Introduction
Microcystins (MCs) are a type of toxins synthesized
by cyanobacteria (1). Among over
200 MC variants, MC-leucine arginine (MC-LR) is considered to be
the most abundant and harmful (2).
It has been reported that MC-LR might be transferred to the food
chain and accumulate in different organisms (3). For example, Sotton et al
(4) found that whitefish
accumulated MC-LR in the body by ingesting foods containing MC-LR
in Lake Hallwil of Switzerland. The World Health Organization
recommends 1 µg/l as a guideline for the maximum tolerated value in
drinking water (5). However, an
increasing number of studies have reported that the concentration
of MC-LR in drinking water is much higher than 1 µg/l in certain
countries, such as Vietnam and China (6,7).
Currently, MC-LR is considered to be a potent carcinogen (8,9). In
a previous study, MC-LR activated MMP expression and promoted
breast cancer cell migration (10). MC-LR also induced
hepatocarcinogenesis in vitro and in nude mice (11). In addition, MC-LR has been shown to
promote prostate epithelial cell proliferation (6). However, the effect of MC-LR on
colorectal cancer (CRC) cells is still poorly documented.
CRC is the third leading cause of cancer death
worldwide (12,13). Old age, dietary patterns and a bad
lifestyle are considered to be risk factors for CRC (13,14).
Previous studies have demonstrated that MC-LR is a carcinogen and
can be transferred to the food chain (3,8).
Therefore, MC-LR may be related to the occurrence and development
of CRC. To date, a few studies have shown that MC-LR promotes CRC
cell migration and metastasis (14,15).
However, to the best of our knowledge, the impact of MC-LR on CRC
cell proliferation has never been studied.
Previous studies have demonstrated that the PI3K/Akt
and Wnt/β-catenin signaling pathways are closely associated with
the occurrence and development of CRC (16,17).
Activation of the PI3K/Akt and Wnt/β-catenin signaling pathways can
promote CRC cell proliferation (18,19).
In addition, these two pathways are important downstream targets of
MC-LR (6,20). Therefore, we speculated that MC-LR
activates the PI3K/Akt and Wnt/β-catenin pathways to promote CRC
cell proliferation. The present study aimed to investigate the
effect of MC-LR on CRC cell proliferation and the underlying
mechanisms. For this purpose, SW620 and HT29 cells were treated
with 50 nM MC-LR and then examined using Cell Counting Kit-8
(CCK-8) and cell colony formation assays to determine whether MC-LR
accelerated cell proliferation. Subsequently, the protein
expression of the pathway key genes was detected using western
blotting. Finally, the pathway inhibitors LY294002 and ICG001 were
used to verify the role of the PI3K/Akt and Wnt/β-catenin pathways
in MC-LR-induced cell proliferation.
Materials and methods
Reagents and antibodies
MC-LR (purity >95%) was purchased from Express
Technology, Co., Ltd. Dulbecco's modified Eagle's Medium (DMEM) and
fetal bovine serum (FBS) were purchased from Thermo Fisher
Scientific, Inc. The nuclear and cytoplasmic protein extraction kit
(cat. no. P0027), CCK-8 kit (cat. no. C0037), enhanced
chemiluminescence (ECL) reaction kit (cat. no. P0018S), LY294002 (a
PI3K/Akt pathway inhibitor; cat. no. S1737), ICG001 (a
Wnt/β-catenin pathway inhibitor; cat. no. SF6827), and anti-PI3K
(p110; cat. no. AF7749), anti-Akt (cat. no. AA326), anti-GSK3β
(cat. no. AF1543), anti-β-catenin (cat. no. AC106), anti-c-myc
(cat. no. AF6513) and anti-cyclin D1 (cat. no. AF1183) antibodies
were purchased from Beyotime Institute of Biotechnology.
Anti-phosphorylated (p)-Akt (Ser473; cat. no. 4060) and
anti-p-GSK3β (Ser9; cat. no. 9322) were purchased from Cell
Signaling Technology, Inc. The HRP-conjugated secondary antibodies
(cat. nos. SA00001-1 and SA00001-2), anti-lamin B (cat. no.
12987-1-AP) and anti-β-actin (cat. no. 20536-1-AP) were purchased
from Wuhuan Sanying Biotechnology.
Cell culture and treatment
SW620 and HT29 cells were obtained from the Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences
and cultured in DMEM with 10% FBS at 37°C with 5% CO2.
SW620 and HT29 cells were cultured for 6 h before MC-LR (dissolved
in DMSO), LY294002 or ICG001 was added. To determine the
appropriate concentration of MC-LR, the cells were treated with
various concentrations of MC-LR (10, 25, 50, 75, 100 and 200 nM)
for 24 h at 37°C, and the relative cell viability was detected by
CCK-8 analysis. To study the effect of MC-LR on cell proliferation,
cells were treated with 50 nM MC-LR for 48 h or 10 days at 37°C,
and then cell proliferation was assessed by CCK-8 and cell colony
formation assays, respectively. In addition, the protein expression
of the key genes of the PI3K/Akt and Wnt/β-catenin pathways was
assessed by western blotting after treating the cells with or
without MC-LR (50 nM), LY294002 (25 µM) or ICG001 (25 µM) for 24 h
at 37°C. The control group was cultured in media containing the
same quantity of DMSO as the treatment group.
CCK-8 and cell colony formation
assay
For the CCK-8 assay, after seeding SW620 and HT29
cells separately in 96-well plates (3,000 cells per well) for 48 h,
CCK-8 was added and incubated for 2 h to assess relative cell
viability, as described in our previous study (5). Light absorbance was examined at 450
nm using a microplate reader, and the optical density values were
used directly to analyze the results of the CCK-8 assay as
previously reported (21,22). For the cell colony formation assay,
SW620 and HT29 cells (400 cells per well) were seeded separately in
6-well plates and incubated for 10 days. Cell colonies were fixed
with methanol for 30 min, and stained with 0.1% (w/v) crystal
violet for 20 min at 37°C. Cell colony counting was performed under
the microscope (>50 cells per cell colony) as previously
described (23).
Western blotting
Western blotting was performed as previously
reported (24). In brief, the
extraction of cell nuclear and cytoplasmic proteins was performed
using a nuclear and cytoplasmic protein extraction kit. After
quantification by BCA method, the protein was subjected to SDS-PAGE
and then transferred to a PVDF membrane. The membrane was blocked
using 5% skimmed milk in phosphate-buffered saline for 1 h at 37°C,
and then incubated with the aforementioned primary antibodies
(1:1,000) at 4°C overnight. After incubating with the
aforementioned secondary antibodies (1:2,000) for 1 h at room
temperature, the protein bands were developed using the ECL kit,
and analyzed using ImageJ software (v1.46; National Institutes of
Health).
Statistical analysis
Data were analyzed by independent samples t-test or
by one-way analysis of variance followed by Tukey's post hoc
analysis, using SPSS 19.0 software (IBM Corp.). All experiments
were performed in triplicate. Data are presented as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MC-LR promotes cell proliferation in
SW620 and HT29 cells
Firstly, the appropriate concentration of MC-LR was
screened for by CCK-8 analysis. The result showed that MC-LR
affected cell viability, and cell viability of SW620 and HT29 cells
was promoted at MC-LR concentrations of 10, 25, 50 and 75 nM,
especially at 50 nM (Fig. 1A).
Therefore, 50 nM MC-LR was used for the further experiments.
Subsequently, CCK-8 and cell colony formation assays were performed
to investigate the effect of MC-LR on cell proliferation. The
result of both assessments showed that MC-LR promoted cell
proliferation significantly in SW620 and HT29 cells compared with
that in the control (P<0.05; Fig.
1B-D).
 | Figure 1.MC-LR promotes cell proliferation in
SW620 and HT29 cells. (A) Cells were treated with different
concentrations of MC-LR (10, 25, 50, 75, 100 and 200 nM) for 24 h,
and the relative cell viability was detected by CCK-8 analysis. (B)
Cells were treated with 50 nM MC-LR for 48 h, and cell
proliferation was tested by CCK-8 analysis. (C) Cells were treated
with 50 nM MC-LR for 10 days, and cell proliferation was assessed
by cell colony formation assay. The results are representative of
three independent experiments. *P<0.05. Error bars indicate SD.
MC-LR, microcystin-leucine arginine; OD, optical density; CCK-8,
Cell Counting Kit-8. |
MC-LR activates the PI3K/Akt and
Wnt/β-catenin pathways in SW620 and HT29 cells
As the PI3K/Akt and Wnt/β-catenin signaling pathways
are closely related to cell proliferation (25–28),
the present study examined whether MC-LR activated the PI3K/Akt and
Wnt/β-catenin pathways in SW620 and HT29 cells. As shown in
Fig. 2, the expression of PI3K and
the level of p-Akt (Ser473) increased significantly in the MC-LR
treatment group compared with that in the control group
(P<0.05). These data demonstrated that MC-LR activated the
PI3K/Akt pathway. Meanwhile, the expression levels of p-GSK3β
(Ser9), β-catenin, c-myc and cyclin D1, the downstream targets of
the Wnt/β-catenin pathway, were also significantly upregulated in
the MC-LR treatment group compared with those in the control group
(P<0.05). These data indicated that MC-LR activated the
Wnt/β-catenin pathway in SW620 and HT29 cells.
MC-LR promotes SW620 and HT29 cell
proliferation by activating the PI3K/Akt/Wnt/β-catenin pathway
To confirm the role of the PI3K/Akt/Wnt/β-catenin
pathway in MC-LR-mediated cell proliferation, LY294002, an
inhibitor of the PI3K/Akt pathway, was used. The results showed
that LY294002 could significantly suppress the overexpression of
p-Akt (Ser473), p-GSK3β (Ser9), β-catenin, c-myc and cyclin D1
induced by MC-LR (P<0.05; Fig. 3A
and B). This indicated that LY294002 neutralized the activation
of the PI3K/Akt/Wnt/β-catenin pathway induced by MC-LR in SW620 and
HT29 cells. Additionally, the results of the CCK-8 and colony
formation analyses showed that LY294002 significantly inhibited
cell proliferation promoted by MC-LR (P<0.05; Fig. 3C and D). These results revealed
that MC-LR activated the PI3K/Akt/Wnt/β-catenin pathway to promote
SW620 and HT29 cell proliferation.
MC-LR activates Wnt/β-catenin through
the PI3K/Akt pathway to promote cell proliferation
In this study, LY294002, as an inhibitor of the
PI3K/Akt pathway, could also significantly inhibit the
Wnt/β-catenin pathway. Thus, we speculated that MC-LR activated
Wnt/β-catenin through the PI3K/Akt pathway in SW620 and HT29 cells.
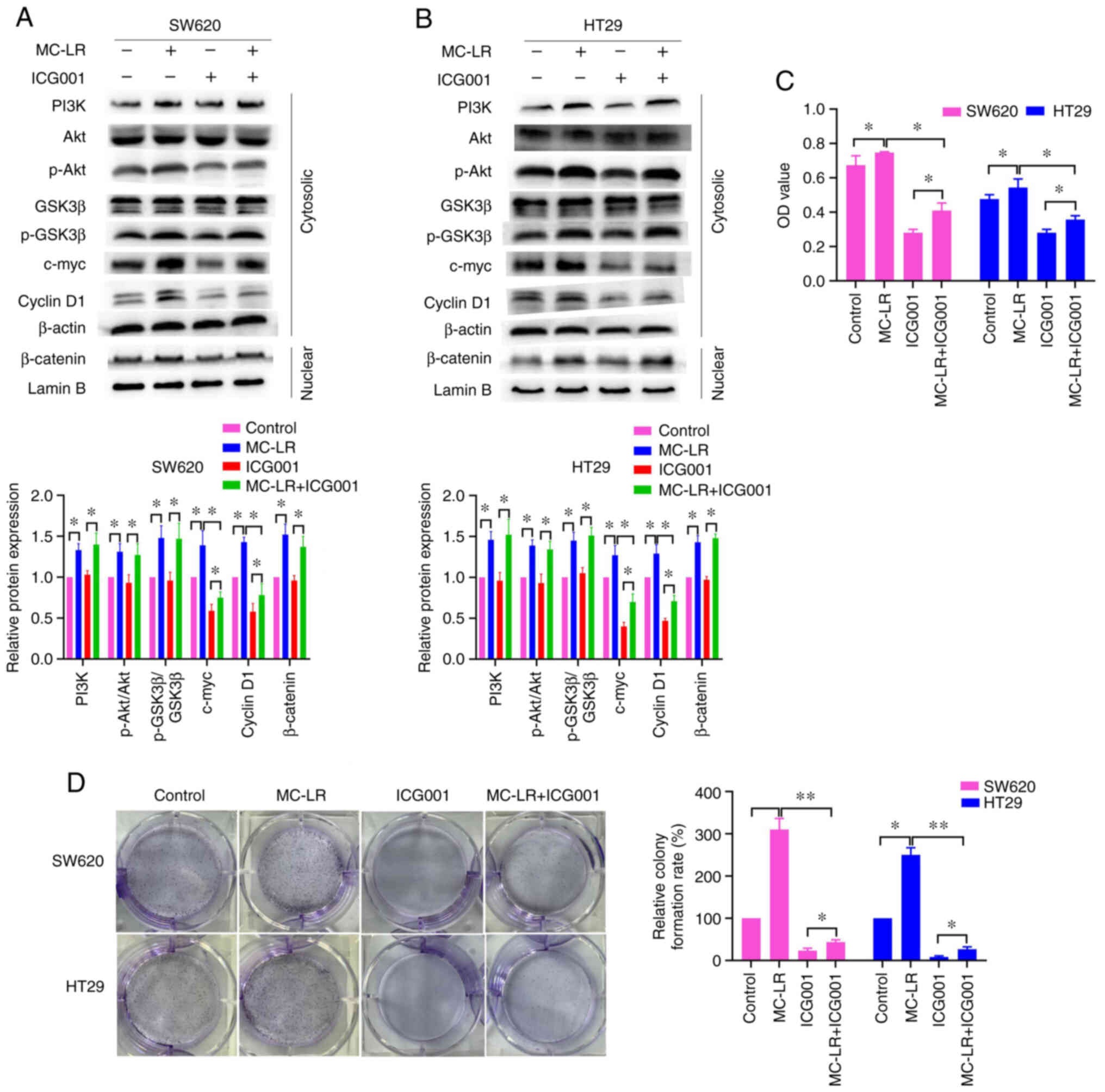
To investigate this, ICG001, an inhibitor of the Wnt/β-catenin
pathway, was used. As shown in Fig. 4A
and B, ICG001 significantly suppressed the overexpression of
c-myc and cyclin D1 induced by MC-LR (P<0.05), but not the
expression of PI3K-related proteins. These results indicated that
ICG001 neutralized the activation of Wnt/β-catenin, rather than the
PI3K/Akt pathway, induced by MC-LR, suggesting that MC-LR activated
Wnt/β-catenin through the PI3K/Akt pathway in SW620 and HT29 cells.
Additionally, the results of the CCK-8 and colony formation
analyses showed that ICG001 significantly suppressed cell
proliferation promoted by MC-LR (P<0.05; Fig. 4C and D). Together, the
aforementioned results suggested that MC-LR activated Wnt/β-catenin
through the PI3K/Akt signaling pathway to promote cell
proliferation.
Discussion
In the present study, the effect of MC-LR on CRC
cell proliferation and the underlying mechanism were investigated.
Firstly, an MC-LR concentration of 50 nM was determined to be
suitable for the subsequent CCK-8 and cell colony formation assays.
Next, the protein expression levels of the key genes of the pathway
were measured using western blotting after treating the cells with
50 nM MC-LR. The results revealed that MC-LR activated the PI3K/Akt
and Wnt/β-catenin pathways. Finally, LY294002 and ICG001 were used
to confirm the role of the PI3K/Akt/Wnt/β-catenin pathway in
MC-LR-promoted cell proliferation. The results showed that MC-LR
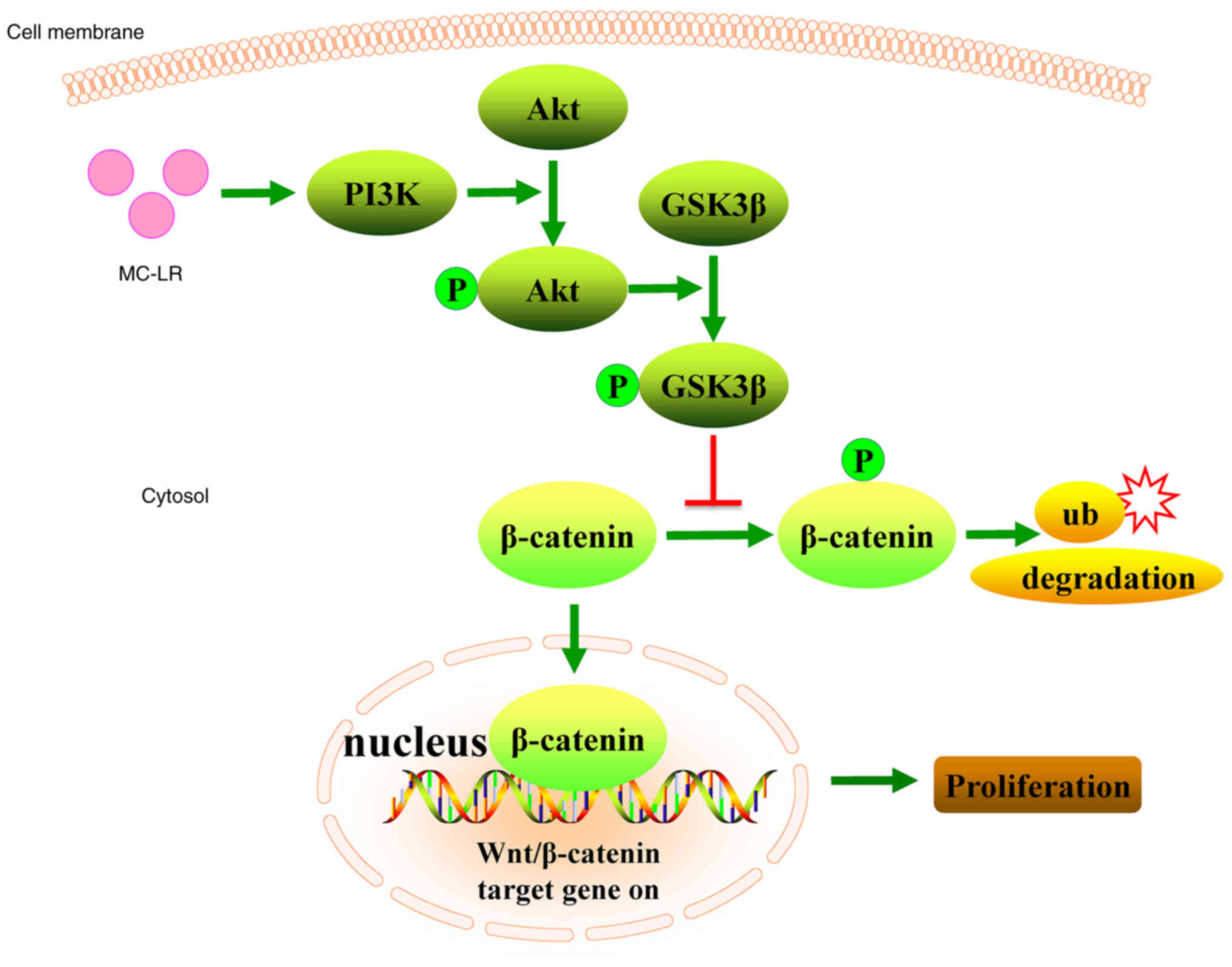
activated the PI3K/Akt pathway and promoted GSK3β phosphorylation
leading to β-catenin accumulation in the cell nucleus, thereby
promoting cell proliferation (Fig.
5). Collectively, the results suggested that MC-LR activated
Wnt/β-catenin through the PI3K/Akt signaling pathway to promote
cell proliferation.
To investigate the impact of MC-LR on CRC cell
proliferation, a suitable concentration of MC-LR needs to be
selected. Several studies have used 25 or 50 nM MC-LR to treat CRC
cells (14,15,29).
For example, Miao et al (14) treated DLD-1, HT29 and SW480 cells
with 12.5, 25 and 50 nM MC-LR. Ren et al (15) treated DLD-1 and HT29 cells with 25
and 50 nM MC-LR. Likewise, in the present study, SW620 and HT29
cells were treated using similar concentrations of MC-LR, and the
results showed that MC-LR could promote cell proliferation of SW620
and HT29 cells at a concentration of 10, 25, 50 and 75 nM,
especially at 50 nM. Therefore, 50 nM MC-LR was determined suitable
for use in further experiments.
It has been reported that MC-LR could accelerate CRC
cell migration and metastasis (14,15).
However, the impact of MC-LR on CRC cell proliferation has never
been studied. In the present study, the results of CCK-8 and cell
colony formation analyses revealed that MC-LR promoted CRC cell
proliferation significantly. Similarly, previous studies found that
MC-LR accelerated cell proliferation in liver, biliary and prostate
epithelial cells (6,30–33).
It was notable that MC-LR promoted cell proliferation at high doses
in these studies. For instance, Liu et al (30) found that MC-LR accelerated HL7702
cell proliferation at 5 and 10 µM. Wang et al (34) reported that MC-LR increased A549
cell proliferation at 1 and 5 µM. In the present study, the results
showed that a low dose of MC-LR (50 nM) significantly increased
cell proliferation, highlighting the role of MC-LR in the malignant
transformation of CRC. Therefore, the underlying mechanisms need to
be investigated.
Previous studies have found that the PI3K/Akt and
Wnt/β-catenin signaling pathways play key roles in cell
proliferation (25–28). As a result, the key molecules of
the PI3K/Akt and Wnt/β-catenin pathways were assessed using western
blotting in the present study. The results showed that MC-LR
increased the expression of PI3K (p110) and p-Akt (Ser473)
significantly. It is well-known that elevated expression of PI3K
(p110) and p-Akt (Ser473) is marker of the PI3K/Akt pathway
activation (35,36). Therefore, this result suggested
that MC-LR activated the PI3K/Akt pathway in CRC cells. In
addition, the present data revealed that MC-LR upregulated the
level of p-GSK3β (Ser9) and the expression of β-catenin, c-myc and
cyclin D1. Previous studies have demonstrated that p-β-catenin
induced by GSK3β is degraded by ubiquitination, and that
non-p-β-catenin is transferred to and accumulates in the cell
nucleus, where it combines with T-cell factor/lymphoid enhancer
factor to activate downstream target genes, such as c-myc and
cyclin D1 (25,37). Therefore, the present results
indicated that MC-LR also activates the Wnt/β-catenin pathway in
CRC cells.
To verify the role of the PI3K/Akt/Wnt/β-catenin
pathway in MC-LR-promoted cell proliferation, LY294002, an
inhibitor of the PI3K/Akt pathway (14,38),
was used to treat the cells. The results showed that LY294002
neutralized the activation of the PI3K/Akt/Wnt/β-catenin pathway,
leading to the inhibition of cell proliferation promoted by MC-LR
in CRC cells. To further study the underlying mechanism, ICG001, an
inhibitor of the Wnt/β-catenin pathway (39), was used to treat the studied cells.
The results revealed that ICG001 neutralized the activation of
Wnt/β-catenin rather than the PI3K/Akt pathway, thereby inhibiting
cell proliferation promoted by MC-LR in the CRC cells. Based on
these results, it was shown that MC-LR activated Wnt/β-catenin
through the PI3K/Akt signaling pathway to promote cell
proliferation. In relation to the present study, Liu et al
(30) reported that MC-LR
increased proliferation by activating the Akt pathway in HL7702
cells. The study by Han et al showed that MC-LR activated
the PI3K/Akt pathway in RM-1 cells (20). Pan et al (6) found that MC-LR activated the
Wnt/β-catenin pathway to promote RWPE-1 cell proliferation. To the
best of our knowledge, this is the first study to find that MC-LR
activated Wnt/β-catenin through the PI3K/Akt pathway, leading to
the promotion of CRC cell proliferation.
In conclusion, the present study demonstrated that
MC-LR increased CRC cell proliferation by activating the
PI3K/Akt/Wnt/β-catenin pathway. The present study provides a novel
insight into the toxicological mechanism of MC-LR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Shandong Province (grant nos. ZR2020MH263 and
ZR2014CL034) and the National Natural Science Foundation of China
(grant no. 81802905).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WF, XZ and ZP designed the experiments and wrote the
manuscript. YT, XY and TY performed the experiments. BL and YL
analyzed the data. All authors read and approved the final
manuscript. YT and XY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu H, Guo X, Liu L, Yan M, Li J, Hou S,
Wan J and Feng L: Simultaneous microcystin degradation and
microcystis aeruginosa inhibition with the single enzyme
microcystinase A. Environ Sci Technol. 54:8811–8820. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou S, Yu Y, Zhang W, Meng X, Luo J, Deng
L, Shi Z and Crittenden J: Oxidation of microcystin-LR via
activation of peroxymonosulfate using ascorbic acid: Kinetic
modeling and toxicity assessment. Environ Sci Technol.
52:4305–4312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng R, Zhu H, Shutes B and Yan B:
Treatment of microcystin (MC-LR) and nutrients in eutrophic water
by constructed wetlands: Performance and microbial community.
Chemosphere. 263:1281392021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sotton B, Guillard J, Anneville O,
Maréchal M, Savichtcheva O and Domaizon I: Trophic transfer of
microcystins through the lake pelagic food web: Evidence for the
role of zooplankton as a vector in fish contamination. Sci Total
Environ. 466:152–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Q, Wang G, Xie Y, Gao Z, Liang Z,
Pan Z, Wang G and Feng W: Mechanical changes and microfilament
reorganization involved in microcystin-LR-promoted cell invasion in
DU145 and WPMY cells. Front Pharmacol. 11:892020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan C, Chen Y, Xu T, Wang J, Li D and Han
X: Chronic exposure to microcystin-leucine-arginine promoted
proliferation of prostate epithelial cells resulting in benign
prostatic hyperplasia. Environ Pollut. 242:1535–1545. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duong TT, Jähnichen S, Le TPQ, Ho CT,
Hoang TK, Nguyen TK, Vu TN and Dang DK: The occurrence of
cyanobacteria and microcystins in the Hoan Kiem Lake and the Nui
Coc reservoir (North Vietnam). Environ Earth Sci. 71:2419–2427.
2014. View Article : Google Scholar
|
|
8
|
Xiao C, Mei F, Ren G, Long L, Chen M, Fang
X, Li J, Li K, Tang Y, Huang T and Deng W: Synergistic effect of
MC-LR and C-terminal truncated HBx on HepG2 cells and their effects
on PP2A mediated downstream target of MAPK signaling pathway. Front
Genet. 11:5377852020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiki H and Suganuma M: Tumor
promoters-microcystin-LR, nodularin and TNF-α and human cancer
development. Anticancer Agents Med Chem. 11:4–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Zhang XX, Qin W, Xu L, Wang T,
Cheng S and Yang L: Effects of microcystin-LR exposure on matrix
metalloproteinase-2/-9 expression and cancer cell migration.
Ecotoxicol Environ Saf. 77:88–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He L, Huang Y, Guo Q, Hui Z, Zheng C, Wang
J, Chen JA, Wang L and Shu W: Chronic microcystin-LR exposure
induces hepatocarcinogenesis via increased gankyrin in vitro and in
vivo. Cell Physiol Biochem. 49:1420–1430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harada S and Morlote D: Molecular
pathology of colorectal cancer. Adv Anat Pathol. 27:20–26. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long J, He Q, Yin Y, Lei X, Li Z and Zhu
W: The effect of miRNA and autophagy on colorectal cancer. Cell
Prolif. 53:e129002020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miao C, Ren Y, Chen M, Wang Z and Wang T:
Microcystin-LR promotes migration and invasion of colorectal cancer
through matrix metalloproteinase-13 up-regulation. Mol Carcinog.
55:514–524. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren Y, Yang M, Meng C, Zhu Q, Zhou L, Qin
W and Wang T: Microcystin-LR promotes epithelial-mesenchymal
transition in colorectal cancer cells through PI3-K/AKT and SMAD2.
Toxicol Lett. 265:53–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He XS, Ye WL, Zhang YJ, Yang XQ, Liu F,
Wang JR, Ding XL, Yang Y, Zhang RN, Zhao YY, et al: Oncogenic
potential of BEST4 in colorectal cancer via activation of PI3K/Akt
signaling. Oncogene. 41:1166–1177. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu C, Wang M and Shi H: Cholesterol
promotes colorectal cancer growth by activating the PI3K/AKT
pathway. J Oncol. 2022:15154162022.PubMed/NCBI
|
|
19
|
Zhang Q, Fei S, Zhao Y, Liu S, Wu X, Lu L
and Chen W: PUS7 promotes the proliferation of colorectal cancer
cells by directly stabilizing SIRT1 to activate the Wnt/β-catenin
pathway. Mol Carcinog. Oct 12–2022.(Epub ahead of print).
View Article : Google Scholar
|
|
20
|
Han R, Zhang L, Gan W, Fu K, Jiang K, Ding
J, Wu J, Han X and Li D: piRNA-DQ722010 contributes to prostate
hyperplasia of the male offspring mice after the maternal exposed
to microcystin-leucine arginine. Prostate. 79:798–812. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang X, Chen L, Liu W, Qiao Q, Wu K, Wen
J, Huang C, Tang R and Zhang X: Involvement of oxidative stress and
cytoskeletal disruption in microcystin-induced apoptosis in CIK
cells. Aquat Toxicol. 165:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan C, Zhang L, Meng X, Qin H, Xiang Z,
Gong W, Luo W, Li D and Han X: Chronic exposure to microcystin-LR
increases the risk of prostate cancer and induces malignant
transformation of human prostate epithelial cells. Chemosphere.
263:1282952021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang Z, Mou Q, Pan Z, Zhang Q, Gao G, Cao
Y, Gao Z, Pan Z and Feng W: Identification of candidate diagnostic
and prognostic biomarkers for human prostate cancer: RPL22L1 and
RPS21. Med Oncol. 36:562019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng W, Zhang M, Wu ZX, Wang JQ, Dong XD,
Yang Y, Teng QX, Chen XY, Cui Q and Yang DH: Erdafitinib
antagonizes ABCB1-mediated multidrug resistance in cancer cells.
Front Oncol. 10:9552020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He S and Tang S: WNT/β-catenin signaling
in the development of liver cancers. Biomed Pharmacother.
132:1108512020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li F, Xie W, Fang Y, Xie K, Liu W, Hou L
and Tan W: HnRNP-F promotes the proliferation of bladder cancer
cells mediated by PI3K/AKT/FOXO1. J Cancer. 12:281–291. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Q, Zhang X, Zai HY, Jiang W, Zhang KJ,
He YQ and Hu Y: circSLC8A1 sponges miR-671 to regulate breast
cancer tumorigenesis via PTEN/PI3k/Akt pathway. Genomics.
113:398–410. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li K, Zhang J, Tian Y, He Y, Xu X, Pan W,
Gao Y, Chen F and Wei L: The Wnt/β-catenin/VASP positive feedback
loop drives cell proliferation and migration in breast cancer.
Oncogene. 39:2258–2274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li K, Huang M, Xu P, Wang M, Ye S, Wang Q,
Zeng S, Chen X, Gao W, Chen J, et al: Microcystins-LR induced
apoptosis via S-nitrosylation of GAPDH in colorectal cancer cells.
Ecotoxicol Environ Saf. 190:1100962020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Wang H, Wang B, Chen T, Wang X, Pu
H, Xu L and Guo Z: Microcystin-LR promotes proliferation by
activating Akt/S6K1 pathway and disordering apoptosis and cell
cycle associated proteins phosphorylation in HL7702 cells. Toxicol
Lett. 240:214–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia X, Guan B, Liao J, Hu X, Fan Y, Li J,
Zhao H, Huang Q, Ma Z, Zhu X, et al: Down-regulation of GCLC is
involved in microcystin-LR-induced malignant transformation of
human liver cells. Toxicology. 421:49–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan M, Gu S, Pan C, Chen Y and Han X:
MC-LR-induced interaction between M2 macrophage and biliary
epithelial cell promotes biliary epithelial cell proliferation and
migration through regulating STAT3. Cell Biol Toxicol. 37:935–949.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Wang B, Huang P, Wang H, Xu K, Wang
X, Xu L and Guo Z: Microcystin-LR promotes cell proliferation in
the mice liver by activating Akt and p38/ERK/JNK cascades.
Chemosphere. 163:14–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Xu K, Wang B, Liu J, Wang X, Xing
M, Huang P, Guo Z and Xu L: Microcystin-LR induces a wide variety
of biochemical changes in the A549 human non-small cell lung cancer
cell line: Roles for protein phosphatase 2A and its substrates.
Environ Toxicol. 32:1065–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan G, Ru Y, Wu K, Yan F, Wang Q, Wang J,
Pan T, Zhang M, Han H, Li X and Zou L: GOLM1 promotes prostate
cancer progression through activating PI3K-AKT-mTOR signaling.
Prostate. 78:166–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han X, Wu P, Li L, Sahal HM, Ji C, Zhang
J, Wang Y, Wang Q, Qian H, Shi H and Xu W: Exosomes derived from
autologous dermal fibroblasts promote diabetic cutaneous wound
healing through the Akt/β-catenin pathway. Cell Cycle. 20:616–629.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng K, Bai J, Li N, Li M, Sun H, Zhang
W, Ge G, Liang X, Tao H, Xue Y, et al: Protective effects of
sirtuin 3 on titanium particle-induced osteogenic inhibition by
regulating the NLRP3 inflammasome via the GSK-3β/β-catenin
signalling pathway. Bioact Mater. 6:3343–3357. 2021. View Article : Google Scholar : PubMed/NCBI
|