Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most
common histological type of pancreatic cancer, which is associated
with chemoresistance (1). PDAC is
characterized by desmoplastic stroma that includes α-smooth muscle
actin (αSMA)-positive fibroblasts called cancer-associated
fibroblasts (CAFs), which promote tumor growth and inhibit drug
delivery (2,3). In recent years, neoadjuvant
chemotherapy (NAC) has been accepted as a new standard strategy for
the surgical management of PDAC (4,5).
Furthermore, clinical trials have shown that surgery followed by
chemotherapy provides better survival benefits than surgery alone
(6). Similar to the conventional
regimen of gemcitabine and S-1 (GS), a regimen of gemcitabine and
nab-paclitaxel (GnP) has been reported to be associated with good
outcomes in terms of progression-free survival and overall survival
(7,8).
Although reports from clinical trials have been
encouraging regarding the treatment of PDAC, there are limitations
regarding the use of radiological examination and pathological
analysis for the determination of the therapeutic effects of NAC.
Even though Response Evaluation Criteria in Solid Tumors is the
accepted method for the determination of tumor progression, it may
overestimate tumor burden (9).
There are a number of grading systems used to determine the
histological therapeutic effects of NAC, including the College of
American Pathologists grading system, Evans grading system and MD
Anderson Cancer Center grading system (10–12).
However, it is difficult to compare grades between the systems
because the grading systems use different criteria (13). Furthermore, radiological evaluation
of treatment response using computed tomography (CT) or magnetic
resonance imaging does not correspond to pathological results
(14,15). Therefore, a new method of
determination that integrates radiological and histological
therapeutic effects is needed.
Our previous study on PDAC not treated with NAC
showed that the time-density curve (TDC) of dynamic
contrast-enhanced CT (CECT) was associated with the histological
characteristics of PDAC, such as densities of cancer cells, CAFs
and microvessels (16). Dynamic
CECT consists of four time phases: Non-contrast, arterial, portal
and equilibrium phases. TDCs represent serial changes in the
contrast effect of tumors at the four time phases. Our previous
study demonstrated that the first slope of the TDC between the
non-contrast and arterial phases was associated with the density of
microvessels, and that the second slope of the TDC between the
arterial and portal phases was associated with the densities of
cancer cells and CAFs. Based on our previous study, it was
hypothesized that TDC changes before and after NAC may be
associated with histological changes caused by NAC. The aim of the
present study was to investigate the histological changes caused by
NAC, and to demonstrate the use of TDC for the determination of the
histological therapeutic effects of NAC for PDAC.
Materials and methods
Patients
A total of 96 patients with PDAC were examined; 46
underwent NAC (NAC group), whereas 50 did not undergo NAC (non-NAC
group). The present study included a non-NAC group because a
comparison between the NAC and non-NAC groups was necessary to
understand the histological changes caused by NAC. Although it was
possible to evaluate the histological therapeutic effects using
specimens treated with NAC alone, evaluating the changes in cancer
stroma and microvessels after NAC would be difficult if the treated
specimens were not compared with non-treated specimens. The
patients underwent surgical treatment at Hirosaki University
Hospital (Hirosaki, Japan) between November 2011 and April 2021.
Written informed consent for the use of clinical records and
pathological specimens was obtained from each patient before
commencement of the study. All of the patients underwent dynamic
CECT; none of them had contrast media allergy or renal function
problems that would prevent them from undergoing CECT. The NAC
group underwent dynamic CECT before and after NAC. Pathological
tumor, node and metastasis (TNM) categories and staging were
conducted according to an up-to-date TNM classification from the
Union for International Cancer Control (eighth edition) (17). Regarding histological
differentiation, tumors were classified as well-differentiated,
moderately differentiated or poorly differentiated according to the
World Health Organization classification of tumors of the digestive
system (fifth edition) (18). A
total of 17 patients in the NAC group were administered 50
mg/m2 S-1 on days 1–14 and 1,000 mg/m2
gemcitabine on days 8 and 15 for two 21-day cycles (GS). A total of
29 patients in the NAC group were administered 75 mg/m2
nab-paclitaxel, followed by 1,000 mg/m2 gemcitabine on
days 1, 8 and 15 for two 28-day cycles (GnP). For patients who
received GS, the dosing period was 2–10 cycles. For patients who
received GnP, the dosing period was 2–15 cycles (Tables SI and SII). The dosing periods were clinically
determined based on preoperative stage and patient condition. At
our institution, the usual dosing period for patients with
resectable pancreatic cancer is two cycles of GS or GnP. In cases
of locally advanced borderline pancreatic cancer, the dosing period
was extended until surgical factors that may complicate radical
resection, such as portal vein invasion or superior mesenteric
nerve plexus invasion, were resolved. The clinicopathological
characteristics of all patients, including age, sex, tumor location
and dosing period of NAC, are summarized in Table I.
 | Table I.Clinicopathological characteristics
of NAC-treated group and NAC-untreated group. |
Table I.
Clinicopathological characteristics
of NAC-treated group and NAC-untreated group.
| Parameter | NAC-treated
group | Non-NAC group | P-value |
|---|
| Number of
patients | 46 | 50 |
|
| Age, years |
|
| 0.237 |
|
≤65 | 20 | 22 |
|
|
>65 | 26 | 48 |
|
| Sex |
|
| 0.683 |
|
Male | 23 | 22 |
|
|
Female | 23 | 28 |
|
| Location, n
(%) |
|
| 0.543 |
|
Head | 27 (58.7) | 26 (52.0) |
|
| Body or
tail | 19 (41.3) | 24 (48.0) |
|
| Histological
differentiation, n (%) |
|
| 0.772 |
|
Well | 3 (6.5) | 5 (10.0) |
|
|
Moderate | 39 (84.8) | 42 (84.0) |
|
|
Poor | 4 (8.7) | 3 (6.0) |
|
| Pathological T
stage, n (%) |
|
| 0.758 |
| T1 | 17 (37.0) | 16 (32.0) |
|
| T2 | 27 (58.7) | 30 (60.0) |
|
| T3 | 2 (4.3) | 4 (8.0) |
|
| Pathological N
stage, n (%) |
|
| 0.575 |
| N0 | 24 (52.2) | 32 (64.0) |
|
| N1 | 19 (41.3) | 16 (32.0) |
|
| N2 | 3 (6.5) | 2 (4.0) |
|
| Clinical M stage, n
(%) |
|
| 1.000 |
| M0 | 46 (100) | 50 (100) |
|
| M1 | 0 (0) | 0 (0) |
|
| TNM stage, n
(%) |
|
| 0.341 |
| Stage
I | 16 (34.8) | 23 (46.0) |
|
| Stage
II | 21 (45.7) | 22 (44.0) |
|
| Stage
III | 9 (19.5) | 5 (10.0) |
|
| Stage
IV | 0 (0) | 0 (0) |
|
| NAC, n (%) |
|
| 1.000 |
| GS | 17 (37.1) | 0 (0) |
|
|
GnP | 29 (62.9) | 0 (0) |
|
| Dosing period
cycles |
|
|
|
| GS | 2-10 | 0 |
|
|
GnP | 2-15 | 0 |
|
Method of determination of
histological therapeutic effect
For the present study, the area of residual tumor
(ART) grading system was adopted as the method of determination of
histological therapeutic effect of NAC. The ART grading system was
adopted because Matsuda et al (13) reported that this system was the most
prognostic method for the determination of the histological
therapeutic effects of NAC for PDAC, whereas other grading systems,
such as the College of American Pathologists, Evans and MD Anderson
Cancer Center grading systems, did not demonstrate significant
associations with patient outcomes. In the present study, the
largest cross-section of tumor tissue stained with hematoxylin and
eosin (H&E) was evaluated in the NAC group according to the ART
grading system using a BX53 light microscope (Olympus Corporation)
with a 10× eyepiece and a 4× objective lens (UPlanSApo 4×; Olympus
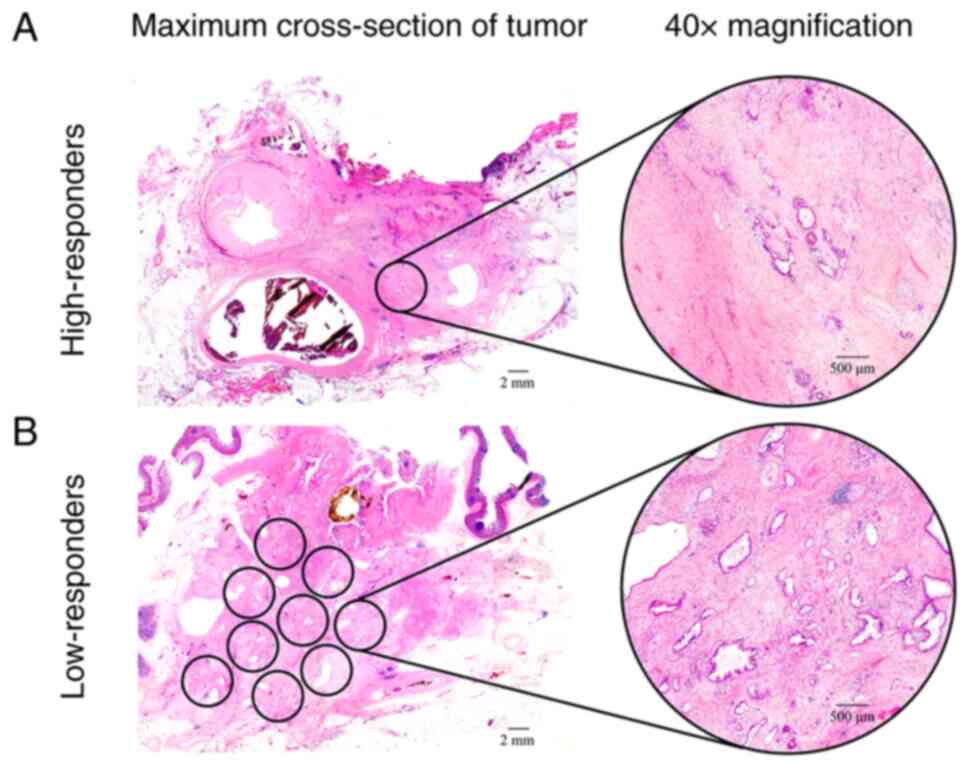
Corporation). High- and low-responders were defined as patients in
whom the ART was less than or equal to three 40× microscopic
fields, and greater than three 40× microscopic fields,
respectively. Treatments for non-viable cancer cells and
histological changes following NAC, including fibrosis, macrophage
aggregates, vascular degeneration and acellular mucous pools, were
adjusted in accordance with the criteria of the ART grading system.
Notably, histological evaluation was performed by expert
pathologists specializing in pancreatobiliary pathology (TY and
HK). Furthermore, all histological evaluations were performed while
these pathologists were blinded to the clinical information.
Surgical specimens
The surgical specimens of the NAC and non-NAC groups
were examined. In our hospital, we routinely sample whole PDAC
tissues for pathological diagnosis. In the NAC group, 27 patients
underwent pancreatoduodenectomy and 19 patients underwent
pancreatosplenectomy. In the non-NAC group, 26 patients underwent
pancreatoduodenectomy and 24 patients underwent
pancreatosplenectomy. No patient underwent total pancreatectomy.
Surgical specimens from pancreatoduodenectomy were sliced axially,
whereas surgical specimens from pancreatosplenectomy were sliced at
right angles to the main pancreatic duct. All surgical specimens
were fixed with 10% formalin at 24°C for 24 h. Tissues were
embedded in paraffin and sliced to a thickness of 4 µm for H&E
staining and immunohistochemistry. H&E staining was performed
using Mayer's hematoxylin for 20 min and with eosin for 5 min.
Histological image analysis
The histological characteristics of the NAC and
non-NAC groups were investigated. The densities of cancer cells,
CAFs, microvessels and stromal collagen fibers in the whole areas
of the tumor were measured in a way similar to that described in
our previous study (16). In the
non-NAC group, the entire maximum cross-section of the tumor was
examined. In the NAC group, ARTs, including viable cancer cells,
were examined. Maximum cross-sections of tumor were divided into
40× microscopic fields using a BX53 light microscope with a 10×
eyepiece and a 4× objective lens (UPlanSApo 4×) and a DP74 digital
camera (Olympus Corporation). Whole images of the largest
cross-sections of tumors with immunohistochemistry and Masson's
trichrome staining were captured using CellSens software (version
2.3 64-bit; Olympus Corporation).
In the present study, ImageJ software [Java 1.6.0_24
(64-bit); National Institutes of Health] was used to analyze the
histological images following immunohistochemistry and Masson's
trichrome staining. The area of cytokeratin AE1/AE3, αSMA and
CD31-positive components was measured in terms of the pixel number
using thresholds with minimum and maximum values of 0 and 120,
respectively. Finally, the average area ratios of positive
components in the whole largest cross-sections of tumors were
measured as the densities of cancer cells, CAFs, microvessels and
stromal collagen fibers.
Immunostaining and Masson's trichrome
staining
To measure the densities of cancer cells, CAFs,
microvessels and stromal collagen fibers, immunohistochemistry
(cytokeratin AE1/AE3, αSMA and CD31) and Masson's trichrome
staining were performed on the slides of the maximum cross-section
of the tumor. Cytokeratin AE1/AE3 is positive in cancer cells and
nontumoral epithelial cells. αSMA is used as a general marker of
CAFs in numerous types of human cancer (19–21).
CD31 is positive in vascular endothelial cells of tumoral and
nontumoral vessels (22). In the
present study, Masson's trichrome staining was used to measure the
density of stromal collagen fibers because it provides better
visualization than immunohistochemistry (23).
Tissue slides were deparaffinized using the
avidin-biotin-peroxidase complex method with Benchmark XT
autoimmunostainer (Roche Tissue Diagnostics; Roche Diagnostics,
Ltd.). Deparaffinized slides were treated with tris-EDTA buffer (pH
7.8) at 95°C for 44 min. The slides were then treated with 5%
non-fat dry milk at 37°C for 15 min to block endogenous peroxides
and proteins. Subsequently, the slides were incubated with primary
antibodies for 60 min at 24°C. The clone numbers and dilution
ratios of the primary antibodies were as follows: Cytokeratin
AE1/AE3 (monoclonal mouse; clone AE1, AE3; 1:100; cat. no. 412811;
Nichirei Bioscience Inc.), αSMA (monoclonal mouse; clone 1A4; cat.
no. M0851; 1:100; Dako; Agilent Technologies, Inc.) and CD31
(monoclonal mouse; clone JC70A; cat. no. M0823; 1:40; Dako; Agilent
Technologies, Inc.). All reaction products of primary antibodies
were visualized using the iVIEW DAB detection kit with a
biotin-conjugated goat anti-mouse immunoglobulin G secondary
antibody (1:1,000; cat. no. 760-091; Roche Tissue Diagnostics;
Roche Diagnostics, Ltd.). Slides were incubated with the secondary
antibody at 37°C for 1 h. Subsequently, counterstaining with
hematoxylin at 37°C for 8 min was performed and staining was
observed under an optical microscope.
For Masson's trichrome staining, tissue slides were
deparaffinized and rehydrated in 100, 95 and 70% alcohol. After
washing in distilled water, the slides were stained in Weigert's
iron hematoxylin working solution for 10 min. After further washing
in distilled water, slides were stained in 1% Biebrich scarlet-acid
fuchsin solution for 15 min. Slides were then differentiated in 5%
phosphomolybdic-phosphotungstic acid solution after washing in
distilled water. Subsequently, slides were directly stained in
aniline blue solution for 10 min without rinsing. After washing in
distilled water, slides were differentiated in 1% acetic acid
solution for 5 min. After further washing in distilled water,
slides were dehydrated quickly in 95% ethanol and cleared in
xylene. Masson's trichrome staining was performed at 24°C and
staining was observed under an optical microscope.
Radiological imaging analysis
All of the patients in the NAC group underwent
dynamic CECT before and after NAC using the fixed protocol at
Hirosaki University Hospital. The protocol was the same as that
described in our previous study (16). Dynamic CECT was conducted using a
64-detector row CT scanner (Discovery CT750 HD; GE Healthcare) with
the following parameters: Detector configuration of 64×0.625, tube
voltage of 120 kV, automatic tube current modulation, collimation
of 40 mm, tube rotation time of 0.5 sec, pitch of 0.8, field of
view of 35×35 cm, image matrix of 512×512, and slice thickness of 5
mm. After obtaining unenhanced images, a non-ionic contrast medium
dose of 600 mgI/kg body weight with an iodine content of 300 mgI/ml
(Iopamiron 300/370, Bayer Yakuhin Ltd.; Omnipaque 300,
Daiichi-Sankyo Co. Ltd.; Iopromide 300/370, Fujifilm Toyama
Chemical Co., Ltd.; Iomelon 350, Eisai Co. Ltd.; Optiray 320, Fuji
Pharma Co., Ltd.) was injected intravenously within 30 sec, and
scanning of the arterial, portal venous and equilibrium phases
began 35–40, 60–70 and 180 sec after the start of contrast medium
injection.
TDCs were drawn using Digital Imaging and
Communications in Medicine data [EV insite R version 3.4.0.0 (PSP
Corporation); or ShadeQuest/ViewR V1.24 (Yokogawa Medical
Solutions)]. The method used to draw regions of interest (ROIs) in
our previous study was used in the present study (16). The ROIs were drawn on the central
areas of tumors, avoiding tumor margins, contrasted vessels and
artificial materials, such as stents. Tumor margins were avoided in
ROIs because histological analysis excluded tumor margins since
they included a number of nontumoral components. After measuring
the number of ROIs on CT for each time phase, the digital imaging
software created TDCs before and after NAC. At our institution, all
patients with PDAC underwent dynamic CECT via a fixed protocol to
limit the influence of patient-related factors, such as body
weight, and cardiac and renal function, which can affect TDCs.
Additionally, concentrations of contrast agents and their
administration rates were adjusted according to patient body
weight, and cardiac and renal function. However, all
patient-related factors could not be completely controlled.
Therefore, in the present study, the slopes of the TDC were adopted
as parameters of radiological images to minimize error factors
because we considered that the changes in the rates of contrast
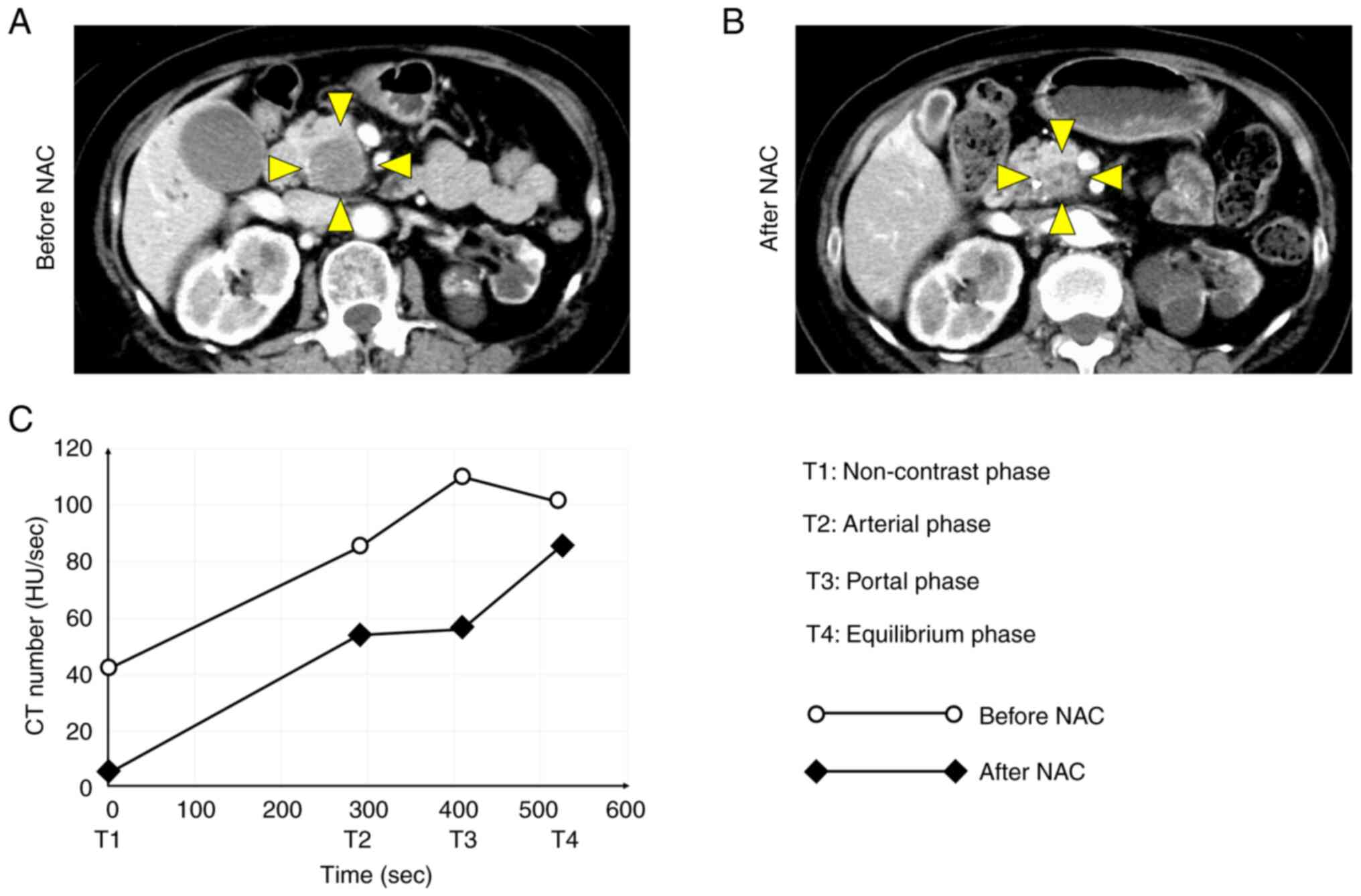
enhancement were more appropriate than individual CT values. δ1 was
defined as the first slope between the non-contrast and arterial
phases before NAC, δ2 was defined as the second slope between the
arterial and portal phases before NAC, and δ3 was defined as the
third slope between the portal and equilibrium phases before NAC.
δ1′ was defined as the first slope after NAC, δ2′ was defined as
the second slope after NAC, and δ3′ was defined as the third slope
after NAC. δδ1 was defined as δ1′ minus δ1, δδ2 was defined as δ2′
minus δ2, and δδ3 was defined as δ3′ minus δ3. CT number [in
Hounsfield unit (HU)] was plotted on the vertical axis of the TDC,
and time phase was plotted on the horizontal axis of the TDC.
Statistical analysis
First, comparisons of the densities of cancer cells,
CAFs, microvessels and stromal collagen fibers between the non-NAC
group, low-responders and high-responders were examined using the
Kruskal-Wallis test, followed by the Steel-Dwass post hoc test.
Second, the differences between δ1 and δ1′, δ2 and δ2′, and δ3 and
δ3′ in low-responders and high-responders were analyzed using
Wilcoxon test. Third, differences in δδ1, δδ2 and δδ3 between
low-responders and high-responders were analyzed using Mann-Whitney
U-test, which was also used for comparison of CAFs and
microvessels, A receiver operating characteristic curve was drawn
to calculate the cutoff value of radiological parameters between
low- and high-responders. Furthermore, Kaplan-Meier curves and
log-rank test were used to analyze recurrence-free survival
according to the ART grading system and radiological parameters.
Contingency tables were analyzed using Fisher's exact test.
All statistical analyses were performed using EZR
version 1.54 (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), which is a modified version of R commander
designed to add statistical functions frequently used in
biostatistics (24).
Results
Clinicopathological characteristics of
the NAC group
The ART grading system classified 29 patients as
low-responders and 17 patients as high-responders. The
clinicopathological characteristics of the NAC group are summarized
in Table II using Pearson's
chi-square test.
 | Table II.Clinicopathological characteristics
of NAC group. |
Table II.
Clinicopathological characteristics
of NAC group.
| Parameter | Low-responder | High-responder | P-value |
|---|
| Number of
patients | 29 | 17 |
|
| Age, years |
|
| 0.533 |
|
≤65 | 10 | 8 |
|
|
>65 | 19 | 9 |
|
| Sex |
|
| 0.542 |
|
Male | 16 | 7 |
|
|
Female | 13 | 10 |
|
| Location, n
(%) |
|
| 0.028 |
|
Head | 21 (72.4) | 6 (35.3) |
|
| Body or
tail | 8 (27.6) | 11 (64.7) |
|
| Histological
differentiation, n (%) |
|
| 0.193 |
|
Well | 1 (3.4) | 2 (11.8) |
|
|
Moderate | 24 (82.8) | 15 (88.2) |
|
|
Poor | 4 (13.8) | 0 (0) |
|
| Pathological T
stage, n (%) |
|
| 0.498 |
| T1 | 9 (31.0) | 8 (47.1) |
|
| T2 | 18 (62.1) | 9 (52.9) |
|
| T3 | 2 (6.9) | 0 (0) |
|
| Pathological N
stage, n (%) |
|
| 0.504 |
| N0 | 16 (55.2) | 8 (47.1) |
|
| N1 | 10 (34.5) | 9 (52.9) |
|
| N2 | 3 (10.3) | 0 (0) |
|
| Clinical M stage, n
(%) |
|
| 1.000 |
| M0 | 29 (100) | 17 (100) |
|
| M1 | 0 (0) | 0 (0) |
|
| TNM stage, n
(%) |
|
|
|
| Stage
I | 9 (31.0) | 7 (41.2) | 0.245 |
| Stage
II | 12 (41.4) | 9 (52.9) |
|
| Stage
III | 8 (27.6) | 1 (5.9) |
|
| Stage
IV | 0 (0) | 0 (0) |
|
| NAC, n (%) |
|
| 1.000 |
| GS | 11 (37.9) | 6 (35.3) |
|
|
GnP | 18 (62.1) | 11 (64.7) |
|
| Dosing period,
cycles |
|
|
|
| GS | 2–8 | 2–10 |
|
|
GnP | 2–12 | 2–15 |
|
Results of histological analysis
The ART grading system classified the NAC group into
high-responders and low-responders. High-responders had ARTs less
than or equal to three 40× microscopic fields. Low-responders had
ARTs greater than three 40× microscopic fields (Fig. 1).
At the time of analyzing histological images,
cytokeratin AE1/AE3 was positive in cancer and nontumoral
epithelial cells on tumor margins. Nontumoral epithelial cells were
excluded following H&E staining. Even though αSMA was positive
in CAFs and vascular smooth muscles, Mann-Whitney U-test showed
that the number of CAFs was significantly greater than the number
of vascular smooth muscles in the NAC and non-NAC groups (data not
shown). Therefore, the effect of smooth muscles on the measurement
were ignored because the number of CAFs was predominantly greater
than the number of vascular smooth muscles.
Immunohistochemical analysis revealed a marked
decrease in cancer cells (Fig. 2B and
G) and CAFs (Fig. 2C and H) in
the high-responders compared with those in the low-responders and
non-NAC group (Fig. 2).
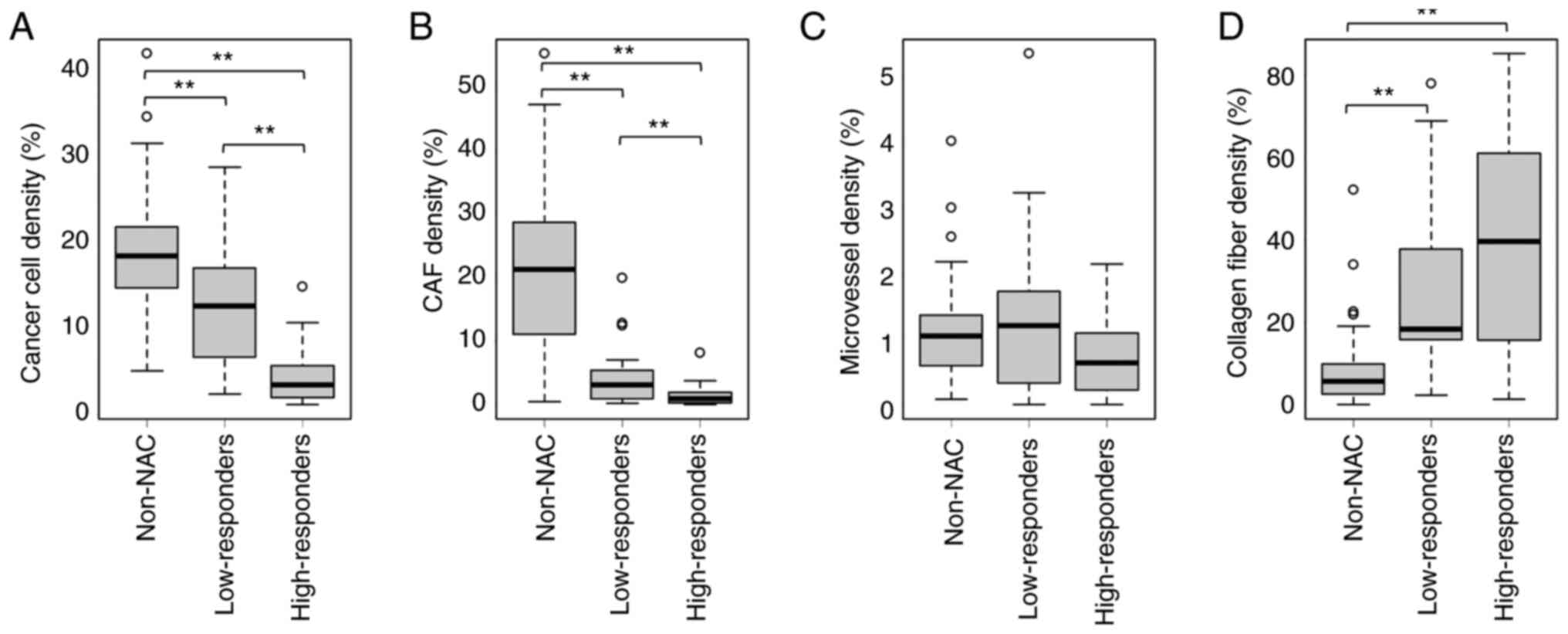
Kruskal-Wallis test revealed that the density of cancer cells was
significantly decreased in order from the non-NAC group to
low-responders to high-responders (Fig.
3A). Similarly, the density of CAFs was significantly decreased
in order from the non-NAC group to low-responders to
high-responders (Fig. 3B). There
were no significant differences in the density of microvessels
between the non-NAC group, low-responders and high-responders
(Fig. 3C). Furthermore, collagen
fiber density was significantly increased in the NAC groups
compared with that in the non-NAC group; however, there was no
significant difference in collagen fiber density between
low-responders and high-responders (Fig. 3D).
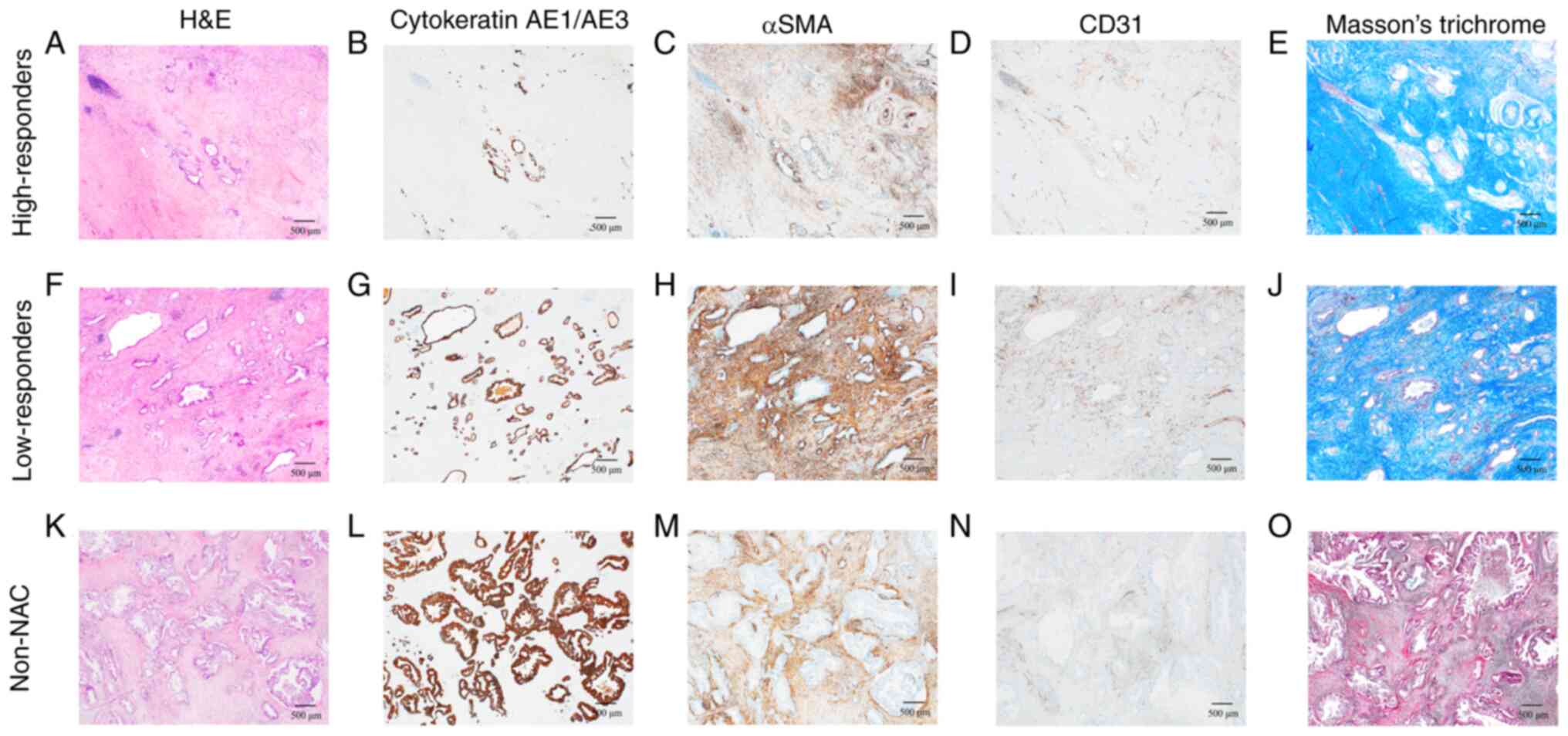 | Figure 2.Representative histological
characteristics of the high-responders, low-responders and non-NAC
group. Cytokeratin AE1/AE3 was positive in cancer cells, αSMA was
positive in CAFs, and CD31 was positive in microvessels. Masson's
trichrome stain was used for staining the collagen fibers.
High-responders exhibited a marked decrease in cancer cells and
CAFs. Low-responders showed a slight decrease in cancer cells.
High-responders' microscopic images (40× magnification) of (A)
H&E staining, (B) cytokeratin AE1/AE3, (C) αSMA, (D) CD31
immunostaining, and (E) Masson's trichrome stain. Low-responders'
microscopic images (40× magnification) of (F) H&E staining, (G)
cytokeratin AE1/AE3, (H) αSMA, (I) CD31 immunostaining, and (J)
Masson's trichrome stain. Non-NAC group's microscopic images (40×
magnification) of (K) H&E staining, (L) cytokeratin AE1/AE3,
(M) αSMA, (N) CD31 immunostaining, and (O) Masson's trichrome
stain. αSMA, α-smooth muscle actin; CAF, cancer-associated
fibroblast; H&E, hematoxylin and eosin; NAC, neoadjuvant
chemotherapy. |
Results of radiological analysis
The curve shape of the TDC was markedly altered
before and after NAC (Fig. 4). In
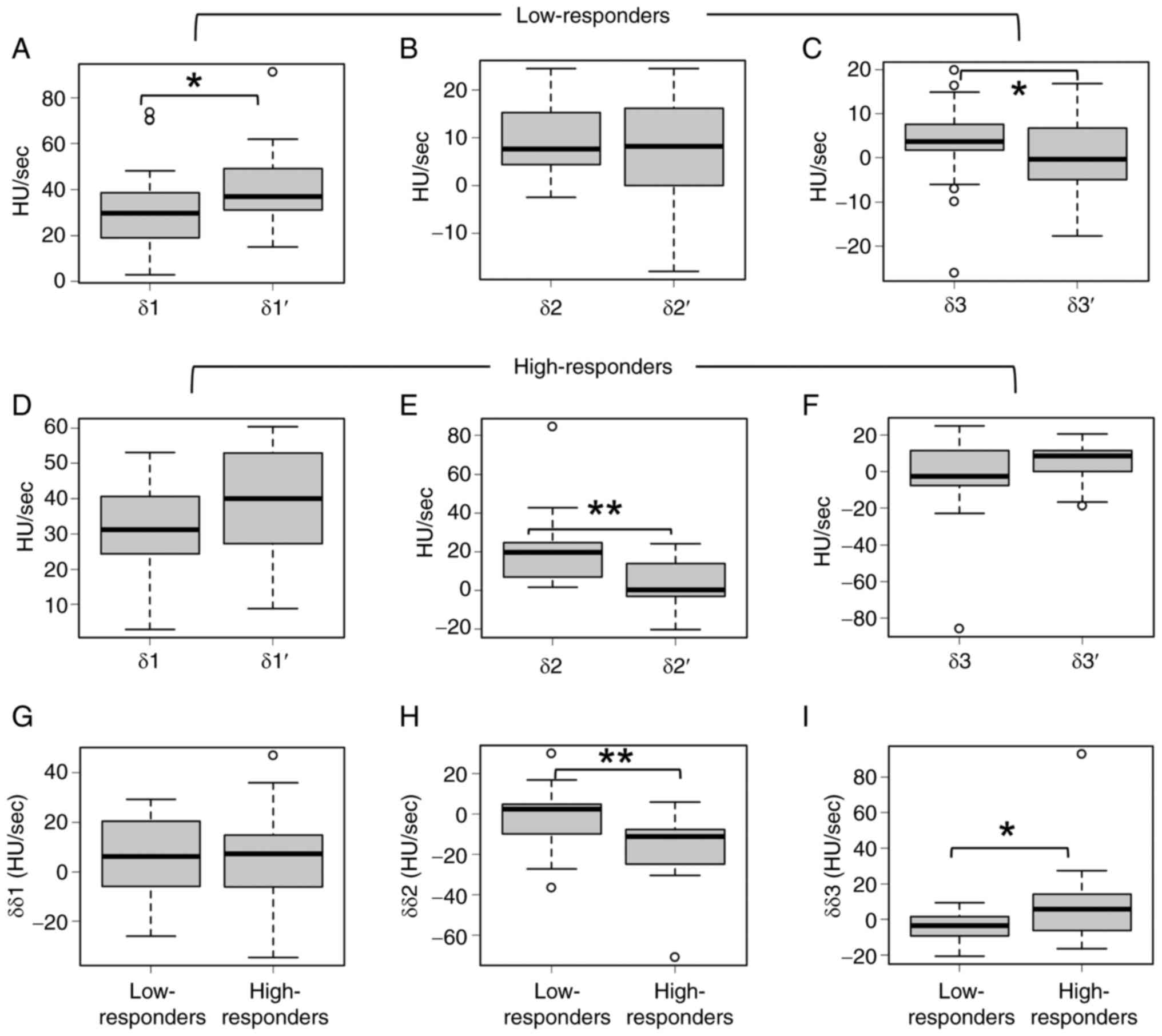
low-responders, δ1′ was significantly higher than δ1 (Fig. 5A), whereas there were no significant
differences between δ2 and δ2′ (Fig.
5B). δ3′ was significantly lower than δ3 (Fig. 5C). In high-responders, there was no
significant difference between δ1 and δ1′ (Fig. 5D), δ2′ was significantly lower than
δ2 (Fig. 5E), and there was no
significant difference between δ3 and δ3′ (Fig. 5F). No significant difference was
noted in δδ1 between low-responders and high-responders (Fig. 5G). Furthermore, δδ2 was
significantly lower and δδ3 was significantly higher in
high-responders than in low-responders (Fig. 5H and I). Receiver operating
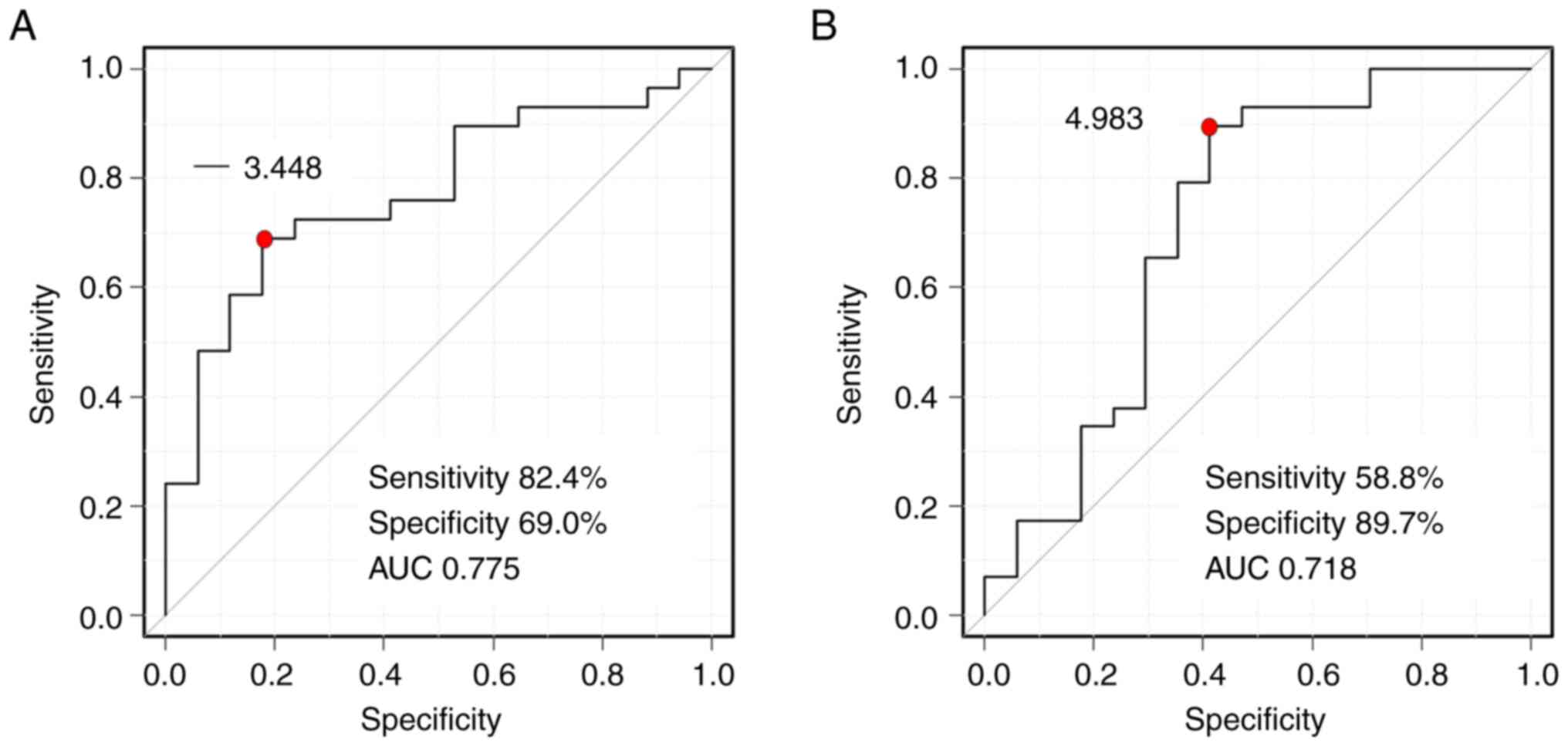
characteristic curve showed that the cutoff values between
low-responders and high-responders were δδ2=−3.448 HU/sec
[sensitivity=82.4%, specificity=69.0%, area under the curve
(AUC)=0.775] and δδ3=4.983 HU/sec (sensitivity=58.8%,
specificity=89.7%, AUC=0.718) (Fig.
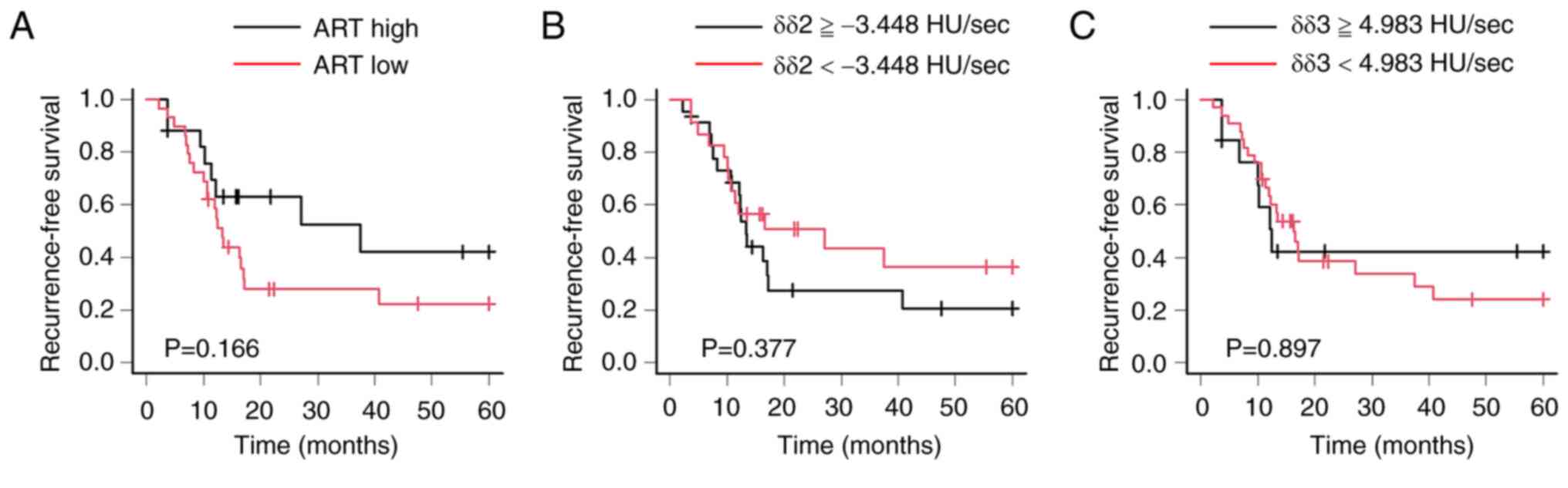
6). Regarding the ART grading system, there was no prognostic
significant difference between high- and low-responders (P=0.166).
In addition, there was no prognostic significant difference between
high and low δδ2; the cutoff value of δδ2 was −3.448 HU/sec.
Furthermore, there was no prognostic significant difference between
high and low δδ3; the cutoff value of δδ3 was 4.983 HU/sec
(Fig. 7).
Discussion
In the present study, the histological differences
between the NAC and non-NAC groups, and the association between the
TDC and histological therapeutic effects were investigated.
Histological examination revealed that NAC effectively reduced the
densities of cancer cells and CAFs, and significantly increased the
density of stromal collagen fibers. Radiological and histological
examinations suggested that δδ2 and δδ3 were associated with a high
histological therapeutic effect based on the ART grading system.
Furthermore, the present study suggested the potential use of TDCs
for the prediction of the histological therapeutic effect of NAC
for PDACs.
Comparison of the histological characteristics of
the NAC and non-NAC groups revealed that NAC reduced the densities
of cancer cells and CAFs, and increased the density of stromal
collagen fibers. In the present study, >50% of the NAC group
consisted of patients who underwent NAC with GnP. Miyashita et
al reported that NAC with GnP reduced the density of CAFs in
patients with PDAC, thereby depleting the tumor stroma (25). Although this study did not focus on
NAC with GnP, it is possible that the strong resistance of tumor
stroma against GnP is associated with the reduction in the density
of CAFs, given that patients who underwent NAC with GnP made up 63%
of the NAC group. Furthermore, an overwhelming proliferation of
stromal collagen fibers was observed in the NAC group. Tissues
exposed to chemotherapy undergo a wound-healing process that
involves deposition of extracellular matrix, which includes various
types of collagen fibers (26). An
association between microvessel density and NAC was not revealed in
the present study. It was reported in several previous studies that
treatment with NAC and anti-vascular endothelial growth factor
drugs, such as bevacizumab, can reduce microvessel density in
primary and metastasized rectal cancer (27,28).
However, to the best of our knowledge, there are no studies on PDAC
that investigated the association between NAC and microvessel
density.
Comparison of TDCs before and after NAC revealed
that δ1′ was significantly higher than δ1 in low-responders. In our
previous study, it was revealed that the first slope of TDCs is
associated with microvessel density (16). Even though there were no significant
differences in microvessel density between low-responders and
high-responders, tumor angiogenesis may have involved the ascent of
the first slope of TDC in low-responders. In high-responders, δ2′
was significantly lower than δ2. Moreover, δδ2 was significantly
lower and δδ3 was significantly higher in high-responders compared
with that in low-responders. In our previous study, it was reported
that the second slope of TDC (δ2) is associated with tumor
cellularity, including the densities of cancer cells and CAFs
(16). The lower δδ2 and the higher
δδ3 in high-responders compared with low-responders may be because
abundant collagen fibers replace cancer cells and CAFs in stromal
areas. Non-ionic contrast agents are typically used for the
diagnosis of pancreatic cancer. They do not distribute into the
cytoplasm of cancer cells and CAFs, but they do distribute into the
extracellular matrix (29). In
general, they leak from microvessels into the intracellular matrix
of tumors between the arterial and portal phases. Stromal collagen
fiber proliferation may have strongly suppressed the leak of
contrast agents from microvessels into intracellular matrix,
thereby lowering the δδ2. Contrast agents are generally resorbed
into microvessels across a concentration gradient between the
portal and equilibrium phases. Furthermore, stromal collagen fiber
proliferation may have strongly suppressed the resorption of
contrast agents from intracellular matrix into microvessels,
leading to an increased δδ3. It was hypothesized that the decrease
in the second slope and the increase in the third slope in
high-responders are not independent phenomena but are sequential
phenomena associated with NAC-induced stromal collagen fiber
proliferation.
The predictive value of tumor markers (such as
CA19-9), PET-CT, perfusion CT and CECT have been reported when
predicting treatment response to NAC for PDAC in previous studies
(30–33). Although the predictive values were
interesting, the sample sizes of the previous studies were smaller
than that of the present study. Additionally, some studies lacked a
detailed description of the histological effects following therapy.
A previous study used the Evans grading system to evaluate the
histological response to NAC. Although this system is considered a
standard method, it is ambiguous (11). Furthermore, as it specifies a
percentage of tumor cell viability or destruction, it is difficult
to determine the viability of degenerative tumor cells (13). In the present study, the ART grading
system and simpler criteria were adopted, which enabled the
detection of cases with an excellent response to NAC. In addition
to the advantages of using the ART grading system, the present
study investigated the histological characteristics of PDAC
with/without NAC using immunohistochemistry. It was revealed that
NAC-induced intense changes in PDAC stroma, because the NAC groups
showed a decrease in CAFs and increase in collagen fibers in the
cancer stroma compared with in the non-NAC group. However, it is
difficult to confirm the stromal changes that affected the TDC. The
results of the present study suggest an association between the
changes in TDC and histological changes after NAC by performing
radiological and histological analyses.
The present study has some limitations. First,
although it was suggested that the proliferation of stromal
collagen fibers affected δδ2 and δδ3, there were no significant
differences in collagen fiber density between the low-responders
and high-responders. This limitation may be due to non-specific
chemotherapy-induced fibrosis, and the presence of various types of
collagen fibers, such as types I, II, III, V, VI, XI, XXIV and
XXVII (34). Collagen fiber
subtypes cannot be identified using Masson's trichrome staining. In
the present study, to prioritize visualization, Masson's trichrome
staining, not immunostaining, was used (23). Second, there was some concern that
necrotic, fibrous and severe inflammatory cancer tissue would
affect the results of TDC. In the present study, it was difficult
to visualize the cancer microenvironment using CT images, which is
a technical limitation. It may be hypothesized that a novel
radiological technique, such as dual-energy CT, would solve this
problem, and this may be a topic of future research. Third, there
were no prognostic significant differences between the ART grading
system, δδ2 and δδ3 in recurrence-free survival. This may be
attributed to the impacts of drug types and dosing periods that
were not considered in the present study because of the limited
number of patients. Studies with larger sample sizes should be
conducted in the future to minimize these limitations.
In conclusion, the histological differences in PDAC
between the NAC and non-NAC groups were identified, and the use of
TDCs of dynamic CECT for the prediction of the histological
therapeutic effects of NAC was suggested. However, it remains
difficult to draw firm conclusions because the present study lacked
a larger cohort. Novel research and radiological techniques, and a
large cohort may allow for the integration of radiological and
histological analyses in future research.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the following
research assistants: Ms. Misaki Ishiyama (Hirosaki University
School of Medicine), Ms. Shizuka Fujio (Hirosaki University School
of Medicine), Ms. Yuri Nakano (Hirosaki University School of
Medicine) and Mr. Yuya Takami (Hirosaki University School of
Medicine).
Funding
The present study was supported by JSPS KAKENHI (grant no.
JP19K16763).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG and TY designed the experiments. SG performed the
experiments and data analysis, wrote the main manuscript and
prepared the figures. HS evaluated the radiological images. SM and
HK contributed to histological evaluation. HO, SK, KO, AN, KI and
KH provided clinical information and interpreted clinical data. TY
and HK confirm the authenticity of all the raw data. All authors
read and approved the final manuscript, and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The research protocol was approved by the ethics
committee of Hirosaki University (approval no. 2022-035). All study
procedures involving human participants were performed in
accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki Declaration
and its later amendments or comparable ethical standards. Written
informed consent for the use of clinical records and pathological
specimens was obtained from each patient before commencement of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
TDC
|
time-density curve
|
|
CECT
|
contrast-enhanced computed
tomography
|
|
NAC
|
neoadjuvant chemotherapy
|
|
CAFs
|
cancer-associated fibroblasts
|
|
αSMA
|
α smooth muscle actin
|
|
ART
|
area of residual tumor
|
References
|
1
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whatcott CJ, Diep CH, Jiang P, Watanabe A,
LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD and Han H:
Desmoplasia in primary tumors and metastatic lesions of pancreatic
cancer. Clin Cancer Res. 21:3561–3568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neesse A, Bauer CA, Öhlund D, Lauth M,
Buchholz M, Michl P, Tuveson DA and Gress TM: Stromal biology and
therapy in pancreatic cancer: Ready for clinical translation? Gut.
68:159–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oba A, Ho F, Bao QR, Al-Musawi MH,
Schulick RD and Del Chiaro M: Neoadjuvant treatment in pancreatic
cancer. Front Oncol. 10:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motoi F and Unno M: Adjuvant and
neoadjuvant treatment for pancreatic adenocarcinoma. Jpn J Clin
Oncol. 50:483–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Versteijne E, van Dam JL, Suker M, Janssen
QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA,
Buijsen J, Busch OR, et al: Neoadjuvant chemoradiotherapy versus
upfront surgery for resectable and borderline resectable pancreatic
cancer: Long-term results of the Dutch randomized PREOPANC trial. J
Clin Oncol. 40:1220–1230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldstein D, El-Maraghi RH, Hammel P,
Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S,
Tabernero J, Teixeira L, et al: nab-Paclitaxel plus gemcitabine for
metastatic pancreatic cancer: Long-term survival from a phase III
trial. J Natl Cancer Inst. 107:dju4132015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Welsh JL, Bodeker K, Fallon E, Bhatia SK,
Buatti JM and Cullen JJ: Comparison of response evaluation criteria
in solid tumors with volumetric measurements for estimation of
tumor burden in pancreatic adenocarcinoma and hepatocellular
carcinoma. Am J Surg. 204:580–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chatterjee D, Katz MH, Rashid A, Wang H,
Iuga AC, Varadhachary GR, Wolff RA, Lee JE, Pisters PW, Crane CH,
et al: Perineural and intraneural invasion in posttherapy
pancreaticoduodenectomy specimens predicts poor prognosis in
patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol.
36:409–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SM, Katz MH, Liu L, Sundar M, Wang H,
Varadhachary GR, Wolff RA, Lee JE, Maitra A, Fleming JB, et al:
Validation of a proposed tumor regression grading scheme for
pancreatic ductal adenocarcinoma after neoadjuvant therapy as a
prognostic indicator for survival. Am J Surg Pathol. 40:1653–1660.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalimuthu SN, Serra S, Dhani N,
Hafezi-Bakhtiari S, Szentgyorgyi E, Vajpeyi R and Chetty R:
Regression grading in neoadjuvant treated pancreatic cancer: An
interobserver study. J Clin Pathol. 70:237–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda Y, Ohkubo S, Nakano-Narusawa Y,
Fukumura Y, Hirabayashi K, Yamaguchi H, Sahara Y, Kawanishi A,
Takahashi S, Arai T, et al: Objective assessment of tumor
regression in post-neoadjuvant therapy resections for pancreatic
ductal adenocarcinoma: Comparison of multiple tumor regression
grading systems. Sci Rep. 10:182782020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrone CR, Marchegiani G, Hong TS, Ryan
DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN,
Blaszkowsky LS, et al: Radiological and surgical implications of
neoadjuvant treatment with FOLFIRINOX for locally advanced and
borderline resectable pancreatic cancer. Ann Surg. 261:12–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagner M, Antunes C, Pietrasz D,
Cassinotto C, Zappa M, Sa Cunha A, Lucidarme O and Bachet JB: CT
evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline
and locally advanced pancreatic adenocarcinoma. Eur Radiol.
27:3104–3116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goto S, Seino H, Yoshizawa T, Morohashi S,
Ishido K, Hakamada K and Kijima H: Time density curve of dynamic
contrast-enhanced computed tomography correlates with histological
characteristics of pancreatic cancer. Oncol Lett. 21:2762021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brierley JD, Gospodarowicz MK and
Wittekind C: The TNM classification of malignant tumours. 8th
edition. Wiley-Blackwell; Oxford: pp. 93–95. 2017
|
|
18
|
WHO Classification of Tumours Editorial
Board, . WHO classification of tumours of the digestive system.
IARC Press; Lyon: pp. 322–332. 2019
|
|
19
|
Inoue C, Miki Y, Saito R, Hata S, Abe J,
Sato I, Okada Y and Sasano H: PD-L1 induction by cancer-associated
fibroblast-derived factors in lung adenocarcinoma cells. Cancers
(Basel). 11:12572019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itou RA, Uyama N, Hirota S, Kawada N, Wu
S, Miyashita S, Nakamura I, Suzumura K, Sueoka H, Okada T, et al:
Immunohistochemical characterization of cancer-associated
fibroblasts at the primary sites and in the metastatic lymph nodes
of human intrahepatic cholangiocarcinoma. Hum Pathol. 83:77–89.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Li S, Zhao Y, Ma P, Cao Y, Liu C,
Zhang X, Wang W, Chen L and Li Y: Cancer-associated fibroblasts
promote the migration and invasion of gastric cancer cells via
activating IL-17a/JAK2/STAT3 signaling. Ann Transl Med. 8:8772020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuda K, Ohga N, Hida Y, Muraki C,
Tsuchiya K, Kurosu T, Akino T, Shih SC, Totsuka Y, Klagsbrun M, et
al: Isolated tumor endothelial cells maintain specific character
during long-term culture. Biochem Biophys Res Commun. 394:947–954.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calvi EN, Nahas FX, Barbosa MV, Calil JA,
Ihara SS, Silva Mde S, Franco MF and Ferreira LM: An experimental
model for the study of collagen fibers in skeletal muscle. Acta Cir
Bras. 27:681–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyashita T, Tajima H, Makino I, Okazaki
M, Yamaguchi T, Ohbatake Y, Nakanuma S, Hayashi H, Takamura H,
Ninomiya I, et al: Neoadjuvant chemotherapy with gemcitabine plus
nab-paclitaxel reduces the number of cancer-associated fibroblasts
through depletion of pancreatic stroma. Anticancer Res. 38:337–343.
2018.PubMed/NCBI
|
|
26
|
Mancini ML and Sonis ST: Mechanisms of
cellular fibrosis associated with cancer regimen-related
toxicities. Front Pharmacol. 5:512014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arimoto A, Uehara K, Tsuzuki T, Aiba T,
Ebata T and Nagino M: Role of bevacizumab in neoadjuvant
chemotherapy and its influence on microvessel density in rectal
cancer. Int J Clin Oncol. 20:935–942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eefsen RL, Engelholm L, Willemoe GL, Van
den Eynden GG, Laerum OD, Christensen IJ, Rolff HC, Høyer-Hansen G,
Osterlind K, Vainer B and Illemann M: Microvessel density and
endothelial cell proliferation levels in colorectal liver
metastases from patients given neo-adjuvant cytotoxic chemotherapy
and bevacizumab. Int J Cancer. 138:1777–1784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Awai K and Date S: Basic knowledge to
achieve optimal enhancement of CT. Nichidoku Iho. 56:13–32.
2011.
|
|
30
|
Harder FN, Jungmann F, Kaissis GA, Lohöfer
FK, Ziegelmayer S, Havel D, Quante M, Reichert M, Schmid RM, Demir
IE, et al: [18F]FDG PET/MRI enables early chemotherapy response
prediction in pancreatic ductal adenocarcinoma. EJNMMI Res.
11:702021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamdy A, Ichikawa Y, Toyomasu Y, Nagata M,
Nagasawa N, Nomoto Y, Sami H and Sakuma H: Perfusion CT to assess
response to neoadjuvant chemotherapy and radiation therapy in
pancreatic ductal adenocarcinoma: Initial experience. Radiology.
292:628–635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdelrahman AM, Goenka AH, Alva-Ruiz R,
Yonkus JA, Leiting JL, Graham RP, Merrell KW, Thiels CA, Hallemeier
CL, Warner SG, et al: FDG-PET predicts neoadjuvant therapy response
and survival in borderline resectable/locally advanced pancreatic
adenocarcinoma. J Natl Compr Canc Netw. 20:1023–1032.e3. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koay EJ, Truty MJ, Cristini V, Thomas RM,
Chen R, Chatterjee D, Kang Y, Bhosale PR, Tamm EP, Crane CH, et al:
Transport properties of pancreatic cancer describe gemcitabine
delivery and response. J Clin Invest. 124:1525–1536. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Myllyharju J and Kivirikko KI: Collagens
and collagen-related diseases. Ann Med. 33:7–21. 2001. View Article : Google Scholar : PubMed/NCBI
|