Introduction
Cancer is one of the leading causes of mortality,
accounting for ~10 million annual deaths worldwide. According to
the latest report, it was estimated that annually, there were
~4,064,000 new cases and ~2,413,500 cancer-associated deaths in
China (1). In the US, there were
~1,958,310 new cancer cases and ~609,820 cancer-associated deaths
in 2023 (2). In addition, cancer
also negatively affects a country's economic growth; the estimated
global economic cost of cancer between 2020 and 2050 is $25.2
trillion (3). Of note, the burden
of cancer shows a continuously increasing trend worldwide, and as
such, cancer prevention and control are becoming an ever more
prominent subject. However, the efficacy of treatments with
traditional therapeutic strategies is limited, and thus, there is
an urgent need to develop novel approaches for cancer
treatment.
Chimeric antigen receptor (CAR)-T-cell therapy is a
revolutionary success in cancer-adoptive cellular immunotherapy.
Adoptive cellular immunotherapy is a promising strategy for curing
cancer completely. Numerous remarkable clinical responses have been
observed in treating certain subsets of B-cell leukemia or lymphoma
with CAR-T cells; however, several challenges limit their
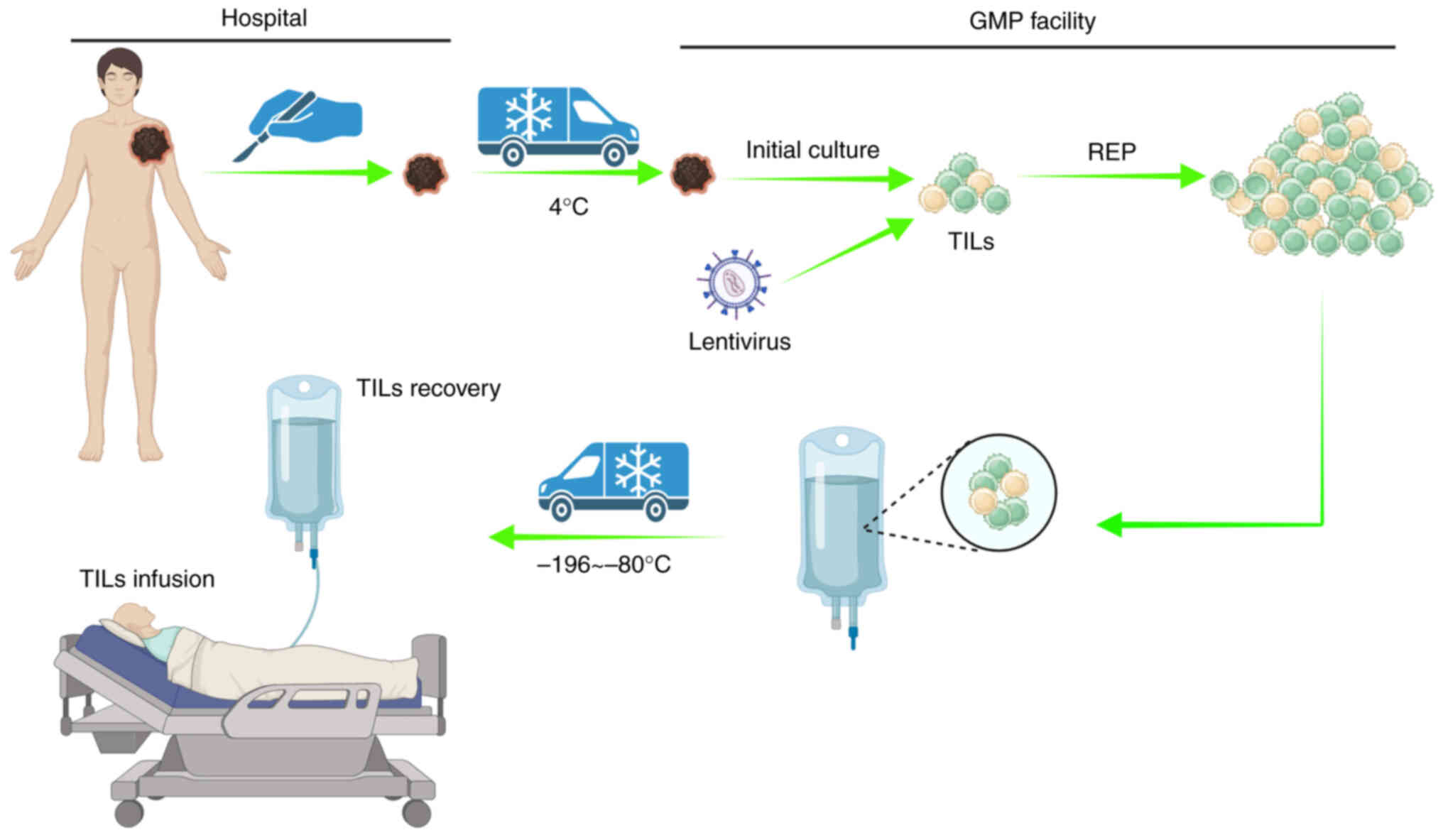
therapeutic efficacy in solid tumors (4). Tumor-infiltrating lymphocyte (TIL)
therapy is a type of adoptive cellular immunotherapy in which TILs
are harvested from tumor tissues. After amplification in
vitro, TILs are infused back into a patient and exert a
specific killing effect on cancerous cells (Fig. 1). Since TILs are autogenous
lymphocytes that are not genetically modified, they exhibit T-cell
receptor (TCR) clone diversity, superior tumor-homing ability and
low off-target toxicity, and thus, there are scarcely any adverse
reactions. Therefore, TIL-based therapy offers unique advantages in
treating solid tumors compared with other adoptive cellular
therapies (5,6).
However, in the tumor microenvironment, given the
antigen heterogeneity and tumor evolution, the application of
TIL-based therapy is still limited. In the present review, the
fundamental studies on TIL-based therapy and clinical trials on
improving the therapeutic effect of TILs are summarized.
Furthermore, the current limitations, challenges and opportunities
of TIL-based therapy are also discussed. Finally, the future
developmental directions of next-generation TIL-based therapy are
highlighted.
Relevant techniques and significant findings
in the developmental trajectory of TIL-based therapy
In 1980, Yron et al (7) reported that lymphoid cells in
suspension derived from tumor tissues were able to expand and grow
continuously in the presence of T-cell growth factor without tumor
cell proliferation. These lymphoid cells exhibited significant
cytotoxic activity towards syngeneic tumor cells and normal
fibroblasts grown in culture but did not lyse normal lymphoid
cells. After 6 years, they reported a novel approach by adopting
immunotherapy towards cancerous cells using TILs. Their results
showed that in combination with cyclophosphamide, treatment with
TILs and IL-2 cured all mice bearing MC-38-induced colon
adenocarcinoma of advanced hepatic metastases, and up to 50% of
mice were cured of advanced pulmonary metastases (8). These data provide a rationale for the
use of TILs in the treatment of patients with advanced cancer.
In 1988, Topalian et al (9) performed a pilot study to investigate
the feasibility and practicality of administering IL-2-expanded
TILs in patients with metastatic cancer; two partial responses to
therapy were observed and one additional patient with breast cancer
experienced a partial regression of disease. In five of six
patients with melanoma, TILs demonstrated lytic activity specific
to the autologous tumor target. No toxic effects were observed that
were directly attributable to TIL infusions. This study represents
an initial attempt to use TILs as a means of immunotherapy in
patients with cancer. Similarly, in 1994, Rosenberg et al
(10) reported that treatment with
TILs and IL-2, with or without cyclophosphamide, resulted in
objective responses (ORs) in approximately one-third of patients
with metastatic melanoma, and the treatment was safely
administered. These results demonstrate the practicality of TILs
for the treatment of patients with melanoma.
In 2008, Tran et al (11) developed an alternative ‘young’ TILs
method; this approach used tumor tissue to rapidly expand TILs for
administration without testing for tumor recognition. These younger
TILs exhibited higher levels of antigen reactivity, longer
telomeres and higher levels of CD27 and CD28. This strategy may
facilitate the widespread application of TILs in tumor
immunotherapy. In 2011, Itzhaki et al (12) described an efficient and reliable
method for generating young-TILs for adoptive transfer therapy.
Treatment with these young TILs resulted in an OR rate (ORR) of
~50% in patients with refractory melanoma (12). Thus, this approach may be adopted
for the treatment of other types of malignancies.
In 2012, Jin et al (13) described a modified method for the
initial culture and rapid expansion of TILs in gas-permeable
flasks. TIL initiation and rapid expansion procedure in
gas-permeable G-Rex flasks required fewer total vessels, less
media, less incubator space and less labor than other approaches.
In addition, TIL culture in G-Rex flasks facilitated the production
of TILs for the treatment of patients. In the same year, Friedman
et al (14) found that at
least 20% of metastatic melanomas contained CD4+
lymphocytes with specific tumor recognition by interferon-γ release
assay. After lymphodepletion, a patient with widespread metastatic
disease was administered TILs containing human leukocyte antigen
(HLA) class II-restricted tumor activity with high-dose IL-2
therapy that mediated the regression of extensive metastatic
disease in the liver and spleen. These results suggest a possible
role for CD4+ cells in the effectiveness of adoptive
cell therapy.
In 2014, Tran et al (15) used a whole-exome sequencing-based
method to demonstrate that TILs from a patient with metastatic
cholangiocarcinoma contained CD4+ T helper type 1 (Th1)
cells recognizing a mutation in the erb-b2 receptor tyrosine kinase
2 interacting protein frequently expressed in cancer. Adoptive
transfer mutation-specific polyfunctional CD4+ Th1 cells
may help a patient achieve prolonged stabilization of disease.
Patients with cancer progression treated with CD4+ Th1
cells have also been shown to exhibit tumor regression. These
results provide novel evidence that CD4+ T cells
recognize and respond to mutated antigens, and this can be
exploited to promote the regression of metastatic epithelial
cancer.
In 2015, Zhang et al (16) genetically engineered TILs to secrete
single-chain IL-12 selectively at the tumor site. The IL-12
encoding gene was driven by a nuclear factor of the activated
T-cell promoter. In this first-in-man trial, administration of
genetically engineered TILs mediated tumor responses in the absence
of IL-2 administration using cell doses 10- to 100-fold lower than
conventional TIL-based treatment. However, due to the toxicity of
secreted IL-12, further improvements are necessary before this
approach can be safely used in the therapy of patients with cancer.
In addition, Beane et al (17) used zinc finger nucleases (ZFNs)
directed against the gene encoding human programmed cell death 1
(PD-1) to genetically edit melanoma TILs, which resulted in a 76%
reduction in PD-1 surface expression. Of note, the genetically
edited TIL product showed improved in vitro effector
function and a significantly increased polyfunctional cytokine
profile compared to unmodified TILs. Furthermore, all donor cells
displayed an effector memory phenotype and expanded on a large
scale in vitro. However, the efficiency and safety of PD-1
gene-edited TILs for the treatment of metastatic melanoma requires
further study.
The adoptive transfer of neoantigen-reactive TILs
can result in tumor regression in patients with cancer. Thus,
neoantigen-specific TCR identification is a key technology for
improving the clinical efficacy of TILs. In 2017, Parkhurst et
al (18) identified 27 TCRs
from 6 patients that specifically recognized 14 neoantigens
expressed by autologous tumor cells. In 2018, Lu et al
(19) developed a new TCR
identification approach, which included the co-culture of TILs with
tandem minigene-transfected or peptide-pulsed autologous
antigen-presenting cells and identification of paired TCR sequences
using single-cell RNA sequencing analysis. Transduced T cells with
these TCRs specifically recognized the neoantigens. This strategy
provides an efficient procedure to identify neoantigen-specific
TCRs for use in future clinical applications for patients with
cancer.
In 2019, Nguyen et al (20) used a modified TIL protocol with a
lower dose of IL-2 in a single-institution phase II clinical trial
in patients with unresectable, metastatic melanoma. Their results
showed that this novel protocol of low-dose IL-2 following adoptive
cell transfer of TILs was feasible and clinically effective
(20).
In 2020, Krishna et al (21) identified a memory-progenitor
CD39-negative stem-like phenotype
(CD39−CD69−) by using high-dimensional
analysis of human adoptive cell therapy products. These TILs were
associated with complete cancer regression and TIL persistence. In
addition, these TILs were capable of self-renewal, expansion,
persistence and a superior antitumor response in vivo.
In 2021, Sinicrope et al (22) reported that lower circulating
adiponectin levels were not only associated with an increase in TIL
densities in colon cancer but also with an enhanced antitumor
immune response.
In 2022, Chamberlain et al (23) used clustered regularly interspersed
short palindromic repeats (CRISPR)-CRISPR associated 9 (Cas9) gene
editing to knock out PD-1 in TILs; an 87.53% reduction in cell
surface PD-1 expression was observed, and PD-1 knockout did not
affect the final fold expansion of TILs. These results demonstrated
that a non-viral, non-plasmid-based CRISPR-Cas9 gene editing method
can be feasibly adopted into a TIL-based adoptive cell transfer
therapy protocol to produce treatment products without any evident
negative effects.
In 2023, Forsberg et al (24) produced CAR-TILs through transducing
a lentiviral vector encoding an anti-HER2 CAR construct. CAR-TILs
were able to eradicate melanoma in patient-derived xenograft mouse
models, even in the absence of antigen presentation by HLA.
Furthermore, the tolerable and anti-tumor activity of CAR-TILs was
also observed in four companion dogs. These results demonstrated
that CAR-TIL therapy is a promising approach for improving the
tumor-targeting capacity of TILs.
Representative techniques and significant findings
in the development of TIL-based therapies are summarized in
Fig. 2.
Molecular and cellular mechanisms underlying
the function of TILs
TCRs are heterodimers comprised of an α and β chain;
the TCR repertoire is highly diverse and allows T cells to
specifically recognize multiple types of major histocompatibility
complex I-presenting tumor antigens. After interaction with tumor
antigens, TCRs complex with CD3ϵ/γ/δ/ζ subunits to ensure signal
transduction and drive the antigen-specific immune response to the
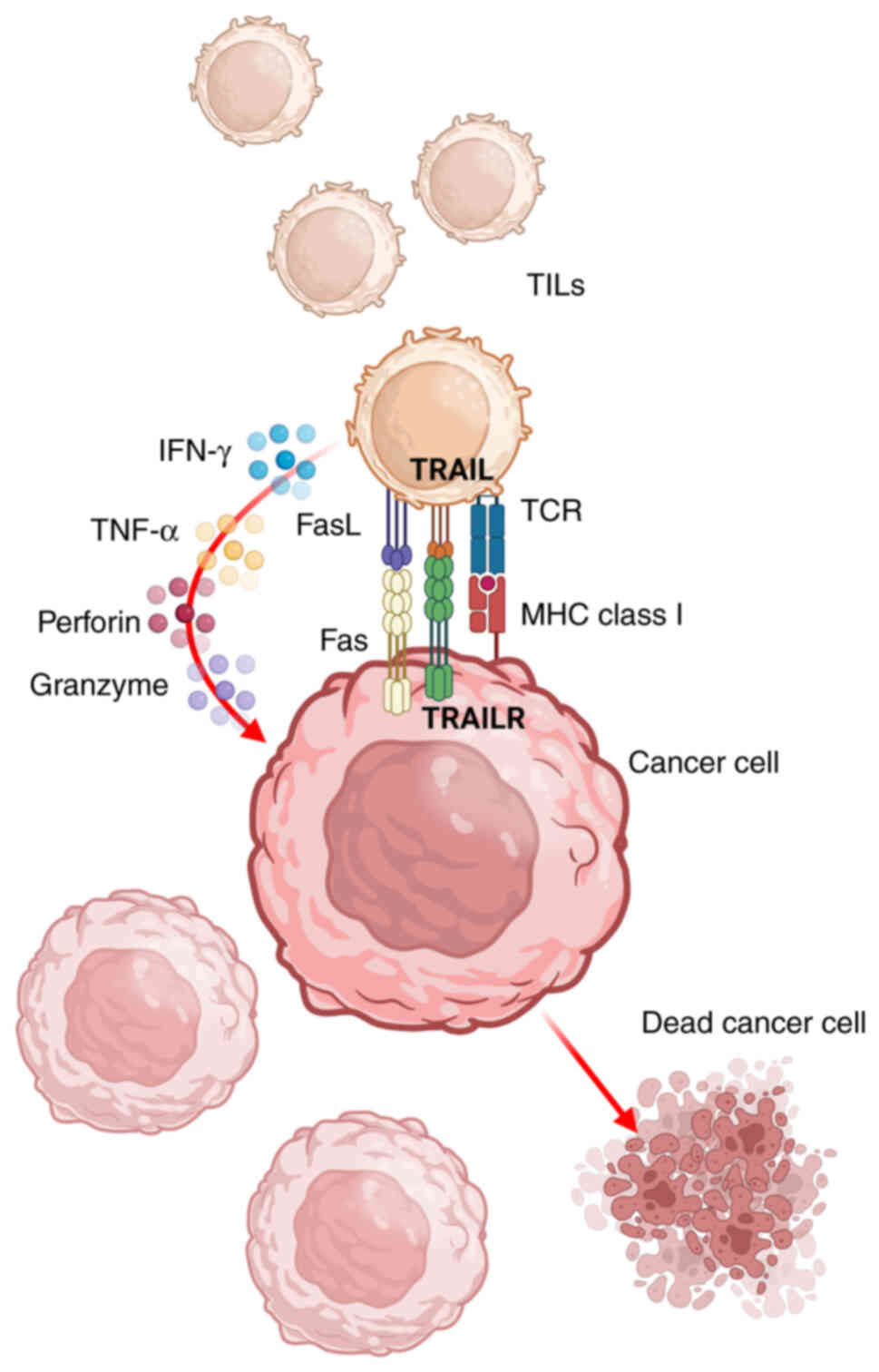
cancer cell (25,26).
The TCR and CD3 complex transduces extracellular
signals to intracellular effectors and thus has an irreplaceable
role in the activation of TILs and their anti-tumor properties. The
activated TILs secrete various cytokines to improve the killing
effect on tumor cells. For example, activated TILs upregulate the
expression of CD107a on the cell surface, which is associated with
cytotoxity and degranulation. Furthermore, the cellular signals
increase the release of IFN-γ, a key effector of TIL anti-tumor
activity (27). Importantly, IFN-γ
can induce apoptosis of both Fas-high and Fas-low cancer cells by
upregulating apoptosis-related proteins, such as caspase-3, Bak and
Fas (28). Activated TILs produce
more TNF-α, perforin and granzyme, which further enhance the
anti-tumor function of TILs (29–31).
TNF-α initiates cancer-cell apoptosis through p53-dependent
pathways by upregulating death receptor-4 and death receptor-5
(32). Interestingly, TNF-α can
also improve the sensitivity of cells to Fas ligand (FasL)-induced
cell death (33). Granzyme and
perforin are released by TILs and have both been shown to exert
anti-cancer properties in several types of cancer. Perforin and
granzyme B prevent the formation of cancer cell foci and induce
cancer-cell apoptosis (34,35). Mechanistically, granzyme B induces
the accumulation of reactive oxygen species, which results in
dysregulated mitochondrial function and cytochrome c release, and
therefore, accelerated cancer cell apoptosis (36,37).
The interaction between TCRs and tumor antigens
upregulates the expression of death ligands on the surface of TILs,
including TNF-related apoptosis-inducing ligand (TRAIL) and FasL.
TRAIL and FasL can recognize TRAIL receptor and Fas on cancer
cells, respectively, and induce cancer-cell apoptosis (38–40).
Binding of TRAIL to its receptor activates caspase family-dependent
apoptosis signaling, such as caspase-8, −10 and −3. The activated
caspase-8 leads to mitochondrial outer membrane permeabilization
via the Bid-Bax/Bak pathway, which results in mitochondrial
disorder and cytochrome C release, accelerating cancer-cell
apoptosis (41). Furthermore, FasL
binds Fas to induce cancer-cell apoptosis through the MAPK, NF-κB
and caspase-8 signaling pathways (42).
Taken together, TILs show powerful anti-tumor
properties via multiple mechanisms (Fig. 3).
Novel strategies for augmenting the
anti-tumor efficacy of TILs
The anti-tumor properties of TILs have innate
advantages, such as high safety, TCR diversity and superior
tumor-homing ability, amongst other benefits (5,6).
However, there remain several challenges regarding their clinical
use. Thus, innovative therapeutic strategies are required to
improve the anti-tumor properties of TILs.
Current challenges in the utilization
of TILs for cancer therapy
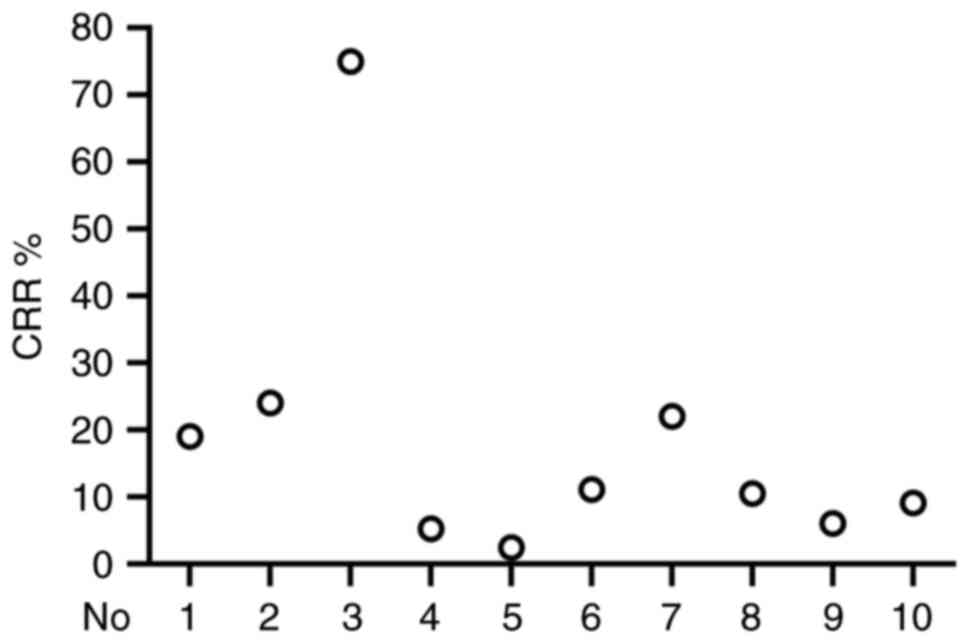
In the present review, 10 clinical trials were
randomly selected and the clinical complete response rate (CRR) was
analyzed. A total of 542 patients with different types of cancer
were treated with TIL therapy and the CRR ranged from 2.4–75% with
an average CRR of 14.15% (Table I)
(43–52). As shown in Fig. 4, there were differences in CRRs
among the different clinical trials. These clinical data indicated
that the effectiveness and stability of TIL-based therapy requires
further improvement.
 | Table I.Previous clinical data of
tumor-infiltrating lymphocytes in cancer treatment (total patients,
n=542). |
Table I.
Previous clinical data of
tumor-infiltrating lymphocytes in cancer treatment (total patients,
n=542).
| No. | First author,
year | Cancer type | Classification | Patients, n | CRR, % | (Refs.) |
|---|
| 1 | Goedegebuure,
1995 | Melanoma | Metastatic
melanoma | 16 | 19 | (43) |
| 2 | Goff, 2016 | Melanoma | Metastatic
melanoma | 101 | 24 | (44) |
| 3 | Huang, 2022 | Cervical
cancer | Locally advanced
cervical cancer | 12 | 75 | (45) |
| 4 | Chesney, 2022 | Melanoma | Advanced melanoma
after progression on immune checkpoint inhibitors | 153 | 5.2 | (46) |
| 5 | Zacharakis,
2022 | Breast cancer | Metastatic breast
cancer, phase II | 42 | 2.4 | (47) |
| 6 | Stevanović,
2019 | Cervical
cancer | HPV-associated
cervical cancer | 18 | 11.11 | (48) |
| 7 | Rosenberg,
2011 | Melanoma | Metastatic
melanoma | 93 | 22 | (49) |
| 8 | Queirolo, 1999 | Melanoma | Metastatic
melanoma | 19 | 10.5 | (50) |
| 9 | Dudley, 2010 | Melanoma | Metastatic
melanoma | 33 | 6 | (51) |
| 10 | Figlin, 1997 | Renal cell
carcinoma | Metastatic renal
cell carcinoma | 55 | 9.1 | (52) |
Mechanistic analysis revealed that the quality of
TILs, expansion ability in vivo, exhaustion,
immunosuppression and function disorder may be the leading causes
of unstable clinical outcomes. Therefore, there is an urgent need
to develop innovative therapeutic strategies to improve TIL-based
anti-tumor therapies by addressing these issues.
Enrichment of functional TILs
Functional disorder of TILs in the tumor
microenvironment results in the uncontrollable proliferation of
malignant cells. Therefore, the enrichment of functional TILs and
infusion of these TILs into patients is the ‘first step’ for
preventing the growth of cancer cells.
Minimizing the TIL culture time and enriching more
‘younger’ TILs is essential to reducing the time it takes to treat
a patient. Dudley et al (51) reported that 58% of patients treated
with CD8+ enriched young TILs and nonmyeloablative
lymphodepletion resulted in an OR and a CRR of 9%. These data
revealed that young TILs may be regarded as a novel source for
clinical application.
Tumor-reactive stem-like TILs are capable of steady
expansion, longer persistence, self-renewal and superior antitumor
properties (21). CD39 and CD69
double negative memory-progenitor stem-like TILs are strongly
associated with TIL persistence in vivo and complete cancer
regression (21). These stem-like
TILs have a critical role in preventing cancer cell immune escape
(53). In addition, induced
pluripotent stem cells (iPSCs) are capable of self-renewal,
maintaining their stemness, and have elongated telomeres.
TIL-derived iPSCs are able to produce less-differentiated and
tumor-specific T cells, which are a high-quality source of
autologous TILs for cancer immunotherapy (54). Islam et al (55) selected CD8+
PD-1+ CD137+ TILs and reprogrammed them into
iPSCs, and the tumor-reactive TCRs of TIL-iPSCs were consistent
with starting TILs. Furthermore, TIL-iPSCs were found to possess
rare tumor antigen-specific TCRs, which were not found in cultured
TILs. This approach may thus be used to enrich TILs that contain
tumor antigen-specific TCRs.
Furthermore, Bhadurihauck et al (56) directly delivered SOX2, Oct-4 and
NANOG proteins into the nucleus of tumor-infiltrating cytotoxic
T-lymphocytes by utilizing a nuclear protein delivery system, which
improved the proliferation and survival of these TICTLs. Teo et
al (57) applied a simulated
infective protocol to transform activated TILs into a
CD45RA+ central memory T-lymphocytes phenotype, which
exhibited elongated telomeres, improved persistence and the
powerful clearance ability of autologous acute myeloid leukemia
blast cells. These approaches provide a promising novel avenue for
tumor immunotherapy.
The anti-tumor properties of TILs can also be
enhanced by optimizing the cell culture conditions. An
overabundance of extracellular potassium suppresses T-cell effector
functions by limiting nutrient uptake while improving the
production of CD8+ T-cells with enhanced in vivo
persistence, stemness and tumor eradication ability (58). Acid accumulation induces methionine
uptake and metabolism disorder by down-regulating the expression of
SLC7A5, which alters H3K27me3 methylation on the promoter regions
of genes related to T-cell stemness, and further increases their
persistence and maintenance of a stem-like memory phenotype, and
improves the anti-tumor properties of T cells in vivo
(59). These mechanistic results
may be used to develop a novel strategy for improving the treatment
outcomes of T cell-based cancer immunotherapy.
Artificial antigen-presenting cells (aAPC) can be
used to rapidly expand the clinical scale of TILs. TILs undergo
rapid expansion under CD64/CD137 aAPC stimulation, and these
aAPC-expanded TILs show a low CD4/CD8 ratio, fewer forkhead box
(FOX)P3+ cells and a larger population of tumor
antigen-specific cells (60).
CD86/CD137L/membrane-bound IL-15 aAPC can significantly increase
the ratio of CD8+T cell populations with effector-memory
phenotype (61).
Collectively, these strategies summarized above can
be used to enrich and expand functional TILs and further improve
the persistence, stemness and survivability of said TILs.
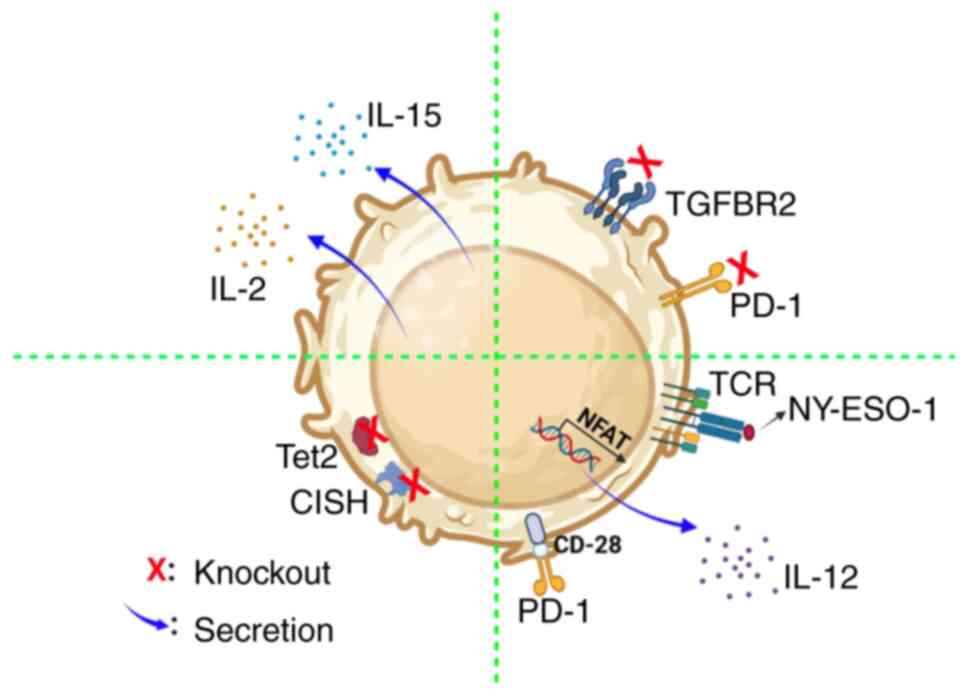
Improved antitumor efficacy of TILs
through gene editing
Gene editing is an effective approach to improving
survival, resistance to immunosuppression and the tumor eradication
ability of modified TILs (Fig.
5).
To increase the survival of TILs, Hsu et al
(62) transduced IL-15 and herpes
simplex virus-thymidine kinase (HSV-TK) genes into TILs and the
transduced TILs secreted IL-15, which significantly prolonged their
survival in vitro following withdrawal of IL-2; meanwhile,
TILs expressing HSV-TK were readily eliminated by low
concentrations of ganciclovir. Of particular importance, the
transduced TILs maintained the ability to specifically recognize
and eradicate melanomas, even without IL-2. In addition, transduced
TILs with IL-2 exhibited prolonged TILs without IL-2
supplementation while preserving their tumor-targeting specificity
(63). These reports show that
genetic modifications could reduce the dependence of exogenous IL-2
and improve the survival of TILs.
For blocking immunosuppressive signals, Beane et
al (17) efficiently reduced
the surface expression of PD-1 in TILs using ZFNs; the effector
function of these TILs was improved and the secretion of
polyfunctional cytokines was also significantly increased.
Importantly, these TILs showed an effector memory phenotype and
rapidly expanded in vitro. Furthermore, using CRISPR-Cas9 to
knockout and reduce PD-1 expression in TILs did not affect TIL
expansion (23). In addition, Fix
et al (64) used CRISPR/Cas9
to mediate transforming growth factor β (TGF-β) receptor 2
knockout, which resulted in TILs that were resistant to TGF-β
signaling-induced immunosuppression, while the expansion
efficiency, phenotype and TCR diversity of TILs were not affected.
These results demonstrated that these methods could be applied to
produce TILs that were resistant to immunosuppressive signals;
these TILs may have significant value in clinical practice.
For improving the anti-tumor properties of TILs,
Robbins et al (65)
transduced T cells with a TCR, which was able to directly act
against New York-esophageal cancer 1 (NY-ESO-1). Adoptive transfer
of TCR-TILs to patients with NY-ESO-1+ tumors showed a
45.5% ORR and 18.2% complete regression in patients with melanoma.
Liu et al (66) constructed
a CAR-TIL with a switch receptor, which contained an optimized PD-1
extracellular domain, and CD28 transmembrane and intracellular
domains. These CAR-TILs were able to easily infiltrate into tumors,
significantly decreased the tumor volume and were resistant to
hypofunction induced by the tumor. Lee et al (67) revealed that ten-eleven
translocation-2 (Tet2) deficiency contributed to the accessibility
of chromatin and the binding of transcription factors, which
enhanced the activation of CD8+ TILs. In addition, Tet2
inactivation increased the effector-like cell population and
improved the anti-tumor effects of TILs. Furthermore, TILs
genetically engineered to produce single-chain IL-12 using an
nuclear factor of activated T-cells promoter led to the
transduction of TILs to selectively secrete IL-12 at the tumor site
to mediate the antitumor effects in the absence of IL-2 (16). Deletion of cytokine-induced SH2
protein using CRISPR/Cas9 improved TIL activation against tumor
neoantigens (68). Similarly,
utilization of CRISPR/Cas9 technology to inactivate SOCS1 resulted
in augmentation of cytokine signal responsiveness and a notable
enhancement in the efficacy of antitumor responses in murine models
(69). These reports revealed that
genetic editing offers a novel approach to improve the tumor
eradication ability of TILs.
Taken together, these studies reveal that genetic
modification can further improve the anti-tumor properties of TILs,
and modification of TILs provides additional opportunities for
designing effective therapeutic strategies to manage solid
tumors.
Combination therapy
Combination therapy is regarded as an effective
strategy for improving the effectiveness of adoptive TIL-based
therapy.
Combination of TILs with oncolytic
viruses (OVs)
OVs are a type of naturally occurring genetically
modifiable virus, which can specifically kill tumor cells without
affecting normal cells. In recent years, OV treatment has shown
promise as a therapeutic approach for tumor patients. Studies have
indicated that combinations with OVs can improve the therapeutic
efficiency of other approaches. For instance, intratumor injection
of IL-2-armed OV resulted in the recruitment and accumulation of
TILs, which have higher tumor specificity and contain fewer
exhausted T cells and regulatory T cells. These TILs lead to tumor
regression and prolong survival of mice with MC38 tumors (70). Mechanistically, OVs significantly
enhance the tumor killing ability of TILs by inducing granzyme B
secretion and increasing the population of cytotoxic cells
(71).
Furthermore, administration of ex vivo tumor
cultures with OVs [adenovirus (Ad)5/3-E2F promoter-24 bp deletion
(E2F-D24)-hTNF-α-internal ribosome entry site (IRES)-hIL-2] induced
the activation of CD4+ and CD8+ TILs and
promoted the secretion of IFN-γ, C-X-C motif chemokine ligand 10,
TNF-α and IL-2, which mediate anti-tumor responses (72). In addition, the combination of OVs
with TILs in hamsters with pancreatic tumors resulted in a CRR of
100%. Interestingly, further study revealed that OVs could
efficiently bind to TILs, and these TILs could carry and deliver
OVs to tumors. As feedback, OVs further enhanced TIL cytotoxicity
(73).
Kudling et al (74) locally injected OVs
(Ad5/3-E2F-D24-hIL-7) into a hamster model with tumors and observed
a significant reduction in tumor growth and increased TIL
infiltration. Mechanistic analysis revealed that oncolytic
adenoviruses promoted the secretion of pro-inflammatory cytokines,
and enhanced the activation and migration of cytotoxic T cells. In
a clinical study, 18 stage IIIc/IV patients with melanoma were
treated with TILs in combination with an adenovirus that expressed
IFN-γ. This combination was tolerated by patients and the overall
ORR was 38.5%, with a disease control rate of 46% (75).
Ye et al (76) developed a novel method to modify
tumor cells with a HSV 1-based OV encoding TNF superfamily member 4
(OX40L) and IL-12 and transformed them into aAPCs. These infected
tumor cells exhibited the features of APCs but with the improved
activation and killing ability of TILs in vitro.
Importantly, the combination administration of OV-OX40L/IL-12 and
TILs led to complete tumor regression. In addition, activated T
cells induced antitumor immunological memory and reprogrammed
macrophages to an anti-tumor phenotype.
Collectively, these reports reveal that OVs not only
improve the anti-tumor ability of TILs but also regulate the tumor
microenvironment. This showcases the promising synergistic effect
of TILs in combination with OVs in tumor therapy and demonstrates
the feasibility of the clinical application of this novel
therapeutic strategy.
TILs in combination with other
drugs
Numerous drugs have been widely used in tumor
treatment to significantly inhibit tumor growth by regulating
multiple pathways. Recent studies discovered that several drugs can
enhance the tumor killing ability of TILs.
Treatment with Ibrutinib plus Rapamycin restores TIL
functions by blocking IL-2-inducible kinase and mTOR pathways. In
addition, this combination therapy facilitated CD45RA re-expression
in TILs and downregulated the expression of exhaustion markers
(77). Ipilimumab plus TILs for
metastatic melanoma treatment was well tolerated, the ORR was
38.5%, four of five patients maintained OR after 1 year and one
achieved CR at 52 months (78). In
addition, bispecific antibodies efficiently improve the killing
effects of TILs on HER-2+ ovarian cells (79). These approaches remodel the
phenotype and enhance the therapeutic effects of TILs.
Combination administration with fibroblast
activation protein (FAP)-4-1BBL and TCR activator notably improves
TIL proliferation, activation and cytotoxicity. Mechanistically,
FAP-4-1BBL induced secretion of IL-13 from TILs, which mediates
tumor cell apoptosis dependent on IL-13α 1/2 receptors and the
STAT6 pathway (31). Furthermore,
the fusion protein PD-1Ab-IL21 promotes the expansion of memory
stem CD8+ T cells, which are tumor-specific and trigger
anti-tumor immune responses (80).
In addition, Pentoxifylline (PTXF) improved the ratio of cytotoxic
TILs, increased IFN-γ levels, upregulated the expression of t-bet,
suppressed the recruitment of regulatory TILs, decreased TGF-β
levels and downregulated the expression of FOXP3. These changes
induced by PTXF contributed to the anti-tumor responses of TILs
(81). These combination strategies
highlight the potential of TILs in combination with other
therapeutics and/or adjuvants to improve the anti-tumor properties
of TILs, and thus deserve further evaluation in clinical
trials.
Conclusions and future perspectives
Successful cancer therapy remains a formidable
challenge worldwide. Currently, traditional therapeutic strategies
fall short in achieving a cure for cancer, resulting in substantial
economic losses.
TIL therapy is an advanced immunotherapy approach
for the treatment of cancer, which involves the collection and
expansion of autologous TILs followed by their reinfusion into
patients to specifically target and eliminate tumor cells.
Currently, TIL therapy has demonstrated significant efficacy in
treating malignant tumors, such as melanoma and colorectal cancer
(44,45). Adoptive TIL therapy represents a
promising strategy for cancer treatment, as TILs exhibit remarkable
diversity in their TCR repertoire, possess tumor-homing
capabilities and exert potent cytotoxic effects. Multiple clinical
trials have consistently demonstrated the safety and efficacy of
this approach (5,6).
However, TIL therapy also encounters several
challenges and issues, including the acquisition of an adequate
quantity and quality of immune cells, optimization of cell
preparation processes, as well as addressing concerns related to
immune evasion and drug resistance. These key issues necessitate
urgent resolution. Furthermore, from a clinical perspective, immune
toxicity resulting from the targeting of normal tissues is a
significant concern due to the potential for immune cells to
inadvertently harm healthy tissue during their attack on tumor
cells. From a production standpoint, the protracted manufacturing
cycle, exorbitant costs and challenging pricing structure
associated with cell therapy impede patient accessibility and
present commercial obstacles for this emerging treatment. Besides
that, the infusion of TILs is ineffective in a subset of patients
with cancer due to tumor heterogeneity and the intricate nature of
the tumor microenvironment (Fig.
4).
Therefore, further enhancement of the anti-tumor
properties of TILs in multiple dimensions is imperative, including
the enrichment of functional TILs, gene editing techniques, and
combination therapies. Gene editing represents one of the most
efficacious strategies for modifying TIL characteristics and
augmenting their tumor-killing potential, thereby paving the way
for future clinical applications and commercialization of TILs. TIL
infusion is a highly promising strategy for cancer treatment.
Addressing clinical unmet needs and developing innovative TIL-based
therapies will accelerate the growth of the TIL industry in tumor
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the New Industry Cultivation
Program of Qingdao (grant no. 23-1-4-xxgg-18-nsh) and the
Technological SMEs Innovation Ability Improvement Project of
Shandong Province (grant no. 2023TSGC0510).
Availability of data and materials
Not applicable.
Authors' contributions
Project administration: ZY, JS, AX and NL.
Writing-original draft preparation: ZY, JS, YF, YZ and AX.
Writing-review and editing: ZY, AX and NL. All authors have read
and agreed to the published version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAR
|
chimeric antigen receptor
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
ZFNs
|
zinc finger nucleases
|
|
PD-1
|
programmed cell death 1
|
|
TCR
|
T-cell receptor
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
FasL
|
Fas ligand
|
|
CRR
|
complete response rate
|
|
iPSCs
|
induced pluripotent stem cells
|
|
aAPC
|
artificial antigen-presenting
cells
|
|
OV
|
oncolytic virus
|
References
|
1
|
Zheng RS, Zhang SW, Sun KX, Chen R, Wang
SM, Li L, Zeng HM, Wei WW and He J: Cancer statistics in China,
2016. Zhonghua Zhong Liu Za Zhi. 45:212–220. 2023.(In Chinese).
PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen S, Cao Z, Prettner K, Kuhn M, Yang J,
Jiao L, Wang Z, Li W, Geldsetzer P, Bärnighausen T, et al:
Estimates and projections of the global economic cost of 29 cancers
in 204 countries and territories from 2020 to 2050. JAMA Oncol.
9:465–472. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11:692021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Sun J, Chen K, Ma P, Lei Q, Xing
S, Cao Z, Sun S, Yu Z, Liu Y and Li N: Perspectives of
tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med.
19:1402021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin B, Du L, Li H, Zhu X, Cui L and Li X:
Tumor-infiltrating lymphocytes: Warriors fight against tumors
powerfully. Biomed Pharmacother. 132:1108732020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yron I, Wood TA Jr, Spiess PJ and
Rosenberg SA: In vitro growth of murine T cells. V. The isolation
and growth of lymphoid cells infiltrating syngeneic solid tumors. J
Immunol. 125:238–245. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenberg SA, Spiess P and Lafreniere R: A
new approach to the adoptive immunotherapy of cancer with
tumor-infiltrating lymphocytes. Science. 233:1318–1321. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Solomon D, Avis FP, Chang AE,
Freerksen DL, Linehan WM, Lotze MT, Robertson CN, Seipp CA, Simon
P, et al: Immunotherapy of patients with advanced cancer using
tumor-infiltrating lymphocytes and recombinant interleukin-2: A
pilot study. J Clin Oncol. 6:839–853. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenberg SA, Yannelli JR, Yang JC,
Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA,
Einhorn JH and White DE: Treatment of patients with metastatic
melanoma with autologous tumor-infiltrating lymphocytes and
interleukin 2. J Natl Cancer Inst. 86:1159–1166. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran KQ, Zhou J, Durflinger KH, Langhan
MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA and Dudley
ME: Minimally cultured tumor-infiltrating lymphocytes display
optimal characteristics for adoptive cell therapy. J Immunother.
31:742–751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itzhaki O, Hovav E, Ziporen Y, Levy D,
Kubi A, Zikich D, Hershkovitz L, Treves AJ, Shalmon B, Zippel D, et
al: Establishment and large-scale expansion of minimally cultured
‘young’ tumor infiltrating lymphocytes for adoptive transfer
therapy. J Immunother. 34:212–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin J, Sabatino M, Somerville R, Wilson
JR, Dudley ME, Stroncek DF and Rosenberg SA: Simplified method of
the growth of human tumor infiltrating lymphocytes in gas-permeable
flasks to numbers needed for patient treatment. J Immunother.
35:283–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedman KM, Prieto PA, Devillier LE,
Gross CA, Yang JC, Wunderlich JR, Rosenberg SA and Dudley ME:
Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J
Immunother. 35:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tran E, Turcotte S, Gros A, Robbins PF, Lu
YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS,
et al: Cancer immunotherapy based on mutation-specific CD4+ T cells
in a patient with epithelial cancer. Science. 344:641–645. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Morgan RA, Beane JD, Zheng Z,
Dudley ME, Kassim SH, Nahvi AV, Ngo LT, Sherry RM, Phan GQ, et al:
Tumor-infiltrating lymphocytes genetically engineered with an
inducible gene encoding interleukin-12 for the immunotherapy of
metastatic melanoma. Clin Cancer Res. 21:2278–2288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beane JD, Lee G, Zheng Z, Mendel M,
Abate-Daga D, Bharathan M, Black M, Gandhi N, Yu Z, Chandran S, et
al: Clinical scale zinc finger nuclease-mediated gene editing of
PD-1 in tumor infiltrating lymphocytes for the treatment of
metastatic melanoma. Mol Ther. 23:1380–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parkhurst M, Gros A, Pasetto A, Prickett
T, Crystal JS, Robbins P and Rosenberg SA: Isolation of T-cell
receptors specifically reactive with mutated tumor-associated
antigens from tumor-infiltrating lymphocytes based on CD137
expression. Clin Cancer Res. 23:2491–2505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu YC, Zheng Z, Robbins PF, Tran E,
Prickett TD, Gartner JJ, Li YF, Ray S, Franco Z, Bliskovsky V, et
al: An Efficient Single-Cell RNA-Seq approach to identify
neoantigen-specific T cell receptors. Mol Ther. 26:379–389. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen LT, Saibil SD, Sotov V, Le MX,
Khoja L, Ghazarian D, Bonilla L, Majeed H, Hogg D, Joshua AM, et
al: Phase II clinical trial of adoptive cell therapy for patients
with metastatic melanoma with autologous tumor-infiltrating
lymphocytes and low-dose interleukin-2. Cancer Immunol Immunother.
68:773–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krishna S, Lowery FJ, Copeland AR,
Bahadiroglu E, Mukherjee R, Jia L, Anibal JT, Sachs A, Adebola SO,
Gurusamy D, et al: Stem-like CD8 T cells mediate response of
adoptive cell immunotherapy against human cancer. Science.
370:1328–1334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinicrope FA, Shi Q, Smyrk TC, Goldberg
RM, Cohen SJ, Gill S, Kahlenberg MS, Nair S, Shield AF, Jahagirdar
BN, et al: Association of adiponectin and vitamin D with tumor
infiltrating lymphocytes and survival in stage III colon cancer.
JNCI Cancer Spectr. 5:pkab0702021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chamberlain CA, Bennett EP, Kverneland AH,
Svane IM, Donia M and Met Ö: Highly efficient PD-1-targeted
CRISPR-Cas9 for tumor-infiltrating lymphocyte-based adoptive T cell
therapy. Mol Ther Oncolytics. 24:417–428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forsberg EMV, Riise R, Saellström S,
Karlsson J, Alsén S, Bucher V, Hemminki AE, Olofsson Bagge R, Ny L,
Nilsson LM, et al: Treatment with Anti-HER2 Chimeric Antigen
receptor tumor-infiltrating lymphocytes (CAR-TILs) Is safe and
associated with antitumor efficacy in mice and companion dogs.
Cancers (Basel). 15:6482023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shafer P, Kelly LM and Hoyos V: Cancer
therapy with TCR-Engineered T Cells: Current strategies,
challenges, and prospects. Front Immunol. 13:8357622022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alcover A, Alarcón B and Di Bartolo V:
Cell biology of T cell receptor expression and regulation. Annu Rev
Immunol. 36:103–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang WC, Zhang ZQ, Li PP, Ma JY, Chen L,
Qian HH, Shi LH, Yin ZF, Sun B and Zhang XF: Anti-tumor activity
and mechanism of oligoclonal hepatocellular carcinoma
tumor-infiltrating lymphocytes in vivo and in vitro. Cancer Biol
Ther. 20:1187–1194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn EY, Pan G, Vickers SM and McDonald JM:
IFN-gammaupregulates apoptosis-related molecules and enhances
Fas-mediated apoptosis in human cholangiocarcinoma. Int J Cancer.
100:445–451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Paola F, Ridolfi R, Riccobon A, Flamini
E, Barzanti F, Granato AM, Mordenti GL, Medri L, Vitali P and
Amadori D: Restored T-cell activation mechanisms in human
tumour-infiltrating lymphocytes from melanomas and colorectal
carcinomas after exposure to interleukin-2. Br J Cancer.
88:320–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Draghi A, Chamberlain CA, Khan S, Papp K,
Lauss M, Soraggi S, Radic HD, Presti M, Harbst K, Gokuldass A, et
al: Rapid identification of the tumor-specific reactive TIL
Repertoire via combined detection of CD137, TNF, and IFNγ,
following recognition of autologous tumor-antigens. Front Immunol.
12:7054222021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trüb M, Uhlenbrock F, Claus C, Herzig P,
Thelen M, Karanikas V, Bacac M, Amann M, Albrecht R, Ferrara-Koller
C, et al: Fibroblast activation protein-targeted-4-1BB ligand
agonist amplifies effector functions of intratumoral T cells in
human cancer. J Immunother Cancer. 8:e0002382020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sano E, Kazaana A, Tadakuma H, Takei T,
Yoshimura S, Hanashima Y, Ozawa Y, Yoshino A, Suzuki Y and Ueda T:
Interleukin-6 sensitizes TNF-α and TRAIL/Apo2L dependent cell death
through upregulation of death receptors in human cancer cells.
Biochim Biophys Acta Mol Cell Res. 1868:1190372021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Faletti L, Peintner L, Neumann S, Sandler
S, Grabinger T, Mac Nelly S, Merfort I, Huang CH, Tschaharganeh D,
Kang TW, et al: TNFα sensitizes hepatocytes to FasL-induced
apoptosis by NFκB-mediated Fas upregulation. Cell Death Dis.
9:9092018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li XY, Li Z, An GJ, Liu S and Lai YD:
Co-expression of perforin and granzyme B genes induces apoptosis
and inhibits the tumorigenicity of laryngeal cancer cell line
Hep-2. Int J Clin Exp Pathol. 7:978–986. 2014.PubMed/NCBI
|
|
35
|
Shi L, Mai S, Israels S, Browne K, Trapani
JA and Greenberg AH: Granzyme B (GraB) autonomously crosses the
cell membrane and perforin initiates apoptosis and GraB nuclear
localization. J Exp Med. 185:855–866. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jacquemin G, Margiotta D, Kasahara A,
Bassoy EY, Walch M, Thiery J, Lieberman J and Martinvalet D:
Granzyme B-induced mitochondrial ROS are required for apoptosis.
Cell Death Differ. 22:862–874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinkoski MJ, Waterhouse NJ, Heibein JA,
Wolf BB, Kuwana T, Goldstein JC, Newmeyer DD, Bleackley RC and
Green DR: Granzyme B-mediated apoptosis proceeds predominantly
through a Bcl-2-inhibitable mitochondrial pathway. J Biol Chem.
276:12060–12067. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Upadhyay R, Boiarsky JA, Pantsulaia G,
Svensson-Arvelund J, Lin MJ, Wroblewska A, Bhalla S, Scholler N,
Bot A, Rossi JM, et al: A critical role for fas-mediated off-target
tumor killing in T-cell immunotherapy. Cancer Discov. 11:599–613.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martínez-Lostao L, Anel A and Pardo J: How
do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res.
21:5047–5056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Golstein P and Griffiths GM: An early
history of T cell-mediated cytotoxicity. Nat Rev Immunol.
18:527–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Montinaro A and Walczak H: Harnessing
TRAIL-induced cell death for cancer therapy: A long walk with
thrilling discoveries. Cell Death Differ. 30:237–249. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Coe GL, Redd PS, Paschall AV, Lu C, Gu L,
Cai H, Albers T, Lebedyeva IO and Liu K: Ceramide mediates
FasL-induced caspase 8 activation in colon carcinoma cells to
enhance FasL-induced cytotoxicity by tumor-specific cytotoxic T
lymphocytes. Sci Rep. 6:308162016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goedegebuure PS, Douville LM, Li H,
Richmond GC, Schoof DD, Scavone M and Eberlein TJ: Adoptive
immunotherapy with tumor-infiltrating lymphocytes and interleukin-2
in patients with metastatic malignant melanoma and renal cell
carcinoma: A pilot study. J Clin Oncol. 13:1939–1949. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Goff SL, Dudley ME, Citrin DE, Somerville
RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM,
Kammula US, et al: Randomized, prospective evaluation comparing
intensity of lymphodepletion before adoptive transfer of
tumor-infiltrating lymphocytes for patients with metastatic
melanoma. J Clin Oncol. 34:2389–2397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang H, Nie CP, Liu XF, Song B, Yue JH,
Xu JX, He J, Li K, Feng YL, Wan T, et al: Phase I study of adjuvant
immunotherapy with autologous tumor-infiltrating lymphocytes in
locally advanced cervical cancer. J Clin Invest. 132:e1577262022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chesney J, Lewis KD, Kluger H, Hamid O,
Whitman E, Thomas S, Wermke M, Cusnir M, Domingo-Musibay E, Phan
GQ, et al: Efficacy and safety of lifileucel, a one-time autologous
tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with
advanced melanoma after progression on immune checkpoint inhibitors
and targeted therapies: Pooled analysis of consecutive cohorts of
the C-144-01 study. J Immunother Cancer. 10:e0057552022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zacharakis N, Huq LM, Seitter SJ, Kim SP,
Gartner JJ, Sindiri S, Hill VK, Li YF, Paria BC, Ray S, et al:
Breast cancers are immunogenic: Immunologic analyses and a phase II
pilot clinical trial using mutation-reactive autologous
lymphocytes. J Clin Oncol. 40:1741–1754. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stevanović S, Helman SR, Wunderlich JR,
Langhan MM, Doran SL, Kwong MLM, Somerville RPT, Klebanoff CA,
Kammula US, Sherry RM, et al: A phase II study of
tumor-infiltrating lymphocyte therapy for human
papillomavirus-associated epithelial cancers. Clin Cancer Res.
25:1486–1493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rosenberg SA, Yang JC, Sherry RM, Kammula
US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF,
Wunderlich JR, et al: Durable complete responses in heavily
pretreated patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Queirolo P, Ponte M, Gipponi M, Cafiero F,
Peressini A, Semino C, Pietra G, Lionetto R, Vecchio S, Ribizzi I,
et al: Adoptive immunotherapy with tumor-infiltrating lymphocytes
and subcutaneous recombinant interleukin-2 plus interferon alfa-2a
for melanoma patients with nonresectable distant disease: A phase
I/II pilot trial. Melanoma Istituto Scientifico Tumori Group. Ann
Surg Oncol. 6:272–278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dudley ME, Gross CA, Langhan MM, Garcia
MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE,
et al: CD8+ enriched ‘young’ tumor infiltrating lymphocytes can
mediate regression of metastatic melanoma. Clin Cancer Res.
16:6122–6131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Figlin RA, Pierce WC, Kaboo R, Tso CL,
Moldawer N, Gitlitz B, deKernion J and Belldegrun A: Treatment of
metastatic renal cell carcinoma with nephrectomy, interleukin-2 and
cytokine-primed or CD8(+) selected tumor infiltrating lymphocytes
from primary tumor. J Urol. 158((3 Pt 1)): 740–745. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jansen CS, Prokhnevska N, Master VA, Sanda
MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R,
Sowalsky AG, et al: An intra-tumoral niche maintains and
differentiates stem-like CD8 T cells. Nature. 576:465–470. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saito H, Iwabuchi K, Fusaki N and Ito F:
Generation of induced pluripotent stem cells from human melanoma
tumor-infiltrating lymphocytes. J Vis Exp. 117:54372016.
|
|
55
|
Islam SMR, Maeda T, Tamaoki N, Good ML,
Kishton RJ, Paria BC, Yu Z, Bosch-Marce M, Bedanova NM, Liu C, et
al: Reprogramming of Tumor-reactive Tumor-infiltrating Lymphocytes
to Human-induced Pluripotent Stem Cells. Cancer Res Commun.
3:917–932. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bhadurihauck A, Li L, Li Q, Wang J and
Xiao Z: Transient exposure to proteins SOX2, Oct-4, and NANOG
immortalizes exhausted tumor-infiltrating CTLs. Biochem Biophys Res
Commun. 473:1255–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Teo YWB, Linn YC, Goh YT, Li S and Ho LP:
Tumor infiltrating lymphocytes from acute myeloid leukemia marrow
can be reverted to CD45RA+ central memory state by reactivation in
SIP (Simulated Infective Protocol). Immunobiology. 224:526–531.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vodnala SK, Eil R, Kishton RJ, Sukumar M,
Yamamoto TN, Ha NH, Lee PH, Shin M, Patel SJ, Yu Z, et al: T cell
stemness and dysfunction in tumors are triggered by a common
mechanism. Science. 363:eaau01352019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cheng H, Qiu Y, Xu Y, Chen L, Ma K, Tao M,
Frankiw L, Yin H, Xie E, Pan X, et al: Extracellular acidosis
restricts one-carbon metabolism and preserves T cell stemness. Nat
Metab. 5:314–330. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ye Q, Loisiou M, Levine BL, Suhoski MM,
Riley JL, June CH, Coukos G and Powell DJ Jr: Engineered artificial
antigen presenting cells facilitate direct and efficient expansion
of tumor infiltrating lymphocytes. J Transl Med. 9:1312011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Forget MA, Malu S, Liu H, Toth C, Maiti S,
Kale C, Haymaker C, Bernatchez C, Huls H, Wang E, et al: Activation
and propagation of tumor-infiltrating lymphocytes on clinical-grade
designer artificial antigen-presenting cells for adoptive
immunotherapy of melanoma. J Immunother. 37:448–460. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hsu C, Abad JD and Morgan RA:
Characterization of human T lymphocytes engineered to express
interleukin-15 and herpes simplex virus-thymidine kinase. J Surg
Res. 184:282–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heemskerk B, Liu K, Dudley ME, Johnson LA,
Kaiser A, Downey S, Zheng Z, Shelton TE, Matsuda K, Robbins PF, et
al: Adoptive cell therapy for patients with melanoma, using
tumor-infiltrating lymphocytes genetically engineered to secrete
interleukin-2. Hum Gene Ther. 19:496–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fix SM, Forget MA, Sakellariou-Thompson D,
Wang Y, Griffiths TM, Lee M, Haymaker CL, Dominguez AL, Basar R,
Reyes C, et al: CRISPR-mediated TGFBR2 knockout renders human
ovarian cancer tumor-infiltrating lymphocytes resistant to TGF-β
signaling. J Immunother Cancer. 10:e0037502022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Robbins PF, Morgan RA, Feldman SA, Yang
JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ,
Mackall CL, et al: Tumor regression in patients with metastatic
synovial cell sarcoma and melanoma using genetically engineered
lymphocytes reactive with NY-ESO-1. J Clin Oncol. 29:917–924. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation CAR T cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lee M, Li J, Li J, Fang S, Zhang J, Vo
ATT, Han W, Zeng H, Isgandarova S, Martinez-Moczygemba M, et al:
Tet2 inactivation enhances the antitumor activity of
tumor-infiltrating lymphocytes. Cancer Res. 81:1965–1976. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Palmer DC, Webber BR, Patel Y, Johnson MJ,
Kariya CM, Lahr WS, Parkhurst MR, Gartner JJ, Prickett TD, Lowery
FJ, et al: Internal checkpoint regulates T cell neoantigen
reactivity and susceptibility to PD1 blockade. Med. 3:682–704.e8.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schlabach MR, Lin S, Collester ZR,
Wrocklage C, Shenker S, Calnan C, Xu T, Gannon HS, Williams LJ,
Thompson F, et al: Rational design of a SOCS1-edited
tumor-infiltrating lymphocyte therapy using CRISPR/Cas9 screens. J
Clin Invest. 133:e1630962023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Feist M, Zhu Z, Dai E, Ma C, Liu Z, Giehl
E, Ravindranathan R, Kowalsky SJ, Obermajer N, Kammula US, et al:
Oncolytic virus promotes tumor-reactive infiltrating lymphocytes
for adoptive cell therapy. Cancer Gene Ther. 28:98–111. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Quixabeira DCA, Jirovec E, Pakola S,
Havunen R, Basnet S, Santos JM, Kudling TV, Clubb JHA, Haybout L,
Arias V, et al: Improving the cytotoxic response of
tumor-infiltrating lymphocytes towards advanced stage ovarian
cancer with an oncolytic adenovirus expressing a human vIL-2
cytokine. Cancer Gene Ther. 30:1543–1553. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Santos JM, Heiniö C, Cervera-Carrascon V,
Quixabeira DCA, Siurala M, Havunen R, Butzow R, Zafar S, de Gruijl
T, Lassus H, et al: Oncolytic adenovirus shapes the ovarian tumor
microenvironment for potent tumor-infiltrating lymphocyte tumor
reactivity. J Immunother Cancer. 8:e0001882020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Santos J, Heiniö C, Quixabeira D, Zafar S,
Clubb J, Pakola S, Cervera-Carrascon V, Havunen R, Kanerva A and
Hemminki A: Systemic delivery of oncolytic adenovirus to tumors
using tumor-infiltrating lymphocytes as carriers. Cells.
10:9782021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kudling TV, Clubb JHA, Quixabeira DCA,
Santos JM, Havunen R, Kononov A, Heiniö C, Cervera-Carrascon V,
Pakola S, Basnet S, et al: Local delivery of interleukin 7 with an
oncolytic adenovirus activates tumor-infiltrating lymphocytes and
causes tumor regression. Oncoimmunology. 11:20965722022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khammari A, Nguyen JM, Saint-Jean M, Knol
AC, Pandolfino MC, Quereux G, Brocard A, Peuvrel L, Saiagh S,
Bataille V, et al: Adoptive T cell therapy combined with
intralesional administrations of TG1042 (adenovirus expressing
interferon-γ) in metastatic melanoma patients. Cancer Immunol
Immunother. 64:805–815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ye K, Li F, Wang R, Cen T, Liu S, Zhao Z,
Li R, Xu L, Zhang G, Xu Z, et al: An armed oncolytic virus enhances
the efficacy of tumor-infiltrating lymphocyte therapy by converting
tumors to artificial antigen-presenting cells in situ. Mol Ther.
30:3658–3676. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang H, Berezowska S, Dorn P, Zens P, Chen
P, Peng RW, Marti TM, Kocher GJ, Schmid RA and Hall SRR:
Tumor-infiltrating lymphocytes are functionally inactivated by
CD90+ stromal cells and reactivated by combined Ibrutinib and
Rapamycin in human pleural mesothelioma. Theranostics. 12:167–185.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mullinax JE, Hall M, Prabhakaran S, Weber
J, Khushalani N, Eroglu Z, Brohl AS, Markowitz J, Royster E,
Richards A, et al: Combination of ipilimumab and adoptive cell
therapy with tumor-infiltrating lymphocytes for patients with
metastatic melanoma. Front Oncol. 8:442018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Oberg HH, Janitschke L, Sulaj V, Weimer J,
Gonnermann D, Hedemann N, Arnold N, Kabelitz D, Peipp M,
Bauerschlag D and Wesch D: Bispecific antibodies enhance
tumor-infiltrating T cell cytotoxicity against autologous
HER-2-expressing high-grade ovarian tumors. J Leukoc Biol.
107:1081–1095. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li Y, Cong Y, Jia M, He Q, Zhong H, Zhao
Y, Li H, Yan M, You J, Liu J, et al: Targeting IL-21 to
tumor-reactive T cells enhances memory T cell responses and
anti-PD-1 antibody therapy. Nat Commun. 12:9512021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kazemi MH, Shokrollahi Barough M,
Momeni-Varposhti Z, Ghanavatinejad A, Zarehzadeh Mehrabadi A,
Sadeghi B and Falak R: Pentoxifylline changes the balance of immune
cell population in breast tumor-infiltrating lymphocytes. Med
Oncol. 40:1682023. View Article : Google Scholar : PubMed/NCBI
|