Introduction
According to the latest cancer data statistics, the
incidence rate and mortality of lung cancer rank second and first
in all malignant tumors, respectively (1,2).
According to the latest available data in China, lung cancer has
the highest incidence and mortality rates. The histological types
of lung cancer are divided into non-small cell lung cancer (NSCLC)
and small cell lung cancer (SCLC), with NSCLC accounting for ~85%
of the total number of lung cancer cases (3,4).
Patients are often in advanced stages at the time of treatment,
with limited treatment options and poor prognosis. The treatment of
lung cancer has attracted widespread attention due to its low
5-year survival that is <15% (5). The treatment of lung cancer mainly
includes surgical resection, radiotherapy, chemotherapy,
immunotherapy and targeted therapy. Targeted therapy offers several
advantages, such as high selectivity and minimal adverse reactions.
With the advent of precision medicine, targeted therapy has been
widely applied in the field of cancer and has become a research
hotspot in recent years. Epidermal growth factor receptor (EGFR) is
a tyrosine kinase receptor, which carries the most common oncogenic
driving mutation in NSCLC. Mutations in this gene significantly
enhance the growth and division of cancer cells. ~15% of Caucasians
and 50% of Asian patients with late-stage NSCLC have mutations in
the EGFR domain (6,7). Among these mutations, the deletion of
exon 19 and the point mutation of exon 21 L858R accounted for 90%
of EGFR mutations (8). The
development of anticancer drugs targeting this specific target has
greatly changed the treatment methods and their prognostic efficacy
for patients with NSCLC. The EGFR target was the first target to be
discovered and applied in lung cancer, and the discovery of this
target is of landmark significance for the treatment of lung
cancer. EGFR-targeted drugs have prolonged the survival time of
patients with lung cancer from >1 year in chemotherapy to ~3
years with the current three generations of targeted therapy. The
incidence of grade 3 or 4 adverse events (AEs) has also decreased
from 61 to 28.7%, and the targeted therapy has significantly
improved the quality of life compared with chemotherapy (9,10). The
present article summarizes and reviews the development history,
mechanism of action and clinical trial data of the above drugs as
first-line and second-line treatments for efficacy and safety
analysis, with the intent to provide new ideas and references for
clinical treatment and scientific research.
History of the EGFR-targeted drugs
The drugs targeting the EGFR, namely EGFR tyrosine
kinase inhibitor (TKI), are divided into first, second, third and
fourth generations (11). The first
and second-generation EGFR-TKIs are used to treat advanced NSCLC
with EGFR-sensitized mutations. The first-generation drugs include
gefitinib and erlotinib among others. The second-generation drugs
include afatinib and dacomitinib among others. Although the
objective response rate (ORR) of the first and second-generation
drugs is very high, which can reach 60–70%, most patients show
resistance after 8–14 months of treatment, and the average
progression-free survival is 9–15 months (12). Therefore, third-generation drugs
have been developed, including osimertinib and almonertinib
(13,14). Currently, there are several targeted
drugs of the third generation that have been introduced worldwide.
Among them, three drugs, namely osimertinib, almonertinib and
furmonertinib, have been authorized for use in China. The
fourth-generation drugs mainly focus on fighting the acquired
resistance that the C797S mutation causes, which is common in
third-generation drugs. These fourth-generation drugs, such as
EAI045 and CH7233163, have not yet been implemented in clinical
practice.
Osimertinib is the world's first third-generation
targeted drug to be launched, which was developed by AstraZeneca in
the United States. In November 2015, patients with NSCLC and T790M
mutations were approved by the Food and Drug Administration (FDA)
for use in previous EGFR TKI treatment progression (15). Rociletinib was developed by Wuhan
Chemstan Biotechnology in China and showed to be not as effective
as osimertinib, and the incidence of hyperglycemia (34%) and ECG
QTc prolongation (11%) in third-grade adverse reactions were
relatively high. Therefore, the FDA voted to postpone the approval
of the drug and ultimately terminated its development in May 2016
(16,17). Olmutinib was developed by Boehringer
Ingelheim and Hammi Pharmaceuticals Co., Ltd. and was approved in
May 2016 in South Korea for the treatment of patients with locally
advanced or metastatic EGFR T790M mutation-positive NSCLC (18). Subsequently, due to limited efficacy
and severe adverse reactions, development was discontinued.
Osimertinib was approved for listing in China in March 2017
(19). Naquotinib was developed by
Astellas, a Japanese company. The development of naquotinib was
also discontinued due to its general efficacy and high incidence of
adverse reactions in the SOLAR trial in May 2017 (16,20).
In November 2018, the 2019 version of the National Comprehensive
Cancer Network guidelines for NSCLC in the United States included
osimertinib as the first-line treatment option for EGFR-positive
patients. Almonertinib is a third-generation targeted drug
developed by Jiangsu Hansoh Pharmaceutical Co., Ltd. It is the
first independently developed third-generation targeted drug in
China. It was launched in March 2020 and is used for the
second-line treatment of patients with EGFR-sensitive mutations and
EGFR T790M mutations. Lazertinib was developed by Yuhan and Janssen
Biotechnology and was approved as a second-line treatment for NSCLC
in South Korea in January 2021 (21). Nazartinib was developed by Novartis
Pharmaceuticals and approved by the Korean Ministry of Drug and
Food Safety in January 2021. Furmonertinib is an irreversible
third-generation EGFR-TKI, independently developed by Shanghai
Allist Pharmaceutical Company in China. Approved for marketing by
NMPA in March 2021, it is used to treat patients with NSCLC who
develop resistance after first or second-generation targeted drug
therapy and have been found to have T790M mutations through genetic
testing (22). In December 2021,
the National Medical Products Administration (NMPA) approved
almonertinib as the first-line treatment for patients with
EGFR-mutated NSCLC (23). In June
2022, furmonertinib was approved as a first-line treatment. At
present, the clinical trials of the fourth generation of targeted
drugs have also achieved favorable results, and we look forward to
the approval of the fourth generation of targeted drugs as soon as
possible. The development process of the aforementioned drugs is
shown in Fig. 1.
Mechanism
EGFR is a member of the ERBB family, a classic cell
surface signaling protein, and the most common mutated oncogene. It
consists of an extracellular ligand binding domain, a hydrophobic
transmembrane region anchored to the cell membrane, and a tyrosine
kinase domain located within the cell (24,25).
EGFR and EGF, Transforming Growth Factors α (TGF α) ligands combine
to form homomorphic or heteromorphic dimers, activating the
tyrosine kinase domain. ATP binds tightly to this domain and
transmits signals to downstream signaling pathways (26,27).
There are four common downstream signal pathways: i) PI3K/AKT; ii)
RAS/RAF/MAPK; iii) JAK/STAT; and iv) PLC/PKC, respectively, as
demonstrated in Fig. 2; in
addition, it also includes SRC, JNK and other signaling pathways
(25,27–30).
EGFR plays a role in promoting cell survival, proliferation,
differentiation and inhibiting apoptosis by triggering the signal
transductions aforementioned.
In NSCLC, EGFR mutations no longer rely on receptor
activation, causing downstream signaling pathways to remain
continuously activated, leading to the long-term existence of
signals that promote proliferation, inhibit apoptosis, and
ultimately lead to carcinogenesis (31). The signals transmitted by
heterodimers are amplified and enhanced, exhibiting stronger
carcinogenicity compared with signals transmitted by homodimers
(32). Small molecule kinase
inhibitors, such as first-generation targeted drugs including
gefitinib and erlotinib, are reversible inhibitors that can
competitively bind to the tyrosine kinase domain with ATP, leading
to a decrease in EGFR's affinity for ATP, thereby blocking signal
transmission, reducing proliferation, promoting apoptosis, and
ultimately inhibiting tumor growth and metastasis (33). However, after a period of treatment,
a T790M mutation occurred. On the one hand, the amino acid changed
from threonine to a larger volume of methionine increasing steric
hindrance, and resulting in weakened binding between the
first-generation targeted drug and EGFR. On the other hand, the
T790M mutation increased the binding ability of the L858R mutant to
ATP, ultimately leading to drug resistance (34). The second generation of targeted
drugs, including afatinib and dacomitinib, is an irreversible
inhibitor, which cannot reach the concentration to overcome the
T790M mutation in the human body. In addition, the second
generation of drugs has low selectivity and combines with wild
EGFR, which brings severe adverse reactions such as diarrhea and
rash to patients; therefore, its clinical use is limited (35). Although the ORR of first-generation
and second-generation drug therapy reached 60–70%, most patients
developed resistance after 8–14 months of treatment, with an
average progression-free survival of 10–14 months (12,36).
The most common resistance mechanism among the others is the T790M
mutation, which accounts for ~50% of all EGFR TKI resistance
mechanisms in patients with NSCLC (37). However, third-generation targeted
drugs have been developed for the T790M mutation, such as
osimertinib, almonertinib and lazertinib (13,14).
These drugs form a covalent bond with the C797 residue on the ATP
binding site, which binds irreversibly and has stronger binding
power (35,38). They have a weak inhibitory effect on
wild EGFR, higher selectivity, and fewer adverse reactions
(39). The mechanism of action and
drug resistance of EGFR-TKI is revealed in Fig. 3.
Third-generation EGFR-TKIs
Osimertinib (also named AZD9291)
The AURA17 (NCT02442349) trial is a phase 2,
open-label, single-arm clinical study in the Asia Pacific
population, evaluating the efficacy and safety of osimertinib as a
second-line treatment for patients with EGFR T790M mutation who had
previously progressed with EGFR-TKI or after chemotherapy (40). According to a data report from the
European Society for Medical Oncology Asia 2018 conference, the ORR
of osimertinib was 62%, the disease control rate (DCR) was 88%, and
the median duration of response (mDoR) was 9.9 months, the median
progression-free survival (mPFS) was 9.7 months, and the median
overall survival (mOS) was 23.2 months. Grade 3 or higher AEs
occurred in 35% of patients. The most common AEs were diarrhea
(29.24%) and leukopenia (12.87%), with grade 3 or higher diarrhea
and rash occurring in only 1% of patients. The aforementioned data
was similar to the data from the AURA2 (41) and AURA3 (42) trials, both of which are global
multicenter trials, indicating that osimertinib showed clinical
efficacy, durability and safety similar to global data, providing
data to support the use of osimertinib in patients with T790M
mutation after EGFR TKI progression in the Asia-Pacific region. The
global data suggest strengthening and improving the clinical
application of osimertinib in China.
The FLAURA (NCT02296125) trial is a Phase 3,
double-blind, randomized, multicenter clinical study, comparing the
efficacy and safety of osimertinib with gefitinib or erlotinib as
first-line treatment in locally advanced or metastatic EGFR
sensitized NSCLC with positive mutations (43). According to the data from the FLAURA
China trial, osimertinib has significantly improved in all aspects
compared with the first-generation drug. The PFS was extended from
9.8 to 17.8 months for the first-generation drug, the mDoR was
extended from 10.9 to 16.4 months, the ORR was increased from 70.8
to 76.1%, the DCR was increased from 95.4 to 97.2% and the mOS was
extended from 25.7 to 33.1 months. The proportion of grade 3 and
aforementioned AEs was 54% in the osimertinib group and 28% in the
other EGFR TKIs group. The common AEs were decreased neutrophil,
platelet and with blood counts, as well as rash. However, changes
in the severity of these AEs did not result in any clinically
significant sequelae. In addition to the aforementioned AEs,
interstitial lung disease (ILD) is a potentially fatal side effect,
but its incidence is only 3.3% (in the aforementioned study, the
incidence of ILD was 3%), and the probability of progression to a
life-threatening state was only 0.5%. There are even fewer reported
and documented cases (44). In
addition, in patients with central nervous system (CNS) metastasis,
mPFS was not reached in the osimertinib group, while it was 13.9
months in the standard EGFR-TKI group and the hazard ratio (HR) was
0.48. CNS progression occurred in 20% of patients in the
osimertinib group vs. 39% of patients in the standard EGFR-TKI
group. The data for osimertinib in China were generally consistent
with the global data, except for the rate of grade 3 or higher AEs.
In the global data, grade ≥3 AEs were 34% in the osimertinib group
and 45% in the standard EGFR-TKI group, but as aforementioned, the
higher rate of AEs with osimertinib was primarily related to the
reporting of laboratory abnormalities, and no new safety concerns
or clinically significant sequelae were identified (45–47).
From the aforementioned data, it was observed that in first-line
treatment, osimertinib offers a markedly greater advantage as a
third-generation targeted drug than the first-generation targeted
drug. Osimertinib can not only significantly prolong the PFS, but
also control the metastasis and progression in the CNS. Therefore,
for patients with EGFR mutation positivity in advanced lung cancer,
osimertinib has established its unshakable position in the targeted
treatment of NSCLC due to its improved efficacy and permeability to
CNS metastatic patients. The subjects of this experiment come from
multiple centers, different countries, and different ethnicities,
and the reliability of the experimental results is higher and more
convincing. The overall data of the FLAURA trial in China revealed
a consistent trend with global data, which has great reference
value for the clinical application and scientific research of
osimertinib in China.
Almonertinib (also named
HS-10296)
The APPLLO (NCT02981108) trial was a phase 2,
open-label, single-arm clinical study in China, evaluating the
efficacy and safety of almonertinib as a second-line treatment for
patients with T790M mutations after disease progression treated
with first and second-generation EGFR-TKI (48,49).
In the aforementioned study, the ORR of almonertinib was 68.9%, DCR
was 93.4%, mDoR was 15.1 months, PFS was 12.4 months and mOS had
not yet been reached. The most common AEs were the elevation of
blood creatine phosphokinase (CPK) level (20.9%), rash (13.9%), and
aspartate transaminase (AST, 12.3%), among which 16.4% were
patients with ≥grade 3 AEs. In addition, almonertinib also has a
favorable blood-brain barrier permeability, which has a favorable
therapeutic effect on patients with CNS metastasis. The CNS-ORR was
60.9%, CNS-DCR was 91.3%, CNS-mDoR was 12.5 months and CNS-mPFS was
11.8 months. It can be concluded that almonertinib is a
third-generation targeted drug with excellent efficacy, strong
tolerability, and improved CNS activity compared with the first and
second-generation drugs. Based on this result, almonertinib was
approved in China for the treatment of patients with NSCLC and EGFR
T790M mutation. The limitations of the aforementioned study include
the lack of a control group and participants from a single ethnic
group, highlighting the need for further research.
The AENEAS (NCT03849768) trial was a phase 3,
double-blind, randomized clinical study in China, comparing the
efficacy and safety of almonertinib and gefitinib as first-line
treatment for NSCLC EGFR mutation-positive patients (49–51).
The ORR and DCR of almonertinib were 73.8 and 93.0%, respectively,
similar to those of the gefitinib group (72.1 and 96.7%,
respectively). However, almonertinib significantly prolonged the
patient's mPFS and mDoR, with mPFS extending from 9.9 to 19.3
months, and mDoR extending from 8.3 to 18.1 months, both extending
for nearly 10 months. The mOS of both experimental groups had not
yet been reached. The most common AEs were elevated blood CPK
(35.5%), elevated AST (29.9%) and elevated alanine transaminase
(ALT) (29.4%). Among them, 36.4% of patients in the almonertinib
group had AEs of grade 3 or above and 35.8% of patients had AEs
common to gefitinib. Notably, almonertinib significantly reduced
the incidence of skin and gastrointestinal AEs and improved the
quality of life of patients by inhibiting wild-type EGFR. In
addition, almonertinib also had favorable efficacy in patients with
CNS metastasis, with a CNS-mPFS of 15.3 months, which is >7
months than the gefitinib group (8.2 months), winning patients a
longer survival period and more treatment opportunities. Compared
with osimertinib, although the mOS of CNS in the osimertinib group
was not reached, the favorable CNS activity of almonertinib was
also demonstrated by the decrease in the HR of almonertinib
compared with osimertinib, which dropped from 0.48 to 0.38. The
aforementioned data indicated that in first-line treatment,
almonertinib can exert greater advantages than the first-generation
targeted drug gefitinib. In conclusion, almonertinib is a
third-generation EGFR TKI with favorable efficacy and tolerability.
The limitation of this trial was that the participants were all of
the same ethnicity, whilst there may be certain differences between
different countries and ethnicities. Additional research is
required before it can be promoted on a global scale.
Furmonertinib (also named
AST2818)
The ALSC003 (NCT03452592) trial was a phase 2,
open-label, single-arm clinical study in China, evaluating the
efficacy and safety of furmonertinib as a second-line treatment in
patients with advanced NSCLC and EGFR T790M mutation (52). The clinical trial demonstrated that
the ORR of furmonertinib was 74%, DCR was 94%, mPFS was 9.6 months
and mDoR was 8.3 months, whereas mOS was not reached. For patients
with CNS metastasis, furmonertinib also had favorable efficacy,
with 66% CNS-ORR and CNS-100% DCR. During treatment, 26% of
patients experienced grade 3 or above AEs, with the most common
being increased γ-glutamyltransferase, AST and ALT. In terms of
mPFS, furmonertinib and osimertinib exhibited similar results, but
both were inferior to almonertinib. In terms of CNS efficacy,
furmonertinib and almonertinib are both third-generation EGFR TKIs
with favorable blood-brain barrier permeability activity. The data
indicated that furmonertinib has favorable efficacy and safety as a
second-line treatment of progression after first-generation or
second-generation drug treatment in patients with NSCLC. The
limitation of this clinical trial lies in the absence of a control
group and a single-subject population. Further verification is
required due to potential variations among different countries and
races.
The FLAG (FURLONG, NCT03787992) trial was a phase 3,
double-blind, randomized clinical study in China, comparing the
efficacy and safety of furmonertinib and gefitinib as first-line
treatment for patients with locally advanced or metastatic EGFR
mutated NSCLC (53,54). ORR was similar to furmonertinib vs.
gefitinib (89 vs. 84%), and DCR was 96 vs. 93%. The mPFS for
furmonertinib was 20.8 months, while that for gefitinib was 11.1
months. Compared with gefitinib, furmonertinib extended the mPFS by
nearly 10 months. The mDoR was also significantly prolonged in the
furmonertinib group from 11.0 to 19.7 months in the gefitinib
group. In the trial, 11% of patients in the furmonertinib group
experienced ≥grade 3 AEs, while 18% were in the gefitinib group.
Among them, the most common AE of higher than grade 3 in the
furmonertinib group was ECG QTc interval prolongation and diarrhea.
In addition, furmonertinib has excellent effects on patients with
CNS metastasis, with 89% CNS-ORR, 96% CNS-DCR, 19.7 months CNS-DoR
and 18.0 months CNS-mPFS. The mPFS of furmonertinib was higher than
that of osimertinib and almonertinib, and the HR was the lowest
among the three, which was sufficient to prove the favorable
efficacy of furmonertinib. At the same time, furmonertinib had
favorable safety, and CNS penetration activity was also improved
compared with the almonertinib group. The aforementioned data
indicated that, compared with first-generation EGFR TKIs,
furmonertinib has a significant therapeutic effect on patients with
advanced EGFR-positive mutations, and has favorable effects on
patients with CNS metastasis. The AEs were reduced compared with
gefitinib, providing an emerging treatment option for patients with
EGFR-positive NSCLC in recent years. The limitations of this trial
were similar to those of the AENEAS trial with almonertinib and
there was a problem of a single-subject population that requires
further research.
Olmutinib (also named HM61713)
The ELUXA1 (NCT02485652) trial was a phase 2,
open-label, single-arm, multicenter clinical study, evaluating the
efficacy and safety of olmutinib as a second-line treatment in
patients with T790M-positive NSCLC (55). In this trial, the ORR of olmutinib
was 51.9%, DCR was 86.4%, mDoR was 12.7 months, mPFS was 9.4 months
and mOS was 19.7 months. The data indicated that the efficacy of
olmutinib was acceptable, but 48.2% of patients have experienced
more than grade 3 drug-related AEs. Due to the high incidence of
AEs and severe skin toxicity (including toxic epidermal necrolysis
and Stevens-Johnson syndrome), further ELUXA trials were
discontinued (56). In April 2018,
the development of olmutinib was terminated (16).
Lazertinib (also named YH25448)
The LASER201 (NCT03046992) trial was a phase 1/2,
open-label, single-arm, multicenter clinical study, evaluating the
efficacy and safety of lazertinib as a second-line treatment in
patients with EGFR mutation-positive advanced NSCLC (57,58).
The results demonstrated that the ORR of lazertinib was 55.3%, DCR
was 89.5%, mDoR was 17.7 months and mPFS was 11.1 months, while mOS
was not reached. Among patients with CNS metastasis, the ORR was
85.7%, DCR was 100%, mDoR was 15.1 months and PFS was 26.0 months.
The most surprising aspect of the data was the mPFS of patients
with CNS metastasis, which exceeds 2 years. Lazertinib exhibited a
very favorable blood-brain barrier penetration effect. Grade 3 or
higher AEs occurred in 34.6% of the patients in the trial. The most
common AEs were rash (38.5%), pruritus (34.6%), paresthesia
(33.3%), headache (28.2%) and muscle spasms (28.2%). In conclusion,
lazertinib had a manageable safety profile and durable antitumor
activity, especially CNS activity, with the longest mPFS among the
third-generation EGFR TKIs.
The LASER301 (NCT04248829) trial was a phase 3,
double-blind, randomized, multicenter clinical study, comparing the
efficacy and safety of lazertinib and gefitinib as first-line
treatment for patients with NSCLC and EGFR mutations (59). The ORR was 76% in both lazertinib
and gefitinib groups. DCR was 93.9%. mDoR was 19.4 months in the
lazertinib group and 8.3 months in the gefitinib group. mPFS was
20.6 months and 9.7 months, respectively, and mOS was not reached.
In patients with baseline brain metastases, mPFS was 16.4 months
with lazertinib and 9.5 months with gefitinib. Treatment-related
AEs of grade 3 or greater occurred in 41% of the patients in the
lazertinib group and 44% of those in the gefitinib group. The most
common AEs were paresthesia (39%), rash (36%), pruritus (27%) and
diarrhea (26%). This trial identified that, compared with
gefitinib, lazertinib significantly improved PFS and had favorable
activity in patients with brain metastases, with a manageable
safety profile. The efficacy of lazertinib was similar to that of
osimertinib, almonertinib and furmonertinib, and all of them
provide improved survival benefits to patients. This trial was the
second, after osimertinib, to successfully compare a
third-generation EGFR-TKI with a first-generation EGFR TKI as
first-line therapy for patients with EGFR-positive mutations. This
trial included both Asian and non-Asian populations, and the
results were more representative. At the same time, the two
subgroups also showed a consistent trend, which provides strong
support for the application of the drug in China in the future.
More surprisingly, according to the latest research results
released at the time of writing, the combination of lazertinib and
amivantamab is expected to break the resistance of Osimertinib
(60).
Nazartinib (also named EGF816)
The NCT02108964 trial was a phase 2, open-label,
single-arm, multicenter clinical study, evaluating the efficacy and
safety of nazartinib in the first-line treatment of patients with
NSCLC (61). The research revealed
that the ORR of nazartinib was 69.0%, DCR was 91.0%, mDoR was 25
months and mPFS was 18 months, while mOS was not reached. In
addition, the drug showed favorable efficacy in patients with brain
metastasis, with CNS-ORR of 67%, CNS-mDoR of 15 months, and
CNS-mPFS of 17 months. In this trial, 31% of patients experienced
grade 3 or above AEs, with the most common being macular papules
(11%), elevated lipase (11%) and hypokalemia (7%). Nazartinib
demonstrated favorable efficacy and controllable safety, and it can
also be well controlled in those patients with baseline brain
metastases and is a promising third-generation EGFR TKI, which is
worth further research and development.
NCT03529084 is a phase 3, open-label, randomized,
multicenter clinical study, comparing the efficacy and safety of
nazartinib vs. gefitinib or erlotinib as first-line treatment in
patients with locally advanced or metastatic NSCLC carrying
EGFR-activated mutations. However, the study was withdrawn before
the subjects were enrolled.
Rociletinib (also named CO1686)
The TIGER-2 trial (NCT02147990) was a phase 2,
open-label, single-arm, multicenter clinical study, evaluating the
safety and efficacy of rociletinib as second-line EGFR TKI in
patients with mutant EGFR NSCLC. The research evidenced that
rociletinib has an improved therapeutic effect when received orally
at a dose of 500 mg compared with a dose of 625 mg. The ORR of the
500 mg dose group was 34.0%, DCR was 76.3%, mDoR was 9.1 months and
mPFS was 5.9 months. Compared with other third-generation targeted
drugs, the therapeutic effect of this drug was not significant.
The TIGER-1 (NCT02186301) trial was a phase 2/3,
open-label, randomized, multicenter clinical study, comparing the
efficacy and safety of rociletinib vs. erlotinib as first-line
treatment for advanced or metastatic NSCLC with EGFR mutations.
Multiple sets of data for the 500 mg group could not be calculated
due to the unavailability of the upper limit, but mPFS and mDoR
were worse in both the 500 and 625 mg groups than in the erlotinib
group, which is a relatively unexpected result. Due to the high
incidence of hyperglycemia (34%) and QT prolongation (11%) among
the tertiary adverse reactions of rociletinib, and the lower
efficacy compared with other third-generation targeted drugs, the
FDA postponed the approval of the drug with a 12:1 vote. After the
results of the TIGER-3 (17) trial
were released, the drug was ultimately discontinued in May 2016 due
to the high incidence of AEs (8,16).
Other third-generation targeted
drugs
The NCT02330367 trial of abivertinib (AC0010) was a
phase 1/2, open-label, single-arm, clinical study in China,
evaluating the efficacy and safety of abivertinib as second-line
treatment in patients with NSCLC who had previously received
treatment and had EGFR T790M mutations. The ORR was 52.2%, DCR was
88.0%, mDoR was 8.5 months, mPFS was 7.5 months, mOS was 24.9
months, and treatment-related AEs of grade 3 or above were 32.6%
(62,63). The most common AEs were elevated ALT
(7.0%), elevated AST (4.8%), diarrhea (4.4%) and neutropenia
(3.5%). The aforementioned data indicated that abivertinib had
favorable therapeutic effects, especially mOS, which had the
longest mPFS among the drugs aforementioned. The NCT03856697 trial
was a phase 3, double-blind, randomized, clinical study in China,
comparing the efficacy and safety of abivertinib vs. gefitinib as
first-line standard treatment EGFR-TKI in advanced NSCLC with
sensitive EGFR mutations, but no results have been published
yet.
The SOLAR (NCT02588261) trial of naquotinib
(ASP8273) was a phase 3, open-label, randomized multicenter
clinical study, comparing the efficacy and safety of naquotinib vs.
gefitinib or erlotinib as first-line treatment. In this trial, the
ORR of naquotinib was 33.0%, DCR was 62.0%, mDoR was ~9.2 months,
mPFS was ~9.3 months, and drug-related grade 3 or above AEs were
46.0%. However, the ORR of the gefitinib or erlotinib group was
47.9% and PFS was 9.6 months. The DCR and mDoR were similar in both
groups. From the results, it can be easily observed that the
efficacy of naquotinib is limited and even cannot reach that of the
gefitinib or erlotinib group, and the toxicity is significant.
Therefore, the trial was terminated by the independent disease
monitoring committee in May 2017. However, in a Phase 2 study
(64), it was found that the ORR
and DCR of naquotinib were 45 and 94%, indicating acceptable
efficacy. However, the number of participants in the study was
relatively small and there may be some deviation. The drug has
currently been discontinued.
The NCT03812809 trial of rezivertinib (BPI-7711) was
a phase 2, open-label, single-arm clinical study in China,
evaluating the efficacy and safety of rezivertinib as second-line
treatment in patients with NSCLC who had advanced and confirmed
EGFR-sensitive mutations and EGFR T790M positive mutations after
previous EGFR-TKI treatment (65).
The results revealed that the ORR of rezivertinib was 64.6%, DCR
was 89.8%, mDoR was 12.5 months, mPFS was 12.2 months and mOS was
23.9 months. The proportion of grade 3 and above AEs in the trial
was 19.9%. In addition, rezivertinib also demonstrated favorable
efficacy in patients with CNS metastasis, with CNS-ORR of 69%,
CNS-DCR of 100% and CNS-mPFS of 16.6 months. In conclusion, as a
second-line treatment, rezivertinib showed favorable antitumor
effect, high safety and favorable CNS penetration activity.
Rezivertinib is a third-generation EGFR TKI with great development
potential in the future. The NCT03866499 trial is a randomized,
double-blind, phase 3 trial that evaluated the efficacy and safety
of rezivertinib compared with gefitinib as a first-line treatment
in NSCLC patients with advanced EGFR mutations. The trial is
expected to be completed by October 2023.
In addition, there are various third-generation
targeted drugs, including mavelertinib (PF-06747775) (65), limertinib (ASK120067) (66,67),
befotertinib (D-0316) (68),
olafertinib (CK-101/RK518), keynatinib (16), SH-1028 (69,70)
and TAS-121 (71), which are all
under development and trial.
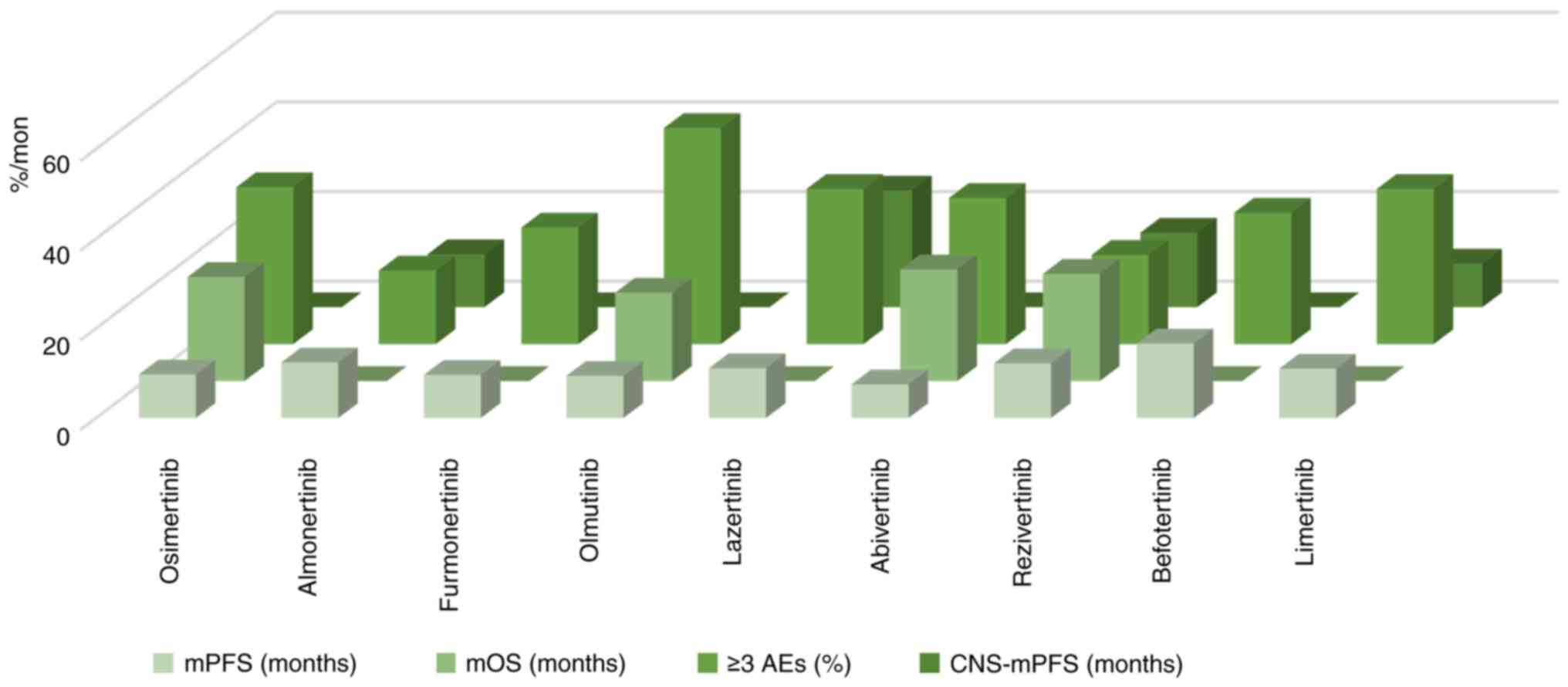
Comparison of drug efficacy and clinical
plan recommendations
By comparing the phase 2 and second-line treatment
trials of third-generation targeted drugs such as the AURA17 trial
and the APOLLO trial, it was found that the data on mPFS and grade
3 and above AEs of almonertinib were more prominent than those of
other third-generation drugs; especially mPFS, which was extended
by 2.7 months compared with osimertinib and 9.8 months compared
with furmonertinib. The probability of grade 3 and above AEs is
currently the lowest among the drugs mentioned in the present
review, which was 8.6% lower than osimertinib and 31.8% lower than
olmutinib. The ORR and DCR of furmonertinib are more prominent and
the ORR of this drug is the only one that exceeds 70% of the other
drugs discussed in the present review. The mDoR of lazertinib was
extended by 7.8 months compared with osimertinib, 2.6 months
compared with almonertinib, and 9.4 months compared with
furmonertinib. In addition, lazertinib has excellent efficacy in
patients with CNS metastasis and the CNS-mPFS is markedly longer
than other drugs. Abivertinib and rezivertinib have also shown
favorable efficacy through longer OS, and rezivertinib has
relatively fewer AEs. Therefore, for patients with NSCLC who can
receive second-line treatment, almonertinib, with its accuracy,
efficiency, and low toxicity, is expected to become the first
choice for patients with T790M mutations who progress after
treatment. In addition, for patients with CNS metastasis,
lazertinib exhibited excellent blood-brain barrier penetration
efficacy in the LASER201 trial, with >2 years of mPFS. If
subsequent or larger trials can confirm these results, lazertinib
may bring greater benefits to patients with brain metastasis and
become the first choice for such patients. China can also consider
introducing and applying lazertinib to domestic patients. The
comparison of the efficacy and safety of the aforementioned drugs
as second-line treatment for NSCLC is demonstrated in Fig. 4 and Table I. The comparison of the AEs of the
aforementioned drugs as second-line treatment for NSCLC is listed
in Table III.
 | Table I.Comparison of the efficacy of the
third generation EGFR-TKIs as second-line treatment. |
Table I.
Comparison of the efficacy of the
third generation EGFR-TKIs as second-line treatment.
|
| mPFS (month) | mOS (month) | ≥3 AEs (%) | CNS-mPFS
(month) |
|---|
| Osimertinib | 9.7 | 23.2 | 35 | - |
| Almonertinib | 12.4 | NA | 16.4 | 11.8 |
| Furmonertinib | 9.6 | NA | 26 | - |
| Olmutinib | 9.4 | 19.7 | 48.2 | - |
| Lazertinib | 11.1 | NA | 34.6 | 26.0 |
| Abivertinib | 7.5 | 24.9 | 32.6 | - |
| Rezivertinib | 12.2 | 23.9 | 19.9 | 16.6 |
| Befotertinib | 16.6 | NA | 29.3 | NA |
| Limertinib | 11.0 | NA | 34.6 | 9.7 |
 | Table III.Comparison of adverse events when
third-generation EGFR-TKIs were used as second-line therapy. |
Table III.
Comparison of adverse events when
third-generation EGFR-TKIs were used as second-line therapy.
|
| Osimertinib | Almonertinib | Furmonertinib | Lazertinib | Abivertinib | Rezivertinib |
|---|
| Leukopenia | 12.87 | 4.51 | - | - | - | 7.1 |
| Diarrhea | 29.24 | 0 | - | 28.2 | 61.2 | 7.5 |
| Nausea | 9.36 | 6.15 | - | 16.7 | 17.2 | - |
| Vomiting | 8.19 | 0 | 5 | 11.6 | 16.7 | 10.6 |
| Neutrophil count
decreased | 7.02 | 5.74 | 6 | - | 23.3 | 18.6 |
| Platelet count
decreased | 11.11 | 9.43 | 6 | - | 24.2 | 23.0 |
| White blood cell
count decreased | 12.28 | 12.3 | 12 | - | 25.6 | 27.9 |
| Rash | 7.60 | 13.9 | - | 38.5 | 37.0 | 8.8 |
| Pruritus | 12.28 | 10.7 | - | 34.6 | - | 8.8 |
| Increased blood
creatine | - | 20.9 | - | - | - | 8.4 |
| Increased alanine
aminotransferase | - | 11.9 | 15 | 12.8 | 64.8 | 11.9 |
| Increased aspartate
aminotransferase | - | 12.3 | 16 | 14.1 | 57.3 | 16.4 |
| Prolonged
electrocardiogram | - | - | 15 | - | 19.4 | 5.3 |
By comparing phase 3 and first-line treatment trials
with third-generation targeted drug trials such as the FLAURA and
AENEAS, the results revealed that third-generation targeted drugs
have improved efficacy, safety, CNS permeability and greater
advantages compared with first-generation targeted drugs. In the
case of third-generation EGFR-TKIs, osimertinib still holds a very
important position as the first-line treatment for NSCLC, and ORR,
DCR and OS all show favorable efficacy. The mPFS of both
almonertinib and furmonertinib was slightly longer than that of
osimertinib, with almonertinib extending by 1.5 months and
furmonertinib extending by 3 months. In addition, furmonertinib
also has excellent therapeutic effects on patients with CNS
metastasis, with an ORR increase of 22% compared with osimertinib
and an mPFS increase of 2.7 months compared with almonertinib. The
biggest advantage of furmonertinib is the extremely low incidence
of AEs, with 11% of patients experiencing grade 3 or above AEs,
which is currently the lowest known rate, with a 17% decrease
compared with osimertinib and a 25.4% decrease compared with
almonertinib. The development of naquotinib was terminated due to
its low efficacy and high adverse reaction rate. The similarity
between the aforementioned third-generation targeted drug trials is
that the control group consists of first-generation targeted drugs
and the experimental group contains third-generation targeted
drugs. The authors of the present review found by comparing the
data that osimertinib, almonertinib and furmonertinib have similar
results and comparable efficacy. For patients with CNS metastasis,
furmonertinib is the best choice. In addition, the lower incidence
of AEs with furmonertinib leads to the conclusion that all three
drugs are effective in patients receiving regular first-line
treatment for NSCLC, while furmonertinib is preferable in patients
with CNS metastasis and those with underlying diseases that cannot
tolerate AEs. Due to the limitations of cross-comparison of data
derived from different trials, further research and verification is
needed. The comparison of the efficacy and safety of the
aforementioned drugs as first-line treatment for NSCLC is shown in
Fig. 5 and Table II. The comparison of the AEs of the
aforementioned drugs as first-line treatment for NSCLC is presented
in Table IV.
 | Table II.Comparison of the efficacy of
third-generation EGFR-TKIs as first-line therapy. |
Table II.
Comparison of the efficacy of
third-generation EGFR-TKIs as first-line therapy.
|
| mPFS (month) | mOS (month) | ≥3 AEs (%) | CNS-mPFS
(month) |
|---|
| Osimertinib | 17.8 | 33.1 | 54 | NA |
| Almonertinib | 19.3 | NA | 36.4 | 15.3 |
| Furmonertinib | 20.8 | NA | 11 | 18.0 |
| Nazartinib | 18 | NA | 31 | 17 |
| Lazertinib | 20.6 | NA | 41 | 16.4 |
 | Table IV.Comparison of adverse events when
third-generation EGFR-TKIs were used as first-line therapy. |
Table IV.
Comparison of adverse events when
third-generation EGFR-TKIs were used as first-line therapy.
|
| Osimertinib | Almonertinib | Furmonertinib | Lazertinib |
|---|
| Leukopenia | 17 | - | - | - |
| Neutropenia | 17 | - | - | - |
| Diarrhea | 24 | 16.4 | 27 | 26 |
| Nausea | 14 | 10.7 | - | - |
| Vomiting | 14 | - | - | - |
| Neutrophil count
decreased | 24 | 13.6 | 11 | - |
| Platelet count
decreased | 28 | 22 | 9 | - |
| White blood cell
count decreased | 41 | 23.8 | 15 | - |
| Rash | 37 | 23.4 | 17 | 36 |
| Pruritus | - | 6.5 | - | 27 |
| Increased blood
creatine | - | 35.5 | 4 | - |
| Increased alanine
aminotransferase | 9 | 29.4 | 29 | 15 |
| Increased aspartate
aminotransferase | 16 | 29.9 | 26 | 11 |
| Prolonged
electrocardiogram | 10 | 10.7 | 9 | - |
The ARCHER 1050 trial compared the efficacy and
safety of dacomitinib and gefitinib as first-line treatment for
patients with EGFR mutation-positive NSCLC (72). In this trial, the ORR was similar to
dacomitinib and gefitinib (75 vs. 72%), with mPFS of 14.7 and 9.2
months, and mDoR of 14.8 and 8.3 months, respectively. In this
regard, the efficacy of dacomitinib is markedly improved compared
with gefitinib, and the drug shows greater benefit in the Asian
subgroup than in the non-Asian subgroup, which is also the basis
for the use of dacomitinib as the first-line treatment for patients
with EGFR mutations. However, grade 3 or higher AEs occurred in 63%
of the patients in the dacomitinib group and 41% in the gefitinib
group. The LUX-Lung7 trial compared the efficacy and safety of
afatinib with those of gefitinib as first-line treatment for
patients with EGFR mutation-positive NSCLC (73). In this trial, afatinib was
associated with some improvement in ORR (70 vs. 56%), DCR (91 vs.
87%), mPFS (11.0 vs. 10.9 months), mDoR (10.1 vs. 8.4 months) and
mOS (27.9 vs. 25.0 months), but the improvement was not
significant. Rates of grade 3 or higher AEs were also similar, 57%
with afatinib and 52% with gefitinib. Although the efficacy of
dacomitinib and afatinib is improved compared with the
first-generation EGFR TKIs, the incidence of grade 3 and above AEs
of these two drugs was higher than that of the first-generation
EGFR TKIs. Therefore, the clinical utilization rate of the
second-generation EGFR TKIs decreases significantly after the
emergence of the third-generation EGFR TKIs.
Advantages and disadvantages of the
third-generation EGFR-TKIs
Osimertinib is the first third-generation targeted
drug to be marketed, which is highly specific and selective
compared with the first and second-generation EGFR TKIs. In
addition, osimertinib also can penetrate the blood-brain barrier
and has a favorable effect on patients with brain metastases.
However, patients using osimertinib may experience a white blood
cell count decrease, which may lead to the development of
infectious diseases, especially bacterial infections. In addition,
rash AE is also not to be ignored. According to the current data,
almonertinib and furmonertinib have improved efficacy and few AEs
compared with Osimertinib; however, the data on these two drugs are
only available in China and there is a lack of global data, which
represents a limitation. The AEs of furmonerftinib and almonertinib
mainly regard the increase of blood CPK, ALT and AST, which may
cause liver injury. The efficacy of lazertinib and osimertinib were
comparable and the first-line trial of lazertinib included both
Asian and non-Asian subgroups, making it more reliable; however,
paresthesia differed from the other grade >3 AEs with lazertinib
and the incidence of pulmonary embolism was relatively high. As a
second-line treatment, nazartinib has significant CNS activity, but
first-line results are not yet available. The advantages and
disadvantages of other drugs will not be discussed in the present
review.
Prospects
In summary, numerous trials revealed that the
third-generation EGFR-TKIs have absolute advantages over the first
generation. The third-generation EGFR-TKIs have favorable efficacy,
controllable safety and strong drug activity against CNS metastasis
in the treatment of patients with advanced EGFR mutations and T790M
mutations in NSCLC. However, each drug also has its advantages and
disadvantages, and suitable drugs should be selected according to
the different conditions of patients. In addition, various
third-generation EGFR-TKIs, including limertinib (ASK120067),
rezivertinib (BPI-7711) and abivertinib (AC0010), are also under
intense research and experimentation. In the future, more effective
targeted drugs will emerge, providing more choices for NSCLC. But
whether it is the first, second or third-generation drugs, drug
resistance will occur after a period of treatment. At present,
there are solutions for drug resistance, such as third-generation
combination with first-generation or second-generation targeted
drug therapy, such as osimertinib combined with gefitinib
(NCT03122717), osimertinib combined with dacomitinib (NCT03810807)
and nazartinib combined with gefitinib (NCT03292133); the
combination of EGFR third-generation targeted drugs with other
targeted drugs, such as osimertinib and anlotinib (74); fourth-generation targeted drugs,
such as EAI045 (75), OBX02-011
(76), LS-106 (77) and CH7233163 (78). The research on the mechanism of
third-generation drug resistance is currently mainly focused on
osimertinib and there is very little research on other drug
resistance mechanisms. Therefore, the research on drug resistance
mechanisms and response strategies after drug resistance remain
urgent challenges that need further attention. Further research and
exploration by medical and scientific researchers are needed to
provide improved solutions for individualized and precise treatment
of tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Inner Mongolia Medical
University Zhiyuan Talent Program (Good Learning Talent Program)
(grant no. ZY0202031), the Inner Mongolia Autonomous Region
‘Grassland Talent’ project youth innovation and entrepreneurship
talent project (grant no. 2022073), the Young Talents of Science
and Technology in Universities of Inner Mongolia Autonomous Region
(grant no. NJYT23050), the Postgraduate Talent Excellence Program
(grant no. YKD2023ZY001), The Health Technology Plan Project of
Inner Mongolia Autonomous Region Health Committee (grant no.
202202156) and the Public hospital reform and high-quality
development demonstration project research fund, gastrointestinal
tumors (grant no. 2023SGGZ114).
Availability of data and materials
Not applicable.
Authors' contributions
ZC, HC and YW were the major contributors in writing
and editing the manuscript. XJ and LS provided direction and
guidance throughout the preparation of this manuscript. JC, JY, CL,
XS and YZ analysis and organized the data. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun C, Gao W, Liu J, Cheng H and Hao J:
FGL1 regulates acquired resistance to Gefitinib by inhibiting
apoptosis in non-small cell lung cancer. Respir Res. 21:2102020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leonetti A, Sharma S, Minari R, Perego P,
Giovannetti E and Tiseo M: Resistance mechanisms to osimertinib in
EGFR-mutated non-small cell lung cancer. Br J Cancer. 121:725–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrews Wright NM and Goss GD:
Third-generation epidermal growth factor receptor tyrosine kinase
inhibitors for the treatment of non-small cell lung cancer. Transl
Lung Cancer Res. 8 (Suppl 3):S247–S264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simeone JC, Nordstrom BL, Patel K and
Klein AB: Treatment patterns and overall survival in metastatic
non-small-cell lung cancer in a real-world, US setting. Future
Oncol. 15:3491–3502. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran PN and Klempner SJ: Profile of
rociletinib and its potential in the treatment of non-small-cell
lung cancer. Lung Cancer (Auckl). 7:91–97. 2016.PubMed/NCBI
|
|
12
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (Review). Int J Oncol.
59:902021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan CS, Kumarakulasinghe NB, Huang YQ, Ang
YLE, Choo JR, Goh BC and Soo RA: Third generation EGFR TKIs:
Current data and future directions. Mol Cancer. 17:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Remon J, Steuer CE, Ramalingam SS and
Felip E: Osimertinib and other third-generation EGFR TKI in
EGFR-mutant NSCLC patients. Ann Oncol. 29 (Suppl_1):i20–i27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper AJ, Sequist LV and Lin JJ:
Third-generation EGFR and ALK inhibitors: Mechanisms of resistance
and management. Nat Rev Clin Oncol. 19:499–514. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F
and Ou SI: Beyond osimertinib: The development of third-generation
EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac
Oncol. 16:740–763. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JC, Reckamp KL, Kim YC, Novello S,
Smit EF, Lee JS, Su WC, Akerley WL, Blakely CM, Groen HJM, et al:
Efficacy and safety of rociletinib versus chemotherapy in patients
with EGFR-Mutated NSCLC: The results of TIGER-3, a phase 3
randomized study. JTO Clin Res Rep. 2:1001142020.PubMed/NCBI
|
|
18
|
Kim ES: Olmutinib: First global approval.
Drugs. 76:1153–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
H L, H X and H Y, . Research on the
third-generation epidermal growth factor receptor tyrosine kinase
inhibitor drugs and their patents. Chin J N Drugs. 31:1553–1559.
2022.
|
|
20
|
Kelly RJ, Shepherd FA, Krivoshik A, Jie F
and Horn L: A phase III, randomized, open-label study of ASP8273
versus erlotinib or gefitinib in patients with advanced stage
IIIB/IV non-small-cell lung cancer. Ann Oncol. 30:1127–1133. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhillon S: Lazertinib: First approval.
Drugs. 81:1107–1113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deeks ED: Furmonertinib: First approval.
Drugs. 81:1775–1780. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch AL, Vellanki PJ, Drezner N, Li X,
Mishra-Kalyani PS, Shen YL, Xia H, Li Y, Liu J, Zirkelbach JF, et
al: FDA approval summary: Osimertinib for adjuvant treatment of
surgically resected non-small cell lung cancer, a collaborative
project orbis review. Clin Cancer Res. 27:6638–6643. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prenzel N, Fischer OM, Streit S, Hart S
and Ullrich A: The epidermal growth factor receptor family as a
central element for cellular signal transduction and
diversification. Endocr Relat Cancer. 8:11–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levantini E, Maroni G, Del Re M and Tenen
DG: EGFR signaling pathway as therapeutic target in human cancers.
Semin Cancer Biol. 85:253–275. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ward RA, Anderton MJ, Ashton S, Bethel PA,
Box M, Butterworth S, Colclough N, Chorley CG, Chuaqui C, Cross DA,
et al: Structure- and reactivity-based development of covalent
inhibitors of the activating and gatekeeper mutant forms of the
epidermal growth factor receptor (EGFR). J Med Chem. 56:7025–7048.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong RF, Zhu ML, Liu MM, Xu YT, Yuan LL,
Bian J, Xia YZ and Kong LY: EGFR mutation mediates resistance to
EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms
to clinical research. Pharmacol Res. 167:1055832021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang L and Fu L: Mechanisms of resistance
to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 5:390–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Q, Yu S, Zhao W, Qin S, Chu Q and Wu
K: EGFR-TKIs resistance via EGFR-independent signaling pathways.
Mol Cancer. 17:532018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soejima K, Yasuda H and Hirano T:
Osimertinib for EGFR T790M mutation-positive non-small cell lung
cancer. Expert Rev Clin Pharmacol. 10:31–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sabbah DA, Hajjo R and Sweidan K: Review
on epidermal growth factor receptor (EGFR) structure, signaling
pathways, interactions, and recent updates of EGFR inhibitors. Curr
Top Med Chem. 20:815–834. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yun CH, Mengwasser KE, Toms AV, Woo MS,
Greulich H, Wong KK, Meyerson M and Eck MJ: The T790M mutation in
EGFR kinase causes drug resistance by increasing the affinity for
ATP. Proc Natl Acad Sci USA. 105:2070–2075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cross DA, Ashton SE, Ghiorghiu S, Eberlein
C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ,
et al: AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated
resistance to EGFR inhibitors in lung cancer. Cancer Discov.
4:1046–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu L, Ke L, Zhang Z, Yu J and Meng X:
Development of EGFR TKIs and options to manage resistance of
Third-generation EGFR TKI osimertinib: Conventional ways and immune
checkpoint inhibitors. Front Oncol. 10:6027622020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lamb YN: Osimertinib: A review in
previously untreated, EGFR Mutation-positive, advanced NSCLC.
Target Oncol. 16:687–695. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh M and Jadhav HR: Targeting non-small
cell lung cancer with small-molecule EGFR tyrosine kinase
inhibitors. Drug Discov Today. 23:745–753. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Patel H, Pawara R, Ansari A and Surana S:
Recent updates on Third generation EGFR inhibitors and emergence of
Fourth generation EGFR inhibitors to combat C797S resistance. Eur J
Med Chem. 142:32–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou C, Wang M, Cheng Y, Li H, Wang J and
Wu YL: AURA17 study of osimertinib in Asia-Pacific patients (pts)
with EGFR T790M-positive advanced non-small cell lung cancer
(NSCLC): Updated phase II results including overall survival (OS).
Ann Oncol. 29:IX1572018. View Article : Google Scholar
|
|
41
|
Goss G, Tsai CM, Shepherd FA, Bazhenova L,
Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, et al:
Osimertinib for pretreated EGFR Thr790Met-positive advanced
non-small-cell lung cancer (AURA2): A multicentre, open-label,
single-arm, phase 2 study. Lancet Oncol. 17:1643–1652. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Papadimitrakopoulou VA, Mok TS, Han JY,
Ahn MJ, Delmonte A, Ramalingam SS, Kim SW, Shepherd FA, Laskin J,
He Y, et al: Osimertinib versus platinum-pemetrexed for patients
with EGFR T790M advanced NSCLC and progression on a prior
EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis.
Ann Oncol. 31:1536–1544. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng Y, He Y, Li W, Zhang HL, Zhou Q,
Wang B, Liu C, Walding A, Saggese M, Huang X, et al: Osimertinib
versus comparator EGFR TKI as First-line treatment for EGFR-mutated
advanced NSCLC: FLAURA China, A randomized study. Target Oncol.
16:165–176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu L, Zhong W, Li A, Qiu Z, Xie R, Shi H
and Lu S: Successful treatment of EGFR T790M-mutant non-small cell
lung cancer with almonertinib after osimertinib-induced
interstitial lung disease: A case report and literature review. Ann
Transl Med. 9:9502021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leighl NB, Karaseva N, Nakagawa K, Cho BC,
Gray JE, Hovey T, Walding A, Rydén A and Novello S:
Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib
or gefitinib in patients with EGFR-mutated advanced non-small-cell
lung cancer. Eur J Cancer. 125:49–57. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reungwetwattana T, Nakagawa K, Cho BC,
Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, et
al: CNS response to osimertinib versus standard epidermal growth
factor receptor tyrosine kinase inhibitors in patients with
untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin
Oncol: Jco2018783118. 2018.doi: 10.1200/JCO.2018.78.3118 (Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu S, Wang Q, Zhang G, Dong X, Yang CT,
Song Y, Chang GC, Lu Y, Pan H, Chiu CH, et al: Efficacy of
aumolertinib (HS-10296) in patients with advanced EGFR T790M+
NSCLC: updated post-national medical products administration
approval results from the APOLLO registrational trial. J Thorac
Oncol. 17:411–422. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shirley M and Keam SJ: Aumolertinib: A
review in non-small cell lung cancer. Drugs. 82:577–584. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III
trial of aumolertinib versus gefitinib as First-line therapy for
locally advanced or metastaticnon-small-cell lung cancer with EGFR
exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Benjamin DJ and Nagasaka M: Freeing the
competition: Will aumolertinib (AENEAS) have a fighting chance
against osimertinib (FLAURA)? J Clin Oncol. 41:742–744. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi Y, Hu X, Zhang S, Lv D, Wu L, Yu Q,
Zhang Y, Liu L, Wang X, Cheng Y, et al: Efficacy, safety, and
genetic analysis of furmonertinib (AST2818) in patients with EGFR
T790M mutated non-small-cell lung cancer: A phase 2b, multicentre,
single-arm, open-label study. Lancet Respir Med. 9:829–839. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Furmonertinib (AST2818) versus
gefitinib as first-line therapy for Chinese patients with locally
advanced or metastatic EGFR mutation-positive non-small-cell lung
cancer (FURLONG): A multicentre, double-blind, randomised phase 3
study. Lancet Respir Med. 10:1019–1028. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Central Nervous system efficacy
of furmonertinib (AST2818) versus gefitinib as first-line treatment
for EGFR-mutated NSCLC: Results from the FURLONG study. J Thorac
Oncol. 17:1297–1305. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park K, Jӓnne PA, Kim DW, Han JY, Wu MF,
Lee JS, Kang JH, Lee DH, Cho BC, Yu CJ, et al: Olmutinib in
T790M-positive non-small cell lung cancer after failure of
first-line epidermal growth factor receptor-tyrosine kinase
inhibitor therapy: A global, phase 2 study. Cancer. 127:1407–1416.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murtuza A, Bulbul A, Shen JP, Keshavarzian
P, Woodward BD, Lopez-Diaz FJ, Lippman SM and Husain H: Novel
Third-generation EGFR tyrosine kinase inhibitors and strategies to
overcome therapeutic resistance in lung cancer. Cancer Res.
79:689–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee J, Hong MH and Cho BC: Lazertinib: On
the Way to Its Throne. Yonsei Med J. 63:799–805. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cho BC, Han JY, Kim SW, Lee KH, Cho EK,
Lee YG, Kim DW, Kim JH, Lee GW, Lee JS, et al: A Phase 1/2 Study of
Lazertinib 240 mg in patients with advanced EGFR T790M-positive
NSCLC after previous EGFR tyrosine kinase inhibitors. J Thorac
Oncol. 17:558–567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cho BC, Ahn MJ, Kang JH, Soo RA,
Reungwetwattana T, Yang JC, Cicin I, Kim DW, Wu YL, Lu S, et al:
Lazertinib versus gefitinib as First-line treatment in patients
with EGFR-mutated advanced non-small-cell lung cancer: Results from
LASER301. J Clin Oncol. 41:4208–4217. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cho BC, Kim DW, Spira AI, Gomez JE, Haura
EB, Kim SW, Sanborn RE, Cho EK, Lee KH, Minchom A, et al:
Amivantamab plus lazertinib in osimertinib-relapsed EGFR-mutant
advanced non-small cell lung cancer: A phase 1 trial. Nat Med.
14:023–02554. 2023.
|
|
61
|
Tan DSW, Kim SW, Ponce Aix S, Sequist LV,
Smit EF, Yang JCH, Hida T, Toyozawa R, Felip E, Wolf J, et al:
Nazartinib for treatment-naive EGFR-mutant non-small cell lung
cancer: Results of a phase 2, single-arm, open-label study. Eur J
Cancer. 172:276–286. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou Q, Wu L, Hu P, An T, Zhou J, Zhang L,
Liu XQ, Luo F, Zheng X, Cheng Y, et al: A Novel Third-generation
EGFR Tyrosine kinase inhibitor abivertinib for EGFR T790M-mutant
Non-small cell lung cancer: A multicenter phase I/II study. Clin
Cancer Res. 28:1127–1135. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang F and Zhou Q: The challenges of
Third-generation EGFR tyrosine kinase inhibitors in the therapy of
advanced NSCLC. J Thorac Oncol. 17:481–486. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Azuma K, Nishio M, Hayashi H, Kiura K,
Satouchi M, Sugawara S, Hida T, Iwamoto Y, Inoue A, Takeda K, et
al: ASP8273 tolerability and antitumor activity in tyrosine kinase
inhibitor-naïve Japanese patients with EGFR mutation-positive
non-small-cell lung cancer. Cancer Sci. 109:2532–2538. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cho BC, Goldberg SB, Kim DW, Socinski MA,
Burns TF, Lwin Z, Pathan N, Ma WD, Masters JC, Cossons N, et al: A
phase 1b/2 study of PF-06747775 as monotherapy or in combination
with Palbociclib in patients with epidermal growth factor receptor
mutant advanced non-small cell lung cancer. Expert Opin Investig
Drugs. 31:747–757. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi Y, Li B, Wu L, Pan Y, Pan Z, Liu Y,
Fan Y, Ji Y, Fang J, Shi Q, et al: Efficacy and safety of
limertinib (ASK120067) in patients with locally advanced or
metastatic EGFR Thr790Met-mutated NSCLC: A multicenter, single-arm,
phase 2b study. J Thorac Oncol. 17:1205–1215. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang T, Qu R, Chan S, Lai M, Tong L, Feng
F, Chen H, Song T, Song P, Bai G, et al: Discovery of a novel
third-generation EGFR inhibitor and identification of a potential
combination strategy to overcome resistance. Mol Cancer. 19:902020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lu S, Zhang Y, Zhang G, Zhou J, Cang S,
Cheng Y, Wu G, Cao P, Lv D, Jian H, et al: Efficacy and safety of
befotertinib (D-0316) in patients with EGFR T790M-mutated NSCLC
that had progressed after prior EGFR tyrosine kinase inhibitor
therapy: A phase 2, multicenter, single-arm, open-label study. J
Thorac Oncol. 17:1192–1204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han L, Zhang X, Wang Z, Zhang X, Zhao L,
Fu W, Liang X, Zhang Z and Wang Y: SH-1028, An irreversible
Third-generation EGFR TKI, overcomes T790M-mediated resistance in
non-small cell lung cancer. Front Pharmacol. 12:6652532021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xiong A, Ren S, Liu H, Miao L, Wang L,
Chen J, Li W, Li R, Wang X, Lu Z, et al: Efficacy and Safety of
SH-1028 in patients with EGFR T790M-positive NSCLC: A multicenter,
single-arm, open-label, phase 2 trial. J Thorac Oncol.
17:1216–1226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ito K, Nishio M, Kato M, Murakami H,
Aoyagi Y, Ohe Y, Okayama T, Hashimoto A, Ohsawa H, Tanaka G, et al:
TAS-121, A selective mutant EGFR inhibitor, shows activity against
tumors expressing various EGFR mutations including T790M and
uncommon mutations G719X. Mol Cancer Ther. 18:920–928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al: Dacomitinib
versus gefitinib as first-line treatment for patients with
EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A
randomised, open-label, phase 3 trial. Lancet Oncol. 18:1454–1466.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lei T, Xu T, Zhang N, Zou X, Kong Z, Wei C
and Wang Z: Anlotinib combined with osimertinib reverses acquired
osimertinib resistance in NSCLC by targeting the c-MET/MYC/AXL
axis. Pharmacol Res. 188:1066682023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jia Y, Yun CH, Park E, Ercan D, Manuia M,
Juarez J, Xu C, Rhee K, Chen T, Zhang H, et al: Overcoming
EGFR(T790M) and EGFR(C797S) resistance with mutant-selective
allosteric inhibitors. Nature. 534:129–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Choi YJ, Kim DS, Hoon Sung Y, Kim DH, Im
K, Lee H, Lee CW, Cho J, Min J, Woo DC, et al: The reversible
fourth-generation EGFR tyrosine kinase inhibitor OBX02-011
overcomes C797S-mediated resistance in lung cancer. Cancer Res. Jun
14–2022.(Epub ahead of print). doi: 10.1158/0008-5472.CAN-22-0394.
View Article : Google Scholar
|
|
77
|
Liu Y, Lai M, Li S, Wang Y, Feng F, Zhang
T, Tong L, Zhang M, Chen H, Chen Y, et al: LS-106, a novel EGFR
inhibitor targeting C797S, exhibits antitumor activities both in
vitro and in vivo. Cancer Sci. 113:709–720. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kashima K, Kawauchi H, Tanimura H,
Tachibana Y, Chiba T, Torizawa T and Sakamoto H: CH7233163
overcomes osimertinib-resistant EGFR-Del19/T790M/C797S mutation.
Mol Cancer Ther. 19:2288–2297. 2020. View Article : Google Scholar : PubMed/NCBI
|