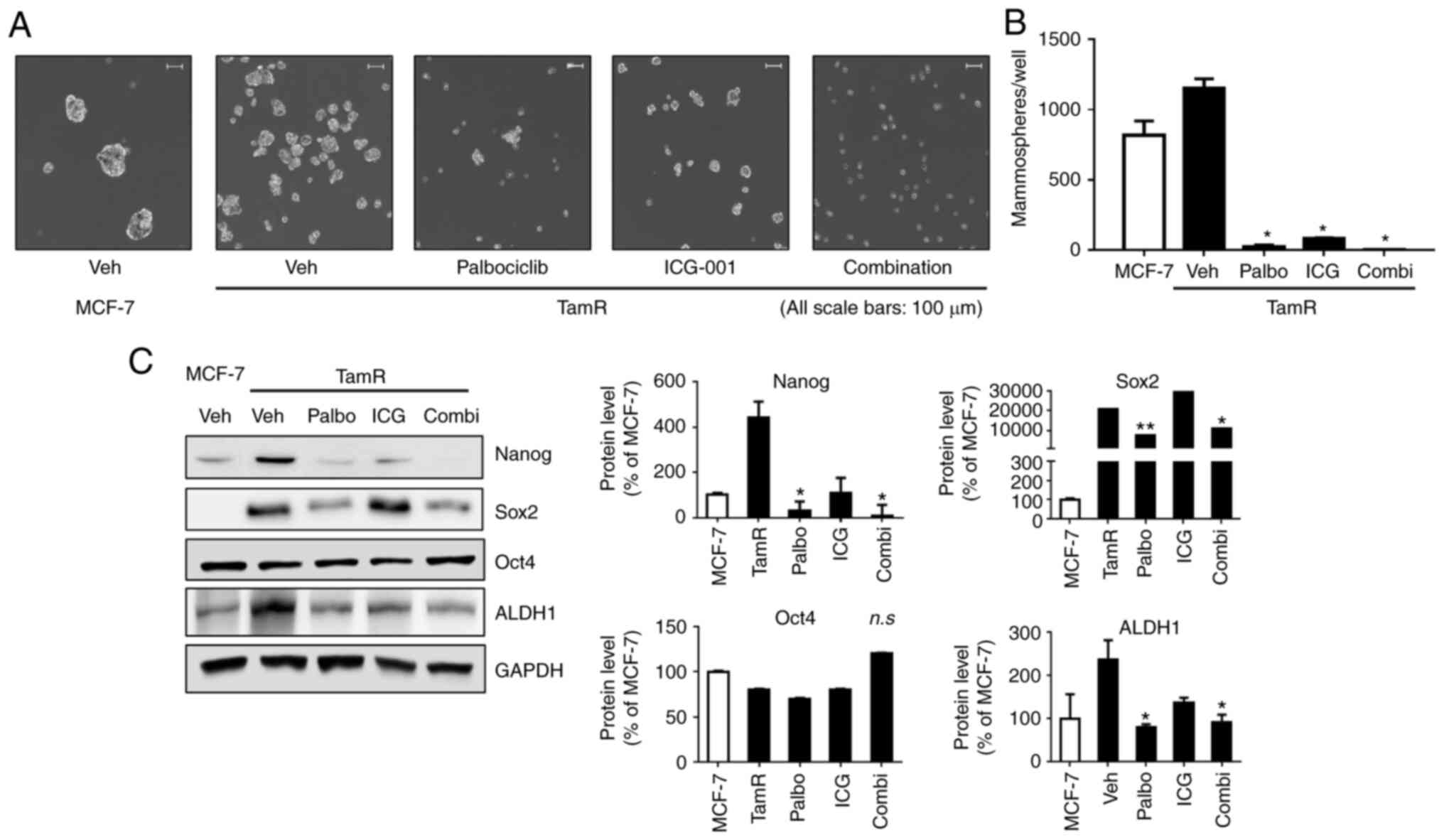 | Figure 4.Combination therapy with palbociclib
and ICG-001 inhibits stem cell-like properties. (A and B) For
sphere formation, cells were seeded in 6-well ultra-low attachment
plates, treated with palbociclib (25 µM) and ICG-001 (50 µM), and
incubated for 7 days in a 37°C CO2 incubator. The number
of mammospheres, with a diameter >60 µm, was counted. The
experiment was performed in triplicate, and the average number of
microspheres was as follows: TamR_P (n=27.50), TamR_I (n=83.50),
and TamR_C (n=5.00). TamR_P, palbociclib; TamR_I, ICG-001; TamR_C,
combination. (C) The expression of protein markers of stem
cell-like properties in cells treated with the drugs was assessed.
Nanog, Sox2, and ALDH1 were downregulated in TamR cells treated
with palbociclib (25 µM) and ICG-001 (50 µM) in combination.
Representative western blots are presented along with densitometric
data. Data were expressed as the mean ± SD of values from three
independent experiments under the same conditions. The statistical
hypothesis was examined using a one-way ANOVA. *P<0.05 and
**P<0.005 vs. TamR vehicle-treated cells. TamR,
tamoxifen-resistant MCF-7; n.s, not significant. |
The mechanism underlying the effect of the
combination treatment was further elucidated. The expression levels
of the markers of stem cell-like properties were determined
(Fig. 4C). The expression levels
of Nanog, Sox2, and ALDH1 were significantly reduced when TamR
cells were treated with palbociclib (25 µM) and ICG-001 (50 µM)
simultaneously, and the suppression of these proteins was similar
in the combination therapy and palbociclib monotherapy.
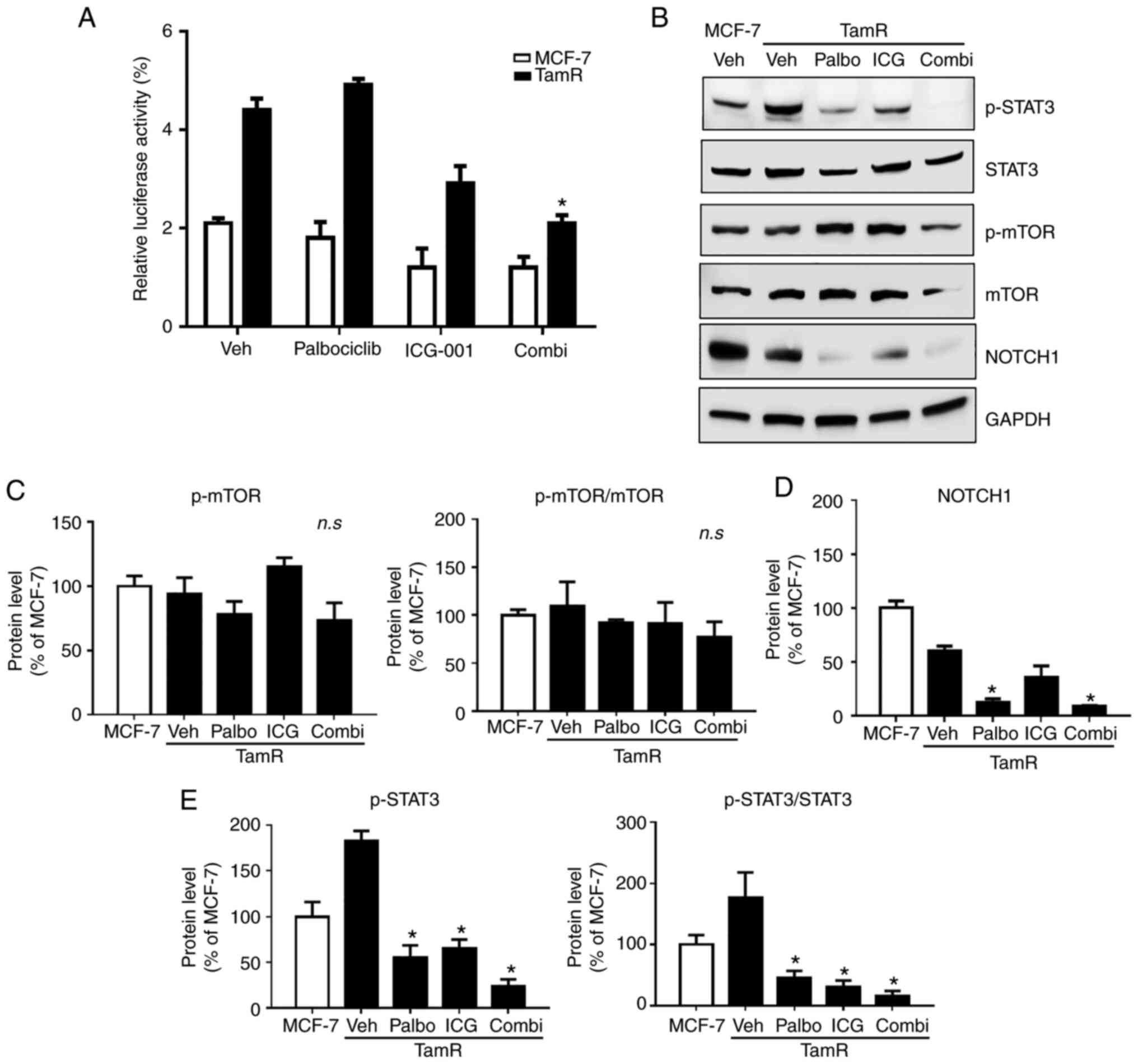
Active β-catenin and p-STAT3 are
significantly associated with the combinatorial effect of
palbociclib and ICG-001 on the reduction in stem cell-like
properties
To investigate the factors responsible for the
reduction in stem cell-like properties, the levels of active
β-catenin and markers of cell signaling pathways were assessed.
First, a luciferase assay was performed to evaluate the inhibitory
effect of palbociclib and ICG-001 on active β-catenin levels
(Fig. 5A). Compared with those in
MCF-7 cells, the levels of active β-catenin levels were
significantly increased in TamR cells. Treatment of TamR cells with
ICG-001 downregulated the levels of active β-catenin compared with
vehicle-treated TamR cells. Moreover, when TamR cells were
simultaneously treated with the two drugs, the levels of active
β-catenin were significantly reduced compared with vehicle-treated
TamR cells, indicating a combinatorial effect. Second, using
western blot analysis, the expression of markers related to cell
signaling pathways was determined (Fig. 5B-E). The level of p-STAT3 in TamR
cells was reduced upon treatment with palbociclib and ICG-001
compared with those in the vehicle-treated cells. The levels of
p-STAT3 were significantly reduced (P<0.05) in the combination
treatment group compared with those in the vehicle-treated cells;
however, the reduction was not significant when compared with the
levels in the palbociclib and ICG-001 treatment groups (P=0.990 and
P=0.978, respectively). No significant change in the expression of
p-mTOR was noted in the different treatment groups. The expression
of NOTCH1 in vehicle-treated TamR cells was reduced compared with
that in MCF-7 cells; therefore, it was difficult to confirm whether
this protein was involved in tamoxifen resistance before the cells
were treated with the drugs. Based on the aforementioned results,
it is strongly considered that the cell signaling pathway proteins,
including p-STAT3, and the decrease in the levels of active
β-catenin, are associated with the reduction in stem cell-like
properties of endocrine-resistant TamR cells treated with the
combination of palbociclib and ICG-001 compared with those of
parental MCF-7 cells.
Suppression of STAT3 does not affect
the level of active β-catenin
To investigate the crosstalk between β-catenin and
STAT3 signaling, changes in the expression of STAT3 and β-catenin
in cells treated with S3I-201, a targeted inhibitor of STAT3, were
evaluated via western blot analysis and luciferase assay (Fig. 6A-C). The appropriate therapeutic
dose of S3I-201 as the concentration of drug resulting in the
IC50 of naïve MCF-7 cells was determined, and the
IC50 value of S3I-201 was 100 µM. When treated with
S3I-201 (100 µM), the expression of p-STAT3 and STAT3 was
downregulated, indicating that S3I-201 worked, as expected.
However, there was no change in the levels of active β-catenin in
the luciferase assay and western blot analysis. Moreover, the
expression of Sox2 was reduced after drug treatment, which shows
its association with the STAT3 signaling pathway. The crosstalk
between STAT3 and β-catenin was also confirmed using the siRNA
transfection technique (Fig. 6D and
E). Following siRNA transfection, reduction in the levels of
p-STAT3 and STAT3 was confirmed using western blot analysis;
however, the levels of β-catenin remained unchanged regardless of
the STAT3 knockdown. Experiments with two different techniques
demonstrated that STAT3 did not affect the levels of active
β-catenin. Therefore, this additional mechanistic insight suggests
that β-catenin may be involved in STAT3 signaling, leading to the
theory that STAT3/Sox2 signaling may be involved in the reduction
in the stem cell-like property.
 | Figure 6.Suppression of STAT3 does not affect
the levels of active β-catenin. (A) The cell viability assay of
MCF-7 and TamR cells treated with S3I-201. Treatment with S3I-201
(100 µM) reduced the viability of MCF-7 (66.7%) and TamR (53.5%)
cells. The experiment was performed in triplicate. (B) Expression
of proteins related to cell signaling pathways and markers of
stem-like properties was investigated. Representative western blots
are presented along with densitometric data. In cells treated with
S3I-201 (100 µM), the expression of Sox2, a marker of stem-like
property, was reduced. Data are expressed as the mean ± SD of
values from three independent experiments under the same
conditions. The statistical hypothesis was examined using a one-way
ANOVA. *P<0.05 and **P<0.005 vs. MCF-7 cells. (C) There was
no inhibitory effect of S3I-201 (100 µM) on the levels of active
β-catenin, as assessed using the luciferase assay. Data are
expressed as the mean ± SD of values from three independent
experiments under the same conditions. (D) Western blot analysis
confirmed the knockdown of STAT3; however, siSTAT3 had no
inhibitory effect on the levels of active β-catenin, as assessed
using the western blot analysis. Representative western blots are
presented along with densitometric data. (E) The results of a
luciferase assay confirmed the change in the levels of active
β-catenin with the use of siSTAT3; there was no inhibitory effect
of siSTAT3 on active β-catenin levels. TamR, tamoxifen-resistant
MCF-7; STAT3, signal transducer and activator of transcription 3;
p-, phosphorylated; si, small interfering; siNC, siRNA negative
control; n.s, not significant. |
Discussion
Endocrine therapy has been the mainstay of
ER+ breast cancer treatment modalities over the last 60
years; however, endocrine resistance is inevitable (1). Recently, CDK4/6 inhibitors and PIK3CA
pathway inhibitors have been used worldwide in clinical settings.
Nevertheless, one-third of patients administered CDK4/6 inhibitors
experienced recurrence within 2 years, and over 70% of patients
treated with palbociclib experienced disease progression by 40
months in the PALOMA2 clinical trial (32). Thus, there is a need to develop
additional therapeutic agents to treat patients with
endocrine-resistant breast cancer.
In the present study, a model is proposed, in which
the inhibition of β-catenin levels in combination with the
administration of CDK4/6 inhibitors, which are, currently, the
standard treatment for endocrine-resistant breast cancer, may be a
reasonable treatment alternative. The levels of β-catenin increased
in the tamoxifen-resistant breast cancer cell line, and inhibition
of β-catenin in the nucleus suppressed stem cell-like properties.
In particular, combining a β-catenin blocker with a conventional
CDK4/6 inhibitor accelerated STAT3 suppression.
Contribution of the JAK/STAT3 signaling pathway to
the hormone resistance mechanism in breast cancer has been reported
in a previous study (33). In
addition, STAT3 is a transcription factor that regulates cell
proliferation and survival and is also involved in the growth of
stem-like cells in breast cancer (14). In the present study, it was
observed that STAT3, similarly to β-catenin, was upregulated in
TamR cells and was associated with tamoxifen resistance (Fig. 1B). In particular, STAT3 may
influence the additive effects of palbociclib and ICG-001 by
reducing the levels of p-STAT3 (Fig.
5B and E). The results demonstrated that p-STAT3 was the main
reason for the combinatorial effect of ICG-001 and palbociclib;
however, this was not statistically significant when compared with
the effects of treatments with palbociclib (P=0.990) and ICG-001
(P=0.978) individually. Nevertheless, in terms of cell viability, a
clear combinatorial effect was observed when compared with the
effects of treatments with the drugs individually. In addition,
ICG-001 sufficiently contributed to the reduction in the levels of
active β-catenin. This suggests that the inhibition of active
β-catenin by IGC-001 and the consequent underactivation of the
STAT3 signaling pathway may have an additive effect on the role of
palbociclib in terms of cell growth inhibition.
Although several studies have been conducted to
elucidate the signaling pathway between β-catenin and STAT3, it
remains unclear (34,35). Armanious et al reported that
STAT3 upregulates the expression of β-catenin and its
transcriptional activity in breast cancer cells. They found a
binding sequence for STAT3 in the β-catenin gene promoter through
DNA sequence analysis and confirmed the amplification product using
chromatin immunoprecipitation (35). In contrast, Yan et al
reported that β-catenin upregulates the expression of STAT3 in
esophageal squamous cell carcinoma. These authors scanned the human
STAT3 promoter and found matching sequences for five T-cell factor
(TCF)-binding protein elements; it was confirmed that TCF4 binds to
the human STAT3 promoter. They performed RT-PCR and western blot
analysis of several esophageal squamous cell lines to demonstrate
that overexpression of β-catenin upregulates the mRNA and protein
levels of STAT3 (34). Recent
studies further support the notion that β-catenin regulates STAT3.
Huang et al reported that β-catenin binds to the predicted
promoter region of STAT3, as assessed using the chromatin
immunoprecipitation assay in prostate cancer, and that a specific
inhibitor of β-catenin (XAV-939) partially reduces its binding
activity (36). Kawasaki et
al reported that LGR5 activates β-catenin in intrahepatic
cholangiocarcinoma, and that activated β-catenin further activates
STAT3 to enhance cancer stem-like properties and
epithelial-mesenchymal transition (37). To elucidate the association between
β-catenin and STAT3, western blot analysis was performed after
treatment of cells with a STAT3 inhibitor or after subjecting them
to STAT3 knockdown to identify the nuclear/cytoplasmic fraction of
β-catenin; however, no changes in the nuclear translocation of
β-catenin were noted (data not shown). Therefore, after treatment
of cells with the STAT3 inhibitor or siSTAT3, the levels of active
β-catenin level were additionally verified using luciferase assay
and were found to be unaltered (Fig.
6). This indicates that STAT3 does not regulate β-catenin
activity. The results of the present study showed a reduction in
the expression of p-STAT3 upon inhibition of β-catenin. Therefore,
it is consistent with previous findings that β-catenin regulates
STAT3 activity (34,36,37).
The present study has some limitations. First, in
the experiments, the known targets were not considered. For
example, the mechanism underlying the activation of cell cycle
regulation by the upregulation of cyclin D1 through β-catenin/TCF
signaling is already known and was, therefore, not verified in the
present study. Comparison of the reduction in the stem-like
properties and cell growth inhibition induced by β-catenin was
focused on, to evaluate the effect of the combination treatment
versus that of the existing CDK4/6 inhibitor. Second, the mechanism
by which CDK4/6 inhibitors reduce the levels of STAT3 was not
clearly elucidated. The IL-6/STAT3 pathway has been reported to be
induced by a mechanism of acquired resistance to CDK4/6 inhibitors
(38). However, the interaction
with STAT3 during initial treatment with CDK4/6 inhibitors remains
unclear. To the best of our knowledge, there are no reports of an
association between treatment with CDK4/6 inhibitors and STAT3
levels. In this context, further experiments should be designed to
evaluate this association.
In conclusion, it was revealed that β-catenin is
activated in endocrine-resistant breast cancer and that the
antitumor effects of conventional CDK4/6 inhibitors are further
potentiated by β-catenin blockers. This suggests that β-catenin
blockers may be a reasonable treatment alternative for
endocrine-resistant breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
The content of this manuscript was presented as a
poster at the 14th Annual Meeting of the Korean Society of Medical
Oncology and 2020 International Conference.
Funding
The present study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MSIT) (grant no. NRF-2017R1D1A1B04035892).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AH and KL contributed to the conception and design
of the experiments. MC contributed to the acquisition of the
experimental data. AH, MC, JJ, HW and KL contributed to the
analysis of the data. AH drafted and revised the manuscript. HW and
KL also revised the manuscript. AH and KL confirm the authenticity
of all raw data. KL assisted with the funding. All authors
critically reviewed, and have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TamR
|
tamoxifen-resistant MCF-7
|
|
CI
|
combination index
|
|
ER
|
estrogen receptor
|
|
CBP
|
CREB-binding protein
|
References
|
1
|
Waks AG and Winer EP: Breast cancer
treatment: A review. Jama. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murphy CG and Dickler MN: Endocrine
resistance in hormone-responsive breast cancer: Mechanisms and
therapeutic strategies. Endocr Relat Cancer. 23:R337–R352. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ziauddin MF, Hua D and Tang SC: Emerging
strategies to overcome resistance to endocrine therapy for breast
cancer. Cancer Metastasis Rev. 33:791–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mills JN, Rutkovsky AC and Giordano A:
Mechanisms of resistance in estrogen receptor positive breast
cancer: Overcoming resistance to tamoxifen/aromatase inhibitors.
Curr Opin Pharmacol. 41:59–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Won HS, Lee KM, Oh JE, Nam EM and Lee KE:
Inhibition of β-catenin to overcome endocrine resistance in
tamoxifen-resistant breast cancer cell line. PLoS One.
11:e01559832016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke R, Tyson JJ and Dixon JM: Endocrine
resistance in breast cancer-An overview and update. Mol Cell
Endocrinol. 418Pt:220–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beaver JA and Park BH: The BOLERO-2 trial:
The addition of everolimus to exemestane in the treatment of
postmenopausal hormone receptor-positive advanced breast cancer.
Future Oncol. 8:651–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodriguez D, Ramkairsingh M, Lin X, Kapoor
A, Major P and Tang D: The central contributions of breast cancer
stem cells in developing resistance to endocrine therapy in
estrogen receptor (ER)-positive breast cancer. Cancers (Basel).
11:10282019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Zhang HW, Sun XF, Guo XH, He YN,
Cui SD and Fan QX: Tamoxifen-resistant breast cancer cells possess
cancer stem-like cell properties. Chin Med J (Engl). 126:3030–3034.
2013.PubMed/NCBI
|
|
11
|
Sakunrangsit N and Ketchart W: Plumbagin
inhibits cancer stem-like cells, angiogenesis and suppresses cell
proliferation and invasion by targeting Wnt/β-catenin pathway in
endocrine resistant breast cancer. Pharmacol Res. 150:1045172019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin S, Cheryan VT, Xu L, Rishi AK and
Reddy KB: Myc mediates cancer stem-like cells and EMT changes in
triple negative breast cancers cells. PLoS One. 12:e01835782017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mukherjee N and Panda CK: Wnt/β-catenin
signaling pathway as chemotherapeutic target in breast cancer: An
update on pros and cons. Clin Breast Cancer. 20:361–370. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44+CD24− stem
cell-like breast cancer cells in human tumors. J Clin Invest.
121:2723–2735. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Damsky WE, Curley DP, Santhanakrishnan M,
Rosenbaum LE, Platt JT, Rothberg BE, Taketo MM, Dankort D, Rimm DL,
McMahon M and Bosenberg M: β-catenin signaling controls metastasis
in Braf-activated Pten-deficient melanomas. Cancer Cell.
20:741–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/β-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu F, Xia Z, Zhang M, Ding J, Feng Y, Wu
J, Dong Y, Gao W, Han Z, Liu Y, et al: SMARCAD1 promotes pancreatic
cancer cell growth and metastasis through Wnt/β-catenin-mediated
EMT. Int J Biol Sci. 15:636–646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Chen H, Zheng P, Zheng Y, Luo Q,
Xie G, Ma Y and Shen L: ICG-001 suppresses growth of gastric cancer
cells and reduces chemoresistance of cancer stem cell-like
population. J Exp Clin Cancer Res. 36:1252017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin P, Wang W, Zhang Z, Bai Y, Gao J and
Zhao C: Wnt signaling in human and mouse breast cancer: Focusing on
wnt ligands, receptors and antagonists. Cancer Sci. 109:3368–3375.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khramtsov AI, Khramtsova GF, Tretiakova M,
Huo D, Olopade OI and Goss KH: Wnt/beta-catenin pathway activation
is enriched in basal-like breast cancers and predicts poor outcome.
Am J Pathol. 176:2911–2920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bilir B, Kucuk O and Moreno CS: Wnt
signaling blockage inhibits cell proliferation and migration, and
induces apoptosis in triple-negative breast cancer cells. J Transl
Med. 11:2802013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv C, Li F, Li X, Tian Y, Zhang Y, Sheng
X, Song Y, Meng Q, Yuan S, Luan L, et al: MiR-31 promotes mammary
stem cell expansion and breast tumorigenesis by suppressing Wnt
signaling antagonists. Nat Commun. 8:10362017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katoh M: Multi-layered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β-catenin signaling activation
(Review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI
|
|
25
|
Arensman MD, Telesca D, Lay AR, Kershaw
KM, Wu N, Donahue TR and Dawson DW: The CREB-binding protein
inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer
Ther. 13:2303–2314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kartha VK, Alamoud KA, Sadykov K, Nguyen
BC, Laroche F, Feng H, Lee J, Pai SI, Varelas X, Egloff AM, et al:
Functional and genomic analyses reveal therapeutic potential of
targeting β-catenin/CBP activity in head and neck cancer. Genome
Med. 10:542018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata H, Harbeck N, Loibl S, Bartlett CH, Zhang K, et al:
Palbociclib in hormone-receptor-positive advanced breast cancer. N
Engl J Med. 373:209–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2-advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Phase III randomized study of ribociclib and fulvestrant
in hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin
Oncol. 36:2465–2472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bijnsdorp IV, Giovannetti E and Peters GJ:
Analysis of drug interactions. Methods Mol Biol. 731:421–434. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JW, Gautam J, Kim JE, Kim JA and Kang
KW: Inhibition of tumor growth and angiogenesis of
tamoxifen-resistant breast cancer cells by ruxolitinib, a selective
JAK2 inhibitor. Oncol Lett. 17:3981–3989. 2019.PubMed/NCBI
|
|
34
|
Yan S, Zhou C, Zhang W, Zhang G, Zhao X,
Yang S, Wang Y, Lu N, Zhu H and Xu N: beta-catenin/TCF pathway
upregulates STAT3 expression in human esophageal squamous cell
carcinoma. Cancer Lett. 271:85–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Armanious H, Gelebart P, Mackey J, Ma Y
and Lai R: STAT3 upregulates the protein expression and
transcriptional activity of β-catenin in breast cancer. Int J Clin
Exp Pathol. 3:654–664. 2010.PubMed/NCBI
|
|
36
|
Huang R, Wang S, Wang N, Zheng Y, Zhou J,
Yang B, Wang X, Zhang J, Guo L, Wang S, et al: CCL5 derived from
tumor-associated macrophages promotes prostate cancer stem cells
and metastasis via activating β-catenin/STAT3 signaling. Cell Death
Dis. 11:2342020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawasaki K, Kuboki S, Furukawa K,
Takayashiki T, Takano S and Ohtsuka M: LGR5 induces β-catenin
activation and augments tumour progression by activating STAT3 in
human intrahepatic cholangiocarcinoma. Liver Int. 41:865–881. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kettner NM, Vijayaraghavan S, Durak MG,
Bui T, Kohansal M, Ha MJ, Liu B, Rao X, Wang J, Yi M, et al:
Combined inhibition of STAT3 and DNA repair in
palbociclib-resistant ER-positive breast cancer. Clin Cancer Res.
25:3996–4013. 2019. View Article : Google Scholar : PubMed/NCBI
|