Introduction
Breast cancer stands out as the most prevalent
malignant tumor affecting women worldwide, with its incidence and
fatality rates escalating annually, as indicated by the 2020 Global
Cancer Report from the International Agency for Research on Cancer
of the World Health Organization (1). Recent global statistics revealed a
staggering 2.26 million new cases of breast cancer, surpassing lung
cancer for the first time to claim the top spot in worldwide
incidence (2). Projections suggest
a continued rise in incidence over the coming decades. Metastatic
spread of cancer cells remains the primary cause of death in breast
cancer cases (3). Triple-negative
breast cancer (TNBC), characterized by the absence of progesterone
receptor, estrogen receptor and human epidermal growth factor
receptor 2, represents a subtype associated with heightened
metastasis, invasiveness and grim prognosis, constituting ~10–15%
of all breast cancer cases (4).
Notably, patients with distant metastases exhibit a dismal 5-year
survival rate of merely 11.2% compared with non-triple-negative
types (5). Chemotherapy remains the
cornerstone of TNBC treatment due to its extensive heterogeneity
and the absence of well-defined molecular targets (4).
Cancer stem-like cells (CSCs) comprise a small
subset of malignant tumor cells possessing stem cell-like
properties. Within TNBCs, there exists a notable enrichment of
breast CSCs (BCSCs), characterized by their capacity for
self-renewal, differentiation and generation of rapidly
proliferating tumor cells, thus driving tumor progression and
metastasis (6). Consequently, BCSCs
are recognized as pivotal contributors to TNBC recurrence and
metastasis (7). However,
conventional chemotherapeutic agents such as Paclitaxel (PTX) often
fail to eradicate BCSCs, thereby increasing the risk of recurrence
(8). Therefore, there is an urgent
need for novel agents targeting BCSCs to enhance the prognosis of
patients with breast cancer.
Ferroptosis, a type of programmed cell death
distinct from apoptosis and necrosis, was defined in 2012 (9). It is characterized by the accumulation
of lipid peroxidation in the cell membrane and high levels of
intracellular reactive oxygen species (ROS). This process involves
four key biological processes: Glutathione (GSH) metabolism, iron
metabolism, lipid metabolism and oxidative stress (10). Iron metabolism plays a critical role
in maintaining the characteristics of CSCs (11). Ferric ions (Fe3+) bind to
transferrin (TF) or TF receptor 1 (TFR1) at the cell surface and
are endocytosed into cells. In endosomes, Fe3+ is
released and reduced to ferrous iron (Fe2+). In the
cytosol, Fe2+ ions constitute a labile intermediate
pool. Free iron is stored in ferritin heavy chain 1 (FTH1) or
exported by ferroportin (FPN). Ferric iron promotes cancer cell
growth by activating the ribonucleotide reductase enzyme, while
ferrous iron enhances lipoxygenase, promoting ferroptosis (12). CSCs predominantly store iron in the
ferric state (ferritin), limiting the availability of ferrous iron
and inhibiting ferroptosis (12).
In breast cancer, CSCs exhibit higher expression levels of TFR1 and
cellular iron compared with non-CSCs (13). Iron chelation abolishes the
expression of CSC surface markers and stemness characteristics
(14). Nuclear factor
erythroid-related factor 2 (NRF2) is an oxidative-stress-responsive
transcription factor that reduces ferroptosis by stimulating the
expression of ferroptosis-suppressing genes, such as glutathione
peroxidase 4 (GPX4), solute carrier family 7 member 11 (SLC7A11)
and ferroptosis suppressor protein 1 (FSP1) (15). Under oxidative conditions, NRF2 is
released from Kelch-like ECH-associated protein 1 (KEAP1) and
translocated to the nucleus.
The exploration of targeted activation of
ferroptosis as a means of treating CSCs holds promise in cancer
therapy (16). Ursolic acid (UA) is
a natural pentacyclic triterpenoid compound with diverse
pharmacological activities against various cancer types (17). Recent studies have shown that UA can
inhibit the growth of TNBC and has effects on metastasis and drug
resistance (18,19). Additionally, UA has been found to
inhibit the stemness characteristics of BCSCs (20). A recent study demonstrated that UA,
when combined with sorafenib, can inhibit cancer cell growth by
inducing apoptosis or SLC7A11-dependent ferroptosis (21). However, the precise effects of UA on
the regulation of ferroptosis and its potential for targeting BCSCs
to inhibit tumor growth remain largely unknown.
In the present study, spheroids of TNBC cells were
treated and mice were xenografted with UA to explore the effects of
UA on BCSCs and its underline mechanism.
Materials and methods
Chemicals and reagents
UA (cat. no. U6753) and deferoxamine (DFO; cat. no.
D9533) were procured from Sigma-Aldrich; Merck KGaA. Z-VAD-FMK
(ZVAD; cat. no. S7023) and ferrostatin-1 (fer; cat. no. S7243) were
obtained from Selleck Chemicals. Chloroquine (CQ; cat. no.
HY-17589A) and PTX (cat. no. HY-B0015) were purchased from
MedChemExpress.
Cell culture
MDA-MB-231 and BT-549 cell lines were sourced from
the Shanghai Institute of Biotechnology, Chinese Academy of
Science. Cell line expansion and cryopreservation followed the
guidelines of the American Pharmacopoeia Commission, with periodic
mycobacterial inspection. MDA-MB-231 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) (HyClone; Cytiva), while
BT-549 cells were cultured in Roswell Park Memorial Institute-1640
media (HyClone; Cytiva). Both cell lines were supplemented with 10%
fetal bovine serum (Bioexplorer, Inc.) and maintained in a humid
environment with 5% CO2 at 37°C. Subsequently, cells
were kept under stem cell conditions using serum-free DMEM/F12
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10 ng/ml
human recombinant epidermal growth factor (EGF) (Invitrogen; Thermo
Fisher Scientific, Inc.). Adherent cells of MDA-MB-231 and BT-549
were seeded into ultra-low attachment 6-well plates (Corning, Inc.)
to form non-adherent spheroids at a density of 8×104
cells/well, with medium replacement every four days.
Fluorescence-activated cell sorter
analysis for CD44/CD24 cells
Non-adherent spheroids were stained with CD44-APC
(cat. no. 397517; BioLegend, Inc.), CD24-FITC (cat. no. 311117;
BioLegend, Inc.) and respective isotype controls. After a 40-min
incubation in the dark, cells were detected using the NovoCyte flow
cytometer (ACEA Bioscience, Inc.) and analyzed by NovoExpress 1.5.8
software (Agilent Technologies, Inc.).
CD44+/CD24−
cells isolation
The manufacturer's protocol for the CD24 Microbead
Kit (cat. no. 130-095-951; Miltenyi Biotec GmbH) was adhered to for
the cultivation of 3×107 MDA-MB-231 and BT-549
mammospheres using a primary antibody against CD24. Subsequently,
the labeled cells were incubated with IgG microbeads (Miltenyi
Biotec GmbH) and underwent magnetic separation using MiniMACS
columns (Miltenyi Biotec GmbH). The purified CD24− cells
were then incubated with CD44 microbeads (cat. no. 130-095-194;
Miltenyi Biotec GmbH), washed and subjected to magnetic separation
once more.
Cell viability assay
Cellular proliferation was monitored according to
the manufacturer's protocol using an optical microscope and
quantitatively assessed via the Cell Counting Kit-8 (CCK-8) assay
(Chongqing Baoguang Biotech Co., Ltd.; http://www.bgbiotech.com/) across various experimental
groups. MDA-MB-231 and BT-549 adherent cells and spheroids were
seeded into 96-well microplates or ultra-low attachment 96-well
plates at a density of 1×104 cells per well. After
incubation at 37°C for 24 h, cells were treated with varying
concentrations of UA or PTX (0, 10, 20, 40 µM) for 24 h,
respectively. Subsequently, 10 µl of CCK-8 reagent was added per
well, and the plates were incubated for 2 h. Cell viability was
determined using the formula: Cell viability
(%)=[(As-Ab)/(Ac-Ab)]x100%, where Ab, As, and Ac respectively
denote the absorbance values at 450 nm wavelength for the blank
medium, experimental group and control group.
Aldehyde dehydrogenase (ALDH) activity
assay
The ALDH assay kit (cat. no. MAK082; Sigma-Aldrich;
Merck KGaA) was employed for assessing ALDH activity, using
1×106 cells per group. The colorimetric product measured
at 450 nm was directly proportional to the ALDH activity present.
The experiments were repeated at least three times.
ROS assay
The ROS assay kit (cat. no. S0033S; Beyotime
Institute of Biotechnology) was utilized. Cells were seeded at a
density of 5×104 cells per well in either a standard
24-well plate or an ultra-low attachment 24-well plate.
Intracellular ROS were measured using the DCFH-DA probe. Images of
randomly selected fields were captured using a fluorescence
microscope. The experiments were repeated at least three times.
GSH and malondialdehyde (MDA)
assay
The GSH (cat. no. KTB1600) and MDA (cat. no.
KTB1050) assay kits were obtained from Abbkine Scientific Co., Ltd.
The relative contents of MDA and GSH were detected following the
manufacturer's recommended protocol. Each group prepared
1×106 cells. The GSH and MDA levels of each group were
determined by spectrophotometer detection at 412, 532 and 600 nm.
The experiments were repeated at least three times.
Iron assay
The iron assay kit (cat. no. ab83366) was supplied
by Abcam. In a physiological context, Fe2+ reacts with
the iron probe to form a stable colored complex exhibiting
absorbance at 593 nm. Additionally, Fe3+ can be reduced
to Fe2+, enabling the accurate measurement of total iron
(both Fe2+ and Fe3+) by adding iron reducer.
The concentration of Fe3+ was determined by subtracting
the Fe2+ content from the total iron level. To assess
the iron content, 2×106 cells were collected from each
experimental group. The experiments were repeated at least three
times.
Western blotting (WB)
Primary antibodies against CD44 (1:1,000; cat. no.
ab189524), CD24 (1:1,000; cat. no. ab179821), TFR1 (1:1,000; cat.
no. ab214039), KEAP1 (1:1,000; cat. no. 10503-2-ap) and LaminB1
(1:1,000; cat. no. 10503-2-ap) were supplied by Abcam, while the
phosphorylated (p-) NRF2 (1:1,000; cat. no. ap1133) antibody was
supplied by ABclonal Biotech Co., Ltd. and NRF2 (1:1,000; cat. no.
12721s) was bought from Cell Signaling Technology, Inc. Total
protein was extracted from cells or tissues of each sample group
using WB and IP cell lysates. Quantification was achieved using the
BCA Protein Assay kit (cat. no. 23227; Thermo Fisher Scientific,
Inc.). Subsequently, protein samples (20 micrograms per well) were
separated by SDS-PAGE on a 10% gel and transferred onto PVDF
membranes via electrophoresis. The membranes were blocked with
blocking buffer (Vazyme Biotech Co., Ltd.) for 30 min at room
temperature, followed by overnight incubation with primary
antibodies at 4°C. The next day, the membranes were incubated with
HRP-conjugated anti-rabbit secondary antibodies (1:10,000; cat. no.
ab6721; Abcam) for 1 h at room temperature. The target protein was
then detected by exposure to an ECL luminescent solution (Shanghai
Yeasen Biotechnology Co., Ltd.). Quantitative protein analysis was
performed using ImageJ 1.8.0 software (National Institutes of
Health).
Preparation of protein
extractions
The Nuclear Extract Kit (cat. no. 40010) was
purchased from Active Motif, Inc. MDA-MB-231 spheroids were plated
in an ultra-low attachment dish (100 mm) at a density of
5×106 cells/well and incubated for 4 h. The cells were
then treated with 20 µM UA, 2 µM fer, or 10 µM DFO for 48 h. After
washing the cells twice with ice-cold PBS containing phosphatase
inhibitors, the cells were harvested by scraping the wells. The
harvested suspension was centrifuged at 200 × g at 4°C for 5 min.
To extract nuclear protein, the cell pellets were initially treated
with hypotonic buffer for 15 min and then centrifuged at 14,000 × g
at 4°C for 30 sec. The supernatants, representing the cytosolic
fractions, were discarded. The pellets, designated as nuclear
fractions, were lysed again in Complete Lysis Buffer (Active Motif,
Inc.). The cell lysates were vortexed every 5 min for a total of 30
min and then centrifuged at 14,000 × g at 4°C for 10 min. The
supernatants were isolated and collected as the nuclear protein
extract.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from MDA-MB-231 and BT-549
cells, as well as their respective sphere cells, using the Tissue
RNA Miniprep Kit (cat. no. BW-R6311; Biomiga, Inc.). According to
the manufacturer's instructions, a total of 500 ng of total RNA
from each sample was used for the reverse transcription reaction
using the HiScript III RT SuperMix (+gDNA wiper) (cat. no. R323-01;
Vazyme Biotech Co., Ltd.). qPCR was performed on cDNA templates
using ChamQ SYBR Color qPCR Master Mix (cat. no. Q431-02; Vazyme
Biotech Co., Ltd.) on a realplex RT-qPCR system (Eppendorf SE). All
qPCR reactions were conducted in a total volume of 10 µl. The qPCR
protocol started with an initial denaturation step at 95°C for 5
min, followed by 40 amplification cycles, each consisting of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, and
extension at 72°C for 30 sec. The primer sequences used for the
analysis of each gene are comprehensively listed in Table SI.
Mammosphere formation
Single cell suspensions of MDA-MB-231 and BT-549
spheroids were prepared by pipetting. In DMEM/F-12 medium
supplemented with 20 ng/ml EGF and 10% B27, the single cell
suspensions were cultured in ultra-low adhesion 6-well plates at a
density of 2×104 cells per well to generate mammospheres
for 7 days. A total of 3–5 randomly selected fields of view per
well were counted, and floating aggregates >50 µm in diameter
were chosen as mammospheres for further analysis. The total
mammosphere area was used for statistical analysis.
In vivo xenograft experiments
Female Balb/C mice, aged 5 weeks and weighing
between 16–18 grams, were obtained from Shanghai Jihui Laboratory
Animal Care Co., Ltd. The mice were housed in specific
pathogen-free conditions with 24–26°C temperature, 50–60% humidity
and 12/12-h light/dark cycle at the Animal Resource Center of the
Shanghai University of Medicine and Health Sciences, where they
were provided with autoclaved water and food ad libitum. The
efficacy of UA in vivo was assessed using an MDA-MB-231
spheroid xenograft model. Animals underwent a one-week
acclimatization period before being used in experiments.
Dissociated MDA-MB-231 spheroids (5×106) were
resuspended in 100 µl PBS and injected subcutaneously into the
right flank of mice. Engrafted mice were monitored for tumor
development through visual inspection until tumor formation
occurred. On the 14th day post-injection, the mice were randomly
assigned to 7 treatment groups, with 5 mice per group. Mice
received intraperitoneal injections of 30 µl 10% dimethyl sulfoxide
(DMSO) (22–24), which was freshly diluted in a saline
containing 0.9% NaCl, containing UA (20 mg/kg body weight, daily)
(25), or PTX (20 mg/kg body
weight, daily) (26), or together
with fer (5 mg/kg body weight, daily) (27) or DFO (100 mg/kg body weight, daily)
(28) for 30 days. Body weight,
tumor mass and the health indexes of mice (strong appetite, bright
eyes, quick reaction, power of muscles) were assessed every 5 days.
Tumor volume was measured using Vernier calipers and calculated
using the following formula: Width2 × length × π/6.
According to the Laboratory animal Guidelines for euthanasia in
China, the criteria used to determine when animals should be
euthanized included: i) The maximum tumor diameter approaching 15
mm; ii) loss of weight >20%; iii) difficulty to move or breath.
When the maximum tumor diameter approached 15 mm (the 30th day of
treatment), mice were administered 2.5% isoflurane for induction of
anesthesia by inhalation for 3 min and then 1.5% isoflurane for
maintenance of anesthesia before being euthanized. Mice were
euthanized by cervical dislocation under anesthesia to ensure
humane conditions for the study. The mice were observed to have no
response, including limb paralysis and no rise and fall of the
chest to confirm death. The duration of the in vivo
experiment was 44 days totally, including 14 days for tumor cells
to proliferate in vivo and 30 days for the treatment with
individual drug. Totally, 35 mice were used in the present study
with no mice succumbing before the end of study.
Immunohistochemistry (IHC)
staining
The expression of KEAP1, NRF2 and TFR1 in cancer
tissues was assessed using IHC staining. The tumors were fixed with
4% paraformaldehyde overnight at 4°C and then embedded in paraffin.
Paraffin sections (4–6 µm) were heated in an oven at 60°C for 60
min and then dehydrated in xylene, followed by a series of ethanol
washes (anhydrous ethanol, 95, 85 and 75% ethanol). Subsequently,
the slices underwent three consecutive washes with PBS. Antigen
retrieval was performed in sodium citrate buffer solution at 120°C
for 15 min. After blocking with goat serum (cat. no. 16210064;
Gibco; Thermo Fisher Scientific, Inc.) for 10 min at room
temperature, the sections were incubated with primary antibodies
against KEAP1 (1:500), NRF2 (1:500) and TFR1 (1:350) overnight at
4°C. The following day, the slices were washed with PBS and then
incubated with biotin-labeled goat anti-rabbit antibody (1:1,000;
cat. no. R-21234; Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C for 1 h. DAB solution was applied to stain the samples,
followed by counterstaining with hematoxylin at room temperature
for 3 min. Finally, images were captured using a light microscope
and quantitatively analyzed by IHC mean optical density (MOD)
values. MOD value=(staining intensity × staining area)/total tissue
area.
Statistical analysis
All data were presented as the mean ± SD from three
independent experimental replicates. Comparison between two groups
was performed using unpaired t-test. Statistical analysis of
multiple groups was performed using one-way analysis of variance
with Tukey's post hoc test using GraphPad Prism 9.0 (GraphPad
Software; Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
BCSCs exhibit less lipid
peroxidation
BCSCs, enriched through the formation of
non-adherent spheroids, exhibit characteristics of CSCs (29). CD44+/CD24−
cells and ALDHhigh cells are widely recognized as BCSCs
(30). In the present study, BCSCs
were collected from MDA-MB-231 and BT-549 cells within non-adherent
spheroids, showing higher CD44 expression and lower CD24 expression
compared with adherent cells, as detected by flow cytometric assay
(Fig. 1A) and WB (Fig. 1B). ALDH activity was significantly
elevated in spheroids compared with adherent cells (Fig. 1C). Moreover, the expression levels
of putative stemness-associated markers CD133, EpCAM and
transcription factors OCT4, Sox2 and c-myc were increased in
spheroids (Fig. 1D). To further
purify the CSCs, CD44+/CD24− cells were
isolated from MDA-MB-231 and BT-549 mammospheres using
magnetic-activated cell sorting (Fig.
S1). The intracellular ROS levels were slightly increased in
spheroids and CD44+/CD24− CSCs with no
significant difference compared with adherent cells (Fig. 1E). Additionally, the amount of GSH,
an intracellular antioxidant, was significantly higher in spheroids
and CD44+/CD24− CSCs than in adherent cells
(Fig. 1F). However, lipid
peroxidation levels were increased in
CD44+/CD24− CSCs compared with adherent
cells, as evidenced by the amount of MDA in these cells (Fig. 1G). These findings suggested that
BCSCs may possess a protective mechanism against lipid
peroxidation.
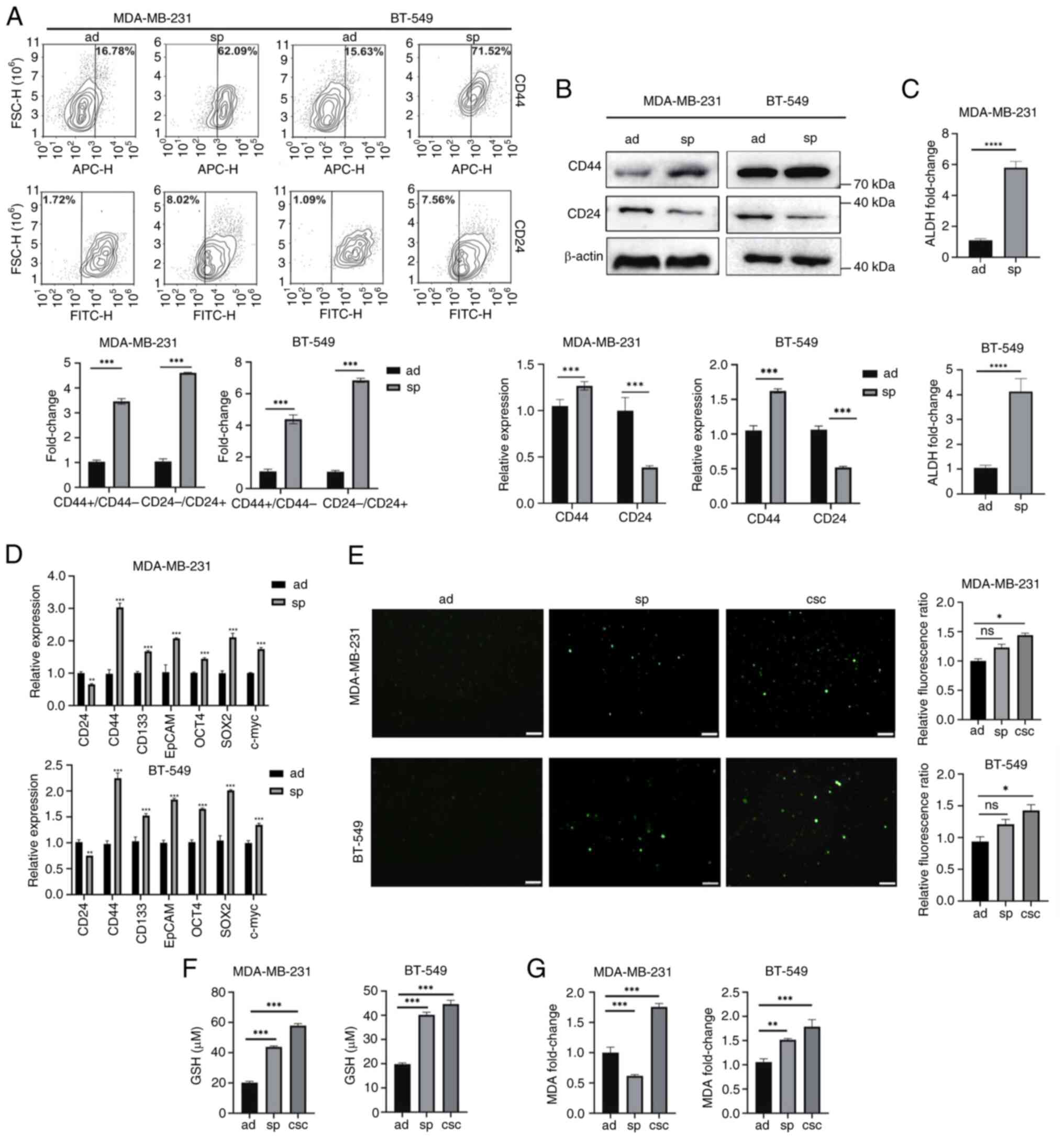 | Figure 1.Breast cancer stem-like cells exhibit
stemness characteristics and less lipid peroxidation. (A)
MDA-MB-231 and BT-549 cells cultured in sp or ad cells were
analyzed for CD44 and CD24 expression using flow cytometry. (B)
Protein expression levels of CD24 and CD44 in ad cells and sp were
detected by western blotting. All protein expression data were
normalized relative to β-actin as a loading control. (C) Detection
of ALDH enzyme activity in ad cells and sp. (D) The mRNA level of
stemness-related genes in ad cells and sp were analyzed by reverse
transcription-quantitative PCR. (E-G) Relative levels of reactive
oxygen species, GSH and MDA in ad cells, sp and
CD44+/CD24− CSCs were measured by commercial
assay kits. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001. Scale bar, 100 µm. sp, spheroids; ad, adherent;
ALDH, aldehyde dehydrogenase; GSH, glutathione; MDA,
malondialdehyde; CSCs, cancer stem cells; ns, not significant
(P>0.05). |
BCSCs undergo less ferroptosis
Given that lipid peroxidation leads to ferroptosis,
an iron-dependent form of cell death, several key regulators of
ferroptosis were examined. Inhibitors of ferroptosis such as
SLC7A11, GPX4 and NRF2 were significantly upregulated in spheroids
and CSCs compared with adherent cells (Fig. 2A), indicating that BCSCs undergo
less ferroptosis. Importantly, spheroids and CSCs exhibited
enhanced iron uptake with higher TFR1 expression, increased iron
storage with higher FTH1 expression, and reduced iron export with
lower FPN expression (Fig. 2A). The
expression of TFR1 was confirmed by WB (Fig. 2B). Indeed, spheroids and CSCs showed
evident iron accumulation in ferric form (Fig. 2C). Conversely, ferrous iron, an
activator of ferroptosis, was significantly lower in spheroids and
CSCs compared with adherent cells (Fig.
2C). Thus, it was indicated that BCSCs might employ a
protective mechanism against ferroptosis to maintain their stemness
characteristics in an iron homeostasis-related manner.
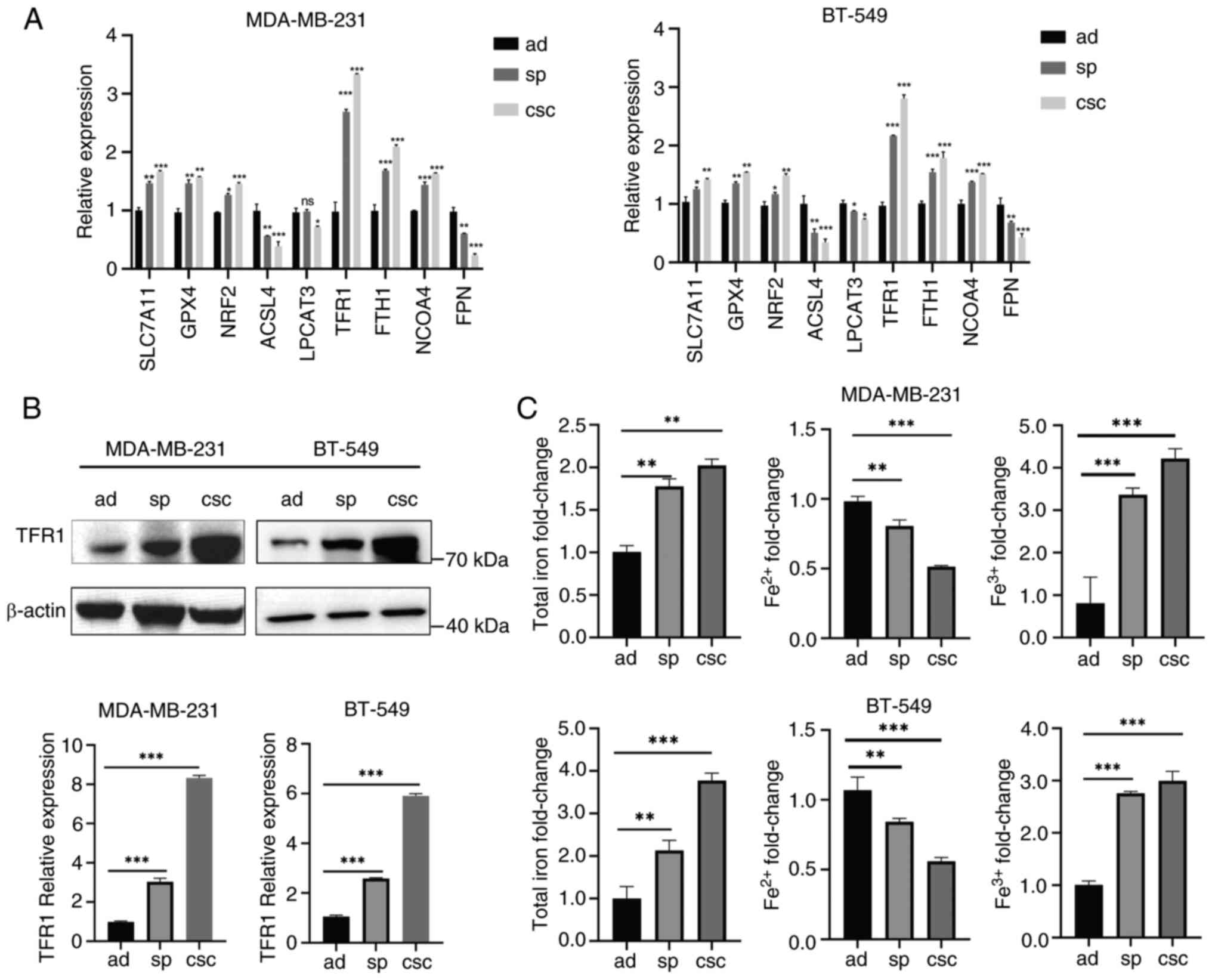 | Figure 2.Breast cancer stem-like cells undergo
less ferroptosis. (A) The mRNA levels of genes involved in
ferroptosis were measured in MDA-MB-231 and BT-549 ad cells, sp and
CD44+/CD24− CSCs by reverse
transcription-quantitative PCR. (B) Protein expression levels of
TFR1 in MDA-MB-231 and BT-549 ad cells, sp and CSCs were detected
by western blotting. (C) The contents of total iron,
Fe2+, and Fe3+ in MDA-MB-231 and BT-549 ad
cells, sp and CD44+/CD24− CSCs were measured
by commercial assay kits. *P<0.05, **P<0.01 and
***P<0.001. ad, adherent; sp, spheroids; CSCs, cancer stem
cells; TFR1, transferrin receptor 1; SLC7A11, solute carrier family
7 member 11; GPX4, glutathione peroxidase 4; NRF2, nuclear factor
erythroid-related factor 2; NCOA4, nuclear receptor coactivator 4;
FPN, ferroportin; ns, not significant (P>0.05). |
UA inhibits breast cancer stem-like
cell stemness characteristics and proliferation through inducing
ferroptosis
Since BCSCs exhibit resistance to traditional
chemotherapy drugs such as PTX, there is an urgent need to develop
novel drugs targeting BCSCs. UA has gained attention as a potent
anticancer agent in various cancers through inducing apoptosis or
ferroptosis (27,31). However, whether UA could target CSCs
by overcoming ferroptosis resistance remains unclear. In the
present study, it was found that PTX and UA had no significant
difference in inhibiting cell proliferation in adherent cells
(Fig. 3A). However, breast cancer
cell spheroids were more sensitive to UA-induced cell death than to
PTX-induced cell death (Fig. 3A),
indicating that UA is more effective than PTX in inducing cell
death in BCSCs. Since the IC50 of UA on MDA-MB-231 and
BT-549 spheroids was 25.8 and 18.6 µM, respectively (Fig. S2A and B), 20 µM of UA were then
used for the treatment of breast cancer cells. To elucidate the
mechanisms by which UA induces cell death in BCSCs, several cell
death inhibitors were added. As demonstrated in Fig. 3B, the caspase inhibitor ZVAD failed
to reverse UA-induced cell death in BCSCs. The autophagy inhibitor
CQ efficiently reversed UA-induced cell death in BCSCs.
Importantly, ferroptosis inhibitors fer (which blocks lipid
peroxidation) and DFO (an iron chelator) significantly reversed
UA-induced cell death in BCSCs. Thus, UA might induce cell death in
BCSCs mainly through ferroptosis. Furthermore, mammosphere
formation, a typical property of CSCs in vitro, was
investigated upon UA treatment. UA significantly reduced the sphere
formation ability of BCSCs (Fig.
3C). Fer and DFO significantly reversed the inhibitory effect
of UA. The expression of stemness markers, CD44 and CD24, was also
analyzed in BCSCs upon UA treatment. UA treatment led to a decrease
in CD44 and an increase in CD24 in BCSCs, which could be reversed
by fer and DFO (Fig. 3D). The
stemness regulators OCT4, SOX2 and c-myc were downregulated by UA,
which could be partly reversed by fer (Fig. 3E). These data indicated that UA
efficiently inhibits stemness characteristics and proliferation of
BCSCs through ferroptosis-related cell death.
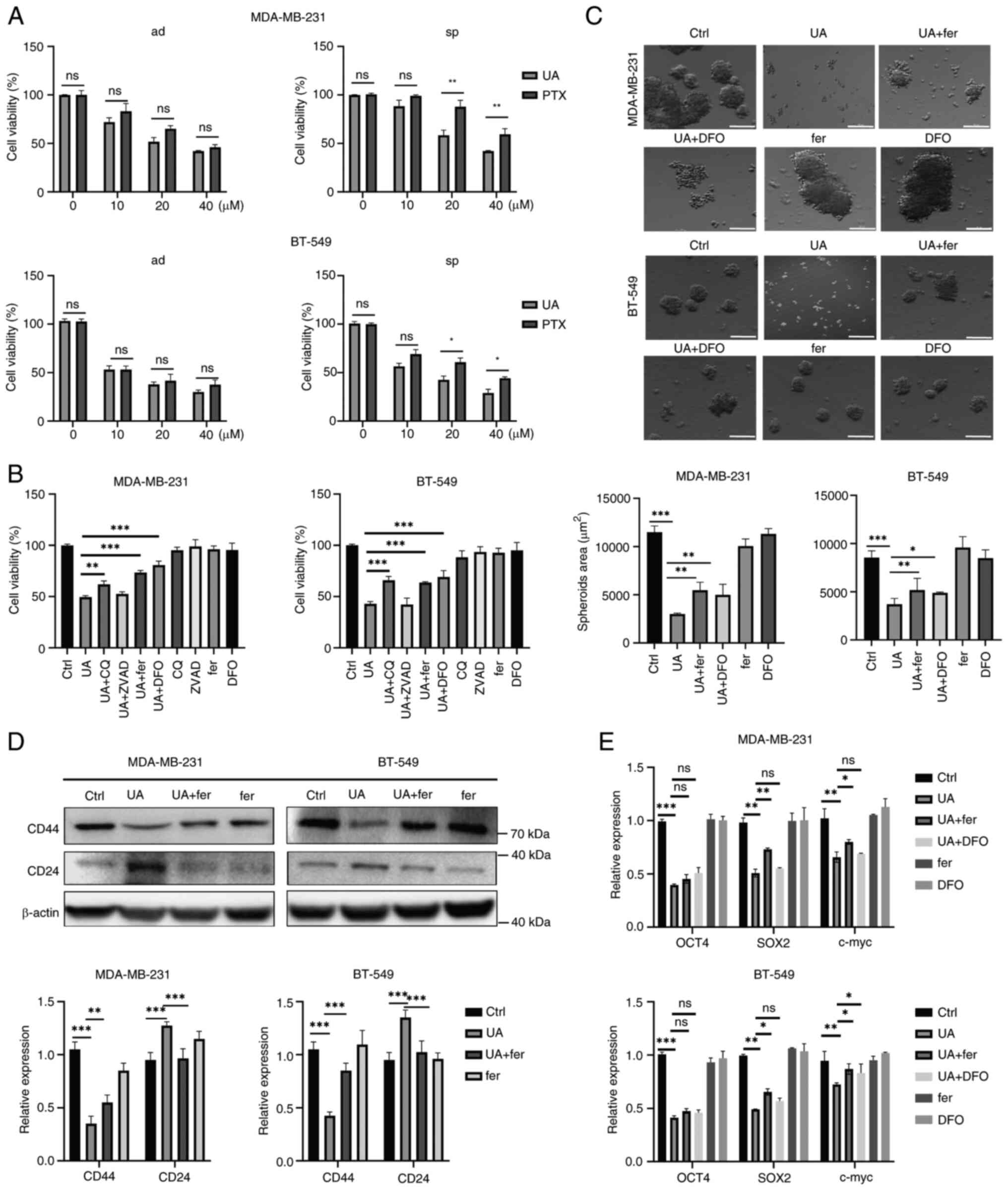 | Figure 3.UA inhibits breast cancer stem-like
cells stemness characteristics and proliferation through inducing
ferroptosis. (A) Effect of UA or PTX on the cellular proliferation
of MDA-MB-231 and BT-549 ad cells and sp using Cell Counting Kit-8
assay. Cells were treated for 24 h at varying concentrations (0,
10, 20, 40 µM). (B) Effects of different death pathway inhibitors
on MDA-MB-231 and BT-549 sp. Cells were treated with UA (20 µM),
ZVAD (10 µM), CQ (10 µM), fer (2 µM) or DFO (10 µM), respectively,
or with combination as indicated for 24 h. (C) Representative
images of mammospheres treated by UA (20 µM) with or without fer (2
µM) or DFO (10 µM) in sp for 24 h. (D) Protein expression levels of
CD24 and CD44 in sp were detected by western blotting after the
treatment of UA and fer for 24 h. (E) The mRNA levels of
stemness-related genes in sp were analyzed by reverse
transcription-quantitative PCR after the treatment of UA and fer
for 24 h. *P<0.05, **P<0.01 and ***P<0.001. Scale bar, 100
µm. UA, ursolic acid; PTX, paclitaxel; ad, adherent; sp, spheroids;
ZVAD, ZVAD, Z-VAD-FMK; CQ, chloroquine; DFO, deferoxamine; fer,
ferrostatin-1; ns, not significant (P>0.05). |
UA induces ferrous iron and ROS
accumulation in BCSCs
To further confirm ferroptosis induced by UA in
BCSCs, intracellular ROS levels, lipid peroxidation levels and the
amount of iron were detected. UA treatment led to a prominent
increase in intracellular ROS in BCSCs (Fig. 4A). The UA-induced ROS eruption in
BCSCs was significantly attenuated by fer and DFO (Fig. 4A). Lipid peroxidation levels, as
indicated by the amount of MDA, were significantly enhanced in
UA-treated BCSCs, which were alleviated by fer and DFO (Fig. 4B). Intracellular total iron and
ferrous iron were significantly increased by UA treatment, and this
increase was efficiently blocked by fer and DFO (Fig. 4C). Several key regulators of iron
metabolism were therefore examined, including TFR1 for ferric iron
uptake, FTH1 for ferrous iron storage, nuclear receptor coactivator
4 (NCOA4) for releasing ferrous iron in the lysosome, and FPN for
iron export. The expression of TFR1, which was upregulated in
BCSCs, was significantly decreased upon UA treatment as detected by
RT-qPCR and WB (Fig. 4D and E). Fer
and DFO efficiently reversed UA-induced TFR1 downregulation,
indicating that TFR1 expression is mainly regulated by
intracellular iron concentration (Fig.
4D). Consistent with the increase in ferrous iron in UA-treated
BCSCs (Fig. 4C), FTH1 and NCOA4
were significantly enhanced upon UA treatment, and this was
reversed by fer and DFO (Fig. 4D).
FPN also increased prominently upon UA treatment and this was
reversed by fer and DFO (Fig. 4D).
Thus, UA-induced ferrous iron accumulation might be related to
ferritin formation and degradation. These data indicated that UA
might induce ferroptosis through stimulating ferrous iron and ROS
accumulation in BCSCs.
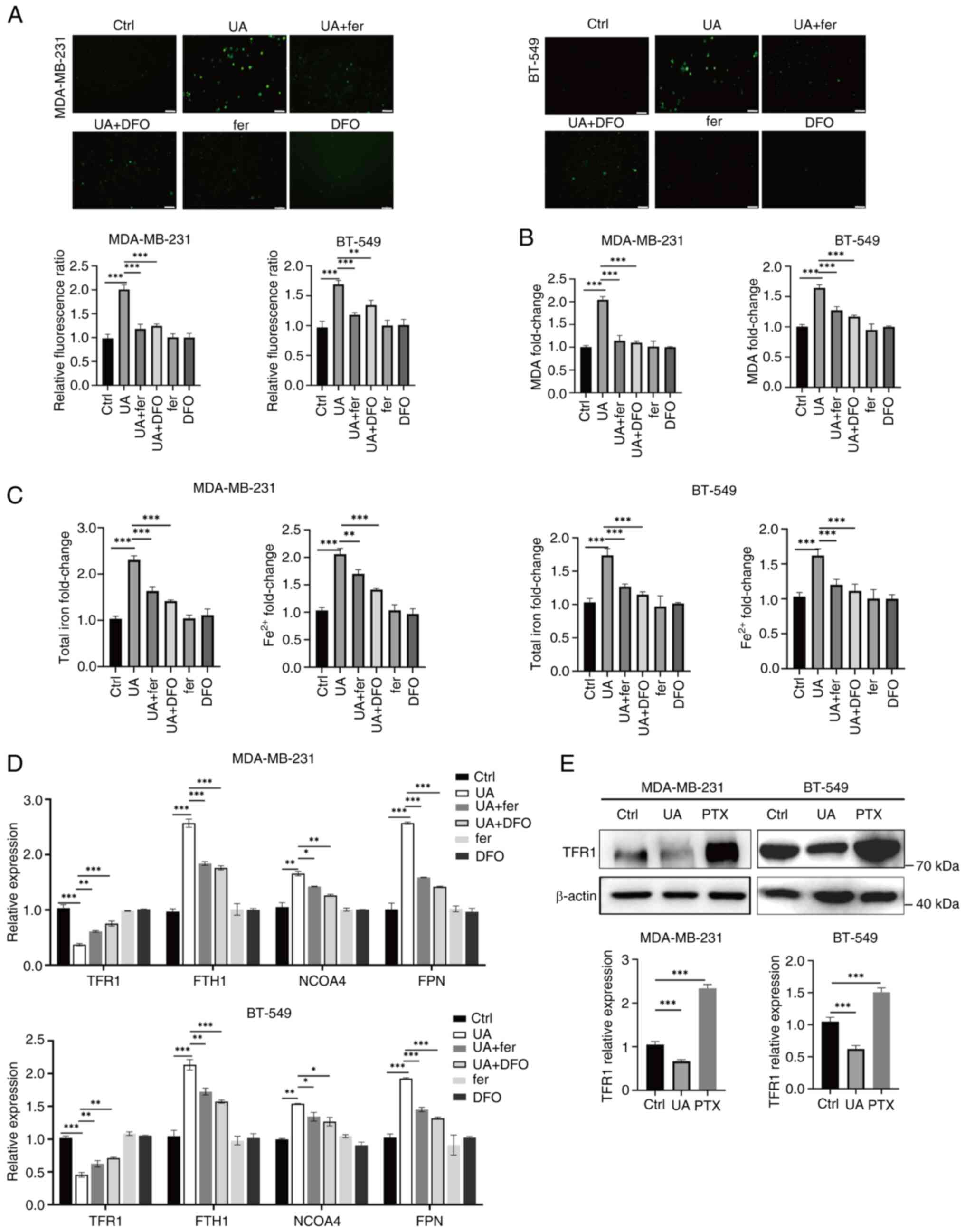 | Figure 4.UA induces ferrous iron and ROS
accumulation in breast cancer stem-like cells. (A) Fluorescence
imaging of ROS (green) in MDA-MB-231 and BT-549 spheroids treated
with UA (20 µM) together with or without ferroptosis inhibitors,
fer (2 µM) and DFO (10 µM) for 24 h. (B and C) Relative levels of
MDA, total iron and Fe2+ in spheroids were measured by
commercial assay kits after the same treatment as in panel A. (D)
The mRNA levels of iron metabolism-related genes TFR1, FTH1, NCOA4
and FPN in spheroids were analyzed by reverse
transcription-quantitative PCR after the same treatment as in panel
A. (E) Protein expression of TFR1 in MDA-MB-231 and BT-549
spheroids treated with UA (20 µM) or PTX (20 µM) for 24 h was
detected by western blotting. *P<0.05, **P<0.01 and
***P<0.001. Scale bar, 40 µm. UA, ursolic acid; ROS, reactive
oxygen species; fer, ferrostatin-1; DFO, deferoxamine; MDA,
malondialdehyde; TFR1, transferrin receptor 1; FTH1, ferritin heavy
chain 1; NCOA4, nuclear receptor coactivator 4; FPN, ferroportin;
PTX, paclitaxel; ns, not significant (P>0.05). |
UA regulates ferroptosis through the
KEAP1-NRF2-mediated pathway in BCSCs
NRF2, the master regulator of the antioxidant
system, is known to play a crucial role in the transcriptional
regulation of ferroptosis by targeting FTH1, TFR1, FSP1, GPX4 and
SLC7A11 (32). KEAP1 acts as a
negative regulator of NRF2 through the ubiquitin-proteasome
pathway. In the present study, it was observed that the expression
of KEAP1 was enhanced by UA treatment and this was reversed by fer
and DFO in MDA-MB-231 spheroids (Fig.
5A). Similarly, the total level of p-NRF2/NRF2 and p-NRF2/NRF2
in the nucleus was decreased by UA treatment as well, and this was
reversed by fer and DFO (Fig. 5B and
C). Thus, UA may induce ferroptosis through stabilizing KEAP1
and inhibiting NRF2 activation. The main ferroptosis-suppressing
pathways were further investigated; namely the GSH/GPX4 pathway and
the FSP1 pathway, which is GPX4 independent. As demonstrated in
Fig. 5D and E, SLC7A11, GPX4 and
FSP1 were significantly reduced upon UA treatment in MDA-MB-231 and
BT-549 spheroids. Fer and DFO efficiently reversed the UA-induced
decline of SLC7A11, GPX4 and FSP1. These results indicated that UA
may induce ferroptosis through the KEAP-NRF2-mediated pathway.
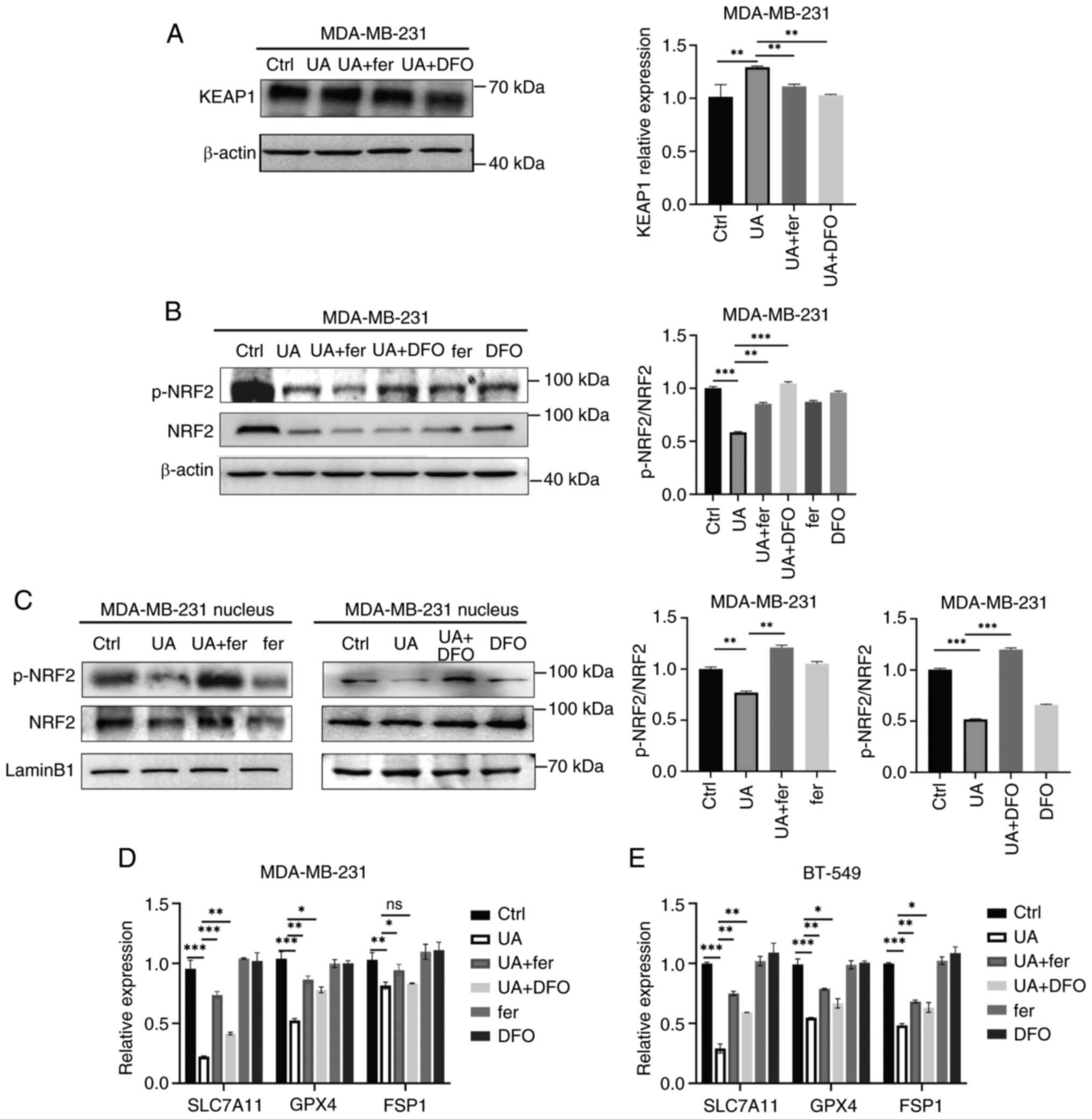 | Figure 5.UA regulates ferroptosis through the
KEAP1-NRF2-mediated pathway in breast cancer stem-like cells. (A)
Protein expression levels of KEAP1 in MDA-MB-231 spheroids treated
with UA (20 µM) together with or without ferroptosis inhibitors,
fer (2 µM) and DFO (10 µM), were detected by western blotting for
48 h. (B) Total proteins and (C) nucleic proteins were collected
from MDA-MB-231 spheroids after the same treatment as in panel B.
p-NRF2 and NRF2 were detected by western blotting. (D and E) The
mRNA levels of SLC7A11, GPX4 and FSP1 in MDA-MB-231 and BT-549
spheroids were analyzed by reverse transcription-quantitative PCR
after the treatment of UA together with or without ferroptosis
inhibitors for 48 h. *P<0.05, **P<0.01 and ***P<0.001. UA,
ursolic acid; KEAP1, Kelch-like ECH-associated protein 1; NRF2,
nuclear factor erythroid-related factor 2; fer, ferrostatin-1; DFO,
deferoxamine; p-, phosphorylated; SLC7A11, solute carrier family 7
member 11; GPX4, glutathione peroxidase 4; FSP1, ferroptosis
suppressor protein 1; ns, not significant (P>0.05). |
UA inhibits proliferation of BCSCs in
mouse xenograft models
A subcutaneous tumor model was established to study
the therapeutic potential of UA in vivo, and tumor growth
was observed periodically (Fig.
6A-C). It was found that compared with DMSO treatment, UA
treatment significantly slowed tumor growth, which was
significantly more efficient than PTX treatment. The inhibitory
effects of UA were attenuated by fer and DFO. Furthermore, TFR1,
KEAP1 and NRF2 expression levels were detected in tumor tissues.
Consistent with WB analyses, the expression levels of TFR1 and NRF2
were declined, and KEAP1 was enhanced by UA treatment, which could
be reversed by fer and DFO administration (Fig. 6D). The schematic model depicting how
UA regulates the KEAP1/NRF2 pathway and ferroptosis is shown in
Fig. 6E.
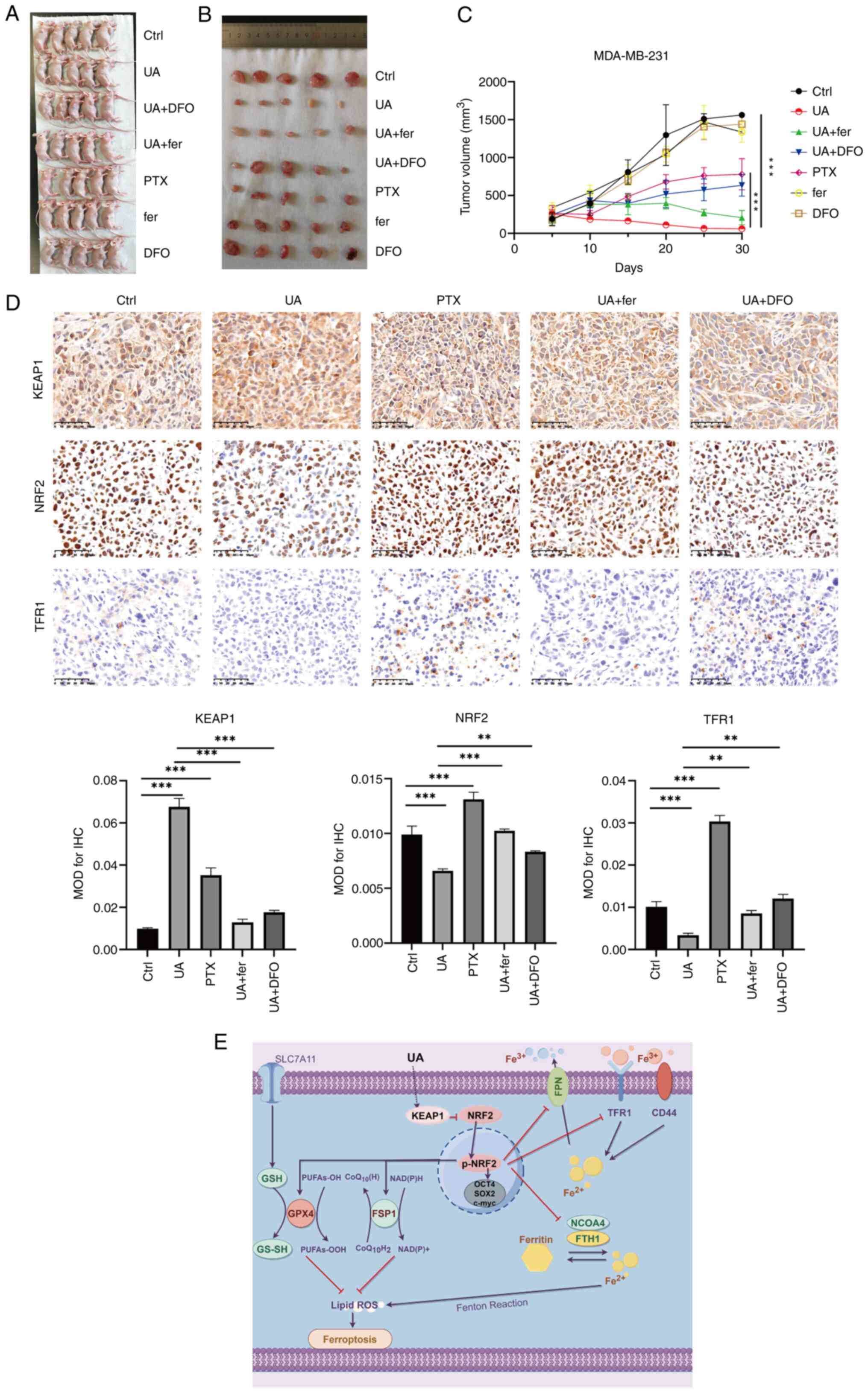 | Figure 6.UA inhibits proliferation of breast
cancer stem-like cells in mouse xenograft models. After the mouse
xenograft model was established, the mice were injected with UA at
20 mg/kg daily, PTX at 20 mg/kg daily, DMSO at 20 mg/kg daily, fer
at 5 mg/kg daily, and DFO at 100 mg/kg daily. (A) Images of the
animals at the time of euthanasia. (B) Images of all the tumors
excised from each animal. (C) Tumor volumes were measured every
five days after subcutaneous injection of 5×106 MDA-MB-231
spheroids. (D) Immunohistochemical staining and mean optical
density values of KEAP1, NRF2 and TFR1 in tumor tissues from
xenografted mice with different treatments as indicated. (E) The
schematic model depicting how UA regulates KEAP1/NRF2 pathway and
ferroptosis drawn by Figdraw 2.0 (https://www.figdraw.com/static/index.html#/).
**P<0.01 and ***P<0.001. Scale bar, 50 μm. UA, ursolic acid;
PTX, paclitaxel; fer, ferrostatin-1; DFO, deferoxamine; KEAP1,
Kelch-like ECH-associated protein 1; NRF2, nuclear factor
erythroid-related factor 2; TFR1, transferrin receptor 1. |
Discussion
Cancer heterogeneity stands as a primary factor
behind drug resistance and tumor recurrence. Within cancer cells,
CSCs represent a distinct subset intimately linked to therapeutic
resistance. Prior investigations have pointed to iron dependency
and ferroptosis dysregulation within CSCs (11). Notably, compared with non-CSCs, CSCs
in breast cancer and glioblastoma exhibit higher expression of TFR
(33). Concurrently, TF levels were
found to be elevated in glioblastoma stem cells and circulating
melanoma cells (34). Previous
findings also indicated that CD44 plays a role in regulating iron
endocytosis, thereby maintaining the mesenchymal state of cells
(35). These insights underscore
the enhanced iron uptake and trafficking observed in CSCs.
Interestingly, despite heightened iron uptake, CSCs predominantly
store iron in the ferric state (ferritin), exhibiting resistance to
ferroptosis, possibly attributed to increased expression of
antioxidants such as SLC7A11 and GSH (36).
In breast cancer cell spheroids and
CD44+/CD24− populations derived from TNBC
cell lines MDA-MB-231 and BT-549, elevated expression of TFR1 and
FTH1 was observed, along with reduced iron export marked by lower
FPN expression. Additionally, spheroids and BCSCs exhibited iron
accumulation primarily in the ferric form. Lipid peroxidation
levels were diminished in spheroids and BCSCs, as evidenced by
reduced levels of MDA. Furthermore, the levels of GSH, a crucial
intracellular antioxidant, were significantly higher in spheroids
and CSCs compared with adherent cells. Importantly, inhibitors of
ferroptosis such as SLC7A11, GPX4 and NRF2 were significantly
upregulated in spheroids and CSCs. In line with previous findings
(33–35), the data of the present study
confirmed that BCSCs exhibit enhanced iron accumulation and lower
ferroptosis levels.
Given the heightened intracellular iron accumulation
in CSCs, they become more susceptible to ferroptosis activators.
Targeting ferroptosis has emerged as a promising strategy to
address CSC-related tumor metastasis and drug resistance (16). Several studies have indicated that
CSCs exhibit increased sensitivity to ferroptosis-inducing agents
(11,36,37).
In the present study, it was observed that TNBC spheroids were more
prone to UA-induced cell death (Fig.
3A). Notably, ferroptosis inhibitors fer (which blocks lipid
peroxidation) and DFO (an iron chelator) efficiently reversed
UA-induced cell death. Furthermore, UA treatment led to a decrease
in CD44 expression, an increase in CD24 expression, and
downregulation of stemness regulators, which could be effectively
attenuated by fer and DFO. The inhibitory effects of UA on BCSCs
were further validated in xenografted mice. Collectively, these
findings highlight UA as a potent ferroptosis inducer capable of
efficiently inhibiting stemness characteristics and proliferation
of BCSCs.
Furthermore, it was demonstrated that UA disrupts
iron homeostasis, leading to intracellular ferrous iron and ROS
accumulation. Several iron metabolism regulators were altered upon
UA treatment. TFR1, which was upregulated in BCSCs, significantly
decreased following UA treatment. Additionally, UA enhanced both
ferritin formation, indicated by FTH1 expression, and degradation,
indicated by NCOA4 expression. These observations suggested that UA
may induce ferroptosis through the alteration of iron homeostasis,
resulting in the release of ferrous iron into the cytosol.
GSH, the most abundant reductant in mammalian cells,
is synthesized from cystine, which is taken up by the system
xc− cystine/glutamate antiporter, a transmembrane
protein complex containing subunits SLC7A11 and SLC3A2 (38). The SCL7A11-GSH-GPX4 axis has been
demonstrated to play a crucial role in suppressing ferroptosis
(10,39). GPX4 is the primary enzyme catalyzing
the reduction of phospholipid hydroperoxides in mammalian cells,
thereby preventing lipid peroxidation and ferroptosis. Previous
genome-wide screenings have unveiled GPX4-independent mechanisms,
such as the FSP1-CoQ10-NAD(P)H pathway, which inhibits ferroptosis
independently of GPX4 (40,41). In investigating the pathway through
which UA regulates ferroptosis, significant reductions in SLC7A11,
GPX4 and FSP1 were observed upon UA treatment in spheroids.
Notably, fer and DFO efficiently reversed the decline of SLC7A11,
GPX4 and FSP1 induced by UA. These results suggested that
UA-induced ferroptosis may be regulated through the SLC7A11/GPX4
and FSP1 pathway in an iron-dependent manner.
To further elucidate the mechanism by which UA
induces ferroptosis, the key regulator of oxidative stress,
KEAP1/NRF2, was investigated. KEAP1 acts as a negative regulator of
NRF2 via the ubiquitin-proteasome pathway. NRF2 regulates the
antioxidant system by transcriptionally regulating several key
regulators involved in ferroptosis, including FTH1, TFR1, FSP1,
GPX4 and SLC7A11 (32). It was
observed that UA treatment enhanced the expression of KEAP1, which
was reversed by fer and DFO in MDA-MB-231 spheroids and tissue
samples from xenografted mice. Furthermore, p-NRF2 levels were
significantly decreased following UA treatment in both MDA-MB-231
spheroids and tissue samples from xenografted mice. These findings
suggested that UA may induce ferroptosis by stabilizing KEAP1 and
inhibiting NRF2 activation.
Through utilization of spheroids and a xenografted
mouse model, it was demonstrated that UA exhibits promising
potential in inhibiting stemness and proliferation of BCSCs by
inducing ferroptosis. These findings expand the scope of clinical
application of UA and provide theoretical support for its use in
the treatment of TNBC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81972252, 81670573, 81830052 and
82127807), the Shanghai Nature Science Foundation (grant no.
22ZR1428000), the National Key Research and Development Program of
China (grant no. 2020YFA0909000) and the Construction Project of
Shanghai Key Laboratory of Molecular Imaging (grant no.
18DZ2260400).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XY, JZ and GH conceived and designed the study. XY,
BL, LZ, MZ, MM and LQ collected and processed samples. XY, JZ, MM
and HY performed data analysis and interpretation. BL, HY and JZ
provided resources. XY, BL, HY, GH and JZ wrote and edited the
manuscript. All authors read and approved the final manuscript. XY
and JZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Animal welfare and experimental procedures were
performed strictly in accordance with high standards for animal
welfare and other related ethical regulations approved by the
Shanghai University of Traditional Chinese Medicine. All animal
experiments were performed in compliance with the ARRIVE
guidelines. The study was approved (approval no.
2022-SZR-18-45010319800509104X) by the Animal Ethics Committee of
Shanghai University of Medicine and Health Sciences (Shanghai,
China). All methods were carried out in accordance with relevant
guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UA
|
ursolic acid
|
|
TNBC
|
triple-negative breast cancer
|
|
TNBCs
|
triple-negative breast cancer
cells
|
|
CSCs
|
cancer stem-like cells
|
|
BCSCs
|
breast CSCs
|
|
PTX
|
paclitaxel
|
|
ROS
|
reactive oxygen species
|
|
GSH
|
glutathione
|
|
TF
|
transferrin
|
|
TFR1
|
TF receptor 1
|
|
FTH1
|
ferritin heavy chain 1
|
|
FPN
|
ferroportin
|
|
NRF2
|
nuclear factor erythroid-related
factor 2
|
|
GPX4
|
glutathione peroxidase 4
|
|
SLC7A11
|
solute carrier family 7 member 11
|
|
FSP1
|
ferroptosis suppressor protein 1
|
|
KEAP1
|
Kelch-like ECH-associated protein
1
|
|
DFO
|
deferoxamine
|
|
ZVAD
|
Z-VAD-FMK
|
|
fer
|
ferrostatin-1
|
|
CQ
|
chloroquine
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
CCK-8
|
Cell Counting Kit-8
|
|
ALDH
|
aldehyde dehydrogenases
|
|
MDA
|
malondialdehyde
|
|
DMSO
|
dimethyl sulfoxide
|
|
NCOA4
|
nuclear receptor coactivator 4
|
|
p-NRF2
|
phosphorylated NRF2
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
Globocan estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houghton SC and Hankinson SE: Cancer
progress and priorities: Breast cancer. Cancer Epidemiol Biomarkers
Prev. 30:822–844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng H, Muthupalani S, Erdman S, Liu H,
Niu Z, Wang TC and Fox JG: Translocation of Helicobacter hepaticus
synergizes with myeloid-derived suppressor cells and contributes to
breast carcinogenesis. Oncoimmunology. 11:20573992022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma P: Biology and management of
patients with triple-negative breast cancer. Oncologist.
21:1050–1062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu JY, Chang CJ and Cheng JS: Survival,
treatment regimens and medical costs of women newly diagnosed with
metastatic triple-negative breast cancer. Sci Rep. 12:7292022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiagarajan PS, Sinyuk M, Turaga SM,
Mulkearns-Hubert EE, Hale JS, Rao V, Demelash A, Saygin C, China A,
Alban TJ, et al: Cx26 drives self-renewal in triple-negative breast
cancer via interaction with NANOG and focal adhesion kinase. Nat
Commun. 9:5782018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toledo-Guzmán ME, Bigoni-Ordóñez GD,
Ibáñez Hernández M and Ortiz-Sánchez E: Cancer stem cell impact on
clinical oncology. World J Stem Cells. 10:183–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin X, Wang Y, Fang K, Guo Z, Lin N and Li
L: The application of nanoparticles in theranostic systems
targeting breast cancer stem cells: Current progress and future
challenges. Stem Cell Res Ther. 14:3562023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu
Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, et al:
Breast cancer stem cells transition between epithelial and
mesenchymal states reflective of their normal counterparts. Stem
Cell Reports. 2:78–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cosialls E, El Hage R, Dos Santos L, Gong
C, Mehrpour M and Hamaï A: Ferroptosis: Cancer stem cells rely on
iron until ‘to die for’ It. Cells. 10:29812021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mai TT, Hamaï A, Hienzsch A, Cañeque T,
Müller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, et al:
Salinomycin kills cancer stem cells by sequestering iron in
lysosomes. Nat Chem. 9:1025–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pandrangi SL, Raju Bagadi SA, Sinha NK,
Kumar M, Dada R, Lakhanpal M, Soni A, Malvia S, Simon S, Chintamani
C, et al: Establishment and characterization of two primary breast
cancer cell lines from young Indian breast cancer patients:
Mutation analysis. Cancer Cell Int. 14:142014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anandhan A, Dodson M, Schmidlin CJ, Liu P
and Zhang DD: Breakdown of an ironclad defense system: The critical
role of NRF2 in mediating ferroptosis. Cell Chem Biol. 27:436–447.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun S, Shen J, Jiang J, Wang F and Min J:
Targeting ferroptosis opens new avenues for the development of
novel therapeutics. Signal Transduct Target Ther. 8:3722023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zafar S, Khan K, Hafeez A, Irfan M,
Armaghan M, Rahman AU, Gürer ES, Sharifi-Rad J, Butnariu M, Bagiu
IC, et al: Ursolic acid: A natural modulator of signaling networks
in different cancers. Cancer Cell Int. 22:3992022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Ma X, Li H, Zhuang J, Feng F, Liu
L, Liu C and Sun C: Identifying the effect of ursolic acid against
triple-negative breast cancer: Coupling network pharmacology with
experiments verification. Front Pharmaco. 12:6857732021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zong L, Cheng G, Zhao J, Zhuang X, Zheng
Z, Liu Z and Song F: Inhibitory effect of ursolic acid on the
migration and invasion of doxorubicin-resistant breast cancer.
Molecules. 27:12822022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mandal S, Gamit N, Varier L, Dharmarajan A
and Warrier S: Inhibition of breast cancer stem-like cells by a
triterpenoid, ursolic acid, via activation of Wnt antagonist, sFRP4
and suppression of miRNA-499a-5p. Life Sci. 265:1188542021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Yu Y, Liu Y, Luo Z, Law BYK, Zheng
Y, Huang X and Li W: Ursolic acid enhances the antitumor effects of
sorafenib associated with Mcl-1-related apoptosis and
SLC7A11-dependent ferroptosis in human cancer. Pharmacol Res.
182:1063062022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DAH,
Glennie MJ, et al: Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stevens MF, Hickman JA, Langdon SP, Chubb
D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA, et
al: Antitumor activity and pharmacokinetics in mice of
8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one
(CCRG 81045; M & B 39831), a novel drug with potential as an
alternative to dacarbazine. Cancer Res. 47:5846–5852.
1987.PubMed/NCBI
|
|
24
|
Yates AG, Weglinski CM, Ying Y, Dunstan
IK, Strekalova T and Anthony DC: Nafamostat reduces systemic
inflammation in TLR7-mediated virus-like illness. J
Neuroinflammation. 19:82022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun N, Zhang RX, Wang Y, Huang ZJ, Han J,
Bao YS, Duan WY, Dong CR, Deng GS and Zhuang G: Effects of ursolic
acid on oxidative stress and inflammatory factors in a rat model of
AR after PM2.5 exposure. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 57:860–867. 2022.(In Chinese). PubMed/NCBI
|
|
26
|
Innocenti F, Danesi R, Di Paolo A, Agen C,
Nardini D, Bocci G and Del Tacca M: Plasma and tissue disposition
of paclitaxel (taxol) after intraperitoneal administration in mice.
Drug Metab Dispos. 23:713–717. 1995.PubMed/NCBI
|
|
27
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang G, Chen Y, Chen J, Chen Z and Jiang
W: Deferoxamine ameliorates compressed spinal cord injury by
promoting neovascularization in rats. J Mol Neurosci. 70:1437–1444.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong Y, Shen S, Zhou Y, Mao F, Guan J,
Lin Y, Xu Y and Sun Q: ALDH1 is a better clinical indicator for
relapse of invasive ductal breast cancer than the
CD44+/CD24-phenotype. Med Oncol. 31:8642014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sandhu SS, Rouz SK, Kumar S, Swamy N,
Deshmukh L, Hussain A, Haque S and Tuli HS: Ursolic acid: A
pentacyclic triterpenoid that exhibits anticancer therapeutic
potential by modulating multiple oncogenic targets. Biotechnol
Genet Eng Rev. 4:1–31. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee J and Hyun DH: The interplay between
intracellular iron homeostasis and neuroinflammation in
neurodegenerative diseases. Antioxidants (Basel). 12:9182023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schonberg DL, Miller TE, Wu Q, Flavahan
WA, Das NK, Hale JS, Hubert CG, Mack SC, Jarrar AM, Karl RT, et al:
Preferential iron trafficking characterizes glioblastoma stem-like
cells. Cancer Cel. 28:441–455. 2015. View Article : Google Scholar
|
|
34
|
Hong X, Roh W, Sullivan RJ, Wong KHK,
Wittner BS, Guo H, Dubash TD, Sade-Feldman M, Wesley B, Horwitz E,
et al: The lipogenic regulator SREBP2 induces transferrin in
circulating melanoma cells and suppresses ferroptosis. Cancer
Discov. 11:678–695. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller S, Sindikubwabo F, Cañeque T, Lafon
A, Versini A, Lombard B, Loew D, Wu TD, Ginestier C,
Charafe-Jauffret E, et al: CD44 regulates epigenetic plasticity by
mediating iron endocytosis. Nat Chem. 12:929–938. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pandrangi SL, Chittineedi P, Chalumuri SS,
Meena AS, Neira Mosquera JA, Sánchez Llaguno SN, Pamuru RR,
Mohiddin GJ and Mohammad A: Role of intracellular iron in switching
apoptosis to ferroptosis to target therapy-resistant cancer stem
cells. Molecules. 27:30112022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|




















