Introduction
Breast cancer (BC) is an epithelial-derived
malignancy occurring in the ducts or lobules of the breast
(1). According to the 2018 Global
Cancer Data report, BC is the most common form of cancer in women
and is second only to lung cancer in terms of cancer-related
mortality (2). Globally, BC is the
most common cancer in women worldwide and accounted for 2.261
million new cases and 685,000 cancer-related deaths in 2020, which
corresponded to 24.5% of all new cancer cases and 15.5% of
cancer-related deaths in women (3).
Based on gene expression profiles with distinct clinical outcomes,
BC can be divided into four subtypes, namely luminal A, luminal B,
human epidermal growth factor receptor 2 (HER2)-enriched and
triple-negative BC (TNBC) (4). TNBC
is a subtype that lacks estrogen receptor, progesterone receptor
and HER2, which accounts for 10–17% of all BC cases (5,6). TNBC
is reported to be a highly heterogeneous form of tumor
characterized by an aggressive clinical course and increased
likelihood of recurrence (7).
Emerging evidence has revealed various roles of
pyroptosis in cancer pathogenesis and progression (8). Li et al demonstrated that
pyroptosis is closely related to the initiation and development of
cervical cancer (9). Wan et
al revealed pyroptosis could trigger antitumor immunity and
represent a promising new strategy to potentiate cancer
immunotherapy (10). Gasdermin D
(GSDMD) expression, together with the upstream components of the
NLRP3 inflammasome complex, has been revealed to be closely
associated with the occurrence of pyroptosis in numerous cancers
(11,12).
Pyroptosis is pro-inflammatory, thus, it could
activate the immune system and suppress tumor immune escape
(13). Therefore, exploring new
targets to enhance pyroptosis may be a new potential strategy to
treat TNBC. Azurocidin 1 (AZU1) is a heparin-binding protein which
has been reported to be aberrantly expressed in various tumors
(14). It is a neutrophil-derived
granule glycoprotein stored in secretory vesicles and azurophil
granules of neutrophils which was discovered and isolated by Shafer
et al (15). As an
inflammatory mediator, AZU1 can increase the permeability of
vascular endothelial cells, thus leading to vascular leakage and
neutrophil extravasation (16). In
addition, it could also activate inflammatory cells to release
pro-inflammatory factors and cause systemic inflammatory response
syndrome in severe cases, such as sepsis, in which the expression
level of AZU1 is positively associated with the severity of disease
(17,18). However, its role in TNBC remains
unclear.
In the present study, the differential expression of
AZU1 in non-BCs and BCs was firstly analyzed using The Cancer
Genome Atlas (TCGA) and TIMER2.0 databases and the expression level
of AZU1 in patients with TNBC was validated using western blot
analysis. Subsequently, exogenous AZU1 was used to stimulate TNBC
cell lines and investigate the proliferation and pyroptosis of AZU1
exerted on TNBC cells. Collectively, the findings of the present
study revealed that AZU1 inhibited the aberrant proliferation of
TNBC through the regulation of pyroptosis.
Materials and methods
Bioinformatic analysis
Pan-cancer analysis was conducted using TIMER2.0
database (http://timer.cistrome.org//). The differential level
of AZU1 between normal tissues and BCs were analyzed using TCGA
database (https://www.cancer.gov/ccg/research/genome-sequencing/tcga).
One-way survival analysis was performed based on Kaplan-Meier
plotter (https://kmplot.com/analysis/).
Overall survival, first progression survival and post-progression
survival were used as indicators for survival analysis.
BC cell culture
MDA-MB-231 (TNBC), MCF-7 (luminal A) and BT-549
(TNBC) cell lines were cultured in high-sugar DMEM or RPMI-1640
containing 10% fetal bovine serum (FBS; all from Zhejiang Ginocell
Biological Technology Co., Ltd.; www.genomcell.com) plus 1% streptomycin/penicillin at
37°C in an environment of 5% CO2. Subsequently, the
cells were pre-treated with 50 µM pyroptosis inhibitor (VX765;
MedChemExpress) which was prepared in DMSO and was further diluted
in cell culture medium for 2 h, followed by the exposure to various
concentrations (0, 250, 500 and 1,000 ng/ml) of AZU1 (Beijing
Solarbio Science & Technology Co., Ltd.) for 24 h at 37°C in an
environment of 5% CO2. Then, 10 µl of Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology) reagent was
added to each well and incubated at 37°C for 2 h to detect the
viability of BC cell lines.
Patients and specimens
The three samples of TNBC paired with adjacent
normal mammary tissues were obtained from surgical specimens of
patients with BC (aged, 67, 47 and 71 years) at Hunan Provincial
People's Hospital (Changsha, China) from September 2021 to August
2022. The inclusion criteria were as follows: i) Patients with
pathologically confirmed TNBC (19); ii) patients >18 years old; iii)
patients with no indication for surgery; and iv) patients with no
acute or chronic inflammation, hematological and autoimmune
diseases. The exclusion criteria were as follows: i) Patients with
other malignant tumors; and ii) patients with severe heart, brain
or kidney diseases. Samples were frozen in liquid nitrogen
immediately after surgical removal and maintained at −80°C until
protein extraction. All studies were approved (approval no.
2021-036) by the Institutional Ethics Committee of Hunan Provincial
People's Hospital (Changsha, China), and written informed consent
was obtained from all the patients.
Protein preparation and western blot
analysis
Proteins were extracted and harvested from
MDA-MB-231, MCF-7 and BT-549 cell lines with radio
immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The isolated proteins were centrifuged at 10,000 ×
g for 10 min at 4°C. A BCA assay was then used to measure protein
concentration and densitometry was detected using microplate reader
(Biotek ELX808). Total protein (20 µg per lane) was separated on a
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a polyvinylidene fluoride membrane (PVDF). The PVDF
membrane was soaked in 5% skim milk which was dissolved in
Tris-buffered saline with Tween-20 (TBST; containing 2% Tween-20)
for 1 h at room temperature. Subsequently, the soaked PVDF membrane
was incubated overnight at 4°C with a rabbit polyclonal antibody
against β-actin (product no. 4970) or GAPDH (product no. 92310;
both from Cell Signaling Technology, Inc.), GSDMD (cat. no.
ab219800; Abcam), p65 NF-κB (cat. no. 310099) and p-p65 NF-κB (cat.
no. 310013; both from Chengdu Zhengneng Biotechnology, Inc.;
www.zen-bio.cn), caspase-1 (cat. no. ab179515;
Abcam), apoptosis-associated speck-like protein containing a CARD
(ASC; cat. no. DF6304; Affinity Biosciences), NLRP3 (cat. no.
A5652; Abclonal Biotech Co., Ltd.), cleaved caspase-3 (product no.
9664), Bax (product no. 2772) and Bcl-2 (4223; all from Cell
Signaling Technology, Inc.) at a dilution of 1:1,000 (for GSDMD,
p65, p-p65, ASC, NLRP3, cleaved caspase-3, Bax and Bcl-2) or
1:2,000 (for GAPDH and β-actin). Subsequently, the membrane was
washed with TBST three times and incubated with a secondary HRP
goat anti-rabbit antibody (cat. no. AS014; Abclonal Biotech Co.,
Ltd.) for 1 h at room temperature at a dilution of 1:5,000.
Similarly, the PVDF membrane was washed three times again using
TBST. Finally, specific bands were visualized using a
chemiluminescent substrate (cat. no. P0018M; Beyotime Institute of
Biotechnology) and detected using the Omega Lum C Gel Imaging
System. The relative expression of proteins was analyzed using
ImageJ 1.48v (National Institutes of Health).
Colony formation assay
For the colony formation assay, 3×102
MDA-MB-231 or BT-549 cells were plated into 60-mm culture plates,
and cultured in DMEM or RPMI-1640 culture medium supplemented with
10% FBS. The cells were then treated with various concentrations of
AZU1 (0, 250, 500 and 1,000 ng/ml). After 14 days of incubation at
37°C in a 5% CO2 environment, cells were fixed with 4%
paraformaldehyde for 30 min and stained with 0.1% crystal violet
for 10 min at room temperature. Finally, the number of colonies
that contained >50 cells were counted manually. All experiments
were performed at least three times.
5-Ethynyl-2′-deoxyuridine (EdU)
proliferation assay
A total of 5×103 MDA-MB-231 or BT-549
cells were seeded into 24-well plates and cultured in DMEM or
RPMI-1640 complete culture medium supplemented with 10% FBS,
followed by treatment with 0, 250, 500, 100 ng/ml of AZU1 for 24 h
at 37°C in a 5% CO2 environment. An EdU proliferation
assay was then used to examine the proliferation capability of
MDA-MB-231 or BT-549 cells, according to the manufacturer's
protocol. Firstly, the cells were incubated with 10 µM EdU (product
no. CX002; Shanghai Epizyme Biotech Co., Ltd.) for 2 h at 37°C in a
5% CO2 environment. Subsequently, the medium was removed
and fixed with 4% paraformaldehyde for 15 min at room temperature.
The cells were then permeabilized with Triton X-100 for 5 min (cat.
no. T8200; Beijing Solarbio Science & Technology Co., Ltd.) and
500 µl Click Additive Solution (included in the EdU kit) was added
at room temperature. A total of 500 µl DAPI solution (cat. no.
D1306; Thermo Fisher Scientific, Inc.) was added after 30 min and
the fluorescence intensity was measured using a fluorescence
microscope at a magnification of 40.
Apoptosis analysis
The apoptosis of MDA-MB-231 or BT-549 cells were
analyzed using flow cytometry. Firstly, the cells were trypsinized
with 0.25% trypsin (Beijing Solarbio Science & Technology Co.,
Ltd.) and washed with 1X PBS twice. Subsequently, the cells were
resuspended in PBS and 5 µl Annexin V-fluorescein isothiocyanate
and propidium iodide [cat. no. AT101; Hangzhou Multisciences
(Lianke) Biotech Co., Ltd.] were added to the sample. After 15 min
of incubation at room temperature, the apoptosis of the cells was
detected using a flow cytometer (CytoFLEX; Beckman Coulter, Inc.).
The acquired data was analyzed using FlowJo v10.8.1 (BD
Biosciences). All experiments were performed at least three
times.
Statistical analysis
All data are presented as the mean ± standard
deviation and were statistically analyzed using GraphPad Prism 8.0
software (GraphPad Software Inc.; Dotmatics). The Student's
unpaired t-test was used to determine statistical significance
between two groups. Statistical analyses for more than two groups
were performed using one-way analysis of variance (ANOVA), followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
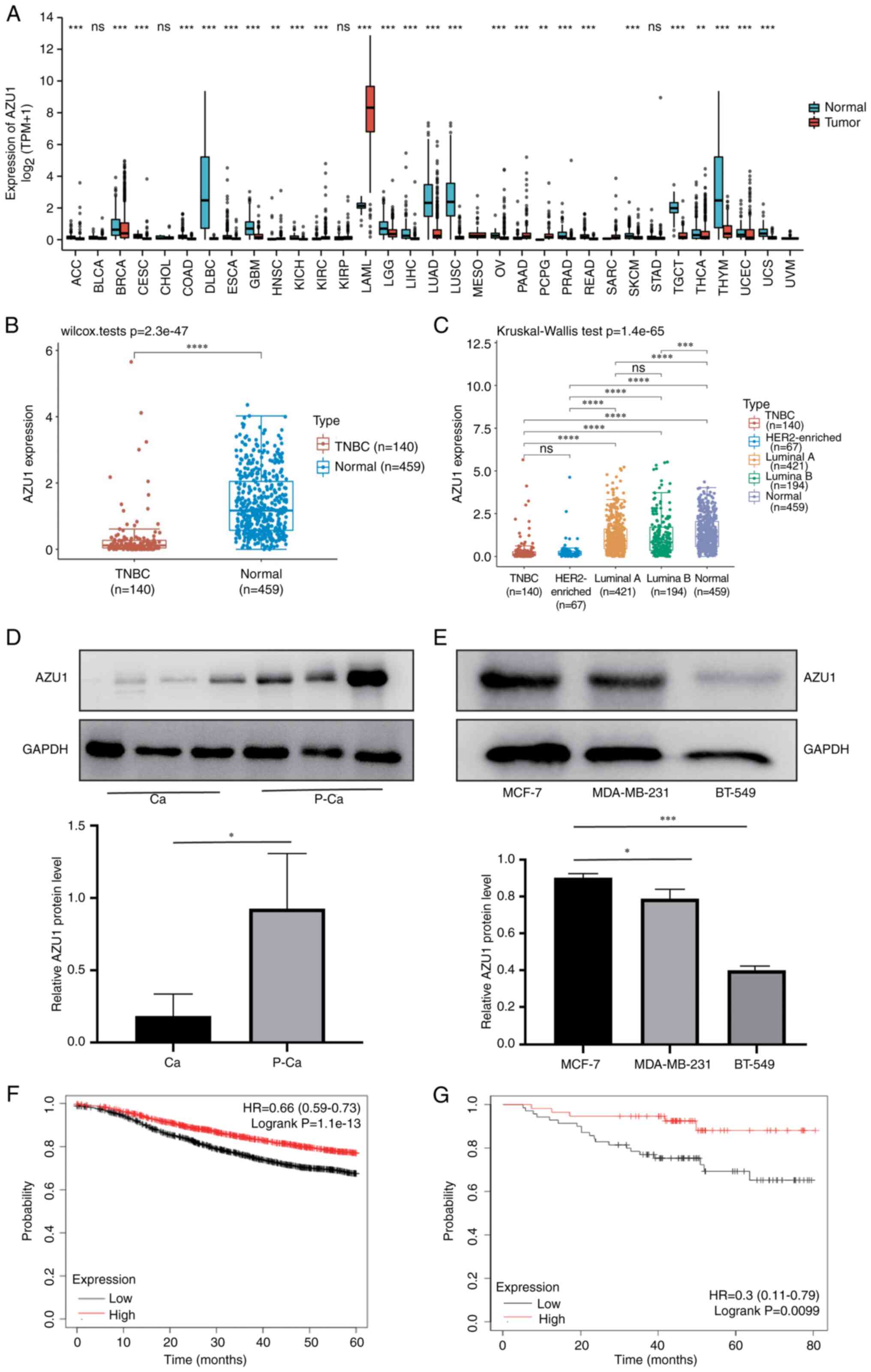
AZU1 level is negatively associated
with the survival rate of TNBC patients
To clarify the role of AZU1 in tumor growth,
pan-cancer analysis was conducted in tumors and adjacent normal
tissues using TIMER2.0. The results revealed that AZU1 mRNA
expression was strongly downregulated in BC, especially in TNBC
(Fig. 1A-C). In order to further
validate its expression, the cancerous tissues (Ca) and
para-cancerous tissues (P-Ca) of patients with TNBC were isolated
for western blot analysis. The results validated the bioinformatics
analysis data (Fig. 1D and E). To
analyze the association between AZU1 expression level and prognosis
of patients with TNBC, survival analysis was performed in the
present study. In Fig. 1F and G,
the abscissa axis represents the survival time and the vertical
axis indicates the survival rate of patients with BC or TNBC. In
addition, the red line indicates the BC or TNBC patients with a
high expression level of AZU1, while the black line indicates the
patients with BC or TNBC with a low expression level of AZU1
(Fig. 1F and G). The results of the
survival analysis revealed that patients with TNBC and a low
expression level of AZU1 exhibited a low survival rate (Fig. 1G). These data suggested that AZU1
exerted a vital role in the progression of TNBC.
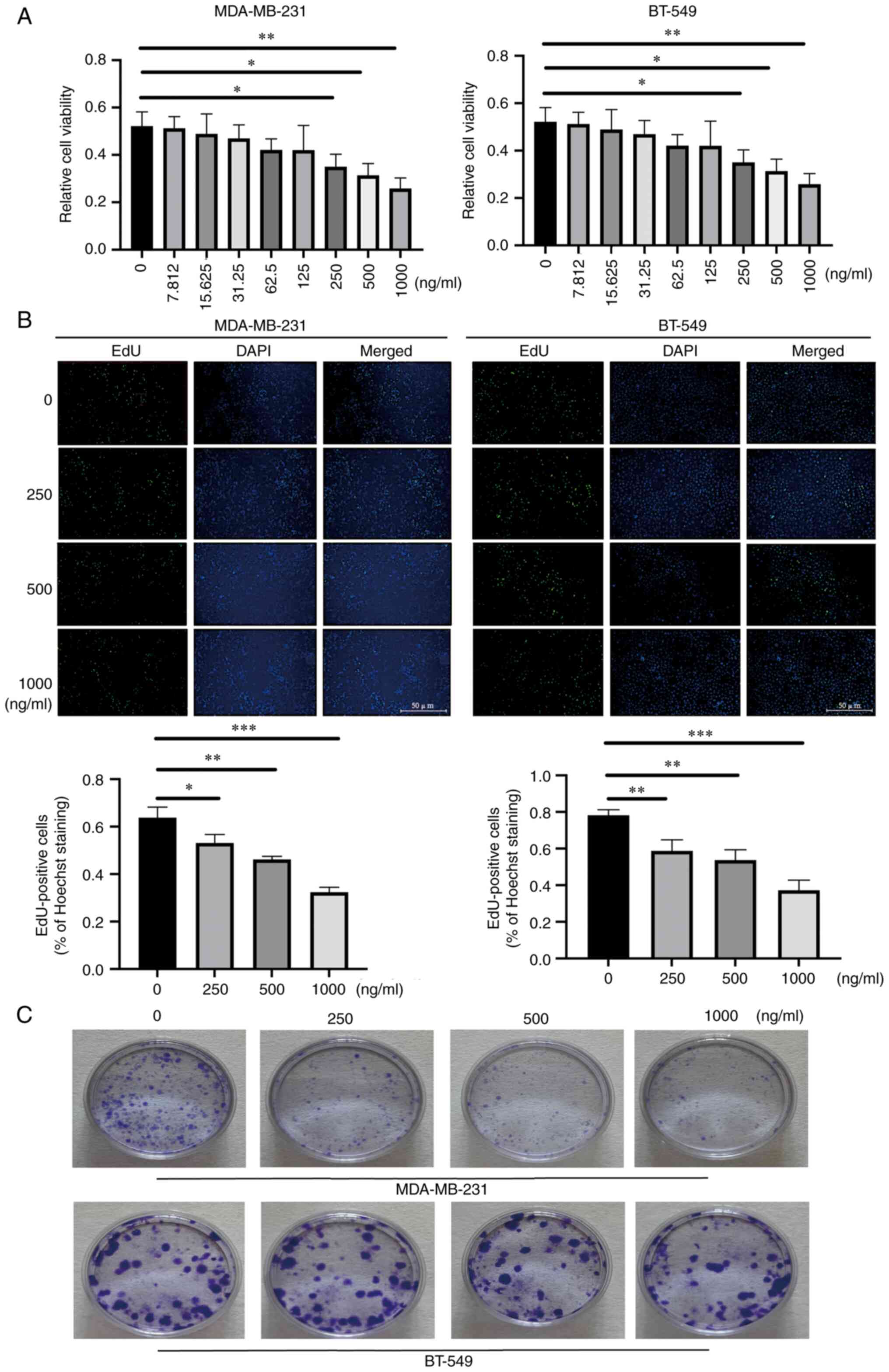
AZU1 inhibits the proliferation and
colony formation of TNBC tumor cells
To further explore the role that AZU1 exerted in the
pathogenesis of TNBC, various concentrations of AZU1 (0, 7.8, 15.6,
31.2, 62.5, 125, 250, 500 and 1,000 ng/ml) were utilized to
stimulate the cell lines of TNBC (MDA-MB-231 and BT-549 cells).
CCK-8 assays demonstrated that a high concentration of AZU1 (above
250 ng/ml) was able to inhibit the proliferation of MDA-MB-231 and
BT-549 cells (Fig. 2A). Therefore,
250, 500 and 1,000 ng/ml AZU1 was used in the subsequent
experiments. The results of the EdU assays also showed that a
higher concentration of AZU1 led to a weak proliferation capability
of the cells (Fig. 2B). The colony
formation assay results were highly similar with the EdU data
(Fig. 2C).
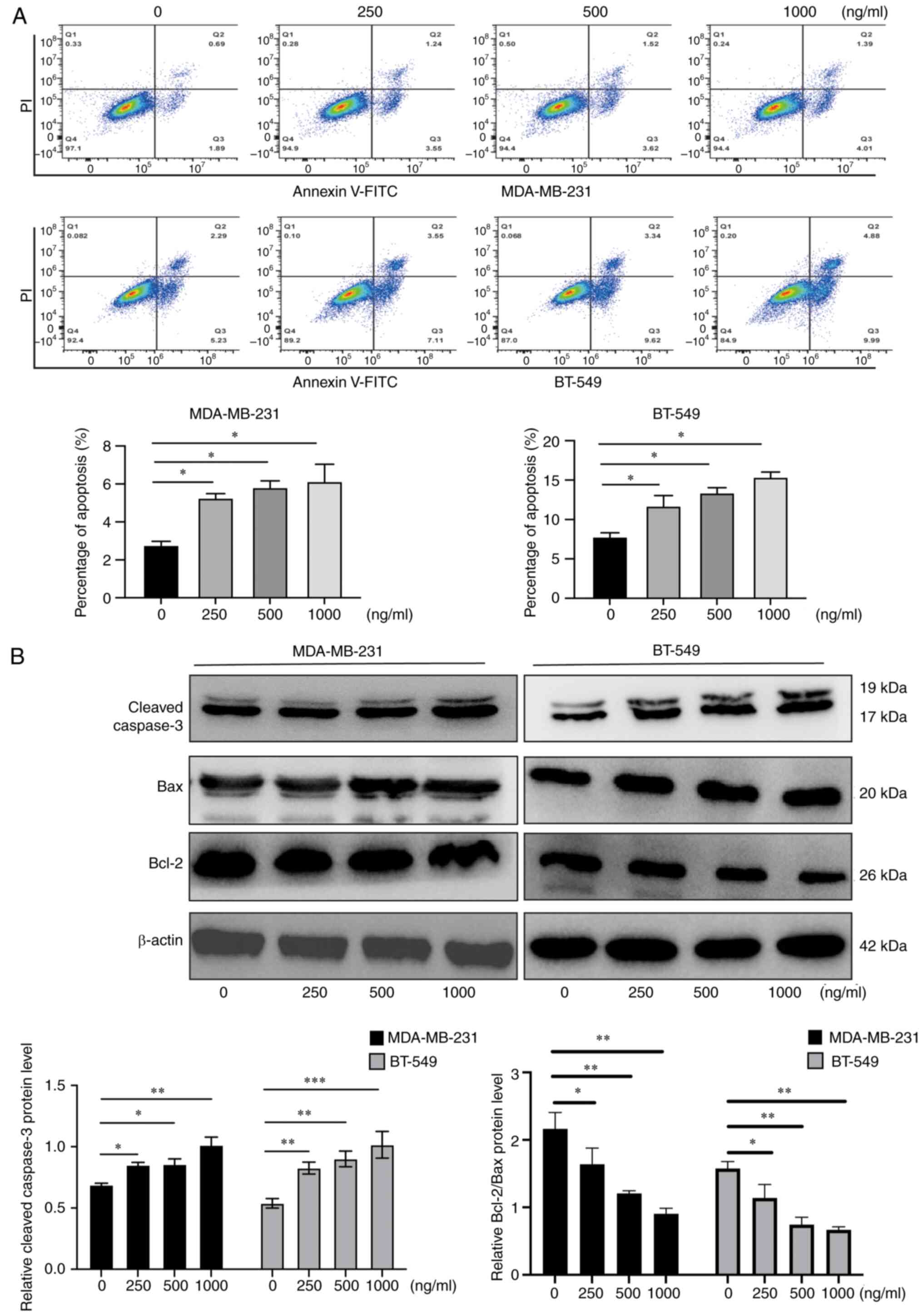
AZU1 promotes apoptosis of TNBC tumor
cells
For further exploration of the underlying mechanism
of AZU1, exerted in TNBC progression, MDA-MB-231 cells and BT-549
cells were treated with various concentrations of AZU1 and flow
cytometry was conducted to detect the apoptosis levels. As revealed
in Fig. 3A, a high concentration of
AZU1 led to an increased percentage of apoptosis (Fig. 3A). Subsequently, the expression
levels of apoptosis associated proteins, including anti-apoptotic
and pro-apoptotic-related proteins, were detected using western
blot analysis. As demonstrated in Fig.
3B, the anti-apoptotic-related protein, Bcl-2, was decreased
following AZU1 treatment, whereas the pro-apoptotic proteins,
cleaved caspase-3 and Bax, were increased in MDA-MB-231 cells and
BT-549 cells. Collectively, the data of the present study showed
that high concentrations of AZU1 promoted the apoptosis of TNBC
cell lines.
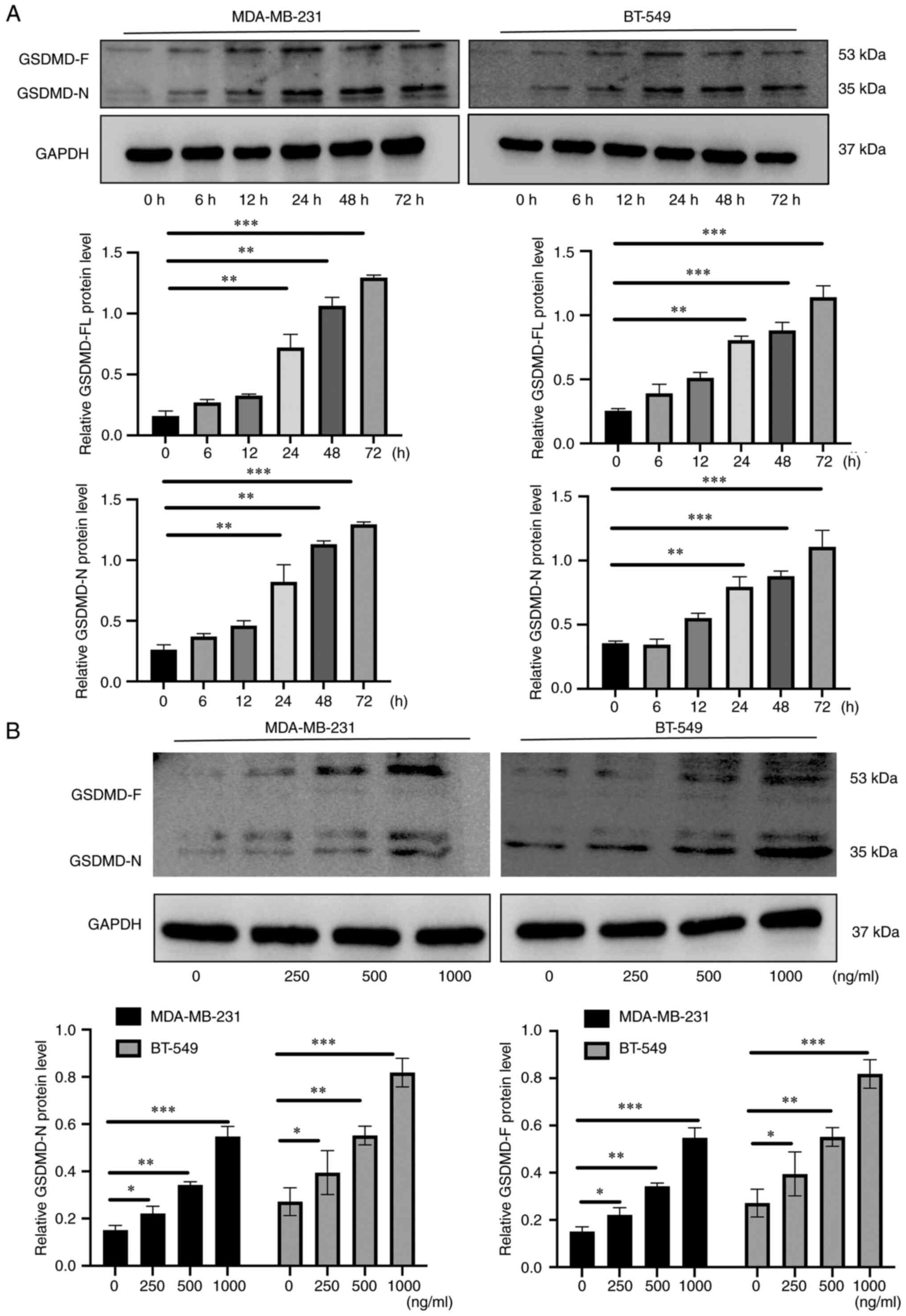
AZU1 induces pyroptosis of TNBC cells
lines
Pyroptosis, a form of programmed cell death, has
been revealed to exert crucial roles in modulating the aberrant
proliferation of tumor cells (20).
As GSDMD is its specific biomarker, the protein level of GSDMD in
TNBC cell lines was investigated. The results revealed that the
effects of AZU1 on the levels of GSDMD, both the full-length GSDMD
(GSDMD-FL) and the N-terminal cleavage product of GSDMD (GSDMD-N),
were dose- and time-dependent, especially after 24 h of the
treatment (Fig. 4A and B).
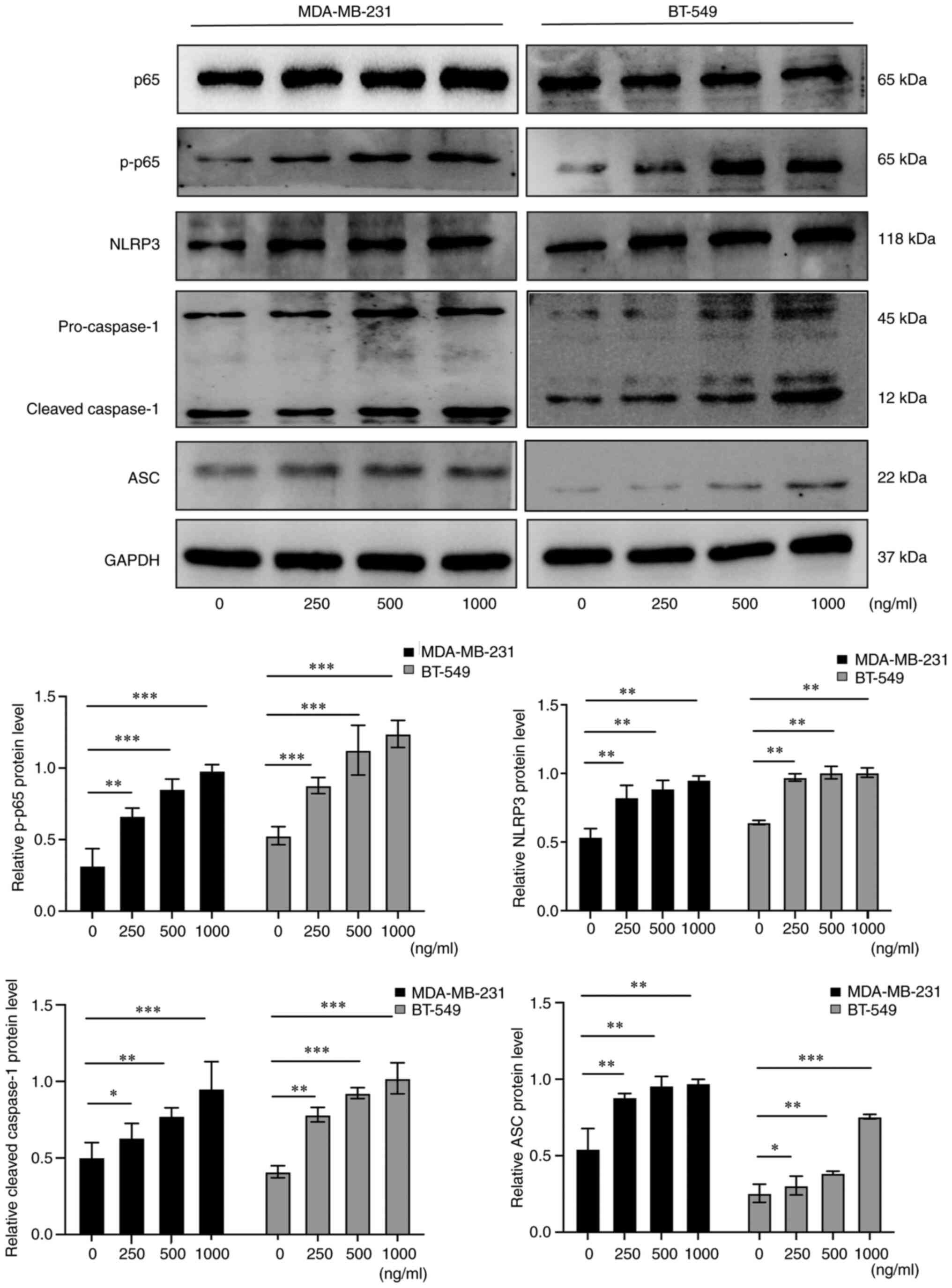
AZU1 promotes the pyroptosis of TNBC
cell lines through the modulation of the NF-κB/NLRP3 axis
As the NF-κB/NLRP3 axis is an effective and
functional regulator of pyroptosis (21), the role that AZU1 exerted on the
NF-κB/NLRP3 axis was subsequently assessed. Firstly, TNBC cell
lines were treated with various concentrations of AZU1 and western
blot analysis was utilized to detect the expression of p-65 NF-κB,
NLRP3, caspase-1 and ASC. As depicted in Fig. 5, p-65 NF-κB, NLRP3, caspase-1 and
ASC protein levels revealed a significant increase under the
treatment of AZU1 after 24 h. These findings indicated that AZU1
promoted the pyroptosis of TNBC cell lines through the modulation
of the NF-κB/NLRP3 axis.
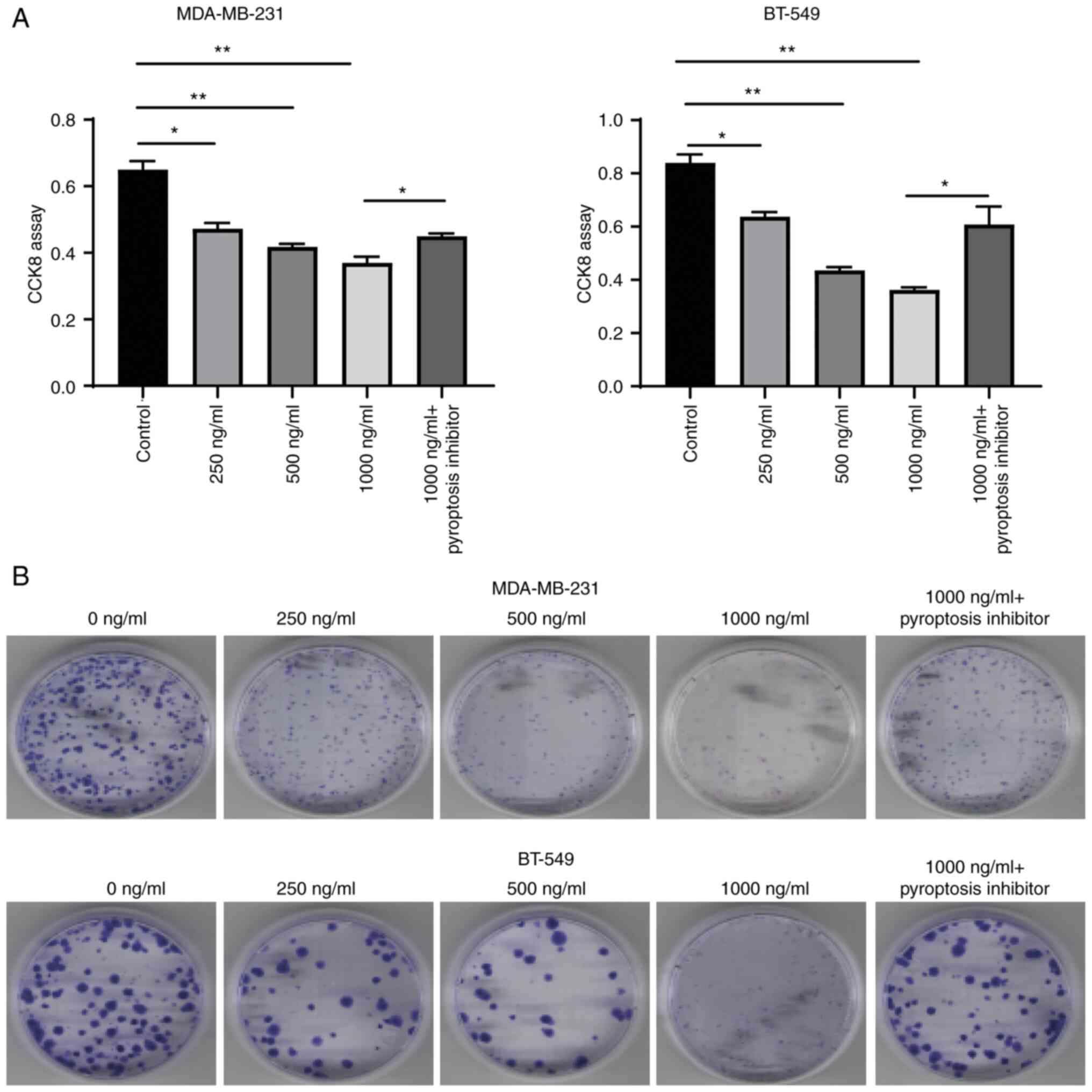
AZU1 inhibits TNBC proliferation
through the modulation of pyroptosis
As it was demonstrated that AZU1 promoted the
pyroptosis of TNBC cell lines through the modulation of the
NF-κB/NLRP3 axis, next, it was further explored whether AZU1
functioned to inhibit the proliferation of TNBC cell lines through
pyroptosis. Therefore, TNBC cell lines were treated with a
pyroptosis inhibitor and CCK-8 along with colony formation assay
were used to assess the proliferation of the cells. The results
revealed that the proliferation of TNBC cell lines was
significantly inhibited when treated with various concentrations of
AZU1, which was partially reversed when the cells were co-incubated
with the pyroptosis inhibitor and AZU1 (Fig. 6). These findings suggested that AZU1
inhibited TNBC proliferation through the modulation of
pyroptosis.
Discussion
Despite the recent great advances in the treatment
of TNBC, an effective treatment remains elusive. Patients with TNBC
are still characterized by a poor prognosis and high recurrence
rate due to the complex pathological features (22). Therefore, it is urgent to identify
the potential molecular mechanism and explore novel therapeutic
strategies. Luminal A is the most frequent BC molecular subtype
(4), thus it was selected as a
control. Furthermore, MDA-MB-231 and BT-549 cell lines are the most
commonly used cell lines in TNBC studies (23), therefore, they were selected for the
experiments conducted. In the present study, two well-known TNBC
cell lines, MDA-MB-231 and BT-549 were used, and preliminarily
revealed that high concentrations of AZU1 exposure induced
GSDMD-dependent pyroptosis through the modulation of the
NF-κB/NLRP3/caspase-1/GSDMD axis. The findings of the present study
provided a novel perspective for exploring the therapeutic
interventions of TNBC.
The tumor microenvironment (TME) is highly complex
and heterogeneous, and is composed of various components, such as
the extracellular matrix, cancer cells and neutrophils (24). It is indispensable for the
initiation and progression of TNBC. The induction of proliferation,
angiogenesis, inhibition of apoptosis, immune suppression and
evasion of immune surveillance are intrinsically linked to the TME
(25). AZU1, has been identified as
a chemoattractant and activator of monocytes and macrophages
(26), as it is stored in the
secretory vesicles and primary granules of neutrophils (27). Tumor-associated macrophages are
known to be attracted by chemokines and cytokines produced by tumor
cells, leading to the construction of the tumor microenvironment
and production of stimuli which contribute to cell pyroptosis
(28). However, to date, a limited
number of studies have revealed its roles in tumor growth. In the
present study, its effect on the pathogenesis of TNBC was
addressed, to the best of the authors' knowledge, for the first
time. The bioinformatics analysis conducted, demonstrated that TNBC
and HER2-enriched patients have a lower expression of AZU1 than
healthy individuals and patients with other subtypes of BC, and the
western blot analysis conducted in the present study validated this
observation. These data indicated that AZU1 played an important
role in the pathogenesis of TNBC. As TNBC has the worst prognosis
among all BC subtypes (7), the
effects of AZU1 exerted in TNBC were investigated. However, the
roles AZU1 played in the HER2-enriched subtype warrant further
investigation. A previous study revealed that AZU1 was able to
decrease the cell viability and increase cell death with increasing
concentrations and treatment time (29). The results of the present study also
demonstrated that the cell viability was suppressed in a time- and
dose-dependent manner by AZU1 exposure. Apoptosis is an important
process for the inhibition of aberrant proliferation (30). During apoptosis, pro-apoptotic
proteins (such as Bcl-2) were unleashed via their upregulation.
When saturated, they can activate Bax, which forms pores that cause
mitochondrial outer membrane permeabilization and result in the
release of cytochrome c (31). Cytochrome c binds apoptotic
peptidase activating factor 1 in the cytosol to form the
apoptosome, which serves as a platform for the activation of
caspase-9, and then continues in order to activate the effector
caspase-3 to dismantle the cell and prepare it for phagocytosis
(32). Therefore, the expression of
Bcl-2, Bax and caspase-3 were detected in the present study. The
results revealed that AZU1, in concentrations over 250 ng/ml,
upregulated the pro-apoptotic-associated protein expression levels
and suppressed the anti-apoptotic-related protein expression
levels, thus resulting in an increase in apoptosis, suggesting that
AZU1 played a role in protection against tumor growth.
Regulation of cell death by AZU1 exposure was
further examined, as the characteristics of cell death are
different from apoptosis morphologically, with cell swelling and
rounding emerging from the plasma membrane (33,34).
The results revealed that high concentrations of AZU1 not only
contributed to apoptosis, but also pyroptosis. To date, numerous
studies have revealed that pyroptosis is a key regulator which
significantly inhibits the aberrant proliferation of tumor cells
(20,35). Previous research has focused on the
in-depth understanding of the underlying regulatory mechanism of
pyroptosis (36). GSDMD is an
important pore-forming protein that has been extensively studied in
pyroptosis (37). In the present
study, it was revealed that the GSDMD N-terminal fragment was
significantly increased in AZU1-treated MDA-MB-231 and BT-549
cells, indicating that GSDMD was cleaved under AZU1 exposure.
Moreover, NLRP3 inflammasome complex activation was observed in
response to AZU1 exposure in vitro, as reflected by the
elevated expression of NLRP3, caspase-1, ASC and p-65 NF-κB.
Collectively, these data provided direct evidence that AZU1 could
induce pyroptosis through the modulation of the p65
NF-κB/NLRP3/caspase-1/GSDMD axis.
In conclusion, it was revealed for the first time,
to the best of the authors' knowledge, that AZU1 exposure promotes
pyroptosis through the modulation of the p65
NF-κB/NLRP3/caspase-1/GSDMD axis in TNBC in vitro. The
findings of the present study unveiled a novel mechanism of
AZU1-induced pyroptosis in TNBC, which may aid in developing new
strategies for therapeutic interventions in TNBC. Although the
current results expanded the knowledge of the authors on the
mechanism of pyroptosis induced by AZU1 exposure, there are still
some limitations in the present study. Since both GSDMD and
gasdermin E (GSDME) are important pyroptotic products (38), only the level of GSDMD-mediated
pyroptosis in TNBC was investigated. Therefore, further research is
required to explore whether GSDME-mediated pyroptosis is involved
in the AZU1-induced antitumor effect of TNBC.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the National
Natural Science Foundation of China (grant no. 82102278), the
Natural Science Foundation of Hunan Province (grant nos.
2021JJ40928 and 2021JJ70090), the Project of Changsha Natural
Science Foundation (grant no. kq2202023), the Huxiang High-Level
Talent Gathering Project (grant no. 2019RS1067) and the Major
Special Project of Hunan Province (grant no. 2020SK1014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YL and YJ conceived and designed the project. SLi
and SLei, WeiweiX and QJ acquired and analyzed the data. FC, SY,
WenX, JC, JW and LZ analyzed and interpreted the data. SLi and SLei
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Informed written consent were obtained from all
patients. The present study was approved (approval no. 2021-036) by
the Institutional Ethics Committee of Hunan Provincial People's
Hospital (Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tharmapalan P, Mahendralingam M, Berman HK
and Khokha R: Mammary stem cells and progenitors: Targeting the
roots of breast cancer for prevention. EMBO J. 38:e1008522019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katsura C, Ogunmwonyi I, Kankam HK and
Saha S: Breast cancer: Presentation, investigation and management.
Br J Hosp Med (Lond). 83:1–7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prat A, Pineda E, Adamo B, Galván P,
Fernández A, Gaba L, Díez M, Viladot M, Arance A and Muñoz M:
Clinical implications of the intrinsic molecular subtypes of breast
cancer. Breast. 24 (Suppl 2):S26–S35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Zhu X, Tang C, Guan X and Zhang W:
Progress and challenges of immunotherapy in triple-negative breast
cancer. Biochim Biophys Acta Rev Cancer. 1876:1885932021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho B, Han Y, Lian M, Colditz GA, Weber
JD, Ma C and Liu Y: Evaluation of racial/ethnic differences in
treatment and mortality among women with triple-negative breast
cancer. JAMA Oncol. 7:1016–1023. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Chen Z, Zhou J, Gu J, Wu H, Jiang
Y, Gao S, Liao Y, Shen R, Miao C and Chen W: NAT10 regulates
neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and
activating STING pathway. Commun Biol. 5:9162022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li K, Qiu J, Pan J and Pan JP: Pyroptosis
and its role in cervical cancer. Cancers (Basel). 14:57642022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan SC, Ye MJ, Yang QC, Zhang T, Zhang MJ,
Ma XB, Xu JM, Wang S, Wu ZZ, Yang LL, et al: Diselenide-based
dual-responsive prodrug as pyroptosis inducer potentiates cancer
immunotherapy. Adv Healthc Mater. 12:e22021352023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Yang T, Xiao J, Xu C, Alippe Y,
Sun K, Kanneganti TD, Monahan JB, Abu-Amer Y, Lieberman J and
Mbalaviele G: NLRP3 inflammasome activation triggers gasdermin
D-independent inflammation. Sci Immunol. 6:eabj38592021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swanson KV, Deng M and Ting JP: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jingushi K, Uemura M, Ohnishi N, Nakata W,
Fujita K, Naito T, Fujii R, Saichi N, Nonomura N, Tsujikawa K and
Ueda K: Extracellular vesicles isolated from human renal cell
carcinoma tissues disrupt vascular endothelial cell morphology via
azurocidin. Int J Cancer. 142:607–617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shafer WM, Martin LE and Spitznagel JK:
Cationic antimicrobial proteins isolated from human neutrophil
granulocytes in the presence of diisopropyl fluorophosphate. Infect
Immun. 45:29–35. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Gennaro A, Kenne E, Wan M, Soehnlein O,
Lindbom L and Haeggström JZ: Leukotriene B4-induced changes in
vascular permeability are mediated by neutrophil release of
heparin-binding protein (HBP/CAP37/azurocidin). FASEB J.
23:1750–1757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naito T, Jingushi K, Ueda K and Tsujikawa
K: Azurocidin is loaded into small extracellular vesicles via its
N-linked glycosylation and promotes intravasation of renal cell
carcinoma cells. FEBS Lett. 595:2522–2532. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun JK, Shen X, Sun XP, Wang X, Zhang WH,
Shi QK and Mu XW: Heparin-binding protein as a biomarker of
gastrointestinal dysfunction in critically ill patients: A
retrospective cross-sectional study in China. BMJ Open.
10:e0363962020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J and Wei K: Necrosulfonamide
reverses pyroptosis-induced inhibition of proliferation and
differentiation of osteoblasts through the NLRP3/caspase-1/GSDMD
pathway. Exp Cell Res. 405:1126482021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan S, Jiang Y, Yu T, Hou C, Xiao W, Xu J,
Wen H, Wang J, Li S, Chen F, et al: Shengjiang San alleviated
sepsis-induced lung injury through its bidirectional regulatory
effect. Chin Med. 18:392023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Y, Hu Y, Zhou N, Yao C, Wu L, Liu L
and Chen F: CAR T-cell therapy for triple-negative breast cancer:
Where we are. Cancer Lett. 491:121–131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie Q, Yang Z, Huang X, Zhang Z, Li J, Ju
J, Zhang H and Ma J: Ilamycin C induces apoptosis and inhibits
migration and invasion in triple-negative breast cancer by
suppressing IL-6/STAT3 pathway. J Hematol Oncol. 12:602019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Del Prete A, Schioppa T, Tiberio L,
Stabile H and Sozzani S: Leukocyte trafficking in tumor
microenvironment. Curr Opin Pharmacol. 35:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blanco M, Collazo-Lorduy A, Yanguas-Casás
N, Calvo V and Provencio M: Unveiling the role of the tumor
microenvironment in the treatment of follicular lymphoma. Cancers
(Basel). 14:21582022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linder A, Soehnlein O and Akesson P: Roles
of heparin-binding protein in bacterial infections. J Innate Immun.
2:431–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher J and Linder A: Heparin-binding
protein: A key player in the pathophysiology of organ dysfunction
in sepsis. J Intern Med. 281:562–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang M, Li J, Gu P and Fan X: The
application of nanoparticles in cancer immunotherapy: Targeting
tumor microenvironment. Bioact Mater. 6:1973–1987. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soehnlein O, Xie X, Ulbrich H, Kenne E,
Rotzius P, Flodgaard H, Eriksson EE and Lindbom L:
Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on
endothelium enhances monocyte arrest under flow conditions. J
Immunol. 174:6399–6405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vervloessem T, Akl H, Tousseyn T, De Smedt
H, Parys JB and Bultynck G: Reciprocal sensitivity of diffuse large
B-cell lymphoma cells to Bcl-2 inhibitors BIRD-2 versus venetoclax.
Oncotarget. 8:111656–111671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mendez DL, Akey IV, Akey CW and Kranz RG:
Oxidized or reduced cytochrome c and axial ligand variants all form
the apoptosome in vitro. Biochemistry. 56:2766–2769. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang L, Li XP, Dai YT, Chen B, Weng XQ,
Xiong SM, Zhang M, Huang JY, Chen Z and Chen SJ: Multidimensional
study of the heterogeneity of leukemia cells in t(8;21) acute
myelogenous leukemia identifies the subtype with poor outcome. Proc
Natl Acad Sci USA. 117:20117–20126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia X, Wang X, Cheng Z, Qin W, Lei L,
Jiang J and Hu J: The role of pyroptosis in cancer: pro-cancer or
pro-‘host’? Cell Death Dis. 10:6502019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zuo Y, Chen L, Gu H, He X, Ye Z, Wang Z,
Shao Q and Xue C: GSDMD-mediated pyroptosis: A critical mechanism
of diabetic nephropathy. Expert Rev Mol Med. 23:e232021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chung C, Seo W, Silwal P and Jo EK:
Crosstalks between inflammasome and autophagy in cancer. J Hematol
Oncol. 13:1002020. View Article : Google Scholar : PubMed/NCBI
|