Introduction
Our previous studies confirmed that cobalt chloride
(CoCl2), chemical reagents, radiotherapy and Chinese
herbal medicines can induce the formation of polyploid giant tumor
cells (PGCCs) by internal replication or cell fusion (1–3). PGCCs
are a subpopulation of cancer cells that contribute to solid tumor
heterogeneity. The size of PGCCs is at least three times larger
than that of regular-sized diploid cancer cells and PGCCs are
multinucleated or have giant nuclei. PGCCs can produce daughter
cells with high proliferation, migration and invasion abilities via
asymmetric division (budding, splitting and bursting). These
daughter cells have strong proliferation, infiltration and
migration abilities (3,4). PGCCs have been observed in a number of
malignant tumors such as breast (5), ovarian (6,7),
colorectal (CRC) (4), non-small
cell lung (8) and prostate cancers
(9). Clinically, PGCCs have been
more frequently observed in high-grade malignancies and metastatic
foci than in low-grade tumors and primary sites. For the same
patients, the number of PGCCs in the recurrent cancer was higher
than that in the original cancer. The number of PGCCs is associated
with a poor prognosis and metastatic recurrence in patients with
malignant tumors (4).
Hypoxia is important in the progression of malignant
tumors and is associated with the formation and maintenance of
cancer stem cells (10,11). CoCl2 is a hypoxia mimic
that stabilizes hypoxia-inducible factor-1 (HIF1α). HIF1α is a key
factor activated in response to hypoxia and mediates the
transcriptional response of local hypoxia in cancer and promotes
tumor progression by altering cellular metabolism (12) and stimulating angiogenesis (13). Our previous results confirmed that
HIF1α is significantly upregulated in PGCCs and their daughter
cells (PDCs) (14). Under
conditions of normal oxygen saturation, HIF1α is rapidly degraded
by ubiquitin protease hydrolysis complex after translation,
resulting in a low HIF1 expression level (15). However, in hypoxic
microenvironments, HIF1α degradation is inhibited. HIF1α and HIF1β
subunits combine to form complexes, which are then transferred to
the nucleus to regulate the transcription of multiple genes and
promote cell adaptation to hypoxia (16). SUMOylation is an important
post-translational modification (PTM) characterized by the covalent
and reversible binding of a small ubiquitin-like modifier (SUMO) to
the target protein, changing the subcellular location of the
protein and maintaining its stability. SUMOylation plays an
important role in epithelial-mesenchymal transition, metastasis,
therapeutic resistance and antitumor immune responses (17).
The present study demonstrated that SUMOylation
could regulate the subcellular location of HIF1α and that nuclear
expression of HIF1α promoted the proliferation, migration and
invasion of PDCs. Microphthalmia-associated transcription factor
(MITF), but not the protein inhibitor of activated STAT protein 4
(PIAS4), regulated the transcription and protein levels of HIF1α
and participates in the regulation of HIF1α SUMOylation, which
occurs at the K391 and K477 amino acid sites of HIF1α in PDCs.
Materials and methods
Cell culture
Human colon cancer cells (Hct116 and LoVo) were
purchased from the American Type Culture Collection. The medium
used was RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum, streptomycin and penicillin
(Gibco; Thermo Fisher Scientific, Inc.) (complete medium). Cells
were cultured in a constant-temperature incubator containing 5%
carbon dioxide at 37°C.
Induction of the formation of PGCCs by
CoCl2
When the cell confluency reached ~80%, 450 µM of
CoCl2 was added to the flask. After treatment for 48 h
(Hct116) and 36 h (LoVo), most regular-sized diploid cancer cells
died and only a few cells with large nuclei (PGCCs) survived. The
surviving cells exhibited multinucleated and mononuclear giant cell
morphology and were highly resistant to hypoxia. Following
treatment, the remaining cells were cultured in a complete medium
without CoCl2. After ~15 days of recovery, the PGCCs
produced daughter cells via asymmetric division. Following three
repeated treatments, 30% of PGCCs and 70% of daughter cells
appeared in the flask and the cells were collected for subsequent
experiments.
Western blotting
Proteins were extracted by RIPA Buffer according to
the manufacturer's instructions (Thermo Fisher Scientific, Inc.).
Protein concentration was determined using Nanodrop (Thermo Fisher
Scientific, Inc.). Protein samples from control cells and PDCs were
separated by 10% SDS-PAGE gel for electrophoresis at a constant
voltage of 80 V. After separating the protein bands, the voltage
was adjusted to 120 V. After the membrane transfer, 5% skimmed milk
was added to the membrane for 1 h at room temperature. According to
the molecular weight of proteins, PVDF membrane was cut prior to
hybridization with different primary antibodies (detailed
information regarding the antibodies is provided in Table I) at 4°C overnight. For the target
proteins with similar molecular weight, a membrane regeneration
solution was used to elute. After washing, the corresponding
secondary antibodies were added and the mixture was shaken at room
temperature for 1 h. After the ECL developer (Shanghai Yeasen
Biotechnology Co., Ltd.) was added, a ChemiDoc imaging system
(Bio-Rad Laboratories, Inc.) was used for development and
observation. ImageJ software (National Institutes of Health; 1.54D)
was used to analyze and calculate the gray value of the
corresponding strip and the expression index of the target protein.
The experiment was independently repeated thrice.
 | Table I.Detailed information of the
antibodies utilized in this study. |
Table I.
Detailed information of the
antibodies utilized in this study.
| Antibody | Company (cat.
no.) | Dilution |
|---|
| Hypoxia inducible
factor 1 α | Abcam
(ab51608) | 1:1,000 (western
blotting); 1:500 |
|
|
|
(immunocytochemical); 1:50 |
|
|
|
(co-immunoprecipitation) |
|
Microphthalmia-associated
transcription | Proteintech Group,
Inc. | 1:1,000 (western
blotting); |
| factor | (13092-1-AP) | 1:1,000
(immunocytochemical) |
| von Hippel-Lindau
disease tumor suppressor | Proteintech Group,
Inc. | 1:1,000 (western
blotting); |
|
| (24756-1-AP) | 1:1,000
(immunocytochemical) |
| Protein inhibitor
of activated STAT protein 4 | Proteintech Group,
Inc. | 1:1,000 (western
blotting); |
|
| (14242-1-AP) | 1:1,000
(immunocytochemical) |
| Small
ubiquitin-like modifier 1 | CST (4930S) | 1:1,000 (western
blotting) |
| Small
ubiquitin-like modifier 23 | CST (4971T) | 1:1,000 (western
blotting) |
| β-actin | OriGene
Technologies, | 1:3,000 (western
blotting) |
|
| Inc. (TA-09) |
|
| GAPDH | Affinity
(AF7021) | 1:3,000 (western
blotting) |
| H3 | Affinity
(BF9211) | 1:1,000 (western
blotting) |
| Anti-Mouse IgG
HRP-linked | CST (7074F) | 1:5,000 (western
blotting) |
| Anti-Rabbit IgG
HRP-linked | CST (7076F) | 1:5,000 (western
blotting) |
Reverse transcription-quantitative
(RT-q) PCR
The primers were designed by Primer 5 (http://www.premierbiosoft.com/primerdesign/). A total
of 2.5×106 cells were collected and total RNA was
extracted using an RNA extraction kit (cat. no. 9190; Takara
Biotechnology) according to the manufacturer's protocols.
Transcription levels of MITF and HIF1α were detected by qPCR. The
PCR conditions were set according to the instructions provided in
the SYBR Green Kit (Shanghai Yeasen Biotechnology Co., Ltd.). The
amplification was performed for 40 cycles (95°C 2 min, 95°C 10 sec
and 60°C 30 sec). The relative amount of each mRNA level was
normalized to that of the β-actin level and the difference in mRNA
level was calculated using the 2−ΔΔCq method (18). Detailed information on the primers
used is provided in Table II. All
experiments were repeated at least three times.
 | Table II.List of primers used. |
Table II.
List of primers used.
| Name | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| Hypoxia inducible
factor 1 α |
GAACGTCGAAAAGAAAAGTCTCG |
CCTTATCAAGATGCGAACTCACA |
|
Microphthalmia-associated transcription
factor |
ACCTGTTACAACAACTCTCGATCTCA |
CTCAGTCCCAGTTCCGAGGTT |
| β-actin |
TGGCACCCAGCACAATGAA |
CTAAGTCATAGTCCGCCTAGAAGCA |
Nuclear and cytoplasmic protein
extraction
After an appropriate amount of cell precipitation
from Hct116 and LoVo control cells and PDCs, 200 µl cytoplasmic
protein extraction reagent A was added to the nuclear protein and
cytoplasmic protein extraction kit (cat. no. P0027, Beyotime
Institute of Biotechnology) and was placed on ice for lysis for 15
min. Then, 10 µl cytoplasmic protein extraction reagent B was
added, placed on ice for cracking for 1 min, vortex-oscillated for
5 sec and then centrifuged at 4°C using centrifuge at 16.2 × g for
15 min. The supernatant comprised the cytoplasmic protein solution,
which was then transferred to a pre-cooled Eppendorf tube. The
remaining precipitate was rinsed with 500 µl phosphate-buffered
saline (PBS) thrice and centrifuged at 0.6 × g at 4°C for 5 min.
Subsequently, 50 µl of nuclear protein extraction reagent was added
and the precipitation was dissolved on ice for 30 min. After
vortex-oscillation every 1 min for 30 sec and centrifugation at
16.2 × g at 4°C for 15 min, the resultant supernatant comprised the
nuclear protein solution. The two parts of the supernatant were
mixed with 1/4 volume of 5× protein loading buffer and then the
protein was denatured at 100°C for 10 min and stored in the
refrigerator at −20°C for subsequent experiments.
Immunocytochemical (ICC) staining
Hct116 and LoVo control cells and PDCs were
inoculated onto a cover slide. When the cell density reached ~70%,
the cells were fixed with methanol at room temperature for 30 min.
Hydrophobic circles were drawn with a neutral oil pen. A
peroxidase-blocking agent was used to treat the cells in the dark
for 15 min and then goat serum-containing working solution was
added, followed by incubation at room temperature for 20 min. The
corresponding primary antibodies (Table
I) were added and incubated at 4°C overnight. The next day,
one-to-two drops of biotin-labeled goat anti-rat/rabbit IgG polymer
were added, followed by incubation at room temperature for 30 min.
A DAB color-developing solution was prepared to observe brown
particles under a microscope at room temperature for 1–2 min. The
color development reaction was stopped after brown staining.
Hematoxylin was used to stain at room temperature for 30 sec,
followed by alcohol gradient-mediated dehydration, the addition of
dimethylbenzene and final mounting with neutral gum.
Plate colony formation assay
The cell samples were diluted to obtain samples with
50, 100 and 150 cells/ml and cultured in 24-well plates with three
repeated pores in each group for 2 weeks. Cells were fixed with
anhydrous methanol for 30 min and stained with 0.1% crystal violet
for 30 min at room temperature. Cell colonies were counted at ×100
magnification (a single colony was defined as that containing
>50 cells). The cell colony formation efficiency was assessed
using the following formula: formation efficiency=number of
clones/number of inoculated cells.
Wound healing assay
A wound healing assay was used to detect cell
migration. Cells (1×105) in the logarithmic growth stage
were cultured in a 6-well plate and three repeat pores were set.
Single-layer cells were scratched uniformly using sterile pipette
tips to create wounds. PBS was used to wash away the detached
cells. The cells were then incubated in serum-free RPMI 1640.
ImageJ software (National Institutes of Health; 1.54D) was used to
outline the migration area and calculate the wound-healing index
according to the following formula: [(the wound area at 0 h)-(the
wound area at the indicated time)]/(the wound area at 0 h). A high
score indicated stronger migration ability.
Transwell migration and invasion
assay
For the Transwell migration assay, 200 µl of
serum-free cell suspension containing 1×105 cells was
added to the upper chamber of the Transwell chamber and 600 µl of
medium containing 20% serum was added to the lower chamber, which
was cultured in an incubator for 24 h. The cells were fixed with
methanol for 30 min and stained with 0.1% crystal violet for 30
min. The cells were observed under an inverted microscope and three
fields (magnification, ×100) were randomly selected, images
captured and cells counted. A total of three duplicate wells were
set for each group of cells. The procedure for the Transwell
invasion experiment was the same as that for the Transwell
migration experiment. The difference was that the Transwell
invasion experiment required a 200 µl sample containing
5×105 cells and the invasion chamber contained Matrigel
(cat. no. 354480; Corning, Inc.). After the cells were added into
the chamber with Matrigel, the plates were incubated for 12 h at
37°C.
Co-immunoprecipitation (Co-IP)
assay
Co-IP was used to determine the interactions of
SUMO1, SUMO2 and HIF1α in Hct116 and LoVo control cells and PGCs
according to the manufacturer's protocols. When the cell density of
the T25 flask reached 80%, the cells were collected in EP tubes.
The cells were lysed using 500 µl IP lysis buffer (Thermo Fisher
Scientific, Inc.) containing a halt protease and phosphatase
inhibitor cocktail (1:100 dilution) for 30 min on ice and then
centrifuged at 16.2 × g at 4°C for 10 min. The supernatant was
transferred to an EP tube containing A/G agarose homogenate of agar
glycoprotein beads and shaken at 4°C for 30 min. After incubation,
the supernatant was divided into three parts: one part was used to
detect the total protein level (input). Primary antibodies
corresponding to rabbit IgG and the target protein were added to
the other two tubes, respectively and maintained at 4°C overnight.
The next day, A/G agarose homogenates of the agar glycoprotein
beads were adsorbed by a magnetic grate and washed by lysis buffer
(Thermo Fisher Scientific, Inc.). The samples containing rabbit IgG
and primary antibodies (Table I) of
the target protein were transferred to the newly washed column and
incubated at 4°C for 2 h. After incubation, the supernatant was
discarded and washed five times with 500 µl IP-specific cracking
buffer. Finally, western blotting was performed to analyze the
samples.
Ginkgolic acid (GA) treatment
CoCl2-treated cells were seeded in
six-well plates until they reached 80% confluence. Approximately 20
µM of GA (15:1, MedChem Express, USA) was added to control cells
and PDCs for 24 h, and the samples were evaluated using western
blotting analysis and other assays described.
Cell viability assay
Methyl linoleate (ML), the main active ingredient of
Sageretia thea, is a major anti-melanin-producing compound that
downregulates MITF expression. To assess cell viability before and
after ML treatment, Hct116 and LoVo PDCs were seeded at a density
of 5,000 cells per well into 96-well plates and incubated at 37°C
for 12 h. The cells were divided into five groups and each group
was independently analyzed in triplicate. The cells were treated
with ML at concentrations of 40, 80, 160 and 320 µM for 12, 24, 48
and 72 h. After incubation, 10 µl of CCK8 (Dojindo Laboratories,
Inc.) reagent was added to each well and incubated at 37°C for 12
h. After adding the CCK8 reagent, the wells were analyzed using a
Bio-Rad microplate reader at a wavelength of 450 nm (Bio-Rad
Laboratories, Inc.). Optical density data are presented as the
means ± standard error of the mean.
Transient short interfering (si)RNA
and plasmid vector transfection
The BLOCK-iT RNAi Designer (https://rnaidesigner.thermofisher.com/rnaiexpress/)
was used to design the siRNA of MITF. A total of three different
siRNA sequences targeting MITF, PIAS4 and negative control siRNA
oligonucleotides were obtained from Shanghai GenePharma Co., Ltd.
The K391R, K477R and empty vector was purchased from Genewiz, Inc.
The cells were inoculated into a 6-well plate and transfected when
the cell confluence reached ~50% at 37°C. According to the
manufacturer's experimental protocol, 5 µl of transfect-Mate
(Shanghai GenePharma Co., Ltd.) and 5 µl of interfering sequence or
plasmids were added to 100 µl of Opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.) to formulate the transfection complexes at room
temperature. After 48 h of transfection, cell samples were
collected to detect the targeted proteins using western blotting.
Detailed information on the siRNA oligonucleotide sequences is
provided in Tables III and
IV.
 | Table III.List of MITF short interfering RNA
used. |
Table III.
List of MITF short interfering RNA
used.
| Name | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| MITF-708 |
GCUAUGCUUACGCUUAACUTT |
AGUUAAGCGUAAGCAUAGCTT |
| MITF-1215 |
GUGGACUAUAUCCGAAAGUTT |
ACUUUCGGAUAUAGUCCACTT |
| MITF-1303 |
GCAUUUGUUGCUCAGAAUATT |
UAUUCUGAGCAACAAAUGCTT |
| MITF-PC |
UGACCUCAACUACAUGGUUTT |
AACCAUGUAGUUGAGGUCATT |
| MITF-NC |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
 | Table IV.List of PIAS4 short interfering RNA
used. |
Table IV.
List of PIAS4 short interfering RNA
used.
| Name | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| PIAS4-315 |
GCCCUGAGCUGUUCAAGAATT |
UUCUUGAACAGCUCAGGGCTT |
| PIAS4-493 |
GCUCUACGGAAAGUACUUATT |
UAAGUACUUUCCGUAGAGCTT |
| PIAS4-1134 |
UCAUCUGUCCGCUGGUGAATT |
UUCACCAGCGGACAGAUGATT |
| PIAS4-PC |
UGACCUCAACUACAUGGUUTT |
AACCAUGUAGUUGAGGUCATT |
| PIAS4-NC |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Statistical analyses
All data and statistical charts were processed using
GraphPad Prism 8.0.2 (Dotmatics) or SPSS Statistics 25 (IBM Corp.)
software. Statistical significance was assessed by comparing mean
values using the Student's t-test for independent groups. An ANOVA
followed by Bonferroni correction was used among different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CoCl2 can induce the
formation of PGCCs
Hct116 and LoVo cells were cultured in a complete
medium and their morphologies are shown in Fig. 1A a and c. Control cells were
epithelioid and oval in shape, with uniform cell distribution and
size. When 450 µM of CoCl2 was added to the flask, most
of the regular-sized diploid cancer cells died and only a few cells
with large nuclei (PGCCs) survived. After ~15 days of recovery,
PGCCs produced daughter cells through asymmetric division (Fig. 1A b and d; Control represents cells
without CoCl2 treatment and treatment represents cells
with CoCl2 treatment).
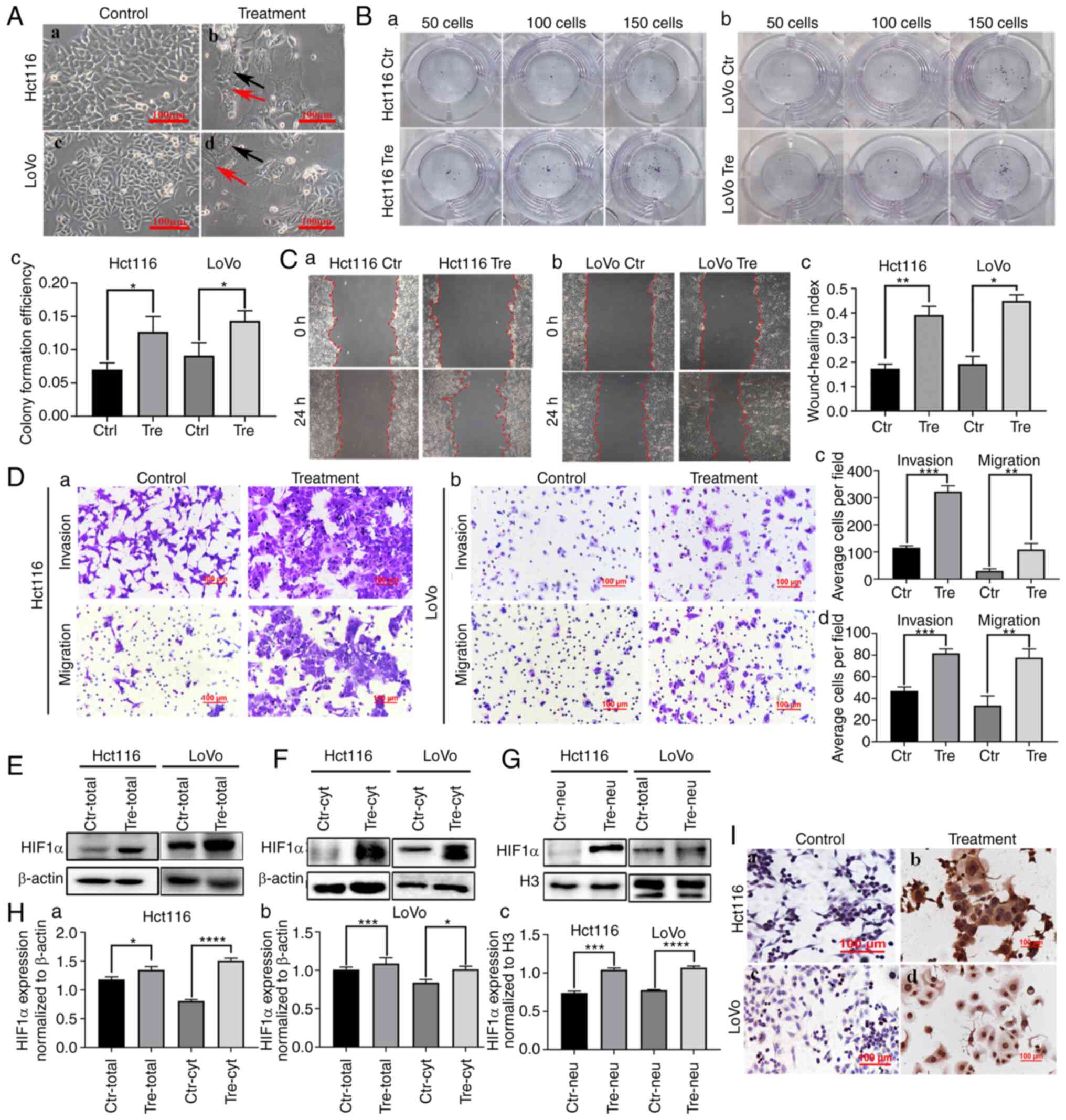 | Figure 1.HIF1α expression in Hct116 and LoVo
cells before and after CoCl2 treatment. (A) Control cells and PDCs
derived from Hct116 and LoVo (magnification, ×100). (a) Hct116
control cells. (b) Hct116 PGCCs and daughter cells. (c) LoVo
control cells. (d) LoVo PGCCs with daughter cells. The black arrow
indicates PGCCs; the red arrow indicates PDCs. (B) Colony formation
of 50, 100 and 150 (a) Hct116 control cells and PDCs, (b) LoVo
control cells and PDCs and (c) statistiacal analysis of Colony
formation efficiency of Hct116 and LoVo cells before and after
CoCl2 treatment. (C) Wound healing assay of (a) Hct116
control cells and (b) LoVo control cells and PDCs at 0 h and 24 h
(magnification, ×100). (c) Statistical analysis of wound healing
index of Hct116 and LoVo cells before and after CoCl2
treatment. (D) The invasion and migration abilities of (a) Hct116
control cells and PDCs and (b) LoVo control cells and PDCs.
Comparison of the average cell number in invasion and migration
assay of (c) Hct116 PDCs and (d) LoVo PDCs before and after
CoCl2 treatment. (E) Total HIF1α expression in Hct116
and LoVo control cells and PDCs. (F) Cytoplasmic and (G) nuclear
HIF1α expression in Hct116 and LoVo control cells and PDCs. (H)
Statistical analysis of total and cytoplasmic HIF1α expression in
(a) Hct116 and (b) LoVo control cells and PDCs and (c) nuclear
HIF1α expression in Hct116 and LoVo control cells. (I)
Immunocytochemical staining of HIF1α in (a) Hct116 control cells,
(b) Hct116 PDCs, (c) LoVo control cells and (d) LoVo PDCs.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. HIF1α,
hypoxia inducible factor 1 alpha; PDCs, daughter cells; PGCCs,
polyploid giant cells; Tre, PGCCs with PDCs; Ctr, control. |
Daughter cells derived from PGCCs had
strong proliferation, migration and invasion abilities
The results of the plate cloning assay demonstrated
that Hct116 and LoVo PDCs had greater proliferative ability than
the control cells (Fig. 1B a and b)
and the differences were statistically significant (Fig. 1Bc). The wound healing assay showed a
significantly higher migration ability of LoVo and HCT116 PDC
compared with that of control cells (Fig. 1C a and b) and the differences were
statistically significant (Fig.
1Cc), indicating that the migration ability of PGCCs and their
progeny cells was stronger than that of the control group.
Additionally, Transwell migration and invasion experiments showed
that PDC had stronger migration and invasion abilities than the
control cells (Fig. 1D).
The expression of HIF1α was
upregulated and the subcellular location was altered in PDCs
In the present study, western blotting and ICC were
used to detect the expression and subcellular location of HIF1α in
Hct116 and LoVo control cells and PDCs. The expression of HIF1α was
higher in PDCs than in the control cells (Fig. 1E). In the control cells, HIF1α was
detected only in the cytoplasm (Fig.
1F). In PDCs, HIF1α was detected in both the cytoplasm
(Fig. 1F) and the nucleus (Fig. 1G). Total, cytoplasmic and nuclear
HIF1α expression levels were significantly higher in PDCs than in
control cells (Fig. 1H). In
addition, immunocytochemical staining demonstrated the expression
and subcellular localization of HIF1α in control cells and PDCs
(Fig. 1I).
HIF1α is modified by SUMOylation in
PDCs
Co-IP was used to detect the interactions between
SUMO1, SUMO2/3 and HIF1α. The total cell lysates of control and
PDCs were immunoprecipitated with an anti-HIF1α antibody (Fig. 2A) and then immunoblotted with
anti-SUMO1 and anti-SUMO2/3 antibodies. The results showed that
HIF1α could bind to SUMO1 and SUMO2/3 in PDCs (Fig. 2B and C).
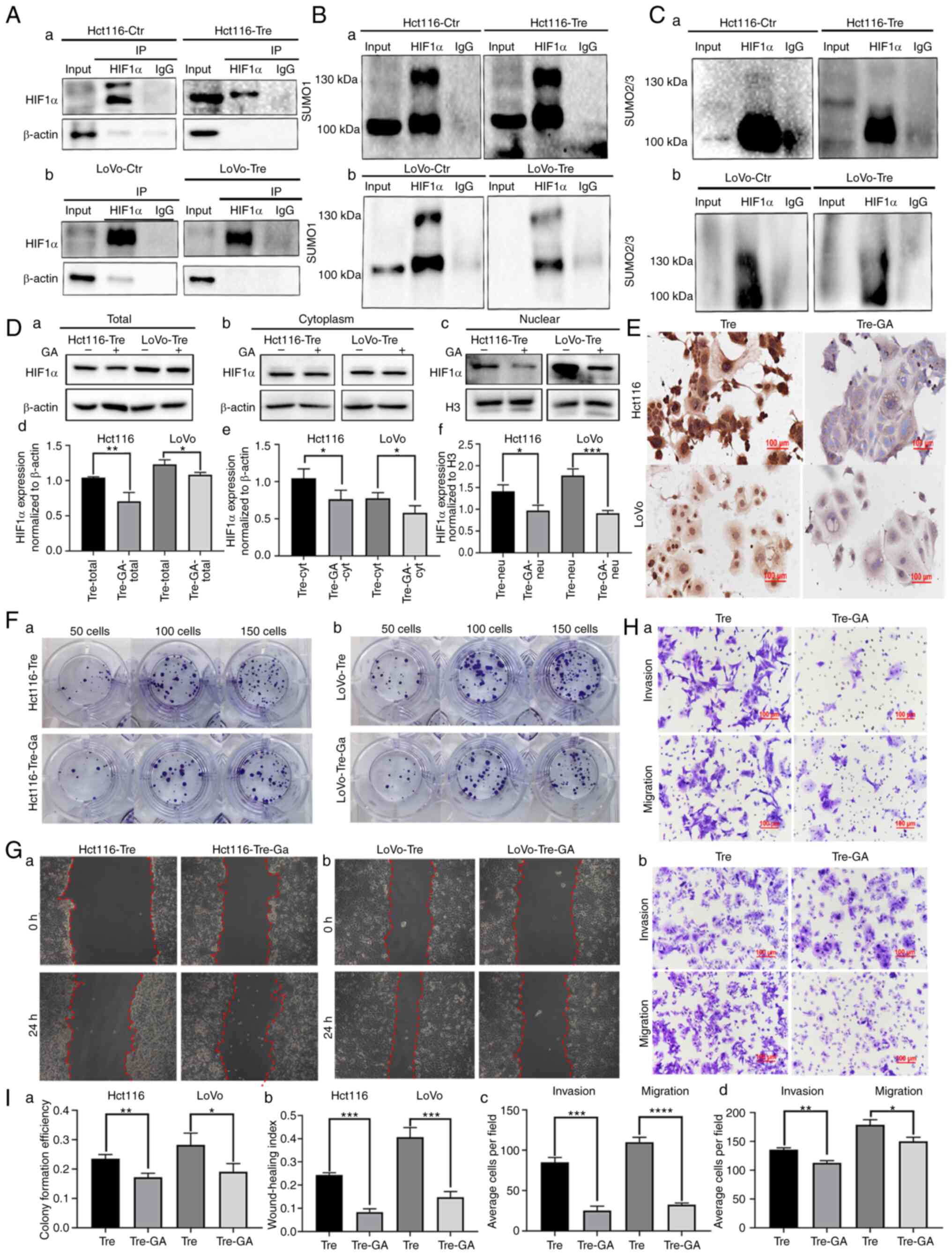 | Figure 2.The nuclear location of HIF1α
modified by SUMOylation regulated the migration, invasion and
proliferation of PDCs. (A) Results of HIF1α co-immunoprecipitation
in (a) Hct116 and (b) LoVo PDCs (anti-HIF1α was used to perform
immunoprecipitation). Total lysates of (a) Hct116 and (b) LoVo
control cells and PDCs were immunoprecipitated with anti-HIF1α and
immunoblotted with (B) anti-SUMO1 and (C) anti-SUMO2/3. (D) (a)
Total, (b) cytoplasmic and (c) nuclear HIF1α expression in Hct116
and LoVo PDCs before and after 20 µM GA treatment. Statistical
analysis of (d) total, (e) cytoplasmic and (f) nuclear HIF1α
expression in Hct116 and LoVo PDCs befoer and after GA treatment.
(E) Immunocytochemical staining of HIF1α in Hct116 and LoVo PDCs
before and after GA treatment. (F) Colony formation of 50, 100 and
150 (a) Hct116 and (b) LoVo PDCs before and after GA treatment. (G)
Wound-healing assay of (a) Hct116 and (b) LoVo PDCs before and
after GA treatment at 0 h and 24 h (magnification, ×100). (H) The
invasion and migration abilities of (a) Hct116 and (b) LoVo PDCs
before and after GA treatment. (I) (a) The differences in colony
formation efficiency of Hct116 and LoVo PDCs before and after GA
treatment. (b) Statistical analysis of wound healing index of
Hct116 and LoVo cells before and after GA treatment. (c) Comparison
of the average cell number in invasion and migration assay of
Hct116 PDCs before and after GA treatment. (d) Comparison of the
average cell number in invasion and migration assay of LoVo PDCs
before and after GA treatment. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001, ns, no significance. HIF1α, hypoxia
inducible factor 1 alpha; PDCs, daughter cells; PGCCs, polyploid
giant cells; GA, ginkgolic acid; Tre, PGCCs with PDCs; Ctr,
control. |
Ginkgolic Acid (GA) treatment
decreased the nuclear expression of HIF1α and inhibited the
migration, invasion and proliferation of PDCs
GA can inhibit the SUMOylation of important proteins
that are critical during the development and progression of
malignant tumors (19). GA directly
binds to the SUMO E1 activating enzyme to inhibit the formation of
the E1-SUMO thioester complex (20). After GA treatment, the total,
cytosolic and nuclear fractions were collected to detect the
expression of HIF1α. In the cytosolic fraction, the expression of
HIF1α in PDCs was slightly inhibited after GA treatment compared
with that in PDCs without GA treatment (Fig. 2D b and e). The total and nuclear
localization of HIF1α was inhibited by GA treatment and the
differences before and after GA treatment in PDCs were
statistically significant (Fig. 2D a,
c, d and f), indicating that SUMOylation might play an
important role in the nuclear localization of HIF1α. Additionally,
ICC staining was performed on the cells before and after GA
treatment. Staining results showed that GA treatment inhibited the
nuclear expression of HIF1α (Fig.
2E).
Functional cell experiments were performed to assess
the effect of GA on PDC migration, invasion and proliferation
before and after GA treatment. Cloning experiments showed that the
number of PDCs decreased after GA treatment (Fig. 2F). The number of colonies of 50, 100
and 150 GA-treated PDCs was reduced compared with that of untreated
Hct116 and LoVo PDCs (Fig. 2F and
Ia). Wound healing experiments showed that the scratched areas
of PDCs before GA treatment were significantly narrower than those
after GA treatment (Fig. 2G and
Ib). The results of the Transwell assay showed that the
migratory and invasive abilities of PDCs treated with GA were
inhibited compared with those of PDCs without GA treatment
(Fig. 2H and I c and d).
Total, cytosolic and nuclear
expression of MITF, PIAS4 and von Hippel-Lindau disease tumor
suppressor (VHL) in control and PDCs
The expression of MITF, PIAS4 and VHL is an
important indicator influencing the expression and subcellular
location of HIF1α. Total, cytosolic and nuclear fractions were
collected to detect the expression of MITF, PIAS4 and VHL in
control cells and PDCs. The total protein levels of MITF, PIAS4 and
VHL were elevated in Hct116 and LoVo PDCs compared with those in
control cells (Fig. 3A and D). In
the cytosolic fraction, the expression of MITF, PIAS4 and VHL in
LoVo PDCs was upregulated (Fig. 3B and
D). After CoCl2 treatment, the expression levels of
MITF, PIAS4 and VHL in the nucleus were also higher than those in
control cells (Fig. 3C and D). For
further verification, ICC staining was performed and the results
showed both nuclear and cytoplasmic expression of MITF, PIAS4 and
VHL. The staining intensity of MITF, PIAS4 and VHL in PDCs was
stronger than that of control cells (Fig. 3E).
 | Figure 3.Total, cytosolic and nuclear
expression of MITF, PIAS4 and VHL in control cells and PDCs.
Western blotting showing the (A) total (B) cytoplasmic and (C)
nuclear protein ex pression of PIAS4, MITF and VHL in Hct116 and
LoVo control cells and PDCs. (D) Statistical analysis of the
expression differences of (a) total, (b) cytoplasmic and (c)
nuclear MITF expression; (d) total, (e) cytoplasmic and (f) nuclear
VHL expression; (g) total, (h) cytoplasmic and (i) nuclear PIAS4
expression in Hct116 and LoVo control and PDCs. (E)
Immunocytochemical staining of PIAS4, MITF and VHL in Hct116 and
LoVo control cells and PDCs. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. PIAS4, protein inhibitor of
activated STAT protein 4; MITF, microphthalmia-associated
transcription factor; VHL, von Hippel-Lindau disease tumor
suppressor PDCs, daughter cells; Tre, polyploid giant cells
PDCs. |
MITF regulates the SUMOylation of
HIF1α
MITF can bind to the HIF1α promoter and stimulate
its transcriptional activity (21).
To confirm whether MITF interacts with HIF1α, Co-IP was performed.
When HIF1α was used as a bait protein and incubated with MITF, MITF
bands appeared in the input and IP groups, indicating that HIF1α
interacted with MITF (Fig. 4A).
Additionally, MITF was knocked down using siRNAs. Following MITF
knockdown, the mRNA expression levels of MITF and HIF1α were
detected by qPCR. As shown in Fig.
4B, the mRNA expression levels of MITF and HIF1α were
significantly decreased in PDCs.
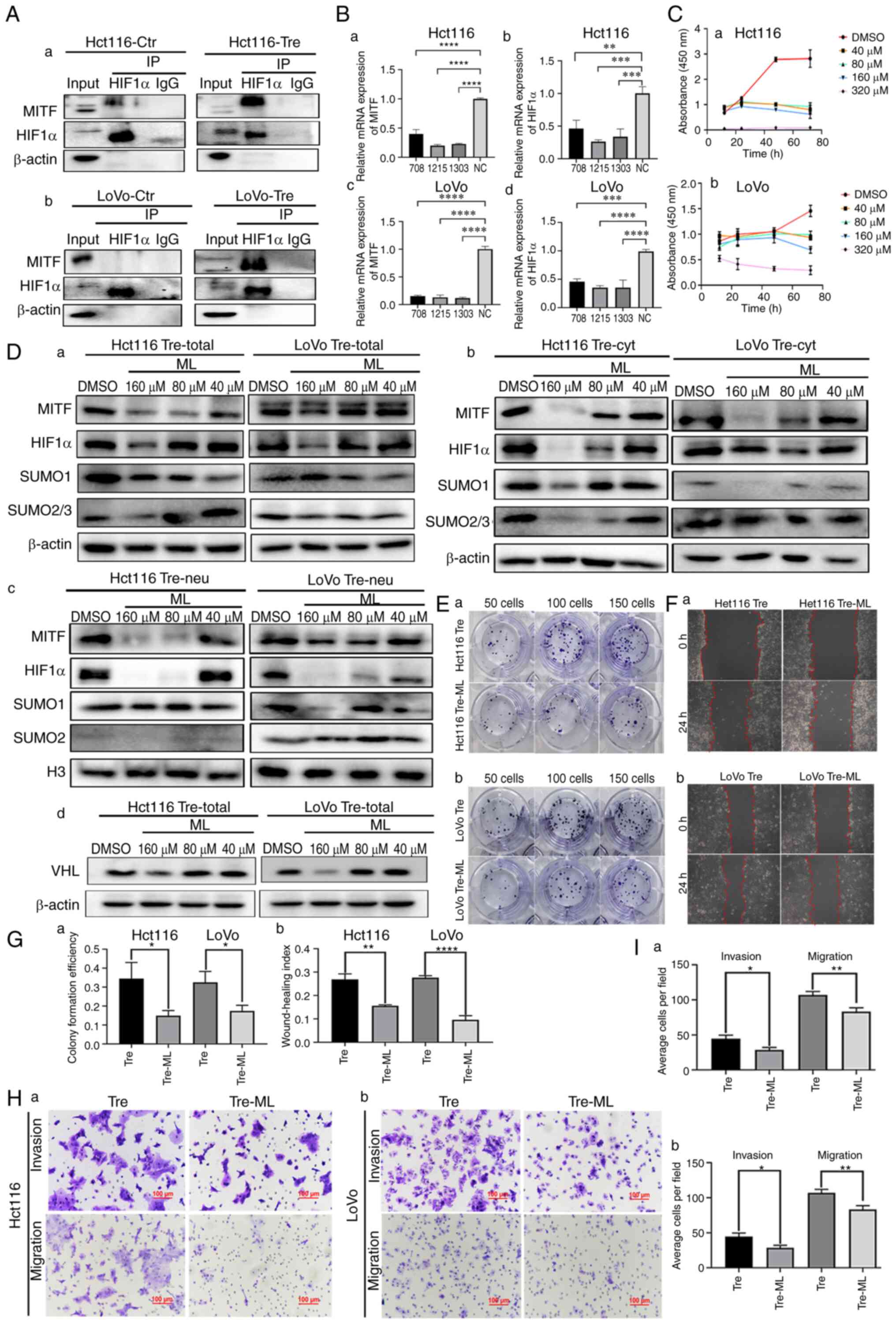 | Figure 4.MITF regulates the SUMOylation of
HIF1α. (A) The interaction between HIF1α and MITF was verified by
Co-IP in (a) Hct116 and (b) LoVo control cells and PDCs. (B) mRNA
expression levels of (a and c) MITF and (b and d) HIF1α were
detected in Hct116 and LoVo PDCs following MITF knockdown. (C) (a)
Hct116 and (b) LoVo PDCs were treated with ML at different
concentrations (40, 80, 160 and 320 µM). At each time point (0, 20,
40, 60 and 80 h), cell viability was assessed using CCK8 assay. (D)
Western blotting showing the (a) total, (b) cytoplasmic and (c)
nuclear expression of MITF, HIF1α, SUMO1 and SUMO2/3 in Hct116 and
LoVo PDCs following treatment with different concentrations of ML.
(d) Western blotting showing the expression of VHL in Hct116 and
LoVo PDCs following treatment with differernt concentrations of ML.
(E) Colony formation of 50, 100 and 150 (a) Hct116 and (b) LoVo
PDCs before and after ML treatment. (F) Wound healing assay of (a)
Hct116 and (b) LoVo PDCs before and after ML treatment at 0 h and
24 h (magnification, ×100). (G) (a) The differences in colony
formation efficiency of Hct116 and LoVo PDCs before and after ML
treatment. (b) Statistical analysis of wound healing index of
Hct116 and LoVo PDCs before and after ML treatment. (H) The
invasion and migration abilities of (a) Hct116 and (b) LoVo PDCs
before and after ML treatment. (I) Comparison of the average cell
number in invasion and migration assay of (a) Hct116 and (b) LoVo
PDCs before and after ML treatment. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. HIF1α, hypoxia inducible factor 1
alpha; MITF, microphthalmia-associated transcription factor; SUMO,
small ubiquitin-like modifier; Co-IP, co-immunoprecipitation; PDCs,
daughter cells; ML, methyl linoleate; PGCCs, polyploid giant cells;
Tre, PGCCs with PDCs; Ctr, control. |
ML, the main active ingredient in S. thea, is
a major anti melanin-producing compound that downregulates MITF
expression. CCK8 was used to screen the appropriate concentration
of ML and 40, 80 and 160 µM were finally selected (Fig. 4C). After ML treatment, the western
blotting results showed that the total, cytosolic and nuclear
expression of HIF1α, SUMO1 and SUMO2/3 was inhibited to varying
degrees. Nuclear expression of HIF1α in PDCs was inhibited,
suggesting that MITF may play an important role in the nuclear
localization of HIF1α. Compared to the control cells, the
expression levels of nuclear SUMO1 and SUMO2/3 also decreased after
ML treatment, indicating that MITF can regulate SUMOylation of
HIF1α and further affect the nuclear location of HIF1α (Fig. 4Da-c). Following ML treatment, the
western blotting results showed that the expression of VHL was
inhibited (Fig. 4Dd). Functional
experiments were also performed on the PDCs before and after ML
treatment. The proliferation, migration and invasion abilities of
PDCs were inhibited after ML treatment, as demonstrated by plate
cloning (Fig. 4E and Ga), wound
healing (Fig. 4F and Gb) and
Transwell experiments (Fig. 4H and
I).
The expression of PIAS4 is not
associated with HIF1α or VHL expression in PDCs
PIAS4 belongs to the PIAS protein family, which are
protein inhibitors that activate STAT proteins. PIAS4 is often
involved in PTM as it acts as a SUMO E3 ligase. VHL is an E3
ubiquitin ligase. PIAS4 mediates the SUMOylation of VHL and reduces
the activity of its ubiquitin E3 ligase, contributing to the
stabilization of HIF1α (22). The
present study demonstrated no significant change in the protein
expression levels of VHL, HIF1α and MITF following the knockdown of
PIAS4 using siRNA (Fig. 5A and Da-c
and Fig. S1). In addition, no
significant differences were observed in the expression of
cytoplasmic and nuclear VHL and HIF1α after PIAS4 knockdown
(Figs. 5B, C and Dd-i and S1).
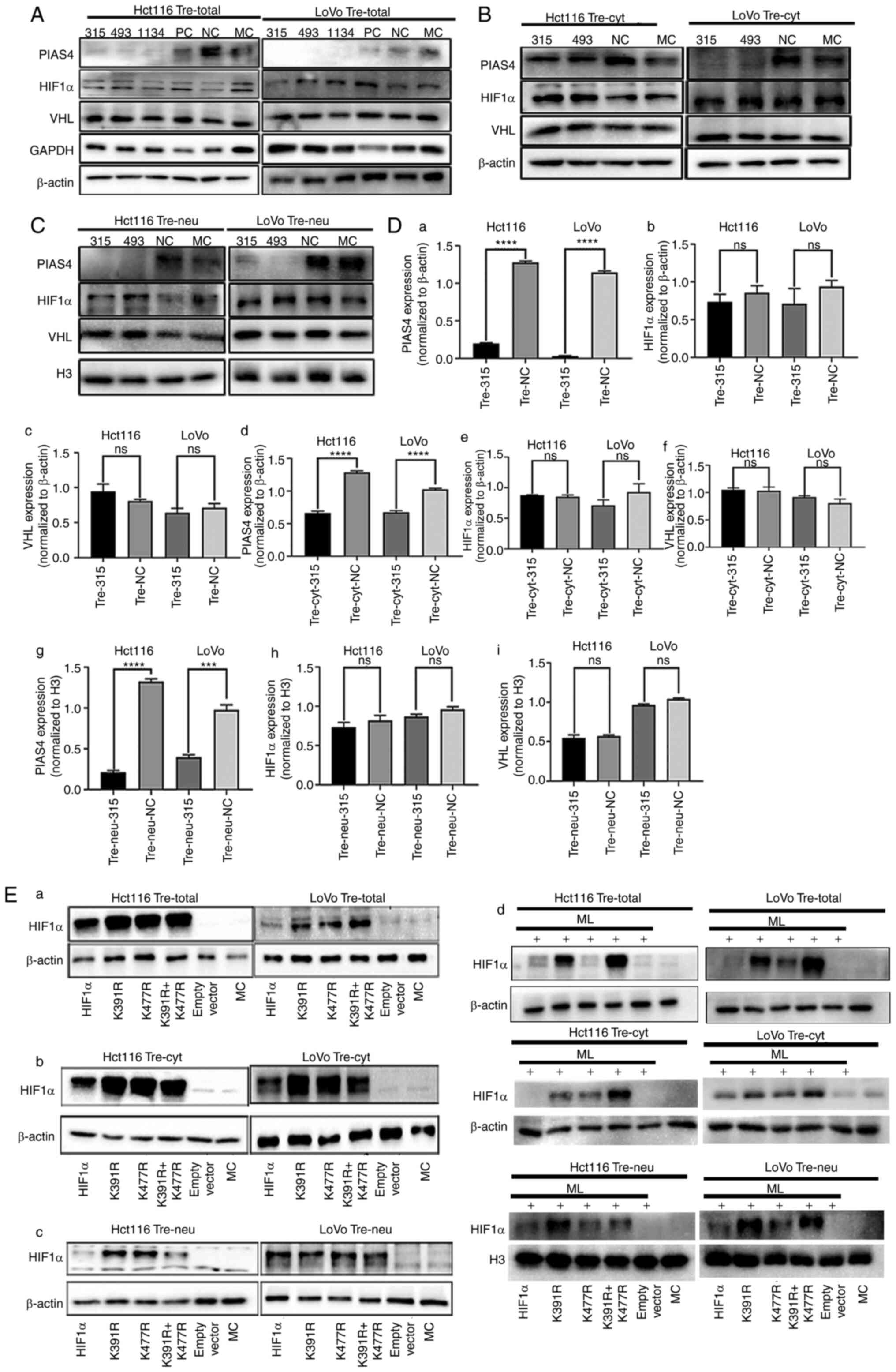 | Figure 5.HIF1α is modified by SUMOylation at
different lysine sites. (A) Expression of total PIAS4, HIF1α and
VHL in Hct116 and LoVo PDCs transfected with siRNA PIAS4-315, 493,
1134, PC, NC and MC, respectively. (B) Expression of cytoplasmic
PIAS4, HIF1α and VHL in Hct116 and LoVo PDCs transfected with siRNA
PIAS4-315, 493, NC and MC, respectively. (C) The expression of
nuclear PIAS4, HIF1α and VHL in Hct116 and LoVo PDCs transfected
with siRNA PIAS4-315, 493, NC and MC, respectively. (D) Histograms
showing the expression of total, plasma and nuclear PIAS4, HIF1α
and VHL in Hct116 and LoVo PDCs and PDCs after PIAS knockdown. (E)
The expression of total, cytoplasmic and nuclear HIF1α was detected
by western blotting Hct116 and LoVo PDCs before and after
transfection with wild type HIF1α, K391R, K477R, K391R and K477R
double mutants. ***P<0.001, ****P<0.0001, ns, no
significance. PIAS4, protein inhibitor of activated STAT protein 4;
HIF1α, Hypoxia inducible factor 1 alpha; VHL, von Hippel-Lindau
disease tumor suppressor; si, short interfering; PDCs, daughter
cells; PC, positive control; NC, negative control; MC, mock
control; PGCCs, polyploid giant cells; Tre, PGCCs with PDCs. |
HIF1α is modified by SUMOylation at
k391 and k477 sites
The proteins that can undergo SUMOylation have a
known consensus motif: ΨKXE (Ψ: a hydrophobic amino acid, K: lysine
residue, X: a variable residue amino acid, E or D: a glutamic acid)
(23). Two lysine sites, K391 and
K477, were revealed by the GSP-SUMO1.0 (The Cuckoo Workgroup)
computer analysis software and the amino acid sequences of the two
sites were 390–393 (LKKE) and 476–479 (LKLE), which met the
characteristics of SUMOylation (Table
V).
 | Table V.GSP-SUMO1.0 prediction of candidate
SUMOylation sites of HIF-1α. |
Table V.
GSP-SUMO1.0 prediction of candidate
SUMOylation sites of HIF-1α.
| No. | Position | Peptide | Score | Cutoff | Type |
|---|
| 1 | 220-224 | KKPPMTC
LVLIC EPIPHPS | 61.735 | 59.29 | SUMO
interaction |
| 2 | 391 |
SSLFDKLKKEPDALT | 29.24 | 16 | SUMOylation |
| 3 | 408-412 | APAAGDT
IISLD FGSNDTE | 59.572 | 59.29 | SUMO
interaction |
| 4 | 477 |
LNQEVALKLEPNPES | 27.474 | 16 | SUMOylation |
| 5 | 635-639 | TKDRMED
IKILI ASPSPTH | 68.227 | 59.29 | SUMO
interaction |
| 6 | 771-775 | NGMEQKT
IILIP SDLACRL | 65.589 | 59.29 | SUMO
interaction |
To determine whether HIF1α is SUMOylated at K391 and
K477 sites, the plasmid with mutated lysine to arginine was
transiently transfected. Compared with the empty plasmid and blank
control groups, the expression level of HIF1α was higher in the
HIF1α overexpression and the mutant groups. The expression level of
HIF1α in the HIF1α overexpression group was lower than that in the
mutant group, which indicated that HIF1α was not degraded in the
mutation group because lysine was mutated to arginine and
ubiquitination could not bind the lysine sites (Fig. 5Ea). The expression level of
cytoplasmic HIF1α in different groups was consistent with that of
total protein (Fig. 5Eb). However,
the expression level of nuclear HIF1α in the K391R+K477R group
decreased, indicating that HIF1α with mutated arginine sites cannot
be modified by SUMOylation or enter the nucleus (Fig. 5Ec). The expression levels of HIF1α
with K391R, K477R, K391R and K477R double mutants in ML-treated
cells are shown in Fig. 5Ed.
To further investigate the effects of mutation of
HIF1α at sites K391 and K477 on cell proliferation, migration and
invasion in PDCs, cell functional experiments, including plate
cloning, wound healing, Transwell migration and invasion assays,
were performed. The results showed that the proliferation (Fig. 6A and Da), migration (Fig. 6B and Db) and invasion (Fig. 6C and E) abilities of Hct116 and LoVo
PDCs after mutation were significantly lower than those in the
non-mutation group.
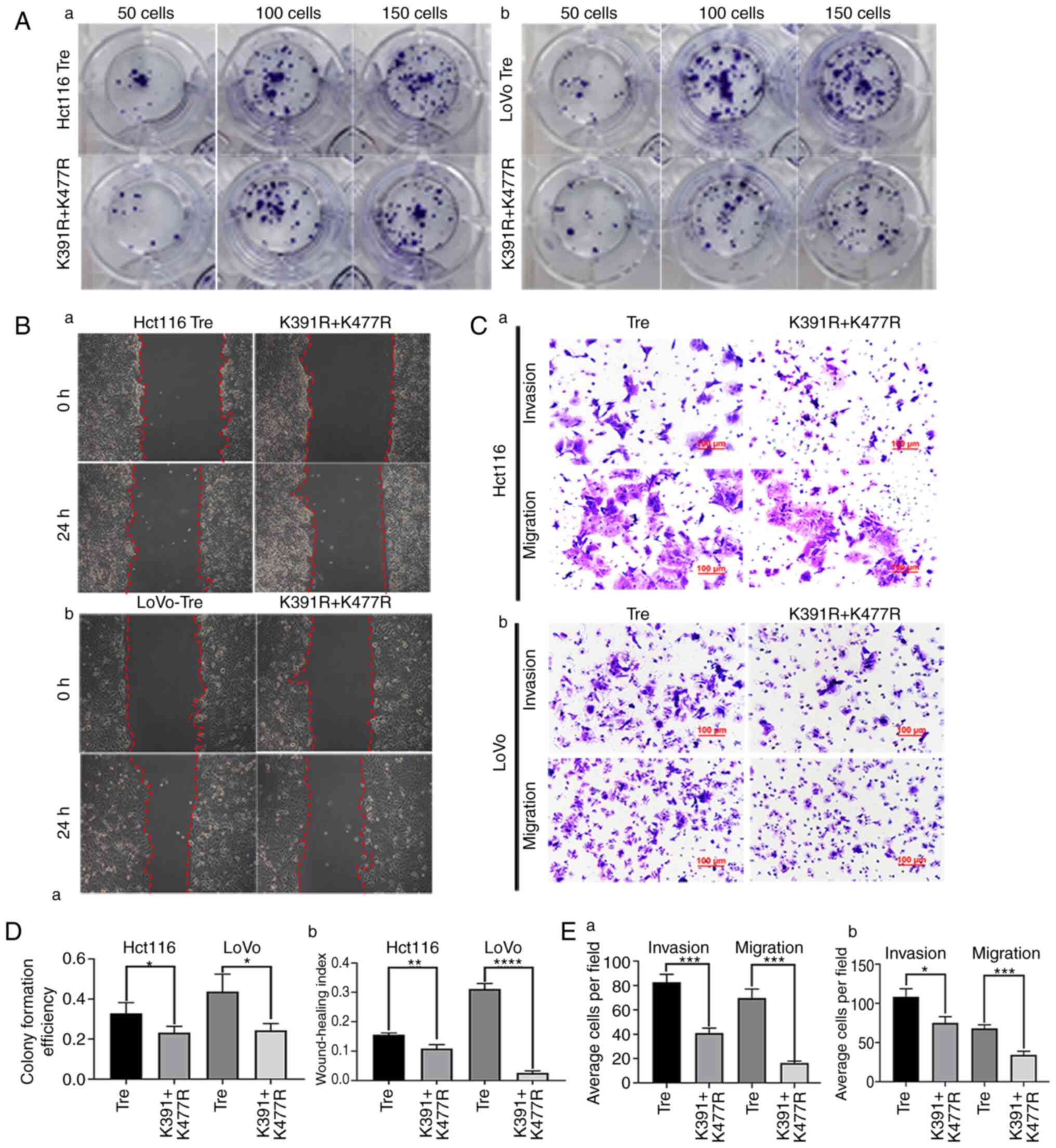 | Figure 6.The mutation of HIF1α at different
lysine sites is associated with the proliferation, migration and
invasion abilities of PDCs. (A) Colony formation of 50, 100 and 150
(a) Hct116 and (b) LoVo PDCs before and after transfection with
K391R and K477R double mutants. (B) Wound healing assay of (a)
Hct116 and (b) LoVo PDCs before and after transfection with K391R
and K477R double mutants at 0 h and 24 h (magnification, ×100). (C)
Invasion and migration abilities of (a) Hct116 and (b) LoVo PDCs
before and after transfection with K391R and K477R double mutants.
(D) (a) Differences in colony formation efficiency and (b)
statistical analysis of wound healing index in Hct116 and LoVo PDCs
before and after transfection with K391R and K477R double mutants.
(E) Comparison of the average cell number in invasion and migration
assay of (a) Hct116 and (b) LoVo PDCs before and after transfection
with K391R and K477R double mutants. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 ns, no significance. PDCs, daughter
cells; PGCCs, polyploid giant cells; Tre, PGCCs with PDCs. |
Discussion
CoCl2, chemical reagents, radiotherapy
and Chinese herbal medicines can induce PGCC formation and the
daughter cells derived from these PGCCs have strong proliferative,
migratory and invasive abilities. PGCCs are more frequently
observed in high-grade malignancies and metastatic foci than in
low-grade tumors and primary sites. PDCs with daughter cells can
exert important effects on the progression of malignant tumors,
including induction of metastasis, chemoresistance and tumor
relapse (24–26). The present study demonstrated that
the expression and subcellular location of HIF1α changed in the
treated cells compared with the control cells. SUMOylation can
affect the stable expression and nuclear localization of HIF1α.
HIF1α modified by SUMOylation could enter the nucleus and play an
important role in regulating the proliferation, migration and
invasion of PDCs. The protein expression level of HIF1α decreased
and nuclear localization was weakened after the use of SUMOylation
inhibitors.
SUMOylation is an important PTM involved in the
development and progression of malignant tumors, such as B-cell
lymphoma (27), multiple myeloma
(28), bladder cancer (29) and CRC (4). The target protein modified by
SUMOylation can regulate the protein-protein interaction and
subcellular location and promote the stability of the target
protein (30). Abnormal regulation
of SUMOylation promotes cancer metastasis, angiogenesis, invasion
and proliferation (31).
SUMOylation is also an important anti-stress mechanism and high
levels of SUMOylation are required for cancer cells to survive
internal and external stresses. Tumor cells become more aggressive
in response to both internal and external stresses. Prevention of
tumor metastasis, recurrence and radiochemotherapy resistance can
be partially achieved by attenuating SUMOylation (32).
In addition, the expression of MITF, PIAS4 and VHL
is a crucial factor affecting the expression and subcellular
location of HIF1α. The total protein levels of MITF, PIAS4 and VHL
were elevated in Hct116 and LoVo PDCs compared with those in
control cells. The qPCR results showed that the mRNA expression
level of HIF1α decreased after MITF knockdown, indicating that MITF
can regulate the expression of HIF1α at the transcriptional level.
MITF has been extensively studied. After inhibiting the expression
of MITF, the expression of HIF1α reduced. MITF can regulate the
SUMOylation modification of HIF1α by affecting the expression of
SUMO1 and SUMO2/3. In addition, MITF can bind to the HIF1α promoter
as a transcription factor to mediate the cAMP effects on the
expression of HIF1α (21). MITF
silencing clearly inhibited the cAMP-induced HIF1α promoter
transactivation (21). MITF is
positively associated with melanocyte survival, proliferation and
differentiation and is involved in cancer progression (33). MITF expression is associated with
poor prognosis in patients with hepatocellular carcinoma (34). Additionally, MITF is involved in
autophagy and cellular homeostasis in lung cancer (35). The overexpression of MITF in human
melanoma cells stimulates the expression of HIF1α mRNA, which plays
a pro-survival role in melanoma (21).
PIAS4 belongs to the PIAS protein family and has
been implicated in a number of biological activities, such as
regulating the DNA-binding activity of transcription factors,
recruiting coactivators and participating in PTM through its SUMO
E3 ligase activity (36). In
synovial sarcoma, PIAS4 mediates SUMOylation, leading to the
overexpression of nuclear receptor coactivator 3, which is critical
for tumor formation (37). In
hepatocellular carcinoma, PIAS4 regulates the SUMOylation of NEMO
(an essential regulator of NF-κB) and activation of NF-κB in
response to DNA damage (38). The
important role of PIAS4 in regulating the growth of pancreatic
cancer cells and enhancing HIF1-α activity by regulating VHL
SUMOylation has garnered our interest (39). The present study proved that the
expression of PIAS4 was not associated with the expression or
subcellular localization of HIF1α in PDCs. Therefore, SUMO E3
ligase PIAS4 may not play a role in regulating the expression of
HIF1α. Additionally, HIF1α was degraded through a VHL-dependent
mechanism. PIAS4 mediates the SUMOylation of VHL and reduces the
activity of its ubiquitin E3 ligase, contributing to the
stabilization of HIF1α (22). In
the present study, the expression levels of total, plasma and
nuclear VHL proteins showed no significant downward trends
following PIAS4 knockdown.
HIF1α can be SUMOylated at K391R and K477R (23). Site-mutated plasmids based on lysine
in the amino acid sequence of HIF1α were designed and the
transfected plasmids were subjected to distinct lysine site
modifications within cellular environments employing a transient
plasmid transfection methodology. The results confirmed that the
total and nuclear protein expression of HIF1α with double mutations
K391R and K477R was lower than that with single mutations K391R and
K477R in PDCs. The proliferation, migration and invasion abilities
of PDCs were weakened when the K391R and K477R sites of HIF1α were
mutated.
In conclusion, the expression of HIF1α increased and
its subcellular localization was altered, which was associated with
the proliferation, migration and invasion abilities of
CoCl2-induced PDCs. HIF1α can undergo SUMOylation at the
lysine residues K391 and K477. MITF can regulate the transcription
and protein levels of HIF1α and participate in the regulation of
HIF1α SUMOylation, but PIAS4 does not regulate HIF1α SUMOylation.
However, molecular mechanism by which HIF1α locates in the nucleus
and regulates the migration and invasion of PDCs is still unclear
and requires further research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants from the
National Science Foundation of China (grant nos. 82173283 and
82103088) and Foundation of the Committee on Science and Technology
of Tianjin (grant nos. 21JCZDJC00230, 21JCYBJC00190 and
21JCYBJC01070).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
Conceptualization and supervision was by SZ.
Research was performed by MZhe, ST and XZ. MZho, YY, YZ, XW, MY and
NL confirmed the authenticity of all the raw data. MZhe and ST
wrote the manuscript. Reviewing and editing was by LR and SZ.
Funding acquisition was by SZ. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CoCl2
|
cobalt chloride
|
|
CRC
|
colorectal cancer
|
|
PTM
|
post-translational modification
|
|
Co-IP
|
co-immunoprecipitation
|
|
GA
|
ginkgolic acid
|
|
HIF1α
|
hypoxia inducible factor 1 alpha
|
|
ICC
|
immunocytochemical
|
|
MITF
|
microphthalmia-associated
transcription factor
|
|
MC
|
mock control
|
|
NC
|
negative control
|
|
PC
|
positive control
|
|
PGCCs
|
polyploid giant cells
|
|
ML
|
methyl linoleate
|
|
PIAS4
|
protein inhibitor of activated STAT
protein 4
|
|
SUMO
|
small ubiquitin-like modifier
|
|
VHL
|
von Hippel-Lindau disease tumor
suppressor
|
References
|
1
|
Fan L, Zheng M, Zhou X, Yu Y, Ning Y, Fu
W, Xu J and Zhang S: Molecular mechanism of vimentin nuclear
localization associated with the migration and invasion of daughter
cells derived from polyploid giant cancer cells. J Transl Med.
21:7192023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Zheng M, Zhang H, Yang X, Fan L, Fu
F, Fu J, Niu R, Yan M and Zhang S: Arsenic trioxide promotes tumor
progression by inducing the formation of PGCCs and embryonic
hemoglobin in colon cancer cells. Front Oncol. 11:7208142021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Mercado-Uribe I, Xing Z, Sun B,
Kuang J and Liu J: Generation of cancer stem-like cells through the
formation of polyploid giant cancer cells. Oncogene. 33:116–128.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Q, Zhang K, Li Z, Zhang H, Fu F, Fu
J, Zheng M and Zhang S: High migration and invasion ability of
PGCCs and their daughter cells associated with the nuclear
localization of S100A10 modified by SUMOylation. Front Cell Dev
Biol. 9:6968712021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nehme Z, Pasquereau S, Haidar Ahmad S, El
Baba R and Herbein G: Polyploid giant cancer cells, EZH2 and Myc
upregulation in mammary epithelial cells infected with high-risk
human cytomegalovirus. EBioMedicine. 80:1040562022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv H, Shi Y, Zhang L, Zhang D, Liu G, Yang
Z, Li Y, Fei F and Zhang S: Polyploid giant cancer cells with
budding and the expression of cyclin E, S-phase kinase-associated
protein 2, stathmin associated with the grading and metastasis in
serous ovarian tumor. BMC Cancer. 14:5762014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bowers RR, Andrade MF, Jones CM,
White-Gilbertson S, Voelkel-Johnson C and Delaney JR: Autophagy
modulating therapeutics inhibit ovarian cancer colony generation by
polyploid giant cancer cells (PGCCs). BMC Cancer. 22:4102022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pustovalova M, Blokhina T, Alhaddad L,
Chigasova A, Chuprov-Netochin R, Veviorskiy A, Filkov G, Osipov AN
and Leonov S: CD44+ and CD133+ non-small cell lung cancer cells
exhibit DNA damage response pathways and dormant polyploid giant
cancer cell enrichment relating to their p53 status. Int J Mol Sci.
23:49222022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilé-Silva A, Lopez-Beltran A, Rasteiro H,
Vau N, Blanca A, Gomez E, Gaspar F and Cheng L: Pleomorphic giant
cell carcinoma of the prostate: Clinicopathologic analysis and
oncological outcomes. Virchows Arch. 482:493–505. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wicks EE and Semenza GL: Hypoxia-inducible
factors: Cancer progression and clinical translation. J Clin
Invest. 132:e1598392022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Konopleva MY and Jordan CT: Leukemia stem
cells and microenvironment: Biology and therapeutic targeting. J
Clin Oncol. 29:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paredes F, Williams HC and San Martin A:
Metabolic adaptation in hypoxia and cancer. Cancer Lett.
502:133–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H, Wang X and Fang B: HIF1A promotes
miR-210/miR-424 transcription to modulate the angiogenesis in
HUVECs and HDMECs via sFLT1 under hypoxic stress. Mol Cell Biochem.
477:2107–2119. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Mercado-Uribe I, Hanash S and Liu
J: iTRAQ-based proteomic analysis of polyploid giant cancer cells
and budding progeny cells reveals several distinct pathways for
ovarian cancer development. PLoS One. 8:e801202013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei J, Yang Y, Lu M, Lei Y, Xu L, Jiang Z,
Xu X, Guo X, Zhang X, Sun H and You Q: Recent advances in the
discovery of HIF-1α-p300/CBP inhibitors as anti-cancer agents. Mini
Rev Med Chem. 18:296–309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du L, Liu W and Rosen ST: Targeting
SUMOylation in cancer. Curr Opin Oncol. 33:520–525. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itokawa H, Totsuka N, Nakahara K, Takeya
K, Lepoittevin JP and Asakawa Y: Antitumor principles from ginkgo
biloba L. Chem Pharm Bull (Tokyo). 35:3016–3020. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu K, Wang X, Li D, Xu D, Li D, Lv Z,
Zhao D, Chu WF and Wang XF: Ginkgolic acid, a SUMO-1 inhibitor,
inhibits the progression of oral squamous cell carcinoma by
alleviating SUMOylation of SMAD4. Mol Ther Oncolytics. 16:86–99.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buscà R, Berra E, Gaggioli C, Khaled M,
Bille K, Marchetti B, Thyss R, Fitsialos G, Larribère L, Bertolotto
C, et al: Hypoxia-inducible factor 1{alpha} is a new target of
microphthalmia-associated transcription factor (MITF) in melanoma
cells. J Cell Biol. 170:49–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai Q, Verma SC, Kumar P, Ma M and
Robertson ES: Hypoxia inactivates the VHL tumor suppressor through
PIASy-mediated SUMO modification. PLoS One. 5:e97202010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bae SH, Jeong JW, Park JA, Kim SH, Bae MK,
Choi SJ and Kim KW: Sumoylation increases HIF-1alpha stability and
its transcriptional activity. Biochem Biophys Res Commun.
324:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haidar Ahmad S, El Baba R and Herbein G:
Polyploid giant cancer cells, cytokines and cytomegalovirus in
breast cancer progression. Cancer Cell Int. 23:1192023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casotti MC, Meira DD, Zetum ASS, Araújo
BC, Silva DRCD, Santos EVWD, Garcia FM, Paula F, Santana GM, Louro
LS, et al: Computational biology helps understand how polyploid
giant cancer cells drive tumor success. Genes (Basel). 14:8012023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alhaddad L, Chuprov-Netochin R,
Pustovalova M, Osipov AN and Leonov S: Polyploid/multinucleated
giant and slow-cycling cancer cell enrichment in response to X-ray
irradiation of human glioblastoma multiforme cells differing in
radioresistance and TP53/PTEN status. Int J Mol Sci. 24:12282023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoellein A, Fallahi M, Schoeffmann S,
Steidle S, Schaub FX, Rudelius M, Laitinen I, Nilsson L, Goga A,
Peschel C, et al: Myc-induced SUMOylation is a therapeutic
vulnerability for B-cell lymphoma. Blood. 124:2081–2090. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Driscoll JJ, Pelluru D, Lefkimmiatis K,
Fulciniti M, Prabhala RH, Greipp PR, Barlogie B, Tai YT, Anderson
KC, Shaughnessy JD Jr, et al: The sumoylation pathway is
dysregulated in multiple myeloma and is associated with adverse
patient outcome. Blood. 115:2827–2834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia QD, Sun JX, Xun Y, Xiao J, Liu CQ, Xu
JZ, An Y, Xu MY, Liu Z, Wang SG and Hu J: SUMOylation pattern
predicts prognosis and indicates tumor microenvironment
infiltration characterization in bladder cancer. Front Immunol.
13:8641562022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai X, Zhang T and Hua D: Ubiquitination
and SUMOylation: Protein homeostasis control over cancer.
Epigenomics. 14:43–58. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bettermann K, Benesch M, Weis S and
Haybaeck J: SUMOylation in carcinogenesis. Cancer Lett.
316:113–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han ZJ, Feng YH, Gu BH, Li YM and Chen H:
The post-translational modification, SUMOylation, and cancer
(review). Int J Oncol. 52:1081–1094. 2018.PubMed/NCBI
|
|
33
|
Levy C, Khaled M and Fisher DE: MITF:
Master regulator of melanocyte development and melanoma oncogene.
Trends Mol Med. 12:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nooron N, Ohba K, Takeda K, Shibahara S
and Chiabchalard A: Dysregulated expression of MITF in subsets of
hepatocellular carcinoma and cholangiocarcinoma. Tohoku J Exp Med.
242:291–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Qin X, Wang B, Xu G, Zhang J, Jiang
X, Chen C, Qiu F and Zou Z: MiTF is associated with chemoresistance
to cisplatin in A549 lung cancer cells via modulating lysosomal
biogenesis and autophagy. Cancer Manag Res. 12:6563–6573. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilkinson KA and Henley JM: Mechanisms,
regulation and consequences of protein SUMOylation. Biochem J.
428:133–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun Y, Perera J, Rubin BP and Huang J:
SYT-SSX1 (synovial sarcoma translocated) regulates PIASy ligase
activity to cause overexpression of NCOA3 protein. J Biol Chem.
286:18623–18632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mabb AM, Wuerzberger-Davis SM and Miyamoto
S: PIASy mediates NEMO sumoylation and NF-kappaB activation in
response to genotoxic stress. Nat Cell Biol. 8:986–993. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chien W, Lee KL, Ding LW, Wuensche P, Kato
H, Doan NB, Poellinger L, Said JW and Koeffler HP: PIAS4 is an
activator of hypoxia signalling via VHL suppression during growth
of pancreatic cancer cells. Br J Cancer. 109:1795–1804. 2013.
View Article : Google Scholar : PubMed/NCBI
|




















