Introduction
Bone tumors either occur in the bone or originate
from various tissue components of bone, irrespective of whether
they are primary malignant or metastatic bone tumors, thereby
seriously affecting the quality of life of patients. Traditional
surgical methods are either incapable of reconstructing the
function of tumor-bearing limbs or can only achieve this with great
difficulty; moreover, these traditional techniques are associated
with numerous complications, all having the effect of bringing
great pain to patients (1,2). The method of photodynamic therapy
(PDT) mainly comprises irradiation with near-infrared light (NIR)
to stimulate the photosensitizer (PS) enriched in the tumor site,
which has the effect of shifting energy to the circumambient
ground-state oxygen molecules, thereby producing reactive oxygen
species (ROS) such as·OH, 1O2,
O2−, etc.; these are then responsible for
producing cytotoxic singlet oxygen (1O2) and
oxidizing biological macromolecules such as lipids, proteins and
DNA in the bone tumor, causing the death of the tumor cells
(3–5). However, one disadvantage of PDT is
that the radiation depth of the common excitation light sources is
<1 cm, and within the physiological safety limits the
concentration of H2O2 is low, which leads to
the poor toxicity of ROS, therefore, this technique is not ideal
for use in deep bone tumors. Considering the particularity of the
location of the bone tumor and the complexity of the tumor
microenvironment (TME), the degree of response to tumor therapies
continues to present a huge challenge. Due to the high penetrative
ability of ultrasound (US), sonodynamic therapy (SDT), however, is
able to make up for the deficiencies of PDT. The toxicity
mechanisms that are instigated through the application of SDT to
kill cells mainly include US cavitation and heating, ROS oxidative
stress mechanisms, mechanical stress damage, apoptosis, and the
comprehensive interactions that occur among the different
mechanisms (6,7). Among the mechanisms, US cavitation
refers to the high shear stress and strong shock waves caused by
US, which enhance the physical damage to the cell membrane (CM) of
the bone tumor, eventually leading to mechanical injury and
necrosis of tumor cells. SDT, as a novel non-invasive method of
bone tumor therapy, was initially proposed by Yumita et al
(8) in 1989. US is applied to
penetrate biological tissues; focused US can specifically be
applied as a non-invasive technique to focus sound energy on deep
tissues, which can lead to the activation of certain
sound-sensitive drugs (such as hemoporphyrins), thereby producing
antitumor effects. US mainly attacks the subcellular organelles of
tumor cells, including mitochondria, rough endoplasmic reticulum
and smooth endoplasmic reticulum, to disrupt the cellular metabolic
processes. The underlying mechanism of SDT in tumor microvessels
comprises destruction of the endothelial cells of tumor-nourishing
vessels, thereby releasing thromboxane, and the resultant formation
of thrombus causes a disruption of the local microcirculation in
tumor tissue, which further leads to ischemic necrosis of the
diseased tissue. SDT also elicits a substantial effect on body
immunity. After the aforementioned process has occurred, in terms
of tumor cell necrosis, the body is capable of producing a large
number of antigens that stimulate antitumor immunity (9–11).
In addition, the technique of SDT is associated with
a number of additional advantages. First, the method is
non-invasive. Making use of the good penetrative ability of US, it
is best to use the therapeutic head of the corresponding body
surface position as the focus. Secondly, the method is targeted.
The acoustic sensitizer has the ‘targeting’ characteristic of high
concentration aggregation in tumor cells and tumor
neovascularization endothelial cells, which results in the serious
damage that is caused being restricted to the lesion area. Thirdly,
the method has a high degree of accuracy. Sound sensitizers can be
selectively concentrated in tumor cells, and accurately located
with the assistance of imaging prior to treatment. Fourthly, the
technique is safe. With the exception of preventable photosensitive
reactions, sound sensitizers exert no effects on hematopoiesis,
immunity or organ function. The fifth advantage is efficiency. SDT
uses a sonochemical reaction to stimulate the immune function of
the body when destroying the diseased tissue, which greatly
improves the effective rate of treatment and reduces the recurrence
rate. Sixthly, the method is repeatable. Patients will not develop
drug resistance, and therefore they may be treated repeatedly.
Finally, and essentially, the technique has the advantage of
synergistic therapy. For patients who experience tumor sequelae
following surgical resection, SDT is capable of eliminating the
remaining tumor cells more thoroughly, thereby reducing tumor
recurrence, and in this manner, radiotherapy and chemotherapy are
combined (12,13). It has been demonstrated that the
sensitizer has both photodynamic and sonodynamic anticancer
effects. SPDT circumvents the typical disadvantages of conventional
methods (i.e., surgery, chemotherapy and radiotherapy), among which
are drug resistance, limitations of the anatomical site, huge
trauma and numerous side effects. However, two disadvantages of
SPDT are that the majority of the sensitizers are toxic to the skin
and that they readily accumulate in the solution, which results in
the disappearance of 1O2 and poor
tumor-targeting specificity. Therefore, there is a demand for the
generation of improved sensitizers that lack these defects
(14–16). Compared with PDT limited penetration
depth of light source and SDT palliative treatment strategy, SPDT
as a new type of highly selective and non-invasive treatment, it
utilizes smaller dose of PS and exert stronger cytotoxicity through
releasing more ROS to destroy the cell membrane, DNA and protein by
NIR or US radiation SPDT not only has a strong ability to actively
target tumors, but also greatly enhances the accumulation of drugs
and regulates the release of sensitizers (14,17).
For a single PDT or SDT, it is a great challenge to deliver
sensitizers and targeted chemotherapeutic drugs to the same tumor
cell, therefore, single PDT or SDT exhibit numerous shortcomings,
such as sensitizer accumulation, enrichment selection and
endogenous hypoxia (18,19). The rapid rise of nanotechnology,
provides an emerging approach to solve the problem of drug
resistance, as nanotechnology can improve the sensitivity of tumors
by inhibiting drug resistance-related proteins. In addition, the
drug delivery system based on nanotechnology breaks through the
biological barrier and provides spatial and temporal control of
drug release (20–22). Nanotechnology artificially activates
and promotes drug internalization. US exposure also triggers the
release of PSs and the synergistic effect of the two strongly
affects the vascular system of the tumor.
Pathological mechanism of PDT/SDT
combination therapy (or SPDT) in bone tumors
The implementation of SPDT is generally based on
metabolic and inherent characteristics of bone tumors as the
targets. The cellular process of ferroptosis, the extracellular
matrix (ECM), the receptor activator of nuclear factor-κB
(RANK)/RANK ligand (RANKL) signaling pathway and combined autophagy
inhibitors are all features that may be targeted via the technique
(Fig. 1). For example, to
illustrate this concept in greater detail, SPDT leads to the
production of ROS, which accumulate in the mitochondria; this not
only induces activation of the iron death pathway marked by
glutathione (GSH) and promotes the production of a high
concentration of the lipid peroxidation product 4-hydroxynonenal
(4-HNE) on the surface of tumor cells, but it also obliterates
RANKL/RANK-mediated bone resorption in the bone tumor, which
effectively inhibits the occurrence and metastasis of bone tumors
(23–25). Furthermore, via US
cavitation-excited mechanical stress, the tumor may be effectively
attacked, and its metastasis obstructed through either breaking the
hard structure of the ECM or pushing targeted nanomaterials into
the soft ECM (26,27).
 | Figure 1.Multimode therapy based on PDT/SDT is
cytotoxic to bone tumor cells. With the exception of the classical
pathway used to induce apoptosis and necrosis of cancer cells, (A)
releasing the hardness of the ECM, among them, (A-a) PDT/SDT
destroys hard ECM, down-regulates the mechanical force of tumor
cells, (A-b) increases the sensitivity of treatment and inhibits
tumor metastasis. (B) Combining autophagy inhibitor therapy, (C)
inhibiting the RANK/RANKL pathway and (D) focusing on ferroptosis,
all provide new directions for potential development strategies.
PDT/SDT, photodynamic therapy combined with sonodynamic therapy;
RANK, receptor activator of nuclear factor-κB; RANKL, RANK ligand;
ECM, extracellular matrix. |
Targeting iron death therapy affords a very
promising avenue for treatment, although it still has certain
shortcomings. The physiological process of ECM degradation is also
accompanied by the deposition of different tumor-specific types of
ECM, resulting in an increase in the density and hardness of the
ECM. The chemical composition and mechanical function of the ECM
changes with the degree of injury, and the induced cascade
reactions subsequently result in alterations to a large number of
immune processes (28,29). In addition, implementing a targeted
therapeutic strategy for RANK/RANKL may also lead to the occurrence
of immune side effects, due to its indiscriminate inhibition of
soluble RANKL and membrane-bound RANKL (30). In the next section, osteosarcoma
(OS) is investigated, as it has the highest incidence and is the
most widely studied in the field of bone tumors.
PDT/SDT are aimed at ECM degradation
in bone tumors
The remarkable feature of OS is the upregulation of
osteoclast activity, which leads to an increase in bone resorption.
Phenotype is determined by the interaction between genes and the
environment. Cells and the surrounding ECM transmit and interact
with each other through physical or biochemical signals. Especially
in bone tumors, the physical forces mainly involved are rigidity,
matrix orientation and cell distance (31–35).
Cells will preferentially migrate to areas with a hard matrix. When
there is a physical force acting on the cell, this traverses the
membrane and is converted into biochemical signals after its
perception, affecting the function of molecular signaling pathways
(Fig. 1A). The destruction of the
ECM by nano-purine-based PDT/SDT, however, reduces the mechanical
force on tumor cells, which causes a marked increase in the
sensitivity of the treatment and the suppression of metastasis. The
sound-sensitive nanosonosensitizer FePO2@HC provides
rich oxygen content for the degradation of deep ECM, expanding its
permeability, whereas PSs reduce the number of cancer-associated
fibroblasts, cells that shape the normal flow of tumor blood
vessels (35,36). Yang et al (37) discovered that circular nanostructure
PA-Apt-CHO-polyethylene glycol, which achieves an efficient imaging
and PDT effect in vivo, is able to effectively overcome the
obstacle of the ECM biological microenvironment in the cells,
target the bone tumor cells, and achieve good clinical
transformation. The pre-existing ECM is repaired via its
replacement with ECM possessing bone-like properties (‘bony ECM’)
via the activation of osteoblasts, which go straight to the soft
tissue and traverse the bone cortex and endosteum (38). However, highly malignant OS is
associated with bone resorption and ‘volcanic vent-like’ bone
boundaries, which, mainly through the dissolution of the pore or
central hollow area, expand and raise the periosteum, thereby
destroying the bone boundary at the same time. The underlying
genetic basis of bone tumors lies in the mutation of the
proto-oncogene Ras and tumor suppressor genes pRB and p53, which
leads to the abnormal functioning of several signaling pathways,
especially the RANK/RANKL, Hippo and Wnt pathways (39–42).
Glycogen synthase kinase-3β and AKT act upstream of Wnt/β-catenin,
and phosphorylation of glycogen synthase promotes downstream
activation of the pathway, targeting the corresponding genes and
biological effects.
Sound sensitizers/PSs associated with
the autophagy depressor
When tumor cells are confronted with different
stress stimuli, the process of autophagy maintains the tumor cells
in their active and living condition through recovering damaged
organelles and disordered folding proteins. Autophagy therefore
offers a stabilizing, regulatory mechanism in cells. Through the
process of autophagy, certain damaged organelles and dysfunctional
proteins, or other components, are degraded in lysosomes, thereby
participating in the cyclic remodeling of intracellular structures
and functional macromolecules. In general, PDT/SDT is blocked by
inefficient production of ROS and protective autophagy activation
(43,44). Therefore, the cascade nanoreactor
based on a sensitizer combined with an autophagy inhibitor is
widely used to enhance the antitumor efficacy. Among them, the
sensitizer can load a variety of nano-enzymes to catalyze the
formation of ROS (Fig. 1B). The
process of autophagy can be divided into three types. First,
macroautophagy, which is the most common type of autophagy, is the
type of autophagy through which spontaneous lipid bilayer
autophagosomes enfold small molecules or damaged organelles, which
are then transported to the lysosomes for degradation (45). Chen et al reported results
suggesting that the PS pyropheophorbide-α methyl ester (MPPa) is
able to exert similar effects (46). Secondly, microautophagy refers to
the process through which the lysosome itself is actively
phagocytized via the invagination of the membrane without the
formation of autophagosomes; in this manner, the substrate is
enfolded and then degraded (18). A
combination of the hydrophobic PS zinc phthalocyanine/bovine serum
albumin with autophagy inhibitors has been revealed to enhance the
extent of PDT/SDT via restricting the expression of programmed
death-ligand 1 (PD-L1) in bone tumors (47). Thirdly, there is molecular
chaperone-mediated autophagy, a process through which the target
protein first binds to the molecular chaperone to form a complex,
and the receptor on the lysosome membrane subsequently specifically
recognizes the complex, which is then degraded. This process occurs
commonly in mammalian cells (48).
Since autophagy induced by PDT/SDT greatly weakens the killing
effect, the synergistic targeting of autophagy inhibitors presents
a novel strategy worthy of further exploration. Nanoparticles (NPs)
themselves can also be used as compounds that influence autophagy,
which have the effect of regulating intracellular oxidative stress
or changing the expression levels of autophagy-associated genes
through the dual regulation of lysosome internal physical and
chemical properties and surface functionalization. L(D)-PAV-gold
NPs mainly produce ROS and activate the lysosome, leading to
autophagy and death. SDT is dependent on US and sound sensitizers
present in the tumor tissue, and at a specific frequency and
intensity, after radiation of the deep tissue of the tumor site for
a certain period of time, the processes of apoptosis and necrosis
are induced through inflexible stress and biochemical reactions,
leading to the successful destruction of the bone tumor, depending
on the penetration depth and the targeting characteristics
(49).
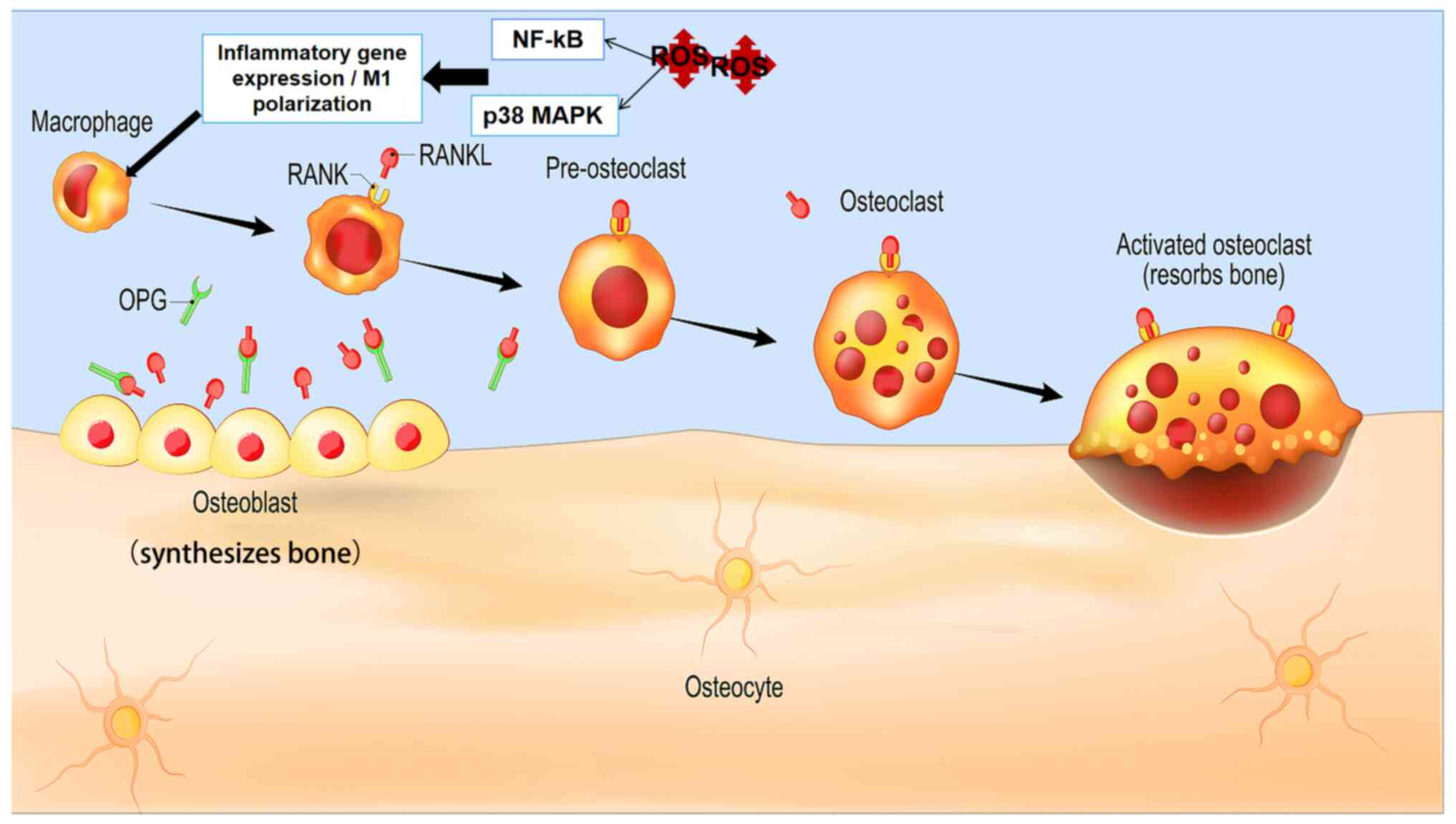
PDT/SDT activates the RANK/RANKL
signaling pathway to disperse the bone tumor
RANK and RANKL exist in osteoblasts, which serve to
promote bone resorption via activating osteoclast signaling.
According to a number of previously published studies (50,51),
the gene expression levels of RANKL and osteoprotegerin (OPG) are
markedly increased following PDT treatment of periodontitis
(52). Although there are numerous
types of osteolytic bone tumors, if the RANKL/RANK pathway is
blocked in the early stage and the bone resorption that is caused
by the tumor is inhibited, the growth of bone tumors can be
effectively prevented (Fig. 1C).
Lai et al (51) demonstrated
that the efficacy of PDT against malignant cancer is closely
associated with the expression levels of RANKL/Yes-associated
protein (YAP). Franco et al (53) further showed that there is a
reduction in the process of osteoclastogenesis. Although research
in this area is only in its infancy, the potential of exploiting
this system for therapeutic interventions is promising. Romagnoli
et al (54) revealed that
the level of OPG influences the redox coupling of GSH/glutathione
disulfide (GSSG), ultimately affecting the formation of
osteoclasts, whereas a negative correlation existed in the ratio of
OPG to GSH. In particular, a physiological decrease in the GSH/GSSG
ratio was identified to promote the formation of osteoclasts during
the final stage of differentiation.
The Hippo/YAP and transcriptional coactivator with
PDZ-binding motif (TAZ) signaling pathways have been demonstrated
to regulate organ size and to maintain the blockade on the
processes of homeostasis and regeneration (55,56).
If the expression is maladjusted and these signaling pathways are
disrupted, the cancer cells are able to repair themselves and
proliferate widely. Verteporfin-PDT binds to YAP/TEAD protein,
which leads to the subsequent inhibition of the Hippo pathway that
drives the progression of sarcoma; very impressive results have
been achieved in inhibiting cancer employing this dual action
mechanism (57). After entering the
nucleus, TEAD transcription factor binds to YAP and TAZ, which
promotes the binding of bromodomain-containing protein 4 (BRD4) and
RNA polymerase II to histones 3 and 4, thereby activating their
biological function. It is worth mentioning that the binding of
BRD4 with acetylated RELA (also known as nuclear factor NF-κB p65
subunit) can also accelerate the osteolytic progression of the
RANK/RANKL pathway. Zhan et al (58) showed that mevalonate is able to
activate the RhoA/YAP or RhoA/ROCK2/LIMK2/YAP signaling pathways,
which, in turn, promote the resistance of bone tumors to PDT
therapy. As a PS of PDT, Vitipofen not only inhibits the
upregulation of the Hippo signaling pathway, but it also binds to
YAP/TEAD protein in the nucleus, which has the effect of fully
inhibiting bone tumor formation (59,60).
PDT/SDT cooperates with ferroptosis to
reinforce bone tumor treatment
In the different stages of cancer progression, GSH
has been revealed to exert a dual effect. During the initial stage
of bone tumorigenesis, intracellular GSH degrades carcinogens and
protects DNA from oxidative damage, which prevents the cells from
becoming cancerous at the outset. Upon aggravation of the
malignancy of the bone tumor, however, cancer cells produce vast
amounts of ROS to meet the needs of tumor metabolism, and
concomitantly, the expression of GSH is markedly upregulated to
counteract the imbalance of intracellular homeostasis; therefore, a
large intracellular concentration of GSH serves to protect against
the oxidative damage of cancer cells (61). Consequently, targeting GSH is an
attractive developmental strategy for the purposes of enhancing the
degree of oxidation, and to improve the therapeutic effects
(Fig. 1D). From the perspective of
GSH synthesis, approaches to accomplish this aim include
suppressing the antiporter system Xc−, and inhibiting
γ-glutamyl transpeptidase, γ-glutamylcysteine ligase or glutathione
synthetase. From the perspective of GSH consumption, it may be
necessary to stimulate GSH oxidation and to promote GSH outflow.
Among these strategies, they are most commonly applied in the field
of bone tumor therapy using the techniques of PDT and SDT.
Based on the balance between GSH and
ROS, identification of the breakthrough point of PDT/SDT in the
elimination of bone tumor
Ferroptosis is a regulatory form of necrosis that is
mediated by iron catalysis and an excessive oxidation of
polyunsaturated fatty acids. In PDT, Fe2+ produces an excessive
amount of hydroxyl-free radicals, which promotes the production of
lipid peroxides. In addition, the level of glutathione peroxidase 4
(GPX4), which regulates the level of lipid peroxides, is
significantly downregulated. Upon exposure to hypoxia in the TME
over a long period of time, GSH, as a ROS scavenging system,
becomes significantly upregulated (62–64).
With the application of SDT technology and the increased level of
oxidative stress, the regulation of the GSH antioxidative system
becomes weakened, which thereby disrupts the redox system; at the
same time, GSH is oxidized to GSSG, which is its final oxidative
form (Fig. 2) (65,66).
Moreover, Mn-MOF-guided SDT exerts antitumor immune effects through
increasing the activity of CD8+ T cells and mature dendritic cells
(DCs) to promote an environment of immunosuppression. In breast
cancer, with treatment with post-transplant cyclophosphamide upon
US or NIR stimulation, SDT/PDT led to a marked downregulation of
GSH and GPX4, resulting in cancer ferroptosis (67–70).
Moreover, with GSH as the target, the administration of
polydopamine-methylene blue (PDA-MB) eliminated the endogenous ROS
scavenging system, thereby enhancing the effect of phototherapy and
effectively inhibiting tumor growth both in vivo and in
vitro (71). There are four
ferroptosis markers triggered by ROS in bone tumors: GPX4,
dihydroorotate dehydrogenase (DHODH), ferroptosis
suppressor-protein 1 (FSP1) and GTP cyclohydrolase 1 (GCH1).
GPX4 maintains its inherent characteristics through
the stabilization of GSH and the activation of cystine transport
system Xc-, which contains two subunits: SLC7A11 and SLC3A2.
Furthermore, additional regulatory systems have been identified to
exist in the cytoplasm of tumors, such as the FSP1/CoQ10 and
GCH1/BH4 systems (72). This
indicates that the GSH-GPX4 system fulfills an important role in
PDT-induced cell death. Its downstream compound is 4-HNE, i.e., one
of the products of lipid peroxidation, and a positive correlation
was identified to exist between the iron level and the 4-HNE
content. Accumulated 4-HNE is a typical lipid peroxidation product
that mainly attacks proteins, DNA and membrane lipids, also
promoting oxidative stress damage in the hippocampus (62,73).
The antioxidant system compound DHODH mediates a protective effect
on ferroptosis via converting ubiquinone into ubiquinol, and
acyl-CoA synthetase long-chain family member 4 (ACSL4), adenosine
monophosphate-activated protein kinase (AMPK)-ACC2 and NF2-YAP are
further signaling pathways that act through controlling PUFA
metabolism and the proportion of phospholipid components in cells
to resist the oxidative damage of CMs, thereby inhibiting the
occurrence of iron death (74–76).
There are two main methods that are employed to kill
bone tumors. One is to increase the number of ROS via inducing DNA
damage and protein oxidation disorder; the other is to increase the
consumption of GSH by amplifying the oxidative pressure and
improving the cancer treatment level (61). In bone tumors, PDT/SDT produces the
requisite ROS, which combine with the PINK1/Parkin signaling
pathway in mitochondria to activate autophagy synergistically
(77). In parallel, ROS activate
the PI3K/AKT/mTOR signaling pathway in generating endoplasmic
reticulum (ER) stress (Fig. 3). As
dysfunctional mitochondria are engulfed via the process of
autophagy, tumor cells are thereby assisted to adapt to external
stimuli, including oxidative stress damage. Therefore, the
implementation of a combined therapy with autophagy inhibitors can
significantly improve the efficiency of killing bone tumors, not
only through the enhancement of oxidative damage, but also through
the promotion of apoptosis (48,78).
Among them, under specific US conditions, cavitation can be divided
into several stages, i.e., nucleation, growth, internal bursting
and collapse. According to its native characteristics, cavitation
can be roughly divided into two types: Stable cavitation and
inertial cavitation (79). When the
microbubble has stable cavitation, its collapse produces the effect
of the massaging the cell, which destroys the integrity of the
plasma membrane through the Vera operation. Experiencing more
intense inertial cavitation, microbubbles can cause membrane
perforation and cytoskeletal rupture. Strong US waves and high
stress finally cause the cell to undergo necrosis. US piercing
produces an extreme temperature, and releases pressure, which will
lead to a variety of biological effects, including increasing the
level of ROS in the cytoplasm, enhancing CM permeability, and
eliciting CM potential depolarization and hyperpolarization.
Hydroxyapatite NPs are employed in bone tissue regeneration by
taking advantage of the implosive power of cavitation bubbles, and
this is effective as the energy of the implosion pushes NPs onto
the surface of damaged bone tissue (80). Following PDT/SDT treatment,
histopathological investigations have provided clear evidence of
bubble clouds and tissue ablation in the affected area, thereby
demonstrating the feasibility of using this technique for the
treatment of bone tumors (81).
Targeting mitochondria and suppressing hypoxia-inducible factor-1α
are essential for improving the PDT/SDT effect (82,83).
In addition, downstream inhibitory effects in
various signaling pathways were also detected in cells treated by
sound pores, including cell cycle arrest, morphological inhibition,
apoptosis and cell necrosis. The use of SPDT significantly enhanced
the therapeutic effects. It was determined that, after implementing
titanium dioxide-induced SPDT, the expression level of
malondialdehyde was significantly increased, whereas the levels of
superoxide dismutase, catalase and GSH were downregulated (84).
In osteolytic OS, bone tumor cells have been
demonstrated to secrete osteoclast active factors, which activate
osteoclasts. Once the balance between the osteoblasts and
osteoclasts is broken, excited osteoclasts secrete acid and
lysozymes, which cause degradation of the bone matrix, leading to
pain via eventual bone resorption for patients (85,86).
The degree of destruction of bone tissue, especially through the
process of progressive osteolysis, is closely associated with the
severity and frequency of breakthrough pain. The RANK/RANKL pathway
is mainly responsible for the genesis of osteoclasts, in which the
upregulation of osteoclast activity and an increase in bone
resorption are mainly based on the imbalance of RANK, RANKL and OPG
(Fig. 4). The RANK/RANKL/OPG
pathway is particularly important in terms of stimulating bone
resorption in osteoclasts.
It is well known that acetylation, methylation and
the associated epigenetic enzymes are able to disrupt the molecular
pathways of normal bone tumors (87,88).
Among them, the genetic modification of methylation is widely
reported to be mediated by the methyltransferase, EZH2-mediated
H3K27me3. Photodynamic regulators have been shown to inhibit H3K27
methylation by inhibiting the epigenetic regulatory factor EZH2,
which stimulates tumor cells to express MHC class I molecules and
release a large amount of C-X-C motif chemokine ligand 10, which
ultimately enhances the immunosuppressive tumor microenvironment of
the tumor cells, and promotes the capability of T cells to
recognize tumors (89,90).
In order to increase the level of ROS to boost the
therapeutic effects of tumor treatment, Liu et al (91) confirmed that, after
mPEG2000-P(HDI-DN)20 had entered the 143B
cells, OS cells were inhibited through promoting the accumulation
of ROS in the presence of GSH.
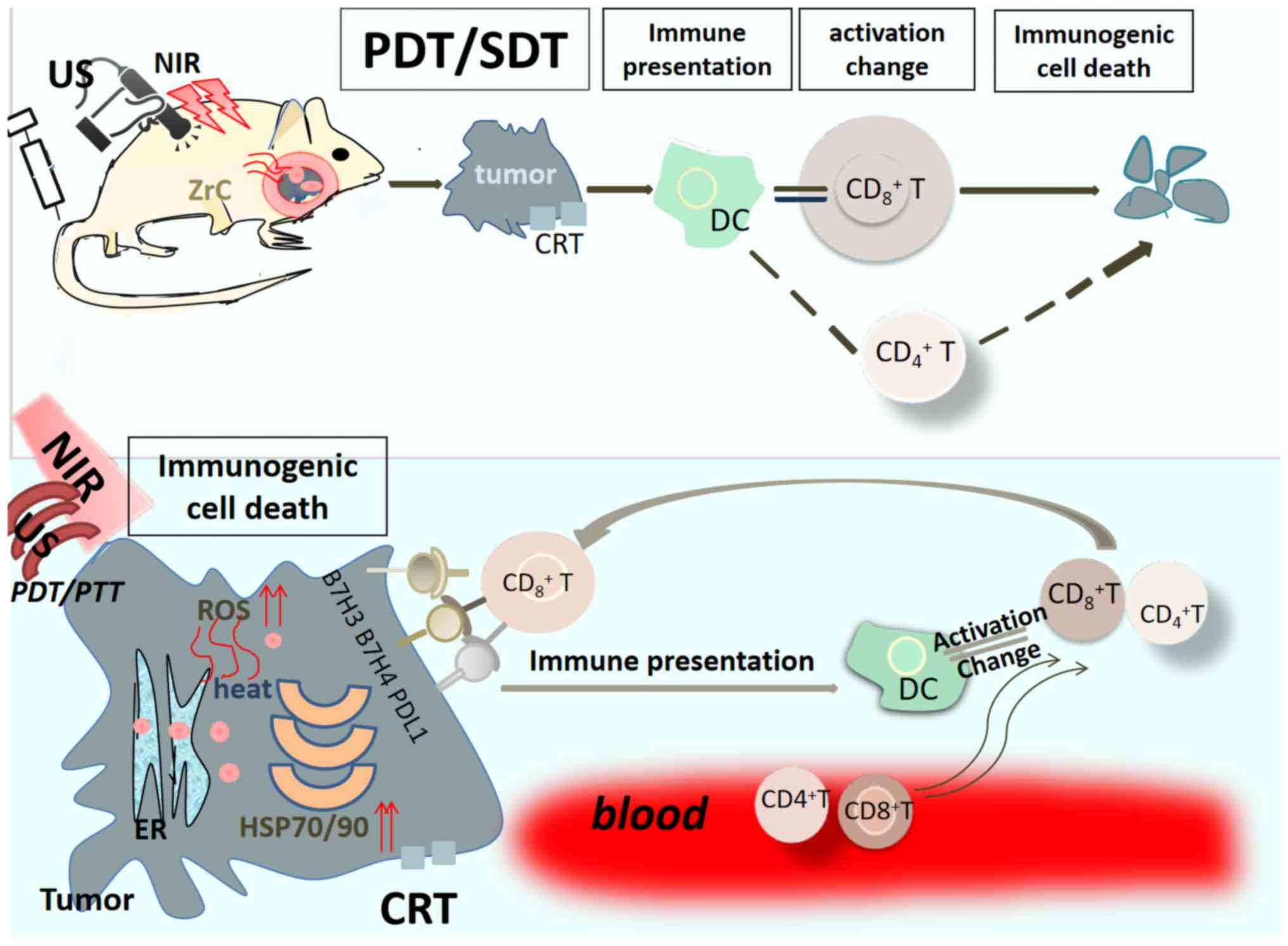
SPDT enhances bone tumor
immunotherapy
When the application of PDT combined with SDT is
successful in terms of destroying the bone tumor, not only are the
processes of apoptosis and necrosis involved, but an immunogenic
reaction is also mediated. For example, treatment of the bone tumor
with black phosphorus (BP) (with its phosphorus element in the BP),
which targets the nucleic acid and cell bilayer membrane skeleton,
upon being radiated by NIR and US, leads to the ‘double killing’ of
the targeted cancer cells; to be specific, the process not only
induces tumoral structural damage through heat, ROS and shock
waves, but an anticancer immune stress response is also stimulated
that originates from the damaged tumor in situ tissue, and
this process is termed photoimmunotherapy (92). Bone tumor cells immediately
endocytose BP and undergo oxidative stress (at the same time
increasing the level of energy metabolism), whereupon BP is
converted into PO43−; this is accompanied by
a massive induction of apoptosis. At higher temperatures, necrosis
is triggered, which both stimulates the outflow of tumor-associated
antigens and promotes the maturation of follicular DCs; this
process greatly enhances the CD8+ T
cell-mediated cytotoxic killing response, which is termed
‘immunogenic cell death’. Moreover, CD8+ T
cells are able to specifically recognize and eliminate the B7
family ligand/receptor molecules B7-H3, B7-H4 and PD-L1, which are
expressed by tumor cells (Fig. 5).
In addition, the infiltration of immune cells, such as natural
killer cells, lymphocytes and DCs, is effectively reversed through
relieving the damage-associated molecular patterns, which includes
calreticulin, the release of ATP, the secretion of high mobility
group box 1 and heat-shock proteins (93). ‘Cold’ tumors are thereby converted
into ‘hot’ tumors, and the body can spontaneously form an immune
system response to target the tumor.
Research on direction-targeted materials of
bone tumor based on PDT
As the number of publications has grown, the
therapeutic materials of PDT for bone tumors may be currently
divided into the categories presented in Table I. MPPa is a second-generation PS
which is used in the treatment of OS, and is has been successfully
shown to induce the apoptosis of MG-63 cells (94). In addition, mTHPC (95), hiporfin-PDT (96), aloe-emodin (97) and PS M007 (98) have been used in conjunction with PDT
to counteract the multiplication of human OS cells in seconds to
restore the function of DCs via upregulating HSP70 (99). The first generation (which has now
been superseded) utilized metal NPs, for example, gold (100), silver and Pt (101). The second generation comprised
carbon materials, including the preparation of PDT nanomaterials
through the combination of graphene oxide (GO) and PEG (102). These have attractive photophysical
and photochemical properties, although their disadvantages are poor
solvability and high crystallinity, which serve as limiting factors
in terms of their loading into most drug delivery systems (DDSSs),
thereby hindering further drug development. PEG-d to carry ZnPc,
thereby achieving π-π interaction (103). In addition, the constituent
PEG-PMAN/ZnPc NPs (PPZ) was revealed in physical experiments in
vivo to markedly increase the production of ROS in OS cells,
resulting in mitochondrial damage and arresting the cell cycle at
the G2/M checkpoint (103). Drug-mediated bone tumor PDT and
photothermal therapy (PTT) mainly comprise 5-aminolevulinic acid
(5-ALA) and synchronous hyperthermia (104).
 | Table I.Representative materials targeting
therapy for bone tumors and their associated mechanisms of PDT. |
Table I.
Representative materials targeting
therapy for bone tumors and their associated mechanisms of PDT.
| PDT
nanomaterials | PDT
photosensitizer | Types of bone
tumors treated | Associated
underlying mechanism that is affected by the treatment | (Refs.) |
|---|
| - | MPPa | MG-63 osteosarcoma
cells | Mitochondrial
apoptosis pathway induces apoptosis, whereas the ROS-JNK signaling
pathway participates in autophagy induced by MPPa-PDT | (79) |
| - | 5,10,15,20-Tetrakis
(metahydroxyphenyl) | Human 143B
cells | Tumor
cell-targeting mechanism that causes antivascular effect and
stimulates the immune system | (80) |
| Carbon materials:
The combination of GO and PEG | - | HOS cells | Attenuates the M2
polarization of macrophages that is induced by IL-4, regulating its
antitumor ability | (87) |
| - | PPZ
nanoparticles | Osteosarcoma
cells | Mitochondrial
damage and cell cycle arrest at the G2/M checkpoint | (88) |
At present, one limitation of available DDSSs is the
difficulty in efficiently transmitting the photothermal agents to
the source of the tumor. The surface of modified silicon dioxide NP
(SLN) has been demonstrated to be adaptable by the CM to construct
a platform (CM/SLN) that is able to target homogenous 143B cells.
Moreover, Zhang et al (105) used indocyanine green at a
PTT-level dose in conjunction with CM/SLN. PDT is also becoming
increasingly popular in the adjuvant therapy of bone tumors, such
as treatment with cisplatin (106).
Current status of SDT-based treatment of
bone tumors
SDT has promising prospects in terms of treating
cancer. Compared with organic nanomaterials, inorganic materials
have the characteristics of stronger skin sensitivity, faster
pharmacokinetics, a higher stability and a faster conversion rate.
At the same time, however, the low conversion efficiency and
biosafety challenges of SDT should also be taken into account
(107,108). The effects of SDT on the growth of
implantable bone tumors has also been widely studied.
5-ALA-mediated SDT caused a marked attenuation of the bone tumor
volume and the vitality of UMR-106 cells, although ROS generation
was promoted (109). The
experimental results obtained confirmed that the expression of
Bcl-2 was downregulated. By contrast, the expression levels of Bax,
p53 and caspase-3 were markedly increased, demonstrating that
ALA-SDT was able to act on the associated mitochondrial
ROS-mediated apoptotic pathway of OS cells. A number of other
studies have produced similar results, including a study of
hematoporphyrin monomethyl ether-sonodynamic therapy on MG-63 OS
cells (94). Geng et al
(110) designed W-doped TiO2
(W-TiO2) nanometer material for the treatment of OS. Oxygen and
Ti-vacancies were introduced into W-TiO2 nanorods to enhance their
sonodynamic properties, and W6+ restored the endogenous GSH to
GSSG. Following a similar principle (and mechanism), other studies
on Cu(II) NS sonosensitizers containing porphyrins, Cu2+, and PEG,
have yielded similar results. In summary, fundamentally changing
the redox microenvironment of the tumor has a great effect in terms
of promoting the curative effect on the bone tumor (111).
Conclusion
PDT produces ROS, which eliminate superficial tumor
tissue according to the toxic oxidation level. In SDT, when the
acoustic sensitizer releases energy, this forms cavitation bubbles
around the cancer cells. After a period of time, the energy
produced by the collapse of cavitation bubbles causes sound, light
and heat damage to the cancer tissue via mechanical stress. The
efficient combination of these can bring about the complete
destruction of primary and metastatic bone tumors. A large number
of studies have demonstrated that targeting GSH consumption
significantly promotes ROS-based therapies (i.e., those containing
PDT, SDT, CDT, ferroptosis, etc.). The present review analyzed the
sources, metabolism and associated functions of GSH in detail. Due
to the special anatomical structure of bone tumors, PDT/SDT
combination therapy has been identified to have an indispensable
role in terms of targeting the ECM and the RANK/RANKL signaling
pathway. In addition, discussions are still ongoing regarding the
different mechanisms of action. In terms of the work of the authors
of the present review, their research is focused on PS/sound
sensitizers in the obliteration and clinical transformation
optimization of bone tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Nature Science
Foundation of China (grant no. 82072985), the Postdoctoral
Scientific Research Developmental Fund of Heilongjiang Province
(grant no. LBH-Q18076), the N10 Found project of Harbin Medical
University Cancer Hospital (grant no. 2017-03), the Outstanding
Youth Foundation of Harbin Medical University Cancer Hospital
(grant no. JCQN-2018-05), the National Nature Science Foundation of
Heilongjiang Province (grant no. YQ2020H036), the Special funds of
central finance to support the development of local Universities
(grant no. 2020GSP04), the Wu-Jieping Medical Foundation (grant no.
320.6750.19089-22,320.6750.19089-48), the Beijing Medical Award
Foundation (grant no. YXJL-2019-1416-0069), Scientific Research
Project of Provincial Health Commission (grant no. 20210808020126),
Joint guidance Project of Provincial Natural Science Foundation
(grant no. LH2021H066), the Hai Yan Youth Fund of Harbin Medical
University Cancer Hospital (grant no. JJQN2021-02), the Fundamental
Research Funds for the Provincial Universities (grant no.
2021-KYYWF-0253) and the Natural Science Foundation of Heilongjiang
(grant no. LH2022H065).
Availability of data and materials
Not applicable.
Authors' contributions
YH, DZ, XY reviewed the literature and wrote the
first draft. CG revised and interpreted the related information. MW
and JB contributed to the conception and design of the manuscript.
GQ and HM supervised the manuscript and provided critical
revisions. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shao R, Wang Y, Li L, Dong Y, Zhao J and
Liang W: Bone tumors effective therapy through functionalized
hydrogels: Current developments and future expectations. Drug
Deliv. 29:1631–1647. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel GW, Biermann JS, Calinescu AA,
Spratt DE and Szerlip NJ: Surgical approach to bone metastases.
Curr Osteoporos Rep. 16:512–518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwiatkowski S, Knap B, Przystupski D,
Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O,
Kotowski K and Kulbacka J: Photodynamic therapy-mechanisms,
photosensitizers and combinations. Biomed Pharmacother.
106:1098–1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji B, Wei M and Yang B: Recent advances in
nanomedicines for photodynamic therapy (PDT)-driven cancer
immunotherapy. Theranostics. 12:434–458. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Lovell JF, Yoon J and Chen X:
Clinical development and potential of photothermal and photodynamic
therapies for cancer. Nat Rev Clin Oncol. 17:657–674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Son S, Kim JH, Wang X, Zhang C, Yoon SA,
Shin J, Sharma A, Lee MH, Cheng L, Wu J and Kim JS: Multifunctional
sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev.
49:3244–3261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu T, Liu Y, Cao Y and Liu Z: Engineering
macrophage exosome disguised biodegradable nanoplatform for
enhanced sonodynamic therapy of glioblastoma. Adv Mater.
34:e21103642022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yumita N, Nishigaki R, Umemura K and
Umemura S: Hematoporphyrin as a sensitizer of cell-damaging effect
of ultrasound. Jpn J Cancer Res. 80:219–222. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan X, Wang H, Wang S, Sun X, Wang L, Wang
W, Shen H and Liu H: Sonodynamic therapy (SDT): A novel strategy
for cancer nanotheranostics. Sci China Life Sci. 61:415–426. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji C, Si J, Xu Y, Zhang W, Yang Y, He X,
Xu H, Mou X, Ren H and Guo H: Mitochondria-targeted and
ultrasound-responsive nanoparticles for oxygen and nitric oxide
codelivery to reverse immunosuppression and enhance sonodynamic
therapy for immune activation. Theranostics. 11:8587–8604. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Huang J, Liu M, Qiu Y, Chen Q,
Zhao T, Xiao Z, Yang Y, Jiang Y, Huang Q and Ai K: Emerging
sonodynamic therapy-based nanomedicines for cancer immunotherapy.
Adv Sci (Weinh). 10:e22043652023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu M, Zhou L, Zheng L, Zhou Q, Liu K, Mao
Y and Song S: Sonodynamic therapy-derived multimodal synergistic
cancer therapy. Cancer Lett. 497:229–242. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zhang X, Yang H, Yu L, Xu Y,
Sharma A, Yin P, Li X, Kim JS and Sun Y: Advanced
biotechnology-assisted precise sonodynamic therapy. Chem Soc Rev.
50:11227–11248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Y, Ye J, Li Z, Chen H and Gao Y:
Recent progress in sono-photodynamic cancer therapy: From developed
new sensitizers to nanotechnology-based efficacy-enhancing
strategies. Acta Pharm Sin B. 11:2197–2219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Wang P, Liu Q and Wang X:
Sinoporphyrin sodium triggered sono-photodynamic effects on breast
cancer both in vitro and in vivo. Ultrason Sonochem. 31:437–448.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Li C, Wang X, Xiong W, Feng X, Liu
Q, Leung AW and Xu C: Anti-metastatic and pro-apoptotic effects
elicited by combination photodynamic therapy with sonodynamic
therapy on breast cancer both in vitro and in vivo. Ultrason
Sonochem. 23:116–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li R, Chen Z, Dai Z and Yu Y:
Nanotechnology assisted photo- and sonodynamic therapy for
overcoming drug resistance. Cancer Biol Med. 18:388–400. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Q, Ma X, Song Y, Chen Q, Jiao Q and
Zhou L: Targeting regulated cell death in tumor nanomedicines.
Theranostics. 12:817–841. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huo J, Jia Q, Huang H, Zhang J, Li P, Dong
X and Huang W: Emerging photothermal-derived multimodal synergistic
therapy in combating bacterial infections. Chem Soc Rev.
50:8762–8789. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Xiao Z, An Y, Han S, Wu W, Wang
Y, Guo Y and Shuai X: Nanodrug with dual-sensitivity to tumor
microenvironment for immuno-sonodynamic anti-cancer therapy.
Biomaterials. 269:1206362021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin X, Song J, Chen X and Yang H:
Ultrasound-activated sensitizers and applications. Angew Chem Int
Ed Engl. 59:14212–14233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang XQ, Wang W, Peng M and Zhang XZ: Free
radicals for cancer theranostics. Biomaterials. 266:1204742021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Wang Z, Xiong Y, Wang C, Deng Q,
Yang T, Xu Q, Yong Z, Yang X and Li Z: A two-pronged strategy to
alleviate tumor hypoxia and potentiate photodynamic therapy by mild
hyperthermia. Biomater Sci. 11:108–118. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phua SZF, Yang G, Lim WQ, Verma A, Chen H,
Thanabalu T and Zhao Y: Catalase-Integrated hyaluronic acid as
nanocarriers for enhanced photodynamic therapy in solid tumor. ACS
Nano. 13:4742–4751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang D, Wu M, Zeng Y, Wu L, Wang Q, Han
X, Liu X and Liu J: Chlorin e6 conjugated poly(dopamine)
nanospheres as PDT/PTT dual-modal therapeutic agents for enhanced
cancer therapy. ACS Appl Mater Interfaces. 7:8176–8187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia Q, Zhang Y, Li Z, Hou X and Feng N:
Red blood cell membrane-camouflaged nanoparticles: A novel drug
delivery system for antitumor application. Acta Pharm Sin B.
9:675–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruiz-Moreno JM, Montero JA, Barile S and
Zarbin MA: Photodynamic therapy and high-dose intravitreal
triamcinolone to treat exudative age-related macular degeneration:
1-Year outcome. Retina. 26:602–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang M, Li J, Gu P and Fan X: The
application of nanoparticles in cancer immunotherapy: Targeting
tumor microenvironment. Bioact Mater. 6:1973–1987. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan H, Liu Y, Gao Z and Huang W: Recent
advances in drug delivery systems for targeting cancer stem cells.
Acta Pharm Sin B. 11:55–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu
Z, Hao Z, Li Z, Liu L and Xie J: Protein nanocage mediated
fibroblast-activation protein targeted photoimmunotherapy to
enhance cytotoxic T cell infiltration and tumor control. Nano Lett.
17:862–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Z, Liu W, Yang Z, Luo Y, Qiao C, Xie
A, Jia Q, Yang P, Wang Z and Zhang R: Sonodynamic-immunomodulatory
nanostimulators activate pyroptosis and remodel tumor
microenvironment for enhanced tumor immunotherapy. Theranostics.
13:1571–1583. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin T, Chen H, Ma A, Pan H, Chen Z, Tang
X, Huang G, Liao J, Zhang B, Zheng M and Cai L: Cleavable
collagenase-assistant nanosonosensitizer for tumor penetration and
sonodynamic therapy. Biomaterials. 293:1219922023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang D, Feng F, Li Q, Wang X and Yao L:
Nanopurpurin-based photodynamic therapy destructs extracellular
matrix against intractable tumor metastasis. Biomaterials.
173:22–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Zhu W, Cheng L, Cai R, Yi X, He J,
Pan X, Yang L, Yang K, Liu Z, et al: Tumor microenvironment
(TME)-activatable circular aptamer-PEG as an effective
hierarchical-targeting molecular medicine for photodynamic therapy.
Biomaterials. 246:1199712020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Tian L, Zhang R, Dong Z, Wang H and
Liu Z: Collagenase-encapsulated pH-responsive nanoscale
coordination polymers for tumor microenvironment modulation and
enhanced photodynamic nanomedicine. ACS Appl Mater Interfaces.
10:43493–43502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuo Q, Ou Y, Zhong S, Yu H, Zhan F and
Zhang M: Targeting GRP78 enhances the sensitivity of HOS
osteosarcoma cells to pyropheophorbide-α methyl ester-mediated
photodynamic therapy via the Wnt/β-catenin signaling pathway. Acta
Biochim Biophys Sin (Shanghai). 53:1387–1397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yalçın CÖ, Barut B, Barut EN, Demirbaş Ü,
Dinçer T, Engin S, Özel A and Sena Sezen F: Photodynamic therapy
effect of morpholinium containing silicon (IV) phthalocyanine on
HCT-116 cells. Photodiagnosis Photodyn Ther. 32:1019752020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang XY, Zhang JG, Zhou QM, Yu JN, Lu YF,
Wang XJ, Zhou JP, Ding XF, Du YZ and Yu RS: Extracellular matrix
modulating enzyme functionalized biomimetic Au
nanoplatform-mediated enhanced tumor penetration and synergistic
antitumor therapy for pancreatic cancer. J Nanobiotechnology.
20:5242022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang SB, Chen ZX, Gao F, Zhang C, Zou MZ,
Ye JJ, Zeng X and Zhang XZ: Remodeling extracellular matrix based
on functional covalent organic framework to enhance tumor
photodynamic therapy. Biomaterials. 234:1197722020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Yu W, Chen M, Zhang B, Zhang L
and Li P: The applications of nanozymes in cancer therapy: Based on
regulating pyroptosis, ferroptosis and autophagy of tumor cells.
Nanoscale. Jun 28–2023.(Epub ahead of print).
|
|
44
|
Li Q, Liu Q, Wang P, Feng X, Wang H and
Wang X: The effects of Ce6-mediated sono-photodynamic therapy on
cell migration, apoptosis and autophagy in mouse mammary 4T1 cell
line. Ultrasonics. 54:981–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo T, Liu T, Sun Y, Liu X, Xiong R, Li H,
Li Z, Zhang Z, Tian Z and Tian Y: Sonodynamic therapy inhibits
palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent
mitophagy. Cell Death Dis. 10:4572019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Y, Yin H, Tao Y, Zhong S, Yu H, Li J,
Bai Z and Ou Y: Antitumor effects and mechanisms of
pyropheophorbide-α methyl ester-mediated photodynamic therapy on
the human osteosarcoma cell line MG-63. Int J Mol Med. 45:971–982.
2020.PubMed/NCBI
|
|
47
|
Lu SL, Wang YH, Liu GF, Wang L, Li Y, Guo
ZY and Cheng C: Graphene oxide nanoparticle-loaded ginsenoside Rg3
improves photodynamic therapy in inhibiting malignant progression
and stemness of osteosarcoma. Front Mol Biosci. 8:6630892021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zou W, Hao J, Wu J, Cai X, Hu B, Wang Z
and Zheng Y: Biodegradable reduce expenditure bioreactor for
augmented sonodynamic therapy via regulating tumor hypoxia and
inducing pro-death autophagy. J Nanobiotechnology. 19:4182021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Peng Z, Yuan L, XuHong J, Tian H, Zhang Y,
Deng J and Qi X: Chiral nanomaterials for tumor therapy: Autophagy,
apoptosis, and photothermal ablation. J Nanobiotechnology.
19:2202021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang M, Hu W, Cai C, Wu Y, Li J and Dong
S: Advanced application of stimuli-responsive drug delivery system
for inflammatory arthritis treatment. Mater Today Bio.
14:1002232022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lai HW, Takahashi K, Nakajima M, Tanaka T
and Ogura SI: Efficiency of aminolevulinic acid (ALA)-photodynamic
therapy based on ALA uptake transporters in a cell
density-dependent malignancy model. J Photochem Photobiol B.
218:1121912021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Su X, Zhuang D, Zhang Y, Lv H, Wang Y,
Luan X and Bi L: Influence of photodynamic therapy on the
periodontitis-induced bone resorption in rat. Lasers Med Sci.
36:675–680. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Franco EJ, Pogue RE, Sakamoto LH,
Cavalcante LL, Carvalho DR and de Andrade RV: Increased expression
of genes after periodontal treatment with photodynamic therapy.
Photodiagnosis Photodyn Ther. 11:41–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Romagnoli C, Marcucci G, Favilli F,
Zonefrati R, Mavilia C, Galli G, Tanini A, Iantomasi T, Brandi ML
and Vincenzini MT: Role of GSH/GSSG redox couple in osteogenic
activity and osteoclastogenic markers of human osteoblast-like
SaOS-2 cells. FEBS J. 280:867–879. 2013.PubMed/NCBI
|
|
55
|
Moya IM and Halder G: Hippo-YAP/TAZ
signalling in organ regeneration and regenerative medicine. Nat Rev
Mol Cell Biol. 20:211–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W,
Ho KK, Qin L, Song H and Mak KK: Reciprocal inhibition of YAP/TAZ
and NF-κB regulates osteoarthritic cartilage degradation. Nat
Commun. 9:45642018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rytlewski JD, Scalora N, Garcia K, Tanas
M, Toor F, Miller B, Allen B, Milhem M and Monga V: Photodynamic
therapy using hippo pathway inhibitor verteporfin: A potential dual
mechanistic approach in treatment of soft tissue sarcomas. Cancers
(Basel). 13:6752021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhan F, He T, Chen Z, Zuo Q, Wang Y, Li Q,
Zhong S and Ou Y: RhoA enhances osteosarcoma resistance to MPPa-PDT
via the Hippo/YAP signaling pathway. Cell Biosci. 11:1792021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang C, Zhu X, Feng W, Yu Y, Jeong K, Guo
W, Lu Y and Mills GB: Verteporfin inhibits YAP function through
up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am J
Cancer Res. 6:27–37. 2015.PubMed/NCBI
|
|
60
|
Zhou A, Fang T, Chen K, Xu Y, Chen Z and
Ning X: Biomimetic activator of sonodynamic ferroptosis amplifies
inherent peroxidation for improving the treatment of breast cancer.
Small. 18:e21065682022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xiao Y, Zhang T, Ma X, Yang QC, Yang LL,
Yang SC, Liang M, Xu Z and Sun ZJ: Microenvironment-responsive
prodrug-induced pyroptosis boosts cancer immunotherapy. Adv Sci
(Weinh). 8:e21018402021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shui S, Zhao Z, Wang H, Conrad M and Liu
G: Non-enzymatic lipid peroxidation initiated by photodynamic
therapy drives a distinct ferroptosis-like cell death pathway.
Redox Biol. 45:1020562021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun J, Du K, Diao J, Cai X, Feng F and
Wang S: GSH and H2 O2 Co-activatable
mitochondria-targeted photodynamic therapy under normoxia and
hypoxia. Angew Chem Int Ed Engl. 59:12122–12128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu G, Fang YZ, Yang S, Lupton JR and
Turner ND: Glutathione metabolism and its implications for health.
J Nutr. 134:489–492. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu Q, Zhan G, Zhang Z, Yong T, Yang X and
Gan L: Manganese porphyrin-based metal-organic framework for
synergistic sonodynamic therapy and ferroptosis in hypoxic tumors.
Theranostics. 11:1937–1952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lai Y, Lu N, Ouyang A, Zhang Q and Zhang
P: Ferroptosis promotes sonodynamic therapy: A
platinum(ii)-indocyanine sonosensitizer. Chem Sci. 13:9921–9926.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhao LP, Chen SY, Zheng RR, Rao XN, Kong
RJ, Huang CY, Liu YB, Tang Y, Cheng H and Li SY: Photodynamic
therapy initiated ferrotherapy of self-delivery nanomedicine to
amplify lipid peroxidation via GPX4 inactivation. ACS Appl Mater
Interfaces. 14:53501–53510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Y, Zhang L, Zhao G, Zhang Y, Zhan F,
Chen Z, He T, Cao Y, Hao L, Wang Z, et al: Homologous targeting
nanoparticles for enhanced PDT against osteosarcoma HOS cells and
the related molecular mechanisms. J Nanobiotechnology. 20:832022.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Meng X, Deng J, Liu F, Guo T, Liu M, Dai
P, Fan A, Wang Z and Zhao Y: Triggered all-active metal organic
framework: ferroptosis machinery contributes to the apoptotic
photodynamic antitumor therapy. Nano Lett. 19:7866–7876. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Di Giorgio E, Ferino A, Choudhary H,
Löffler PMG, D'Este F, Rapozzi V, Tikhomirov A, Shchekotikhin A,
Vogel S and Xodo LE: Photosensitization of pancreatic cancer cells
by cationic alkyl-porphyrins in free form or engrafted into POPC
liposomes: The relationship between delivery mode and mechanism of
cell death. J Photochem Photobiol B. 231:1124492022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu F, Liu Y, Wu Y, Song D, Qian J and Zhu
B: Chlorin e6 and polydopamine modified gold nanoflowers for
combined photothermal and photodynamic therapy. J Mater Chem B.
8:2128–2138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang Y, Xu Y, Guo X, Wang L, Zeng J, Qiu
H, Tan Y, Chen D, Zhao H and Gu Y: Enhanced antimicrobial activity
through the combination of antimicrobial photodynamic therapy and
low-frequency ultrasonic irradiation. Adv Drug Deliv Rev.
183:1141682022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Aksel M, Bozkurt-Girit O and Bilgin MD:
Pheophorbide a-mediated sonodynamic, photodynamic and
sonophotodynamic therapies against prostate cancer. Photodiagnosis
Photodyn Ther. 31:1019092020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang X, Chen Y, Yang X, Cheng L, He Z, Xin
Y, Huang S, Meng F, Zhang P and Luo L: Activation of ALOX12 by a
multi-organelle-orienting photosensitizer drives ACSL4-independent
cell ferroptosis. Cell Death Dis. 13:10402022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fekrazad R, Seraj B, Chiniforush N,
Rokouei M, Mousavi N and Ghadimi S: Effect of antimicrobial
photodynamic therapy on the counts of salivary Streptococcus mutans
in children with severe early childhood caries. Photodiagnosis
Photodyn Ther. 18:319–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chang M, Hou Z, Wang M, Wang M, Dang P,
Liu J, Shu M, Ding B, Al Kheraif AA, Li C and Lin J: Cu2
MoS4/Au heterostructures with enhanced catalase-like
activity and photoconversion efficiency for primary/metastatic
tumors eradication by phototherapy-induced immunotherapy. Small.
16:e19071462020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Martins WK, Santos NF, Rocha CS, Bacellar
IOL, Tsubone TM, Viotto AC, Matsukuma AY, Abrantes ABP, Siani P,
Dias LG and Baptista MS: Parallel damage in mitochondria and
lysosomes is an efficient way to photoinduce cell death. Autophagy.
15:259–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu Y, Dong Z, Zhang R, Wang Z, Shi Y, Liu
M, Yang J, Yang T, Zhang R, Wang T, et al: Sonodynamic therapy
reduces cardiomyocyte apoptosis through autophagy activated by
reactive oxygen species in myocardial infarction. Free Radic Biol
Med. 195:36–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rogowska-Tylman J, Locs J, Salma I,
Woźniak B, Pilmane M, Zalite V, Wojnarowicz J, Kędzierska-Sar A,
Chudoba T, Szlązak K, et al: In vivo and in vitro study of a novel
nanohydroxyapatite sonocoated scaffolds for enhanced bone
regeneration. Mater Sci Eng C Mater Biol Appl. 99:669–684. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Arnold L, Hendricks-Wenger A,
Coutermarsh-Ott S, Gannon J, Hay AN, Dervisis N, Klahn S, Allen IC,
Tuohy J and Vlaisavljevich E: Histotripsy ablation of bone tumors:
Feasibility study in excised canine osteosarcoma tumors. Ultrasound
Med Biol. 47:3435–3446. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang J, Zhao Z, Liu Y, Cao X, Li F, Ran H,
Cao Y and Wu C: ‘Mito-Bomb’: A novel mitochondria-targeting
nanosystem for ferroptosis-boosted sonodynamic antitumor therapy.
Drug Deliv. 29:3111–3122. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tian Z, Liu H, Zhao Y, Wang X, Ren H,
Zhang F, Li P, Zhang P, Wang J and Yao W: Secondary pneumothorax as
a potential marker of apatinib efficacy in osteosarcoma: A
multicenter analysis. Anticancer Drugs. 32:82–87. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Antonioli M, Di Rienzo M, Piacentini M and
Fimia GM: Emerging mechanisms in initiating and terminating
autophagy. Trends Biochem Sci. 42:28–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kara O, Seseogullari Dirihan R, Sayin Ozel
G, Tezvergil Mutluay A and Usumez A: Inhibition of cathepsin-K and
matrix metalloproteinase by photodynamic therapy. Dent Mater.
37:e485–e492. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wan Y, Fu LH, Li C, Lin J and Huang P:
Conquering the hypoxia limitation for photodynamic therapy. Adv
Mater. 33:e21039782021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhao L, Rao X, Huang C, Zheng R, Kong R,
Chen Z, Yu X, Cheng H and Li S: Epigenetic reprogramming of carrier
free photodynamic modulator to activate tumor immunotherapy by EZH2
inhibition. Biomaterials. 293:1219522023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu
C, Li X, Wu D, Xia S, Chen J, et al: Acetylation of KLF5 maintains
EMT and tumorigenicity to cause chemoresistant bone metastasis in
prostate cancer. Nat Commun. 12:17142021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
MacDonald IJ, Tsai HC, Chang AC, Huang CC,
Yang SF and Tang CH: Melatonin inhibits osteoclastogenesis and
osteolytic bone metastasis: Implications for osteoporosis. Int J
Mol Sci. 22:94352021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sardoiwala MN, Kushwaha AC, Dev A,
Shrimali N, Guchhait P, Karmakar S and Roy Choudhury S:
Hypericin-loaded transferrin nanoparticles induce PP2A-regulated
BMI1 degradation in colorectal cancer-specific chemo-photodynamic
therapy. ACS Biomater Sci Eng. 6:3139–3153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kaundal B, Srivastava AK, Sardoiwala MN,
Karmakar S and Choudhury SR: A NIR-responsive indocyanine
green-genistein nanoformulation to control the polycomb epigenetic
machinery for the efficient combinatorial photo/chemotherapy of
glioblastoma. Nanoscale Adv. 1:2188–2207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu P, Zhang W, Deng J, Zheng Y, Weng J,
Yu F, Wang D, Zheng M, Kang B and Zeng H: Chain-shattering
polymeric sulfur dioxide prodrug micelles for redox-triggered gas
therapy of osteosarcoma. J Mater Chem B. 10:5263–5271. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dong W, Wang H, Liu H, Zhou C, Zhang X,
Wang S and He L: Potential of black phosphorus in immune-based
therapeutic strategies. Bioinorg Chem Appl. 2022:37900972022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Huang W, Gao Y, Wang J, Ding P, Yan M, Wu
F, Liu J, Liu D, Guo C, Yang B and Cao W: Plasmonic enhanced
reactive oxygen species activation on low-work-function tungsten
nitride for direct near-infrared driven photocatalysis. Small.
16:e20045572020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang Q, Ou YS, Tao Y, Yin H and Tu PH:
Apoptosis and autophagy induced by pyropheophorbide-α methyl
ester-mediated photodynamic therapy in human osteosarcoma MG-63
cells. Apoptosis. 21:749–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Meier D, Botter SM, Campanile C, Robl B,
Gräfe S, Pellegrini G, Born W and Fuchs B: Foscan and foslip based
photodynamic therapy in osteosarcoma in vitro and in intratibial
mouse models. Int J Cancer. 140:1680–1692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sun M, Zhou C, Zeng H, Puebla-Osorio N,
Damiani E, Chen J, Wang H, Li G, Yin F, Shan L, et al:
Hiporfin-mediated photodynamic therapy in preclinical treatment of
osteosarcoma. Photochem Photobiol. 91:533–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tu P, Huang Q, Ou Y, Du X, Li K, Tao Y and
Yin H: Aloe-emodin-mediated photodynamic therapy induces autophagy
and apoptosis in human osteosarcoma cell line MG-63 through the
ROS/JNK signaling pathway. Oncol Rep. 35:3209–3215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou YK, Wu WZ, Zhang L, Yang CH and Wang
YP: Effect of M007 mediated photodynamic therapy on proliferation
of human osteosarcoma MG63 cells in vitro. Sichuan Da Xue Xue Bao
Yi Xue Ban. 43:41–45. 2012.(In Chinese). PubMed/NCBI
|
|
99
|
Zhang F, Zhu Y, Fan G and Hu S:
Photodynamic therapy reduces the inhibitory effect of osteosarcoma
cells on dendritic cells by upregulating HSP70. Oncol Lett.
16:5034–5040. 2018.PubMed/NCBI
|
|
100
|
Bu Y, Huang R, Li Z, Zhang P, Zhang L,
Yang Y, Liu Z, Guo K and Gao F: Anisotropic truncated octahedral Au
with Pt deposition on arris for localized surface plasmon
resonance-enhanced photothermal and photodynamic therapy of
osteosarcoma. ACS Appl Mater Interfaces. 13:35328–35341. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xiong S, Xiong G, Li Z, Jiang Q, Yin J,
Yin T and Zheng H: Gold nanoparticle-based nanoprobes with enhanced
tumor targeting and photothermal/photodynamic response for therapy
of osteosarcoma. Nanotechnology. 32:1551022021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Deng X, Liang H, Yang W and Shao Z:
Polarization and function of tumor-associated macrophages mediate
graphene oxide-induced photothermal cancer therapy. J Photochem
Photobiol B. 208:1119132020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yu W, Ye M, Zhu J, Wang Y, Liang C, Tang
J, Tao H and Shen Y: Zinc phthalocyanine encapsulated in polymer
micelles as a potent photosensitizer for the photodynamic therapy
of osteosarcoma. Nanomedicine. 14:1099–1110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yanase S, Nomura J, Matsumura Y, Nagai K,
Kinoshita M, Nakanishi H, Ohnishi Y, Tokuda T and Tagawa T:
Enhancement of the effect of 5-aminolevulinic acid-based
photodynamic therapy by simultaneous hyperthermia. Int J Oncol.
27:193–201. 2005.PubMed/NCBI
|
|
105
|
Zhang J, Miao Y, Ni W, Xiao H and Zhang J:
Cancer cell membrane coated silica nanoparticles loaded with ICG
for tumour specific photothermal therapy of osteosarcoma. Artif
Cells Nanomed Biotechnol. 47:2298–2305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kusuzaki K, Murata H, Matsubara T,
Miyazaki S, Shintani K, Seto M, Matsumine A, Hosoi H, Sugimoto T
and Uchida A: Clinical outcome of a novel photodynamic therapy
technique using acridine orange for synovial sarcomas. Photochem
Photobiol. 81:705–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Heymann PG, Ziebart T, Kämmerer PW, Mandic
R, Saydali A, Braun A, Neff A and Draenert GF: The enhancing effect
of a laser photochemotherapy with cisplatin or zolendronic acid in
primary human osteoblasts and osteosarcoma cells in vitro. J Oral
Pathol Med. 45:803–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
He Z, Du J, Miao Y and Li Y: Recent
developments of inorganic nanosensitizers for sonodynamic therapy.
Adv Healthc Mater. e23002342023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu X, Li W, Geng S, Meng QG and Bi ZG:
Apoptosis induced by sonodynamic therapy in human osteosarcoma
cells in vitro. Mol Med Rep. 12:1183–1188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Geng B, Yang X, Li P, Shi W, Pan D and
Shen L: W-doped TiO2 nanorods for multimode tumor
eradication in osteosarcoma models under single ultrasound
irradiation. ACS Appl Mater Interfaces. 13:45325–45334. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang H, Guo J, Lin W, Fu Z, Ji X, Yu B, Lu
M, Cui W, Deng L, Engle JW, et al: Open-shell nanosensitizers for
glutathione responsive cancer sonodynamic therapy. Adv Mater.
34:e21102832022. View Article : Google Scholar : PubMed/NCBI
|