Introduction
Iron is an essential nutrient that facilitates cell
proliferation and growth (1), and
it can contribute to tumor growth (1). Campbell et al (2) demonstrated that exposure to iron
oxide dust tripled the incidence of pulmonary tumors in mice.
Richmond et al (3) showed
that intramuscular injection of iron-dextran induced sarcoma in
rats. Hann et al (4)
reported that the growth rate of tumor xenografts could be
influenced by levels of dietary iron. Regarding bladder cancer,
there have been few studies concerning the association between
bladder cancer and iron. Seligman et al (5) reported that bladder cancer cellular
proliferation was dependent on iron. Therefore, chelating iron,
such as desferrioxamine (DFO), has been evaluated for its antitumor
effects (1,6), including in bladder cancer (5). Seligman et al (5) demonstrated that DFO inhibited
proliferation of bladder cancer cells. Although iron chelators have
shown great potential in preclinical cancer models (6), they can cause adverse side-effects,
such as infection and gastrointestinal bleeding (7). Therefore, these challenges must be
overcome to improve the therapeutical efficacy of iron chelators
for tumor treatment (7).
Iron is used in cells for DNA synthesis (cell
proliferation), heme synthesis in mitochondria, and iron storage as
ferritin (1). Compared with
healthy cells, cancer cells have inhibited or defective heme
synthesis in mitochondria (8–10).
Considering these factors, it appears that iron contributes to
tumor growth by its use in DNA synthesis and its storage in cancer
cells. Therefore, activation of heme synthesis with consequent
reduction of iron in mitochondria may potentially be a new
treatment for cancer without the use of an iron chelator.
In bladder cancer, photodynamic diagnosis using
5-aminolevurinic acid (5-ALA) is widely used in clinical practice
(11). 5-ALA is distributed
ubiquitously in mammalian cells and is a precursor of heme, which
is essential in aerobic energy metabolism and the
electron-transport system (12).
In cancer cells, 5-ALA causes significantly higher accumulation of
fluorescent endogenous porphyrins, mainly protoporphyrin IX, than
in healthy cells due to certain effects, such as decreased
ferrochelatase activity (8,12).
The enzyme ferrochelatase converts protoporphyrin IX into heme with
iron (13). Treatment with 5-ALA
significantly increases protoporphyrin IX in cancer cells and
activates heme synthesis (14).
Therefore, the aim of the present study was to determine whether
treatment with 5-ALA has antitumor effects in bladder cancer by
reduction of mitochondrial iron without using chelating iron
through activation of heme synthesis.
Materials and methods
Cell culture
Two human bladder cancer cell lines, T24 (ATCC no.
HTB-4 derived from an undifferentiated grade 3 carcinoma) and
MGH-U3 (a generous gift from Dr. H. LaRue at Laval University
Cancer Research Centre, Quebec, Canada; derived from a grade 1
tumor), were maintained in RPMI-1640 growth medium (Nissui
Pharmaceutical Co., Ltd.) supplemented with 10% fetal bovine serum
(FBS) (ICN Biomedicals, Inc.), 100 U/ml penicillin, and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a standard
humidified incubator at 37°C in a 5% CO2 atmosphere for
24 h.
Cell toxicity
T24 and MGH-U3 cells were seeded into 96-well plates
at 2×103 cells/well and incubated overnight. The growth
medium was removed, and serum plus medium with or without 5-ALA
(0.1 and 1 mM; SBI Phramaceuticals Co., Ltd.) under dark conditions
was applied. Cells at 48 h were assessed using a Cell Counting
Kit-8 (Dojindo Laboratories, Inc.) according to the manufacturer's
protocol. The absorbance was measured at 490 nm with a reference at
630 nm using an Infinite 200M PRO microplate auto-reader (Tecan
Group, Ltd.). Experiments were performed three times with duplicate
samples.
Apoptosis analysis
Annexin V assays using Muse® Annexin V
& Dead Cell Assay Kit (cat. no. MCH100105; Merck KGaA)
following the manufacturer's instructions were performed. Briefly,
after treatment with T24 and MGH-U3 cells (1×105 cells)
with 1 mM 5-ALA for 9 h, the detached and adherent cells were
collected and incubated with Annexin V and 7-amino-actinomycin D, a
dead cell marker, for 20 min at room temperature in the dark. The
events for live, early, and late apoptotic cells were counted with
the Muse™ Cell Analyzer (software version. 1.5.0.0; Merck
KGaA).
Transfection of small interfering RNA
(siRNA)
T24 and MGH-U3 cells in 6-well plates at
1×105 cells/well were transfected with synthesized siRNA
ferrochelatase (si-FECH; cat. no. sc-60631; Santa Cruz
Biotechnology, Inc.) or siRNA negative control (si-NC; cat. no.
4390843; Invitrogen; Life Technologies; Thermo Fisher Scientific,
Inc.) with 50 pmol of siRNA and 5 µl of Lipofectamine 2000 (Life
Technologies; Thermo Fisher Scientific, Inc.) in 6-well plates
according to the manufacturer's instructions at 37°C for 48 h.
Following transfection, protein was extracted, and the expression
of ferrochelatase was measured in each cell line by western
blotting.
Evaluation of Fe2+ in
mitochondria
In 6-well plates at 1×105 cells/well, T24
and MGH-U3 cells transfected with si-FECH or si-NC were treated
with 0, 0.1 and 1 mM 5-ALA in an RPMI-1640 growth medium
supplemented with 10% FBS and incubated for 48 h. Fe2+
in mitochondria was then evaluated using Mito-FerroGreen (Dojindo
Laboratories, Inc.) according to the manufacturer's protocol.
Fluorescence of Fe2+ in mitochondria was evaluated using
a fluorescence microscope (EVOS FL Auto; Life Technologies; Thermo
Fisher Scientific, Inc.). The intensity ratio was evaluated by
measuring the intensity divided by the number of cells in an ×400
field of vision in the three views that exhibited the strongest
intensity detected by three observers (YN, TO and YO). ImageJ
software (version 1.8.0_172; National Institutes of Health) was
used for the quantitative assessments.
Cell cycle analysis
Following transfection of siRNA into T24 and MGH-U3,
the cells in 6-well plates at 1×105 cells/well were
treated with 0 mM, 0.1 mM, or 1 mM 5-ALA for 48 h. The cell cycle
was analyzed using a Muse™ Cell Cycle Kit (MilliporeSigma)
following the manufacturer's instructions. The results were
analyzed using the Muse™ Cell Analyzer. Experiments were performed
three times.
Western blotting
Proteins were extracted using RIPA buffer (cat. no.
R0278, Sigma-Aldrich; Merck KGaA), which contained 20 mM of
Tris-HCl (pH 7.5), 150 mM of NaCl, 1% of NP-40, 1% of sodium
deoxycholate, 2.5 mM of sodium pyrophosphate, 1 mM of
Na3VO4, 1 mM of phenylmethylsulfonyl
fluoride, and 1 µg/ml of leupeptin in T24 and MGH-U3 cells
transfected with siRNA. Protein concentrations were quantified
using a Protein Assay BCA kit (Nacalai Tesque, Inc.), and
immunoblotting was performed as previously described (15). Total protein of 10 µg was diluted
with sodium dodecyl (SDS) loading buffer containing 2.5% of
β-mercaptoethanol, boiled at 95°C for 5 min, and electrophoresed
onto 10% SDS-polyacrylamide gels using a Mini-Protean Tetra Cell
(Bio-Rad Laboratories, Inc.) at 200 V for 35 min. Gels were
subjected to transfer onto polyvinylidene difluoride membranes
(Hybond-P; GE Healthcare; Cytiva) using a semidry transfer
apparatus (Trans-Blot SD Semi-Dry Transfer Cell; Bio-Rad
Laboratories, Inc.) at 15 V for 45 min. Following blocking in
Tris-buffered saline (pH 7.6) that contained 5% skim milk for 1 h
at room temperature, the membrane was incubated overnight at 4°C
with an anti-ferritin heavy chain rabbit monoclonal antibody
(product code ab75972; dilution 1:1,000; Abcam), an
anti-ferrochelatase mouse monoclonal antibody (cat. no. sc-377377;
dilution 1:100; Santa Cruz Biotechnology, Inc.) or an anti-actin
mouse monoclonal antibody (cat. no. A2066; dilution 1:3,000;
Sigma-Aldrich; Merck KGaA), which was used as an internal loading
control, followed by 1 h with horseradish peroxidase-conjugated
goat anti-mouse IgG (cat. no. SA00001-1; dilution 1:10,000) or
anti-rabbit IgG antibody (cat. no. SA00001-2; dilution 1:10,000;
both from ProteinTech Group, Inc.) at room temperature. Finally,
the bound secondary antibody was detected using the SuperSignal
West Pico Chemiluminescent Substrate (Pierce Chemical; Thermo
Fisher Scientific, Inc.). Band densities were quantified using
ImageJ (version 1.8.0_172). Protein levels were calculated in
reference to the protein levels of actin. Experiments were
performed three times.
Bladder tumor mouse model
A total of eight C57BL/6J male mice (5 weeks old; 20
g) were obtained from OrientalBioService, Inc. Treatment was
started 2 weeks later. The mice were kept in a temperature (24°C)-
and humidity (60%)-controlled room, with a 12/12-h light/dark
cycle, and food and water were provided ad libitum. The
eight mice were randomly divided into two groups as follows:
Control group, four mice received 0.05% N-butyl-N-(4-hydroxybutyl)
nitrosamine (BBN) in drinking water for 20 weeks to develop
muscle-invasive bladder cancer; and 5-ALA group, four mice received
0.05% BBN plus 6 mM ALA (16) in
drinking water for 20 weeks. Following treatment for 20 weeks, all
mice were euthanized by exsanguination under anesthesia with 2–3%
isoflurane and their tissues were harvested for the subsequent
experiments. The animal study was approved (approval no. 11921,
2017/2/23) by the Ethics Committee on Animal Research of Nara
Medical University (Nara, Japan). All animal experiments were
conducted in accordance with the Guidelines for the Welfare of
Animals in Experimental Neoplasia (17).
Immunohistochemical (IHC)
staining
All resected bladders were filled with 150 µl of 10%
neutral-buffered formalin, and all specimens were fixed in 10%
neutral-buffered formalin at room temperature for 48 h. Paraffin
blocks were cut into 5-µm thickness and placed on SuperFrost Plus
microslides (Thermo Fisher Scientific, Inc.). Sections were
deparaffinized and antigen retrieval was carried out in citric acid
buffer (pH 6.0) using an autoclave. IHC staining was performed
using a Histofine SAB-PO kit (Nichirei Biosciences, Inc.) according
to the manufacturer's instructions. Briefly, slides were treated
with 1% hydrogen peroxide in methanol at room temperature for 10
min to block endogenous peroxidase activity. The slides were
incubated overnight at 4°C with rabbit monoclonal antibodies
against ferritin (product code ab75972, dilution 1:50; Abcam);
rabbit polyclonal antibodies against cytokeratin 20 (CK20) (cat.
no. bs-1588R; dilution 1:100; Bioss Antibodies); rabbit polyclonal
antibodies against survivin (cat. no. 10508-1-AP; dilution 1:500;
ProteinTech Group, Inc.), which is specific to the G2/M phase in
cells; or rabbit monoclonal antibodies against proliferating cell
nuclear antigen (PCNA) (product code ab92552; dilution 1:100;
Abcam), which is specific to the S phase in cells. The slides were
then incubated at room temperature for 10 min with 100 µl of the
secondary antibody [anti-mouse and anti-rabbit IgG antibodies,
included in the Histofine SAB-PO kit (Nichirei Biosciences, Inc.),
at 1 µl/ml]. The slides were counterstained with Meyer's
hematoxylin (Muto Pure Chemicals Co., Ltd.) at room temperature for
1 min, dehydrated, and sealed with a cover slide. Immunoreactive
cancer cells were counted based on five independent high-power
microscopic fields (HPF; magnification, ×400; 0.0625
µm2), and the number of positive cells was divided by
the total number of cancer cells (1–100%) using a microscope (EVOS
FL Auto) by two investigators (SH and YM) to quantify the
expression level of ferritin, CK20, survivin, and PCNA in cancer
cells.
Statistical analysis
PRISM software version 7.00 (GraphPad Software,
Inc.) was used for the statistical analysis. The data are presented
as the mean ± standard deviation (SD). The unpaired t-test
was used for binary comparisons between two groups. A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
Cell cytotoxicity
In the T24 and MGH-U3 cells, treatment with 5-ALA
inhibited cell viability relative to that in the samples not
treated with 5-ALA. The IC50 values of T24 and MGH-U3 by
5-ALA were 2.5 and 3.7 mM, respectively (Fig. 1).
Apoptosis analysis
To evaluate the effect of apoptosis induced by
photodynamic therapy, apoptosis was evaluated. Early apoptosis was
not higher in the T24 (Fig. 2A) or
MGH-U3 (Fig. 2B) cells treated
with 1 mM 5-ALA than in the samples treated without 5-ALA.
Cell cytotoxicity in cells with gene
silencing of ferrochelatase
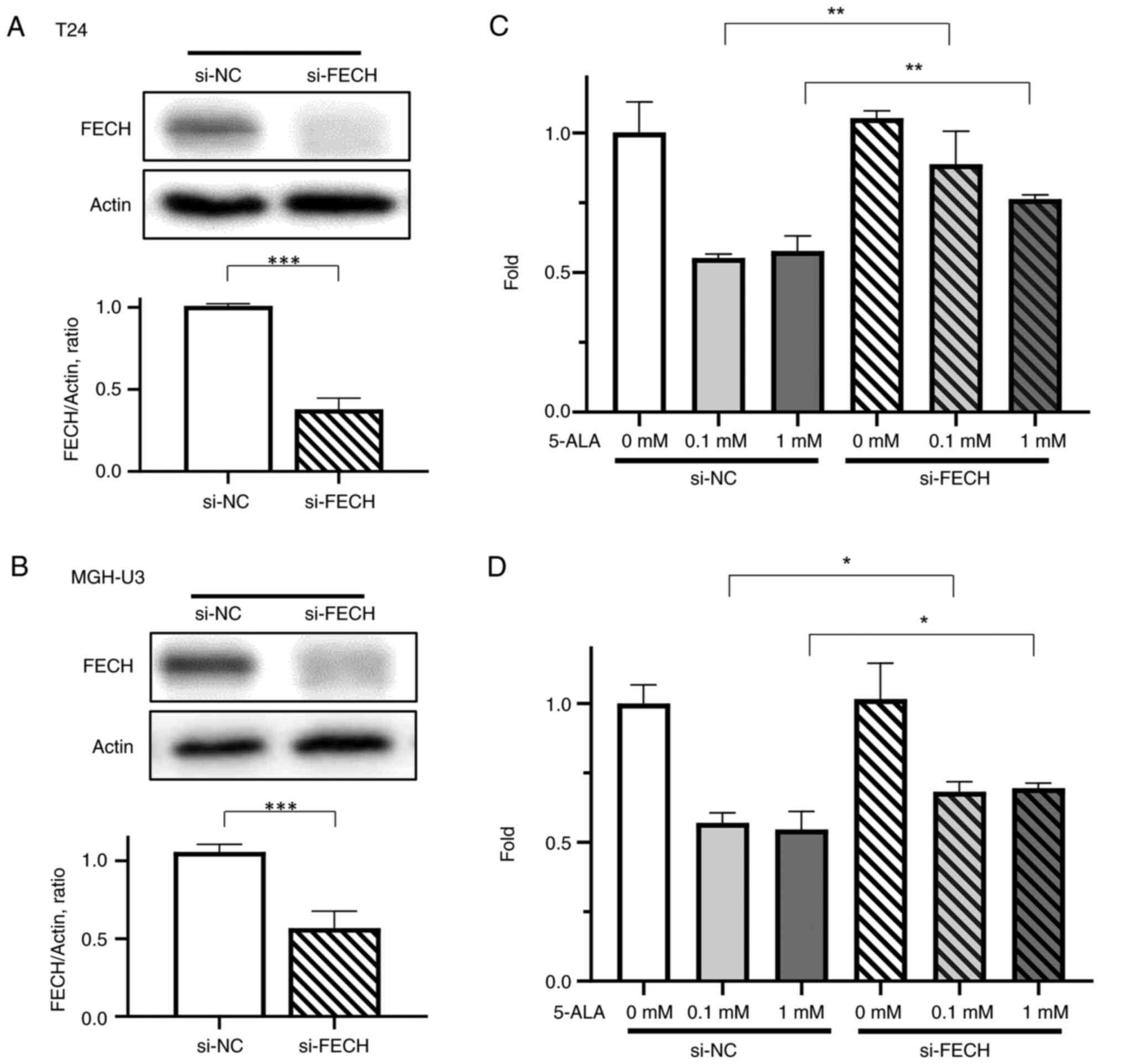
Gene silencing of ferrochelatase was demonstrated
successfully in T24 (Fig. 3A) and
MGH-U3 (Fig. 3B) cells. To confirm
the effect of silencing of ferrochelatase, a cell cytotoxicity
assay was performed. Inhibition of cell viability by 5-ALA was
significantly decreased by silencing of ferrochelatase in T24
(Fig. 3C) and MGH-U3 (Fig. 3D) cells treated with 0.1 and 1 mM
5-ALA.
Fe2+ in mitochondria
The expression of mitochondrial Fe2+ in
T24 (Fig. 4) and MGH-U3 (Fig. 5) cells treated with the si-NC
decreased when treated with 0.1 mM in T24 and MGH-U3 cells, and 1
mM 5-ALA in T24 and MGH-U3 cells. The effect of 5-ALA, which
decreased the expression of Fe2+ in mitochondria in T24
(Fig. 4) and MGH-U3 (Fig. 5) cells, was significantly
suppressed by gene silencing of ferrochelatase.
Ferritin
The expression of ferritin in T24 (Fig. 6A and B) and MGH-U3 (Fig. 6C and D) cells treated with si-NC
was decreased by treatments with 0.1 mM in MGH-U3 cells, and 1 mM
5-ALA in T24 and MGH-U3 cells. The effect of 5-ALA in T24 (Fig. 6A and B) and MGH-U3 (Fig. 6C and D) cells, which decreased the
expression of ferritin, was significantly suppressed by gene
silencing of ferrochelatase.
Cell cycle
The percentage of the S phase in T24 and MGH-U3
cells treated with si-NC was increased by treatments with 0.1 mM in
MGH-U3 cells and 1 mM 5-ALA in T24 and MGH-U3 cells (Figs. 7 and 8). The effect of 5-ALA in T24 and MGH-U3
cells, which increased the percentage of the S phase, was
significantly suppressed by gene silencing of ferrochelatase after
treatment with 1 mM 5-ALA (Figs. 7
and 8).
Effect of oral intake of 5-ALA in mice
treated with BBN
The body weight (mean, 28.3±1.5 g) of the mice
treated with ALA at 20 weeks of treatment was not significantly
different from that of the mice in the control group (median,
30±1.4 g) (Fig. 9A). Liver
fibrosis, necrosis, or filtration of lymphocytes or plasma cells
was not detected in the liver of mice treated with 5-ALA (Fig. 9B). The bladder weights were lower
in mice treated with 5-ALA (mean, 0.089±0.009 g) than in mice not
treated with 5-ALA (median, 0.14±0.058 g) although the difference
was not significant. Bladder cancer was not found in two out of the
four mice treated with 5-ALA, and muscle-invasive cancer was not
found in the other two mice (Table
I). Between the mouse with T1 high grade bladder cancer treated
with ALA and without ALA, the expression of ferritin was
significantly lower in the mice treated with 5-ALA (positive cells:
mean, 18.5±6.6%) than in the mice in the control group (positive
cells: mean, 38.3±6.9%), the expression of PCNA (marker of the S
phase in the cell cycle) was significantly higher in the mice
treated with 5-ALA (positive cells: median, 29.5±6.4%) than in the
control group (positive cells: median, 15.3±6.2%), the expression
of survivin in the mice treated with 5-ALA (positive cells: median,
35.8±4.8%) was not significantly different from that in the mice in
the control group (positive cells: median, 35.3±6.7%), and the
expression of CK20 was significantly lower in the mice treated with
5-ALA (positive cells: mean 30.5±5.9%) than that in the mice in the
control group (positive cells: mean 56.8±4.9%) (Fig. 10).
 | Table I.Pathological findings in the bladders
of mice. |
Table I.
Pathological findings in the bladders
of mice.
| Treatment | T stage | Grade | LVI | INF | Body weight
(g) | Bladder weight
(g) |
|---|
| Control | a | Low grade | 0 | - | 29 | 0.084 |
|
| 1 | High grade | 0 | c | 30 | 0.088 |
|
| 2b | High grade | 1 | c | 32 | 0.168 |
|
| 3a | High grade | 1 | c | 29 | 0.201 |
| 5-ALA | No malignancy | - | - | - | 30 | 0.082 |
|
| No malignancy | - | - | - | 27 | 0.081 |
|
| CIS | High grade | 0 | - | 27 | 0.099 |
|
| 1 | High grade | 0 | b | 29 | 0.093 |
Discussion
The results of the present study demonstrated that
5-ALA inhibited the viability of bladder cancer cells, decreased
the level of Fe2+ in mitochondria, and increased the
expression of ferritin and the percentage of cells in the S phase
of the cell cycle. Furthermore, by silencing ferrochelatase, which
converts Fe2+ and protoporphyrin IX to heme (13), the effects of inhibition of
viability by 5-ALA and the decreased level of Fe2+ in
mitochondria by 5-ALA were reduced, and the increased expression of
ferritin and percentage of cells in the S phase of the cell cycle
were also decreased. These results indicated that 5-ALA inhibited
the viability of bladder cancer cells by reducing iron in
mitochondria through activation of heme synthesis. Previous studies
(18,19) have shown the biological
significance of heme metabolites, the mechanism of protoporphyrin
IX accumulation in tumor cells, and the therapeutic potential of
ALA-induced photodynamic therapy alone and combined with
hyperthermia and immunotherapy in cancer treatment. The present
study, to the best of our knowledge, is the first to demonstrate
the possibility of 5-ALA as an antitumor agent targeting iron in
bladder cancer cells without the use of an iron chelator, and
decreasing mitochondrial iron through the activation of heme
synthesis and inhibiting bladder cancer cell viability.
Chelating iron in cancer cells has been reported to
contribute to inhibition of DNA synthesis (1,20), S
phase arrest (21,22), G1/S arrest (23), and cell proliferation.
Additionally, in the present study, S phase arrest was observed
upon treatment with 5-ALA, and by silencing ferrochelatase, S phase
arrest induced by 5-ALA was weakened. These results indicated that
5-ALA activated heme synthesis in bladder cancer cells, which
converted mitochondrial Fe2+ to heme resulting in
inhibition of DNA synthesis and S phase arrest from inhibition of
cell viability. The data support the theory that 5-ALA inhibits the
viability of bladder cancer cells by reducing mitochondrial iron
through activation of heme synthesis without the use of an iron
chelator.
The expression of ferritin was weaker in the mice
treated with 5-ALA than in those not treated with 5-ALA.
Additionally, the expression of PCNA, which is specific to the S
phase in cells, and not survivin, which is specific to the G2/M
phase in cells, was higher in the mice treated with 5-ALA. Ferritin
is a form of stored iron (1,6), and
a decrease in ferritin results in a shortage of iron in bladder
cancer cells. Therefore, the results of the in vivo study
were consistent with those of the in vitro study.
Furthermore, bladder cancer was not found in two mice treated with
5-ALA. This phenomenon is not explained fully by inhibition of cell
viability caused by 5-ALA. Ferritin protein has been reported to
have a key role in nuclear factor-κB (24), which is a widely expressed
transcription factor involved in cancer and related to cancer
development and progression (25).
Therefore, the effect of decreased ferritin by 5-ALA may have
another antitumor effect that decreases the iron level in cancer
cells, which particularly could have affected the in vivo
results in the present study, because half of the mice treated with
5-ALA did not develop bladder cancer.
We had assumed that the mechanisms of the antitumor
effect of 5-ALA on bladder cancer were due to photodynamic therapy
(26) or apoptosis by oxidative
phosphorylation (12). However,
when bladder cancer cells were treated with 5-ALA, apoptosis was
not observed. The percentage of the late apoptotic cells was higher
in T24 cells treated with 5-ALA than that in T24 cells without
5-ALA, but was not caused by apoptosis due to the same percentages
of early apoptosis in the samples treated with or without 5-ALA
even 6 h after ALA treatment (data not shown). Furthermore, the
effect of photodynamic therapy was not observed in bladder cancer
cells with suppressed ferrochelatase expression, although the
effect of photodynamic therapy reportedly is enhanced by
suppression of ferrochelatase (15). Therefore, the main mechanism
underlying the antitumor effect for bladder cancer by 5-ALA is
considered to mainly involve decreasing of mitochondrial iron
through activation of heme synthesis and inhibiting bladder cancer
cell viability.
The viability of T24 cells was higher than that of
MGH-U3 cells, and the expressions of mitochondrial Fe2+
and ferritin in T24 cells appeared to be higher than those in
MGH-U3 cells. The expression of ferrochelatase was lower in T24
cells than that in MGH-U3 cells, being consistent with the finding
of a previous study (8). This
result indicated that heme synthesis is more defective in T24 cells
than in MGH-U3 cells. Furthermore, the antitumor effect of 5-ALA
appeared to be weaker in T24 cells than that in MGH-U3 cells. The
results of the present study indicated that 5-ALA can be more
effective in cells that express more ferrochelatase.
The present study has some limitations that should
be considered. First, only two types of bladder cancer cell lines
were evaluated. The effect of 5-ALA, which inhibited the viability
of bladder cancer cells by activating heme synthesis, may be in
various types of cancers, especially in cancers where chelating
iron has been reported to exert an antitumor effect. Therefore, the
present mechanism should be evaluated in different cancer cell
lines in future studies. Second, the number of mice used in the
in vivo study was small. However, animal use guidelines
recommend that the number of animals used in experiments be as
limited as possible. Because the results showed significant
differences between groups, an additional in vivo study was
not performed.
In conclusion, the present study demonstrated that
5-ALA decreased iron levels in bladder cancer and inhibited bladder
cancer cell viability. These results indicated that 5-ALA may
potentially be used as a new anticancer agent for bladder cancer
without the use of an iron chelator.
Acknowledgements
The authors would like to thank SBI Pharmaceuticals
Co., Ltd. (Tokyo, Japan) for providing 5-aminolevurinic acid used
in this study.
Funding
The present study was supported by Ministry of Education,
Culture, Sports, Sciences and Technology of Japan, and
Grants-in-Aid for Science Research (C) JSPS KAKENHI grant no.
16K11026 (YN).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YN and KF conceived and designed the study. YN, TO
and SO performed the cell cytotoxicity assay and analyzed and
interpretated the data. YT and TF performed the apoptosis analysis
and analyzed and interpretated the data. YN, TO and YO performed
the Fe2+ in mitochondria assay and analyzed and
interpretated the data. SO performed the western blotting and
transfection of small interfering RNA. TF and SO performed cell the
cycle assay and YN, TF and SO analyzed and interpretated the data.
YN, SH, YM, KI, KO and MM performed the in vivo study and
analyzed and interpretated the data. YN, NT and KF confirm the
authenticity of all the raw data. YN wrote the original draft of
the manuscript. KF and NT contributed to the writing, review and
editing of the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The animal study was approved by the Committee on
Animal Research of Nara Medical University (approval no. 11921,
2017/2/23). All animal experiments were conducted in accordance
with the Guidelines for Welfare of Animals in Experimental
Neoplasia. This study did not include patient participation or
analysis of patient data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campbell JA: Effects of precipitated
silica and of iron oxide on the incidence of primary lung tumors in
mice. Br Med J. 2:275–280. 1940. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richmond HG: Induction of sarcoma in the
rat by iron-dextran complex. Br Med J. 1:947–949. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hann HW, Stahlhut MW and Blumberg BS: Iron
nutrition and tumor growth: Decreased tumor growth in
iron-deficient mice. Cancer Res. 48:4168–4170. 1988.PubMed/NCBI
|
|
5
|
Seligman PA, Schleicher RB, Siriwardana G,
Domenico J and Gelfand EW: Effects of agents that inhibit cellular
iron incorporation on bladder cancer cell proliferation. Blood.
82:1608–1617. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin L, Chen H, Zhao R, Zhu M and Nie G:
Nanomedicine targets iron metabolism for cancer therapy. Cancer
Sci. 113:828–837. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corcé V, Gouin SG, Renaud S, Gaboriau F
and Deniaud D: Recent ad-vances in cancer treatment by iron
chelators. Bioorg Med Chem Lett. 26:251–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakai Y, Tatsumi Y, Miyake M, Anai S,
Kuwada M, Onishi S, Chihara Y, Tanaka N, Hirao Y and Fujimoto K:
Expression of ferrochelatase has a strong correlation in
protoporphyrin IX accumulation with photodynamic detection of
bladder cancer. Photodiagn Photodyn Ther. 13:225–232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Datta SN, Loh CS, MacRobert AJ, Whatley SD
and Matthews PN: Quantitative studies of the kinetics of
5-aminolaevulinic acid-induced fuorescence in bladder transitional
cell carcinoma. Br J Cancer. 78:1113–1118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
el-Sharabasy MM, el-Waseef AM, Hafez MM
and Salim SA: Porphyrin metabolism in some malignant diseases. Br J
Cancer. 65:409–412. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakai Y, Inoue K, Tsuzuki T, Shimamoto T,
Shuin T, Nagao K, Matsuyama H, Oyama M, Furuse H, Ozono S, et al:
Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using
fluorescence cystoscopy for non-muscle-invasive bladder cancer: A
multicenter phase III study. Int J Urol. 25:723–729. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown GC: Nitric oxide and mitochondrial
respiration. Biochim Biophys Acta. 1411:351–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miura M, Ito K, Hayashi M, Nakajima M,
Tanaka T and Ogura S: The effect of 5-aminolevulinic acid on
cytochrome P450-mediated prodrug activation. PLoS One.
10:e01317932015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyake M, Ishii M, Kawashima K, Kodama T,
Sugano K, Fujimoto K and Hirao Y: siRNA-mediated knockdown of the
heme synthesis and degradation pathways: Modulation of treatment
effect of 5-aminolevulinic acid-based photodynamic therapy in
urothelial cancer cell lines. Photochem Photobiol. 85:1020–1027.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hara T, Koda A, Nozawa N, Ota U, Kondo H,
Nakagawa H, Kamiya A, Miyashita K, Itoh H, Nakajima M and Tanaka T:
Combination of 5-aminolevulinic acid and ferrous ion reduces plasma
glucose and hemoglobin A1c levels in Zucker diabetic fatty rats.
FEBS Open Bio. 6:515–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Workman P, Balmain A, Hickman JA, McNally
NJ, Rohas AM, Mitchison NA, Pierrepoint CG, Raymond R, Rowlatt C,
Stephens TC, et al: UKCCCR guidelines for the welfare of animals in
experimental neoplasia. Lab Anim. 22:195–201. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinoda Y, Kato D, Ando R, Endo H,
Takahashi T, Tsuneoka Y and Fujiwara Y: Systematic review and
meta-analysis of in vitro anti-human cancer experiments
investigating the use of 5-aminolevulinic acid (5-ALA) for
photodynamic therapy. Pharmaceuticals (Basel). 14:2292021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung M, Mertens C, Tomat E and Brüne B:
Iron as a central player and promising target in cancer
progression. Int J Mol Sci. 20:2732019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lederman HM, Cohen A, Lee JW, Freedman MH
and Gelfand EW: Deferoxamine: A reversible S-phase inhibitor of
human lymphocyte proliferation. Blood. 64:748–753. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harima H, Kaino S, Takami T, Shinoda S,
Matsumoto T, Fujisawa K, Yamamoto N, Yamasaki T and Sakaida I:
Deferasirox, a novel oral iron chelator, shows antiproliferative
activity against pancreatic cancer in vitro and in vivo. BMC
Cancer. 16:7022016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vazana-Barad L, Granot G, Mor-Tzuntz R,
Levi I, Dreyling M, Nathan I and Shpilberg O: Mechanism of the
antitumoral activity of deferasirox, an iron chelation agent, on
mantle cell lymphoma. Leuk Lymphoma. 54:851–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pham CG, Bubici C, Zazzeroni F, Papa S,
Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C,
et al: Ferritin heavy chain upregulation by NF-kappaB inhibits
TNFalpha-induced apoptosis by suppressing reactive oxygen species.
Cell. 119:529–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karin M: Nuclear factor kappaB in cancer
development and progression. Nature. 25:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fedi B: Photodinamic effect and
fluorescence in the diagnosis and therapy of the cancer of the
bladder. Boll Soc Ital Biol Sper. 53:1138–1144. 1977.PubMed/NCBI
|