Introduction
Head and neck squamous cell carcinoma (HNSCC)
comprise a heterogeneous group of cancers derived from the oral
cavity, nasopharynx, oropharynx, hypopharynx, and larynx (1). An estimated 65,000 new cases of HNSCC
are diagnosed each year in the USA, where it is the seventh most
common cancer (2). Tobacco,
alcohol consumption, and human papillomavirus (HPV) infection are
known risk factors for HNSCC (3–5).
HNSCC remains a lethal disease despite concerted efforts to improve
treatment options, including surgery, chemotherapy, targeted
therapy and immunotherapy (1,6–8). The
5-year survival rate for HNSCC decreases drastically with cancer
stage, from ~80% in early stages (I–II) to only ~40% for advanced
stages (III–IV) (9–11).
The epidermal growth factor receptor (EGFR) remains
the best molecule for HNSCC-targeted therapy, given its high
frequency of overexpression in this disease, regardless of stage or
HPV status (12–14). Targeting EGFR with either an
antibody (e.g., cetuximab) or small molecule inhibitor (e.g.,
erlotinib) has been extensively investigated in clinical trials and
has been approved by the FDA for the treatment of patients with
advanced HNSCC (7,15–18).
However, clinical efficacy is modest, and acquired resistance to
EGFR inhibitors often occurs over time (1,7,19,20).
Thus, additional therapeutics with improved efficacy that could
potentially overcome this resistance are urgently needed (21,22).
Immunotoxin (IT)-based therapy combines cell surface
binding ligands or antibody-based single-chain fragment variable
(scFv) with a peptide toxin and represents another cancer treatment
option (23–25). A novel bivalent diphtheria toxin
(DT)-based EGF fusion toxin (bi-EGF-IT) with improved efficacy and
remarkably less off-target toxicity than the monovalent EGF-IT
(mono-EGF-IT) in human HNSCC cell lines and HNSCC mouse models
established in immunocompromised mice was recently developed by the
authors (26). In the present
study, the efficacy and toxicity of human bi-EGF-IT was further
evaluated in immunocompetent mice in the presence of host immunity.
CD25 is highly expressed on the surface of tumor-infiltrating
effector regulatory T cells (Tregs) (27). It was hypothesized that a
bispecific fusion toxin containing both EGF and interleukin-2
(bis-EGF/IL2-IT) would achieve a dual advantage for treatment of
EGFR+ HNSCC via both EGF-based targeted therapy and
IL2-based immunotherapy (via Treg depletion). In addition, IL-2
(Aldesleukin, PROLEUKIN®) was the first FDA-approved
cancer immunotherapy to stimulate immune system for metastatic
renal cell carcinoma in 1992 and melanoma in 1998 and was approved
as a combination treatment for neuroblastoma in 2015 (28). Based on the aforementioned
information, a bis-EGF/IL2-IT was generated and it was compared
with other two EGF fusion toxins, i.e., mono-EGF-IT and bi-EGF-IT
in immunocompetent mice in the presence of host immunity.
Materials and methods
DNA construct for the bispecific human
EGF/IL2 fusion toxin
Codon-optimized human IL2 DNA was synthesized by
GenScript with a BamHI site at the N-terminus and an
EcoRI site at the C-terminus. This DNA replaced the second
human EGF in the bivalent human EGF fusion toxin DNA construct
(bi-EGF-IT) between the BamHI and EcoRI sites,
yielding the bispecific human EGF-IL2 fusion toxin DNA construct
(bis-EGF/IL2-IT). The human EGF domain and human IL2 were linked by
three tandem G4S linkers (G4S)3. A tag of 6
histidines (6 × His tag) was added to the C terminus to facilitate
protein purification (Fig. 1).
 | Figure 1.Schematic diagrams of the 3 EGF
fusion toxins. Mono-EGF-IT, monovalent human EGF fusion toxin;
bi-EGF-IT, bivalent human EGF fusion toxin; bis-EGF/IL2-IT,
bispecific human EGF/IL2 fusion toxin; DT, diphtheria toxin; hEGF,
human epidermal growth factor; hIL2, human interleukin-2;
G4S, four glycine residues and one serine residue; His,
histidine; N, N-terminal; C, C-terminal. |
Expression of the bispecific human
EGF/IL2 fusion toxin
The human EGF/IL2-IT DNA construct was linearized
and transformed into the DT-resistant Pichia pastoris yeast
strain (29) using the Gene Pulser
Xcell Electroporation System (Bio-Rad Laboratories, Inc.). The
transformed cells were spread onto YPD agar plates (1% yeast
extract, 2% peptone, 1.5% agar and 2% dextrose) containing 100
µg/ml zeocin and incubated at 30°C for 3–4 days. A total of 6
colonies were randomly picked and cultured in 5 ml YPD medium at
30°C for 24 h with shaking at 250 rpm and then in YPG medium (1%
yeast extract, 2% peptone, 1% glycerol) for another 24 h. The
induction of EGF/IL2-IT was carried out in 2 ml BMMYC medium (1%
yeast extract, 2% peptone, 100 mM potassium phosphate, pH 7.0,
1.34% yeast nitrogen base without amino acids, 4×10–5%
biotin, 0.5% methanol, and 1% casamino acids) for 48 h at 25°C with
shaking at 225 rpm. Methanol (0.5%) was added twice daily to
maintain the methanol level. Antifoam (Emerald Performance
Materials LLC) was added to all growth and induction media at a
concentration of 0.02%. Phenylmethylsulfonyl fluoride (PMSF, 1 mM;
MilliporeSigma) was added to inhibit protein degradation during the
induction phase. Penicillin (100 U/ml) and streptomycin (100 µg/ml)
were added to all growth and induction media to inhibit bacterial
contamination. The culture supernatants were analyzed using 4–12%
SDS-PAGE gels. One human bis-EGF/IL2-IT clone was selected for
large-scale expression. The Excella E24 incubator shaker
(Eppendorf) was used for large-scale expression. The seed culture
was prepared by inoculating a single colony into YPD medium and
then incubating at 25°C overnight with shaking at 225 rpm. Next, 5%
of the seed culture was transferred to 1-L PYREX shake flasks
containing 250 ml YPD medium and cultured at 30°C and 250 rpm for
24 h. The cells were centrifuged at 491 × g for 5 min, and the cell
pellet was resuspended in 250 ml YPG medium and cultured at 30°C
for 24 h with shaking at 250 rpm. For the induction phase, the
cells were centrifuged at 491 × g for 5 min, and the cell pellet
was resuspended in 125 ml BMMYC induction medium and induced at
25°C for 48 h with shaking at 225 rpm. Methanol (0.5%) was added
twice daily to maintain the methanol level. After the induction,
the yeast cells were pelleted by centrifugation at 1,692 × g for 10
min at 4°C. The supernatant was used for first-step protein
purification. Antifoam, PMSF, and penicillin/streptomycin were
added to the expression medium, as described for the small-scale
preparation.
Purification of the bispecific human
EGF/IL2 fusion toxin
First-step purification of bis-EGF/IL2-IT was
carried out using Ni-Sepharose™ 6 fast flow resin. The resin was
packed in an XK50 column (Cytiva), equilibrated with 20 mM Tris-HCl
(pH 7.4), 0.5 M NaCl, and 5 mM imidazole. The sample was loaded
onto the equilibrated column in the same buffer. The column was
washed with this buffer, and then the bound proteins were eluted
with 20 mM Tris-HCl pH 7.4, 0.5 M NaCl and 500 mM imidazole. The
purification fractions were analyzed using 4–12% SDS-PAGE gels. The
fractions containing bis-EGF/IL2-IT were pooled and dialyzed
against 20 mM Tris-HCl pH 8.0, 1 mM EDTA, and 5% glycerol using
Spectra/Por membrane tubing with a 3.5 kDa cut-off (Repligen) at
4°C with stirring. The dialysis buffer was replaced once. Strong
anion exchange resin Poros 50 HQ (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was packed in an XK16/20 column (Cytiva) for
second-step purification. The column was equilibrated with 20 mM
Tris-HCl pH 8.0, 1 mM EDTA, and 5% glycerol. The dialyzed sample
was loaded onto the column and washed with the same buffer. The
bound protein was eluted with 100- and 200-mM sodium borate, then
200 mM sodium borate plus 50 mM NaCl (250 mM total salt) in 20 mM
Tris-HCl pH 8.0, 1 mM EDTA, and 5% glycerol. The purified fractions
were analyzed using 4–12% SDS-PAGE gels. The fractions containing
the human bis-EGF/IL2-IT were pooled and dialyzed using Spectra/Por
membrane tubing (3.5 kDa cut-off) against PBS plus 5% glycerol at
4°C with stirring. The dialysis buffer was replaced once. The
protein concentration was measured using the Pierce BCA Protein
Assay Kit (Thermo Fisher Scientific, Inc.). Mono-EGF-IT, bi-EGF-IT,
DT390 (truncated DT), and anti-murine PD-1 IT (mPD1-IT) were also
expressed and purified using the same DT-resistant yeast Pichia
pastoris expression system (26).
Biotin-labeling of the three EGF
fusion toxins
The 3 EGF fusion toxins (mono-EGF-IT, bi-EGF-IT, and
bis-EGF/IL2-IT) were labeled with EZ-Link Sulfo-NHS Biotin (Thermo
Fisher Scientific, Inc.). NHS-Biotin (1 mg) was added to 1 mg of
each of the 3 EGF fusion toxins, and the mixtures were incubated
for 2 h at 4°C with shaking. The samples were transferred to a
Slide-A-Lyzer dialysis cassette (10 kDa MWCO, 0.5–3 ml, Thermo
Fisher Scientific, Inc.) and dialyzed against 1X PBS for 24 h at
4°C with stirring. The dialysis buffer was replaced once.
Cells, western blotting, and
antibodies
The murine EGFR+ HNSCC tumor cell line
MOC2, established from a carcinogen-induced oral SCC in a C57BL/6
mouse (30), was purchased from
Kerafast, Inc. The cell line was authenticated by STR (short tandem
repeat) and was free of pathogens. Purified fusion toxins were
analyzed using 4–12% SDS-PAGE gels. The gels were stained with Gel
Code Blue Staining Reagent (Thermo Fisher Scientific, Inc.) and
mounted with DryEase Mini Cellophane (Thermo Fisher Scientific,
Inc.). Western blot analysis was performed as previously described
(31). Briefly, proteins (~1 µg in
15 µl per sample) were separated on 4–12% SDS-PAGE gels and
transferred onto nitrocellulose membranes (Thermo Fisher
Scientific, Inc.). The membranes were blocked with 5% blotting
grade blocker non-fat dry milk (Bio-Rad Laboratories, Inc.) in 1X
PBS, 0.02% Tween 20 for 1 h with shaking and washed once with 1X
PBS, pH 7.4, 0.2% Tween 20 at room temperature with shaking. The
three EGF fusion toxins were detected using mouse anti-DT, mouse
anti-human EGF, or mouse anti-human IL2 primary antibodies
(1:1,000) and rat anti-mouse IgG-HRP as the secondary antibody
(1:4,000). The proteins were detected using the TMB
(Tetramethylbenzidine) membrane peroxidase substrate (SeraCare; LGC
Clinical diagnostics). The antibodies used in the present study are
listed in Table SI.
The binding affinities of the three
human EGF fusion toxins to murine EGFR+ HNSCC MOC2
cells
MOC2 cells were stained at 4°C for 30 min with
biotinylated mono-EGF-IT, bi-EGF-IT, or bis-EGF/IL2-IT at a range
of concentrations (0.01 to 200 nM). The biotinylated anti-human
EGFR mAb (Santa Cruz Biotechnology, Inc.) was used as a positive
control at a final concentration of 36 nM. Negative control cells
were stained at 4°C for 30 min only with streptavidin-PE (SA-PE) at
a final concentration of 1.5 ng/µl. Flow cytometry was carried out
using a CytoFLEX Flow cytometer (Beckman Coulter, Inc.), and data
were analyzed using FlowJo software v10 (FlowJo LLC). Biotinylated
mPD1-IT was used as a biotinylated protein control.
KD determination
Binding of mono-EGF-IT, bi-EGF-IT or bis-EGF/IL2-IT
to murine EGFR+ HNSCC MOC2 cells was performed using a
wide range of concentrations of biotinylated mono-EGF-IT,
bi-EGF-IT, or bis-EGF/IL2-IT. The KDs were determined
from the flow cytometric data using nonlinear regression with the
saturation binding equation (GraphPad Prism 9.0.0). The mean
fluorescence intensities (MFI) were plotted vs. the biotinylated
mono-EGF-IT, bi-EGF-IT, or bis-EGF/IL2-IT concentration. Nonlinear
regression was based on the equation Y=Bmax × X/(KD +
X), where Y=MFI at a given biotinylated EGF fusion toxin
concentration after subtracting the background, X=biotinylated EGF
fusion toxin concentration, and Bmax=the maximum specific binding
in the same units as Y.
In vitro efficacy
The effect of mono-EGF-IT, bi-EGF-IT, and
bis-EGF/IL2-IT on the viability of murine EGFR+ HNSCC
MOC2 cells was determined using the CellTiter-Glo®
Luminescent Cell Viability Assay kit (Promega Corporation), as
previously described (32). This
assay measures the luminescence produced by ATP production from
metabolically active cells. Increasing concentrations of the
mono-EGF-IT, bi-EGF-IT and bis-EGF/IL2-IT cause cell death and a
corresponding reduction in ATP-related fluorescence. Luminescence
was measured using a BioTek Synergy LX Multi-Mode Reader. DT390 was
included as negative control.
In vivo efficacy
C57BL/6 mice were purchased from Jackson
Laboratories (Bar Harbor, USA). The animal experiments were
approved (approval no. 00866) by the Institutional Animal Care and
Use Committee (IACUC) of the University of Colorado Anschutz
Medical Campus (Aurora, USA). The housing temperature was 22°C and
the housing atmosphere was set at 35% humidity. The light/dark
cycle was 14–10 h, respectively, (6 am to 8 pm on) and the lighting
is at 50% strength unless over-ridden for 15 min durations.
Standard feed provided was Envigo Teklad Global Diets 2920X (19%
protein, 6% Fat). Water was freely accessed via water bottle or
water valve in the cage. C57BL/6 mice (6–8 weeks old, 20–25 g) were
divided into five treatment groups: i) DT390, negative control; ii)
mono-EGF-IT; iii) bi-EGF-IT; iv) bis-EGF/IL2-IT and v) erlotinib,
positive control at a previously reported dose (33,34).
In total, 209 mice (105 males and 104 females) were used for the
present study.
A total of 3 complementary syngeneic HNSCC tumor
models were used to evaluate in vivo efficacy in
immunocompetent C57BL/6 mice, including subcutaneous (SC) tumors,
orthotopic tongue SCC, and experimental metastasis models. For the
SC tumor model, murine EGFR+ HNSCC MOC2 cells (8×106 in
200 µl DMEM) were injected SC into the right flank. For the
orthotopic tongue SCC model, C57BL/6 mice were anesthetized with
isoflurane, and the MOC2 cells (1×106 cells in 50 µl
DMEM) were injected into the tongue. For the experimental
metastasis model, MOC2 cells (1×106 cells in 200 µl DMEM) were
injected intravenously via the tail vein. For all 3 models, the
tumor-bearing mice were randomly divided with equal sex
distribution into the five treatment groups on day 3
post-inoculation.
Treatment commenced for the SC tumors and orthotopic
tongue SCC on day 4 post-tumor cell inoculation of the MOC2 tumor
cells and on day 10 for the experimental metastasis model.
Mono-EGF-IT, bi-EGF-IT, bis-EGF/IL2-IT, or DT390 (50 µg/kg) were
administered by intraperitoneal injection. Erlotinib (20 mg/kg) was
administered via intragastric gavage. All treatments were
administered once daily for 10 consecutive days. The tumors were
measured using digital Vernier calipers every 3 days, as previously
described (26,35). The tumor volume was calculated
according to the formula: Volume
(mm3)=[lengthx(widthx2)]/2. Quantification of lung
metastases was performed by counting the number of metastases
regardless of tumor size and by determining the percentage of tumor
burden in 3 independent microscopic fields at ×5 magnification.
The specific criteria (i.e., humane endpoints) used
in the present study to determine when animals should be euthanized
were: i) Tumor size reaching 2 cm in any diameter, ii) Body weight
loss being over 15% compared with the littermate controls at any
time point, iii) Mice reaching to moribund state or having
difficulty to eat soft food or drink water, iv) Ulcerated tumors
and v) Self-mutilation, hypothermia, or difficulty of breath. The
maximal duration of the experiments for the present study was 3
months. The animal health and behavior were monitored once daily,
and twice daily in the late stage of the experiments. Soft food
such as moist chow was provided for animals in poor health
condition. If their condition deteriorated further, the animals
were euthanized. Mice were anesthetized with inhalation of 5%
isoflurane when injection of the tumor cells to the tongues or oral
gavage of Erlotinib. The method of euthanasia used was
CO2 inhalation plus approved secondary method such as
cervical dislocation and bilateral thoracotomy. After the second
euthanasia method, cessation of breath and heartbeat was verified
as mouse death.
Statistical analysis
IC50s were determined using nonlinear
regression (GraphPad Prism 9.0.0; GraphPad Software, Inc.). The
P-values for the survival curves were calculated using the
Mantel-Cox log-rank test (GraphPad Prism 9.0.0). The P-values for
other comparisons were calculated using the unpaired two-tailed
Student's t-test (GraphPad Prism 9.0.0). P<0.05 was considered
to indicate a statistically significant difference.
Results
Generation of the bispecific human
EGF/IL2 fusion toxin
Monovalent (mono-EGF-IT) and bivalent (bi-EGF-IT)
human EGF fusion toxins were previously generated using a unique
DT-resistant yeast Pichia pastoris expression system
(26). In the current study, the
second human EGF was replaced by the codon-optimized human IL-2 DNA
in the bi-EGF-IT DNA construct to produce the bispecific human
EGF/IL2 fusion toxin (bis-EGF/IL2-IT) DNA construct (26). Bis-EGF/IL2-IT was produced using
the Pichia pastoris expression system (26). The final purification yield of the
bis-EGF/IL2-IT was ~10 mg per liter of harvested supernatant.
Purified bis-EGF/IL2-IT was analyzed using SDS-PAGE and western
blotting with monoclonal antibodies (mAb) against DT, human EGF,
and human IL2. The expected molecular weight of 66.5 kDa was
detected for bis-EGF/IL2-IT (Lane 4, Fig. 2A-D). Mono-EGF-IT (50.1 kDa) and
bi-EGF-IT (57.2 kDa) were detected in lanes 2 and 3 when using
either anti-DT or anti-human EGF mAbs (Fig. 2A-C) but not when using the
anti-human IL2 mAb (Fig. 2D).
In vitro binding affinities and
efficacy of the three EGF fusion toxins against murine HNSCC
cells
The binding affinities of the three EGF fusion
toxins were evaluated in the murine HNSCC cell line MOC2, derived
from a squamous cell carcinoma of the oral cavity in a murine oral
carcinogenesis model (30). MOC2
cells express EGFR (EGFR+), which is shown by a shift in
the flow cytometric parameters using a mAb against mouse EGFR
(Fig. 3). It was revealed that
biotinylated mono-EGF-IT, bi-EGF-IT, and bis-EGF/IL2-IT could bind
to murine MOC2 cells in a dose-dependent fashion, with a
KD of 12.0 nM for mono-EGF-IT, 10.5 nM for bi-EGF-IT,
and 5.2 nM for bis-EGF/IL2-IT (Fig. 3A
and B). The amino acid sequence homology is 69.81% between
human and murine EGF and 62.75% between human and murine IL2.
 | Figure 3.Binding affinity for the 3 EGF fusion
toxins to murine EGFR-positive head and neck squamous cell
carcinoma MOC2 cells and their effect on cell viability. (A) The
binding affinities of mono-EGF-IT, bi-EGF-IT, and bis-EGF/IL2-IT to
MOC2 cells were determined by flow cytometry. Negative controls
included cells only, SA-PE only, biotin PD1-IT (biotinylated
anti-murine PD-1 IT as control for background protein
biotinylation), and an isotype control. Anti-murine EGFR mAb was
used as a positive control. The data are representative of three
individual experiments. (B) KD determination for the
binding of the three EGF fusion toxins to MOC2 cells. The mean
fluorescence intensities from flow cytometry were plotted over a
range of concentrations of biotinylated mono-EGF-IT, bi-EGF-IT, and
bis-EGF/IL2-IT. The KDs were calculated based on the
nonlinear regression fit. (C) The effect of the three EGF fusion
toxins on MOC2 cell viability using the CellTiter-Glo®
Luminescent Cell Viability Assay (DT390, negative control, blue;
mono-EGF-IT, red; bi-EGF-IT, green; bis-EGF/IL2-IT, orange).
Y-axis: inhibition rate of the cell viability by determining the
number of viable cells based on quantifying the ATP present.
X-axis: concentrations of the three EGF fusion toxins.
Cycloheximide (1.25 mg/ml) was used as a positive control. The
negative control contained cells without fusion toxin. The data are
from three individual experiments. The error bars indicate SD. IT,
immunotoxin. |
The in vitro efficacy of the three EGF fusion
toxins against the MOC2 cells was assessed using the
CellTiter-Glo® Luminescent Cell Viability Assay. All 3
EGF fusion toxins effectively reduced cell viability of murine
EGFR+ HNSCC MOC2 cells with IC50s of
1.53×10–10 M, 2.15×10–10 M, and
7.22×10–10 M for mono-EGF-IT, bi-EGF-IT, and
bis-EGF/IL2-IT, respectively. The IC50 for the negative
control DT390 was 7.69×10–7 M (Fig. 3C).
Efficacy of the three EGF fusion
toxins against the syngeneic SC MOC2 tumor model
The in vivo efficacy of the three EGF fusion
toxins was first evaluated using the syngeneic SC MOC2 tumor model.
Beginning 4 days after tumor cell inoculation, mice were treated
daily for 10 consecutive days with mono-EGF-IT, bi-EGF-IT, or
bis-EGF/IL2-IT (50 µg/kg) by intraperitoneal injection or 20 mg/kg
erlotinib by intragastric gavage. The tumor-bearing mice were
sacrificed on day 18, and all the tumors were collected. As
demonstrated in Fig. 4, all 3 EGF
fusion toxins and erlotinib significantly reduced tumor growth
compared with the DT390 control group. Furthermore, bis-EGF/IL2
significantly reduced the tumor volume compared with mono-EGF-IT
(Fig. 4A and B).
 | Figure 4.Antitumor efficacy of the three EGF
fusion toxins against a syngeneic SC tumor model. Murine
EGFR+ head and neck squamous cell carcinoma MOC2 cells
were injected SC into the right flank. Tumor-bearing mice were
treated with DT390 (negative control, n=16), mono-EGF-IT (n=11),
bi-EGF-IT (n=16), bis-EGF/IL2-IT (n=15), or erlotinib (positive
control, n=16) once daily for ten consecutive days beginning on day
4 after tumor cell injection. Mice were sacrificed on day 18 after
tumor cell injection. (A) Images of the harvested tumors on day 18.
(B) Quantification of tumor volume (mean ± SEM): DT390,
460.8±29.82; mono-EGF-IT, 339.7±16.48; bi-EGF-IT, 311.7±23.01;
bis-EGF/IL2-IT, 251.5±25.19; erlotinib, 346.5±24.81. *P<0.05,
***P<0.001 and ****P<0.0001. IT, immunotoxin. |
Efficacy of the three EGF fusion
toxins against the syngeneic orthotopic tongue SCC mouse model
To mimic the clinical manifestation of HNSCC, MOC2
tumor cells was injected into the tongues of C57BL/6 mice.
Beginning 4 days after tumor cell inoculation, the mice were
treated with mono-EGF-IT, bi-EGF-IT, or bis-EGF/IL2-IT (50 µg/kg)
by intraperitoneal injection or 20 mg/kg erlotinib by intragastric
gavage daily for ten consecutive days. Compared with DT390 and
mono-EGF-IT, bi-EGF-IT, bis-EGF/IL2-IT, and erlotinib significantly
prolonged the median survival time from 13 days (DT390 and
mono-EGF-IT) to 15 (bis-EGF/IL2-IT, and erlotinib) or 16.5 days
(bi-EGF-IT) (Fig. 5A and Table I). To further characterize the
effect of the three EGF fusion toxins on murine tongue SCC tumor
incidence and volume, this experiment was repeated and mice were
sacrificed 9 days after tumor cell injection. At this time point,
the numbers of tongue SCC tumors occurring were significantly less
(1/5) in the bi-EGF-IT, bis-EGF/IL2-IT and erlotinib groups than in
the mono-EGF-IT (4/5) and DT390 control groups (5/5) (Fig. 5B). Similarly, the total tongue SCC
volume in the bi-EGF-IT, bis-EGF/IL2-IT, and erlotinib groups were
significantly smaller than those in the mono-EGF-IT or DT390 groups
(Fig. 5C).
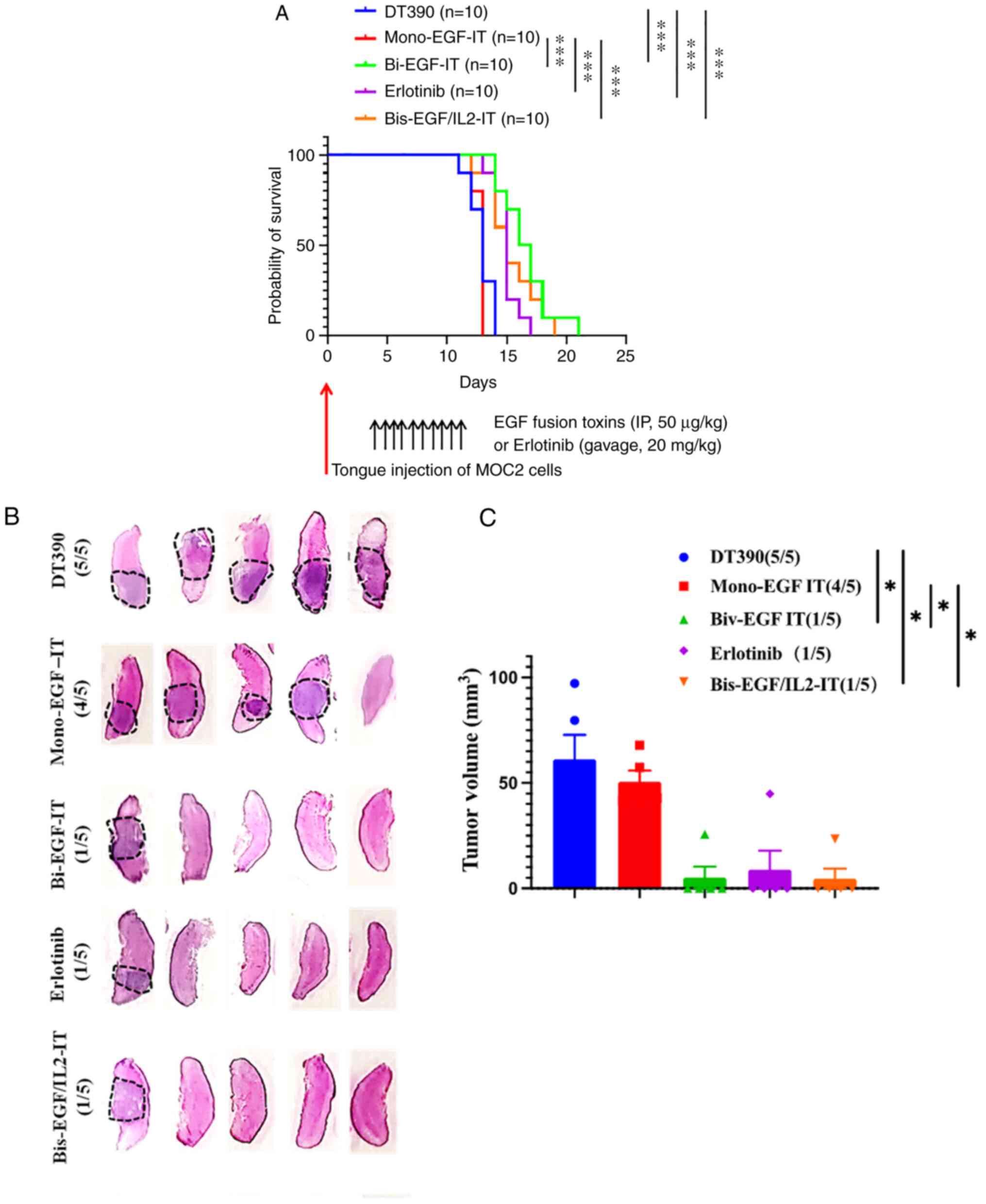 | Figure 5.Antitumor efficacy of the three EGF
fusion toxins against a syngeneic orthotopic tongue SCC mouse
model. (A) Kaplan-Meier survival curves for mice with tongue SCC
following treatment with the three EGF fusion toxins.
EGFR+ HNSCC MOC2 cells were injected into the tongue of
C57BL/6 mice on day 0. Tumor-bearing mice were treated with DT390
(negative control, blue, n=10), mono-EGF-IT (red, n=10), bi-EGF-IT
(green, n=10), bis-EGF/IL2-IT (orange, n=10), or erlotinib
(positive control, purple, n=10) once daily for ten consecutive
days starting on day 4 after tumor cell injection. The timeline and
detailed schedules for tumor cell injection and treatments are
shown under the survival curves. The vertical arrows indicate the
days the tumor cells (red) or treatments (black) were administered.
(B) Histological images of tongues with SCC following treatment
with the three EGF fusion toxins. The tongue SCCs are circled with
black dotted lines. The incidence of tongue SCC is shown in
parentheses. Murine EGFR+ HNSCC MOC2 cells were injected
into the tongues of a second cohort of C57BL/6 mice and treated
with DT390, mono-EGF-IT, bi-EGF-IT, bis-EGF/IL2-IT, or erlotinib
(n=5 for each group). The mice were euthanized, and the tongues
were harvested on day 9 after tumor cell injection. (C)
Quantification of total tumor volume for each treatment group. The
incidence of tongue SCC is shown in parentheses. *P<0.05 and
***P<0.001. SCC, squamous cell carcinoma; HNSCC, head and neck
squamous cell carcinoma; IT, immunotoxin. |
 | Table I.Median survival days for syngeneic
tongue squamous cell carcinoma and lung metastasis mouse models
following treatment. |
Table I.
Median survival days for syngeneic
tongue squamous cell carcinoma and lung metastasis mouse models
following treatment.
|
| Median survival
days |
|---|
|
|
|
|---|
| Tumor model | DT390 | Mono-EGF-IT | Bi-EGF-IT | Bis-EGF/IL2-IT | Erlotinib |
|---|
| Orthotopic tongue
model | 13 | 13 | 16.5 | 15 | 15 |
| Lung metastasis
model | 19 | 29 | 47.5 | 41 | 38 |
Efficacy of the three EGF fusion
toxins against lung metastasis in a syngeneic mouse model
To evaluate the effect of the three EGF fusion
toxins on lung metastasis, MOC2 tumor cells were injected
intravenously into C57BL/6 mice. Beginning 10 days post-tumor cell
injection, mice were treated with mono-EGF-IT, bi-EGF-IT,
bis-EGF/IL2-IT, or DT390 (50 µg/kg) by intraperitoneal injection or
20 mg/kg erlotinib by intragastric gavage daily for ten consecutive
days. All 3 EGF fusion toxins and erlotinib significantly prolonged
the median survival time from 19 days (DT390) to 29 (mono-EGF-IT),
47.5 (bi-EGF-IT), 41 (bis-EGF/IL2-IT), or 38 (erlotinib) days
(Fig. 6A and Table I). Moreover, bi-EGF-IT,
bis-EGF/IL2-IT, and erlotinib were more potent than mono-EGF-IT,
with bi-EGF-IT being the most efficacious (Fig. 6A).
 | Figure 6.In vivo efficacy of the 3 EGF
fusion toxins against lung metastasis in a syngeneic mouse model.
(A) Kaplan-Meier survival curves for mice with lung metastasis
following treatment with the three EGF fusion toxins. Murine
EGFR+ HNSCC MOC2 cells were intravenously injected into
C57BL/6 mice via the tail vein, and the mice were then treated with
DT390 (blue, n=8), mono-EGF-IT (red, n=8), bi-EGF-IT (green, n=6),
bis-EGF/IL2-IT (orange, n=6), or erlotinib (purple, n=6) once daily
for 10 consecutive days starting on day 10 after tumor cell
injection. The timeline and detailed schedules for tumor cell
injection and treatments are shown under the survival curves. The
vertical arrows indicate the days the tumor cells (red) or
treatments (black) were administered. (B) Histological images of
lung metastases following treatment with the 3 EGF fusion toxins or
erlotinib. The lung metastases are circled with black dotted lines.
The incidence of metastases is shown in parentheses. Murine
EGFR+ HNSCC MOC2 cells were intravenously injected into
C57BL/6 mice via the tail vein of a second cohort of C57BL/6 mice
and treated with DT390 (n=5), mono-EGF-IT (n=5), bi-EGF-IT (n=5),
bis-EGF/IL2-IT (n=6), or erlotinib (n=5). The mice were euthanized,
and the lungs were harvested on day 18 after the tumor cell
injection. Scale bar, 100 µm. (C) Quantification of the number of
lung metastases per mouse. (D) Quantification of the percentage of
lung metastatic tumor burden per microscopic field per mouse. A
total of 3 microscopic fields at ×5 magnification were used to
calculate the percentage of tumor occupied in each microscopic
field. ns=non-significant; *P<0.05, **P<0.01 and
***P<0.001. HNSCC, head and neck squamous cell carcinoma; IT,
immunotoxin. |
To further assess the efficacy of the three EGF
fusion toxins against metastasis, the metastasis study was
repeated, and all mice were sacrificed 18 days after tumor cell
inoculation. As revealed in Fig.
6B-D, the numbers and sizes of the metastases (shown as the
percentage of tumor burden) in the lungs of the mice treated with
bi-EGF-IT, bis-EGF/IL2-IT, or erlotinib were significantly reduced
compared with the DT390 and mono-EGF-IT groups.
Comparison of mono-EGF-IT, bi-EGF-IT
and bis-EGF/IL2-IT toxicity
Similar to the previous observation in NSG mice
(26), C57BL/6 mice treated with
mono-EGF-IT appeared generally unhealthy and lethargic compared
with those treated with either bi-EGF-IT or bis-EGF/IL2-IT. The
body weight of mono-EGF-IT group was declined compared with those
in bi-EGF-IT and bis-EGF/IL2-IT groups although with no statistical
significance (Fig. S1A). Necropsy
of mice treated with mono-EGF-IT revealed moderate amounts of
hemorrhagic fluid throughout the abdominal cavity. The stomach and
duodenum were mildly distended with ingesta, while the remainder of
the intestines was relatively empty except for multifocal regions
of dark ingesta. The liver was diffusely pale and enlarged. The
kidneys were diffusely and bilaterally pale. By contrast, the
kidneys, liver, heart, and gastrointestinal organs were
macroscopically normal in mice treated with either bi-EGF-IT or
bis-EGF/IL2-IT (Fig. S1B).
Discussion
In the present study, our previous work was extended
evaluating the efficacy and toxicity of mono-EGF-IT and bi-EGF-IT
(26) to immunocompetent HNSCC
mouse models. In addition, a novel fusion toxin, bis-EGF/IL2-IT,
was generated to allow targeting of the tumor and its immune
microenvironment. The results showed that all 3 EGF-IT exhibited a
variety of efficacies in reducing tumor size (SC tumor), tumor
incidence, tumor volume (orthotopic tongue model), lung metastasis,
and prolonged survival in the syngeneic HNSCC mouse models with
intact immune systems. Both bi-EGF-IT and bis-EGF/IL2-IT improved
efficacies and reduced toxicity compared with mono-EGF-IT in the 3
immunocompetent syngeneic HNSCC mouse models. Bis-EGF-IL2-IT was
superior in reducing tumor size compared with bi-EGF-IT, whereas
the latter was superior to the former in prolonging survival days
in the metastatic model. It was hypothesized that the immunotherapy
function of bis-EGF/IL2-IT may be more effective in reducing tumor
size, and the improved targeted therapy function of bi-EGF-IT may
be more effective in inhibiting metastasis to prolong the survival
in the metastatic model. Nonetheless, these data indicated that
both bi-EGF-IT and bis-EGF/IL2-IT are more potent and less toxic
than mono-EGF-IT.
The superiority of bi-EGF-IT over mono-EGF-IT is
consistent with a previous study by the authors testing the
efficacy and toxicity of these 2 fusion toxins in immunocompromised
HNSCC mouse models (26). The
improved binding affinity of the bivalent version may be one reason
for this observed difference. Notably, the bis-EGF/IL2-IT performed
nearly equivalent to bi-EGF-IT in efficacy and toxicity.
Bis-EGF/IL2-IT was developed to examine the hypothesis that
targeting both tumor and immune microenvironment could improve the
efficacy. Indeed, bis-EGF/IL2-IT was more efficacious than
mono-EGF-IT. HNSCC, either HPV+ or HPV-, is
one of the cancer types with the highest infiltration of Tregs,
which significantly contributes to immune suppression in the tumor
microenvironment (36). Targeting
IL2 receptor boosts the antitumor immune response via Treg
depletion, as reported for a study using the FDA-approved
Ontak® (human IL2 fusion toxin) (37) and shown in a previous study by the
authors (38).
Initially, the murine EGF fusion toxins was planned
to make for the studies in the syngeneic mouse tumor models.
However, since the human EGF fusion toxins could bind to the murine
EGFR+ MOC2 tumor cells, it was decided to directly
assess the in vitro and in vivo efficacy of human EGF
fusion toxins against murine EGFR+ HNSCC MOC2 tumor
cells.
The limitation and future direction of the present
study are as follows: i) Although the syngeneic HNSCC mouse model
is immunocompetent, the cell line used is derived from murine
experimental HNSCC model, which has the limitation of human
relevance. A humanized HNSCC mouse model, which allows assessing
human HNSCC cell lines or cell lines from patient-derived xenograft
in the immunocompetent environment, will be ideal to address both
human relevance and immunocompetent microenvironment; ii) Although
bis-EGF/IL2-IT demonstrated the best in vivo efficacy, the
in vitro efficacy was not as well as expected. It was
hypothesized that, except for the EGF-based targeted therapy
function, IL2 domain of bis-EGF/IL2-IT may also stimulate immune
response and deplete tumor-infiltrating effector Tregs in
vivo. However, the in vitro efficacy assay may only
detect the targeted therapy function, not the immunotherapy
function of bis-EGF/IL2-IT; iii) The effect of bis-EGF/IL2-IT on
tumor microenvironment is hypothetical. A comprehensive
characterization of tumor microenvironment will mechanistically
support the hypothesis and is warranted; iv) There was only one
murine OSCC cell line available for use in the present study.
Although expressed, the EGFR levels in the MOC2 cell line may not
be high enough to observe an improved response to the treatment
efficacy of the human EGF fusion toxins. More syngeneic murine
HNSCC models with a variety of EGFR expression levels may provide
more information on EGFR expression in response to treating human
EGF fusion toxins in HNSCC mouse models in the presence of host
immunity; v) Given the superiority of bi-EGF-IT and bis-EGF/IL2-IT
over mono-EGF-IT, a combination of bivalent and bispecific
approaches should enhance both binding and the anticancer immune
response and may further improve the performance of EGF-IT in the
treatment of HNSCC.
In summary, a novel bis-EGF/IL2-IT was generated and
its efficacy and toxicity were examined, together with mono-EGF-IT
and bi-EGF-IT, in the treatment of syngeneic HNSCC mouse models
with intact immune systems. Both bis-EGF/IL2-IT and bi-EGF-IT were
more effective and less toxic than mono-EGF-IT, suggesting that
targeting the immune microenvironment or enhancing binding affinity
to EGF will improve the performance of EGF-IT in the treatment of
HNSCC mouse models.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors upon a
reasonable request.
Authors' contributions
YQ, SLL and ZhiW designed the experiments. YQ, ZQ,
ZhaW, YC, LL and HZ performed the experiments. YQ, DM, EAP, SLL and
ZhiW analyzed and interpreted the data. YQ, DM, EAP, SLL and ZhiW
wrote and reviewed the manuscript. All authors read and approved
the final manuscript. YQ, SLL and ZhiW confirmed the authenticity
of all the raw data.
Ethics approval and consent to
participate
The animal experiments were approved (approval no.
00866) by the Institutional Animal Care and Use Committee (IACUC)
of the University of Colorado Anschutz Medical Campus (Aurora,
USA).
Patient consent for publication
Not applicable.
Competing interests
ZhiW is the founder of Rock Immune LLC. The
remaining authors declare that they have no competing
interests.
References
|
1
|
Chow LQM: Head and neck cancer. N Engl J
Med. 382:60–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wyss A, Hashibe M, Chuang SC, Lee YC,
Zhang ZF, Yu GP, Winn DM, Wei Q, Talamini R, Szeszenia-Dabrowska N,
et al: Cigarette, cigar, and pipe smoking and the risk of head and
neck cancers: Pooled analysis in the International head and neck
cancer epidemiology consortium. Am J Epidemiol. 178:679–690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, et al: Human papillomavirus and rising oropharyngeal
cancer incidence in the United States. J Clin Oncol. 29:4294–4301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gillison ML, Chaturvedi AK, Anderson WF
and Fakhry C: Epidemiology of human papillomavirus-positive head
and neck squamous cell carcinoma. J Clin Oncol. 33:3235–3242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pfister DG, et al: NCCN clinical practice
guideline in oncology-head and neck cancers, version 1. 2019 March
6–2019.2019.
|
|
7
|
Cramer JD, Burtness B, Le QT and Ferris
RL: The changing therapeutic landscape of head and neck cancer. Nat
Rev Clin Oncol. 16:669–683. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cramer JD, Burtness B and Ferris RL:
Immunotherapy for head and neck cancer: Recent advances and future
directions. Oral Oncol. 99:1044602019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
NCI, . SEER Database: Cancer Stat Facts:
Oral cavity and pharynx cancer, laryngeal cancer. 2009-2015.
|
|
10
|
Cohen EEW, LaMonte SJ, Erb NL, Beckman KL,
Sadeghi N, Hutcheson KA, Stubblefield MD, Abbott DM, Fisher PS,
Stein KD, et al: American cancer society head and neck cancer
survivorship care guideline. CA Cancer J Clin. 66:203–239. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trivedi S and Ferris RL: Epidermal growth
factor receptor-targeted therapy for head and neck cancer.
Otolaryngol Clin North Am. 54:743–749. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forastiere AA and Burtness BA: Epidermal
growth factor receptor inhibition in head and neck cancer-more
insights, but more questions. J Clin Oncol. 25:2152–2155. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saba NF, Chen ZG, Haigentz M, Bossi P,
Rinaldo A, Rodrigo JP, Mäkitie AA, Takes RP, Strojan P, Vermorken
JB and Ferlito A: Targeting the EGFR and immune pathways in
squamous cell carcinoma of the head and neck (SCCHN): Forging a new
alliance. Mol Cancer Ther. 18:1909–1915. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muratori L, La Salvia A, Sperone P and Di
Maio M: Target therapies in recurrent or metastatic head and neck
cancer: State of the art and novel perspectives. A systematic
review. Crit Rev Oncol Hematol. 139:41–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bardelli A and Jänne PA: The road to
resistance: EGFR mutation and cetuximab. Nat Med. 18:199–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jelinek MJ and Vokes EE: Epidermal growth
factor receptor blockade in head and neck cancer: What remains? J
Clin Oncol. 37:2807–2814. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Byeon HK, Ku M and Yang J: Beyond EGFR
inhibition: Multilateral combat strategies to stop the progression
of head and neck cancer. Exp Mol Med. 51:1–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saada-Bouzid E and Le Tourneau C: Beyond
EGFR targeting in SCCHN: Angiogenesis, PI3K, and other molecular
targets. Front Oncol. 9:742019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pastan I, Hassan R, Fitzgerald DJ and
Kreitman RJ: Immunotoxin therapy of cancer. Nat Rev Cancer.
6:559–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JS, Jun SY and Kim YS: Critical issues
in the development of immunotoxins for anticancer therapy. J Pharm
Sci. 109:104–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akbari B, Farajnia S, Ahdi Khosroshahi S,
Safari F, Yousefi M, Dariushnejad H and Rahbarnia L: Immunotoxins
in cancer therapy: Review and update. Int Rev Immunol. 36:207–219.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Z, Qiu Y and Wang Z, Zhang H, Lu L, Liu
Y, Mathes D, Pomfret EA, Gao D, Lu SL and Wang Z: A novel
diphtheria toxin-based bivalent human EGF fusion toxin for
treatment of head and neck squamous cell carcinoma. Mol Oncol.
15:1054–1068. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onda M, Kobayashi K and Pastan I:
Depletion of regulatory T cells in tumors with an anti-CD25
immunotoxin induces CD8 T cell-mediated systemic antitumor
immunity. Proc Natl Acad Sci USA. 116:4575–4582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raeber ME, Sahin D and Boyman O:
Interleukin-2-based therapies in cancer. Sci Transl Med.
14:eabo54092022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu YY, Woo JH and Neville DM: Targeted
introduction of a diphtheria toxin resistant mutation into the
chromosomal EF-2 locus of Pichia pastoris and expression of
immunotoxin in the EF-2 mutants. Protein Expr Purif. 30:262–274.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Judd NP, Winkler AE, Murillo-Sauca O,
Brotman JJ, Law JH, Lewis JS Jr, Dunn GP, Bui JD, Sunwoo JB and
Uppaluri R: ERK1/2 regulation of CD44 modulates oral cancer
aggressiveness. Cancer Res. 72:365–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peraino JS, Schenk M, Li G, Zhang H,
Farkash EA, Sachs DH, Huang CA, Duran-Struuck R and Wang Z:
Development of a diphtheria toxin-based recombinant porcine IL-2
fusion toxin for depleting porcine CD25+ cells. J
Immunol Methods. 398–399. 33–43. 2013.PubMed/NCBI
|
|
32
|
Zheng Q and Wang Z, Zhang H, Huang Q,
Madsen JC, Sachs DH, Huang CA and Wang Z: Diphtheria toxin-based
anti-human CD19 immunotoxin for targeting human CD19+
tumors. Mol Oncol. 11:584–594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fletcher EV, Love-Homan L, Sobhakumari A,
Feddersen CR, Koch AT, Goel A and Simons AL: EGFR inhibition
induces proinflammatory cytokines via NOX4 in HNSCC. Mol Cancer
Res. 11:1574–1584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stanam A, Gibson-Corley KN, Love-Homan L,
Ihejirika N and Simons AL: Interleukin-1 blockade overcomes
erlotinib resistance in head and neck squamous cell carcinoma.
Oncotarget. 7:76087–76100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du L, Chen X, Cao Y, Lu L, Zhang F,
Bornstein S, Li Y, Owens P, Malkoski S, Said S, et al:
Overexpression of PIK3CA in murine head and neck epithelium drives
tumor invasion and metastasis through PDK1 and enhanced TGFβ
signaling. Oncogene. 35:4641–4652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mandal R, Şenbabaoğlu Y, Desrichard A,
Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA and
Morris LG: The head and neck cancer immune landscape and its
immunotherapeutic implications. JCI Insight. 1:e898292016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar P, Kumar A, Parveen S, Murphy JR and
Bishai W: Recent advances with Treg depleting fusion protein toxins
for cancer immunotherapy. Immunotherapy. 11:1117–1128. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Zheng Q, Zhang H, Bronson RT,
Madsen JC, Sachs DH, Huang CA and Wang Z: Ontak-like human IL-2
fusion toxin. J Immunol Methods. 448:51–58. 2017. View Article : Google Scholar : PubMed/NCBI
|




















