Introduction
The immune system plays an integral role in the
recognition and elimination of tumor cells, and the natural killer
(NK) group 2 member D (NKG2D) receptor is a pivotal player in this
intricate cellular interaction network (1,2). In
humans, NKG2D is expressed on NK cells and some T cells, such as
NKT cells, γδT cells, and CD8+ T cells. It is a type II
transmembrane receptor belonging to the C-type lectin-like dimeric
family (1). Binding of the NKG2D
receptor to its ligand (NKG2DL) activates NK cells, resulting in
the release of cytotoxic granules containing perforin and
granzymes, which induce target cell lysis (3). In addition, it induces the secretion
of various cytokines and chemokines, amplifies specific immune
responses, and exerts antiviral and antitumor effects (4). NKG2DL also acts as a costimulatory
molecule that promotes T-cell activation.
In the human genome, NKG2DLs are major
histocompatibility complex (MHC) class I polypeptide-related
sequence A/B (MICA/B) and UL16 binding proteins 1-6 (5,6). These
are also termed retinoic acid early transcript 1 (RAET1) and are
homologous to the mouse ligands of the NKG2D receptor Raet1a-d. The
correspondences are as follows: RAET1I=ULBP1, RAET1H=ULBP2,
RAET1N=ULBP3, RAET1E=ULBP4, RAET1G=ULBP5, and RAET1L=ULBP6
(5). MICA/B is highly polymorphic,
with 280 MICA and 47 MICB protein sequences recorded to date
[IPD-IMGT/HLA Database (ebi.ac.uk)], and these differing
polymorphisms may influence their affinity for binding to NKG2D,
subsequently affecting the NKG2D receptor/NKG2DL axis and altering
NK cell activity. This rich MICA/B polymorphism plays a significant
role in organ transplantation, immune system diseases, and tumor
immunity (6). These ligands are
markedly upregulated in stressed cells, tumor cells, and cells
infected with viruses or bacteria, thereby facilitating immune
system activation (7).
NK and CD8+T cells complement each other
in tumor immune responses. MHC class I molecules are often
underexpressed or not expressed in tumor cells, making it
impossible to activate the cytotoxic functions of CD8+T
cells (8,9). However, their absence can enhance NK
cell activation. Nevertheless, tumor cells can simultaneously
downregulate both MHC-I molecules and NKG2DL expression, thereby
diminishing the immune surveillance capabilities of NK cells and
leading to tumor immune evasion (10). One method to achieve this is to
convert membrane-bound NKG2DL into soluble NKG2DL (sNKG2DL)
molecules (5). This not only
reduces the amount of NKG2DL on the tumor cell surface,
competitively obstructing immune cell recognition of membrane-bound
NKG2DL, but also promotes internalization of the NKG2D receptor
(11). This hinders the recognition
of cancer cells by NK and CD8+T cells, thereby
facilitating tumor progression and metastasis (11). By understanding the release
mechanisms of these soluble forms in depth, new therapeutic
strategies can be identified to bolster immune cell recognition and
eradication of tumor cells.
Therefore, understanding the specific roles and
regulatory mechanisms of sNKG2DLs in tumor immune evasion is
paramount for both basic research and clinical translation. The
present study aimed to elucidate the mechanisms underlying the
production of sNKG2DL and its influencing factors. The role of
sNKG2DL in tumorigenesis, progression, and immune evasion, with a
keen focus on its mechanism of action and potential as a
therapeutic target is comprehensively analyzed. In addition, its
applications in oncological clinical diagnosis and immune-related
treatments are explored, offering new perspectives for research on
the NKG2DL family and the development of clinical treatment
strategies.
Regulation of NKG2DL expression in tumor
cells at multiple levels
Under physiological conditions, MICA/B is primarily
expressed in gastrointestinal epithelial cells, potentially owing
to bacterial stimulation. This is crucial for activating intestinal
γδ T cells, promoting gut immunity (12). RT-PCR has revealed ULBP transcripts
in various tissues, including the heart, brain, lungs, liver,
testes, lymph nodes, thymus, tonsils and bone marrow (13).
Under cellular stress, NKG2DL is expressed in
various tissues and tumor cells. Heat shock treatment can
significantly enhance the expression of MICA and MICB on intestinal
epithelial cells and can boost the lytic ability of γδ T cells
(12,14).
In tumor cells, with the overactivation of oncogenes
and strong proliferation signals, there can be DNA damage responses
(1). Hypoxia in the tumor
environment can also induce heat shock responses (11). These stress reactions can regulate
NKG2DL expression at multiple levels, leading to its expression on
the cell surface and mediating the killing action of NK cells
(11).
Upstream of the MICA and MICB initiation codons,
there are sequences that can bind to transcription factors, heat
shock factor 1 and Sp1, responding to proliferative signals and
participating in heat shock responses (15). DNA damage and replication stress can
activate sensors, such as ataxia telangiectasia mutated (ATM) and
ataxia telangiectasia and Rad3 related (ATR), further activating
downstream p53. Within ULBP1 and ULBP2 genes, there
are p53 response elements (16,17)
that can bind to p53 and increase transcription. ATM and ATR also
promote MICA transcription by activating NF-κB (18,19).
Research has revealed that although most tissue cells do not
express NKG2DL under healthy conditions, most do transcribe MICA
and MICB (20), indicating that
post-transcriptional regulation is of significant importance for
MICA/B. Various miRNAs that target NKG2DL have been identified.
Stern-Ginossar et al (21)
found constitutively expressed miRNAs in cells and proposed that
when the stress-induced transcription of MICA/B surpasses the level
that miRNAs can silence, MICA/B is expressed on the cell surface.
There are also inducible miRNAs; for instance, interferon gamma
(IFN-γ) can downregulate MICA expression by inducing miR-520b
(22), and p53 can induce miR-34
a/c targeting ULBP2 (23). This
inhibition may serve as a mechanism for preventing excessive NKG2DL
expression. As aforementioned, while ATM and ATR can promote the
expression of MICA/B and ULBP, they can also downregulate their
synthesis by inducing the expression of miRNA through activating
IFN-γ and p53.
As tumors progress, the expression of NKG2DL on the
cell surface is downregulated, facilitating immune evasion. At the
transcriptional level, NKG2DL mRNA synthesis is regulated by
epigenetic modifications. Overexpression of histone deacetylases
(HDACs) is observed in tumor tissues. The use of HDAC inhibitors
enhances the transcription of NKG2DL and increases the cytotoxicity
of NK cells by augmenting acetylation of histone H3 at the
promoters of MICA and MICB (24).
MUC1-C, a pivotal molecule in oncogenesis triggered by chronic
inflammation, suppresses the transcription of MICA and MICB by
promoting DNA methylation and histone H3 lysine 27 (H3K27)
methylation at their promoter regions (25). Gliomas with isocitrate dehydrogenase
(IDH) mutations produce the oncometabolite 2-hydroxyglutarate,
which inhibits the expression of various NKG2DLs by facilitating
DNA methylation (26).
Additionally, transforming growth factor-beta (TGF-β) selectively
reduces the mRNA levels of MICA, ULBP2 and ULBP4 in gliomas. At the
post-transcriptional level, several miRNAs, including miR-34a/c,
miR-10b, miR-20a, miR-93, miR-106b and miR-519a-3p, actively
contribute to the downregulation of NKG2DL expression (23,27–29).
IFN-γ induces miR-520b, leading to the downregulation of MICA mRNA
across multiple tumor cell lines (22). Following translation, NKG2DL is
transported to the cell membrane, where its soluble forms, produced
by shedding from the cell surface, contribute to immune
suppression, as elaborated further below.
Mechanism of conversion of NKG2DL from
membrane protein to a soluble form
NKG2DLs are converted into soluble molecules via
three main pathways: Μetalloprotease-mediated proteolytic shedding,
lipid raft-mediated exosome release, and RNA selective splicing
(2,5). The principal release mechanism for
each NKG2DL is influenced by their molecular structure differences.
MICA/B is structurally similar to human MHC class I molecules,
featuring three extracellular domains, α1, α2 and α3, alongside a
transmembrane region and a cytoplasmic tail (6). However, their α1 and α2 do not bind to
antigenic peptides and lack the β2m microglobulin. The ULBP family,
on the other hand, has only two domains, α1 and α2. ULBP1-3 and
ULBP6 do not have a transmembrane domain and are anchored to the
plasma membrane via a GPI-anchor, while ULBP4 and ULBP5, similar to
MICA/B, possess both a transmembrane region and a cytoplasmic tail
(1). MICA/B and ULBP2 are more
susceptible to degradation by metalloproteases due to their
molecular structures (6). ULBP3,
being a GPI-anchored protein, is primarily released into exosomes
through lipid rafts. Research on ULBP4 and ULBP5 has primarily
focused on RNA selective splicing (30). Currently, there is no evidence
indicating the primary release mode of ULBP1. Only trace amounts of
soluble ULBP1 (sULBP1) have been detected. This may be due to the
short half-life of ULBP1 on the cell membrane, which is rapidly
internalized and degraded rather than being released via exosomes
(31). Similarly, MICB has a short
half-life in the plasma membrane and is rapidly internalized and
degraded (32).
Proteolytic shedding of NKG2DL
membrane proteins mediated by metalloproteases
ADAM and MMP are zinc-dependent metalloproteinases
belonging to the metzincin superfamily. These are the main
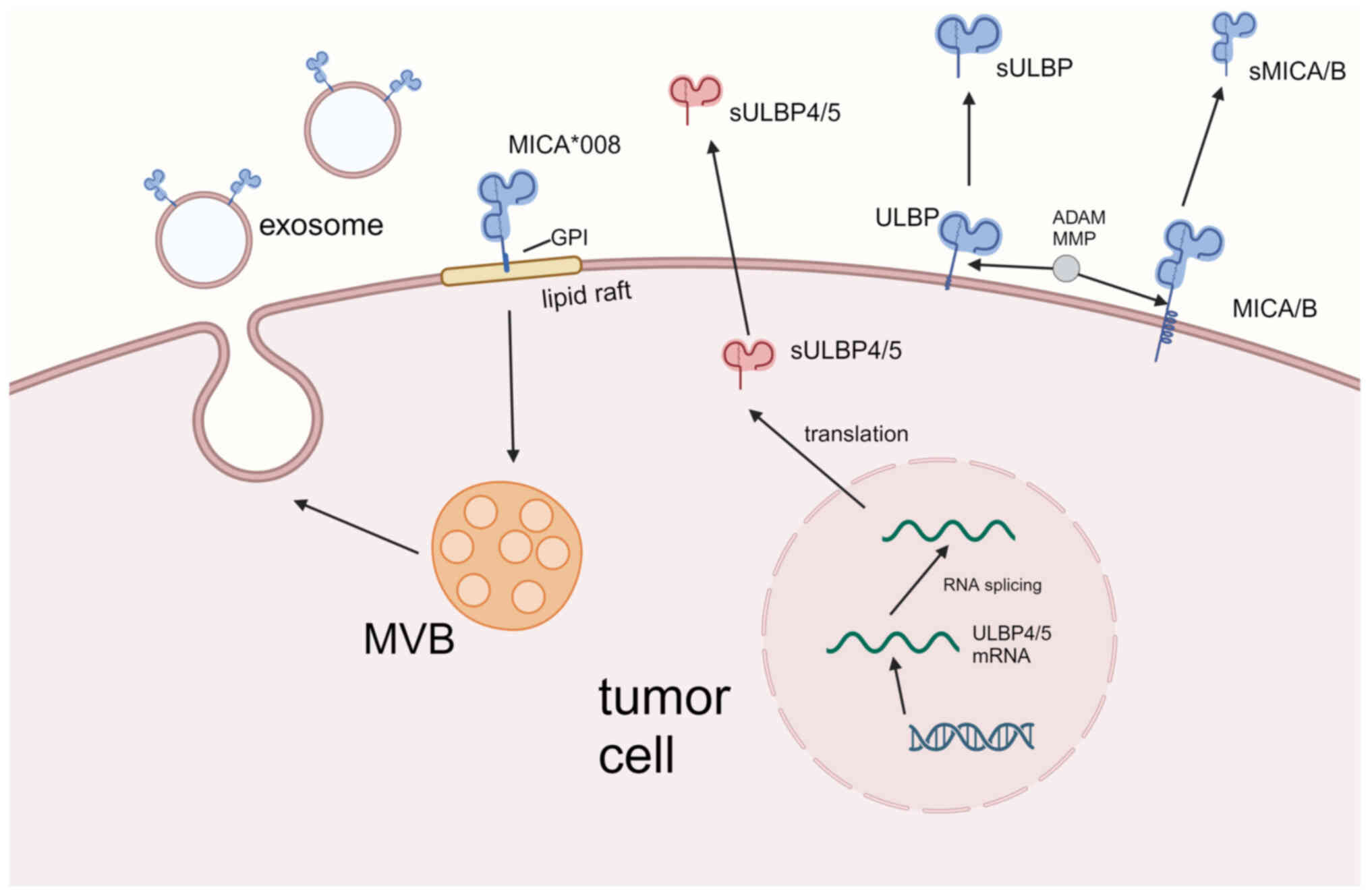
substances involved in the shedding of NKG2DL (Fig. 1). The catalytic domains of these two
enzymes have features conserved within the zincin enzyme family.
The first is a catalytic sequence, HEXXHXXGXXH/D, that binds zinc
ions, where three histidine residues coordinate with the zinc ion,
and the substrate forms a fourth coordination bond. This is
followed by a 1,4-β turn containing glutamic acid (Met-turn) that
helps stabilize the catalytic process (33). Humans express 21 ADAMs, of which 13
are catalytically active. ADAM 9, 10, 12, 15, 17 and 19 are widely
expressed in somatic cells and hydrolyze various extracellular
regions of membrane proteins (34).
MMPs are mainly secreted from cells, degrade various proteins in
the extracellular matrix, and participate in tissue remodeling.
Membrane-bound MMPs (MT-MMP) are anchored to the plasma membrane
via transmembrane or GPI-anchoring domains (35).
ADAM10 and ADAM17 [also known as tumor necrosis
factor alpha (TNF-α) converting enzyme] are involved in the
shedding process of MICA from the plasma membrane. Under stress
conditions, such as malignancy, the expression of MICA increases
and endoplasmic reticulum protein 5 (ERp5) translocates from the
endoplasmic reticulum to the plasma membrane surface, thereby
activating ADAM. In the α3 domain of MICA, there is a conserved
6-peptide sequence between two cysteine residues, allowing ERp5 to
form a complex with MICA. Through this interaction, a transient
disulfide bond is formed, leading to conformational changes
(36,37). This structural change allows ADAM10
and ADAM17 to approach MICA, and catalyze the hydrolysis between
the α3 domain and transmembrane region of MICA, leading to its
shedding from the plasma membrane, resulting in soluble MICA
(sMICA). Studies on the carboxyl-terminus of sMICA have revealed
that the ADAM cleavage site is not fixed, and the efficiency of
ADAM-induced shedding is positively correlated with the length of
the stalk region (38).
Hydrolysis of NKG2DL by the metalloproteinases ADAM
and MMP has been widely studied. Previous research indicates that
ADAM10 and ADAM17 have shedding effects on various NKG2DLs,
including MICA, MICB and ULBP2. By contrast, the hydrolytic
activity of ULBP3 is low and there is no definitive evidence that
metalloproteinases directly participate in ULBP1 hydrolysis
(38–41). Numerous studies have shown that ADAM
and MMP can cause NKG2DL shedding in various tumors; however, most
experiments have used broad-spectrum metalloproteinase inhibitors
without careful differentiation (34,38–41).
Tissue inhibitors of metalloproteinases (TIMPs) are
endogenously synthesized metalloproteinase inhibitors. Of note,
four TIMPs (TIMP1, TIMP2, TIMP3, and TIMP4) inhibit the activities
of MMP and ADAM. Increased TIMP3 expression reduces the activity of
ADAM17, thereby reducing the release of sMICA, sMICB and sULBP2
(42).
Recruitment of NKG2DL to lipid rafts
and release into exosomes
The transfer of NKG2DL to exosomes and its release
into the serum is another way it is shed from the plasma membrane
(Fig. 1). Detergent-resistant
membrane domains (DRMs) are membrane microdomains on the plasma
membrane that cannot be fully dissolved using nonionic detergents,
such as Triton X-100, at low temperatures. They are also rich in
cholesterol and sphingolipids. GPI-anchored proteins (GPI-APs) are
highly enriched in these areas. When cells are stimulated, these
regions form large, stable lipid rafts that facilitate protein
interactions and signal transduction on the membrane (43). the composition of exosomes is
related to lipid rafts as they have similar lipid components and
are enriched in GPI-APs (44).
Additionally, due to mutations in the transmembrane region of
MICA*008, its tail is truncated and acquires a GPI-anchoring
sequence, allowing its release through exosomes (45). MICA, MICB and ULBPs have also been
observed in exosomes (41,46). However, only ULBPs are GPI-anchored,
whereas MICA and MICB have a transmembrane region and a cytoplasmic
tail.
Transmembrane proteins, such as MICA, MICB and
ADAM17, also accumulate in lipid rafts. In these areas, ADAM17 has
high hydrolytic activity on various membrane proteins.
Palmitoylation of cysteine residues in the cytoplasmic tails of
MICA and MICB signals their location in DRMs, and inhibiting
palmitoylation effectively inhibits shedding (40,47).
However, this inhibitory effect was incomplete, indicating that
shedding still occurred outside the DRMs.
Typically, the lipid bilayer in lipid raft regions
is thicker than that in other areas of the plasma membrane. Hence,
transmembrane proteins located in the lipid rafts may encounter
hydrophobic mismatches. This phenomenon was observed
experimentally. Although both MICA and ULBP1-3 co-localized with
lipid raft regions, the association of MICA with DRMs was much
lower than that of ULBP3 (48).
Uniquely, despite ULBP2 being a GPI-AP, it
predominantly becomes soluble through metalloprotease-mediated
proteolysis. ULBP2 is more susceptible to metalloprotease attack
compared with ULBP3, and the presence of metalloprotease inhibitors
significantly increases ULBP2 in exosomes while reducing it on the
cell surface (41).
RNA selective splicing causes NKG2DL
to lose its transmembrane region and be released
extracellularly
ULBP4 (RAET1E) and ULBP5 (RAET1G) are members of the
RAET1 family that contain transmembrane regions. In 2004, Bacon
et al (49), while studying
RAET1G, discovered alternative splicing that led to truncation of
the transmembrane region, predicting that this would turn the
molecule into a soluble form, and named it RAET1G2. Subsequently,
in 2009, research confirmed that both RAET1G and RAET1G2 were
expressed in various tumor cells. Truncated soluble RAET1G2 can
reduce the expression NKG2D in NK cells, leading to
immunosuppression (30). In 2007,
Cao et al (50) discovered
an alternative splice form of RAET1E and named it RAET1E2, which
similarly results in the loss of the transmembrane region, becoming
a soluble molecule (Fig. 1) and
reducing the cytotoxicity of NK cells.
Main regulatory factors in the production of
sNKG2DL
The transformation of NKG2DL into its soluble ligand
is a complex process governed by a multitude of factors. ADAM and
MMP, two key enzymes, play crucial roles in the ligand-shedding
process and represent critical regulatory points. Subsequently, the
factors influencing the shedding and release of NKG2DL in tumor
cells are presented (Table I).
 | Table I.Regulatory factors and mechanisms of
sNKG2DL. |
Table I.
Regulatory factors and mechanisms of
sNKG2DL.
|
| Regulatory
factors | Mechanisms |
Metalloproteinases | Ligands | Effects | (Refs.) |
|---|
| Polymorphism | MICA-A5.1 | GPI anchor | - | sMICA↑ | n.d. | (52,53) |
|
| SNP rs1051792 | Increased | - |
| NKG2D on NK | (51,55) |
|
| SNP rs2596542 | expression |
|
| cells↓ | (56) |
|
|
| of MICA |
|
| n.d. |
|
| Stress | Hypoxia stress | HIF | ADAM10 | sMICA↑, | NK
cytotoxicity↓, | (57,58) |
|
|
|
|
| sMICB↑ | NKG2D on NK
cells↓ |
|
|
| Genotoxic | Cellular | ADAM9, ADAM10 | sMICA↑, | NK
cytotoxicity↓, | (60) |
|
| stress | senescence |
| sMICB↑ | NKG2D on NK
cells↓ |
|
| TME Cells | CAF | Secreted MMP | MMP2, MMP9 | sMICA↑, | NK
cytotoxicity↓, | (62) |
|
|
|
|
| sMICB↑ | MICA/B on
tumor |
|
|
|
|
|
|
| cells↓ |
|
|
| Platelet | n.d. | ADAM10, ADAM17 | sMICA↑, | NK
cytotoxicity↓ | (63) |
|
|
|
|
| sMICB↑, |
|
|
|
|
|
|
| sULBP1↑, |
|
|
|
|
|
|
| sULBP3↑ |
|
|
|
| LN MSC | n.d. | ERp5↑, ADAM10↑ | sMICA↑, | T cytotoxicity↓,
NKG2D | (64) |
|
|
|
|
| sULBP3↓ | on T cells↓ |
|
| Molecules | IL-1β | n.d. | ADAM9 | sMICA↑ | NK
cytotoxicity↓ | (66) |
|
| TGF-β | n.d. | ADAM17 | sMICA↑, | T
cytotoxicity↓ | (67,68) |
|
|
|
|
| sULBP2↑ |
|
|
|
| IDO1 | IDO1-Kyn-AhR | ADAM10 | sMICA↑ | NK
cytotoxicity↓ | (69) |
|
| MUC1-C | ERp5 RAB27A | ADAM10, ADAM17 | sMICA↑, | NK
cytotoxicity↓ | (25) |
|
|
|
|
| sMICB↑ |
|
|
Polymorphism of MICA gene affects the
production of sMICA
Being the most polymorphic member of the
non-classical MHC class I gene family, the polymorphism of the
MICA gene not only affects the quantity of MICA on the cell
membrane and its affinity for the NKG2D receptor, but also
influences the shedding of MICA (51). Exon 5, which encodes the MICA
transmembrane region, contains short tandem repeat sequences (GCT)
that encode for alanine (52). The
MICA-A5.1 gene (also known as MICA*008) has a
purine insertion after the second GCT, leading to a frameshift
mutation and a premature stop codon, giving MICA-A5.1
a GPI anchor point and the shortest transmembrane domain compared
with that of other MICA proteins (52). Although the shorter transmembrane
domain reduces the sensitivity of MICA-A5.1 to MMP (52), the GPI anchor prioritizes embedding
into lipid rafts, thus recruiting MICA-A5.1 to exosomes and
releasing more sMICA through the exosomes (53). MMP inhibitors, while inhibiting
hydrolytic shedding of MICA, promote the exosome pathway, leading
to the release of more sMICA (54).
This indicates that drugs that suppress MICA shedding (such as MMP
inhibitors) may not be as effective in patients with MICA-A5.1.
The association between MICA single nucleotide
polymorphisms (SNPs) and sMICA levels has also garnered attention.
SNPs in MICA can regulate its expression, and the degree of MICA
expression is often correlated with sMICA levels. For instance, the
SNP rs1051792 causes the 129th amino acid site in the MICA α2
domain to change from valine (Val) to methionine (55). In patients with multiple myeloma
(51), those with the
MICA-129Val/Val genotype release more sMICA, which is linked to the
increased expression and quantity of MICA. In rs2596542 (A/G), the
G allele promotes MICA expression, leading to an increase in sMICA
generation (56).
Stress and elevated levels of
sNKG2DL
NKG2DL is rarely expressed in normal tissues. When
cells are subjected to various stress stimuli, such as oncogenic
factors, infections, physical injuries, or inflammatory responses,
they enhance NKG2DL expression through various mechanisms (1). For instance, when cells undergo heat
stress, heat shock factor 1 binds to the heat shock element in the
MIC promoter sequence, thereby promoting the expression of MICA and
MICB through the heat shock response. This leads to increased
expression of MICA/B membrane proteins in tumor cells (15). However, in leukemia and lymphoma,
heat and oxidative stress promote the secretion of MICA, ULBP1 and
ULBP2 in exosomes, thus reducing NK cell cytotoxicity (46).
Contrary to most stress stimuli that elevate NKG2DL
membrane protein levels and boost tumor immunity, hypoxia induced
by the tumor microenvironment promotes the production of sNKG2DL,
exacerbating immune suppression. Under hypoxic conditions,
hydroxylation of hypoxia-inducible factor (HIF)-α in tumor cells is
inhibited. The non-hydroxylated HIF-α accumulates and binds with
HIF-β, collectively acting on the hypoxia-responsive element of the
ADAM10 gene, elevating ADAM10 expression and
promoting MICA shedding (57).
Upregulation of circ_0000977 modulates this process during hypoxia
(58). Circ_0000977, acting as a
miRNA sponge, binds to miR-153, reducing the inhibitory effect of
miR-153 on HIF-α and ADAM10 (58).
Targeting this pathway, nitric oxide (NO) mimetics, such as
glyceryl trinitrate and DETA-NO, can promote the degradation of
HIF-α, reducing MICA shedding induced by hypoxia. They activate the
NO-cyclic guanosine monophosphate-protein kinase G pathway,
enhancing prolyl hydroxylase-mediated degradation of HIF1-α,
interfering with the accumulation of HIF-α under hypoxic
conditions, thereby decreasing ADAM expression (58).
Genotoxic stress, such as that caused by
chemotherapeutic drugs and ionizing radiation, can produce reactive
oxygen species, leading to DNA damage, protein misfolding, and
inactivation. This overactivates the extracellular signal-regulated
kinase pathway, triggering cellular senescence (59). During cell cycle arrest, senescent
cells activate and secrete a senescence-associated secretory
phenotype, including metalloproteinases (59), which can stimulate tumor
progression. In a neuroblastoma model, low doses of
chemotherapeutic drugs induced cellular senescence and stimulated
lncRNA MALAT1 to bind to miR-92a-3p. This reduces the inhibitory
effect of miR-92a-3p on ADAM10 and promotes the shedding of MICA
and MICB (60). However, drugs,
such as sorafenib (61), have the
opposite effect in hepatocellular carcinoma, inhibiting ADAM9 and
ADAM10 and reducing MICA shedding. Therefore, it is crucial to
assess the effects of drugs on NKG2DL shedding carefully.
Production of sNKG2DL is regulated by
the tumor microenvironment
The tumor microenvironment plays an important role
in the development and metastasis of tumors, and the generation of
sNKG2DL is regulated by cells and molecules in the tumor
microenvironment. Cancer-associated fibroblasts promote tumor cell
migration by reshaping the microenvironment. Research has shown
that cancer-associated fibroblasts secrete high levels of active
MMPs, causing MICA/B shedding from the surfaces of melanoma cells
(62). Platelets release exosomes
containing soluble ADAM10 and ADAM17 into tumor cells, facilitating
the shedding of NKG2DL from the tumor cell surface (63). In lymph node mesenchymal stromal
cells, both transcription and expression levels of ERp5 and ADAM10
were revealed to be elevated, promoting the shedding of MICA and
ULBP3 from the surface of lymph node mesenchymal stromal cells and
Reed-Sternberg cells (64).
Metalloproteinases, which are essential enzymes for
the generation of sNKG2DL, influence sNKG2DL production based on
changes in their expression and content. Cytokines in the TME
affect the expression of metalloproteinases through various
signaling pathways and transcription factors. IFN-γ affects MMP
expression via STAT, TGF-β through Smad, and IL-1β and TNF-α
through the mitogen-activated protein kinase pathway (65). Thus, cytokines may influence the
shedding of NKG2DL through these pathways. IL-1β facilitates
ADAM9-mediated NKG2DL cleavage (66). TGF-β induces ADAM17 mRNA expression,
promoting sMICA and sULBP2 production (67,68).
Furthermore, indoleamine 2,3-dioxygenase 1 regulates ADAM10
expression via the indoleamine 2,3-dioxygenase 1-Kyn-AhR pathway,
thereby inducing sMICA shedding and immune evasion (69).
Morimoto et al (25) found that MUC1-C suppresses MICA/B
expression and promotes sMICA/B production through various
pathways. MUC1-C can activate NF-κB, promoting H3K27 trimethylation
mediated by EZH2 and methylation of the MICA/B promoter region by
DNA methyltransferase, thus suppressing MICA/B expression. Another
possible mechanism is that MUC1-C facilitates MICA/B shedding
mediated by ERp5. Notably, RAB27A plays an important role in
exosome secretion. MUC1-C also forms a complex with RAB27A, which
effectively promotes the release of exosomes containing MICA/B.
In summary, ADAM and MMP are the core enzymes
responsible for producing sNKG2DL and are critical targets for
regulating sNKG2DL. Their roles in tumor development, invasion, and
related regulatory factors have been extensively studied. Although
the precise mechanisms by which certain regulatory factors affect
sNKG2DL are yet to be elucidated, their potential impact on sNKG2DL
production cannot be overlooked. Considering the multifaceted roles
of ADAM and MMP in regulating tumor growth, inducing apoptosis, and
promoting invasion, targeted interventions against these
proteinases may yield more pronounced antitumor effects.
sNKG2DL is a key molecule in tumor immune
evasion
NKG2D is an activating receptor of NK cells and a
coactivating receptor of CD8+T cells (1). Thus, within tumor tissues, activation
of the NKG2D-NKG2DL axis is crucial for maintaining immune
surveillance and inhibiting tumor development. In addition to
suppressing NKG2DL expression, tumor cells evade the immune system
by producing or secreting sNKG2DL.
The reduction of membrane-bound NKG2DL on the
surface of tumor cells diminishes the recognition and attack of
these cells by NK cells (70).
Tumor cells employ mechanisms such as proteolysis to convert
membrane-bound NKG2DL into sNKG2DL, which is then released into the
tumor microenvironment, directly resulting in a decrease in
membrane-bound NKG2DL levels (71).
Studies have demonstrated that inhibiting MMPs can reduce the
release of sMICA and promote the accumulation of MICA on the tumor
cell surface (72). Additionally,
sNKG2DL exerts immunosuppressive effects in other ways (Fig. 2). sNKG2DL can inhibit the
interaction between NKG2D on the tumor cell membrane and NKG2DL on
immune cells, inducing the internalization of the NKG2D receptor
and impairing the immunological function of NK cells (73). Beyond its effects on NK cells,
sNKG2DL also targets other immune cells within the tumor
microenvironment, further promoting immunosuppression.
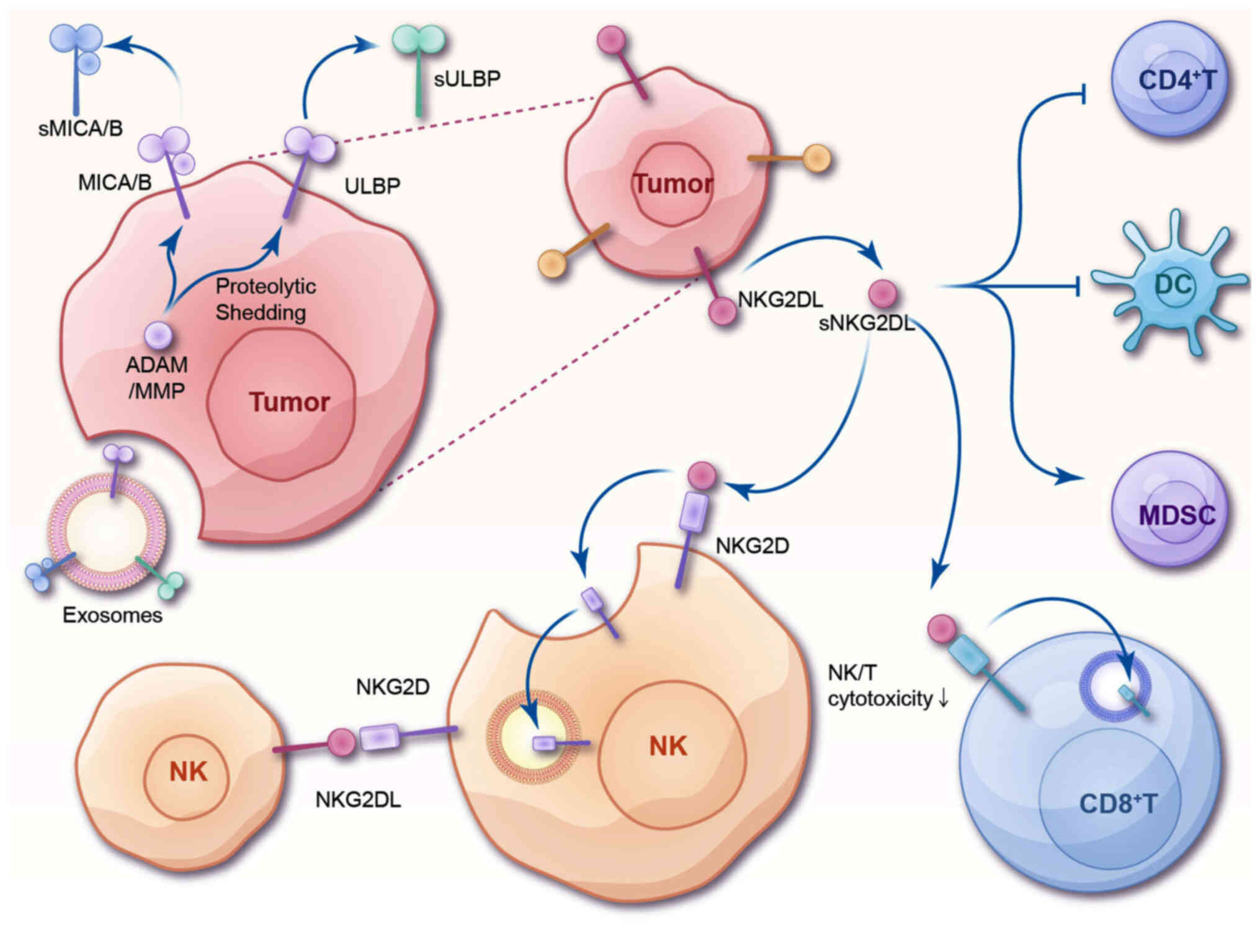 | Figure 2.Potential mechanism of
sNKG2DL-induced immunosuppression. Tumor cells generate sNKG2DL,
including sMICA/B and sULBP, through the ADAM/MMP-mediated
proteolytic cleavage of NKG2DL or by secreting sNKG2DL via
exosomes. The binding of sNKG2DL to NKG2D receptors on the surface
of NK cells and CD8+T cells induces the internalization
and downregulation of NKG2D, leading to a decrease in cytotoxicity.
Additionally, sNKG2DL present on exosomes can be transferred to the
membrane of NK cells, prompting these cells to attack each other.
sNKG2DL also inhibits DC and CD4+T cells, while
promoting the activation of myeloid-derived suppressor cells.
sNKG2DL, soluble natural killer group 2 member D ligand; sMICA/B,
soluble MHC class I polypeptide-related sequence A/B; sULBP,
soluble UL16 binding protein; ADAM, a disintegrin and
metalloproteinase; MMP, matrix metalloproteinase; NK, natural
killer. |
sMICA competitively binds to the NKG2D receptor,
blocking the binding of MICA on the tumor cell membrane to NKG2D.
sNKG2DL consistently binds to NKG2D, prompting the internalization
of NKG2D on the surface of NK cells (73) or CD8+T cells (74), leading to a decrease in cell surface
NKG2D content (Fig. 2) and
weakening the immune rejection effect against tumors. After binding
to NKG2D, sMICA activates the ubiquitin ligase c-Cbl, promotes
DAP10 ubiquitination, directs the internalization of NKG2D, and
sends it to lysosomes for NKG2D degradation (73). This process results in a 50%
reduction in cytolytic activity of NK cells relative to those not
exposed to sMICA (73,75). Compared to culturing NK cells from
AML patients without sULBP1, the presence of sULBP1 leads to a 40%
reduction in NKG2D expression on the surface of NK cells (71). However, high-affinity sNKG2DL,
similar to sMULT1 in mice, can reduce the internalization of cell
membrane NKG2D due to persistent binding to tumor cell NKG2DL,
thereby increasing the content of NKG2D on the NK cell membrane and
enhancing the function of NK cells (76). In humans, both MICA/B are
low-affinity receptors, suggesting that affinity may influence
whether sNKG2DL induces NKG2D internalization. In addition, the
production mode of sNKG2DL also affects the internalization of
NKG2D, and sNKG2DL secreted by exosomes can cause more NKG2D
internalization (41). sULBP3
secreted by exosomes can cause ~15% more downregulation of NKG2D
than sULBP2 produced by proteolytic cleavage (P<0.05), resulting
in more severe immunosuppression (41). Apart from the differences in the
ligands themselves, the reason for this may be that sNKG2DL on the
exosome membrane is multimeric, with a higher affinity and longer
binding time with NKG2D.
A recent study has found that MICA*008 secreted by
exosomes can be transferred to the surface of NK cells, inducing
mutual attacks between NK cells, leading to an increase in NK cell
death, and providing new insights into the mechanisms by which
sNKG2DL causes immune evasion (77).
Innate and specific immunity continuously regulates
the intensity of the immune system through different cytokines and
chemokines. sNKG2DL influences other immune cells in the tumor
microenvironment, resulting in severe immunosuppression. sNKG2DL
inhibits the activation of CD4+T cells and DC cells by
NK cells (Fig. 2) (78). The sMICA/B-neutralizing antibody
B10G5 stimulates the expression of DC co-stimulatory molecules CD80
and CD86 (79). After binding with
NKG2D, sMICB can activate the STAT signaling pathway of MDSC and
promote the proliferation and accumulation of MDSC (80). For CD8+T cells, sMICA/B
activates caspase3/7 through the FasL/Fas pathway, leading to the
degradation of CD3ζ, destroying the stability of CD3ζ, and thus
damaging the signal transmission of the TCR/CD3 complex (79).
Clinical significance of soluble
ligands
This section discusses the role of sNKG2DLs as
biomarkers across various cancers. Elevated levels of sNKG2DL,
including sMICA/B and sULBP variants, have shown diagnostic and
prognostic relevance. As detailed in Table II, these biomarkers contribute to
tumor diagnosis, malignancy assessment, and prognosis prediction,
enhancing clinical outcomes in oncology.
 | Table II.Clinical significance of soluble
ligands. |
Table II.
Clinical significance of soluble
ligands.
| Tumor | Type of
sNKG2DL | Level | Diagnostic
value | Assessing tumor
malignancy | Progression and
prognosis | (Refs.) |
|---|
| Lung cancer | sMICA | High level | + | - | + | (97,98) |
|
| sULBP2 | High level | + | - | + | (81) |
| Pancreatic ductal
adenocarcinoma | sMICA/B | High level | + | - | + | (83) |
| Head and neck
squamous cell | sNKG2DLs | High level | + | - | + | (99) |
| carcinoma | sMICA | High level | - | - | + | (100) |
| Hepatocellular
carcinoma | sMICA | High level | - | + | + | (89,101,102) |
|
| sMICA/B | High level | - | - | + | (92) |
| Breast cancer | sMICA | High level | - | + | + | (103,104) |
| Renal cell
carcinoma | sMICA | High level | - | + | + | (85,105) |
| Cervical
adenocarcinoma | sMICA | High level | - | + | + | (106) |
| Prostate
cancer | sMICA | High level | - | + | + | (88) |
| Pancreatic
cancer | sMICA/B | High level | - | + | - | (107) |
|
| sMICA | High level | - | + | + | (108,109) |
| Nasopharyngeal
carcinoma | sMICA | High level | - | + | - | (110) |
| Oral squamous cell
carcinoma | sMICA | High level | - | + | + | (111) |
| Cervical
cancer | sMICA | High level | - | - | + | (112) |
| Chronic lymphocytic
leukemia | sULBP2 | High level | - | - | + | (113) |
| Melanoma | sULBP1 | High level | - | - | + | (114) |
|
| sULBP2 | High level | - | - | + | (94) |
| Multiple
myeloma | sMICA | High level | - | - | + | (115) |
sNKG2DL as a tumor-related
biomarker
Immunological molecular markers play a vital role in
tumor diagnosis and are increasingly used in clinical practice.
Numerous studies have shown significant elevations in sNKG2DL
levels in the sera of various patients with cancer, suggesting its
potential as an auxiliary diagnostic biomarker for tumors. It has
been proven to be effective in some cancers (81). Particularly in pancreatic ductal
adenocarcinoma, CA19-9 (a commonly used clinical biomarker for
pancreatic cancer diagnosis) levels can increase under several
benign conditions, affecting its specificity (82). However, using sMICA and sMICB as
biomarkers has the potential to overcome the diagnostic limitations
of CA19-9 with higher specificity and sensitivity (83,84).
Nevertheless, distinguishing between malignant and benign kidney
tumors using sMICA alone has limited applicability (85). Moreover, a study with 512
participants with various cancers concluded that when potential
confounding factors were considered, the sMICA level was a valuable
tool for differential diagnosis and treatment efficacy evaluation
(86). However, the same conclusion
has not been drawn for sMICB (87).
Whether the level of sNKG2DL has diagnostic value
depends on the tumor type. However, despite the limited number of
studies, sNKG2DL has demonstrated great potential for auxiliary or
early diagnosis of some cancers and can be used to differentiate
between benign and malignant tumors (81–87).
Additionally, multiple experiments have suggested combining
detection with other biomarkers to improve results.
sNKG2DL levels for assessing tumor
malignancy
Serum sNKGDL levels have been observed to be closely
associated with higher tumor grading and staging (86,87).
It has now been observed in various cancers and serves as a
biomarker to assist in determining tumor malignancy or in early
screening (88,89). Notably, a report on liver cancer
observed that the concentration of sMICA/B decreased in the T4
stage or poorly differentiated tumors (G3). This outcome might be
due to severe impairment of liver cell function in patients with
end-stage cancer, such as protein synthesis disorders, or it might
be due to post-transcriptional regulation, such as proteasomal
degradation (90).
Although no studies have explicitly indicated that
serum sNKG2DL levels can be used clinically for tumor grading and
staging, the significant correlation between them suggests great
potential in this regard. However, a study has noted that
serum-sNKG2DL is a superior indicator of systemic tumor
manifestations than local tumor differentiation (91). Their roles in grading and staging,
however, require further evaluation.
sNKG2DL levels for predicting tumor
progression and prognosis
sNKG2DL reflects biological behaviors centered on
cancer cells and the state of tumor immune surveillance,
potentially serving as a predictive factor for rapid tumor
development, lymph node metastasis, or distant metastasis. sNKGDLs
have been found to correlate with tumor size, progression, and/or
metastasis (84,88). For instance, Qiu et al
(85) found that sMICA
concentration was significantly associated with lymph node
metastasis, distant metastasis, and vascular invasion in renal cell
carcinoma. Given the current absence of reliable noninvasive serum
biomarkers for the diagnosis and monitoring of patients with renal
cell carcinoma, this noninvasive detection method appears to have
an advantage (85). Additionally,
sNKG2DL may also be related to the transformation of precancerous
lesions into tumors, as it has been observed to increase under
various precancerous conditions (92,93).
For example, MICA shedding is a crucial determinant of host
immunity in the progression from monoclonal gammopathy of
undetermined significance (a precancerous condition) to mature
multiple myeloma (93).
Furthermore, sNKGDLs can be used to predict tumor
prognosis by correlating them with overall survival, recurrence
rates, treatment outcomes, and drug resistance, among other
indicators. Strong correlations have already been observed in
numerous cancers (81,94), with some being considered
independent indicators of poor prognosis. Notably, Paschen et
al (94) observed that sULBP
could serve as a prognostic biomarker, outperforming the already
established melanoma serum marker B100P. However, comparisons are
still lacking in a number of cancers, and the broader implications
of sNKG2DL require further evaluation. In a meta-analysis, Zhao
et al concluded that sMICA/B is a marker of tumor prognosis
and immunotherapy efficacy, corroborating the views expressed in
the present review (95). However,
some studies have indicated that sNKGDLs are not related to tumor
size, metastasis, or poor prognosis (86,96).
This may be due to factors, such as detection methods or sample
sources, suggesting the need for a standardized detection criterion
to make results comparable across different laboratories (8).
sNKG2DLs in drug development and
treatment
The development of therapies targeting sNKG2DLs
represents a dynamic frontier in cancer treatment. Advances in
NKG2D-CAR-T and CAR-NK cell therapies, alongside monoclonal
antibodies and targeted therapeutic approaches, exemplify
innovative strategies to enhance immune response against tumors.
These approaches focus on overcoming the immunosuppressive effects
of sNKG2DLs and enhancing the cytotoxic activity of immune cells.
An overview of various drugs targeting sNKG2DLs, their mechanisms,
and clinical indications, illustrating the breadth of current and
potential applications in hematological and solid tumor
malignancies is presented in Table
III.
 | Table III.Drugs targeting sNKG2DL. |
Table III.
Drugs targeting sNKG2DL.
| Category | Drug | Mechanism | Indication | (Refs.) |
|---|
| NKG2D CAR-T | CM-CS1 T-cell | NKG2D-modified
autologous | Acute myeloid
leukemia, | (117) |
|
|
| CAR-T cells | myelodysplastic
syndrome, multiple myeloma |
|
| NCT02203825 | KD-025 | NKG2DL-targeting
CAR-T | Colorectal
cancer | NCT05382377 |
|
|
|
| cells | Early Phase1 |
|
| IMC008 | NKG2D-modified
autologous | Advanced solid
tumor | NCT05837299 |
|
|
| CAR-T cells
targeting |
| Phase 1 |
|
|
| CLDN18.2 |
|
|
| NKG2D CAR-NK | MS-Ig | NKG2D-modified
autologous | CD20(+) lymphoma
cells | (118) |
|
|
| CAR NK cells
targeting CD20 |
|
|
|
| NKG2D CAR-NK | NKG2DL-targeting
CAR | Refractory
metastatic colorectal | NCT05213195 |
|
|
| NK cells | cancer | Phase 1 |
|
| NKG2D CAR-NK | NKG2DL-targeting
CAR | Multiple
myeloma | NCT06379451 |
|
|
| NK cells |
| Early Phase 1 |
| Monocloning | 7C6 | Targeting MICA α3
domain to | Melanoma, multiple
myeloma, | (119,121,122) |
| Antibody |
| prevent its
shedding |
cholangiocarcinoma |
|
|
| 5C6 and 1D11 | Targeting MICA α3
domain to prevent its shedding | Lymphoma | (120) |
|
| B10G5 | Targeting soluble
NKG2DL | Prostate cancer,
melanoma | (124,125) |
|
| CLN-619 | Targeting
MICA/MICB | Multiple
myeloma | NCT06381141 |
|
|
|
|
| phase 1 |
|
| DM919 | Targeting
MICA/MICB | Solid tumors | NCT06328673 |
|
|
|
|
| Phase 1 |
| HDACi | Valproic acid | Inhibition of
histone | Pancreatic cancer,
Acute myeloid | (128,129) |
|
|
| deacetylase | leukemia |
|
|
| Sodium
butyrate | Inhibition of
histone | Multiple
myeloma | (135) |
|
|
| deacetylase |
|
|
|
Metalloproteinase | ADAMi | Inhibition of
ADAM10 | Hodgkin
lymphoma | (131) |
| Inhibitor |
| expression |
|
|
|
| MMPi | Inhibition of MMP
expression | Multiple
myeloma | (135) |
| Other drugs | Gemcitabine | Inhibition of
ADAM10 | Hepatocellular
carcinoma, | (137) |
|
|
| expression | Pancreatic
cancer |
|
|
| phenylarsine | Inhibition of the
protein | Multiple
myeloma | (135) |
|
| oxide | disulfide isomerase
ERp5 |
|
|
|
| Disulfiram | Inhibition of
ADAM10 | Hepatocellular
carcinoma | (138) |
|
|
| expression |
|
|
Immunotherapeutic approaches
Association between NKG2D-CAR-T cell therapy and
sNKG2DL
NKG2D-CAR-T cell therapy is gaining increasing
attention. While NK cells are not restricted by MHC and have
relatively weak antitumor capabilities due to insufficient
migration to tumor cells, T cells can effectively kill tumor cells;
however, their recognition is limited by T-cell receptor (TCR).
NKG2D-CAR-T cells effectively combine the advantages of both NK and
T cells, exhibiting strong targeted cytotoxicity against various
tumors (Fig. 3) (116). As NKG2DL is rarely expressed in
normal tissues, the likelihood of autoimmune reactions or long-term
toxicity is low (117). The
efficacy of NKG2D-CAR-T cells depends on the expression level of
NKG2DL. Therefore, they may be less effective against tumor cells
that express low levels of NKG2DL, or may shed them as soluble
ligands. However, Zhang et al (116) observed that sMICA, even at
concentrations markedly higher than those of serum, maintains full
functionality, suggesting that NKG2D-CAR-T cell therapy can, to
some extent, overcome the immunosuppressive effects of soluble
ligands.
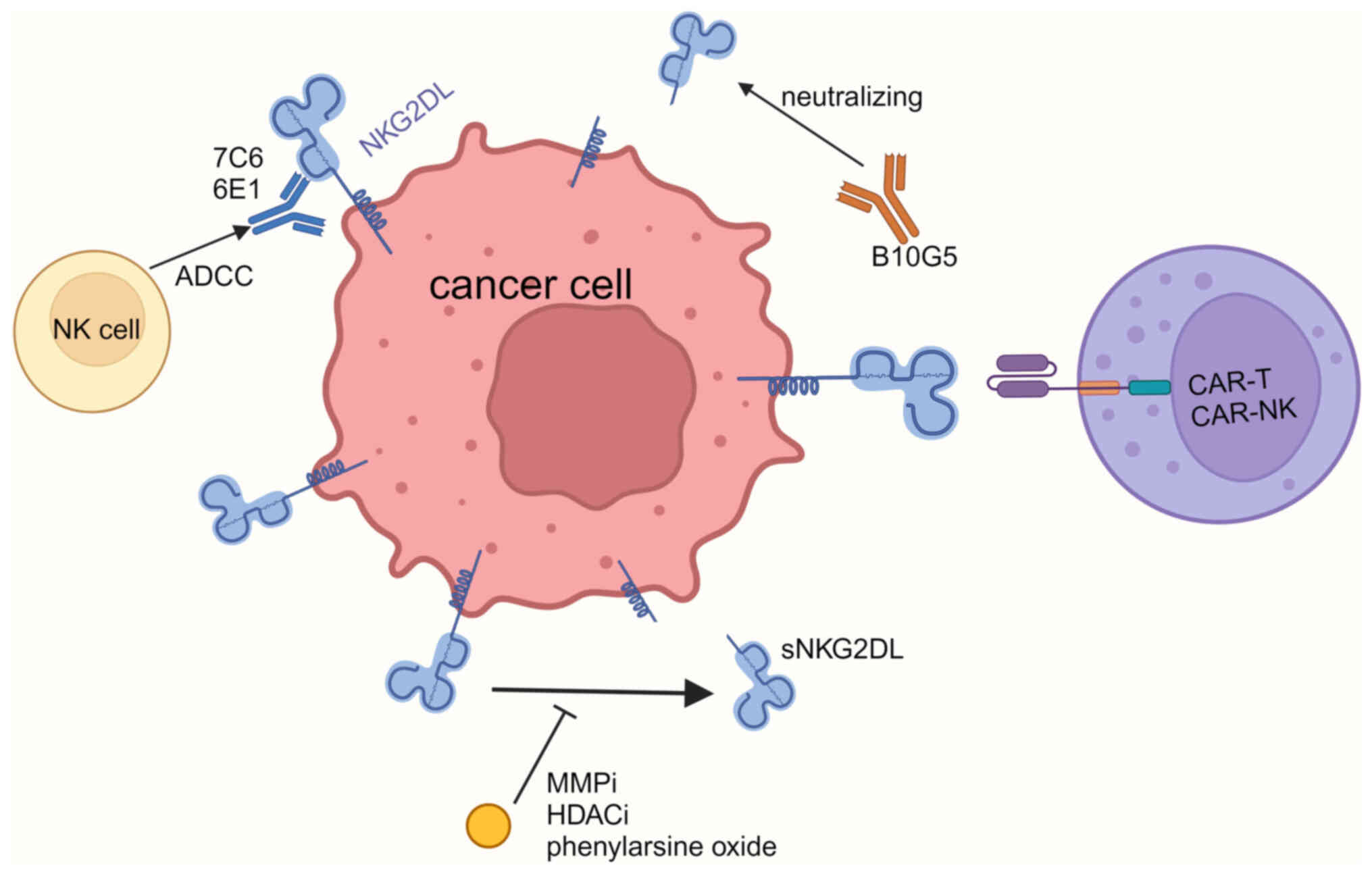 | Figure 3.Treatment targeting sNKG2DL. mAb 7C6
and 6E1 can bind to MICA α3 domain, which inhibits the shedding of
MICA, and triggers immune cells to kill tumor cells through ADCC.
mAb B10G5 neutralizes sMICA. MMPi, HDACi and disulfiram can inhibit
the shedding of MICA. NKG2D-CAR-T/NK targets NKG2DL and kills tumor
cells. sNKG2DL, soluble natural killer group 2 member D ligand;
mAb, monoclonal antibody; MICA, MHC class I polypeptide-related
sequence A; ADCC, antibody-dependent cellular cytotoxicity; sMICA,
soluble MICA; MMPi, matrix metalloproteinase inhibitor; HDACi,
histone deacetylase inhibitor; CAR, chimeric antigen receptor; NK,
natural killer; NKG2DL, natural killer group 2 member D ligand. |
Nevertheless, both CAR-T and CAR-NK cell therapies
unavoidably face challenges, such as the need for ex vivo
expansion and modification, and relatively high costs. Recently,
Liu et al (118) developed
a new soluble NK-CAR for hematological malignancies, termed MS-Ig.
One end can bind to CD20 on tumor cells and the other end can bind
to the NKG2D receptor on NK cells, thus mediating targeted
cytotoxicity. This approach does not require personalized treatment
and reduces toxicity caused by immune cell infusion (118).
Targeting membrane-bound MICA/B with
mAbs to prevent its shedding enhances the cytotoxic effect of NK
cells
mAbs targeting the MICA α3 domain, such as 7C6 and
6E1, can inhibit the shedding of MICA/B and increase its surface
expression, suppressing tumor metastasis (Fig. 3) (119–121). When used in conjunction with HDAC
inhibitors, HDAC inhibitors enhance the expression of the
MICA/B gene, whereas MICA/B antibodies stabilize proteins
synthesized on the cell surface, thereby achieving improved
antitumor effects (121,122). Additionally, the Fcγ segment of
the mAb can mediate the antibody-dependent cellular cytotoxicity
effect, triggering immune cells to kill target cells (121). Since the α3 domain is relatively
conserved, it can overcome the effects of MICA/B polymorphism
(119). Recently, Badrinath et
al (123) designed a tumor
vaccine targeting the MICA α3 domain, which can induce the body to
produce antibodies against the MICA α3 domain, inhibiting the
proteolytic shedding of MICA/B from tumor cells, enhancing the
cytotoxic function of NK cells, and increasing the cDC1-mediated
cross-presentation of tumor antigens to CD8+T cells,
inducing responses against MICA/B in both CD4+ and
CD8+ T cells (123).
mAbs targeting sNKG2DL neutralize and
reduce immunosuppressive effects
The mAb targeting sMICA, B10G5, is considered to
neutralize free sMIC in the plasma, thus alleviating the
immunosuppressive effect of sMIC (Fig.
3). Although B10G5 can also recognize membrane-bound MIC, it
binds at a site different from that of NKG2D, thus it does not
interfere with the function of NKG2D. On the contrary, owing to its
antibody-dependent cellular cytotoxicity action, it may enhance the
reactivity of NK cells toward tumor cells (124). Basher et al (125) observed that the monotherapy with
B10G5 to clear sMIC effectively controlled tumor growth and
eliminated lung metastasis. Additionally, since the membrane-bound
MIC is already low in patients in the late stages of cancer, the
effect of inhibiting its shedding is minimal; thus, using the
neutralizing antibody B10G5 can have a superior effect at this
stage (124).
High-affinity soluble ligands
upregulate membrane NKG2D expression and affect NK cell
activity
MULT1 is a ligand for NKG2D in mice, and compared
with MICA/B, it is a high-affinity, soluble ligand. It can
upregulate the expression of membrane NKG2D and prevent NK cells
from being desensitized by sNKG2DL, thus emerging as a potential
mechanism to enhance antitumor immunity. In an experimental design
developed by Deng et al (76), cells were generated to induce the
production of secMULT1 (an extracellular fragment of HA-MULT1
secreted by induced fibroblasts). It was found to be resistant in
various mouse models, in which secMULT1 mobilized or activated
antitumor effector cells. As the adjustment of NK cell reactivity
by sNKG2DLs depends on their affinity to NKG2D, the preclinical
development of this new class of candidate drugs may reveal new
pharmacokinetics and pharmacodynamic guidelines (126).
Targeted therapeutic approaches
Reducing sNKG2DL production using HDAC
inhibitors
HDAC inhibitors have been approved by the FDA for
the treatment of hematological malignancies and play a role in
reducing the generation of sNKG2DL (Fig. 3). When used alone or in combination
with mAbs and cytotoxic chemotherapy drugs, HDCA inhibitors may
exert antitumor effects by increasing MICA expression and
downregulating metalloproteinase activity, thereby inhibiting
sNKG2DL production (127–129). However, due to their significant
side effects, the use of HDAC inhibitors is limited. Enhancing
selectivity might improve their clinical value (130).
Reducing sNKG2DL production by
inhibiting metalloproteinase activity
Given the critical role of ADAM and MMP in the
release of sNKG2DL, specific inhibition of ADAM and MMP may enhance
the recognition of tumor cells by the immune system. Multiple
studies have observed a reduction in sNKG2DL content in
supernatants after treatment with metalloproteinase inhibitors
(72,119,122), with some studies concurrently
observing elevated levels of membrane-bound NKG2DL (Fig. 3) (60,131).
However, most experiments remain at the preclinical cell-level
stage, and studies on the in vivo efficacy and potential
side effects are either insufficient or absent. Furthermore, TIMPs,
which are endogenous proteinase inhibitors, inhibit MMPs when their
expression is upregulated. Fatty acid amide hydrolase inhibitors
(132) and demethylating agents
(azacytidine and decitabine) (42)
inhibited sNKG2DL shedding by upregulating expression of TIMPs.
Targeted therapies that inhibit multiple enzymes and thus reduce
sNKG2DL production are feasible approaches. However, some
inhibitors have been reported to have side effects or toxicity,
which limit the application of this method (133).
Reducing sNKG2DL production by
inhibiting protein disulfide isomerase
Experiments have observed that phenylarsine oxide,
an inhibitor of the protein disulfide isomerase ERp5, can inhibit
the release of sNKG2DL (Fig. 3).
However, its effective concentrations are toxic. The combination of
phenylarsine oxide with HDAC inhibitors and MMP inhibitors may have
a synergistic effect (134,135).
Discussion
The present review examined the soluble forms of
NKG2DLs released from the cell membrane into the extracellular
environment. Their generation mechanisms, such as cleavage by MMPs
and ADAMs, and their release via exosomes were explored, and the
factors influencing their formation, such as cellular stress and
the tumor microenvironment were outlined. Studies have shown that
sNKG2DLs can inhibit NKG2D receptors, diminish NK cell activity,
and affect their ability to monitor tumors (1,5).
Clinically, their increase correlates with higher tumor staging and
poor prognosis, whereas some drugs or treatments can decrease their
production, alleviate the immunosuppressive effects, and promote
antitumor responses (95,136).
Tumors and their microenvironments are intricate,
and the impact of various cells and cytokines on the production of
soluble ligands remains unclear. The extensive polymorphisms of the
MICA family and their implications for tumor immunity require
detailed analysis. The complexity of extracellular vesicles makes
research particularly challenging, especially because the ligands
on exosomes may have even more crucial immunosuppressive roles in
the tumor microenvironment (41).
From a mechanism standpoint, the generation of these soluble
ligands appears more as ‘byproducts’ caused by tumor progression,
such as the activation of MICA/B and expression of ULBPs,
activation of MMPs and ADAMs, and exosome release. Thus, it remains
unclear whether these ligands play pivotal roles in tumor
progression.
Circulating NK cells and the NKG2D-NKG2DL axis play
vital roles in the surveillance and suppression of tumor
metastasis. Elevated sNKG2DL levels can be observed in the serum of
most patients with cancer, and those with higher levels usually
exhibit higher malignancy and worse prognosis. Hence, serum sNKG2DL
levels have the potential to be used as immunological molecular
markers for tumor diagnosis, malignancy evaluation, and prognostic
prediction. Monitoring serum sNKG2DL levels, especially during the
early tumor stages, can be invaluable. As a non-invasive,
cost-effective, and repeatable circulating biomarker, serum sNKG2DL
levels overcome the limitations of other tumor markers, especially
when tumors are deep-seated or when patients cannot undergo
biopsies (85,95). Additionally, as aforementioned,
various genotypes influence sMICA production, potentially affecting
tumor progression and prognosis. Evaluating the MICA genotypes of
patients may offer new insights (51,56).
However, serum sNKG2DL levels are significantly
elevated across various tumors, resulting in low specificity. Their
primary value may be in assessing tumor malignancy and predicting
metastasis, or perhaps in combination with other tumor markers.
Questions regarding the detection efficiency, inter-individual
variability, and appropriate threshold values remain unresolved.
Additionally, there is a scarcity of related clinical studies, and
most have issues, such as small sample sizes or singular sample
origins, indicating a gap before wide clinical application.
The effects of immunotherapy on soluble ligand
production have opened new avenues for therapeutic interventions
targeting these ligands. Numerous immunotherapies targeting the
NKG2D-NKG2DL axis are currently being developed. These treatments
enhance cellular recognition, inhibit ligand shedding, and remove
existing soluble ligands that exert antitumor effects. This offers
a novel approach for determining whether existing immunotherapies
can be adapted to target the NKG2D-NKG2DL axis for broader
recognition and fewer side effects. However, many of these
therapies are in the early stages of development, and their
efficacy and safety in humans remain unclear. Further clinical
trials are required to determine their roles in cancer
treatment.
In summary, this review thoroughly examined the
production and multifaceted roles of sNKG2DL in the tumor
microenvironment. This underscores the immunosuppressive ability of
sNKG2DL, its potential as a diagnostic and prognostic marker, and
its emerging role as a therapeutic target. Despite the existing
limitations and areas needing further exploration, the evidence
strongly attests to the significance of sNKG2DL in tumor immunity.
Future research should probe the potential of targeting sNKG2DL in
tumor immunotherapy, providing a scientific foundation for
integrated tumor immune treatments.
Acknowledgements
We would like to acknowledge Professor Yizhou Zou,
Department of Immunology, School of Basic Medical Science, Central
South University (Changsha, China) for his assistance with the
language editing of this manuscript. In addition, Figs. 2 and 3 were created using BioRender.com.
Funding
This work was supported by the Hunan Provincial Science and
Technology Innovation Plan Project (grant no. 2021SK50801).
Availability of data and materials
Not applicable.
Authors' contributions
SH, ZQ, FW contributed to the drafting and editing
of the manuscript. YK contributed to drawing the figures and
editing the manuscript. BR designed, revised and finalized the
manuscript. All authors contributed toward the drafting and
revising, and agreed to submit the present study. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y,
Wang Y, Xiong F, Guo C, Li Y, et al: Natural killer group 2D
receptor and its ligands in cancer immune escape. Mol Cancer.
18:292019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan G, Spillane KM and Maher J: The role
and regulation of the NKG2D/NKG2D ligand system in cancer. Biology
(Basel). 12:10792023.PubMed/NCBI
|
|
3
|
Ullrich E, Koch J, Cerwenka A and Steinle
A: New prospects on the NKG2D/NKG2DL system for oncology.
Oncoimmunology. 2:e260972013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raulet DH: Roles of the NKG2D
immunoreceptor and its ligands. Nat Rev Immunol. 3:781–790. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zingoni A, Molfetta R, Fionda C, Soriani
A, Paolini R, Cippitelli M, Cerboni C and Santoni A: NKG2D and its
ligands: ‘One for all, all for one’. Front Immunol. 9:4762018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tchacrome I, Zhu Q, Saleh MA and Zou Y:
Diseases association with the polymorphic major histocompatibility
complex class I related chain a: MICA gene. Transpl Immunol.
75:1016652022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maurer S, Zhong X, Prada BD, Mascarenhas J
and de Andrade LF: The latest breakthroughs in immunotherapy for
acute myeloid leukemia, with a special focus on NKG2D ligands. Int
J Mol Sci. 23:159072022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campos-Silva C, López-Borrego S, Felgueres
MJ, Esteso G and Vales-Gomez M: NKG2D ligands in liquid biopsy: The
importance of soluble and vesicle-bound proteins for immune
modulation. Crit Rev Immunol. 42:21–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lanier LL: NKG2D receptor and its ligands
in host defense. Cancer Immunol Res. 3:575–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Touboul R, Zaravinos A and Bonavida B:
Defective natural killer cells in melanoma: Role of NKG2D in
pathogenesis and immunotherapy. Crit Rev Immunol. 41:45–76. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones AB, Rocco A, Lamb LS, Friedman GK
and Hjelmeland AB: Regulation of NKG2D stress ligands and its
relevance in cancer progression. Cancers (Basel). 14:23392022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Groh V, Bahram S, Bauer S, Herman A,
Beauchamp M and Spies T: Cell stress-regulated human major
histocompatibility complex class I gene expressed in
gastrointestinal epithelium. Proc Natl Acad Sci USA.
93:12445–12450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cosman D, Müllberg J, Sutherland CL, Chin
W, Armitage R, Fanslow W, Kubin M and Chalupny NJ: ULBPs, novel MHC
class I-related molecules, bind to CMV glycoprotein UL16 and
stimulate NK cytotoxicity through the NKG2D receptor. Immunity.
14:123–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Groh V, Steinle A, Bauer S and Spies T:
Recognition of stress-induced MHC molecules by intestinal
epithelial gammadelta T cells. Science. 279:1737–1740. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venkataraman GM, Suciu D, Groh V, Boss JM
and Spies T: Promoter region architecture and transcriptional
regulation of the genes for the MHC class I-related chain A and B
ligands of NKG2D. J Immunol. 178:961–969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Textor S, Fiegler N, Arnold A, Porgador A,
Hofmann TG and Cerwenka A: Human NK cells are alerted to induction
of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1
and ULBP2. Cancer Res. 71:5998–6009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Simon M, Seluanov A and Gorbunova
V: DNA damage and repair in age-related inflammation. Nat Rev
Immunol. 23:75–89. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin D, Lavender H, Soilleux EJ and
O'Callaghan CA: NF-κB regulates MICA gene transcription in
endothelial cell through a genetically inhibitable control site. J
Biol Chem. 287:4299–4310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schrambach S, Ardizzone M, Leymarie V,
Sibilia J and Bahram S: In vivo expression pattern of MICA and MICB
and its relevance to auto-immunity and cancer. PLoS One.
2:e5182007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stern-Ginossar N, Gur C, Biton M, Horwitz
E, Elboim M, Stanietsky N, Mandelboim M and Mandelboim O: Human
microRNAs regulate stress-induced immune responses mediated by the
receptor NKG2D. Nat Immunol. 9:1065–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yadav D, Ngolab J, Lim RSH, Krishnamurthy
S and Bui JD: Cutting edge: Down-regulation of MHC class I-related
chain A on tumor cells by IFN-gamma-induced microRNA. J Immunol.
182:39–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinemann A, Zhao F, Pechlivanis S, Eberle
J, Steinle A, Diederichs S, Schadendorf D and Paschen A: Tumor
suppressive microRNAs miR-34a/c control cancer cell expression of
ULBP2, a stress-induced ligand of the natural killer cell receptor
NKG2D. Cancer Res. 72:460–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato N, Tanaka J, Sugita J, Toubai T,
Miura Y, Ibata M, Syono Y, Ota S, Kondo T, Asaka M and Imamura M:
Regulation of the expression of MHC class I-related chain A, B
(MICA, MICB) via chromatin remodeling and its impact on the
susceptibility of leukemic cells to the cytotoxicity of
NKG2D-expressing cells. Leukemia. 21:2103–2108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morimoto Y, Yamashita N, Daimon T, Hirose
H, Yamano S, Haratake N, Ishikawa S, Bhattacharya A, Fushimi A,
Ahmad R, et al: MUC1-C is a master regulator of MICA/B NKG2D ligand
and exosome secretion in human cancer cells. J Immunother Cancer.
11:e0062382023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Rao A, Sette P, Deibert C,
Pomerantz A, Kim WJ, Kohanbash G, Chang Y, Park Y, Engh J, et al:
IDH mutant gliomas escape natural killer cell immune surveillance
by downregulation of NKG2D ligand expression. Neuro Oncol.
18:1402–1412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsukerman P, Stern-Ginossar N, Gur C,
Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky
N, Bar-Mag T, et al: MiR-10b downregulates the stress-induced cell
surface molecule MICB, a critical ligand for cancer cell
recognition by natural killer cells. Cancer Res. 72:5463–5472.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Codo P, Weller M, Meister G, Szabo E,
Steinle A, Wolter M, Reifenberger G and Roth P: MicroRNA-mediated
down-regulation of NKG2D ligands contributes to glioma immune
escape. Oncotarget. 5:7651–7662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Breunig C, Pahl J, Küblbeck M, Miller M,
Antonelli D, Erdem N, Wirth C, Will R, Bott A, Cerwenka A and
Wiemann S: MicroRNA-519a-3p mediates apoptosis resistance in breast
cancer cells and their escape from recognition by natural killer
cells. Cell Death Dis. 8:e29732017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eagle RA, Flack G, Warford A,
Martínez-Borra J, Jafferji I, Traherne JA, Ohashi M, Boyle LH,
Barrow AD, Caillat-Zucman S, et al: Cellular expression,
trafficking, and function of two isoforms of human ULBP5/RAET1G.
PLoS One. 4:e45032009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernández-Messina L, Reyburn HT and
Valés-Gómez M: A short half-life of ULBP1 at the cell surface due
to internalization and proteosomal degradation. Immunol Cell Biol.
94:479–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agüera-González S, Boutet P, Reyburn HT
and Valés-Gómez M: Brief residence at the plasma membrane of the
MHC class I-related chain B is due to clathrin-mediated
cholesterol-dependent endocytosis and shedding. J Immunol.
182:4800–4808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gomis-Rüth FX: Structural aspects of the
metzincin clan of metalloendopeptidases. Mol Biotechnol.
24:157–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai
Z, Mann HH, Strong RK, Groh V and Spies T:
Disulphide-isomerase-enabled shedding of tumour-associated NKG2D
ligands. Nature. 447:482–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Lundgren AD, Singh P, Goodlett DR,
Plymate SR and Wu JD: An six-amino acid motif in the alpha3 domain
of MICA is the cancer therapeutic target to inhibit shedding.
Biochem Biophys Res Commun. 387:476–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Waldhauer I, Goehlsdorf D, Gieseke F,
Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG
and Steinle A: Tumor-associated MICA is shed by ADAM proteases.
Cancer Res. 68:6368–6376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Waldhauer I and Steinle A: Proteolytic
release of soluble UL16-binding protein 2 from tumor cells. Cancer
Res. 66:2520–2526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boutet P, Agüera-González S, Atkinson S,
Pennington CJ, Edwards DR, Murphy G, Reyburn HT and Valés-Gómez M:
Cutting edge: The metalloproteinase ADAM17/TNF-alpha-converting
enzyme regulates proteolytic shedding of the MHC class I-related
chain B protein. J Immunol. 182:49–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fernández-Messina L, Ashiru O, Boutet P,
Agüera-González S, Skepper JN, Reyburn HT and Valés-Gómez M:
Differential mechanisms of shedding of the
glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol
Chem. 285:8543–8551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raneros AB, Minguela A, Rodriguez RM,
Colado E, Bernal T, Anguita E, Mogorron AV, Gil AC,
Vidal-Castiñeira JR, Márquez-Kisinousky L, et al: Increasing TIMP3
expression by hypomethylating agents diminishes soluble MICA, MICB
and ULBP2 shedding in acute myeloid leukemia, facilitating NK
cell-mediated immune recognition. Oncotarget. 8:31959–31976. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brown DA: Lipid rafts, detergent-resistant
membranes, and raft targeting signals. Physiology (Bethesda).
21:430–439. 2006.PubMed/NCBI
|
|
44
|
de Gassart A, Geminard C, Fevrier B,
Raposo G and Vidal M: Lipid raft-associated protein sorting in
exosomes. Blood. 102:4336–4344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ashiru O, Boutet P, Fernández-Messina L,
Agüera-González S, Skepper JN, Valés-Gómez M and Reyburn HT:
Natural killer cell cytotoxicity is suppressed by exposure to the
human NKG2D ligand MICA*008 that is shed by tumor cells in
exosomes. Cancer Res. 70:481–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hedlund M, Nagaeva O, Kargl D, Baranov V
and Mincheva-Nilsson L: Thermal- and oxidative stress causes
enhanced release of NKG2D ligand-bearing immunosuppressive exosomes
in leukemia/lymphoma T and B cells. PLoS One. 6:e168992011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Agüera-González S, Gross CC,
Fernández-Messina L, Ashiru O, Esteso G, Hang HC, Reyburn HT, Long
EO and Valés-Gómez M: Palmitoylation of MICA, a ligand for NKG2D,
mediates its recruitment to membrane microdomains and promotes its
shedding. Eur J Immunol. 41:3667–3676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eleme K, Taner SB, Onfelt B, Collinson LM,
McCann FE, Chalupny NJ, Cosman D, Hopkins C, Magee AI and Davis DM:
Cell surface organization of stress-inducible proteins ULBP and
MICA that stimulate human NK cells and T cells via NKG2D. J Exp
Med. 199:1005–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bacon L, Eagle RA, Meyer M, Easom N, Young
NT and Trowsdale J: Two human ULBP/RAET1 molecules with
transmembrane regions are ligands for NKG2D. J Immunol.
173:1078–1084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao W, Xi X, Hao Z, Li W, Kong Y, Cui L,
Ma C, Ba D and He W: RAET1E2, a soluble isoform of the UL16-binding
protein RAET1E produced by tumor cells, inhibits NKG2D-mediated NK
cytotoxicity. J Biol Chem. 282:18922–18928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zingoni A, Vulpis E, Cecere F, Amendola
MG, Fuerst D, Saribekyan T, Achour A, Sandalova T, Nardone I, Peri
A, et al: MICA-129 dimorphism and soluble MICA are associated with
the progression of multiple myeloma. Front Immunol. 9:9262018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Toledo-Stuardo K, Ribeiro CH, Canals A,
Morales M, Gárate V, Rodríguez-Siza J, Tello S, Bustamante M,
Armisen R, Matthies DJ, et al: Major histocompatibility complex
class I-related chain A (MICA) allelic variants associate with
susceptibility and prognosis of gastric cancer. Front Immunol.
12:6455282021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ashiru O, López-Cobo S, Fernández-Messina
L, Pontes-Quero S, Pandolfi R, Reyburn HT and Valés-Gómez M: A GPI
anchor explains the unique biological features of the common
NKG2D-ligand allele MICA*008. Biochem J. 454:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
López-Cobo S, Campos-Silva C and
Valés-Gómez M: Glycosyl-phosphatidyl-inositol (GPI)-anchors and
metalloproteases: Their roles in the regulation of exosome
composition and NKG2D-mediated immune recognition. Front Cell Dev
Biol. 4:972016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Isernhagen A, Schilling D, Monecke S, Shah
P, Elsner L, Walter L, Multhoff G and Dressel R: The
MICA-129Met/Val dimorphism affects plasma membrane expression and
shedding of the NKG2D ligand MICA. Immunogenetics. 68:109–123.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kumar V, Kato N, Urabe Y, Takahashi A,
Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H, et
al: Genome-wide association study identifies a susceptibility locus
for HCV-induced hepatocellular carcinoma. Nat Genet. 43:455–458.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
57
|
Barsoum IB, Hamilton TK, Li X, Cotechini
T, Miles EA, Siemens DR and Graham CH: Hypoxia induces escape from
innate immunity in cancer cells via increased expression of ADAM10:
role of nitric oxide. Cancer Res. 71:7433–7441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ou ZL, Luo Z, Wei W, Liang S, Gao TL and
Lu YB: Hypoxia-induced shedding of MICA and HIF1A-mediated immune
escape of pancreatic cancer cells from NK cells: Role of
circ_0000977/miR-153 axis. RNA Biol. 16:1592–1603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gorgoulis V, Adams PD, Alimonti A, Bennett
DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K,
Ferbeyre G, et al: Cellular senescence: Defining a path forward.
Cell. 179:813–827. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Hu R, Xi B, Nie D, Xu H and Liu
A: Mechanisms of senescence-related NKG2D ligands release and
immune escape induced by chemotherapy in neuroblastoma cells. Front
Cell Dev Biol. 10:8294042022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kohga K, Takehara T, Tatsumi T, Ishida H,
Miyagi T, Hosui A and Hayashi N: Sorafenib inhibits the shedding of
major histocompatibility complex class I-related chain A on
hepatocellular carcinoma cells by down-regulating a disintegrin and
metalloproteinase 9. Hepatology. 51:1264–1273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ziani L, Safta-Saadoun TB, Gourbeix J,
Cavalcanti A, Robert C, Favre G, Chouaib S and Thiery J:
Melanoma-associated fibroblasts decrease tumor cell susceptibility
to NK cell-mediated killing through matrix-metalloproteinases
secretion. Oncotarget. 8:19780–19794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maurer S, Kropp KN, Klein G, Steinle A,
Haen SP, Walz JS, Hinterleitner C, Märklin M, Kopp HG and Salih HR:
Platelet-mediated shedding of NKG2D ligands impairs NK cell
immune-surveillance of tumor cells. Oncoimmunology. 7:e13648272017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zocchi MR, Catellani S, Canevali P,
Tavella S, Garuti A, Villaggio B, Zunino A, Gobbi M,
Fraternali-Orcioni G, Kunkl A, et al: High ERp5/ADAM10 expression
in lymph node microenvironment and impaired NKG2D ligands
recognition in Hodgkin lymphomas. Blood. 119:1479–1489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kohga K, Tatsumi T, Tsunematsu H, Aono S,
Shimizu S, Kodama T, Hikita H, Yamamoto M, Oze T, Aketa H, et al:
Interleukin-1β enhances the production of soluble MICA in human
hepatocellular carcinoma. Cancer Immunol Immunother. 61:1425–1432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu Y, Jiang F, Zheng X, Katakowski M,
Buller B, To SS and Chopp M: TGF-β1 promotes motility and
invasiveness of glioma cells through activation of ADAM17. Oncol
Rep. 25:1329–1335. 2011.PubMed/NCBI
|
|
68
|
Eisele G, Wischhusen J, Mittelbronn M,
Meyermann R, Waldhauer I, Steinle A, Weller M and Friese MA:
TGF-beta and metalloproteinases differentially suppress NKG2D
ligand surface expression on malignant glioma cells. Brain.
129:2416–2425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fang X, Guo L, Xing Z, Shi L, Liang H, Li
A, Kuang C, Tao B and Yang Q: IDO1 can impair NK cells function
against non-small cell lung cancer by downregulation of NKG2D
ligand via ADAM10. Pharmacol Res. 177:1061322022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Del Toro-Arreola S, Arreygue-Garcia N,
Aguilar-Lemarroy A, Cid-Arregui A, Jimenez-Perez M, Haramati J,
Barros-Nuñez P, Gonzalez-Ramella O, Del Toro-Arreola A,
Ortiz-Lazareno P, et al: MHC class I-related chain A and B ligands
are differentially expressed in human cervical cancer cell lines.
Cancer Cell Int. 11:152011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hilpert J, Grosse-Hovest L, Grünebach F,
Buechele C, Nuebling T, Raum T, Steinle A and Salih HR:
Comprehensive analysis of NKG2D ligand expression and release in
leukemia: Implications for NKG2D-mediated NK cell responses. J
Immunol. 189:1360–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Arai J, Goto K, Otoyama Y, Nakajima Y,
Sugiura I, Kajiwara A, Tojo M, Ichikawa Y, Uozumi S, Shimozuma Y,
et al: Leukotriene receptor antagonists enhance HCC treatment
efficacy by inhibiting ADAMs and suppressing MICA shedding. Cancer
Immunol Immunother. 70:203–213. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Molfetta R, Quatrini L, Zitti B, Capuano
C, Galandrini R, Santoni A and Paolini R: Regulation of NKG2D
expression and signaling by endocytosis. Trends Immunol.
37:790–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Clayton A and Tabi Z: Exosomes and the
MICA-NKG2D system in cancer. Blood Cells Mol Dis. 34:206–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Molfetta R, Quatrini L, Capuano C,
Gasparrini F, Zitti B, Zingoni A, Galandrini R, Santoni A and
Paolini R: c-Cbl regulates MICA-but not ULBP2-induced NKG2D
down-modulation in human NK cells. Eur J Immunol. 44:2761–2770.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Deng W, Gowen BG, Zhang L, Wang L, Lau S,
Iannello A, Xu J, Rovis TL, Xiong N and Raulet DH: Antitumor
immunity. A shed NKG2D ligand that promotes natural killer cell
activation and tumor rejection. Science. 348:136–139. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vulpis E, Loconte L, Peri A, Molfetta R,
Caracciolo G, Masuelli L, Tomaipitinca L, Peruzzi G, Petillo S,
Petrucci MT, et al: Impact on NK cell functions of acute versus
chronic exposure to extracellular vesicle-associated MICA: Dual
role in cancer immunosurveillance. J Extracell Vesicles.
11:e121762022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jinushi M, Takehara T, Tatsumi T,
Hiramatsu N, Sakamori R, Yamaguchi S and Hayashi N: Impairment of
natural killer cell and dendritic cell functions by the soluble
form of MHC class I-related chain A in advanced human
hepatocellular carcinomas. J Hepatol. 43:1013–1020. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang J, Liu D, Li G, Staveley-O'Carroll
KF, Graff JN, Li Z and Wu JD: Antibody-mediated neutralization of
soluble MIC significantly enhances CTLA4 blockade therapy. Sci Adv.
3:e16021332017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiao G, Wang X, Sheng J, Lu S, Yu X and Wu
JD: Soluble NKG2D ligand promotes MDSC expansion and skews
macrophage to the alternatively activated phenotype. J Hematol
Oncol. 8:132015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yamaguchi K, Chikumi H, Shimizu A, Takata
M, Kinoshita N, Hashimoto K, Nakamoto M, Matsunaga S, Kurai J,
Miyake N, et al: Diagnostic and prognostic impact of serum-soluble
UL16-binding protein 2 in lung cancer patients. Cancer Sci.
103:1405–1413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Duffy MJ, Sturgeon C, Lamerz R, Haglund C,
Holubec VL, Klapdor R, Nicolini A, Topolcan O and Heinemann V:
Tumor markers in pancreatic cancer: A European group on tumor
markers (EGTM) status report. Ann Oncol. 21:441–447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chung HW and Lim JB: Clinical significance
of serum levels of immune-associated molecules, uric acid and
soluble MHC class I chain-related molecules A and B, as diagnostic
tumor markers for pancreatic ductal adenocarcinoma. Cancer Sci.
102:1673–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chung HW, Jang S and Lim JB: Clinical
implications and diagnostic usefulness of correlation between
soluble major histocompatibility complex class I chain-related
molecule a and protumorigenic cytokines in pancreatic ductal
adenocarcinoma. Cancer. 119:233–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Qiu Y, Zhao YK, Yuan GJ and Zhu QG:
Clinical significance of soluble major histocompatibility complex
class I chain-related a in renal cell carcinoma patients. Asian Pac
J Cancer Prev. 14:5651–5655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Holdenrieder S, Stieber P, Peterfi A,
Nagel D, Steinle A and Salih HR: Soluble MICA in malignant
diseases. Int J Cancer. 118:684–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Holdenrieder S, Stieber P, Peterfi A,
Nagel D, Steinle A and Salih HR: Soluble MICB in malignant
diseases: Analysis of diagnostic significance and correlation with
soluble MICA. Cancer Immunol Immunother. 55:1584–1589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu JD, Higgins LM, Steinle A, Cosman D,
Haugk K and Plymate SR: Prevalent expression of the
immunostimulatory MHC class I chain-related molecule is
counteracted by shedding in prostate cancer. J Clin Invest.
114:560–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jiang X, Huang JF, Huo Z, Zhang Q, Jiang
Y, Wu X, Li Y, Jiang G, Zeng L, Yan XX, et al: Elevation of soluble
major histocompatibility complex class I related chain A protein in
malignant and infectious diseases in Chinese patients. BMC Immunol.
13:622012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mantovani S, Varchetta S, Mele D, Donadon
M, Torzilli G, Soldani C, Franceschini B, Porta C, Chiellino S,
Pedrazzoli P, et al: An anti-MICA/B antibody and IL-15 rescue
altered NKG2D-dependent NK cell responses in hepatocellular
carcinoma. Cancers (Basel). 12:35832020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kshersagar J, Damle MN, Bedge P, Jagdale
R, Tardalkar K, Jadhav D, Jagadale S, Toro Y, Sharma R and Joshi
MG: Downregulation of MICA/B tumor surface expressions and
augmented soluble MICA serum levels correlate with disease stage in
breast cancer. Breast Dis. 41:471–480. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kohga K, Takehara T, Tatsumi T, Ohkawa K,
Miyagi T, Hiramatsu N, Kanto T, Kasugai T, Katayama K, Kato M and
Hayashi N: Serum levels of soluble major histocompatibility complex
(MHC) class I-related chain A in patients with chronic liver
diseases and changes during transcatheter arterial embolization for
hepatocellular carcinoma. Cancer Sci. 99:1643–1649. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jinushi M, Vanneman M, Munshi NC, Tai YT,
Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR and
Dranoff G: MHC class I chain-related protein A antibodies and
shedding are associated with the progression of multiple myeloma.
Proc Natl Acad Sci USA. 105:1285–1290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Paschen A, Sucker A, Hill B, Moll I,
Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, et
al: Differential clinical significance of individual NKG2D ligands
in melanoma: Soluble ULBP2 as an indicator of poor prognosis
superior to S100B. Clin Cancer Res. 15:5208–5215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhao Y, Chen N, Yu Y, Zhou L, Niu C, Liu
Y, Tian H, Lv Z, Han F and Cui J: Prognostic value of MICA/B in
cancers: A systematic review and meta-analysis. Oncotarget.
8:96384–96395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xu X, Rao GS, Groh V, Spies T, Gattuso P,
Kaufman HL, Plate J and Prinz RA: Major histocompatibility complex
class I-related chain A/B (MICA/B) expression in tumor tissue and
serum of pancreatic cancer: Role of uric acid accumulation in
gemcitabine-induced MICA/B expression. BMC Cancer. 11:1942011.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang LP, Niu H, Xia YF, Han YL, Niu P,
Wang HY and Zhou QL: Prognostic significance of serum sMICA levels
in non-small cell lung cancer. Eur Rev Med Pharmacol Sci.
19:2226–2230. 2015.PubMed/NCBI
|
|
98
|
Xing S, Zhu Y and Sun Y: Serum sMICA as
biomarker in detection of non-small-cell lung carcinoma. Br J
Biomed Sci. 75:50–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Weil S, Memmer S, Lechner A, Huppert V,
Giannattasio A, Becker T, Müller-Runte A, Lampe K, Beutner D, Quaas
A, et al: Natural killer group 2D ligand depletion reconstitutes
natural killer cell immunosurveillance of head and neck squamous
cell carcinoma. Front Immunol. 8:3872017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen JL, Chang CC, Huang YS, Kuo HY, Chen
TY, Wang CW, Kuo SH and Lin YL: Persistently elevated soluble MHC
class I polypeptide-related sequence A and transforming growth
factor-β1 levels are poor prognostic factors in head and neck
squamous cell carcinoma after definitive chemoradiotherapy. PLoS
One. 13:e02022242018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li JJ, Pan K, Gu MF, Chen MS, Zhao JJ,
Wang H, Liang XT, Sun JC and Xia JC: Prognostic value of soluble
MICA levels in the serum of patients with advanced hepatocellular
carcinoma. Chin J Cancer. 32:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cheung PF, Yip CW, Wong NC, Fong DY, Ng
LW, Wan AM, Wong CK, Cheung TT, Ng IO, Poon RT, et al:
Granulin-epithelin precursor renders hepatocellular carcinoma cells
resistant to natural killer cytotoxicity. Cancer Immunol Res.
2:1209–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Roshani R, Boroujerdnia MG, Talaiezadeh AH
and Khodadadi A: Assessment of changes in expression and
presentation of NKG2D under influence of MICA serum factor in
different stages of breast cancer. Tumour Biol. 37:6953–6962. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Madjd Z, Spendlove I, Moss R, Bevin S,
Pinder SE, Watson NF, Ellis I and Durrant LG: Upregulation of MICA
on high-grade invasive operable breast carcinoma. Cancer Immun.
7:172007.PubMed/NCBI
|
|
105
|
Zhao YK, Jia CM, Yuan GJ, Liu W, Qiu Y and
Zhu QG: Expression and clinical value of the soluble major
histocompatibility complex class I-related chain A molecule in the
serum of patients with renal tumors. Genet Mol Res. 14:7233–7240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Samuels S, Ferns DM, Meijer D, van
Straalen JP, Buist MR, Zijlmans HJ, Kenter GG and Jordanova ES:
High levels of soluble MICA are significantly related to increased
disease-free and disease-specific survival in patients with
cervical adenocarcinoma. Tissue Antigens. 85:476–483. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Märten A, von Lilienfeld-Toal M, Büchler
MW and Schmidt J: Soluble MIC is elevated in the serum of patients
with pancreatic carcinoma diminishing gammadelta T cell
cytotoxicity. Int J Cancer. 119:2359–2365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen J, Xu H and Zhu XX: Abnormal
expression levels of sMICA and NKG2D are correlated with poor
prognosis in pancreatic cancer. Ther Clin Risk Manag. 12:11–18.
2015.PubMed/NCBI
|
|
109
|
Duan X, Deng L, Chen X, Lu Y, Zhang Q,
Zhang K, Hu Y, Zeng J and Sun W: Clinical significance of the
immunostimulatory MHC class I chain-related molecule A and NKG2D
receptor on NK cells in pancreatic cancer. Med Oncol. 28:466–474.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ben Chaaben A, Ouni N, Douik H, Ayari F,
Abaza H, Mamoghli T, Harzallah L, Fortier C, Boukouaci W,
Krishnamoorthy R, et al: Soluble MICA and anti-MICA antibodies as
biomarkers of nasopharyngeal carcinoma disease. Immunol Invest.
49:498–509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tamaki S, Sanefuzi N, Kawakami M, Aoki K,
Imai Y, Yamanaka Y, Yamamoto K, Ishitani A, Hatake K and Kirita T:
Association between soluble MICA levels and disease stage IV oral
squamous cell carcinoma in Japanese patients. Hum Immunol.
69:88–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Arreygue-Garcia NA, Daneri-Navarro A, del
Toro-Arreola A, Cid-Arregui A, Gonzalez-Ramella O, Jave-Suarez LF,
Aguilar-Lemarroy A, Troyo-Sanroman R, Bravo-Cuellar A, Delgado-Rizo
V, et al: Augmented serum level of major histocompatibility complex
class I-related chain A (MICA) protein and reduced NKG2D expression
on NK and T cells in patients with cervical cancer and precursor
lesions. BMC Cancer. 8:162008. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nückel H, Switala M, Sellmann L, Horn PA,
Dürig J, Dührsen U, Küppers R, Grosse-Wilde H and Rebmann V: The
prognostic significance of soluble NKG2D ligands in B-cell chronic
lymphocytic leukemia. Leukemia. 24:1152–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Maccalli C, Giannarelli D, Capocefalo F,
Pilla L, Fonsatti E, Di Giacomo AM, Parmiani G and Maio M:
Immunological markers and clinical outcome of advanced melanoma
patients receiving ipilimumab plus fotemustine in the NIBIT-M1
study. Oncoimmunology. 5:e10710072015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rebmann V, Schütt P, Brandhorst D, Opalka
B, Moritz T, Nowrousian MR and Grosse-Wilde H: Soluble MICA as an
independent prognostic factor for the overall survival and
progression-free survival of multiple myeloma patients. Clin
Immunol. 123:114–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang T, Barber A and Sentman CL:
Generation of antitumor responses by genetic modification of
primary human T cells with a chimeric NKG2D receptor. Cancer Res.
66:5927–5933. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Baumeister SH, Murad J, Werner L, Daley H,
Trebeden-Negre H, Gicobi JK, Schmucker A, Reder J, Sentman CL,
Gilham DE, et al: Phase I trial of autologous CAR T cells targeting
NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer
Immunol Res. 7:100–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu R, Luo Q, Luo W, Wan L, Zhu Q, Yin X,
Lu X, Song Z, Wei L, Xiang Z and Zou Y: A soluble NK-CAR mediates
the specific cytotoxicity of NK cells toward the target
CD20+ lymphoma cells. Aging Dis. 13:1576–1588. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ferrari de Andrade L, Tay RE, Pan D, Luoma
AM, Ito Y, Badrinath S, Tsoucas D, Franz B, May KF Jr, Harvey CJ,
et al: Antibody-mediated inhibition of MICA and MICB shedding
promotes NK cell-driven tumor immunity. Science. 359:1537–1542.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Du C, Bevers J III, Cook R, Lombana TN,
Rajasekaran K, Matsumoto M, Spiess C, Kim JM and Ye Z: MICA immune
complex formed with alpha 3 domain-specific antibody activates
human NK cells in a Fc-dependent manner. J Immunother Cancer.
7:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Alves da Silva PH, Xing S, Kotini AG,
Papapetrou EP, Song X, Wucherpfennig KW, Mascarenhas J and Ferrari
de Andrade L: MICA/B antibody induces macrophage-mediated immunity
against acute myeloid leukemia. Blood. 139:205–216. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ferrari de Andrade L, Kumar S, Luoma AM,
Ito Y, Alves da Silva PH, Pan D, Pyrdol JW, Yoon CH and
Wucherpfennig KW: Inhibition of MICA and MICB shedding elicits
NK-cell-mediated immunity against tumors resistant to cytotoxic T
cells. Cancer Immunol Res. 8:769–780. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Badrinath S, Dellacherie MO, Li A, Zheng
S, Zhang X, Sobral M, Pyrdol JW, Smith KL, Lu Y, Haag S, et al: A
vaccine targeting resistant tumours by dual T cell plus NK cell
attack. Nature. 606:992–998. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lu S, Zhang J, Liu D, Li G,
Staveley-O'Carroll KF, Li Z and Wu JD: Nonblocking monoclonal
antibody targeting soluble MIC revamps endogenous innate and
adaptive antitumor responses and eliminates primary and metastatic
tumors. Clin Cancer Res. 21:4819–4830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Basher F, Dhar P, Wang X, Wainwright DA,
Zhang B, Sosman J, Ji Z and Wu JD: Antibody targeting tumor-derived
soluble NKG2D ligand sMIC reprograms NK cell homeostatic survival
and function and enhances melanoma response to PDL1 blockade
therapy. J Hematol Oncol. 13:742020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Narni-Mancinelli E and Vivier E: Shed
NKG2D ligand boosts NK cell immunity. Cell Res. 25:651–652. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yamanegi K, Yamane J, Kobayashi K, Ohyama
H, Nakasho K, Yamada N, Hata M, Fukunaga S, Futani H, Okamura H and
Terada N: Downregulation of matrix metalloproteinase-9 mRNA by
valproic acid plays a role in inhibiting the shedding of MHC class
I-related molecules A and B on the surface of human osteosarcoma
cells. Oncol Rep. 28:1585–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Miyashita T, Miki K, Kamigaki T, Makino I,
Tajima H, Nakanuma S, Hayashi H, Takamura H, Fushida S, Ahmed AK,
et al: Low-dose valproic acid with low-dose gemcitabine augments
MHC class I-related chain A/B expression without inducing the
release of soluble MHC class I-related chain A/B. Oncol Lett.
14:5918–5926. 2017.PubMed/NCBI
|
|
129
|
Diermayr S, Himmelreich H, Durovic B,
Mathys-Schneeberger A, Siegler U, Langenkamp U, Hofsteenge J,
Gratwohl A, Tichelli A, Paluszewska M, et al: NKG2D ligand
expression in AML increases in response to HDAC inhibitor valproic
acid and contributes to allorecognition by NK-cell lines with
single KIR-HLA class I specificities. Blood. 111:1428–1436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ho TCS, Chan AHY and Ganesan A: Thirty
years of HDAC inhibitors: 2020 Insight and hindsight. J Med Chem.
63:12460–12484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Camodeca C, Nuti E, Tepshi L, Boero S,
Tuccinardi T, Stura EA, Poggi A, Zocchi MR and Rossello A:
Discovery of a new selective inhibitor of A disintegrin and
metalloprotease 10 (ADAM-10) able to reduce the shedding of NKG2D
ligands in Hodgkin's lymphoma cell models. Eur J Med Chem.
111:193–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sekiba K, Otsuka M, Seimiya T, Tanaka E,
Funato K, Miyakawa Y and Koike K: The fatty-acid amide hydrolase
inhibitor URB597 inhibits MICA/B shedding. Sci Rep. 10:155562020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu J and Khalil RA: Matrix
metalloproteinase inhibitors as investigational and therapeutic
tools in unrestrained tissue remodeling and pathological disorders.
Prog Mol Biol Transl Sci. 148:355–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Huang B, Sikorski R, Sampath P and Thorne
SH: Modulation of NKG2D-ligand cell surface expression enhances
immune cell therapy of cancer. J Immunother. 34:289–296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Nwangwu CA, Weiher H and Schmidt-Wolf IGH:
Increase of CIK cell efficacy by upregulating cell surface MICA and
inhibition of NKG2D ligand shedding in multiple myeloma. Hematol
Oncol. 35:719–725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fuertes MB, Domaica CI and Zwirner NW:
Leveraging NKG2D ligands in immuno-oncology. Front Immunol.
12:7131582021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Xie X, Zhou Y, Wang X, Guo J, Li J, Fan H,
Dou J, Shen B and Zhou C: Enhanced antitumor activity of
gemcitabine by polysaccharide-induced NK cell activation and immune
cytotoxicity reduction in vitro/vivo. Carbohydr Polym. 173:360–371.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Goto K, Arai J, Stephanou A and Kato N:
Novel therapeutic features of disulfiram against hepatocellular
carcinoma cells with inhibitory effects on a disintegrin and
metalloproteinase 10. Oncotarget. 9:18821–18831. 2018. View Article : Google Scholar : PubMed/NCBI
|