Introduction
Epithelial carcinoma occasionally contains elements
reminiscent of trophoblastic differentiation, such as
syncytiotrophoblasts and areas resembling choriocarcinoma (1). Immunohistochemical studies have
revealed the expression of β-human chorionic gonadotropin (β-hCG)
and other trophoblastic hormones in syncytiotrophoblast-like and
cytotrophoblast-like cells (1,2).
Moreover, non-giant cancer cells in humans may express hCG in
primary carcinoma of the bladder, upper urinary tract, prostate,
colon, lung, testis and gynecological malignancies. Thus, the
expression of β-hCG suggests aberrant differentiation of cancer
cells toward a gestational trophoblastic phenotype (1–6).
Ectopic expression of β-hCG is usually associated with poor
clinicopathological indicators and unfavorable clinical outcomes
(3,4,7–10). In
previous analyses using β-hCG as a biomarker, carcinoma with
trophoblastic differentiation was revealed to be significantly
associated with poor differentiation (2,4,10–15),
advanced tumor stage (2,4,9,10,13),
early hematogenous spread (7,10),
chemoresistance (16,17), radioresistance (3,8,10,18)
and shorter disease-specific overall survival (4,8). The
most well-known example of trophoblastic differentiation in human
cancer is observed in urothelial carcinoma (UC) with trophoblastic
differentiation (UCTD) (4).
In vitro studies have indicated that
overexpression of β-hCG promotes the migration and invasion of
ovarian cancer cells, and facilitates their metastasis into
peritoneal xenografts (19).
Moreover, upregulation of β-hCG can promote the transition of
cancer cells from an epithelial phenotype with relevant biomarkers
to a loosely adherent, motile phenotype with mesenchymal markers
[epithelial-mesenchymal transition (EMT)], and can reduce their
adhesive ability (19,20). In a mouse model of human colorectal
cancer (CRC), β-hCG-overexpressing cells were shown to exhibit
increased invasiveness, migratory ability and metastatic potential
(20).
EMT is the dynamic transition of cells from an
epithelial to a mesenchymal phenotype. This transition has been
identified as the main driver of tumor progression and metastasis
(21). During normal placentation,
three main trophoblast populations exist: Cytotrophoblast stem
cells and their differentiated derivatives, syncytiotrophoblasts
and extravillous cytotrophoblasts. Syncytiotrophoblasts primarily
exhibit the epithelial phenotype, whereas extravillous
cytotrophoblasts undergo EMT, initially forming multilayered cell
columns and then (in humans) deeply infiltrating the maternal
decidual stroma and blood vessels (22). In epithelial carcinoma, EMT
initiates the dissociation of cancer cells from primary tumors, and
these cells subsequently migrate and disseminate to distant sites.
To the best of our knowledge, the molecular mechanisms underlying
trophoblastic differentiation in nongestational human cancer remain
unclear. In an autopsy study, only tumor cells with trophoblastic
differentiation, but not UC cells, were identified at multiple
metastatic sites after treatment with gemcitabine and oxaliplatin
(7), supporting the potential
resistance of UCTD to standard chemotherapy. Thus, identification
of the mechanisms underlying β-hCG-mediated EMT may facilitate the
development of targeted therapy against tumor progression through
aberrant trophoblastic differentiation. The present review
summarizes the literature on the molecular pathogenesis of β-hCG in
nontrophoblastic human cancer, with the aim of accelerating the
development of targeted therapies for trophoblastic differentiation
in human cancer.
Histopathology of trophoblastic
differentiation and diagnosis
There is a spectrum of trophoblastic differentiation
in human cancer, from syncytiotrophoblasts, areas resembling
choriocarcinoma, pure choriocarcinoma to other trophoblastic
tumors, such as epithelioid trophoblastic tumors (8). The tumor cells exhibit hyperchromatic
trophoblastic-like cells with deep eosinophilic cytoplasm that
usually appear in the periphery of the tumor nests or infiltrate
the interstitium. Tumors may also have scattered multinucleate
giant cells and well-defined syncytiotrophoblastic cells or
syncytiotrophoblastic cells that wrap around mononuclear tumor
cells that resemble cytotrophoblasts (4). In contrast with the inner mass of
conventional carcinoma cells, which have pale to eosinophilic
cytoplasm, the architecture is similar to the normal anatomical
relationship in chorionic villi. Only tumors showing definite
immunostaining for β-hCG are diagnosed as trophoblastic
differentiation phenotype of nongestational carcinoma (4,8).
Trophoblastic differentiation in UC
As per the current classification criteria outlined
by the World Health Organization, UCTD includes UC with giant cells
resembling syncytiotrophoblastic giant cells and, rarely, those
that are indistinguishable from choriocarcinoma (23). To the best of our knowledge, the
incidence of UCTD has not been investigated thoroughly. In our
recent cohort study, UCTD was detected in 47 of 859 patients (5.5%)
and 65 of 635 patients (10.2%) with bladder and upper urinary tract
UC, respectively (4). The incidence
rate was significantly lower than that (19–36%) reported in
small-scale studies (1,2,24).
This inconsistency in incidence rate may be because of our strict
inclusion criteria regarding trophoblastic histological features
compared with the b-hCG immunostaining data used in earlier
studies. Our previous study also demonstrated that β-hCG can be
expressed in not only UC with syncytiotrophoblast-like and
choriocarcinoma-like features, but also in conventional UC
(8). Few studies have investigated
the expression of other trophoblastic hormones, such as human
placental lactogen (hPL), pregnancy-specific β-1-glycoprotein and
placental alkaline phosphatase (24). By contrast, the expression of
pituitary hormones with structures similar to that of hCG, such as
luteinizing hormone (LH), follicle- stimulating hormone (FSH) and
growth hormone, was not found in UCTD (2).
Clinical significance of trophoblastic
differentiation in UC
UCTD in the bladder is usually detected as
nonpapillary, multiple and large (>3 cm) tumors with
muscle-invasion and nodal-metastasis at diagnosis (4). In addition, the risks of recurrence,
progression and mortality are significantly higher than
conventional UC (1,4). Regarding immunohistochemistry, the
nonfocal pattern of b-hCG expression is a key predictor of poor
prognosis (4). The aforementioned
findings corroborate those of other studies indicating the
associations of trophoblastic differentiation, either of
syncytiotrophoblasts or b-hCG-positive cells, with higher grades of
UC and advanced stages of disease (1,8,24).
Notably, elevated levels of b-hCG in the serum may be detected in
20–76% of the total number of patients with advanced-stage disease
or metastasis (23,25–28).
Several studies have reported the potential of b-hCG as a biomarker
of radioresistance and chemoresistance (3,7,18);
however, contradictory findings have also been reported (23,25,27).
Trophoblastic differentiation in
nonurothelial carcinoma and its clinical significance
In nonurothelial carcinoma, trophoblastic
differentiation is frequently observed in gestational trophoblastic
tumors and ovarian germ cell tumors. In addition, sporadic cases of
trophoblastic differentiation have been reported in oral cavity,
head and neck, lung, stomach, colorectum, prostate, breast and
gynecological tract carcinoma (5,9–13,16,20,29–48).
In a study on squamous cell carcinoma of the oral
cavity, β-hCG expression (range, 0.5–5%) was detected in 29 of 45
patients (64%) with oral cancer (11); β-hCG expression had a positive
association with tumor differentiation. Furthermore, β-hCG
expression has been observed in various histological subtypes of
lung cancer, including neuroendocrine carcinoma, squamous cell
carcinoma, adenocarcinoma and giant cell carcinoma (12,16,49).
Trophoblastic hormone immunoreactivity was previously detected in
31% (28/90) of all lung carcinoma cases, regardless of histological
differentiation (12).
Although β-hCG has procarcinogenic activities in
other types of carcinoma, its role in breast cancer remains
controversial. This hormone reportedly inhibits the proliferation
and induces the differentiation of human breast cancer cells in
vitro (50). Paradoxically, a
recent study revealed enhanced proliferation and poor
differentiation of β-hCG-expressing breast cancer cells, which
translated into higher colonization and invasion abilities of these
cells (51). In breast cancer
cells, the BRCA1 mutation reportedly promotes β-hCG-mediated
tumorigenesis through TGF-βRII signaling (52).
A previous study showed that β-hCG-producing cells
can be found in the normal gastric mucosa, particularly the pylorus
(31). β-hCG expression has been
detected in 6.0–8.2% of all cases of gastric carcinoma, and the
rate is even as high as 53% in some reports (32,33).
In general, the presence of β-hCG-positive cells or an elevated
level of β-hCG in the serum is associated with poor
differentiation, adverse prognosis and advanced tumor stage
(9); however, contradictory
findings have been reported (34).
In ovarian epithelial cancer, elevated levels of
serum β-hCG may be detected in both benign (27.6%) and malignant
(67%) neoplasms (13), leading to
false-positive pregnancy test results (53). Immunohistochemical expression of
β-hCG tends to be higher in intermediate- to high-grade ovarian
tumors compared with in low-grade tumors; however, the expression
does not vary across the histological subtypes of ovarian cancer.
Although a study reported higher expression rates of β-hCG in stage
III (Federation of Gynecology and Obstetrics) ovarian mucinous
carcinomas than in stage I carcinoma, no prominent association was
discovered between β-hCG expression and overall survival (13). Regarding molecular mechanisms, β-hCG
overexpression reportedly promotes the transformation and
tumorigenesis of human ovarian epithelial cells (54). The association of trophoblastic
differentiation with high-grade cancer and disease progression has
also been observed in endometrial cancer (5,48).
In summary, β-hCG expression is a well-documented
phenomenon in nongestational carcinoma of different organs, and may
even be observed in some normal tissues and benign tumors. In most
cases, the trophoblastic phenotype is associated with poor
differentiation, advanced tumor stage and poor prognosis.
Prognostic impact of trophoblastic
differentiation in human cancer
In terms of prognostic implications, patients with
carcinoma showing aberrant trophoblastic differentiation have been
reported to have unfavorable clinical outcomes (3–5,7–10,16).
Specifically, the phenotype has been associated with early
hematogenous spread (7,10,15),
higher risk of chemoresistance (16,17) or
radioresistance (3,8,10,18),
and shorter disease-specific overall survival. Using β-hCG as a
biomarker, our recent study revealed a higher risk of recurrence
(P=0.005), progression (P<0.0001) and patient death
(P<0.0001) for UCTD than for traditional, high-grade UC of the
bladder (4). Notably, patients with
UCTD and with circumferential, infiltrative or diffuse patterns of
β-hCG expression have been reported to have poorer disease-specific
overall survival than those with focal β-hCG expression.
In addition to expression in primary tumors,
elevated β-hCG serum levels have been observed in sporadic cases of
carcinoma with trophoblastic differentiation in UC (1,7,14),
squamous cell anal cancer (30),
gastric cancer (9), non-small cell
carcinoma of the lung (36),
pulmonary pleomorphic carcinoma (46), endometrial adenocarcinoma (5,48),
lymphoepithelioma-like carcinoma and squamous cell carcinoma of the
cervix (55). However, the actual
incidence of abnormal laboratory results for this entity has not
been thoroughly examined. In our experience, most UCTDs showing a
focal pattern of β-hCG expression have normal serum levels
(unpublished data). Nevertheless, the levels of serum β-hCG appear
to change with treatment (5–7,30,48)
and are associated with clinical outcome (5,9,16).
Specifically, a serum β-HCG level ≥4 IU/l prior to chemotherapy has
been reported to be a significant prognostic factor for patients
with advanced gastric cancer (hazard ratio 1.7; 95% confidence
interval 2.8–1.1) (9). Serum β-hCG
elevation (≥5 mIU/ml) in patients with non-small cell lung cancer
showing trophoblastic differentiation is also significantly
associated with chemoresistance (16). Taken together, these findings
indicated that serum β-hCG measurement may have potential as a
marker of clinical response or prognosis, and thus should be
applied as a potential biomarker during follow-up after
surgery.
hCG: Gene, protein and structure
The α-subunit of hCG is encoded by a single gene
(CGA) on chromosome 6q21.1–23 (56), whereas the β-subunit is encoded by
six nonallelic genes (CGB1, CGB2, CGB3, CGB5, CGB7 and
CGB8) on chromosome 19q13.3 (57). CGB4, which is adjacent to the
aforementioned CGB cluster, encodes the β-subunit of LH.
CGB6 is an allelic variant of CGB7, whereas
CGB9 is an allelic variant of CGB3. To the best of
our knowledge, the functions of CGB1 and CGB2 remain
unknown; these genes may be pseudogenes (57). The expression of CGB genes is
upregulated to some extent in the first trimester of pregnancy,
which suggests a role in implantation (58). A protein encoded by type I genes
(CGB6 and CGB7) contains an alanine residue at
position 117, whereas β-hCG, which is encoded by type II genes
(CGB3, CGB9, CGB5 and CGB8), contains an aspartic
acid residue at this position. The effect of this heterogeneity on
the function and immunoreactivity of β-hCG remains unknown. Type I
genes are expressed primarily in benign nontrophoblastic tissues,
whereas type II genes are expressed in trophoblastic and malignant
tissues (59).
The hormone hCG comprises 237 amino acids and
belongs to the glycoprotein hormone family, which includes LH,
thyroid-stimulating hormone (TSH) and FSH. The protein members of
the aforementioned family are heterodimers comprising α- and
β-subunits. The α-subunit contains 92 amino acids and is common
across all family members. By contrast, the β-subunit of hCG
exhibits varying degrees of homology with other family members (LH,
80–85%; FSH, 36%; TSH, 46%) (60–62).
The homology between hCG and LH indicates their common biological
function; both bind to the same receptor, the LH/hCG receptor
(63). By contrast, FSH and TSH
bind to structurally similar but distinct receptors. The β-subunit
of hCG contains 145 amino acids, whereas that of LH contains 121
amino acids; this difference originates from a 24-amino-acid-long
extension in hCG, known as the C-terminal peptide (61,64).
The α-subunit of hCG comprises two N-linked
oligosaccharides (linked to the N atom of asparagine), whereas its
β-subunit comprises two N-linked oligosaccharides and four O-linked
oligosaccharides (linked to the O atom of serine) (65). The O-linked and N-linked
oligosaccharides contain saccharides ranging from trisaccharides to
pentasaccharides, and exhibit monoantennary to triantennary
structures (66).
Posttranslationally modified hCG variants are complex and have
three dimeric isoforms: Regular hCG, hyperglycosylated hCG (hCG-H)
and sulfated hCG (hCG-S) (62,67).
hCG-H is a glycoprotein with excessive branching and complex hCG
oligosaccharide side chains. Although hCG and hCG-H share an amino
acid sequence, they are distinct glycoproteins with completely
different oligosaccharide structures (66). The levels of hCG-H are high in early
pregnancy (68) and show a higher
trend in malignant diseases than in normal pregnancy. Various
aberrant glycosylations have been detected in tumor-derived hCG
(69), which has led to the
generation of a diverse molecular weight spectra. In one study, the
average molecular weight of hCG was determined to be 37,500 Da
through matrix-assisted laser desorption/ionization time-of-flight
mass spectrometry (70); the
molecular weights of α-hCG and β-hCG were 14,000 and 23,500 Da,
respectively.
hCG-S originates from the pituitary gland and
appears to mimic the activities of LH during the menstrual cycle;
LH stimulates ovarian granulosa and theca cells to produce relevant
hormones, and facilitates follicular growth, luteinization and
ovulation (62,67,71).
Both hCG and hCG-S act through the LH/hCG receptor, whereas hCG-H
acts through the TGF-β receptor as an autocrine and paracrine
factor (62).
Trophoblasts represent a key source of hCG.
Syncytiotrophoblasts secrete hCG, whereas cytotrophoblasts secrete
hCG-H. Cole et al (72)
reported the following five common forms of hCG: hCG, hCG-H, hCG-S,
free β-hCG and free β-hCG-H (72).
They classified these proteins into group 1 (proteins produced by
placental syncytiotrophoblasts and pituitary gonadotropes) and
group 2 (proteins produced by placental cytotrophoblasts and human
cancer cells). Group 1 proteins are hormones that act through the
hCG/LH receptor, and are central to the menstrual cycle and
pregnancy, whereas group 2 proteins perform autocrine functions by
antagonizing the TGF-β receptor and are critical in advanced
malignancies (67,72).
Structurally, hCG can be classified as intact hCG,
α-hCG, β-hCG, partially degraded or nicked hCG and β-hCG, and a
β-core fragment (73); the
International Federation of Clinical Chemistry has approved this
classification.
Regulation of hCG expression
The expression and production of hCG may be
regulated by various hormones [corticosteroids, progesterone and
gonadotropin-releasing hormone (GnRH)], growth factors (epidermal
growth factor, placental growth hormone, leukemia inhibitory factor
and vascular endothelial growth factor), cytokines (interleukin-6
and tumor necrosis factor-α), ligands of peroxisome
proliferator-activated receptors (PPARs), and homeobox genes
(62,74–77).
Levels of α-hCG and β-hCG expression vary in normal
placenta, with the α/β ratio ranging from 1.7 in the first
trimester, to 12 once the pregnancy has reached full term (78). Therefore, the regulatory mechanisms
of α-hCG and β-hCG expression may be different. Knowledge regarding
the pathways involved in the upregulation of hCG genes
remains limited. Cyclic adenosine monophosphate (cAMP) appears to
regulate the transcription of the α- and β-subunits of hCG in the
placenta and choriocarcinoma (79,80) by
regulating the 5′ cAMP response element (CRE) enhancers of the
promoters of both genes through different mechanisms (81,82).
α-hCG
The promoter of the α-subunit gene contains the
following five regulatory elements: A trophoblast-specific element
(TSE), an α-activating element (αACT), a tandem or duplicated CRE,
a junctional regulatory element (JRE) and a CCAAT box. These
elements have been categorized into the following three domains:
The upstream regulatory domain (URE; TSE and αACT), tandem CRE and
the downstream regulatory domain (JRE and CCAAT box) (83–88).
Tissue-specific expression of α-hCG has been demonstrated in
trophoblasts on the basis of limited or specific tissue
distribution of the binding proteins and regulation of the
aforementioned elements (88).
The exact locations of αACT and TSE overlap
slightly; the sequence of αACT between −161 and −142 overlaps that
of TSE between −182 and −159 (89).
Furthermore, two regulatory sequences can be noted within this
region: A downstream domain located between −172 and −151 and an
upstream domain located between −177 and −156 (86). This structure indicates that these
regions are activated by at least two types of binding proteins
that may be specifically expressed by either pituitary or placental
cells (90).
The tandem CRE is responsible for the binding of
CRE-binding proteins (CREBs), which belong to the basic region
leucine zipper family of proteins. In villous trophoblasts,
activating transcription factor (ATF)-1 is more extensively
involved in the binding of CRE and, to some extent, CREB-1, than
the other members of the ATF/CREB family, such as ATF-2, ATF-3,
ATF-4 and CREB-2 (81). In humans,
CREB binding may be dependent on URE binding (89). URE-1/αACT binds to members of the
ubiquitous GATA family of DNA-binding proteins (91), whereas URE-2/TSE binds to
TSE-binding proteins (TSEBs) (92).
β-hCG
The expression of CGB among patients is
heterogeneous, and the magnitude of expression has varied across
studies, possibly because they focused on various pregnancy
trimesters (93,94). CGB expression also varies
across tumors and normal tissues. For example, the expression
levels of type II genes (CGB3, CGB9, CGB5 and CGB8)
are higher in bladder tumors than in the normal urothelium
(95).
Similar to the α-hCG promoter, the β-hCG promoter
comprises a tandem CRE (two repeated CREs), where c-Jun suppresses
β-hCG expression (96). At least
two additional TSE elements have been reported to cluster in the
regulatory region of CGB5. The genes encoding the α- and
β-subunits of hCG may be coordinately regulated by TSEBs (92). Other transcription factors involved
in CGB expression include ETS protooncogene 2 (ETS2),
activating protein 2 (AP2), promoter selective transcription factor
(SP)1, SP3, octamer-binding transcription factor (OCT)3/OCT4,
PPARγ, P53 and metastasis-associated protein 3 (MTA3) (97–103).
In human choriocarcinoma and murine cells, ETS2
enhances the transcription of CGB5 through activation of the
rat sarcoma virus/mitogen-activated protein kinase pathway, and the
primary effects of cAMP on the β-hCG promoter are mediated through
the proximal ETS2 enhancer (97).
AP2, SP1 and SP3 are the key regulators of basal CGB
transcription in placental trophoblast cells (103,104). AP2 and SP1 play distinct roles in
the regulation of basal activity and cAMP-responsiveness of the
β-hCG promoter (105), whereas SP3
suppresses its basal transcription by inhibiting SP1 (105). The expression levels of
CGB3-CGB9 have been reported to be considerably
higher in ovarian cancer cells than in healthy ovarian cells
(103). CGB1 and
CGB2 transcripts have been detected in 20% of all ovarian
cancer tissue sample, but not in control samples. This may have
resulted from demethylation of CGB promoter regions, an
increased level of transcription factor AP2 (TFAP2)-α, and a
decreased level of SP3 in ovarian tumors (103). OCT3 and OCT4 are essential for
maintaining embryonic cells in an undifferentiated state; these
transcription factors may silence hCG expression in choriocarcinoma
cells (99).
The treatment of trophoblasts with PPARγ or
retinoid X receptor (RXR)α ligands increases the levels of
CGB transcript, hCG and β-hCG (106). PPARγ/RXRα heterodimers directly
bind to the regulatory region of CGB5 (106). Activation of PPARγ enhances the
transcript levels of α-hCG and β-hCG in villous trophoblasts, but
reduces the level of hCG in invasive extravillous trophoblasts
(107). Shalom-Barak et al
(108) and Peng et al
(109) suggested that PPARγ is
distinctly expressed in various trophoblast subsets and during
trophoblast stem cell differentiation. p53 selectively
induces the expression of CGB7; a p53-responsive element has been
identified in the promoter of CGB7 (101). MTA3, a chromatin-remodeling
protein, acts directly on the CGB5 promoter and suppresses
CGB5 expression in trophoblasts (102). The deregulation of MTA3 has
previously been associated with pre-eclampsia (102).
In addition to transcription factors, various
epigenetic mechanisms (methylation) are involved in the regulation
of CGB expression in the placenta and cancer cells (110,111). Allelic polymorphism for
methylation sensitivity in the promoter of CGB5 have been
shown to be associated with a high risk of miscarriage (112). The deregulation of epigenetic
mechanisms with altered CGB expression has also been
associated with an increased risk of early pregnancy loss (104).
Biological effects of β-hCG upregulation in
human cancer
Proliferation
To explore the biological effects of trophoblastic
differentiation in human cancer, in vitro experiments have
been performed using several human cancer models. Ectopic
expression of β-hCG appears to enhance the in vitro
proliferation of ovarian (54) and
stomach (34) cancer cells, but not
that of CRC cells (20,35). Similar effects were noted on the
in vivo proliferation of primary ovarian cancer cells
(54), but not that of CRC
(20,35) or bladder (113) cancer cells. Table I summarizes the various biological
effects of β-hCG upregulation in human cancer.
 | Table I.Biological effects of β-hCG
overexpression in human cancer. |
Table I.
Biological effects of β-hCG
overexpression in human cancer.
| First author,
year | Cancer | Experi-mental
model | Materials | Activated
pathway | Poor prognosis | High
expression | Invasion | Migration | Proliferation | Metastasis | EMT | Morphology
change | Tumorigenesis | Antiapoptosis |
Chemoresistance | Angiogenesis | Recurrence
marker | (Refs.) |
|---|
| Sengodan, 2017 | Breast cancer | In
vitro | MCF7 cells,
MDAMB-231 cells, SKBR3 cells, T47D cells | TGFβRII
signaling | N/A | V | V | V | N/A | N/A | V | N/A | V | N/A | N/A | N/A | N/A | (52) |
| Sengodan, 2017 |
| In vivo | BRCA1 conditional
knockout mouse model | Mutated/defective
BRCA1 activates β-hCG expression | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (52) |
| Kawamata, 2018 | Colorectal
carcinoma | In
vitro | Caco-2 cells, LoVo
cells, HCA7 cells, WiDr cells, T84 cells | EMT via TGF-β
signaling pathway | N/A | N/A | V | V | X | N/A | V | V | N/A | N/A | N/A | N/A | N/A | (20) |
| Li, 2018 |
|
| HCT-116 cells,
HT-29 cells, SW480 cells | N/A | N/A | N/A | V | V | X | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (35) |
| Lundin, 2001 |
| In vivo | Primary tumor | N/A | V | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (40) |
| Kawamata, 2018 |
|
| Primary tumor | EMT via TGFN/Aβ
signaling pathway | V | V | V | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (20) |
| Kawamata, 2018 |
|
| LoVo-GFP cells,
LoVo-hCGβ cells | EMT via TGF-β
signaling pathway | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | V | N/A | N/A | V | N/A | (20) |
| Li, 2018 |
|
| Primary tumor | N/A | V | N/A | V | V | X | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (35) |
| Konishi, 2018 |
|
| Primary tumor | N/A | V | N/A | N/A | N/A | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | (38) |
| Białas, 2020 | Esophageal
carcinoma | In vivo | Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (118) |
| Li, 2013 | Glioblastoma | In
vitro | U87MG cells | ERK1/2 | N/A pathway | N/A | V | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | V | (114) |
| Białas, 2020 | Head and neck
cancer | In vivo | Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (118) |
| Li, 2013 | Lung cancer | In vivo | Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (114) |
| Noda, 1990 | Non-small cell lung
cancer | In vivo | Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (39) |
| Arano, 1994 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (41) |
| Okutur, 2010 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (42) |
| Seder, 2017 |
|
| Preoperative
serum | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | V | (43) |
| Wong, 2015 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (44) |
| Khobta, 2012 |
|
| Primary tumor,
preoperative plasma | N/A | N/A | V | V | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (36) |
| Khattri, 2011 |
|
| Primary tumor,
preoperative serum | N/A | N/A | V | V | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (37) |
| Szturmowicz,
1999 |
|
| Primary tumor,
preoperative serum | N/A | V | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (16) |
| Vicier, 2013 |
|
| Preoperative
serum | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (45) |
| Dinis de Sousa,
2021 |
|
| Primary tumor,
preoperative serum | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (46) |
| Liu, 2017 | Ovarian cancer | In
vitro | ES-2 cells, SKOV3
cells | N/A | N/A | N/A | V | V | N/A | N/A | V | V | N/A | N/A | N/A | N/A | N/A | (19) |
| Sengodan, 2017 |
|
| OVCAR8 cells | BRCA1 regulation on
β-hCG | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (52) |
| Guo, 2011 |
|
| T29 cells, T29-hCG
cells, T80 cells, T80-hCG cells | N/A | N/A | N/A | N/A | N/A | V | N/A | N/A | N/A | N/A | V | N/A | N/A | N/A | (54) |
| Śliwa, 2019 |
| In vivo | Primary tumor | Demethylation
regulating gene expression | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (103) |
| Liu, 2017 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | V | V | N/A | N/A | N/A | N/A | N/A | (19) |
| Liu, 2017 |
|
| ES-2 cells, SKOV3
cells | N/A | N/A | N/A | N/A | N/A | N/A | V | N/A | V | V | N/A | N/A | N/A | N/A | (19) |
| Guo, 2011 |
|
| T29 cells, T29-hCG,
T80 cells and T80-hCG cells were injected into an athymic nude
mouse model | N/A | N/A | N/A | N/A | N/A | V | N/A | N/A | N/A | V | V | N/A | N/A | N/A | (54) |
| Guo, 2011 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (54) |
| Sengodan, 2017 | Prostate
cancer | In
vitro | DU145 cells | BRCA1 regulation on
β-hCG | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (52) |
| Wu. 2006 |
|
| DU145 cells, PC3
cells | N/A | N/A | N/A | V | V | N/A | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | (115) |
| Li, 2013 |
|
| DU145 cells | ERK1/2 pathway | N/A | N/A | V | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (116) |
| Szturmowicz,
1995 | Small cell lung
cancer | In vivo | Preoperative
serum | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | V | N/A | N/A | (138) |
| Butler, 2000 | Urothelial
carcinoma | In
vitro | T24 cells, SCaBER
cells, J82 cells, 5637 cells, RT112 cells | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | V | V | N/A | N/A | N/A | (113) |
| Białas, 2020 |
| In vivo | Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (118) |
| Cheng, 2021 |
|
| Primary tumor | N/A | V | V | V | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | V | (4) |
| Hoshi, 2018 |
|
| Postoperative
serum | N/A | V | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | V | N/A | N/A | (10) |
| Rajabi, 2013 |
|
| Preoperative serum,
urine | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (53) |
| Armah, 2007 |
|
| Primary tumor | N/A | V | V | N/A | N/A | N/A | V | N/A | N/A | N/A | N/A | V | N/A | N/A | (120) |
| Shimada, 2006 |
|
| Primary tumor,
preoperative serum | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (14) |
| Regalado, 2004 |
|
| Primary tumor | N/A | N/A | V | V | N/A | N/A | V | N/A | N/A | N/A | N/A | V | N/A | N/A | (117) |
| Ramakumar,
1998 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (47) |
| Oyasu, 1994 |
|
| Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (139) |
| Białas, 2020 | Uterine corpus
endometrial carcinoma | In vivo | Primary tumor | N/A | N/A | V | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | (118) |
Apoptosis
Overexpression of β-hCG inhibits the in
vitro apoptosis of ovarian and bladder cancer cells (113), as well as that of cells obtained
from the xenografts of nude mice (54). In vitro β-hCG treatment of
bladder UC exerts antiapoptotic effects (113).
Migration
β-hCG reportedly stimulates the in vitro
migration of BRCA1-mutant breast cancer (52), CRC (20,35),
glioblastoma (114), ovarian
cancer (19) and prostate cancer
cells (115,116).
Invasion
β-hCG promotes in vitro cell invasion in
BRCA1-mutant breast cancer (52), CRC (20,35),
ovarian cancer (19), prostate
cancer (115,116) and glioblastoma (114). β-hCG expression has also been
strongly associated with tumor invasion in primary CRC (20,35),
non-small cell lung cancer (36,37)
and bladder cancer (4,117).
EMT
Ectopic expression of β-hCG in cancer cells appears
to induce in vitro EMT in BRCA1-mutant breast cancer
(52), CRC (20) and ovarian cancer (19). β-hCG has been reported to be
essential for in vivo modulation of EMT in mouse tumor
xenograft models of CRC (20), CRC
metastasis (20,38), large cell carcinoma (39) and lung squamous cell carcinoma
(118).
Angiogenesis
Xenografts of CRC with aberrant β-hCG expression
have been demonstrated to harbor a higher density of microvessels
than control tumors in mice (20).
The fifth subunit of β-hCG, CGB5, may also promote tumor growth and
vasculogenic mimicry by activating the LH receptor signal
transduction pathway (119).
Metastasis
Overexpression of β-hCG is strongly associated with
increased metastatic potential in CRC (20,35),
non-small cell lung cancer (36,37),
ovarian cancer (19) and bladder
cancer (4,117,120).
Tumorigenesis
Overexpression of β-hCG has been reported to
enhance tumorigenesis in mouse tumor xenograft models of
BRCA1-mutant breast cancer (52), CRC (20), ovarian cancer (19,54)
and bladder cancer (113).
Taken together, these findings indicated that the
molecular mechanisms underlying the effects of β-hCG on increased
tumorigenesis include promotion of cell proliferation,
differentiation, vasculogenesis, EMT and metastasis.
The hCG-mediated signaling pathways
TGF-β signaling pathway
In an early study conducted using CRC as a model,
LoVo and HCA-7 cells were transfected with β-hCG (20). Ectopic expression of β-hCG was shown
to upregulate zinc finger protein SNAI1 (SNAIL), zinc finger
protein SNAI2, twist-related protein 1 (TWIST) and
phosphorylated-SMAD2, but to downregulate epithelial cadherin in
hCGβ-transfected HCA-7 cells compared with in control cells. The
phosphorylation of SMAD2, which was activated through stimulation
of TGF-β receptors during EMT, was also promoted. Together, these
results indicated that β-hCG may induce EMT through the TGF-β
signaling pathway. Fig. 1 depicts
the β-hCG-mediated signaling pathways discussed in the present
review.
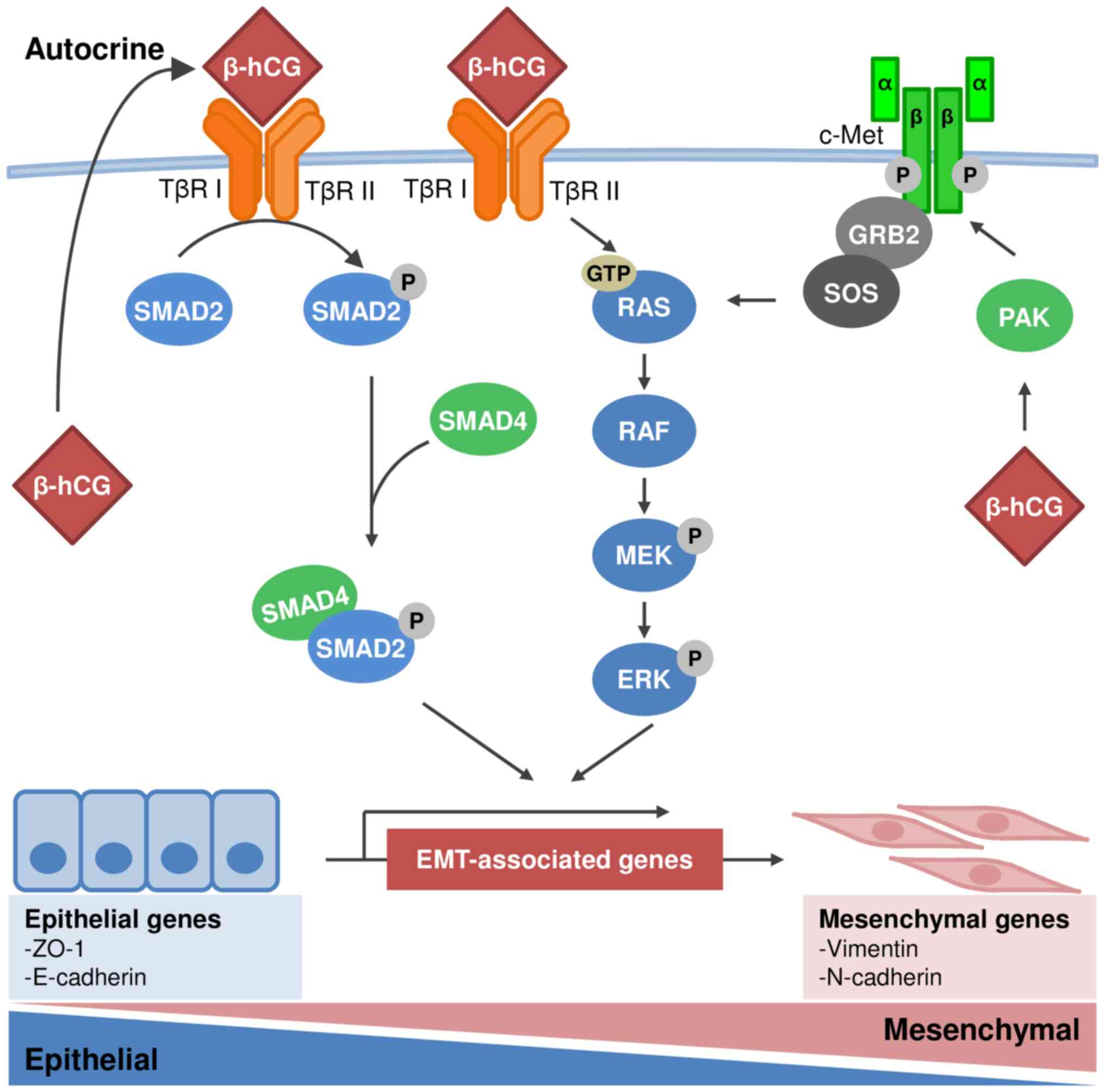 | Figure 1.Schematic diagram of β-hCG-mediated
signaling pathways in human cancer. To the best of our knowledge,
the molecular mechanisms underlying β-hCG-mediated tumor
progression have not yet been elucidated. The literature suggests
that overexpression of β-hCG is involved in activation of the
TGF-β/SMAD, MAPK/ERK and c-Met signaling pathways, resulting in the
EMT of cancer cells in humans. β-hCG binds to the receptor for
TGF-β, leading to activation of the SMAD and MAPK/ERK pathways. In
addition, β-hCG upregulates c-Met through PAK signaling to activate
the MAPK/ERK pathway. Ectopic expression of β-hCG induces EMT in
carcinoma cells with upregulation of vimentin and the
downregulation of E-cadherin. β-hCG, β-human chorionic
gonadotropin; TβR I, transmembrane serine/threonine kinase type II
receptor; TβR II, transmembrane serine/threonine kinase type I
receptor; P, phosphorylated; EMT, epithelial-mesenchymal
transition. |
EMT-related signaling pathway
A recent cell line and mouse model experiment
revealed that β-hCG promotes the proliferation of gastric cancer
cells in vitro through activation of the c-Met (121).
ERK1/2 signaling pathway
In human glioblastoma, β-hCG has been shown to
upregulate the expression of phosphorylated-ERK1/2 in U87MG cells
in a dose- and time-dependent manner (114). In addition, the ERK/matrix
metalloproteinase 2 (MMP2) signaling pathway is involved in the
β-hCG-mediated metastasis of epithelial ovarian cancer cells
(122). This observation was
supported by an in vitro experiment performed using the
DU145 prostate cancer cell line (116).
Expression of non-hCG biomarkers associated
with trophoblastic differentiation
In addition to hCG, the other trophoblastic markers
that are used for diagnostic purposes include hPL, melanoma cell
adhesion molecule (MCAM; also called CD146), p63, mucin (MUC)-4,
human leukocyte antigen (HLA)-G, cytokeratin 18, inhibin A,
GATA-binding protein 3 (GATA3), HSD3B1 and spalt-like transcription
factor 4 (SALL4) (123–128).
The expression of these markers varies across
trophoblast types, namely, cytotrophoblasts, syncytiotrophoblasts
and intermediate trophoblasts (ITs); the latter two types may be
derived from cytotrophoblasts. ITs may be further subtyped into
villous ITs (villi are anchored to the basal plate through
trophoblastic columns), implantation site ITs and chorionic-type
(chorion laeve) ITs (129).
Each subtype of IT has a different immunohistochemical profile and
malignant counterparts. For example, hCG, HSD3B1, hPL, inhibin,
MCAM, SALL4, HLA-G, MUC4 and p63 are expressed in the malignant
villous ITs; hPL, MUC-4, HSD3B1, HLA-G and CD146 are expressed in
the malignant implantation site ITs, with limited expression of hCG
and inhibin; while tumor cells of chorionic-type IT origin
diffusely express HSD3B1, HLA-G, p63, cyclin E, inhibin A and
GATA3, with occasional expression of CD146 and hPL (130).
In general, SALL4 is specifically expressed in
mononuclear cytotrophoblasts, in contrast to hCG, which is
expressed mainly in syncytiotrophoblasts. HSD3B1 is regarded as a
pantrophoblastic marker. HLA-G is useful for all three subtypes of
IT. CD146 and hPL are highly specific for implantation site ITs,
whereas p63 and PLAP are highly specific for chorionic ITs
(129,131).
Notably, some of the aforementioned markers have
been detected in nontrophoblastic tumors. For example, one small
study (n=16) revealed that hCG was expressed in 93% of patients
with UCTD (8). In this study, not
only the trophoblastic component but also the UC component
expressed hCG, at a rate as high as 85% (8); HSD3B1 was also expressed in the
trophoblastic component of all but one case (8). By contrast, SALL4 expression was
variable, with a 50% staining rate in trophoblasts and a 43%
staining rate in the UC component of hCG-positive cases (8).
Although supplementary potential markers for
trophoblastic differentiation have been identified, the application
of some of these markers remains debatable, partly because of the
difficulty associated with determining the trophoblastic lineage of
candidate cells (132). The
proposed characteristics of primary first-trimester trophoblasts
include the expression of a specific set of protein markers
(cytokeratin 7, GATA3 and TFAP2-γ), the HLA class I expression
profile, the methylation of ELF5 and the expression of microRNAs
(miRNAs) from the chromosome 19 miRNA cluster (132).
Options for targeted therapy
As a target for cancer vaccines, hCG has been
explored for decades (133). An
early investigation revealed the potential of a monoclonal antibody
against β-hCG (6H1) in the inhibition of tumor growth in
vitro and in vivo (134). The CDX-1307 vaccine (also called
B11-hCG-β) was developed to target β-hCG-expressing bladder cancer
cells (135). This vaccine
comprises a B11 monoclonal antibody against the mannose receptor of
antigen-presenting cells fused to β-hCG. In a phase I clinical
trial involving patients with cancer, CDX-1307 was found to be well
tolerated. It induced substantial β-hCG-specific cellular and
humoral immune responses when co-administered with GM-CSF and the
Toll-like receptor agonists Resiquimod (R848) and poly-ICLC
(135). However, enrollment for
the early phase II trial was slow and the study was terminated
prematurely (https://clinicaltrials.gov/ct2/show/record/NCT01094496)
(136).
Combination therapies can be used as alternatives.
Through its direct and collaborative effects with Toll-like
receptor ligands and accessory cell-secreted cytokines, hCG was
shown to mediate chemoresistance in gonadotropin-sensitive tumors
in a mouse study (137). The
coadministration of curcumin and an anti-hCG vaccine (β-hCG
conjugated to tetanus toxoid) to mice carrying syngeneic tumors
resulted in considerably improved animal survival (137).
On the basis of the aforementioned molecular
alterations, inhibitors of type I and II TGF-β receptors appear to
successfully reverse the biological effects and overexpression of
SNAIL and TWIST induced by β-hCG in human CRC (20). Moreover, an ERK1/2 inhibitor could
reduce the expression of MMP2, invasion of human glioblastoma cells
(114) and motility of prostate
cancer cells in vitro (116). Taken together, these results
indicated that both ERK1/2 and MMP2 are potential targets in
precision therapy for β-hCG-related cancer progression.
Future perspectives
The understanding of the molecular basis of
β-hCG-mediated tumorigenesis in human cancer remains incomplete.
Information regarding the mechanisms underlying trophoblastic
differentiation in human cancer may facilitate the development of
personalized therapy for patients with cancer. With the advancement
of research, targeting the constituent(s) of β-hCG-mediated EMT and
angiogenesis may improve current therapeutic regimens for patients
with epithelial cancer with trophoblastic differentiation.
Acknowledgements
Not applicable.
Funding
This manuscript was supported by research grants [grant nos.
MOST 108-2320-B-006-050-MY3, MOST 110-2314-B-006-083 and MOST
111-2320-B-006-025] from the Ministry of Science and Technology,
Taiwan, and grants [grant nos. NCKUH-11204052 and NCKUH-11208006]
from the National Cheng Kung University Hospital, Taiwan.
Availability of data and materials
Not applicable.
Authors' contributions
CC, YLC, YWW, TL, HWC, CWH. KCL, YCO, CAC, CLH, CTL
and NHC performed literature research. CC, YLC, CTL and NHC wrote
the original draft. CC, CTL, WLC and NHC revised the article. YLC
generated the original figure and table. CTL and NHC performed
visualization. Data authentication is not applicable. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Campo E, Algaba F, Palacin A, Germa R,
Sole-Balcells FJ and Cardesa A: Placental proteins in high-grade
urothelial neoplasms. An immunohistochemical study of human
chorionic gonadotropin, human placental lactogen, and
pregnancy-specific beta-1-glycoprotein. Cancer. 63:2497–2504. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dirnhofer S, Koessler P, Ensinger C,
Feichtinger H, Madersbacher S and Berger P: Production of
trophoblastic hormones by transitional cell carcinoma of the
bladder: Association to tumor stage and grade. Hum Pathol.
29:377–382. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moutzouris G, Yannopoulos D, Barbatis C,
Zaharof A and Theodorou C: Is beta-human chorionic gonadotrophin
production by transitional cell carcinoma of the bladder a marker
of aggressive disease and resistance to radiotherapy? Br J Urol.
72:907–909. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng HL, Chou LP, Tsai HW, Lee CT, Wang
YW, Chung-Liang H, Ou JH, Tsai YS and Chow NH: Urothelial carcinoma
with trophoblastic differentiation: Reappraisal of the clinical
implication and immunohistochemically features. Urol Oncol.
39:732.e17–732.e23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradley CS, Benjamin I, Wheeler JE and
Rubin SC: Endometrial adenocarcinoma with trophoblastic
differentiation. Gynecol Oncol. 69:74–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman RL, Lindberg G, Muller CY, Miller
DS and Hameed A: Ectopic production and localization of beta-human
chorionic gonadotropin in lymphoepithelioma-like carcinoma of the
cervix: A case report. Int J Gynecol Pathol. 19:179–182. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchida M, Kawai K, Kurobe M, Ikeda A,
Kandori S, Endo T, Miyagawa T, Kojima T, Tsutsumi M and Nishiyama
H: Metastases of urothelial carcinoma with trophoblastic
differentiation that responded to combination chemotherapy with
gemcitabine and oxaliplatin: A case report. Hinyokika Kiyo.
64:55–61. 2018.(In Japanese). PubMed/NCBI
|
|
8
|
Przybycin CG, McKenney JK, Nguyen JK, Shah
RB, Umar SA, Harik L, Shih IM and Cox RM: Urothelial carcinomas
with trophoblastic differentiation, including choriocarcinoma:
Clinicopathologic series of 16 cases. Am J Surg Pathol.
44:1322–1330. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Webb A, Scott-Mackie P, Cunningham D,
Norman A, Andreyev J, O'Brien M and Bensted J: The prognostic value
of serum and immunohistochemical tumour markers in advanced gastric
cancer. Eur J Cancer. 32A:63–68. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoshi S, Numahata K, Morozumi K, Katumata
Y, Kuromoto A, Takai Y, Hoshi K, Bilim V and Sasagawa I: Bladder
cancer metastasis producing beta-human chorionic gonadotropin,
squamous cell carcinoma antigen, granulocyte-colony stimulating
factor, and parathyroid hormone-related protein. IJU Case Rep.
2:47–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhalang K, Kafrawy AH and Miles DA:
Immunohistochemical study of the expression of human chorionic
gonadotropin-beta in oral squamous cell carcinoma. Cancer.
85:757–762. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dirnhofer S, Freund M, Rogatsch H,
Krabichler S and Berger P: Selective expression of trophoblastic
hormones by lung carcinoma: Neurendocrine tumors exclusively
produce human chorionic gonadotropin alpha-subunit (hCGalpha). Hum
Pathol. 31:966–972. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lenhard M, Tsvilina A, Schumacher L, Kupka
M, Ditsch N, Mayr D, Friese K and Jeschke U: Human chorionic
gonadotropin and its relation to grade, stage and patient survival
in ovarian cancer. BMC Cancer. 12:22012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimada K, Nakamura M, Ishida E and
Konishi N: Urothelial carcinoma with plasmacytoid variants
producing both human chorionic gonadotropin and carbohydrate
antigen 19-9. Urology. 68:891.e7–e10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Wang J, Zhang W, Wang D, Wang X
and Liang X: Case report: Urothelial carcinoma of the renal pelvis
with trophoblastic differentiation: A rare case report and review
of literature. Pathol Oncol Res. 29:16108562023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szturmowicz M, Slodkowska J, Zych J,
Rudzinski P, Sakowicz A and Rowinska-Zakrzewska E: Frequency and
clinical significance of beta-subunit human chorionic gonadotropin
expression in non-small cell lung cancer patients. Tumour Biol.
20:99–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cook AM, Huddart RA, Jay G, Norman A,
Dearnaley DP and Horwich A: The utility of tumour markers in
assessing the response to chemotherapy in advanced bladder cancer.
Br J Cancer. 82:1952–1957. 2000.PubMed/NCBI
|
|
18
|
Martin JE, Jenkins BJ, Zuk RJ, Oliver RT
and Baithun SI: Human chorionic gonadotrophin expression and
histological findings as predictors of response to radiotherapy in
carcinoma of the bladder. Virchows Arch A Pathol Anat Histopathol.
414:273–277. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu N, Peng SM, Zhan GX, Yu J, Wu WM, Gao
H, Li XF and Guo XQ: Human chorionic gonadotropin β regulates
epithelial-mesenchymal transition and metastasis in human ovarian
cancer. Oncol Rep. 38:1464–1472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawamata F, Nishihara H, Homma S, Kato Y,
Tsuda M, Konishi Y, Wang L, Kohsaka S, Liu C, Yoshida T, et al:
Chorionic gonadotropin-β modulates epithelial-mesenchymal
transition in colorectal carcinoma metastasis. Am J Pathol.
188:204–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto MA, Huang RYJ, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vićovac L and Aplin JD:
Epithelial-mesenchymal transition during trophoblast
differentiation. Acta Anat (Basel). 156:202–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Comperat EM, Netto GJ and Tsuzuki T:
Invasive urothelial carcinoma. In: WHO Classification of of
Tumours: Urinary and Male Genital Tumours. Vol 8. 5th edition.
IARC; Lyon: pp. 150–165. 2022
|
|
24
|
Moldavsy M, Sazbon A, Kuchersky N and
Turani H: Screening for transitional cell carcinoma of the bladder
with trophoblastic differentiation in Upper Galilee. Harefuah.
134:260–263. 3363351998.(In Hebrew). PubMed/NCBI
|
|
25
|
Dexeus F, Logothetis C, Hossan E and
Samuels ML: Carcinoembryonic antigen and beta-human chorionic
gonadotropin as serum markers for advanced urothelial malignancies.
J Urol. 136:403–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iles RK, Jenkins BJ, Oliver RT, Blandy JP
and Chard T: Beta human chorionic gonadotrophin in serum and urine.
A marker for metastatic urothelial cancer. Br J Urol. 64:241–244.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Douglas J, Sharp A, Chau C, Head J, Drake
T, Wheater M, Geldart T, Mead G and Crabb SJ: Serum total hCGβ
level is an independent prognostic factor in transitional cell
carcinoma of the urothelial tract. Br J Cancer. 110:1759–1766.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iles RK: Ectopic hCGbeta expression by
epithelial cancer: Malignant behaviour, metastasis and inhibition
of tumor cell apoptosis. Mol Cell Endocrinol. 260–262. 264–270.
2007.PubMed/NCBI
|
|
29
|
Gehring C, Siepmann T, Heidegger H and
Jeschke U: The controversial role of human chorionic gonadotropin
in the development of breast cancer and other types of tumors.
Breast. 26:135–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pokharel K, Gilbar PJ, Mansfield SK, Nair
LM and So A: Elevated beta human chorionic gonadotropin in a
non-pregnant female diagnosed with anal squamous cell carcinoma. J
Oncol Pharm Pract. 26:1266–1269. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manabe T, Adachi M and Hirao K: Human
chorionic gonadotropin in normal, inflammatory, and carcinomatous
gastric tissue. Gastroenterology. 89:1319–1325. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito H and Tahara E: Human chorionic
gonadotropin in human gastric carcinoma. A retrospective
immunohistochemical study. Acta Pathol Jpn. 33:287–296.
1983.PubMed/NCBI
|
|
33
|
Yakeishi Y, Mori M and Enjoji M:
Distribution of beta-human chorionic gonadotropin-positive cells in
noncancerous gastric mucosa and in malignant gastric tumors.
Cancer. 66:695–701. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murhekar KM, Anuratha JN, Majhi U and
Rajkumar T: Expression of human chorionic gonadotropin beta in
gastric carcinoma: A retrospective immunohistochemical study.
Indian J Med Paediatr Oncol. 30:99–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Yin M, Song W, Cui F, Wang W, Wang S
and Zhu H: B subunit of human chorionic gonadotropin promotes tumor
invasion and predicts poor prognosis of early-stage colorectal
cancer. Cell Physiol Biochem. 45:237–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khobta N, Tomasini P, Garcia ME, Garcia S
and Barlesi F: β-Human chorionic gonadotropin (HCG) dosage and lung
cancer: A pitfall when screening patients for clinical trials. Bull
Cancer. 99:1065–1068. 2012.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khattri S, Vivekanandarajah A, Varma S and
Kong F: Secretion of beta-human chorionic gonadotropin by non-small
cell lung cancer: A case report. J Med Case Rep. 5:192011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Konishi Y, Kawamata F, Nishihara H, Homma
S, Kato Y, Tsuda M, Kohsaka S, Einama T, Liu C, Yoshida T, et al:
Tumor budding and human chorionic gonadotropin-β expression
correlate with unfavorable patient outcome in colorectal carcinoma.
Med Oncol. 35:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noda Y, Simodaira M, Ito H, Gonda H, Okada
N, Suzuki M and Kaneko M: Two cases of human chorionic
gonadotropin-producing large cell carcinoma of the lung accompanied
with gynecomastia. Nihon Kyobu Shikkan Gakkai Zasshi. 28:781–785.
1990.(In Japanese). PubMed/NCBI
|
|
40
|
Lundin M, Nordling S, Lundin J, Alfthan H,
Stenman UH and Haglund C: Tissue expression of human chorionic
gonadotropin beta predicts outcome in colorectal cancer: A
comparison with serum expression. Int J Cancer. 95:18–22. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arano Y, Shimizu J, Murakami S, Hayashi Y,
Kobayashi K, Sekido N, Morita K, Mochiki Y, Tomita S and Watanabe
Y: A female case of adenocarcinoma of the lung producing human
chorionic gonadotropin. Kyobu Geka. 47:485–487. 1994.(In Japanese).
PubMed/NCBI
|
|
42
|
Okutur K, Hasbal B, Aydin K, Bozkurt M,
Namal E, Oz B, Kaynak K and Demir G: Pleomorphic carcinoma of the
lung with high serum beta-human chorionic gonadotropin level and
gynecomastia. J Korean Med Sci. 25:1805–1808. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seder CW, Arndt AT, Jordano L, Basu S,
Fhied CL, Sayidine S, Chmielewski GW, Gallo K, Liptay MJ and Borgia
JA: Serum biomarkers may prognosticate recurrence in node-negative,
non-small cell lung cancers less than 4 centimeters. Ann Thorac
Surg. 104:1637–1643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wong YP, Tan GC, Aziz S, Pongprakyun S and
Ismail F: Beta-human chorionic gonadotropin-secreting lung
adenocarcinoma. Malays J Med Sci. 22:76–80. 2015.PubMed/NCBI
|
|
45
|
Vicier C, Tabouret E, Tallet A, Gonçalves
A, Chetaille B, Viens P and Madroszyk A: BetaHCG secretion by a
pulmonary adenocarcinoma. World J Surg Oncol. 11:2282013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dinis de Sousa M, Barata M, Miranda AR,
Sequeira P, Oliveira A, Xavier L and Mansinho H: Beta-HCG secretion
by a pulmonary pleomorphic carcinoma: A case report. Respir Med
Case Rep. 34:1015282021.PubMed/NCBI
|
|
47
|
Ramakumar S, Cheville JC and Zincke H:
Urothelial carcinoma of the bladder with choriocarcinomatous
differentiation A report of two cases and review of the literature.
Urol Oncol. 4:39–42. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pesce C, Merino MJ, Chambers JT and
Nogales F: Endometrial carcinoma with trophoblastic
differentiation. An aggressive form of uterine cancer. Cancer.
68:1799–1802. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Attanoos RL, Papagiannis A, Suttinont P,
Goddard H, Papotti M and Gibbs AR: Pulmonary giant cell carcinoma:
Pathological entity or morphological phenotype? Histopathology.
32:225–231. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liao XH, Wang Y, Wang N, Yan TB, Xing WJ,
Zheng L, Zhao DW, Li YQ, Liu LY, Sun XG, et al: Human chorionic
gonadotropin decreases human breast cancer cell proliferation and
promotes differentiation. IUBMB Life. 66:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dando I, Carmona-Carmona CA and Zampieri
N: Human chorionic gonadotropin-mediated induction of breast cancer
cell proliferation and differentiation. Cells. 10:2642021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sengodan SK, Nadhan R, Nair RS, Hemalatha
SK, Somasundaram V, Sushama RR, Rajan A, Latha NR, Varghese GR,
Thankappan RK, et al: BRCA1 regulation on β-hCG: A mechanism for
tumorigenicity in BRCA1 defective breast cancer. Oncogenesis.
6:e3762017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rajabi B, Khoury J, Brewer C and Goodman
OB Jr: Urothelial bladder carcinoma with choriocarcinomatous
differentiation presenting with a false-positive pregnancy test. Am
J Med Sci. 346:256–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo X, Liu G, Schauer IG, Yang G,
Mercado-Uribe I, Yang F, Zhang S, He Y and Liu J: Overexpression of
the β subunit of human chorionic gonadotropin promotes the
transformation of human ovarian epithelial cells and ovarian
tumorigenesis. Am J Pathol. 179:1385–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mustafa A, Bozdag Z, Tepe NB and Ozcan HC:
An unexpected reason for elevated human chorionic gonadotropin in a
young woman. Cervical squamous carcinoma. Saudi Med J. 37:905–907.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fiddes JC and Goodman HM: The gene
encoding the common alpha subunit of the four human glycoprotein
hormones. J Mol Appl Genet. 1:3–18. 1981.PubMed/NCBI
|
|
57
|
Boorstein WR, Vamvakopoulos NC and Fiddes
JC: Human chorionic gonadotropin beta-subunit is encoded by at
least eight genes arranged in tandem and inverted pairs. Nature.
300:419–422. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rull K and Laan M: Expression of
beta-subunit of HCG genes during normal and failed pregnancy. Hum
Reprod. 20:3360–3368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bellet D, Lazar V, Bièche I, Paradis V,
Giovangrandi Y, Paterlini P, Lidereau R, Bedossa P, Bidart JM and
Vidaud M: Malignant transformation of nontrophoblastic cells is
associated with the expression of chorionic gonadotropin beta genes
normally transcribed in trophoblastic cells. Cancer Res.
57:516–523. 1997.PubMed/NCBI
|
|
60
|
Lapthorn AJ, Harris DC, Littlejohn A,
Lustbader JW, Canfield RE, Machin KJ, Morgan FJ and Isaacs NW:
Crystal structure of human chorionic gonadotropin. Nature.
369:455–461. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stenman UH, Tiitinen A, Alfthan H and
Valmu L: The classification, functions and clinical use of
different isoforms of HCG. Hum Reprod Update. 12:769–784. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nwabuobi C, Arlier S, Schatz F,
Guzeloglu-Kayisli O, Lockwood CJ and Kayisli UA: hCG: Biological
functions and clinical applications. Int J Mol Sci. 18:20372017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schmitt EJ, Barros CM, Fields PA, Fields
MJ, Diaz T, Kluge JM and Thatcher WW: A cellular and endocrine
characterization of the original and induced corpus luteum after
administration of a gonadotropin-releasing hormone agonist or human
chorionic gonadotropin on day five of the estrous cycle. J Anim
Sci. 74:1915–1929. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pierce JG and Parsons TF: Glycoprotein
hormones: Structure and function. Annu Rev Biochem. 50:465–495.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Morgan FJ, Birken S and Canfield RE: The
amino acid sequence of human chorionic gonadotropin. The alpha
subunit and beta subunit. J Biol Chem. 250:5247– 5258. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Elliott MM, Kardana A, Lustbader JW and
Cole LA: Carbohydrate and peptide structure of the alpha- and
beta-subunits of human chorionic gonadotropin from normal and
aberrant pregnancy and choriocarcinoma. Endocrine. 7:15–32. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cole LA: Biological functions of hCG and
hCG-related molecules. Reprod Biol Endocrinol. 8:1022010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kovalevskaya G, Birken S, Kakuma T, Ozaki
N, Sauer M, Lindheim S, Cohen M, Kelly A, Schlatterer J and
O'Connor JF: Differential expression of human chorionic
gonadotropin (hCG) glycosylation isoforms in failing and continuing
pregnancies: Preliminary characterization of the hyperglycosylated
hCG epitope. J Endocrinol. 172:497–506. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kobata A and Takeuchi M: Structure,
pathology and function of the N-linked sugar chains of human
chorionic gonadotropin. Biochim Biophys Acta. 1455:315–326. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Birken S, Berger P, Bidart JM, Weber M,
Bristow A, Norman R, Sturgeon C and Stenman UH: Preparation and
characterization of new WHO reference reagents for human chorionic
gonadotropin and metabolites. Clin Chem. 49:144–154. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Birken S, Maydelman Y, Gawinowicz MA,
Pound A, Liu Y and Hartree AS: Isolation and characterization of
human pituitary chorionic gonadotropin. Endocrinology.
137:1402–1411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cole LA: hCG, the wonder of today's
science. Reprod Biol Endocrinol. 10:242012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stenman UH, Bidart JM, Birken S, Mann K,
Nisula B and O'Connor J: Standardization of protein
immunoprocedures. Choriogonadotropin (CG). Scand J Clin Lab Invest
Suppl. 216:42–78. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Khodr G and Siler-Khodr TM: The effect of
luteinizing hormone-releasing factor on human chorionic
gonadotropin secretion. Fertil Steril. 30:301–304. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Szilágyi A, Benz R and Rossmanith WG: The
human first-term placenta in vitro: Regulation of hCG secretion by
GnRH and its antagonist. Gynecol Endocrinol. 6:293–300. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wilson EA and Jawad MJ: Stimulation of
human chorionic gonadotropin secretion by glucocorticoids. Am J
Obstet Gynecol. 142:344–349. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Murthi P, Kalionis B, Cocquebert M,
Rajaraman G, Chui A, Keogh RJ, Evain-Brion D and Fournier T:
Homeobox genes and down-stream transcription factor PPARγ in normal
and pathological human placental development. Placenta. 34:299–309.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Boothby M, Kukowska J and Boime I:
Imbalanced synthesis of human choriogonadotropin alpha and beta
subunits reflects the steady state levels of the corresponding
mRNAs. J Biol Chem. 258:9250–9253. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ringler GE, Kao LC, Miller WL and Strauss
JF III: Effects of 8-bromo-cAMP on expression of endocrine
functions by cultured human trophoblast cells. Regulation of
specific mRNAs. Mol Cell Endocrinol. 61:13–21. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Burnside J, Nagelberg SB, Lippman SS and
Weintraub BD: Differential regulation of hCG alpha and beta subunit
mRNAs in JEG-3 choriocarcinoma cells by 8-bromo-cAMP. J Biol Chem.
260:12705–12709. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Knöfler M, Saleh L, Strohmer H, Husslein P
and Wolschek MF: Cyclic AMP- and differentiation-dependent
regulation of the proximal alphaHCG gene promoter in term villous
trophoblasts. Mol Hum Reprod. 5:573–580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fenstermaker RA, Milsted A, Virgin JB,
Miller WL and Nilson JH: The transcriptional response of the human
chorionic gonadotropin beta-subunit gene to cAMP is cycloheximide
sensitive and is mediated by cis-acting sequences different from
that found in the alpha-subunit gene. Mol Endocrinol. 3:1070–1076.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Deutsch PJ, Jameson JL and Habener JF:
Cyclic AMP responsiveness of human gonadotropin-alpha gene
transcription is directed by a repeated 18-base pair enhancer.
Alpha-promoter receptivity to the enhancer confers
cell-preferential expression. J Biol Chem. 262:12169–12174. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Delegeane AM, Ferland LH and Mellon PL:
Tissue-specific enhancer of the human glycoprotein hormone
alpha-subunit gene: Dependence on cyclic AMP-inducible elements.
Mol Cell Biol. 7:3994–4002. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bokar JA, Keri RA, Farmerie TA,
Fenstermaker RA, Andersen B, Hamernik DL, Yun J, Wagner T and
Nilson JH: Expression of the glycoprotein hormone alpha-subunit
gene in the placenta requires a functional cyclic AMP response
element, whereas a different cis-acting element mediates
pituitary-specific expression. Mol Cell Biol. 9:5113–5122. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jameson JL, Albanese C and Habener JF:
Distinct adjacent protein-binding domains in the glycoprotein
hormone alpha gene interact independently with a cAMP-responsive
enhancer. J Biol Chem. 264:16190–16196. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jameson JL and Hollenberg AN: Regulation
of chorionic gonadotropin gene expression. Endocr Rev. 14:203–221.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Budworth PR, Quinn PG and Nilson JH:
Multiple characteristics of a pentameric regulatory array endow the
human alpha-subunit glycoprotein hormone promoter with trophoblast
specificity and maximal activity. Mol Endocrinol. 11:1669–1680.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pittman RH, Clay CM, Farmerie TA and
Nilson JH: Functional analysis of the placenta-specific enhancer of
the human glycoprotein hormone alpha subunit gene. Emergence of a
new element. J Biol Chem. 269:19360–19368. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cole LA: Human chorionic gonadotropin and
associated molecules. Expert Rev Mol Diagn. 9:51–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Steger DJ, Hecht JH and Mellon PL:
GATA-binding proteins regulate the human gonadotropin alpha-subunit
gene in the placenta and pituitary gland. Mol Cell Biol.
14:5592–5602. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Steger DJ, Büscher M, Hecht JH and Mellon
PL: Coordinate control of the alpha- and beta-subunit genes of
human chorionic gonadotropin by trophoblast-specific
element-binding protein. Mol Endocrinol. 7:1579–1588. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Talmadge K, Boorstein WR, Vamvakopoulos
NC, Gething MJ and Fiddes JC: Only three of the seven human
chorionic gonadotropin beta subunit genes can be expressed in the
placenta. Nucleic Acids Res. 12:8415–8436. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bo M and Boime I: Identification of the
transcriptionally active genes of the chorionic gonadotropin beta
gene cluster in vivo. J Biol Chem. 267:3179–3184. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hotakainen K, Lintula S, Jarvinen R, Paju
A, Stenman J, Rintala E and Stenman UH: Overexpression of human
chorionic gonadotropin beta genes 3, 5 and 8 in tumor tissue and
urinary cells of bladder cancer patients. Tumour Biol. 28:52–56.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pestell RG, Hollenberg AN, Albanese C and
Jameson JL: c-Jun represses transcription of the human chorionic
gonadotropin alpha and beta genes through distinct types of CREs. J
Biol Chem. 269:31090–31096. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ghosh D, Ezashi T, Ostrowski MC and
Roberts RM: A central role for Ets-2 in the transcriptional
regulation and cyclic adenosine 5′-monophosphate responsiveness of
the human chorionic gonadotropin-beta subunit gene. Mol Endocrinol.
17:11–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Knöfler M, Saleh L, Bauer S, Galos B,
Rotheneder H, Husslein P and Helmer H: Transcriptional regulation
of the human chorionic gonadotropin beta gene during villous
trophoblast differentiation. Endocrinology. 145:1685–1694. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu L and Roberts RM: Silencing of the
gene for the beta subunit of human chorionic gonadotropin by the
embryonic transcription factor Oct-3/4. J Biol Chem.
271:16683–16689. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fournier T, Guibourdenche J, Handschuh K,
Tsatsaris V, Rauwel B, Davrinche C and Evain-Brion D: PPARγ and
human trophoblast differentiation. J Reprod Immunol. 90:41–49.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sohr S and Engeland K: The tumor
suppressor p53 induces expression of the pregnancy-supporting human
chorionic gonadotropin (hCG) CGB7 gene. Cell Cycle. 10:3758–3767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen Y, Miyazaki J, Nishizawa H, Kurahashi
H, Leach R and Wang K: MTA3 regulates CGB5 and Snail genes in
trophoblast. Biochem Biophys Res Commun. 433:379–384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Śliwa A, Kubiczak M, Szczerba A, Walkowiak
G, Nowak-Markwitz E, Burczyńska B, Butler S, Iles R, Białas P and
Jankowska A: Regulation of human chorionic gonadotropin beta
subunit expression in ovarian cancer. BMC Cancer. 19:7462019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Głodek A, Kubiczak MJ, Walkowiak GP,
Nowak-Markwitz E and Jankowska A: Methylation status of human
chorionic gonadotropin beta subunit promoter and TFAP2A expression
as factors regulating CGB gene expression in placenta. Fertil
Steril. 102:1175–1182.e8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Johnson W and Jameson JL: AP-2 (activating
protein 2) and Sp1 (selective promoter factor 1) regulatory
elements play distinct roles in the control of basal activity and
cyclic adenosine 3′,5′-monophosphate responsiveness of the human
chorionic gonadotropin-beta promoter. Mol Endocrinol. 13:1963–1975.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tarrade A, Schoonjans K, Guibourdenche J,
Bidart JM, Vidaud M, Auwerx J, Rochette-Egly C and Evain-Brion D:
PPAR gamma/RXR alpha heterodimers are involved in human CG beta
synthesis and human trophoblast differentiation. Endocrinology.
142:4504–4514. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Handschuh K, Guibourdenche J, Cocquebert
M, Tsatsaris V, Vidaud M, Evain-Brion D and Fournier T: Expression
and regulation by PPARgamma of hCG alpha- and beta-subunits:
Comparison between villous and invasive extravillous trophoblastic
cells. Placenta. 30:1016–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shalom-Barak T, Zhang X, Chu T, Timothy
Schaiff W, Reddy JK, Xu J, Sadovsky Y and Barak Y: Placental PPARγ
regulates spatiotemporally diverse genes and a unique metabolic
network. Dev Biol. 372:143–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Peng L, Yang H, Ye Y, Ma Z, Kuhn C, Rahmeh
M, Mahner S, Makrigiannakis A, Jeschke U and von Schönfeldt V: Role
of peroxisome proliferator-activated receptors (PPARs) in
trophoblast functions. Int J Mol Sci. 22:4332021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Campain JA, Gutkin DW and Cox GS:
Differential DNA methylation of the chorionic gonadotropin
beta-subunit multigene family. Mol Endocrinol. 7:1331–1346. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Grigoriu A, Ferreira JC, Choufani S,
Baczyk D, Kingdom J and Weksberg R: Cell specific patterns of
methylation in the human placenta. Epigenetics. 6:368–379. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Uusküla L, Rull K, Nagirnaja L and Laan M:
Methylation allelic polymorphism (MAP) in chorionic gonadotropin
beta5 (CGB5) and its association with pregnancy success. J Clin
Endocrinol Metab. 96:E199–E207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Butler SA, Ikram MS, Mathieu S and Iles
RK: The increase in bladder carcinoma cell population induced by
the free beta subunit of human chorionic gonadotrophin is a result
of an anti-apoptosis effect and not cell proliferation. Br J
Cancer. 82:1553–1556. 2000.PubMed/NCBI
|
|
114
|
Li Z, Du L, Li C and Wu W: Human chorionic
gonadotropin β induces cell motility via ERK1/2 and MMP-2
activation in human glioblastoma U87MG cells. J Neurooncol.
111:237–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wu W and Walker AM: Human chorionic
gonadotropin beta (HCGbeta) down-regulates E-cadherin and promotes
human prostate carcinoma cell migration and invasion. Cancer.
106:68–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li Z, Li C, Du L, Zhou Y and Wu W: Human
chorionic gonadotropin β induces migration and invasion via
activating ERK1/2 and MMP-2 in human prostate cancer DU145 cells.
PLoS One. 8:e545922013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Regalado JJ: Mixed micropapillary and
trophoblastic carcinoma of bladder: Report of a first case with new
immunohistochemical evidence of urothelial origin. Hum Pathol.
35:382–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Białas P, Śliwa A, Szczerba A and
Jankowska A: The study of the expression of CGB1 and CGB2 in human
cancer tissues. Genes (Basel). 11:10822020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gao S, Fan C, Huang H, Zhu C, Su M and
Zhang Y: Effects of HCG on human epithelial ovarian cancer
vasculogenic mimicry formation in vivo. Oncol Lett.
12:459–466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Armah HB and Parwani AV: Sarcomatoid
urothelial carcinoma with choriocarcinomatous features: First
report of an unusual case. Urology. 70:812.e11–e14. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhao R, Zhang T, Xi W, Sun X, Zhou L, Guo
Y, Zhao C and Bao Y: Human chorionic gonadotropin promotes cell
proliferation through the activation of c-Met in gastric cancer
cells. Oncol Lett. 16:4271–4278. 2018.PubMed/NCBI
|
|
122
|
Wu W, Gao H, Li X, Peng S, Yu J, Liu N,
Zhan G, Zhu Y, Wang K and Guo X: β-hCG promotes epithelial ovarian
cancer metastasis through ERK/MMP2 signaling pathway. Cell Cycle.
18:46–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Singer G, Kurman RJ, McMaster MT and Shih
IM: HLA-G immunoreactivity is specific for intermediate trophoblast
in gestational trophoblastic disease and can serve as a useful
marker in differential diagnosis. Am J Surg Pathol. 26:914–920.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Shih IM: The role of CD146 (Mel-CAM) in
biology and pathology. J Pathol. 189:4–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Banet N, Gown AM, Shih IM, Kay Li Q, Roden
RB, Nucci MR, Cheng L, Przybycin CG, Nasseri-Nik N, Wu LS, et al:
GATA-3 expression in trophoblastic tissues: An immunohistochemical
study of 445 cases, including diagnostic utility. Am J Surg Pathol.
39:101–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mao TL, Kurman RJ, Jeng YM, Huang W and
Shih IM: HSD3B1 as a novel trophoblast-associated marker that
assists in the differential diagnosis of trophoblastic tumors and
tumorlike lesions. Am J Surg Pathol. 32:236–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Stichelbout M, Devisme L, Franquet-Ansart
H, Massardier J, Vinatier D, Renaud F and Kerdraon O: SALL4
expression in gestational trophoblastic tumors: A useful tool to
distinguish choriocarcinoma from placental site trophoblastic tumor
and epithelioid trophoblastic tumor. Hum Pathol. 54:121–126. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kaur B and Sebire NJ: Gestational
trophoblastic tumours and non-neoplastic trophoblastic lesions:
morphology and immunocytochemistry to refine the diagnosis. Diagn
Histopathol. 25:53–65. 2019. View Article : Google Scholar
|
|
129
|
Heller DS: Update on the pathology of
gestational trophoblastic disease. APMIS. 126:647–654. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Cheung AN, Hui P and Shih I: Gestational
trophoblastic disease. WHO classification of tumours: Female
genital tumours. Vol 4. 5th edition. IARC; Lyon: pp. 310–333.
2020
|
|
131
|
Shih IM: Trophogram, an
immunohistochemistry-based algorithmic approach, in the
differential diagnosis of trophoblastic tumors and tumorlike
lesions. Ann Diagn Pathol. 11:228–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lee CQE, Gardner L, Turco M, Zhao N,
Murray MJ, Coleman N, Rossant J, Hemberger M and Moffett A: What is
trophoblast? A combination of criteria define human first-trimester
trophoblast. Stem Cell Reports. 6:257–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Triozzi PL and Stevens VC: Human chorionic
gonadotropin as a target for cancer vaccines. Oncol Rep. 6:7–17.
1999.PubMed/NCBI
|
|
134
|
Yu N, Xu W, Jiang Z, Cao Q, Chu Y and
Xiong S: Inhibition of tumor growth in vitro and in vivo by a
monoclonal antibody against human chorionic gonadotropin beta.
Immunol Lett. 114:94–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Morse MA, Bradley DA, Keler T, Laliberte
RJ, Green JA, Davis TA and Inman BA: CDX-1307: A novel vaccine
under study as treatment for muscle-invasive bladder cancer. Expert
Rev Vaccines. 10:733–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rayn KN, Hale GR, Grave GP and Agarwal PK:
New therapies in nonmuscle invasive bladder cancer treatment.
Indian J Urol. 34:11–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sahoo S, Singh P, Kalha B, Singh O and Pal
R: Gonadotropin-mediated chemoresistance: Delineation of molecular
pathways and targets. BMC Cancer. 15:9312015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Szturmowicz M, Wiatr E, Sakowicz A,
Slodkowska J, Roszkowski K, Filipecki S and Rowinska-Zakrzewska ER:
The role of human chorionic gonadotropin beta subunit elevation in
small-cell lung cancer patients. J Cancer Res Clin Oncol.
121:309–312. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Oyasu R, Nan L, Smith DP and Kawamata H:
Human chorionic gonadotropin beta-subunit synthesis by
undifferentiated urothelial carcinoma with syncytiotrophoblastic
differentiation. Arch Pathol Lab Med. 118:715–717. 1994.PubMed/NCBI
|















