Introduction
Non-Hodgkin lymphoma (NHL) is a type of cancer of
the lymphatic system that can be divided into B-, T- or natural
killer-cell NHL (1). Among the
types of NHL, B-cell NHL affects up to ~85% of all patients, and
diffuse large B-cell lymphoma (DLBCL) is the most prevalent and
aggressive subtype. Moreover, T-cell lymphoma represents only
10–15% of cases (2). Compared with
Hodgkin lymphoma (HL), NHL is more complex due to different cell
origins, diverse biological characteristics and clinical pathology.
Notably, patients with NHL have a poor prognosis (3). According to the systematic analysis
for the Global Burden of Diseases Study 2017, ~488,000 patients
were newly diagnosed with NHL (4),
and 248,000 deaths occurred worldwide (5). In 2016, it was estimated that 68,500
new patients were diagnosed with NHL and 37,600 NHL-related deaths
occurred in China (6). Treatment
options, including chemotherapeutic agents (rituximab,
cyclophosphamide, adriamycin, vincristine and prednisone), targeted
therapy, stem cell transplantation and chimeric antigen receptor
T-cell therapy, have improved the clinical outcomes and prognosis
of patients with NHL (3). However,
relapse or refractory disease leads to unsatisfactory results,
particularly in certain subtypes of lymphoma. In primary central
nervous system (CNS) lymphoma, a rare and aggressive subtype of
NHL, the blood-brain barrier (BBB) is a major obstacle for
successful clinical treatment. Therefore, further investigations
into novel therapeutic agents are required.
Artesunate (ART), a semisynthetic compound of
artemisinin, has been recommended by the World Health Organization
for the front-line treatment of malaria, particularly cerebral
malaria (7,8). Notably, ART may be a potential option
for the treatment of diseases affecting the CNS. A previous study
demonstrated that ART suppresses glioma through the regulation of
mevalonate metabolism and the promotion of cell senescence
(9). Moreover, previous studies
have revealed that ART exerts profound anticancer biological
activities in various types of solid tumors and hematological
malignancies, both in vitro and in vivo (10,11).
ART can also inhibit tumor growth via endoplasmic reticulum (ER)
stress and unfolded protein response (UPR) pathways in lymphoma
in vitro and in vivo (12). Studies have also revealed that ART
can induce ferroptosis in glioblastoma and renal cell carcinoma
cells (13,14). In addition, ART has been
administered for the treatment of numerous types of cancer in
clinical trials, including lung (15), cervical (16), breast (17) and colorectal cancer (18). These results suggested that ART
might exhibit potential as an anticancer therapy due to the
associated biological activity and tolerability.
Sorafenib (SOR) is a multikinase molecular
inhibitor, which exerts anticancer activities through inhibiting
cell proliferation, inducing apoptosis and inhibiting tumor
angiogenesis in various cancer models, including in hepatocellular
carcinoma (19), leukemia (20) and lymphoma (21). SOR has been shown to synergistically
induce apoptosis when combined with vorinostat via downregulation
of the antiapoptotic protein, myeloid cell leukemia-1 (MCL-1), in
T-cell lymphoma (22). Moreover,
SOR exerts antitumor effects on tumors of CNS (23), thus indicating that SOR, similar to
ART, may be a candidate for the treatment of CNS disorders.
The results of our previous study demonstrated that
ART could induce ferroptosis in DLBCL cells (24). Notably, ART promotes antitumor
effects via numerous mechanisms (25); however, monotherapy exhibits limited
efficacy, and combined treatment is recommended for the treatment
of lymphoma. ART exhibits potential for the treatment of cancer and
other diseases (26), including
diseases of the CNS, due to a higher concentration of ART in the
brain (27), which indicates a high
BBB permeability. SOR also exhibits potential in the treatment of
diseases of the CNS (28). Thus,
the present study aimed to investigate the combined effects of ART
and SOR, and to explore the associated mechanisms in NHL cells.
These treatments may exhibit potential as effective strategies in
the treatment of NHL, and this study may provide a novel
theoretical basis for the treatment of CNS-related diseases in the
future.
Materials and methods
Reagents
ART (cat. no. MB7316), SOR (cat. no. MB1666), and
Cell Counting Kit-8 (CCK-8; cat. no. MA0218) were purchased from
Dalian Meilun Biology Technology Co., Ltd. Erastin (Era; cat. no.
HY-15763), ferrostatin-1 (Fer; cat. no. HY-100579) and rapamycin
(Rapa; cat. no. HY-10219) were purchased from MedChemExpress.
Monodansylcadaverine (MDC; cat.no. 30432) was purchased from
Sigma-Aldrich; Merck KGaA. BODIPY-C11 581/591 (cat. no. RM02821)
was purchased from ABclonal Biotech Co., Ltd. According to the
manufacturers' protocols, products (ART, SOR, Era, Fer, Rapa, MDC
and BODIPY-C11) were dissolved in DMSO and stored at −20°C.
Plasmids (pLKO.1-Neo, cat. no. SHC001, originally from
Sigma-Aldrich; Merck KGaA; psPAX2, cat. no. 12260, originally from
Addgene, Inc.; and pMD2.G, cat. no. 12259, originally from Addgene,
Inc.) were obtained from the Institute of Hematology, West China
Hospital, Sichuan University (Chengdu, China). The short hairpin
RNAs (shRNAs) [shRNA (sh)-signal transducer and activator of
transcription 3 (STAT3),
5′-CCGGGCACAATCTACGAAGAATCAACTCGAGTTGATTCTTCGTAGATTGTGCTTTTTG-3′;
non-targeting negative control,
5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTTG-3′]
were synthesized by TsingKe Biological Technology.
Cell culture
Human lymphoma cell lines (U2932, cat. no. ACC 633;
SU-DHL4, cat. no. CRL-2957; SU-DHL6, cat. no. CRL-2959; and Jurkat,
cat. no. CRL-2898), a mouse lymphoma cell line (EL4, cat. no.
TIB-39) and 293T (cat. no. CRL-11268) cells were obtained from the
Institute of Hematology, West China Hospital, Sichuan University,
and provided by Professor Yongqian Jia. U2932 was originally from
the Leibniz Institute DSMZ and the other cell lines were originally
from the ATCC. U2932, SU-DHL4 and SU-DHL6 cells were cultured in
RPMI-1640 medium (cat. no. SH30809; HyClone; Cytiva) supplemented
with 20% FBS (cat. no. 900-108; Gemini Bio Products); Jurkat and
EL4 cells were cultured in RPMI-1640 medium supplemented with 10%
FBS; and 293T cells were cultured in DMEM/high glucose medium (cat.
no. SH30243.01; HyClone; Cytiva) supplemented with 10% FBS. All
cells were treated with 1% penicillin/streptomycin (cat. no.
SV30010; HyClone; Cytiva) and maintained at 37°C in an atmosphere
containing 5% CO2. U2932, SU-DHL4 and SU-DHL6 are B-cell
lymphoma cell lines, and Jurkat is T-cell lymphoma cell line. Not
all lymphoma cell lines were used in each experiment.
Antibodies
The primary antibodies used were as follows:
Anti-cleaved poly (ADP-ribose) polymerase (PARP; cat. no. A5034;
1:1,000), anti-p62 (cat. no. A5180; 1:1,000), anti-LC3B-I/II (cat.
no. A5202; 1:1,000), anti-ERK1/2 (ERK; cat. no. A5029; 1:1,000),
anti-phosphorylated (p)-ERK (cat. no. A5036; 1:1,000), anti-MEK1/2
(MEK; cat. no. A5606; 1:1,000), anti-p-MEK (cat. no. A5191;
1:1,000), anti-glutathione peroxidase 4 (GPX4; cat. no. A5569;
1:1,000), anti-ATG5 (cat. no. A5745; 1:1,000) and anti-ferritin
heavy chain 1 (FTH1; cat. no. A5654; 1:1,000) (all from Bimake). In
addition, anti-STAT3 (cat. no. 4904; 1:2,000), anti-p-STAT3
(Tyr727; cat. no. 9134; 1:1000), anti-AKT (cat. no. 2938; 1:1,000),
anti-p-AKT (cat. no. 9018; 1:1,000) and anti-Bcl-XL (cat. no. 2764;
1:1,000) were purchased from Cell Signaling Technology, Inc.
Anti-cleaved caspase-3 (cat. no. WL02117; 1:500), P70S6K (cat. no.
WL03839; 1:500) and anti-p-P70S6K (cat. no. WL04213; 1:500)
antibodies were purchased from Wanleibio Co., Ltd. Anti-CD31 (cat.
no. GB11063; 1:500) was purchased from Wuhan Servicebio Technology
Co., Ltd., and anti-β-actin (cat. no. TA-09; 1:2,000),
HRP-conjugated anti-rabbit IgG (cat. no. ZB-2306) and
HRP-conjugated anti-mouse IgG (cat. no. ZB-2305) secondary
antibodies were purchased from OriGene Technologies, Inc.
Cell viability assay
Cells (1×104/well; U2932, SU-DHL4,
SU-DHL6 and Jurkat) were inoculated in a 96-well plate with 100 µl
medium, and were treated with ART (1, 2, 3, 4 and 5 µM for SU-DHL4
and SU-DHL6; 1, 5, 10, 15 and 20 µM for U2932 and Jurkat,) and SOR
(2.5, 5, 10, 15 and 20 µM) at different concentrations for 48 h at
37°C, or combined with or without Rapa (5 µM)/Era (1 µM)/Fer (5 µM)
for 24 h at 37°C. Subsequently, CCK-8 (10 µl) was added to each
well and incubated for 2 h at 37°C. Subsequently, the optical
density (OD) of each well was detected at a wavelength of 450 nm
(SpectraMax 190; Molecular Devices, LLC). Cell viability was
calculated using the following equation: Cell viability (%)=[(OD
treated-OD blank)/(OD control-OD
blank)] ×100. The combination indices were analyzed
using CompuSyn software (v1.0; ComboSyn, Inc.).
Apoptosis analysis
Cells, cultured in a 6-well plate
(1×105/well; U2932, SU-DHL4, SU-DHL6 and Jurkat), were
harvested and detected using the Annexin V-647/PI Cell Apoptosis
Detection kit (cat. no. Y6026; Suzhou Yuheng Biotechnology Co.,
Ltd). According to the manufacturer's instructions, each sample was
resuspended in 300 µl binding buffer with 2 µl Annexin V and 1 µl
PI, and then incubated for 15 min at room temperature in the dark.
Sample acquisition and analysis were performed using a flow
cytometer (Cytoflex; Beckman Coulter, Inc.) and FlowJo software
10.0 (FlowJo LLC).
Reactive oxygen species (ROS)
assay
Cells, cultured in a 6-well plate
(1×105/well; U2932, SU-DHL4 and Jurkat), were collected
and stained with 5 µM diluted dichlorodihydrofluorescein diacetate
(cat. no. S0033S; Beyotime Institute of Biotechnology), followed by
incubation for 30 min in the dark at 37°C according to the
manufacturer's instructions. Cell acquisition and analysis were
performed using a flow cytometer, as aforementioned.
JC-1 assay
Mitochondrial membrane potential (MMP) was measured
using a JC-1 Kit (cat. no. M8650; Beijing Solarbio Science &
Technology Co., Ltd.). According to the manufacturer's
instructions, cells, cultured in a 6-well plate
(1×105/well; U2932, SU-DHL4 and Jurkat), were stained
with JC-1 dye and incubated for 30 min in the dark at 37°C. The
fluorescence intensity was measured and analyzed using a flow
cytometer, as aforementioned.
MDC staining assay
Autophagic vacuoles in cells were detected using MDC
staining. Briefly, cells, cultured in a 6-well plate
(1×105/well; U2932, SU-DHL4 and Jurkat), were collected
and stained with 50 µM MDC in 1X PBS at 37°C for 30 min.
MDC-stained cells were detected and analyzed using a flow
cytometer, as aforementioned.
Lipid peroxidation assay
Changes in lipid peroxidation were detected using
BODIPY-C11 581/591. Cells, cultured in a 6-well plate
(1×105/well; U2932, SU-DHL4 and Jurkat), were harvested,
washed, stained with 50 µM C11-BODIPY and incubated at 37°C for 1 h
in the dark. Subsequently, cells were detected and the mean
fluorescence intensity was quantified using a flow cytometer, as
aforementioned.
Malondialdehyde (MDA) assay
The relative levels of MDA were qualitatively
detected using a Lipid Peroxidation MDA Assay kit (cat. no. S0131S;
Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Briefly, proteins were extracted from
U2932 and Jurkat cells (1×107) using lysis buffer, and
supernatants were mixed with TBA detection solution, heated for 15
min in a boiling water bath, cooled to room temperature and
centrifuged (1,000 × g for 10 min at room temperature).
Subsequently, the supernatants were transferred to a 96-well plate.
The optical density was detected at a wavelength of 532 nm, and MDA
content was calculated according to a standard curve and normalized
to total protein levels.
GSH assay
The levels of GSH were evaluated using a GSH assay
kit (cat. no. BC1175; Beijing Solarbio Science & Technology
Co., Ltd) according to the manufacturer's protocol. Briefly, cells
(1×107; U2932 and Jurkat) were collected and homogenized
in solution I, followed by centrifugation. The supernatants were
added into a 96-well plate, then incubated at room temperature for
2 min with solution II and solution III, along with standards. The
optical density was detected at a wavelength of 412 nm (SpectraMax
190; Molecular Devices, LLC). GSH levels were calculated according
to the standard curve and normalized to total protein levels.
Western blot analysis
Cells were collected and lysed in RIPA lysis buffer
(cat. no. HY-K1001; MedChemExpress) supplemented with protease
inhibitors (cat. no. P1260; Beijing Solarbio Science &
Technology Co., Ltd.) and PMSF (cat. no. P0100; Beijing Solarbio
Science & Technology Co., Ltd) on ice for 30 min. Protein
concentration was measured using a BCA protein assay kit (cat. no.
CW0014S; CoWin Biosciences). Protein samples (20–30 µg/lane) were
separated by SDS-PAGE on 8–12% gels and transferred onto
nitrocellulose membranes (cat. no. BSP0161; Pall Life Sciences).
Membranes were subsequently blocked with 5% (w/v) skimmed milk at
room temperature for 1 h and were incubated with primary antibodies
at different dilutions according to the manufacturers' protocols
overnight at 4°C. After primary antibody incubation, the membranes
were washed with 1X TBS-0.1% Tween-20 three times and were
incubated with HRP-conjugated secondary antibodies (1:5,000) at
room temperature for 1 h. After further washing, signals were
visualized using an Ultra High Sensitivity ECL kit (cat. no.
HY-K1005; MedChemExpress) on a Tanon 5200 chemiluminescence image
analysis system (Tanon Science and Technology Co., Ltd).
Immunohistochemical (IHC) staining
assay
Tumor tissues and organs were extracted from mice
and fixed in 4% paraformaldehyde for 24 h at room temperature. IHC
staining was performed using an immunohistochemistry kit (cat. no.
G1215; Wuhan Servicebio Technology Co., Ltd.). Briefly, after
dehydrating in a gradient alcohol series and permeabilizing with
xylene, tissues were embedded in paraffin and cut into 5-µm
sections. After dewaxing and hydration, organ tissue sections were
stained using a hematoxylin/eosin staining kit (cat. no. G1120;
Beijing Solarbio Science & Technology Co., Ltd.). The tumor
tissue sections were also dewaxed and rehydrated, and antigen
retrieval was performed in boiled saline sodium citrate buffer for
10 min. The tissue sections were then blocked with 5% FBS at 37°C
for 15 min and stained with an anti-CD31 antibody (1:1,000)
overnight at 4°C. Subsequently, sections were detected using an IHC
detection kit; the slides were incubated with secondary antibody
(1:200) at 37°C for 1 h, and processed with 3,3′-diaminobenzidine.
Slides were observed under an optical microscope (Olympus
Corporation) and analyzed using Image-Pro plus software (version
6.0; Media Cybernetics, Inc.)
RNA interference
According to the manufacturer's protocol, briefly,
293T cells were seeded in 10 cm dishes at a cell density of 60–70%
and cultured overnight. The medium was replaced 6 h before
transfection. Then pLKO.1-shRNA (10 µg), psPAX2 (7.5 µg) and pMD2.G
(2.5 µg) plasmids, mixed with CaCl2 (2 M; 62.5 µl; cat.
no.C4901; Merck KGaA) and HBSS (2X HBSS; 500 µl; cat. no. 14065056;
Thermo Fisher Scientific, Inc.) were co-transfected into 293T cells
for 48 h using calcium phosphate co-precipitation at 37°C. Viral
supernatants were centrifuged at 1,000 × g for 10 min at 4°C,
filtered through a 0.45-µm filter. For infection, U2932 and SU-DHL4
cells (2×105/well) were seeded in a 6-well plate
overnight and 1 ml of viral supernatant was added, then cells were
cultured at 37°C for 48 h. Next cells were selected with 1 µg/ml
puromycin (cat. no. P8230; Beijing Solarbio Science &
Technology Co., Ltd.). Infection efficiency was evaluated by
western blotting. The concentration of puromycin used for
maintenance was also 1 µg/ml.
Xenograft model analysis
To establish lymphoma xenograft models, a total of
20 female C57/BL/6 mice (weight, 20±2 g; age, 6–8 weeks) were
purchased from Beijing Huafukang Biotechnology Co., Ltd. Mice were
maintained in the West China Animal Experiment Center of Sichuan
University. All mice had free access to food and water, and were
maintained in stress-free, hygienic and animal-friendly conditions
(temperature, 22°C; humidity, 60%) under a 12-h light/dark cycle.
All experimental procedures were approved by the Institutional
Animal Care and Use Committee of Sichuan University (ethics
approval no. 20211284A). Mice were injected subcutaneously with
5×105 EL4 cells resuspended in 100 µl serum-free medium
(n=5 mice/group). Tumor size was measured using electronic
digital calipers, and the tumor volume (mm3) was
calculated as (length × width2)/2. When the tumor volume
reached 50–100 mm3 after 5 days, mice were randomly
divided into four groups and administered normal saline (NS, the
control), ART (90 mg/kg), SOR (30 mg/kg) or the combined treatment
of both ART and SOR (ART 90 mg/kg and SOR 30 mg/kg). Drugs were
injected intraperitoneally every day until tumors reached 2 cm in
diameter or had ulcerated, and mice were euthanized using cervical
dislocation. Tumor tissues, livers and spleens were extracted from
mice. Death of the mice was verified by cessation of heartbeat and
breathing. The duration of the animal experiment was 2 weeks and no
mice died during the experiment. The body weight, fur condition and
general survival status were observed and recorded daily.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. GraphPad Prism 8 (Dotmatics) was
used for statistical analysis. A one-way ANOVA with Tukey's post
hoc test was used for multi-group comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
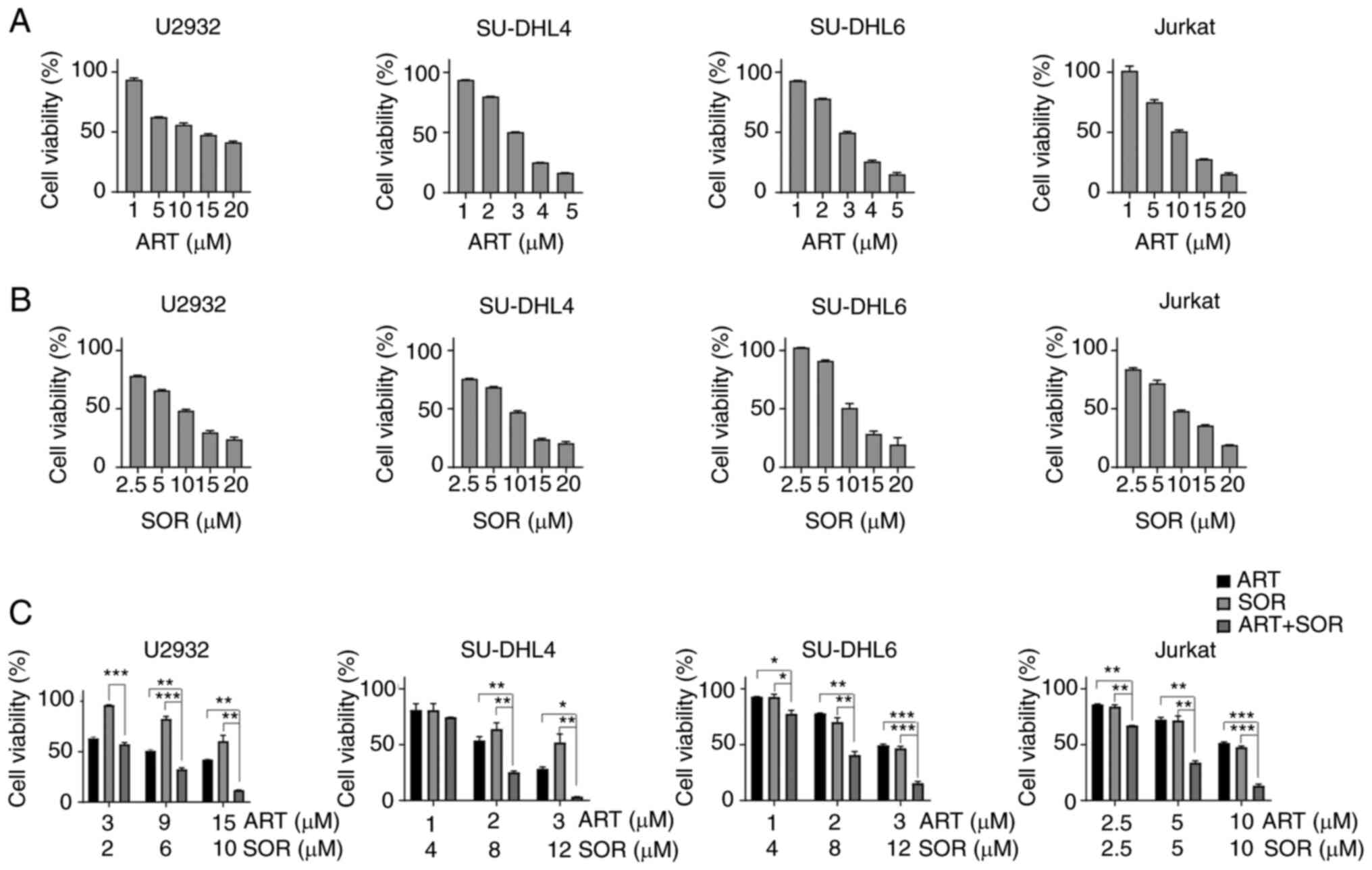
ART and SOR synergistically inhibit
the viability of NHL cells
To investigate the inhibitory effects of ART and SOR
on lymphoma cell lines, the cells were treated with ART and SOR at
different concentrations for 48 h. As shown in Fig. 1A and B, cell viability decreased in
a dose-dependent manner when exposed to ART or SOR in U2932,
SU-DHL4, SU-DHL6 and Jurkat cells. Subsequently, the present study
aimed to examine whether ART and SOR exhibited synergistic effects
on NHL cells. As shown in Fig. 1C,
lymphoma cells treated with a combination of ART and SOR displayed
a significant reduction in cell viability compared with that in
groups treated with either ART or SOR alone. In addition, the
combination indices were <1 in U2932 and Jurkat cells, which
revealed the synergistic inhibitory effect of ART and SOR on the
viability of NHL cells (Fig.
S1A).
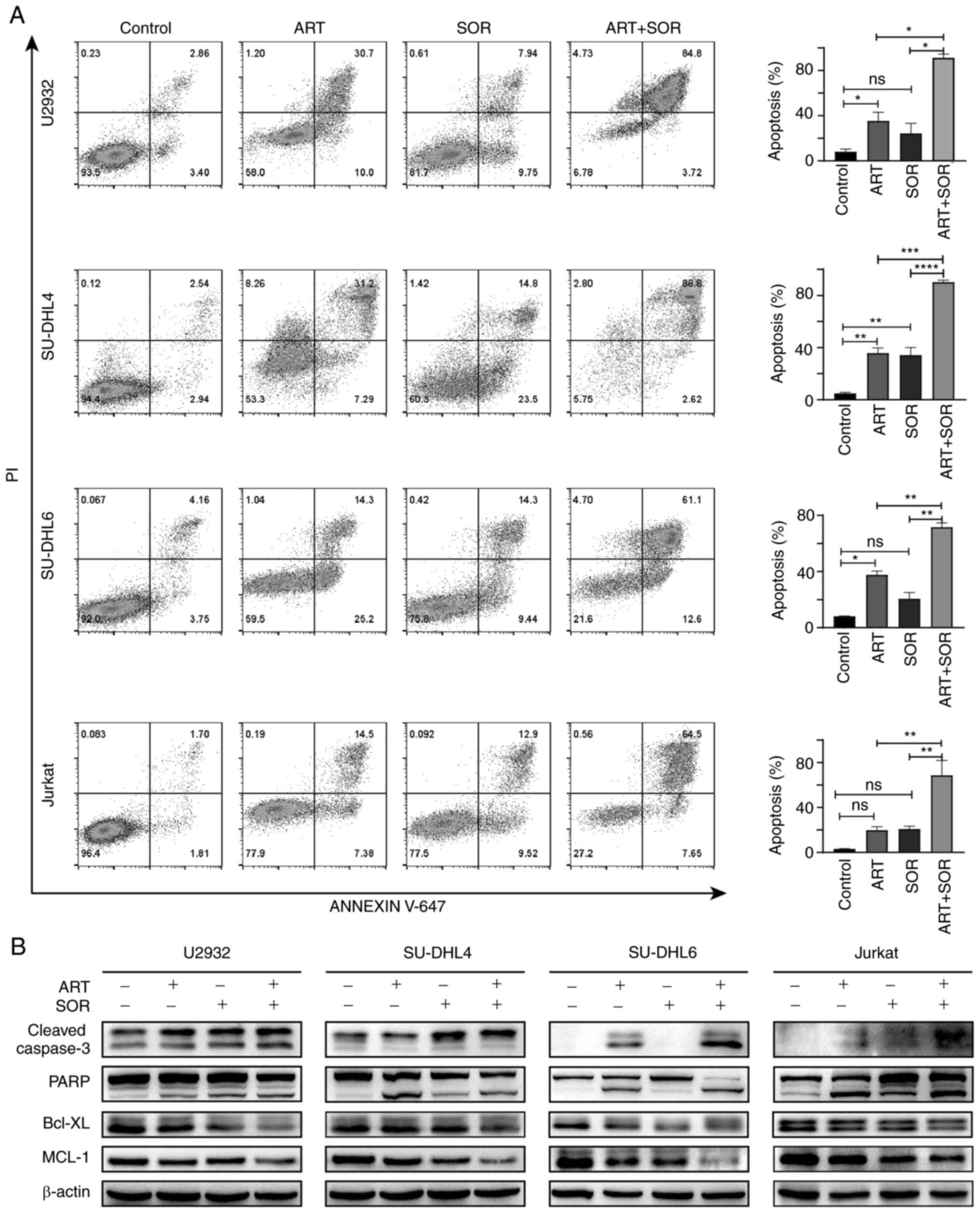
ART facilitates the SOR-induced
apoptosis of NHL cells
To further determine the mechanism underlying the
synergistic anti-lymphoma effects of ART and SOR, cell apoptosis
induced by ART and SOR was assessed using flow cytometry. As shown
in Fig. 2A, ART or SOR treatment
induced the early and late apoptosis of lymphoma cells, but this
was not obvious. Moreover, the combined treatment of ART and SOR
significantly promoted the apoptosis of NHL cells compared with ART
or SOR treatment alone. Notably, western blot analysis was
performed to further verify these results. As shown in Fig. 2B, the expression levels of cleaved
caspase-3 and PARP were increased, whereas Bcl-XL and MCL-1
expression levels were markedly decreased in the combined treatment
group compared with those in the groups treated with either ART or
SOR alone. These findings suggested that ART promoted SOR-induced
apoptosis in NHL cells.
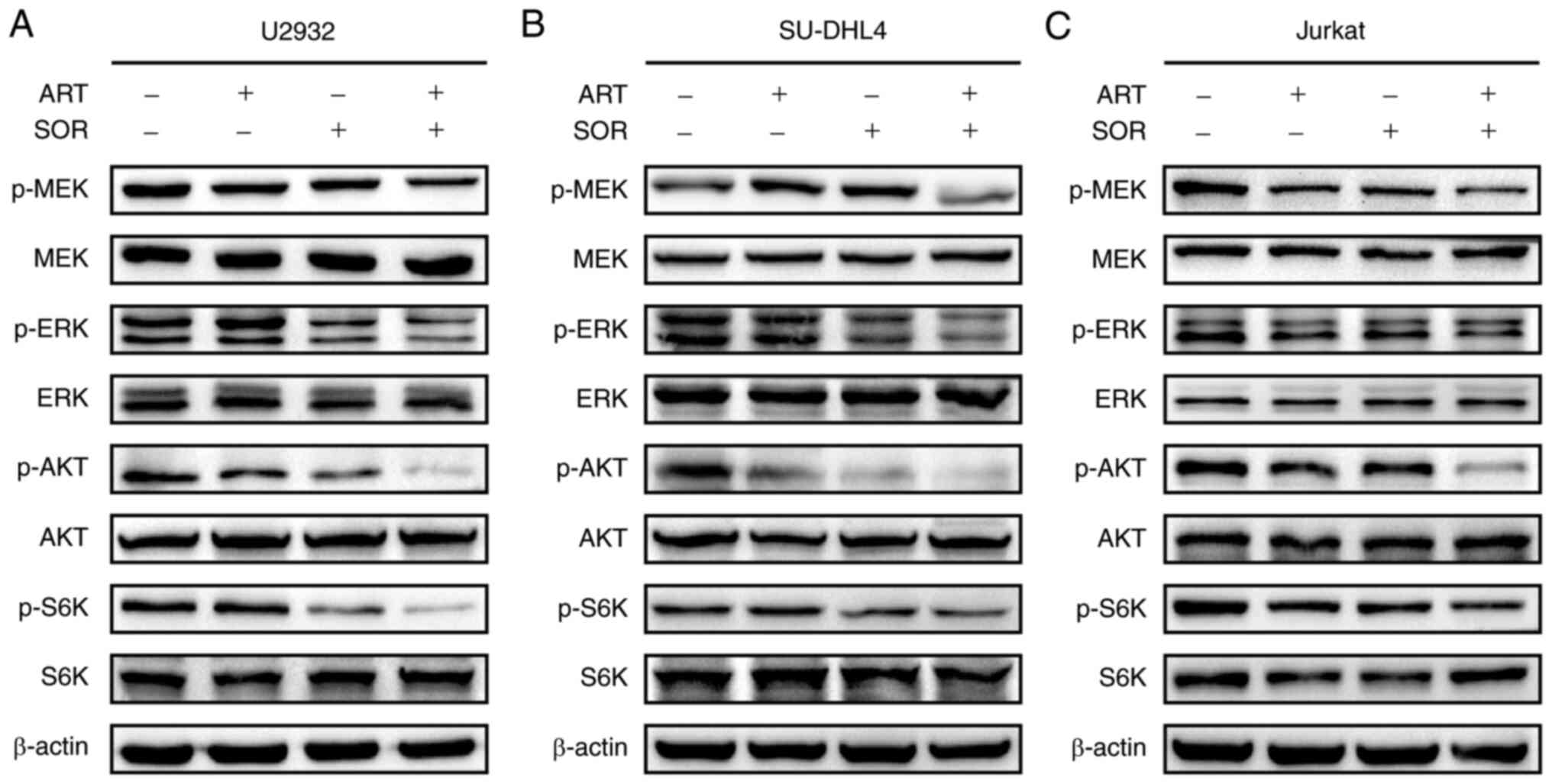
ART and SOR synergistically inhibit
AKT and MEK/ERK pathways in NHL cells
The results of a previous study demonstrated that
ART inhibits tumor cell proliferation via regulation of the AKT
signaling pathway (29).
Subsequently, whether the combined treatment of ART and SOR
affected AKT and MEK/ERK pathways was assessed in U2932, SU-DHL4
and Jurkat cells. The results of western blot analysis demonstrated
that the protein expression levels of p-AKT, p-P70S6K, p-MEK and
p-ERK were reduced following ART or SOR treatment alone; however,
these reductions were minor. Notably, the expression levels of the
aforementioned proteins were more markedly reduced following the
combined treatment of ART and SOR in lymphoma cells (Fig. 3). These results suggested that the
combined treatment of ART and SOR exerted inhibitory effects on AKT
and MEK/ERK pathways in NHL cells.
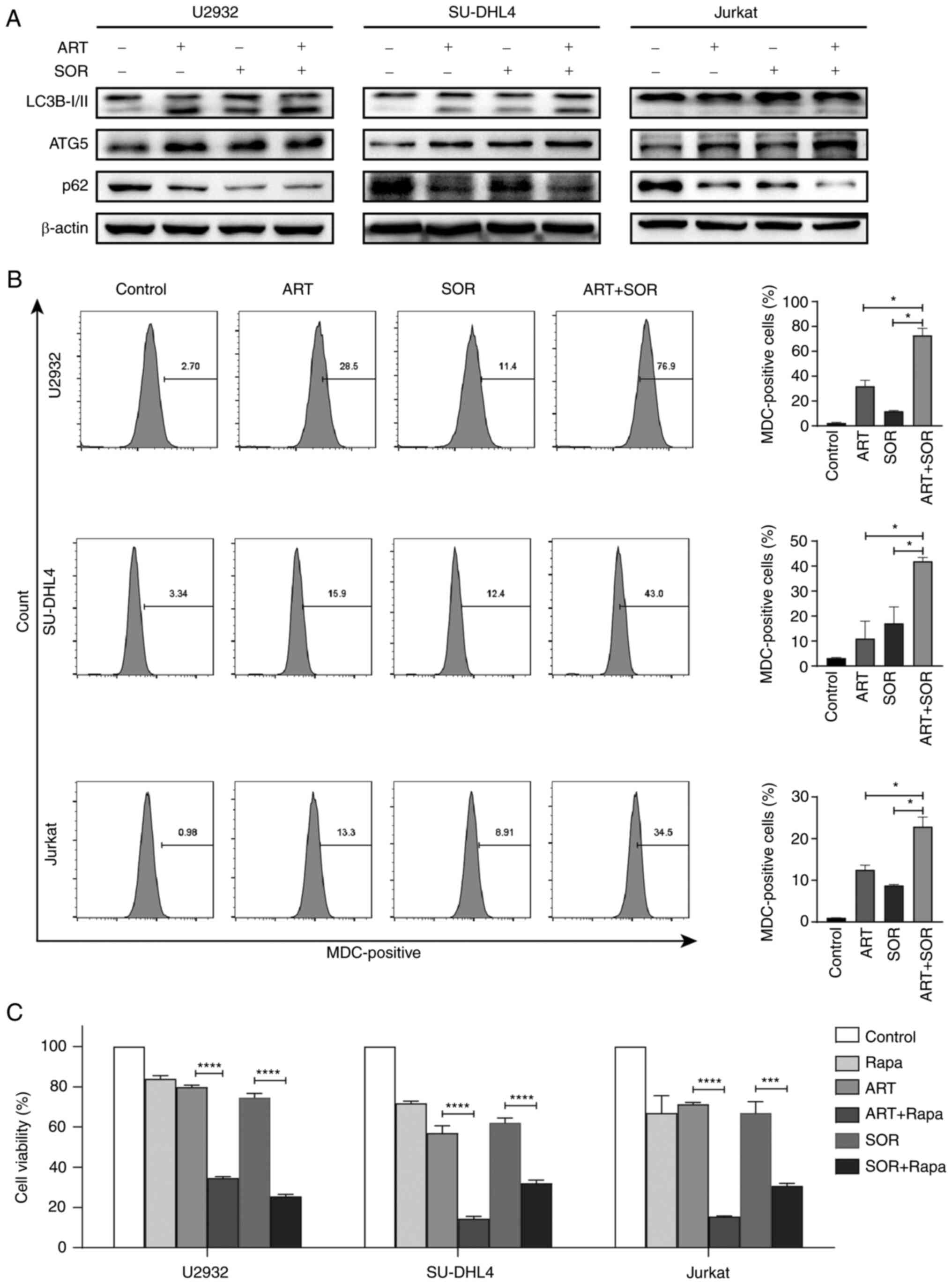
ART and SOR induce autophagy in NHL
cells
The results of our previous study demonstrated that
ART induced autophagy in DLBCL cell lines (29). Subsequently, whether the combined
treatment of ART and SOR synergistically induced autophagy in
lymphoma cells was investigated in the present study. As shown in
Fig. 4A, the expression levels of
LC3B-I/II and ATG5 were increased, whereas those of p62 were
decreased following the combined treatment of ART and SOR compared
with those in groups treated with ART or SOR alone. In addition,
the MDC staining assay was performed to detect the levels of acidic
vesicular organelles (30)
following exposure to ART and/or SOR treatment for 24 h. Consistent
with the aforementioned results, the fluorescence intensity of MDC
was increased following ART or SOR treatment, but this was not
significant, and a greater increase was observed in the combined
treatment group compared with that in groups treated with ART or
SOR alone (Fig. 4B). Notably, Rapa,
an inducer of autophagy, enhanced the inhibitory effects of ART or
SOR on the viability of U2932, SU-DHL4 and Jurkat cells (Fig. 4C). These results indicated that the
synergistic inhibition induced by ART and SOR partially involved
autophagy in lymphoma cells.
ART and SOR induce ferroptosis in NHL
cells
It has been reported that both ART and SOR are
ferroptosis inducers (31). Thus,
the present study aimed to investigate whether ART and SOR induced
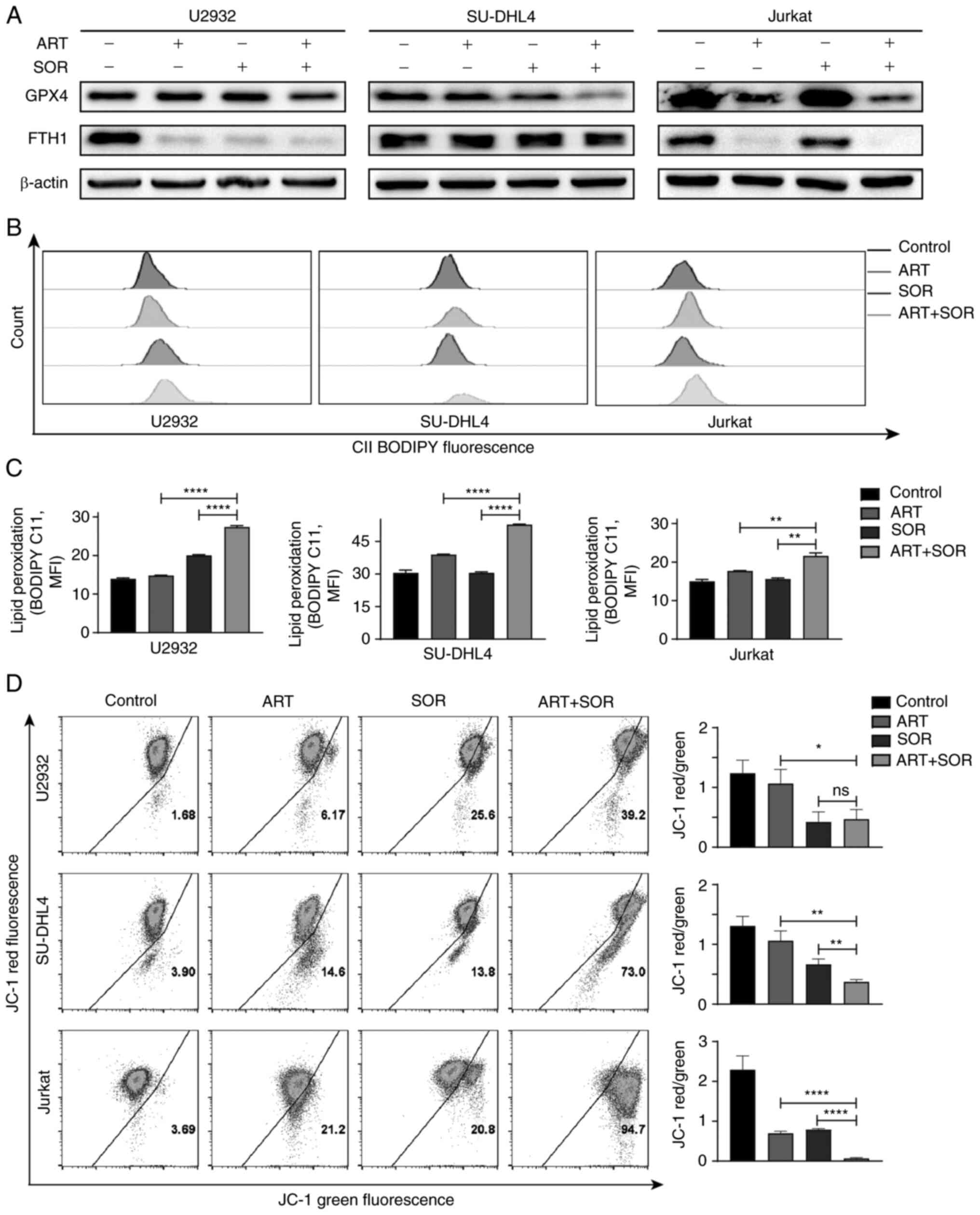
ferroptosis in lymphoma cells. As shown in Fig. 5A, the expression levels of GPX4 and
FTH1 were decreased following exposure to ART or SOR alone;
however, these reductions were minor. Notably, a more marked
reduction in the expression levels of the aforementioned proteins
was observed following treatment with ART and SOR in combination
compared with those in groups treated with ART or SOR alone. Levels
of lipid peroxidation were detected using BODIPY-C11, and the
results demonstrated that the combined treatment of ART and SOR
induced a higher production of lipid peroxidation than groups
treated with ART or SOR alone (Fig. 5B
and C). Moreover, the levels of ROS accumulation were markedly
increased to various extents following ART or SOR treatment, and
ROS generation was significantly higher in the combined treatment
group compared with that in groups treated with ART or SOR alone
(Fig. S1B and C). Notably, the
contents of MDA were significantly higher following the combined
administration of ART and SOR compared with that in groups treated
with ART or SOR alone (Fig. S1D).
However, the levels of GSH were significantly lower in the combined
treatment group compared with those in groups treated with ART or
SOR alone (Fig. S1E). In addition,
MMP was decreased following exposure to ART or SOR in lymphoma
cells, but this was not significant; notably, more monomers were
detected, which indicated a lower ratio of JC-1 aggregates and
monomers occurred in drug-treated cells. A markedly lower level of
MMP was also observed in the combined treatment group compared with
that in groups treated with ART or SOR alone (Fig. 5D).
To further investigate the role of ferroptosis
induced by ART and SOR, cells were co-treated with or without a
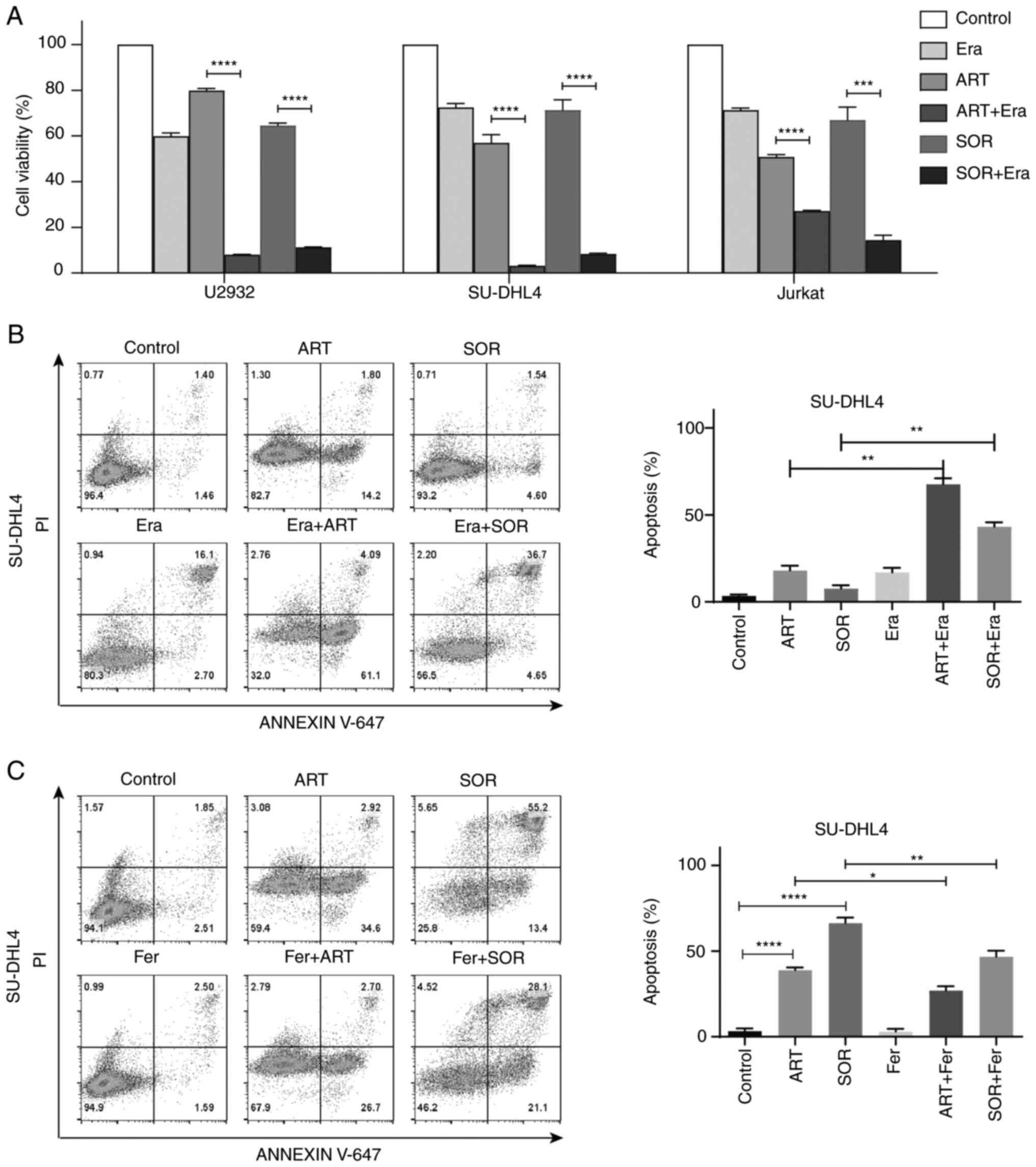
ferroptosis inducer or inhibitor. As shown in Fig. 6A, Era further inhibited cell
viability following treatment with ART or SOR. Moreover,
co-treatment with Era markedly increased the levels of apoptosis in
ART- or SOR-treated cells (ART: 5 µM, SOR: 5 µM) (Fig. 6B). By contrast, ferrostatin-1
partially reduced apoptosis induced by ART or SOR (ART: 10 µM, SOR:
10 µM; higher concentration used to ensure effect) in SU-DHL4 cells
(Fig. 6C). These results suggested
that ART and SOR synergistically induced ferroptosis, and
ferroptosis may contribute to apoptosis induced by ART and SOR in
NHL cells.
ART and SOR induce ferroptosis via
regulation of STAT3 in NHL cells
The results of previous studies have demonstrated
that STAT3 regulates ferroptosis (32,33).
Therefore, whether STAT3 was involved in the inhibitory effects
induced by a combination of ART and SOR was investigated in the
present study. The results of the western blot analysis
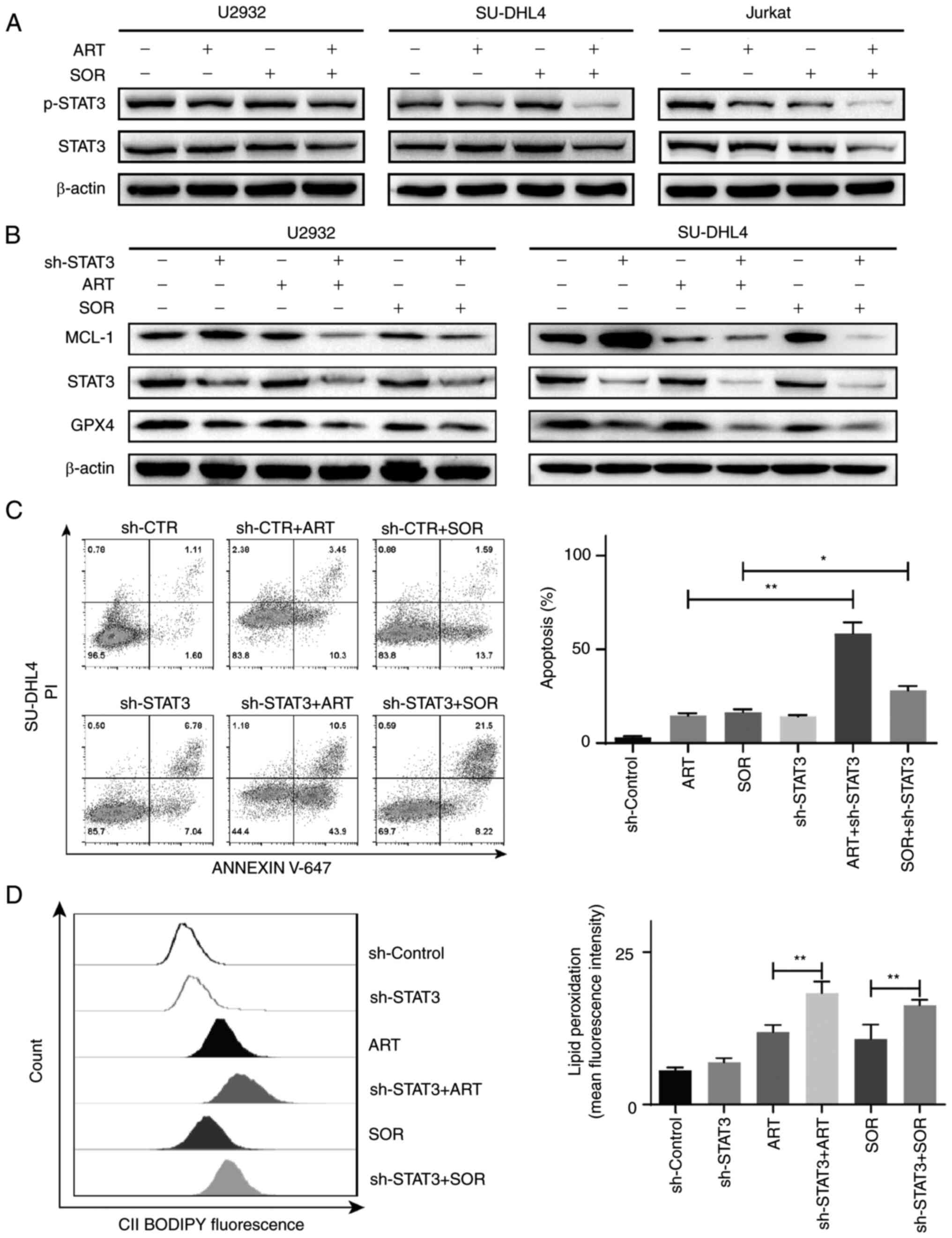
demonstrated that the combined treatment of ART and SOR inhibited
p-STAT3 protein expression compared with that in groups treated
with ART or SOR alone (Fig. 7A).
Subsequently, to further validate the role of STAT3 in ART- and
SOR-induced ferroptosis in lymphoma cells, the expression of STAT3
was inhibited using RNA interference (Fig. S1F), as previously described
(29). As shown in Fig. 7B, the expression levels of MCL-1 and
GPX4 were markedly decreased following ART or SOR treatment in
cells transfected with sh-STAT3 compared with those in the
sh-Control cells. In addition, the apoptotic rates induced by ART
and SOR were higher in the sh-STAT3 groups than those in the
sh-Control groups in SU-DHL4 cells, whereas there was no
significant difference in apoptosis between cells treated with
ART/SOR and the control group (Fig.
7C). Moreover, the production of lipid peroxidation was
increased in cells treated with ART or SOR and transfected with
sh-STAT3 compared with that in the sh-Control groups (Fig. 7D). Collectively, these results
suggested that STAT3 regulated ART- and SOR-induced apoptosis and
ferroptosis in lymphoma cells.
Combined treatment of ART and SOR
inhibits tumor growth in vivo
Subsequently, the anti-lymphoma effects of ART and
SOR were investigated in vivo. EL4 cells were subcutaneously
injected into C57BL/6 mice. In Fig.
S2, the in vitro results shown that ART/SOR could also
inhibit cell viability, induce apoptosis, ROS and lipid
peroxidation accumulation in EL4 cells. After the tumors were
palpable, mice were randomly divided into four groups and treated
intraperitoneally with NS (control), ART, SOR or ART + SOR. As
shown in Fig. 8A and B, tumor
growth was suppressed following ART and SOR administration. In
addition, compared with groups treated with ART or SOR alone, tumor
volumes in the ART + SOR treatment group were significantly
reduced. Moreover, compared with that in groups treated with ART or
SOR alone, the weight of tumors was also smaller in the combined
treatment group (Fig. 8C).
Consistent with the in vitro results, the expression levels
of GPX4, FTH1 and p-STAT3 were reduced in the ART + SOR treatment
group compared with those in the groups treated with ART or SOR
alone (Fig. 8D). In addition, IHC
staining was performed to measure the effect of ART and SOR on
angiogenesis. Reduced CD31-positive areas were observed in the ART
or SOR groups compared with those in the NS group. Moreover, ART +
SOR exhibited a markedly synergistic effect on inhibiting tumor
angiogenesis compared with either ART or SOR monotherapy (Fig. 8E and F). However, the body weights
of mice did not change significantly among the four groups
(Fig. 8G). Finally, the liver and
spleen tissues were removed from the mice and stained with
hematoxylin and eosin. There were no marked changes among the four
groups, which indicated that the combination treatment did not lead
to apparent toxicity (Fig. 8H).
Hence, the results demonstrated that the combination of ART and SOR
performed synergistic effects and was well tolerated in
vivo.
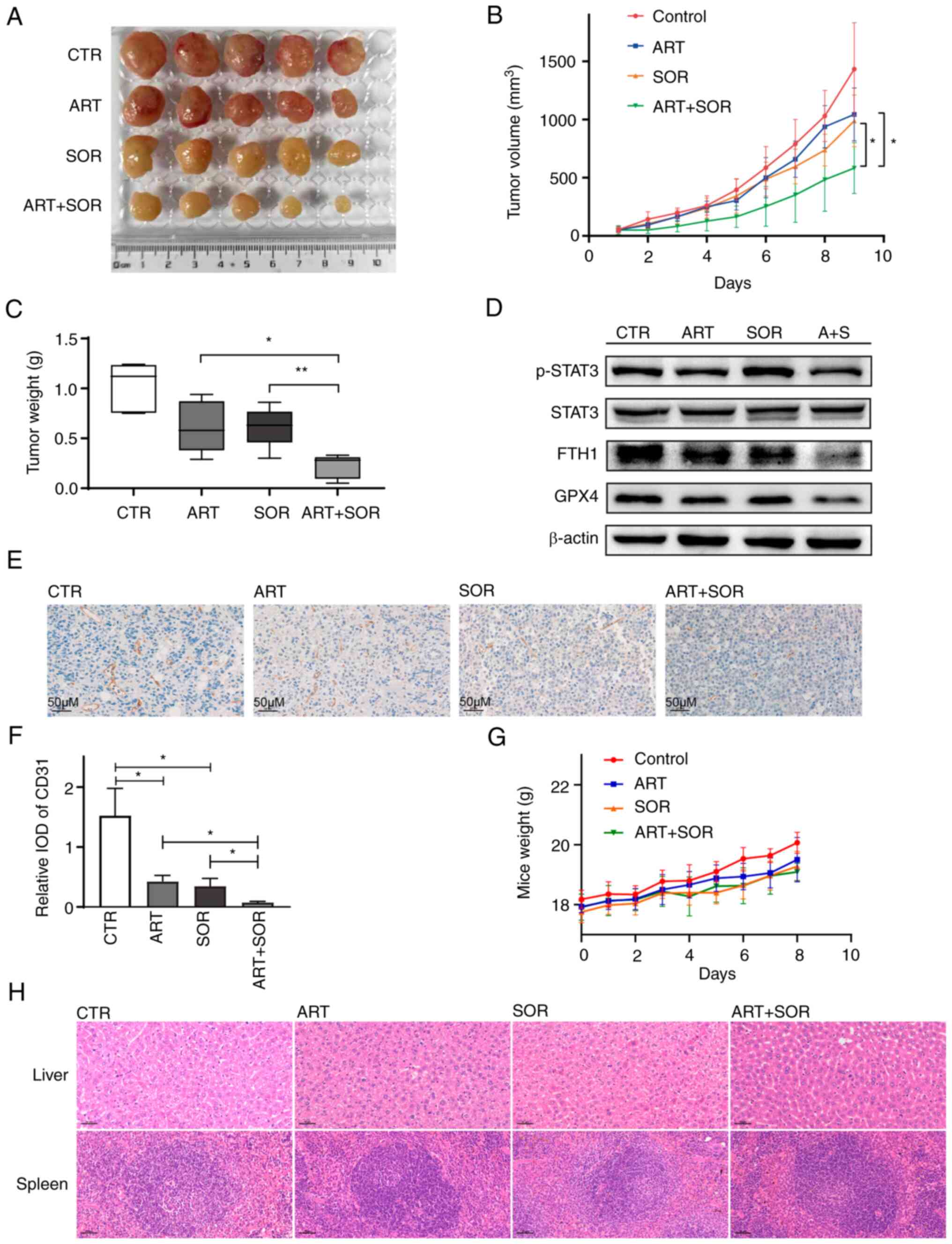 | Figure 8.ART/SOR synergistically inhibit tumor
growth in vivo. (A) Tumor tissues were isolated after 1 week
of treatment. (B) Tumor volumes were calculated and plotted. (C)
Tumor weight was calculated. (D) Western blotting was used to
detect the protein expression levels in tumor tissues. (E and F)
Expression of CD31 protein was evaluated in paraffin-embedded
sections by immunohistochemical staining. (G) Body weight of mice.
(H) Morphology of livers and spleens stained with hematoxylin and
eosin. β-actin was used as a standard. Scale bars, 50 µm.
*P<0.05, **P<0.01. ART, artesunate; CTR, control; FTH1,
ferritin heavy chain 1; GPX4, glutathione peroxidase 4; p-,
phosphorylated; SOR, sorafenib; STAT3, signal transducer and
activator of transcription 3; IOD, integrated optical density. |
Discussion
The results of the present study demonstrated that,
in combination, ART and SOR synergistically exerted anti-lymphoma
effects in vitro and in vivo. The combined treatment
of ART and SOR synergistically suppressed cell viability, and
induced cell apoptosis, autophagy and ferroptosis in NHL cells. In
addition, STAT3 played an important role in ART/SOR-induced
apoptosis and ferroptosis. Furthermore, results of the in
vivo analysis suggested that the combined treatment of ART and
SOR synergistically delayed subcutaneous tumor growth and inhibited
angiogenesis.
Results of previous studies demonstrated that
treatment with ART alone, or in combination with other clinical
drugs, could exert potent antitumor activity in lymphoma in
vitro and in vivo (34–36),
and these results were also observed following treatment with SOR
(37,38). In addition, SOR has been shown to
suppress the MAPK/ERK and AKT pathways in lymphoma cells (39), and to synergize with Rapa to inhibit
NHL cell proliferation (40). When
in combination with perifosine, an AKT inhibitor, SOR can induce
mitochondrial cell death via regulation of tribbles homologue 3
expression in HL (41). Notably,
AKT and MAPK signaling pathways are activated in
methotrexate-resistant primary CNS lymphoma-derived cells,
highlighting that AKT or MAPK inhibitors may increase sensitivity
to methotrexate in CNS lymphoma cells (42). The results of the present study also
demonstrated that ART enhanced the inhibitory effects of SOR on the
protein expression levels of p-AKT, p-MEK and p-ERK in lymphoma
cells, indicating that the combined treatment of ART and SOR may
exert anti-lymphoma effects on the AKT and MAPK signaling
pathways.
The results of our previous study demonstrated that
ART induced autophagy in DLBCL cells (29); however, it is inconclusive as to
whether autophagy promotes or inhibits the antitumor effect induced
by ART (43,44). Notably, autophagy also plays a
paradoxical role in the anticancer effect of SOR (45,46).
Moreover, results of the present study demonstrated that ART and
SOR induced autophagy in lymphoma cells. In addition, Rapa, an
autophagy inducer, enhanced ART- and SOR-induced inhibition of cell
proliferation, which is consistent with the results of previous
studies (40,44). These data indicated that ART and SOR
induced autophagy, which may, at least partly, lead to synergistic
inhibitory effects on lymphoma cells.
Ferroptosis is a novel form of non-apoptotic cell
death. Previous studies have focused on the potential of
ferroptosis induction in cancer therapy (47,48).
Notably, both ART and SOR can induce ferroptosis in tumor cells
(49). DLBCL cells have also been
reported to be one of the most sensitive types of cells to
ferroptosis in distinct tissues (50). The results of the present study
demonstrated that ferroptosis induction occurred in ART- and
SOR-treated cells via downregulation of GPX4 and FTH1 expression
levels. Notably, iron accumulation, a marker of ferroptosis
(51), which can further reflect
the existence of ferroptosis, was not analyzed in the present
study; however, the other main indicator of ferroptosis, lipid
peroxidation, was increased following treatment with ART and SOR.
Imidazole ketone erastin induces ferroptosis and inhibits lymphoma
in vitro and in vivo (52). In addition, the present study
demonstrated that Era enhanced ART- and SOR-induced inhibition of
cell viability and promoted apoptosis. These results indicated that
ferroptosis was involved in the ART- and SOR-induced synergistic
inhibition of NHL cells. However, the association between
ferroptosis and apoptosis is complex. Ferroptosis is different
from, but seems to be linked to apoptosis. For example, inhibiting
GPX4 activation can not only promote ferroptosis but also sensitize
cells to apoptosis (53). Whereas
Bcl-2, an anti-apoptotic protein, can be suppressed by ferroptosis
inhibitors, which indicates that the role of Bcl-2 in apoptosis and
ferroptosis remains elusive (54).
The results of the present study demonstrated that Era promoted
ART- or SOR-induced apoptosis, whereas ferrostatin-1 decreased the
apoptosis induced by ART or SOR in SU-DHL4 cells. These findings
indicated that there may be crosstalk between apoptosis and
ferroptosis induced by ART and SOR. A previous study demonstrated
that the UPR and ER stress induced by ferroptotic agents serve
important roles between ferroptosis and apoptosis (55). Moreover, the results of a previous
study revealed that ART can exert anti-lymphoma activity in
malignant B cells via UPR and ER stress (12). Therefore, ferroptosis may promote
apoptosis through the ER stress pathway in NHL cells treated with
ART and SOR in combination. However, these results require further
investigation.
STAT3 is an oncogenic driver in T- or B-cell
lymphoma, and aberrant and constitutive activation of STAT3
predicts a poor prognosis (56,57).
In addition, dimethyl fumarate treatment induces ferroptosis via
inhibition of the NF-κB and STAT3 signaling pathways in DLBCL
(33). The results of the present
study also demonstrated that ART and SOR treatment downregulated
the expression of p-STAT3, and STAT3 knockdown downregulated the
protein expression levels of GPX4 and MCL-1 in U2932 and SU-DHL4
cells. These data suggested that the combination of ART and SOR
induced ferroptosis and apoptosis in NHL cells via regulation of
the STAT3 pathway. Although it has been demonstrated that STAT3
could regulate ferroptosis by mediating GPX4 expression, it remains
unclear whether STAT3 transcriptionally or post-transcriptionally
regulates GPX4 expression. Notably, the specific role of STAT3 in
ferroptosis and apoptosis in lymphoma remains to be fully
elucidated.
In conclusion, the results of the present study
demonstrated that ART and SOR synergistically suppressed cell
viability, and induced cell apoptosis, autophagy and ferroptosis
in vitro. Notably, the STAT3 pathway exhibited a crucial
association with apoptosis and ferroptosis induced by ART and SOR
in NHL cells. In addition, ART and SOR synergistically inhibited
tumor growth and decreased angiogenesis in vivo. These
findings provide preclinical evidence for the potential application
of ART and SOR in the treatment of NHL. Monotherapy is limited in
the treatment of lymphoma; therefore, ART in combination with other
chemical agents may exhibit potential in the treatment of NHL, as
well as in the treatment of CNS lymphoma. Moreover, ferroptosis may
also act as a potential target in the treatment of lymphoma or
hematological malignancies in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ and YC designed the study. YC performed the
experiments, analyzed the data and drafted the manuscript. HT, FW,
PW, JG, XZ, ZH and ZZ analyzed the data and designed the figures.
YJ and YC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of Sichuan University
(ethics approval no. 20211284A).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the world health organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shankland KR, Armitage JO and Hancock BW:
Non-hodgkin lymphoma. Lancet. 380:848–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armitage JO, Gascoyne RD, Lunning MA and
Cavalli F: Non-hodgkin lymphoma. Lancet. 390:298–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GBD 2017 Disease, Injury Incidence and
Prevalence Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990–2017: A systematic
analysis for the global burden of disease study 2017. Lancet.
392:1789–1858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
GBD 2017 Causes of Death Collaborators, .
Global, regional, and national age-sex-specific mortality for 282
causes of death in 195 countries and territories, 1980–2017: A
systematic analysis for the global burden of disease study 2017.
Lancet. 392:1736–1788. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu W, Liu J, Song Y, Zeng X, Wang X, Mi
L, Cai C, Wang L, Ma J and Zhu J; Union for China Leukemia
Investigators of the Chinese Society of Clinical Oncology; Union
for China Lymphoma Investigators of the Chinese Society of Clinical
Oncology, : Burden of lymphoma in China, 2006–2016: An analysis of
the global burden of disease study 2016. J Hematol Oncol.
12:1152019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White NJ: Qinghaosu (artemisinin): The
price of success. Science. 320:330–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song X, Wei W, Cheng W, Zhu H, Wang W,
Dong H and Li J: Cerebral malaria induced by plasmodium falciparum:
Clinical features, pathogenesis, diagnosis, and treatment. Front
Cell Infect Microbiol. 12:9395322022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei S, Liu L, Chen Z, Yin W, Liu Y, Ouyang
Q, Zeng F, Nie Y and Chen T: Artesunate inhibits the mevalonate
pathway and promotes glioma cell senescence. J Cell Mol Med.
24:276–284. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raza A, Ghoshal A, Chockalingam S and
Ghosh SS: Connexin-43 enhances tumor suppressing activity of
artesunate via gap junction-dependent as well as independent
pathways in human breast cancer cells. Sci Rep. 7:75802017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikawa C, Senba M and Mori N: Evaluation
of artesunate for the treatment of adult T-cell leukemia/lymphoma.
Eur J Pharmacol. 872:1729532020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Våtsveen TK, Myhre MR, Steen CB, Wälchli
S, Lingjærde OC, Bai B, Dillard P, Theodossiou TA, Holien T, Sundan
A, et al: Artesunate shows potent anti-tumor activity in B-cell
lymphoma. J Hematol Oncol. 11:232018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song Q, Peng S, Che F and Zhu X:
Artesunate induces ferroptosis via modulation of p38 and ERK
signaling pathway in glioblastoma cells. J Pharmacol Sci.
148:300–306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Markowitsch SD, Schupp P, Lauckner J,
Vakhrusheva O, Slade KS, Mager R, Efferth T, Haferkamp A and
Juengel E: Artesunate inhibits growth of sunitinib-resistant renal
cell carcinoma cells through cell cycle arrest and induction of
ferroptosis. Cancers (Basel). 12:31502020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang ZY, Yu SQ, Miao LY, Huang XY, Zhang
XP, Zhu YP, Xia XH and Li DQ: Artesunate combined with vinorelbine
plus cisplatin in treatment of advanced non-small cell lung cancer:
A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao.
6:134–138. 2008.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trimble CL, Levinson K, Maldonado L,
Donovan MJ, Clark KT, Fu J, Shay ME, Sauter ME, Sanders SA, Frantz
PS and Plesa M: A first-in-human proof-of-concept trial of
intravaginal artesunate to treat cervical intraepithelial neoplasia
2/3 (CIN2/3). Gynecol Oncol. 157:188–194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Hagens C, Walter-Sack I, Goeckenjan M,
Storch-Hagenlocher B, Sertel S, Elsässer M, Remppis BA, Munzinger
J, Edler L, Efferth T, et al: Long-term add-on therapy
(compassionate use) with oral artesunate in patients with
metastatic breast cancer after participating in a phase I study
(ARTIC M33/2). Phytomedicine. 54:140–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krishna S, Ganapathi S, Ster IC, Saeed ME,
Cowan M, Finlayson C, Kovacsevics H, Jansen H, Kremsner PG, Efferth
T and Kumar D: A randomised, double blind, placebo-controlled pilot
study of oral artesunate therapy for colorectal cancer.
EBioMedicine. 2:82–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther. 5:872020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ravandi F, Alattar ML, Grunwald MR, Rudek
MA, Rajkhowa T, Richie MA, Pierce S, Daver N, Garcia-Manero G and
Faderl S: Phase 2 study of azacytidine plus sorafenib in patients
with acute myeloid leukemia and FLT-3 internal tandem duplication
mutation. Blood. 121:4655–4662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibson JF, Foss F, Cooper D, Seropian S,
Irizarry D, Barbarotta L and Lansigan F: Pilot study of sorafenib
in relapsed or refractory peripheral and cutaneous T-cell lymphoma.
Br J Haematol. 167:141–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kießling MK, Nicolay JP, Schlör T, Klemke
CD, Süss D, Krammer PH and Gülow K: NRAS mutations in cutaneous T
cell lymphoma (CTCL) sensitize tumors towards treatment with the
multikinase inhibitor sorafenib. Oncotarget. 8:45687–45697. 2017.
View Article : Google Scholar
|
|
23
|
Hamed HA, Tavallai S, Grant S, Poklepovic
A and Dent P: Sorafenib/regorafenib and lapatinib interact to kill
CNS tumor cells. J Cell Physiol. 230:131–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Wang F, Wu P, Gong S, Gao J, Tao
H, Shen Q, Wang S, Zhou Z and Jia Y: Artesunate induces apoptosis,
autophagy and ferroptosis in diffuse large B cell lymphoma cells by
impairing STAT3 signaling. Cell Signal. 88:1101672021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Efferth T: From ancient herb to modern
drug: Artemisia annua and artemisinin for cancer therapy. Semin
Cancer Biol. 46:65–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruwizhi N, Maseko RB and Aderibigbe BA:
Recent advances in the therapeutic efficacy of artesunate.
Pharmaceutics. 14:5042022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao KC and Song ZY: Distribution and
excretion of artesunate in rats. Proc Chin Acad Med Sci Peking
Union Med Coll. 4:186–188. 1989.PubMed/NCBI
|
|
28
|
Clavreul A, Roger E, Pourbaghi-Masouleh M,
Lemaire L, Tétaud C and Menei P: Development and characterization
of sorafenib-loaded lipid nanocapsules for the treatment of
glioblastoma. Drug Deliv. 25:1756–1765. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng FB and Qiu HY: Effects of artesunate
on chondrocyte proliferation, apoptosis and autophagy through the
PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid
arthritis. Biomed Pharmacother. 102:1209–1220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomé MP, Filippi-Chiela EC, Villodre ES,
Migliavaca CB, Onzi GR, Felipe KB and Lenz G: Ratiometric analysis
of acridine orange staining in the study of acidic organelles and
autophagy. J Cell Sci. 129:4622–4632. 2016.PubMed/NCBI
|
|
31
|
Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv
H, AlQudsy LHH and Shang P: Ferroptosis, a novel pharmacological
mechanism of anti-cancer drugs. Cancer Lett. 483:127–136. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Gong M, Zhang W, Mo J, Zhang S,
Zhu Z, Wang X, Zhang B, Qian W, Wu Z, et al: Thiostrepton induces
ferroptosis in pancreatic cancer cells through STAT3/GPX4
signalling. Cell Death Dis. 13:6302022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmitt A, Xu W, Bucher P, Grimm M,
Konantz M, Horn H, Zapukhlyak M, Berning P, Brändle M, Jarboui MA,
et al: Dimethyl fumarate induces ferroptosis and impairs
NF-κB/STAT3 signaling in DLBCL. Blood. 138:871–884. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Guo X, Yue W, Wang J, Yang J and
Chen J: Artemether suppresses cell proliferation and induces
apoptosis in diffuse large B cell lymphoma cells. Exp Ther Med.
14:4083–4090. 2017.PubMed/NCBI
|
|
35
|
Cheng C, Wang T, Song Z, Peng L, Gao M,
Hermine O, Rousseaux S, Khochbin S, Mi JQ and Wang J: Induction of
autophagy and autophagy-dependent apoptosis in diffuse large B-cell
lymphoma by a new antimalarial artemisinin derivative, SM1044.
Cancer Med. 7:380–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang N, Zeng GZ, Yin JL and Bian ZX:
Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects
ferroptosis in burkitt's lymphoma. Biochem Biophys Res Commun.
519:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xargay-Torrent S, López-Guerra M,
Montraveta A, Saborit-Villarroya I, Rosich L, Navarro A,
Pérez-Galán P, Roué G, Campo E and Colomer D: Sorafenib inhibits
cell migration and stroma-mediated bortezomib resistance by
interfering B-cell receptor signaling and protein translation in
mantle cell lymphoma. Clin Cancer Res. 19:586–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Locatelli SL, Cleris L, Stirparo GG,
Tartari S, Saba E, Pierdominici M, Malorni W, Carbone A, Anichini A
and Carlo-Stella C: BIM upregulation and ROS-dependent necroptosis
mediate the antitumor effects of the HDACi givinostat and sorafenib
in hodgkin lymphoma cell line xenografts. Leukemia. 28:1861–1871.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carlo-Stella C, Locatelli SL, Giacomini A,
Cleris L, Saba E, Righi M, Guidetti A and Gianni AM: Sorafenib
inhibits lymphoma xenografts by targeting MAPK/ERK and AKT pathways
in tumor and vascular cells. PLoS One. 8:e616032013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ramakrishnan V, Timm M, Haug JL, Kimlinger
TK, Halling T, Wellik LE, Witzig TE, Rajkumar SV, Adjei AA and
Kumar S: Sorafenib, a multikinase inhibitor, is effective in vitro
against non-Hodgkin lymphoma and synergizes with the mTOR inhibitor
rapamycin. Am J Hematol. 87:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Locatelli SL, Giacomini A, Guidetti A,
Cleris L, Mortarini R, Anichini A, Gianni AM and Carlo-Stella C:
Perifosine and sorafenib combination induces mitochondrial cell
death and antitumor effects in NOD/SCID mice with Hodgkin lymphoma
cell line xenografts. Leukemia. 27:1677–1687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takashima Y, Hayano A and Yamanaka R:
Metabolome analysis reveals excessive glycolysis via PI3K/AKT/mTOR
and RAS/MAPK signaling in methotrexate-resistant primary CNS
lymphoma-derived cells. Clin Cancer Res. 26:2754–2766.
2020.PubMed/NCBI
|
|
43
|
Jiang F, Zhou JY, Zhang D, Liu MH and Chen
YG: Artesunate induces apoptosis and autophagy in HCT116 colon
cancer cells, and autophagy inhibition enhances the
artesunateinduced apoptosis. Int J Mol Med. 42:1295–1304.
2018.PubMed/NCBI
|
|
44
|
Zhou X, Chen Y, Wang F, Wu H, Zhang Y, Liu
J, Cai Y, Huang S, He N, Hu Z and Jin X: Artesunate induces
autophagy dependent apoptosis through upregulating ROS and
activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem
Biol Interact. 331:1092732020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shimizu S, Takehara T, Hikita H, Kodama T,
Tsunematsu H, Miyagi T, Hosui A, Ishida H, Tatsumi T, Kanto T, et
al: Inhibition of autophagy potentiates the antitumor effect of the
multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J
Cancer. 131:548–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heqing Y, Bin L, Xuemei Y and Linfa L: The
role and mechanism of autophagy in sorafenib targeted cancer
therapy. Crit Rev Oncol Hematol. 100:137–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Tan H, Daniels JD, Zandkarimi F,
Liu H, Brown LM, Uchida K, O'Connor OA and Stockwell BR: Imidazole
ketone erastin induces ferroptosis and slows tumor growth in a
mouse lymphoma model. Cell Chem Biol. 26:623–633.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tang D, Tang D, Kang R, Berghe TV,
Vandenabeele P and Kroemer G: The molecular machinery of regulated
cell death. Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee Y, Lee DH, Choudry HA, Bartlett DL and
Lee YJ: Ferroptosis-induced endoplasmic reticulum stress:
Cross-talk between ferroptosis and apoptosis. Mol Cancer Res.
16:1073–1076. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu F, Wang KB and Rui L: STAT3 activation
and oncogenesis in lymphoma. Cancers (Basel). 12:192019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lobello C, Tichy B, Bystry V, Radova L,
Filip D, Mraz M, Montes-Mojarro IA, Prokoph N, Larose H and Liang
HC: STAT3 and TP53 mutations associate with poor prognosis in
anaplastic large cell lymphoma. Leukemia. 35:1500–1505. 2021.
View Article : Google Scholar : PubMed/NCBI
|