Introduction
Metformin, with the title of ‘magic drug’, plays an
important role in treating diseases such as diabetes, cancer,
anxiety, obesity, cardiovascular diseases and others (1). However, due to the high concentration
required for its antitumor effect, the clinical trial results of
metformin in treating cancer are not optimal (2,3). The
derivative of metformin, phenformin, has improved antitumor
activity compared with metformin and has attracted widespread
attention. One of the main reasons for its high antitumor activity
is that phenformin has a benzene ring, making it more hydrophobic
and easier to penetrate cell membranes than metformin (4). A study on the molecular mechanism of
biguanides targeting mammalian respiratory chain complex I
suggested that the benzene ring structure enables it to stably bind
to Q channel of mitochondrial complex I through van der Waals
interactions, resulting in a stronger inhibitory effect on
mitochondrial complex I (5).
Another important factor is that metformin requires the organic
cation transporters (OCT) in order to enter cells, while phenformin
does not (4). This expands the
application scope of phenformin, making it still effective in some
cell types with low expression of OCT, such as in melanoma cell
lines (6). Therefore, due to its
improved activity and adaptability, phenformin is considered to
have more clinical development value than metformin.
It is well known that phenformin is a typical
agonist of AMP-activated protein kinase (AMPK). The effect of
phenformin on cancer cell proliferation is mainly achieved by
inhibiting complex I of the mitochondrial respiratory chain to
activate AMPK and block the mammalian target of rapamycin (mTOR)
pathway, thereby affecting protein synthesis, tumor angiogenesis,
epithelial-mesenchymal transition (EMT), cell cycle arrest and
proliferation inhibition (4,7). In
addition, another category of molecular mechanisms that is not
dependent on AMPK has been reported (8,9)
(Fig. 1). For instance, phenformin
blocks the mTOR signaling pathway by inhibiting Rag GTPase without
the involvement of AMPK (9).
Moreover, phenformin exerts antitumor effects by acting on the
tumor microenvironment (TME) (10,11) or
self-renewal of cancer stem cells (CSCs) (12). The latest research has also revealed
a new mechanism through which phenformin induces autophagic cell
death in cancer cells by inducing endoplasmic reticulum (ER) stress
without relying on AMPK (13). The
complex diversity of antitumor mechanisms of phenformin further
highlights its notable clinical application potential.
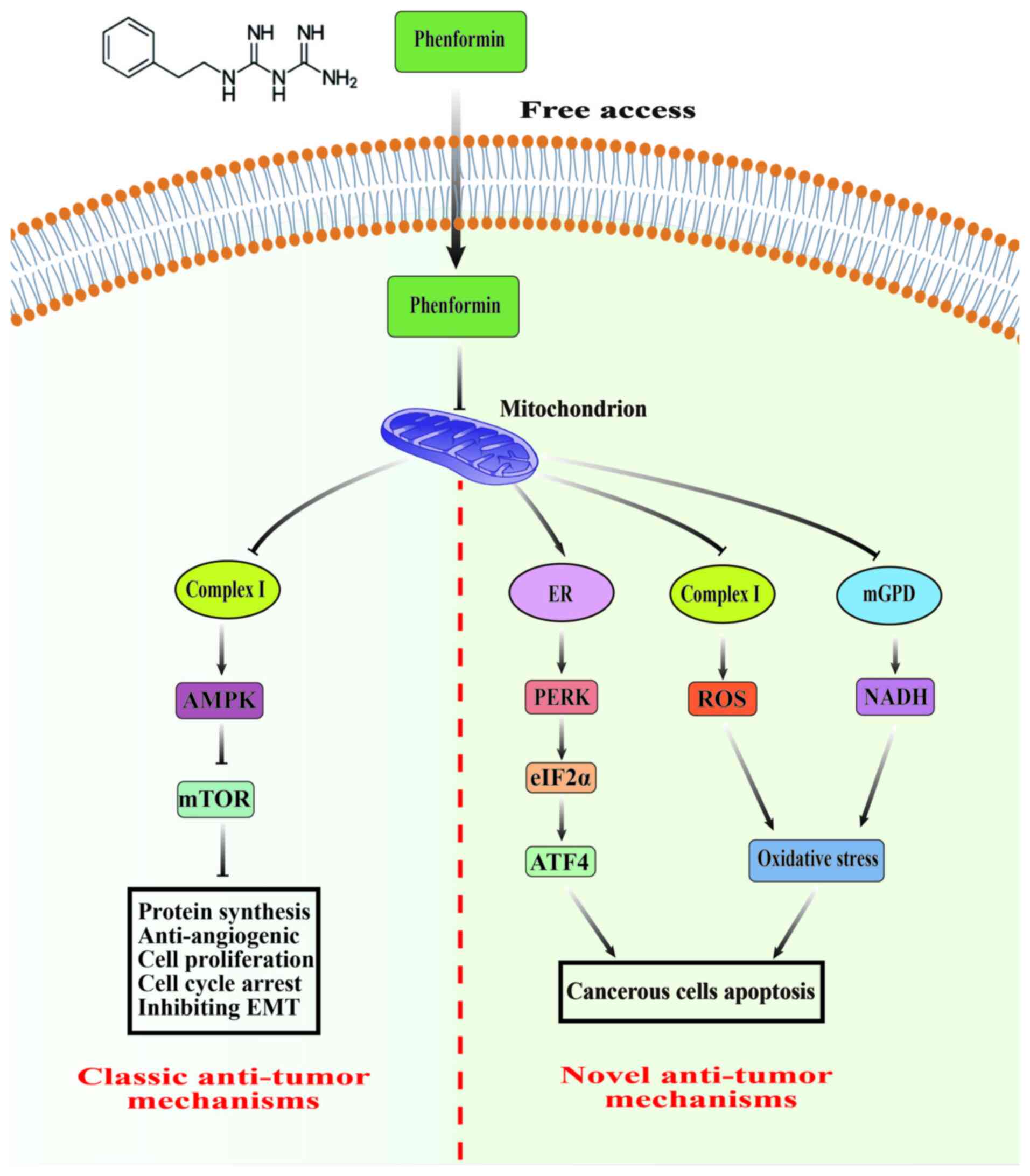 | Figure 1.Main antitumor mechanisms of
phenformin. The classic antitumor mechanism of phenformin is to
activate AMPK and block the mTOR pathway by inhibiting
mitochondrial respiratory chain complex I, thereby affecting
protein synthesis, tumor angiogenesis, EMT, cell cycle arrest and
inhibition of proliferation. In addition, phenformin can promote
cancer cell apoptosis by inducing ER stress or oxidative stress.
AMPK, AMP-activated protein kinase; mTOR, mammalian target of
rapamycin; EMT, epithelial mesenchymal transition; ER, endoplasmic
reticulum; mGPD, mitochondrial glycerol-3-phosphate dehydrogenase;
ROS, reactive oxygen species; NADH, nicotinamide adenine
dinucleotide. |
Resistance and low response rates to antineoplastic
drugs are common in clinical therapy (14,15).
It has been shown that phenformin enhances the sensitivity or
alleviates resistance of drugs, including chemotherapy drugs
(16,17), radiotherapy (18), targeted therapeutic drugs (19,20)
and immune checkpoint inhibitors (10). More specifically, marked
breakthroughs of phenformin combined with dalafinib/trimetinib have
been made in clinical trials of melanoma (21). In addition to considering its
efficacy, the lactic acid toxicity of phenformin has also been a
concern. Although phenformin was withdrawn from the market in the
late 1970s due to the risk of lactic acidosis (22), phenformin has relatively low
toxicity (64 cases of lactate acidosis per 100,000 patients)
compared with other cancer treatments (23). In addition, some achievements have
been made in reducing the lactate toxicity of phenformin. For
example, the administration of 2-deoxyglucose (24) or oxamate (25) can markedly alleviate the symptoms of
lactic acid poisoning caused by phenformin. In several
epidemiological studies, a relationship between phenformin and
diminished incidence and mortality of cancer in patients with type
2 diabetes has been revealed (26,27).
These studies indicated that phenformin is an anticancer agent with
great clinical value.
The present review primarily presents novel
advancement in the antitumor mechanism of phenformin, summarizes
the current treatment status of monotherapy and combination therapy
of phenformin in various tumors, and analyzes and discusses the
future direction of phenformin in the field of cancer treatment,
aiming to provide theoretical foundations and insights for the
successful application of phenformin in the clinical treatment of
cancers.
Antitumor mechanisms of phenformin
The direct target of phenformin remains unclear, and
its antitumor effects are principally achieved through disrupting
mitochondrial function, influencing TME and CSCs. Next, an in-depth
analysis of the antitumor mechanisms of phenformin is
presented.
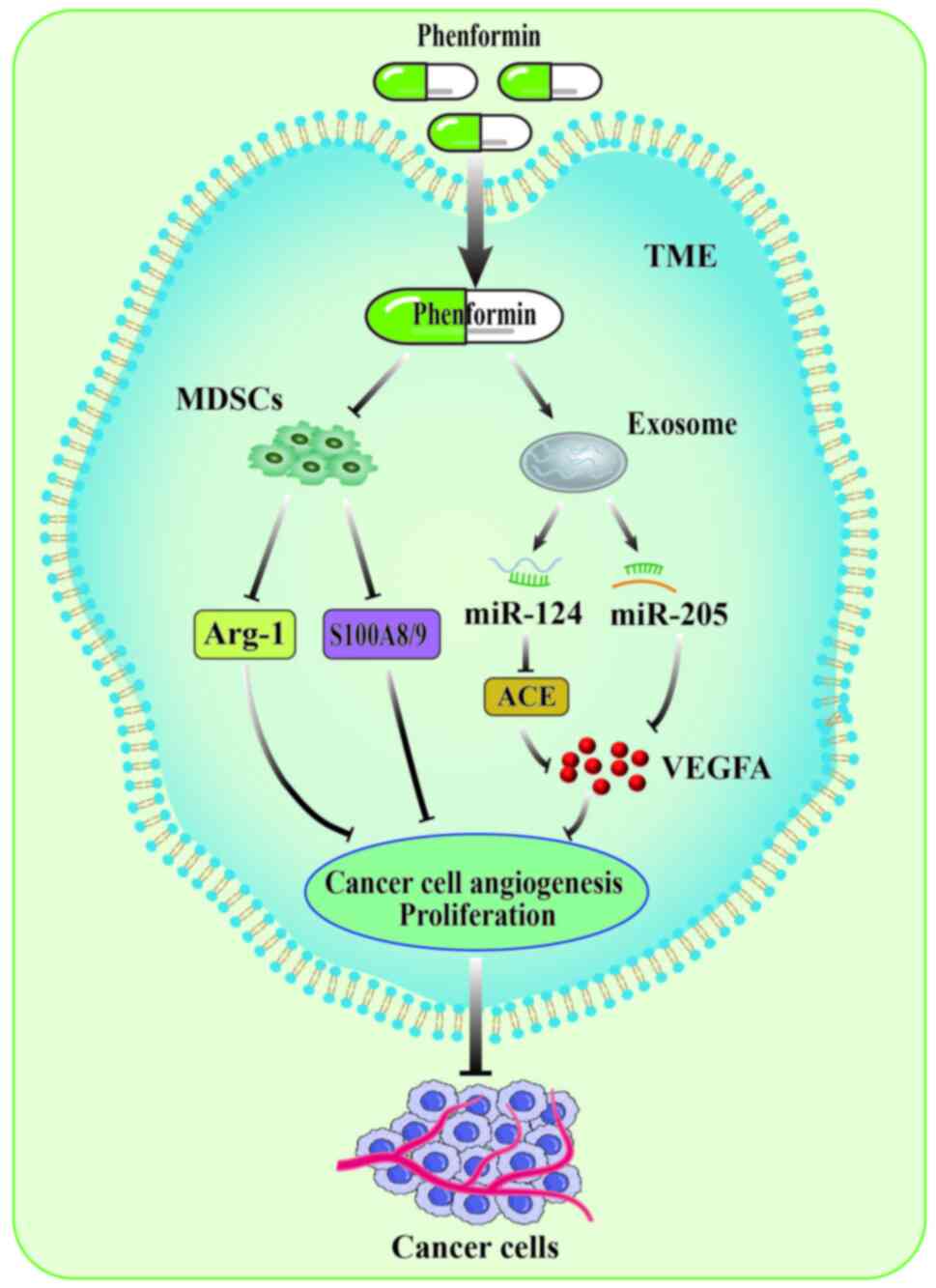
Regulating TME
The TME refers to the living environment around
tumors, encompassing adjacent blood vessels, immune cells,
fibroblasts, signaling molecules and extracellular matrix
components (28). According to
their different functions, the immune cells in the TME can be
categorized into tumor-promoting and -inhibiting cells, which play
distinct roles in various stages of tumor progression (29). The prototypical representatives of
tumor-promoting immune cells are myeloid-derived suppressor cells
(MDSCs), which possess the ability to inhibit immune cell response.
MDSCs not only promote tumor angiogenesis by mediating JAK2/STAT3
signaling to activate VEGFA and MMP9 production (30), but also induce EMT in cancer cells
by mediating the TGF-β, EGF and HGF signaling pathways, thereby
promoting cancer cell metastasis (31,32).
In a murine melanoma model, phenformin selectively decreased the
accumulation of G-MDSCs in the spleen and tumor and downregulated
the expression levels of Arg-1 and S100A8/9 in MDSC. The
combination therapy of phenformin and anti-programmed death-1
(PD-1) antibody exhibits a synergistic effect by inducing
CD8+T cell infiltration (10). This work has received the attention
of researchers and has been reviewed (33). Due to the prominent performance of
phenformin in inhibiting melanoma cells in preclinical studies,
researchers conducted a phase I clinical trial of phenformin
combined with other drugs to treat patients with melanoma. In line
with preclinical investigations, a reduction in tumor-infiltrating
MDSCs was also observed in patients treated with phenformin,
indicating that phenformin may enhance the immune recognition of
melanoma cells (21). Phenformin
plays a particularly vital role in the tumor immune
microenvironment (TIME) based on preclinical studies and clinical
trials.
Tumor cell-derived exosomal microRNAs (miRNAs/miR),
a marked substance in TME, serve as messengers to transmit signals
between cells (34). Exosomal
miRNAs are important in regulating tumor growth, invasion,
metastasis and angiogenesis (35).
Therefore, tumor cell-derived exosomal miRNAs are currently popular
antitumor targets. Targeting exosomal miRNAs is also deemed an
effective avenue for tumor therapy. Phenformin plays a crucial role
in regulating exosomal miRNA. For instance, Zhuang et al
(11) discovered that phenformin
markedly upregulated oral squamous cell carcinoma cells
(OSCC)-derived exosomal miR-1246 and miR-205, subsequently
mediating the ACE signaling pathway and downregulating VEGFA
expression, thereby inhibiting angiogenesis in vascular endothelial
cells. These studies indicated that phenformin is an effective
anticancer strategy by inhibiting tumor angiogenesis affecting
TME.
In conclusion, the aforementioned studies have
indicated that the target of action of phenformin is not only
focused on cancer cells, but also extends to the extracellular
environment, including the TIME and intercellular communication
(Fig. 2). Both preclinical and
clinical trials demonstrated that phenformin effectively reduces
tumor-promoting immune cells known as MDSCs, highlighting the
marked regulatory mechanism of phenformin in the TIME. However, the
TME is intricate, and it remains unclear whether phenformin exerts
potential effects on other constituents, such as macrophages. In
addition, exosomal miRNAs derived from tumor cells are considered
promising targets for disease diagnosis and treatment as they can
accurately reflect crucial information originating from the tumor
cells. However, the limited abundance and diversity of exosomal
miRNAs is challenging for precise quantification in tumor
diagnosis. If they are implemented as diagnostic and therapeutic
targets in clinical practice, numerous issues remain to be
addressed such as the urgent need for diagnostic methods with
heightened sensitivity and fidelity and appropriate drug delivery
systems to introduce drugs into extracellular vesicles.
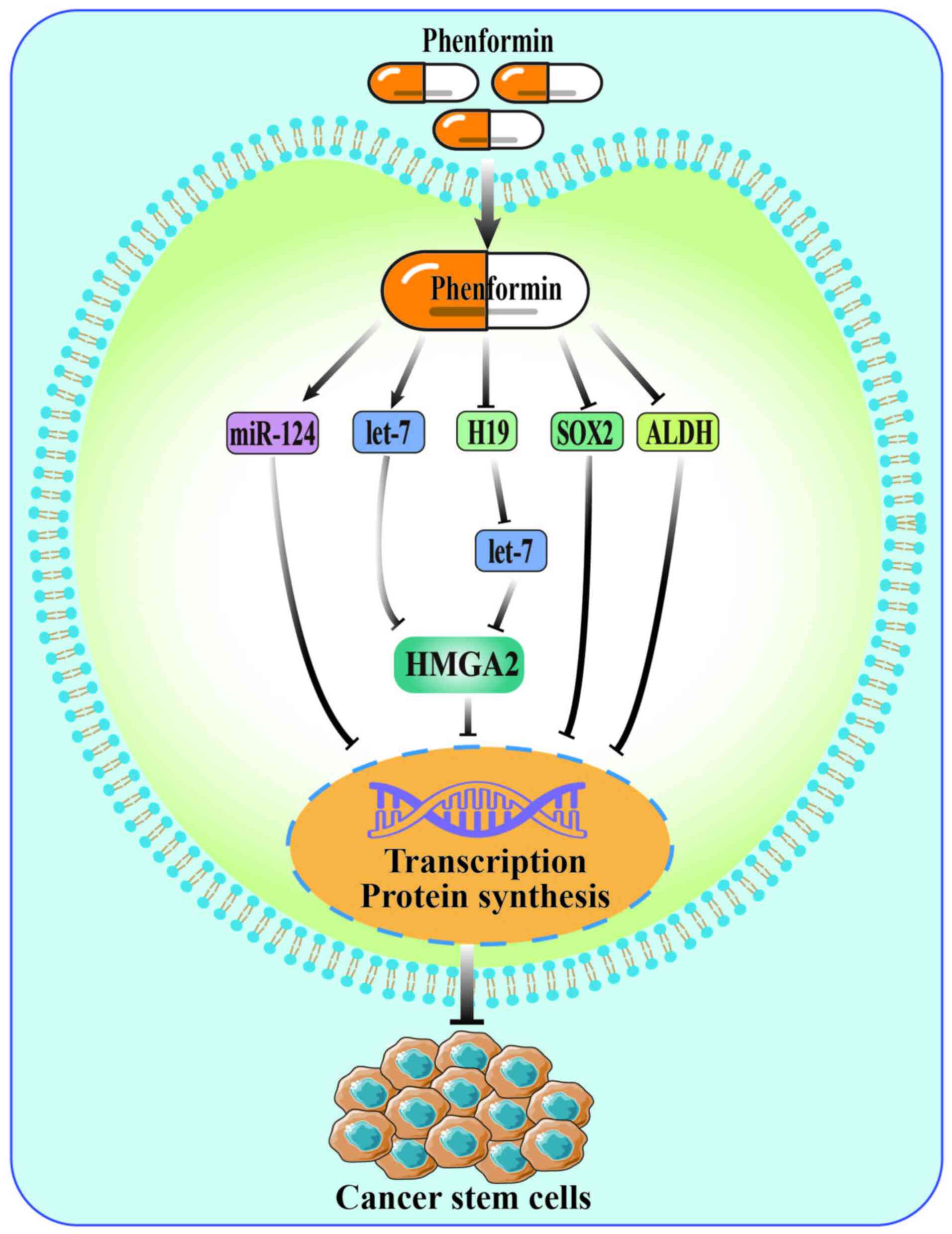
Inhibiting the self-renewal of
CSCs
CSCs are a subgroup of cancer cells. Despite limited
quantity, they possess the ability to regenerate, multiply
infinitely and differentiate in multiple directions. There is a
correlation between CSCs and tumor invasion, metastasis, drug
resistance, as well as relapse post-treatment (36). Therefore, inhibiting self-renewal of
CSCs is considered a potential treatment for tumors. Phenformin has
been shown to impede the self-renewal of CSCs effectively, and its
mechanism of action primarily involves non-coding RNA and stem cell
markers (12,37). According to a study by Jiang et
al (12), phenformin
effectively restrained the self-renewal of glioma stem cells by
directly upregulating the miR-124 pathway or activating let-7, a
tumor suppressor miRNA, to inhibit the HMGA2 pathway. SOX2, a stem
cell marker, was downregulated by phenformin, thereby inhibiting
melanoma stem cell properties (37). In addition, researchers have also
shown that high expression of SOX2 was associated with aldehyde
dehydrogenase (ALDH) overexpression in melanoma cells, and
downregulating ALDH by phenformin notably inhibited the
self-renewal of CSCs (37,38). This indicates that phenformin is an
effective tumor metabolism regulator, and targeting cancer cell
metabolism is another strategy to inhibit self-renewal of CSCs.
Taken together, phenformin inhibits the self-renewal
of CSCs mainly by affecting non-coding RNA and stem cell markers
(Fig. 3). Additionally, cancer
cells often activate alternative signaling pathways to sustain
their survival during targeted drug treatment. For instance, in
melanoma cells treated with BRAF or MEK inhibitors, it was shown
that cancer cells were resistant to drugs by producing higher
levels of ALDH (39). As it was
aforementioned, phenformin downregulates ALDH and inhibits the
self-renewal of CSCs. This indicates that combining phenformin with
BRAF or MEK inhibitors may be a promising strategy for overcoming
resistance to BRAF or MEK inhibition in melanoma cells.
Inducing cellular stress response
During the malignant growth process of tumor cells,
environmental factors continuously trigger cellular stress
responses, thereby affecting their growth status (40). Research has found that phenformin
can induce stress responses in cancer cells, such as oxidative
stress and ER stress, thereby inhibiting the growth and
proliferation of tumor cells. Nicotinamide adenine dinucleotide
(NADH), a reduced form of nicotinamide adenine dinucleotide, is
closely associated with maintaining cell proliferation,
differentiation, energy metabolism and cell protection (41). Studies have shown that phenformin
regulates NADH production by acting on mitochondria, inducing cell
apoptosis (42,43). According to Kim et al
(43), phenformin exerted
antiproliferative effects in cancer cells by inhibiting
mitochondrial complex I and subsequently downregulating the
NAD/NADH ratio, thereby disrupting redox homeostasis and reducing
intracellular aspartic acid levels. In addition, phenformin
inhibits mitochondrial mGPD, a component of glycero-phosphate
shuttling, prompting the cell to synthesize NADH, which induces the
formation of the Gli1/CtBP2 complex, further inhibiting
transcription and translation of Hedgehog and ultimately
suppressing cancer cell proliferation (8). These studies indicated that the
antitumor mechanism of phenformin is closely related to the
disruption of cellular redox status.
The formation of reactive oxygen species (ROS),
unstable molecules containing oxygen, occurs as a byproduct of
oxygen consumption and cellular metabolism (44,45).
The overabundance of ROS in tumor cells results in oxidative
stress, thereby causing cytotoxic consequences (45). Phenformin inhibits mitochondrial
complex I and induces excessive production of ROS, resulting in
oxidative stress and DNA damage, ultimately triggering cell
apoptosis (46,47). In glioma cells, Wang et al
(48) also found that phenformin
could induce the production of ROS, resulting in ROS imbalance. By
contrast, the ROS inhibitor NAC attenuates the induced apoptosis
ability of phenformin. These findings suggested a close association
between the anticancer effects of phenformin and the generation of
ROS. Hence, using phenformin to enhance ROS production and induce
oxidative stress holds promise as a potential approach for cancer
treatment.
Phenformin not only induces oxidative stress, but
also inhibits tumor growth by inducing ER stress. The ER stress is
involved in various important biochemical processes of tumor cells,
such as cell cycle, DNA damage and repair, cell apoptosis and
autophagy, through pathways such as IRE1, PERK and ATF6 (49,50).
The latest research shows that phenformin promotes OSCC autophagy
by inducing ER response. From a mechanistic perspective, phenformin
induces ER stress activation of the PERK/eIF2 α/ATF4 axis pathway
to enhance DDIT4 and NIBAN1 expression without relying on AMPK,
leading to mTOR inhibition to promote autophagy and inhibit OSCC
cell proliferation (13). This
indicates that targeting the ER stress pathway to induce cancer
cell apoptosis is another novel antitumor mechanism of phenformin,
providing new ideas for innovative tumor treatment.
Affecting cancer cell metabolism
Cancer cells often maintain high value-added rates
by altering their metabolism, such as increasing macromolecular
biosynthesis, accelerating ATP production (51). Phenformin affects cancer cell
metabolism by acting on different signaling pathways (52). As a central regulatory factor of
metabolism, the mTOR signaling pathway is overactivated in most
cancers (53). The activation of
mTOR contributes to sustaining cellular survival by promoting
anabolic metabolism or suppressing catabolic processes (54). Phenformin plays an essential role in
modulating anabolic and catabolic processes by phosphorylating
downstream proteins of AMPK or regulating gene expression (55). For instance, phenformin activates
AMPK and reduces S6 protein phosphorylation to block the mTOR
signaling pathway, suppressing protein synthesis and inducing
autophagy, further leading to cell cycle arrest and apoptosis
(56). Beyond that, previous
research has demonstrated that metformin augments lipolysis by
activating AMPK, thereby impeding the proliferation of cancer cells
(57). However, whether phenformin
can mediate AMPK activation and affect the lipid metabolism of
cancer cells needs to be further verified. At present, it is
unclear whether phenformin exerts antitumor effects by affecting
glucose metabolism. It is noteworthy that respiratory stress
induced by phenformin promotes aerobic glycolysis and
glucose-dependent states, enabling cells to resist mitochondrial
dysfunction (58). In this case,
phenformin mediates an increase in glycolytic flux, thereby
reducing the sensitivity of cancer cells to phenformin. Therefore,
the combination of phenformin and glycolytic flux inhibitors such
as dichloroacetate (12) or oxamate
(46) is a promising antitumor
strategy.
Except for material metabolism, phenformin also
affects the energy metabolism of cancer cells. The inhibition of
mitochondrial complex I by phenformin reduces ATP production,
consequently inducing the indirect activation of AMPK to regulate
energy metabolism in tumor cells (59,60).
In certain tumors where the specific tumor suppressor LKB1 is
mutated or inactivated, such as non-small cell lung cancer (NSCLC)
(61,62) and lymphoma (63), obstruction of AMPK activation by
phenformin induces energy metabolic stress, giving rise to
apoptosis through ATP depletion in tumor cells. Therefore,
phenformin can be used as an ATP-depleting cytotoxic agent to
eradicate LKB1-deficient tumors efficiently. This cytotoxicity
exclusively targets tumors with LKB1 mutations while it does not
affect healthy tissue, which contributes to enhancing the
therapeutic index and mitigating toxic side effects (64). These studies suggested that the
induction of energy metabolic stress in cancer cells by phenformin
may represent a feasible cancer treatment option.
In summary, phenformin exerts a distinctive role in
the metabolic processes of tumor cells. However, tumor metabolism
constitutes an intricate network system. When a metabolic pathway
of tumor cells is inhibited, it can maintain growth by activating
other compensatory metabolic pathways. The application of
phenformin in metabolic combination therapy leads to complementary
inhibition of tumor metabolism processes, such as phenformin
combined with MCT1 inhibitor AZD3965 (65) or glycolytic inhibitor Gnetin H
(66). This is a potential strategy
for cancer treatment, which can achieve multi-channel blockade of
tumor substances and energy metabolism pathways. Furthermore, it is
imperative to devise strategies that avoid the potential harm
inflicted on healthy cells. The integration of rapidly advancing
technologies, such as metabolomics, spatial metabolomics,
lipidomics, proteomics and others are crucial for elucidating the
mechanisms underlying aberrant tumor metabolism and identifying
specific molecular targets.
Blocking the cancer cell cycle
An eminent characteristic of cancer development is
the aberrant activation of cyclins that instigate the unbridled
proliferation of cancer cells. Hence, cell cycle regulators have
become attractive targets for anticancer therapy (67). It is noteworthy that phenformin
exhibits the ability to inhibit cyclins or cyclin kinases, thereby
impeding the progression of the tumor cell cycle. For instance,
phenformin induces the upregulation of P21, a cyclin-dependent
kinase suppressor protein, leading to cell cycle arrest in
glioblastoma (GBM) cells (68). In
addition, phenformin downregulates cyclin D1 by inhibiting the
MAPK/ERK signaling pathway, leading to the increase of breast
cancer cells in the G1 phase (69).
Jackson et al (56) also
observed a notable downregulation of cyclin D1 and CDK4, and an
upregulation of P21 in ovarian cancer cells upon treatment with
phenformin, giving rise to alterations in the cell cycle of ovarian
cancer. These studies suggested that phenformin exhibits potential
as an antitumor agent by regulating cell cycle factor-induced cell
cycle arrest.
Inhibiting tumor angiogenesis
Tumor blood vessels, as conduits for the
transportation of tumor nutrients, facilitate the escape of tumor
cells and make tumor angiogenesis a pivotal indicator of solid
tumor growth, invasion and metastasis (70). Hypoxia and VEGF are essential
factors affecting angiogenesis. Solid tumors frequently exhibit
elevated proangiogenic factor VEGF expression levels (71). In addition, mTORC1 facilitates tumor
angiogenesis by promoting HIF-1α synthesis through signal
transduction pathways involving S6K1, 4E-BP1 and STAT3. AMPK
activator effectively suppresses mTOR signaling and inhibits tumor
angiogenesis (72). Jaidee et
al (73) demonstrated that
phenformin effectively restrained mTOR activity through the
activation of AMPK, consequently impeding the HIF-1α signal pathway
and reducing VEGF expression; this ultimately inhibited
angiogenesis in bile duct cancer, but did not affect normal
angiogenesis. Beyond that, phenformin has shown the ability to
impede the expression of proangiogenic factors and suppress
angiogenesis in both in vitro and in vivo models of
KRAS-mutated NSCLC by effectively suppressing the ERK pathway
(74). These positive results
indicated that inhibition of tumor angiogenesis by phenformin
offers remarkable therapeutic advantages in the treatment of
tumors.
Suppressing EMT
The process by which epithelial tumor cells lose
their adhesion ability, then gain the migration ability of
mesenchymal cells and promote metastasis and drug resistance is
called EMT, which is closely associated with the initiation,
development and metastasis of tumor cells (75). The TGF-β/Smad and insulin-like
growth factor (IGF) signaling pathway play a pivotal role in
inducing EMT (76,77). TGF-β receptor 2 signaling is widely
expressed in numerous types of cancer and prominently influences
the EMT process in cancer cells. Lin et al (78) found that phenformin effectively
suppressed TGF-β-induced EMT by activating AMPK. Furthermore, Park
et al (79) confirmed that
phenformin decreases the expression of intermediate mesenchymal
markers such as N-cadherin and vimentin, and EMT regulators
including Snail, Twist, Slug and Zeb1 in colorectal cancer cells.
The observed downregulation of these markers and regulators further
impedes TGF-β-induced EMT. In addition, it has been reported that
IGF facilitated EMT by mediating the PI3K and MAPK pathways,
enhancing the invasive ability of breast cancer cells (77). In ErbB2-overexpressing breast cancer
cells, phenformin can block the IGF1 receptor signaling pathway by
activating AMPK, thus impeding the EMT process (76). These studies suggested that
phenformin can inhibit the EMT process by activating AMPK to
regulate TGF-β and IGF signal transduction, which is a new strategy
to treat tumors.
Applications of phenformin in various
cancers
The antitumor effects of phenformin have been
extensively validated through in vitro and in vivo
studies across versatile cancer types, including, lung cancer
(74,80–82),
skin cancer (83), hepatoma
(84), breast cancer (26), pancreatic cancer (85), ovarian cancer (86), prostate cancer (87), colon adenocarcinoma (88) and others. Next, a comprehensive
review of the recent research progress of phenformin in various
cancer types is presented, and the therapeutic effects and
underlying mechanisms of phenformin in combination with clinical
drugs in the treatment of tumors are systematically summarized
(Fig. 4).
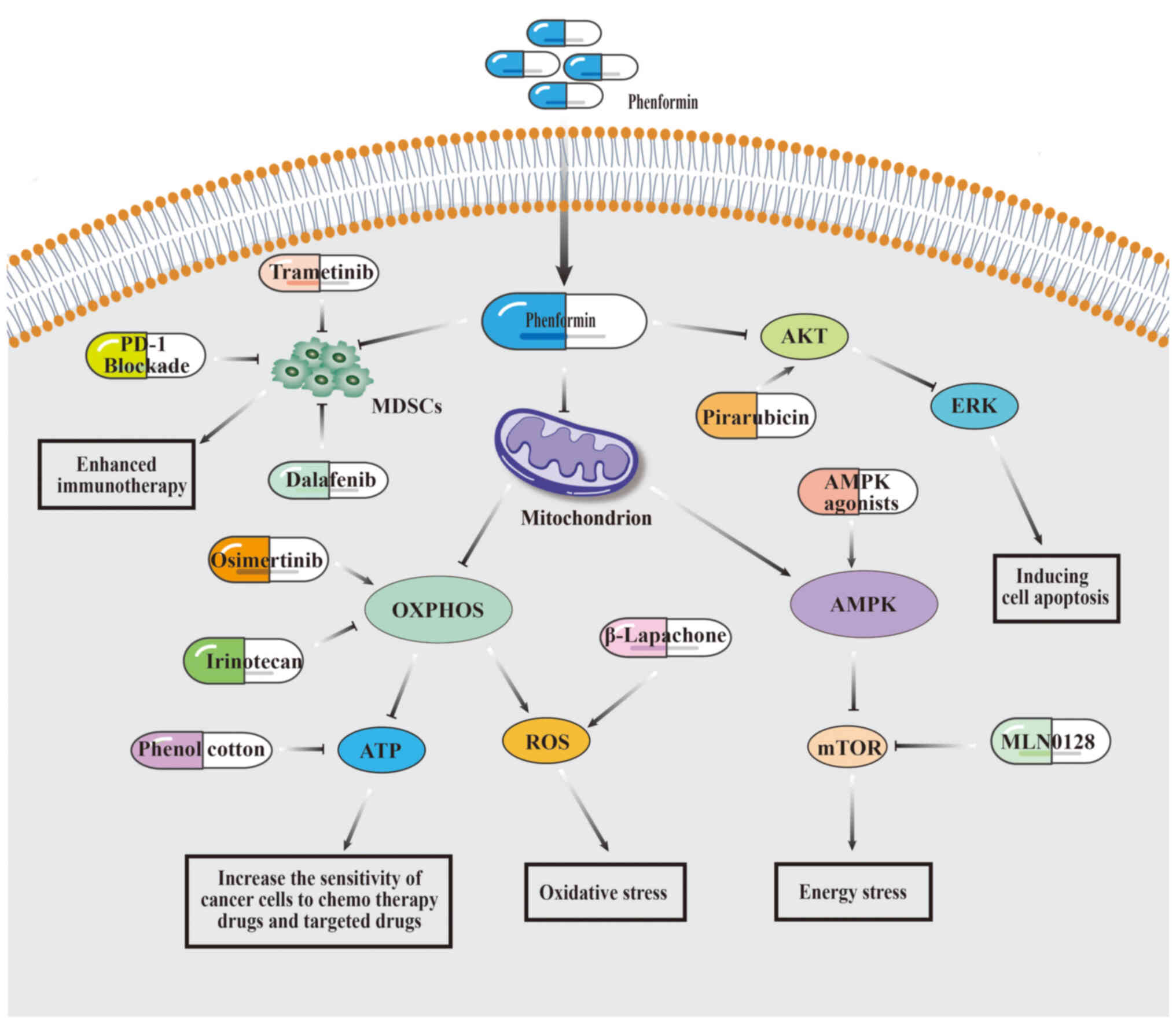 | Figure 4.Strategies and mechanisms of
combination therapy with phenformin. By inhibiting OXPHOS or
activating AMPK, phenformin induces energy stress or oxidative
stress in cancer cells, thereby enhancing the sensitivity of cancer
cells to chemotherapy drugs, targeted drugs, or other clinical
agents. Additionally, phenformin suppresses MDSCs in immune cells
to augment the antitumor efficacy of immunotherapy. Moreover,
phenformin exhibits the potential to overcome cancer cell
resistance to chemotherapy agents through its inhibitory effects on
the AKT/ERK pathway. MDSCs, myeloid-derived suppressor cells; ERK,
extracellular signal-regulated kinase; AMPK, AMP-activated protein
kinase; mTOR, mammalian target of rapamycin; OXPHOS, oxidative
phosphorylation. |
Lung cancer
Lung cancer is one of the most common malignancies,
and has relatively high morbidity and mortality rates. The
treatment efficacy for lung cancer remains unsatisfactory owing to
drug resistance and specific gene mutations in the treatment
process (89,90). Epidermal growth factor
receptor-tyrosine kinase inhibitors (EGFR-TKIs) are a primary class
of targeted therapeutics for the management of lung cancer.
However, prolonged usage can lead to the emergence of drug
resistance (91). It has previously
been established that in NSCLCs, which have acquired resistance to
EGFR-TKIs, there is a metabolic shift from glycolysis to oxidative
phosphorylation (OXPHOS) (19,43).
Notably, phenformin-induced inhibition of OXPHOS resulted in redox
imbalance, suppressing tumor metabolic abnormalities, and
ultimately triggering the death of acquired EGFR-TKI-resistant
NSCLC cells (43). Furthermore,
Martin et al (19)
discovered that combining osimertinib with phenformin delayed the
resistance of tumor cells to osimertinib. In addition to mitigating
drug resistance, phenformin enhances the sensitivity of
chemotherapy drugs. For instance, Lee et al (16) identified that the co-administration
of phenol cotton and phenformin synergistically inhibited OXPHOS
and reduced intracellular ATP levels in irinotecan-resistant NSCLC
cells, thereby inducing cell death and enhancing the sensitivity of
cells to irinotecan. Additionally, a small number of preclinical
studies have certified that phenformin exhibited the potential to
augment the sensitivity of NSCLC towards radiation therapy
(18), as well as reinforce the
sensitivity of Ym155, a mitochondrial inhibitor (92). These studies indicated that
phenformin can reverse drug resistance in NSCLC and enhance
efficacy by inhibiting OXPHOS, providing new ideas for addressing
issues such as drug resistance and low efficacy in clinical cancer
treatment.
KRAS represents the most frequently mutated gene in
lung adenocarcinoma, but no effective KRAS-targeted therapy has
been developed (93). As it was
aforementioned, it has been discovered that phenformin displayed
inhibitory effects on the proliferation of lung cancer cells
harboring KRAS mutations or LKB1 deficiency (62,94).
Momcilovic et al (62)
revealed that the combination of phenformin and mTOR kinase
inhibitor MLN0128 hindered further the survival of KRAS/LKB1
co-mutated NSCLC cells. Knockout of the ALDH1L1 gene enhances the
inhibitory effect of phenformin on KRAS mutation-driven lung cancer
cell proliferation (94). In
addition, LKB1 deficiency attenuates the sensitivity of cancer
cells to the MEK inhibitor selumetinib in KRAS-mutated NSCLC. The
antitumor effect is markedly enhanced when phenformin is combined
with selumetinib (95). These
preclinical studies suggested that whether administered alone or in
combination, phenformin may be a promising drug for the treatment
of specific gene-mutated lung cancer.
In conclusion, phenformin primarily enhances the
susceptibility of lung cancer cells to chemoradiation, targeted
therapies and other clinical drugs by impeding mitochondrial
OXPHOS. This indicates that the dual treatment of targeting
tumorigenic growth signals and cancer metabolism stands for a novel
and efficacious strategy. Although phenformin is emerging in lung
cancer treatment, current studies have primarily focused on lung
cancer cells and small animal models. Further investigations
involving large model animals and human subjects are imperative to
address drug resistance mechanisms and enhance the sensitization
effects of phenformin. Regardless, phenformin manifests promising
potential for the treatment of advanced lung cancer.
Skin cancer
Melanoma is an infrequent and perilous form of skin
cancer (96). Some mutated genes in
melanoma, such as BRAF, NRAS and NF1, activate the RAS/RAF/MEK/ERK
signaling pathway, resulting in uncontrolled tumor growth and
proliferation (97). However,
prolonged use of inhibitors targeting these signaling pathways
increases the chances of developing drug resistance. It has been
reported that phenformin can suppress RAF/MEK/ERK signaling by
activating AMPK, thereby impeding the proliferation of melanoma
cells (6,98). The combination of phenformin and the
BRAF inhibitor PLX4720 displays a synergistic effect in suppressing
the viability of BRAF mutant melanoma cells (6). In addition, phenformin and ERK
inhibitor SCH772984 also synergistically inhibit the proliferation
of NF1 mutant melanoma cells and accelerate apoptosis of cells
(99). These inhibitors, along with
phenformin, work together to inhibit the cell growth and
proliferation signaling pathway mTOR, thereby synergistically
exerting antitumor effects. Additionally, melanoma cells exposed to
BRAF and ERK inhibitors chronically exhibit heightened sensitivity
towards OXPHOS (100).
Consequently, when used with these inhibitors, phenformin
demonstrates superior inhibitory efficacy compared with its
standalone usage.
Recent preclinical studies and clinical trials have
shown that the combination of phenformin and immune checkpoint
inhibitors is a favorable strategy for treating melanoma.
Phenformin selectively reduces the accumulation of G-MDSCs in the
spleen and tumors of melanoma mouse models, thereby enhancing the
anti PD-1 antibody combination therapy effect (33). The aforementioned study has shed
light on the potential impact of phenformin on the TME. Building
upon the efficacy of phenformin in inhibiting melanoma cells, a
phase I b clinical trial was conducted to assess the safety and
effectiveness of combining phenformin with dalafenib/trametinib
(BRAF inhibitor/MEK inhibitor) in patients diagnosed with
BRAFV600E/K mutant melanoma (NCT03026517). In this study, shrinkage
of tumor lesions was found in 56% of patients. In addition,
phenformin was also observed to reduce tumor-promoting immune cells
MDSCs and enhance immune recognition of melanoma cells, confirming
the results of preclinical studies (21). The occurrence of lactate toxicity,
accompanied by symptoms of vomiting/nausea, was observed in 2 out
of the 18 treated patients, and it disappeared upon discontinuation
of medication. Consequently, considering the potential toxicity of
phenformin, further investigation is warranted to determine the
optimal dosage.
The aforementioned studies reveal the considerable
role of phenformin in the treatment of skin cancer, particularly
melanoma. The clinical trial provides recommendations for the
dosage of phenformin in subsequent phase II clinical trials. It
suggests that the combination of phenformin and immune checkpoint
inhibitors is a potential treatment for melanoma. This will advance
the progress of phenformin towards clinical application.
Hepatoma
The high heterogeneity exhibited by hepatocellular
carcinoma (HCC) brings a substantial challenge to its treatment
(101). A low response rate is a
prevalent phenomenon observed in current therapeutic approaches.
Notably, the efficacy of phenformin in enhancing the sensitivity of
clinical drugs and mitigating drug resistance in HCC has been
observed. Huang et al (20)
demonstrated that sorafenib and phenformin suppressed HCC cell
proliferation by concurrently targeting the CRAF/ERK and
PI3K/AKT/mTOR signaling pathways. More importantly, the combination
of the two did not result in weight loss or liver and kidney
toxicity in mice, suggesting that the combination of sorafenib and
phenformin is a safe and effective strategy for the treatment of
HCC. Furthermore, Veiga et al (102) also provided evidence supporting
the role of phenformin in inducing mitochondrial dysfunction,
enhancing the sensitivity of HCC cells to mTOR inhibitors, and
effectively controlling tumor burden in mouse models. These studies
indicated that phenformin primarily increases its inhibitory effect
on liver cancer cells by inhibiting the mTOR signaling pathway and
inducing mitochondrial damage. The liver is a crucial metabolic
organ, and phenformin has a potential role in regulating tumor
metabolism. Thus, the combination of phenformin with inhibitors
targeting critical enzymes involved in metabolic pathways like PKM2
and ALDH may potentially enhance the therapeutic efficacy against
HCC.
Breast cancer
The incidence rate of breast cancer ranks highest
among female malignancies; the mortality rate is second, while the
treatment options for breast cancer remain limited (103,104). Previous studies have confirmed
that phenformin showed inhibitory effects on the development and
progression of breast tumors by modulating angiogenesis, cell
proliferation, apoptosis and EMT in breast cancer cells and animal
models (69,105). The antitumor safety of phenformin
was verified in xenotransplantation of breast cancer mice (26). Furthermore, phenformin is also used
in conjunction with other pharmaceutical agents for the management
of breast cancer. For instance, Totten et al (47) demonstrated that inflammatory
mediators in cancer induced the activation of STAT1 signaling,
thereby upregulating the expression of reactive oxygen scavenger
NQO1 and attenuating the inhibitory effect of phenformin on breast
cancer cells. The combination of the NQO1 inhibitor β-lapadone and
phenformin evoked an elevation in oxidative stress, rendering the
breast cancer xenograft tumor model susceptible to phenformin. The
findings suggested that phenformin manifests antitumor efficacy in
both cellular and murine models, offering valuable insights for the
development of breast cancer treatment.
Other types of cancers
In recent years, phenformin has been found to have
potential inhibitory effects on various types of cancer, including
bladder cancer (17), leukemia
(106,107), brain tumor (25,108),
pancreatic cancer (59,85,109,110) and others. The chemotherapeutic
agent pirarubicin stimulated Akt and ERK phosphorylation, giving
rise to resistance in bladder cancer. Peng et al (17) demonstrated that phenformin
effectively sensitized bladder cancer cells to pirarubicin by
inhibiting AKT and ERK signaling pathways. In a mouse model of T
cell acute lymphoblastic leukemia, the combination of mitochondrial
antioxidants and phenformin efficiently reduced the leukemia burden
(106). Furthermore, in mouse
models of PTEN-deficient T-cell lymphoma and human T-all/T-LL
cancer cells, the combined administration of phenformin, metformin
and the AMPK agonist AICAR synergistically activated AMPK, while
inhibiting the mTOR signaling pathway, thereby exhibiting potent
anti-leukemic properties (107).
In pancreatic ductal adenocarcinoma with high OXPHOS, phenformin
induces a transition to a low OXPHOS state by inhibiting
mitochondria, and acts as a sensitization agent for the
chemotherapy drug gemcitabine, further enhancing the antitumor
effect of gemcitabine (59). In
addition, the combination of phenformin and phenol cotton displays
a double inhibitory effect in GBM, pancreatic cancer and other
malignancies (118–110). In
particular, Park et al (108) also discovered that the combination
of phenformin, phenol cotton and the chemotherapy drug temozolomide
in the treatment of GBM resulted in intracellular energy deficiency
and induced apoptosis, offering a novel strategy for addressing
resistance and recurrence issues in GBM. The aforementioned studies
indicate that phenformin mainly reverses tumor resistance or
synergizes efficacy by inhibiting OXPHOS or mTOR signaling pathways
in various cancers.
In summary, based on preclinical investigations of
multiple types and levels of tumor, phenformin exhibits robust
antitumor activity. In combination therapy specifically, phenformin
demonstrates unique advantages in mitigating drug resistance and
enhancing drug sensitivity. The latest strategies for the
combination therapy of phenformin are summarized in Table I.
 | Table I.The combination strategies of
phenformin for tumor treatment. |
Table I.
The combination strategies of
phenformin for tumor treatment.
| First author,
year | Combined drugs | Cancer type | Antitumor
mechanism | References |
|---|
| Martin et
al, 2016 | Osimertinib | NSCLC | Inhibit OXPHOS,
induce REDOX imbalance, and accelerate cell death in
osimertinib-resistant NSCLC cells. | (19) |
| Lee et al,
2018 | Phenol cotton +
Irinotecan | NSCLC | The synergistic
inhibition of OXPHOS gives rise to a reduction in ATP production
and triggers cellular apoptosis. | (16) |
| Wang et al,
2015 | Radiotherapy | NSCLC | Activation of AMPK
or enhancement of endoplasmic reticulum stress enhances NSCLC
response to ionizing radiation treatment. | (18) |
| Mondal et
al, 2022 | BMP inhibitor +
Ym155 | NSCLC | Synergistically
induce AIF caspase-independent cell death in lung cancer cells by
activating AMPK. | (92) |
| Zhang et al,
2017 | Selumetinib | NSCLC | Synergistically
inhibit phosphorylated ERK and S6 levels, leading to apoptosis
induction. | (95) |
| Momcilovic et
al, 2015 | Kinase inhibitor
MLN0128 | NSCLC | The induction of
energy stress promotes the acceleration of cellular apoptosis. | (62) |
| Yuan et al,
2013 | BRAF inhibitor
PLX4720 | Melanoma | The activation of
AMPK suppresses mTOR signaling and triggers apoptosis in BRAF
mutant melanoma cells. | (6) |
| Trousil et
al, 2017 | ERK inhibitor
SCH772984 | Melanoma | The activation of
AMPK suppresses mTOR signaling, thereby impeding proliferation and
promoting apoptosis in NF1 mutant melanoma cells. | (99) |
| Chapman et
al, 2023 | Dabrafenib +
Trametinib | Melanoma | Activation of AMPK
tempts ROS, selectively inhibiting MDSCs in mice and enhancing the
sensitivity of Dabrafenib/Trametinib in patients with BRAF V600
mutant melanoma. | (21) |
| Li and Xiang,
2022 | Anti-PD-1
antibody | Melanoma | Reduce the
population of G-MDSCs and potentiate the antitumor efficacy of
anti-PD-1 antibodies in melanoma cells. | (33) |
| Huang et al,
2021 | Sorafenib | HCC | Simultaneous
targeting of the CRAF/ERKand PI3K/AKT/mTOR pathways exhibits a more
pronounced inhibitory effect on the proliferation of HCC
cells. | (20) |
| Veiga et al,
2018 | mTOR dual
inhibitor | HCC | Induce
mitochondrial dysfunction, trigger compensatory glycolytic
transformation, and markedly augment the susceptibility of HCC
cells to dual mTOR inhibitors. | (102) |
| Totten et
al, 2021 | NQO1 inhibitor
β-Lapachone | Breast cancer | Increase the level
of ROS, thereby exerting further inhibitory effects on the
proliferation of breast cancer cells. | (47) |
| Peng et al,
2019 | Pirarubicin | BC | Reversal of
pirarubicin-induced AKT and ERK phosphorylation, circumvention of
pirarubicin resistance in BC cells, and facilitation of cellular
apoptosis. | (17) |
| Kong et al,
2020 | MitoTEMPO | Leukemia | Induce
comprehensive stress response and decrease proliferation of cell
proliferation. | (106) |
| Rosilio et
al, 2013 | Metformin +
AICAR | Leukemia | The activation of
AMPK suppresses mTOR signaling and impedes the proliferation of
PTEN-deficient T-cell lymphoma in mice and human T-ALL/T-LL cancer
cells. | (107) |
| Masoud et
al, 2020 | Gefitinib | PDAC | Inhibition of
mitochondrial complex I results in a transition of cells to a low
OXPHOS state, thereby augmenting the antitumor efficacy of
gemcitabine against high OXPHOS tumors. | (59) |
| Lee et al,
2021 | Phenol cotton | PDAC | Reduce ATP and
accelerate cell apoptosis. | (109,110) |
| Park et al,
2018 |
| GMB |
|
|
Discussion
In recent years, phenformin has gradually become a
‘star’ antitumor drug, which has marked clinical significance.
Phenformin has been used to treat type 2 diabetes, and it has the
basis of pharmacokinetics and relatively complete human data. In
addition, phenformin showed notable tumor inhibition effect in a
variety of tumors, and its lactic acid toxicity was controllable,
which met the requirements of clinical drugs with high efficiency
and low toxicity. From the analysis of the results of preclinical
studies and clinical trials, compared with a single drug regimen,
the combined application of phenformin as an adjunct drug with
clinical treatment regimens, such as chemotherapy, radiotherapy,
targeted therapy and immunotherapy, ensures safety and efficacy by
decreasing the dosage of phenformin, which further enriched and
optimized clinical cancer treatment strategies and provided more
selectivity for cancer treatment.
Despite the positive outcomes of phenformin as an
anticancer agent in numerous preclinical trials, there is still a
long way to go to promote the successful use of phenformin in
clinical oncology treatment. Therefore, the future can be optimized
from the following two aspects discussed below. From a
pharmaceutical perspective, by optimizing how phenformin enters
cells, its antitumor activity can also be considerably enhanced.
For instance, the use of CD147 nanoparticles loaded with phenformin
(81) or graphene drug carriers for
targeted delivery of phenformin (111) can effectively and expeditiously
transport phenformin to the site of lesion, thereby facilitating
enhancement in the antitumor efficacy of phenformin. Furthermore,
to enhance the antitumor efficacy of phenformin, it is vital to
consider structural modifications of phenformin from a
pharmacochemical perspective. Over the past few years, researchers
have focused on synthesizing highly effective and low-toxic
derivatives of phenformin to optimize the antitumor activity of
phenformin. The novel phenformin derivatives 2-(2-chlorophenyl)
ethyl biguanide (2-Cl-phen) (112–114) and IM156 (115) exhibited superior efficacy in
inhibiting tumor growth. IM156 has undergone a phase I clinical
trial (NCT03272256) in patients with advanced solid tumors and
lymphoma. Consequently, from the perspectives of pharmaceutics and
pharmaco-chemistry, developing specific tumor-targeting drug
delivery materials and optimizing the structure of phenformin are
future research directions to enhance the antitumor activity of
phenformin.
To sum up, in order to promote the application and
clinical treatment of tumors with phenformin, efforts need to be
made in the following aspects: i) The delivery system of phenformin
requires optimization or use of novo dosage forms for precise
packaging and delivering it to tumor lesions, aiming to mitigate
toxic side effects; ii) the structure of the target of phenformin
needs to be analyzed to refine the synthesis of high-efficiency and
low-toxicity phenformin derivatives; iii) the mechanisms underlying
drug resistance and insensitivity in tumors need to be investigated
from a clinical perspective, and novel combination therapy
strategies for phenformin need to be identified; and iv) despite
positive anticancer effects observed in preclinical studies and
phase I trials, it is urgent to augment the sample size for phase
II and III clinical trials to comprehensively assess lactic acid
toxicity and therapeutic efficacy at recommended dosages.
In conclusion, as a member of the biguanide family,
phenformin has shown superior antitumor effects than metformin in
various cancer cell lines and xenograft animal models, garnering
considerable attention from researchers. Phenformin principally
affects a series of processes in tumor occurrence and development
by inducing mitochondrial dysfunction or acting on TME and CSCs,
such as affecting cancer cell metabolism, inducing cancer cell
stress response, inhibiting tumor angiogenesis, EMT, blocking cell
cycle and others. The multifaceted diversity of antitumor
mechanisms exhibited by phenformin further broadens its range of
applications. It is noteworthy that phenformin reverses tumor
resistance to clinical drugs or synergistically increases efficacy
by inhibiting oxidative OXPHOS or mTOR signaling pathways,
providing a new strategy for clinical cancer treatment. Moreover,
it has been observed in clinical trials that the combination of
phenformin with clinical drugs effectively reduced MDSCs in
patients, which will further promote the study of the combined
regimen of phenformin and immunotherapy. In conclusion, substantial
evidence supports the promising potential of phenformin as an
effective anticancer drug, and it is worth further studying its
mechanism of action, which will pave the way for phenformin to
enter the clinical treatment of tumors and provide a broader
strategy for cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82172653), the Scientific Research
Program of FuRong Laboratory (grant no. 2023SK2096) and the Key
Project of Developmental Biology and Breeding from Hunan (grant no.
2022XKQ0205).
Availability of data and materials
Not applicable.
Authors' contributions
QZ reviewed the literature and drafted the original
manuscript. DL designed and reviewed the manuscript. XY reviewed
the manuscript and provided funding acquisition. All authors read
and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Zhang M, Deng D and Zhu X: The
function, mechanisms, and clinical applications of metformin:
Potential drug, unlimited potentials. Arch Pharm Res. 46:389–407.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ,
Ennis M, Lemieux J, Ligibel JA, Hershman DL, Mayer IA, Hobday TJ,
et al: Effect of metformin vs. placebo on invasive Disease-Free
survival in patients with breast cancer: The MA.32 randomized
clinical trial. JAMA. 327:1963–1973. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galal MA, Al-Rimawi M, Hajeer A, Dahman H,
Alouch S and Aljada A: Metformin: A Dual-role player in cancer
treatment and prevention. Int J Mol Sci. 25:40832024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
García Rubiño ME, Carrillo E, Ruiz Alcalá
G, Domínguez-Martín A, A Marchal J and Boulaiz H: Phenformin as an
anticancer agent: Challenges and prospects. Int J Mol Sci.
20:33162019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bridges HR, Blaza JN, Yin Z, Chung I,
Pollak MN and Hirst J: Structural basis of mammalian respiratory
complex I inhibition by medicinal biguanides. Science. 379:351–357.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo
C, Lee JH, Shen CH, Bosenberg MW, McMahon M, Cantley LC and Zheng
B: Phenformin enhances the therapeutic benefit of BRAF(V600E)
inhibition in melanoma. Proc Natl Acad Sci USA. 110:18226–18231.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Swanson KD and Zheng B:
Therapeutic repurposing of biguanides in cancer. Trends Cancer.
7:714–730. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Magno L, Manni S, Di Pastena F, Coni S,
Macone A, Cairoli S, Sambucci M, Infante P, Moretti M, Petroni M,
et al: Phenformin inhibits Hedgehog-dependent tumor growth through
a Complex I-independent redox/corepressor module. Cell Rep.
30:1735–1752.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalender A, Selvaraj A, Kim SY, Gulati P,
Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et
al: Metformin, independent of AMPK, inhibits mTORC1 in a rag
GTPase-dependent manner. Cell Metab. 11:390–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH, Li M, Trousil S, Zhang Y, Pasca di
Magliano M, Swanson KD and Zheng B: Phenformin inhibits
Myeloid-derived suppressor cells and enhances the Anti-tumor
activity of PD-1 blockade in melanoma. J Invest Dermatol.
137:1740–1748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang D, Wang S, Liu G, Liu P, Deng H,
Sun J, Liu C, Leng X, Zhang Q, Bai F, et al: Phenformin suppresses
angiogenesis through the regulation of exosomal microRNA-1246 and
microRNA-205 levels derived from oral squamous cell carcinoma
cells. Front Oncol. 12:9434772022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang W, Finniss S, Cazacu S, Xiang C,
Brodie Z, Mikkelsen T, Poisson L, Shackelford DB and Brodie C:
Repurposing phenformin for the targeting of glioma stem cells and
the treatment of glioblastoma. Oncotarget. 7:56456–56470. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang D, Wang S, Deng H, Shi Y, Liu C,
Leng X, Zhang Q, Bai F, Zheng B, Guo J, et al: Phenformin activates
ER stress to promote autophagic cell death via NIBAN1 and DDIT4 in
oral squamous cell carcinoma independent of AMPK. Int J Oral Sci.
16:352024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nussinov R, Tsai CJ and Jang H: Anticancer
drug resistance: An update and perspective. Drug Resist Updat.
59:1007962021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan
T, Yang W, Tian C, Miao Z, Wang T, et al: Small molecules in
targeted cancer therapy: Advances, challenges, and future
perspectives. Signal Transduct Target Ther. 6:2012021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee S, Lee JS, Seo J, Lee SH, Kang JH,
Song J and Kim SY: Targeting mitochondrial oxidative
phosphorylation abrogated irinotecan resistance in NSCLC. Sci Rep.
8:157072018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng M, Deng J, Zhou S, Xiao D, Long J,
Zhang N, He C, Mo M and Yang X: Dual inhibition of
Pirarubicin-induced AKT and ERK activations by phenformin
sensitively suppresses bladder cancer growth. Front Pharmacol.
10:11592019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Xia S and Zhu Z: Synergistic
effect of phenformin in non-small cell lung cancer (NSCLC) ionizing
radiation treatment. Cell Biochem Biophys. 71:513–518. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martin MJ, Eberlein C, Taylor M, Ashton S,
Robinson D and Cross D: Inhibition of oxidative phosphorylation
suppresses the development of osimertinib resistance in a
preclinical model of EGFR-driven lung adenocarcinoma. Oncotarget.
7:86313–86325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang L, Xiao D, Wu T, Hu X, Deng J, Yan
X, Wu J, Xu S, Yang X and Li G: Phenformin synergistically
sensitizes liver cancer cells to sorafenib by downregulating
CRAF/ERK and PI3K/AKT/mTOR pathways. Am J Transl Res. 13:7508–7523.
2021.PubMed/NCBI
|
|
21
|
Chapman PB, Klang M, Postow MA, Shoushtari
AN, Sullivan RJ, Wolchok JD, Merghoub T, Budhu S, Wong P, Callahan
MK, et al: Phase Ib trial of phenformin in patients with
V600-mutated melanoma receiving dabrafenib and trametinib. Cancer
Res Commun. 3:2447–2454. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nattrass M and Alberti KG: Biguanides.
Diabetologia. 14:71–74. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stang M, Wysowski DK and Butler-Jones D:
Incidence of lactic acidosis in metformin users. Diabetes Care.
22:925–927. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lea MA, Chacko J, Bolikal S, Hong JY,
Chung R, Ortega A and Desbordes C: Addition of 2-deoxyglucose
enhances growth inhibition but reverses acidification in colon
cancer cells treated with phenformin. Anticancer Res. 31:421–426.
2011.PubMed/NCBI
|
|
25
|
Altinoz MA and Ozpinar A: Oxamate
targeting aggressive cancers with special emphasis to brain tumors.
Biomed Pharmacother. 147:1126862022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Appleyard MV, Murray KE, Coates PJ,
Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR and
Thompson AM: Phenformin as prophylaxis and therapy in breast cancer
xenografts. Br J Cancer. 106:1117–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chowdhury TA: Diabetes and cancer. QJM.
103:905–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anari F, Ramamurthy C and Zibelman M:
Impact of tumor microenvironment composition on therapeutic
responses and clinical outcomes in cancer. Future Oncol.
14:1409–1421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Ji YR and Lee YM: Crosstalk
between angiogenesis and immune regulation in the tumor
microenvironment. Arch Pharm Res. 45:401–416. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gabrilovich DI: Myeloid-derived suppressor
cells. Cancer Immunol Res. 5:3–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toh B, Wang X, Keeble J, Sim WJ, Khoo K,
Wong WC, Kato M, Prevost-Blondel A, Thiery JP and Abastado JP:
Mesenchymal transition and dissemination of cancer cells is driven
by myeloid-derived suppressor cells infiltrating the primary tumor.
PLoS Biol. 9:e10011622011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shrihari GT: Innate and adaptive immune
cells in Tumor microenvironment. Gulf J Oncolog. 1:77–81. 2021.
|
|
33
|
Li Q and Xiang M: Metabolic reprograming
of MDSCs within tumor microenvironment and targeting for cancer
immunotherapy. Acta Pharmacol Sin. 43:1337–1348. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mathieu M, Martin-Jaular L, Lavieu G and
Théry C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao Y, Dong Q, Li J, Zhang K, Qin J, Zhao
J, Sun Q, Wang Z, Wartmann T, Jauch KW, et al: Targeting cancer
stem cells and their niche: Perspectives for future therapeutic
targets and strategies. Semin Cancer Biol. 53:139–155. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petrachi T, Romagnani A, Albini A, Longo
C, Argenziano G, Grisendi G, Dominici M, Ciarrocchi A and Dallaglio
K: Therapeutic potential of the metabolic modulator phenformin in
targeting the stem cell compartment in melanoma. Oncotarget.
8:6914–6928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo Y, Dallaglio K, Chen Y, Robinson WA,
Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris
DA, et al: ALDH1A isozymes are markers of human melanoma stem cells
and potential therapeutic targets. Stem Cells. 30:2100–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sarvi S, Crispin R, Lu Y, Zeng L, Hurley
TD, Houston DR, von Kriegsheim A, Chen CH, Mochly-Rosen D, Ranzani
M, et al: ALDH1 Bio-activates nifuroxazide to eradicate ALDH(High)
Melanoma-Initiating cells. Cell Chem Biol. 25:1456–1469.e6. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kültz D: Molecular and evolutionary basis
of the cellular stress response. Annu Rev Physiol. 67:225–257.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao W, Wang RS, Handy DE and Loscalzo J:
NAD(H) and NADP(H) redox couples and cellular energy metabolism.
Antioxid Redox Signal. 28:251–272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Contenti J, Guo Y, Mazzu A, Irondelle M,
Rouleau M, Lago C, Leva G, Tiberi L, Ben-Sahra I, Bost F, et al:
The mitochondrial NADH shuttle system is a targetable vulnerability
for Group 3 medulloblastoma in a hypoxic microenvironment. Cell
Death Dis. 14:7842023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim S, Im JH, Kim WK, Choi YJ, Lee JY, Kim
SK, Kim SJ, Kwon SW and Kang KW: Enhanced sensitivity of nonsmall
cell lung cancer with acquired resistance to epidermal growth
factor Receptor-Tyrosine kinase inhibitors to phenformin: The roles
of a metabolic shift to oxidative phosphorylation and redox
balance. Oxid Med Cell Longev. 2021:54283642021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta
P, Wei L, Ashby CR Jr, Yang DH and Chen ZS: Modulating ROS to
overcome multidrug resistance in cancer. Drug Resist Updat.
41:1–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miskimins WK, Ahn HJ, Kim JY, Ryu S, Jung
YS and Choi JY: Synergistic anti-cancer effect of phenformin and
oxamate. PLoS One. 9:e855762014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Totten SP, Im YK, Cepeda Cañedo E, Najyb
O, Nguyen A, Hébert S, Ahn R, Lewis K, Lebeau B, La Selva R, et al:
STAT1 potentiates oxidative stress revealing a targetable
vulnerability that increases phenformin efficacy in breast cancer.
Nat Commun. 12:32992021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Meng Y, Zhang S, Wu H, Yang D, Nie
C and Hu Q: Phenformin and metformin inhibit growth and migration
of LN229 glioma cells in vitro and in vivo. Onco Targets Ther.
11:6039–6048. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Di Conza G and Ho PC: ER Stress responses:
An emerging modulator for innate immunity. Cells. 9:6952020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng C, Geng F, Cheng X and Guo D: Lipid
metabolism reprogramming and its potential targets in cancer.
Cancer Commun (Lond). 38:272018.PubMed/NCBI
|
|
53
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Q, Liu S, Zhai A, Zhang B and Tian G:
AMPK-Mediated regulation of lipid metabolism by phosphorylation.
Biol Pharm Bull. 41:985–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jackson AL, Sun W, Kilgore J, Guo H, Fang
Z, Yin Y, Jones HM, Gilliam TP, Zhou C and Bae-Jump VL: Phenformin
has anti-tumorigenic effects in human ovarian cancer cells and in
an orthotopic mouse model of serous ovarian cancer. Oncotarget.
8:100113–100127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lettieri Barbato D, Vegliante R, Desideri
E and Ciriolo MR: Managing lipid metabolism in proliferating cells:
New perspective for metformin usage in cancer therapy. Biochim
Biophys Acta. 1845:317–324. 2014.PubMed/NCBI
|
|
58
|
Khan H, Anshu A, Prasad A, Roy S, Jeffery
J, Kittipongdaja W, Yang DT and Schieke SM: Metabolic rewiring in
response to biguanides is mediated by mROS/HIF-1a in malignant
lymphocytes. Cell Rep. 29:3009–3018.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Masoud R, Reyes-Castellanos G, Lac S,
Garcia J, Dou S, Shintu L, Abdel Hadi N, Gicquel T, El Kaoutari A,
Diémé B, et al: Targeting mitochondrial complex I overcomes
chemoresistance in high OXPHOS pancreatic cancer. Cell Rep Med.
17:1001432020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bridges HR, Sirviö VA, Agip AN and Hirst
J: Molecular features of biguanides required for targeting of
mitochondrial respiratory complex I and activation of AMP-kinase.
BMC Biol. 14:652016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shackelford DB, Abt E, Gerken L, Vasquez
DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS
and Shaw RJ: LKB1 inactivation dictates therapeutic response of
non-small cell lung cancer to the metabolism drug phenformin.
Cancer Cell. 23:143–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Momcilovic M, McMickle R, Abt E, Seki A,
Simko SA, Magyar C, Stout DB, Fishbein MC, Walser TC, Dubinett SM
and Shackelford DB: Heightening energetic stress selectively
targets LKB1-Deficient non-small cell lung cancers. Cancer Res.
75:4910–4922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Izreig S, Gariepy A, Kaymak I, Bridges HR,
Donayo AO, Bridon G, DeCamp LM, Kitchen-Goosen SM, Avizonis D,
Sheldon RD, et al: Repression of LKB1 by miR-17~92 Sensitizes
MYC-Dependent lymphoma to biguanide treatment. Cell Rep Med.
1:1000142020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hardie DG and Alessi DR: LKB1 and AMPK and
the cancer-metabolism link-ten years after. BMC Biol. 11:362013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dalton KM, Lochmann TL, Floros KV, Calbert
ML, Kurupi R, Stein GT, McClanaghan J, Murchie E, Egan RK,
Greninger P, et al: Catastrophic ATP loss underlies a metabolic
combination therapy tailored for MYCN-amplified neuroblastoma. Proc
Natl Acad Sci USA. 118:e20096201182021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Singh S, De Carlo F, Ibrahim MA, Penfornis
P, Mouton AJ, Tripathi SK, Agarwal AK, Eastham L, Pasco DS,
Balachandran P and Claudio PP: The oligostilbene Gnetin H is a
Novel glycolysis inhibitor that regulates thioredoxin interacting
protein expression and synergizes with OXPHOS inhibitor in cancer
cells. Int J Mol Sci. 24:77412023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Suski JM, Braun M, Strmiska V and Sicinski
P: Targeting cell-cycle machinery in cancer. Cancer Cell.
39:759–778. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Caraci F, Chisari M, Frasca G, Chiechio S,
Salomone S, Pinto A, Sortino MA and Bianchi A: Effects of
phenformin on the proliferation of human tumor cell lines. Life
Sci. 74:643–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu Z, Ren L, Liu C, Xia T, Zha X and Wang
S: Phenformin induces cell cycle change, apoptosis, and
Mesenchymal-Epithelial transition and regulates the
AMPK/mTOR/p70s6k and MAPK/ERK pathways in breast cancer cells. PLoS
One. 10:e01312072015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dodd KM, Yang J, Shen MH, Sampson JR and
Tee AR: mTORC1 drives HIF-1α and VEGF-A signalling via multiple
mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene.
34:2239–2250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jaidee R, Kongpetch S, Senggunprai L,
Prawan A, Kukongviriyapan U and Kukongviriyapan V: Phenformin
inhibits proliferation, invasion, and angiogenesis of
cholangiocarcinoma cells via AMPK-mTOR and HIF-1A pathways. Naunyn
Schmiedebergs Arch Pharmacol. 393:1681–1690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang ZD, Wei SQ and Wang QY: Targeting
oncogenic KRAS in non-small cell lung cancer cells by phenformin
inhibits growth and angiogenesis. Am J Cancer Res. 5:3339–3349.
2015.PubMed/NCBI
|
|
75
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Guo Z, Zhao M, Howard EW, Zhao Q, Parris
AB, Ma Z and Yang X: Phenformin inhibits growth and
epithelial-mesenchymal transition of ErbB2-overexpressing breast
cancer cells through targeting the IGF1R pathway. Oncotarget.
8:60342–60357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Walsh LA and Damjanovski S: IGF-1
increases invasive potential of MCF 7 breast cancer cells and
induces activation of latent TGF-β1 resulting in epithelial to
mesenchymal transition. Cell Commun Signal. 9:102011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lin H, Li N, He H, Ying Y, Sunkara S, Luo
L, Lv N, Huang D and Luo Z: AMPK Inhibits the Stimulatory Effects
of TGF-β on Smad2/3 Activity, Cell Migration, and
Epithelial-to-Mesenchymal Transition. Mol Pharmacol. 88:1062–1071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Park JH, Kim YH, Park EH, Lee SJ, Kim H,
Kim A, Lee SB, Shim S, Jang H, Myung JK, et al: Effects of
metformin and phenformin on apoptosis and epithelial-mesenchymal
transition in chemoresistant rectal cancer. Cancer Sci.
110:2834–2845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chuang CH, Dorsch M, Dujardin P, Silas S,
Ueffing K, Hölken JM, Yang D, Winslow MM and Grüner BM: Altered
mitochondria functionality defines a metastatic cell state in lung
cancer and creates an exploitable vulnerability. Cancer Res.
81:567–579. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pereira-Nunes A, Ferreira H, Abreu S,
Guedes M, Neves NM, Baltazar F and Granja S: Combination therapy
with CD147-Targeted nanoparticles carrying phenformin decreases
lung cancer growth. Adv Biol (Weinh). 7:e23000802023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tong X, Chen Y, Zhu X, Ye Y, Xue Y, Wang
R, Gao Y, Zhang W, Gao W, Xiao L, et al: Nanog maintains stemness
of Lkb1-deficient lung adenocarcinoma and prevents gastric
differentiation. EMBO Mol Med. 13:e126272021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhou Q, Kim SH, Pérez-Lorenzo R, Liu C,
Huang M, Dotto GP, Zheng B and Wu X: Phenformin promotes
keratinocyte differentiation via the Calcineurin/NFAT pathway. J
Invest Dermatol. 141:152–163. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu T, Zhou S, Qin M, Tang J, Yan X, Huang
L, Huang M, Deng J, Xiao D, Hu X, et al: Phenformin and
ataxia-telangiectasia mutated inhibitors synergistically
co-suppress liver cancer cell growth by damaging mitochondria. FEBS
Open Bio. 11:1440–1451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rajeshkumar NV, Yabuuchi S, Pai SG, De
Oliveira E, Kamphorst JJ, Rabinowitz JD, Tejero H, Al-Shahrour F,
Hidalgo M, Maitra A, et al: Treatment of pancreatic cancer
Patient-Derived xenograft panel with metabolic inhibitors reveals
efficacy of phenformin. Clin Cancer Res. 23:5639–5647. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gunaydin B, Yigitturk G and Elbe H:
Cytotoxic effects of Phenformin on ovarian cancer cells: Expression
of HIF-1α and PDK1 in the hypoxic microenvironment. Rom J Morphol
Embryol. 64:355–361. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jiménez-Vacas JM, Herrero-Aguayo V,
Montero-Hidalgo AJ, Sáez-Martínez P, Gómez-Gómez E, León-González
AJ, Fuentes-Fayos AC, Yubero-Serrano EM, Requena-Tapia MJ, López M,
et al: Clinical, cellular, and molecular evidence of the additive
antitumor effects of biguanides and statins in prostate cancer. J
Clin Endocrinol Metab. 106:e696–e710. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee B, Lee C, Moon HM, Jo SY, Jang SJ and
Suh YA: Repurposing metabolic inhibitors in the treatment of colon
adenocarcinoma Patient-Derived Models. Cells. 12:28592023.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu L, Leng D, Cun D, Foged C and Yang M:
Advances in combination therapy of lung cancer: Rationales,
delivery technologies and dosage regimens. J Control Release.
260:78–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Recondo G, Facchinetti F, Olaussen KA,
Besse B and Friboulet L: Making the first move in EGFR-driven or
ALK-driven NSCLC: First-generation or next-generation TKI? Nat Rev
Clin Oncol. 15:694–708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mondal A, Roberge J, Gilleran J, Peng Y,
Jia D, Akel M, Patel Y, Zoltowski H, Doraiswamy A and Langenfeld J:
Bone morphogenetic protein inhibitors and mitochondria targeting
agents synergistically induce apoptosis-inducing factor (AIF)
caspase-independent cell death in lung cancer cells. Cell Commun
Signal. 20:992022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Román M, Baraibar I, López I, Nadal E,
Rolfo C, Vicent S and Gil-Bazo I: KRAS oncogene in non-small cell
lung cancer: Clinical perspectives on the treatment of an old
target. Mol Cancer. 17:332018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lee SH, Jeon Y, Kang JH, Jang H, Lee H and
Kim SY: The combination of loss of ALDH1L1 function and phenformin
treatment decreases tumor growth in KRAS-Driven lung cancer.
Cancers (Basel). 12:13822020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang J, Nannapaneni S, Wang D, Liu F,
Wang X, Jin R, Liu X, Rahman MA, Peng X, Qian G, et al: Phenformin
enhances the therapeutic effect of selumetinib in KRAS-mutant
non-small cell lung cancer irrespective of LKB1 status. Oncotarget.
8:59008–59022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dildar M, Akram S, Irfan M, Khan HU,
Ramzan M, Mahmood AR, Alsaiari SA, Saeed AHM, Alraddadi MO and
Mahnashi MH: Skin cancer detection: A review using deep learning
techniques. Int J Environ Res Public Health. 18:54792021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang AX and Qi XY: Targeting
RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB Life.
65:748–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Petti C, Vegetti C, Molla A, Bersani I,
Cleris L, Mustard KJ, Formelli F, Hardie GD, Sensi M and Anichini
A: AMPK activators inhibit the proliferation of human melanomas
bearing the activated MAPK pathway. Melanoma Res. 22:341–350. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Trousil S, Chen S, Mu C, Shaw FM, Yao Z,
Ran Y, Shakuntala T, Merghoub T, Manstein D, Rosen N, et al:
Phenformin enhances the efficacy of ERK Inhibition in NF1-Mutant
melanoma. J Invest Dermatol. 137:1135–1143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pollak M: Targeting oxidative
phosphorylation: Why, when, and how. Cancer Cell. 23:263–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bertuccio P, Turati F, Carioli G,
Rodriguez T, La Vecchia C, Malvezzi M and Negri E: Global trends
and predictions in hepatocellular carcinoma mortality. J Hepatol.
67:302–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Veiga SR, Ge X, Mercer CA,
Hernández-Álvarez MI, Thomas HE, Hernandez-Losa J, Ramón Y Cajal S,
Zorzano A, Thomas G and Kozma SC: Phenformin-Induced mitochondrial
dysfunction sensitizes hepatocellular carcinoma for dual inhibition
of mTOR. Clin Cancer Res. 24:3767–3780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Libson S and Lippman M: A review of
clinical aspects of breast cancer. Int Rev Psychiatry. 26:4–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Orecchioni S, Reggiani F, Talarico G,
Mancuso P, Calleri A, Gregato G, Labanca V, Noonan DM, Dallaglio K,
Albini A and Bertolini F: The biguanides metformin and phenformin
inhibit angiogenesis, local and metastatic growth of breast cancer
by targeting both neoplastic and microenvironment cells. Int J
Cancer. 136:E534–E544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kong H, Reczek CR, McElroy GS, Steinert
EM, Wang T, Sabatini DM and Chandel NS: Metabolic determinants of
cellular fitness dependent on mitochondrial reactive oxygen
species. Sci Adv. 6:eabb72722020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Rosilio C, Lounnas N, Nebout M, Imbert V,
Hagenbeek T, Spits H, Asnafi V, Pontier-Bres R, Reverso J, Michiels
JF, et al: The metabolic perturbators metformin, phenformin and
AICAR interfere with the growth and survival of murine
PTEN-deficient T cell lymphomas and human T-ALL/T-LL cancer cells.
Cancer Lett. 336:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Park HH, Park J, Cho HJ, Shim JK, Moon JH,
Kim EH, Chang JH, Kim SY and Kang SG: Combinatorial therapeutic
effect of inhibitors of aldehyde dehydrogenase and mitochondrial
complex I, and the chemotherapeutic drug, temozolomide against
glioblastoma tumorspheres. Molecules. 26:2822021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lee JS, Lee H, Woo SM, Jang H, Jeon Y, Kim
HY, Song J, Lee WJ, Hong EK, Park SJ, et al: Overall survival of
pancreatic ductal adenocarcinoma is doubled by Aldh7a1 deletion in
the KPC mouse. Theranostics. 11:3472–3488. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Park J, Shim JK, Kang JH, Choi J, Chang
JH, Kim SY and Kang SG: Regulation of bioenergetics through dual
inhibition of aldehyde dehydrogenase and mitochondrial complex I
suppresses glioblastoma tumorspheres. Neuro Oncol. 20:954–965.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Alhourani A, Førde JL, Nasrollahzadeh M,
Eichacker LA, Herfindal L and Hagland HR: Graphene-based phenformin
carriers for cancer cell treatment: A comparative study between
oxidized and pegylated pristine graphene in human cells and
zebrafish. Nanoscale Adv. 4:1668–1680. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Narise K, Okuda K, Enomoto Y, Hirayama T
and Nagasawa H: Optimization of biguanide derivatives as selective
antitumor agents blocking adaptive stress responses in the tumor
microenvironment. Drug Des Devel Ther. 8:701–717. 2014.PubMed/NCBI
|
|
113
|
Oh-Hashi K, Irie N, Sakai T, Okuda K,
Nagasawa H, Hirata Y and Kiuchi K: Elucidation of a novel
phenformin derivative on glucose-deprived stress responses in HT-29
cells. Mol Cell Biochem. 419:29–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Oh-Hashi K, Matsumoto S, Sakai T, Nomura
Y, Okuda K, Nagasawa H and Hirata Y: Elucidating the rapid action
of 2-(2-chlorophenyl)ethylbiguanide on HT-29 cells under a serum-
and glucose-deprived condition. Cell Biol Toxicol. 34:279–290.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Janku F, Beom SH, Moon YW, Kim TW, Shin
YG, Yim DS, Kim GM, Kim HS, Kim SY, Cheong JH, et al:
First-in-human study of IM156, a novel potent biguanide oxidative
phosphorylation (OXPHOS) inhibitor, in patients with advanced solid
tumors. Invest New Drugs. 40:1001–1010. 2022. View Article : Google Scholar : PubMed/NCBI
|