Cancer is the leading cause of mortality worldwide
and has become a major concern with increasing age and changing
lifestyle habits. The predominant cause of mortality by cancer is
the occurrence of distant metastases and for most cancers, bone is
a common site for metastasis. For example, it is estimated that
~70% of patients with advanced breast cancer and over 70% of
patients with advanced prostate cancer develop bone metastases
(BM). In addition, in patients with metastatic prostate cancer, the
probability of BM is as high as 90% (1). BM not only increases the economic
burden on healthcare systems and patients but also significantly
reduces patient quality of life and survival rates. In addition, it
frequently affects the spine, ribs, pelvis and femur, leading to a
higher incidence of spinal cord compression, pathological fractures
and intractable pain (2). In
addition, complications related to BM are significant, typically
occurring every 3–6 months on average. These complications reduce
patient quality of life and mortality is frequently associated with
unresolved skeletal complications. The prognosis of metastatic bone
disease varies based on the primary cancer site, with patients with
breast and prostate cancer often surviving for years, while
patients with lung cancer typically survive only a few months
(3). However, current clinical
treatments for BM are limited, highlighting the need to evaluate
new therapeutic targets.
For tumor metastasis, primary tumor cells must first
undergo epithelial-mesenchymal transition (EMT) to invade
surrounding tissues and enter the microvasculature of the blood or
lymphatic system (4). Cancer cells
in the bloodstream can spread to distant organs, settling in the
metastatic microenvironment. They may become dormant or proliferate
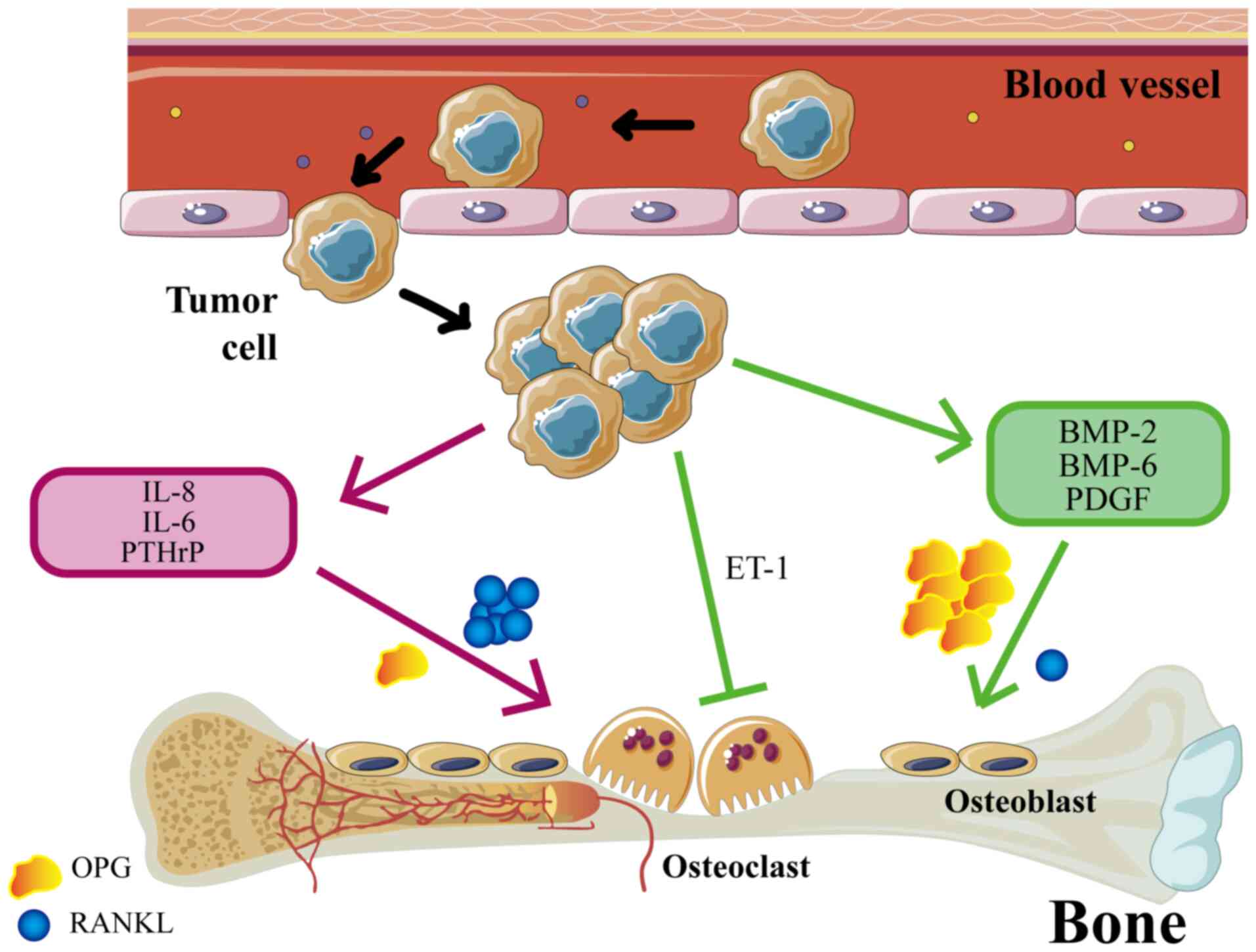
there, eventually forming secondary tumors (5). The occurrence of BM is associated with
osteoblasts and osteoclasts (OCs) in the bone microenvironment
(Fig. 1). BM can be categorized
into osteolytic and osteogenic subtypes. In addition, NF-κB ligand
receptor-activating factors (RANKL) are key mediators of
osteoclastogenesis (6). Tumor cells
in osteolytic BM can stimulate OCs and promote their
differentiation by secreting cytokines such as TNF, RANKL,
prostaglandins, leukemia inhibitory factor and IL-6, −8, −11, −15
and −17. Tumor cells can upregulate OC function by increasing the
ratio of RANKL to osteoprotegerin (OPG) (7). OCs-mediated bone matrix degradation
releases various cytokines and growth factors that promote tumor
cell growth (7). Colony-stimulating
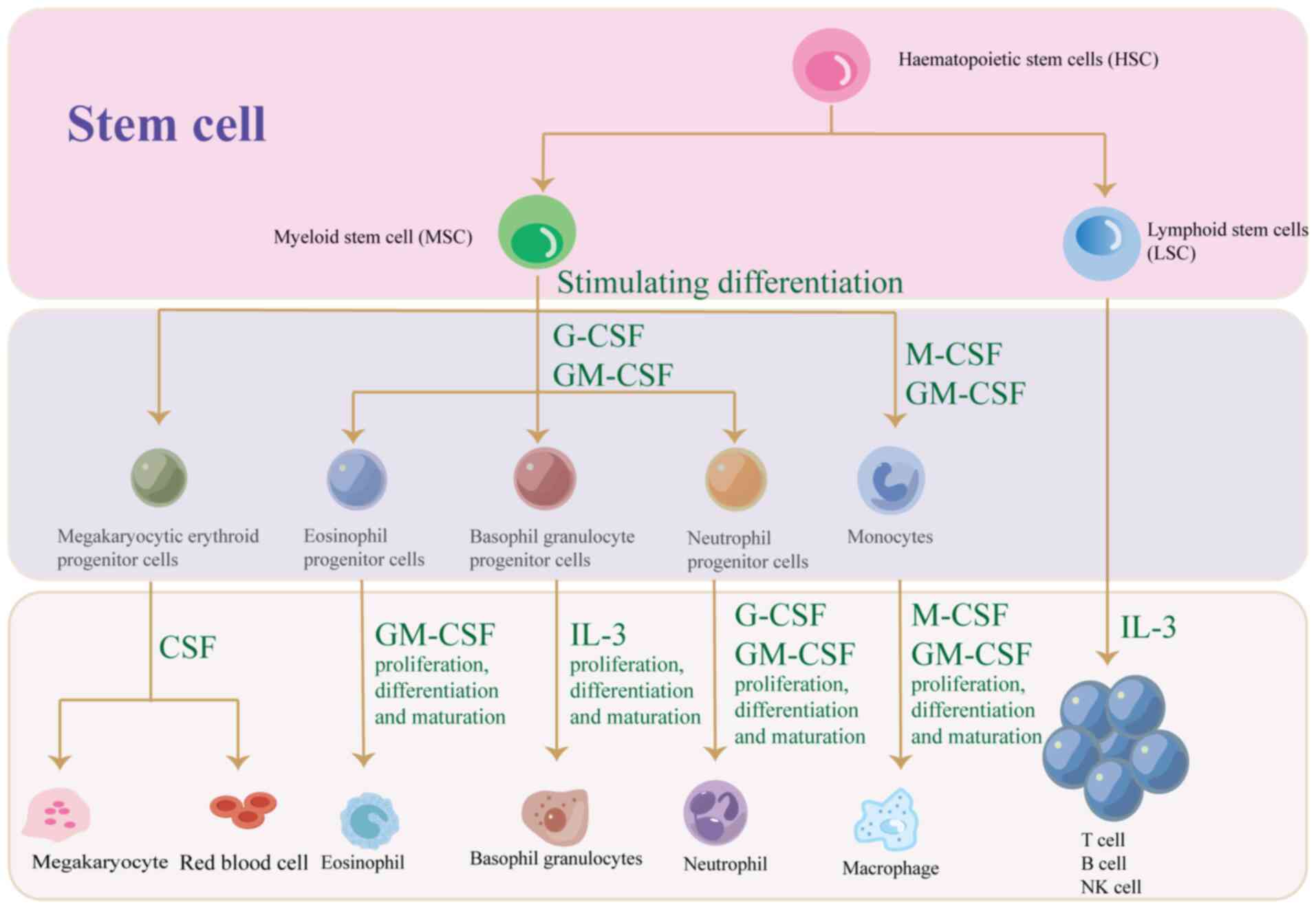
factors (CSF) are a group of cytokines responsible for hemopoiesis,
blood cell function regulation and maintaining homeostasis and
overall immunity (8). CSF plays a
crucial role in regulating blood cell function, given the
instability and short lifespan of these cells (9). The CSFs are of a number of types and
mainly include granulocyte-CSF (G-CSF), macrophage-CSF (M-CSF),
granulocyte-macrophage CSF (GM-CSF) and multipotent CSF (multi-CSF)
(10). In addition, CSF has
profound effects on the development of granulocytes, macrophages
and lymphocytes. The present article reviews the CSF family, its
relationship to BM, the underlying mechanisms involved and the
preclinical applications of CSF.
The G-CSF is a 30 kDa glycosylated peptide primarily
produced by monocytes and macrophages (13). G-CSF acts mainly on granulocytes.
Through binding to its receptor (G-CSFR), G-CSF activates signaling
pathways such as Janus tyrosine kinase (JAK)/signal transducers and
activators of transcription (STAT), Mitogen-activated protein
kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT), ultimately leading to the differentiation of
hematopoietic stem cells towards the granulocyte lineage and the
promotion of neutrophil production (8). G-CSF promotes the proliferation,
differentiation, maturation and release of neutrophils while also
enhancing the chemotaxis, phagocytosis and bactericidal capacity of
mature granulocytes (13). G-CSF
downregulates the inflammatory response by inhibiting the
production of pro-inflammatory cytokines in activated monocytes and
macrophages and by regulating peripheral lymphokine levels
(14). Studies have shown that
elevated G-CSF often indicates a poor cancer prognosis. G-CSF also
stimulates proliferation and metastasis in various cancer cells
(15). For example, G-CSF promotes
the progression of skin cancer and head and neck squamous cell
carcinoma through autocrine and paracrine mechanisms (16,17).
Similarly, G-CSF stimulates tumor progression by suppressing innate
and adaptive immunity while also promoting angiogenesis and tumor
growth (18). While G-CSF can
inhibit human meningioma development, it promotes cancer cell
proliferation through angiogenesis in other types of cancer
(19). G-CSF is significantly
correlated with the aggressiveness of cancer cells. For example,
G-CSF increases the invasiveness of lung cancer cells through
autocrine secretion (20). An in
vivo study showed that G-CSF-induced pre-metastatic
microenvironment was more favorable for cancer cell migration and
increased the expression of MMP-9, S100A8 and S100A9 pro-metastatic
molecules through Ly6G+Ly6C expression (21). Elevated G-CSF receptor expression in
patients with cancer is associated with an increased risk of
advanced metastasis (15). A
clinical study compared serum G-CSF levels between patients with
non-small cell lung cancer and normal individuals and revealed that
G-CSF was significantly elevated in patients with lung cancer,
which significantly decreased after the surgery. Therefore, it was
inferred that G-CSF is important in diagnosing non-small cell lung
cancer (22). Clinical studies have
demonstrated that tumor-derived G-CSF is associated with poor
patient prognosis. In addition, the patients with retroperitoneal
tumor have significantly elevated G-CSF and develop liver and
kidney metastases 3 months after primary tumor resection, BM after
8 months and succumbed after 17 months (23). Notably, in a mouse model of breast
cancer BM, G-CSF is found to create a metastasis-promoting tumor
microenvironment and G-CSF inhibition attenuated bone marrow
vascular remodeling and BM incidence (24).
M-CSF is a hematopoietic growth factor that plays a
key role in regulating mature myeloid cell populations (25). It comprises various cells, including
endothelial, fibroblasts, OCs, smooth muscle and macrophages. M-CSF
primarily acts on the monocyte-macrophage cell line, promoting
monocyte and macrophage production and regulating functions such as
antigen presentation, phagocytosis, cytokine secretion and tissue
repair. M-CSF stimulates the differentiation of myeloid progenitor
cells into monocytes, macrophages, dendritic cells and osteoblasts.
M-CSF promotes macrophage growth and function by binding to its
receptor (CSF-1R) and activating PI3K and MAPK signaling pathways
(26). It has been shown in animal
studies that daily administration of recombinant human G-CSF
enhances the recovery of stem cells, progenitor cells and blood
neutrophils in mice (27). GM-CSF
and M-CSF promote the survival and activation of macrophages,
neutrophils and eosinophils, as well as dendritic cell maturation.
However, certain M-CSF variants specifically promote the survival,
proliferation and differentiation of macrophage lineages (28). M-CSF and GM-CSF differ in their
roles in regulating macrophage differentiation phenotypes (25). M-CSF stimulates the M1 macrophage
phenotype, while GM-CSF promotes M2 phenotype activation (29). In addition, M-CSF modulates
macrophage phenotype and regulates their function in the tumor
microenvironment (30). M-CSF
facilitates tumor invasion, metastasis and immune evasion by
modulating tumor-associated macrophages (TAMs) in the tumor
microenvironment. Studies have shown that M-CSF levels are elevated
in cancer, inflammation and autoimmune diseases. Animal cancer
models reveal that M-CSF antibody administration or inhibition of
CSF-1R improves inflammation and cancer metastasis (31–33).
M-CSF has been closely associated with breast cancer
BM. In a mouse model of breast cancer, M-CSF gene knockdown reduced
the occurrence of distant metastasis. However, M-CSF gene knockdown
did not affect cancer cell proliferation or development. By
contrast, mice with M-CSF gene overexpression exhibit a significant
increase in late-stage cancer and lung metastases (34). A clinical study revealed
significantly elevated serum M-CSF levels in patients with head and
neck tumors, as well as in patients with advanced prostate and
breast cancer with BM (35). A
clinical trial in 1996 found that serum M-CSF levels are
significantly higher in patients with metastatic breast cancer
compared with those with primary breast cancer (36). In an in vivo experiment, it
was reported that the expression of osteoblasts and OCs in the bone
microenvironment can be altered by reducing the expression of
M-CSF, thereby inhibiting the occurrence of breast cancer BM
(37,38). In addition, M-CSFR phosphorylation
reduces bone tumor growth and inhibits osteolysis. Breast cancer BM
has also been associated with mesenchymal stem cells (MSCs). In
addition, the MCSs of patients with advanced breast cancer had
worse self-renewal and proliferation ability than normal
individuals. The reduced function of MSCs may be attributed to the
increased expression of pro-osteoclastogenic genes, such as CCL-2,
MMP-9 and M-CSF (39). M-CSF has
been found to influence BM in prostate cancer. A study indicated
that in a mouse model of prostate cancer BM, osteoblasts in the
bone microenvironment could be altered by the inhibition of RANKL
and M-CSF, reducing BM occurrence (40). These results were consistent with
another animal experiment which indicated that OCs inhibition in a
mouse model of prostate BM decreases M-CSF expression which
decreases the occurrence of BM and bone destruction (41). A clinical study compared serum M-CSF
levels between patients with prostate cancer with BM, patients with
prostate cancer without metastases and healthy individuals. There
were no significant differences in M-CSF between healthy subjects
and patients with prostate cancer without metastases. However,
serum M-CSF levels were significantly higher in patients with
prostate cancer with BM compared with those without metastases
(42). The study concluded that the
occurrence of prostate cancer BM is related to the M-CSF/M-CSFR
signaling pathway (42). Similar
findings were observed in lung cancer BM. In vitro and in
vivo studies have both demonstrated that osteoclastogenesis can
be promoted by upregulating M-CSF and RANKL in human lung
adenocarcinoma A549 cells, leading to an increased BM development
(43,44). M-CSF inhibition reduces the
M-CSF/RANKL-induced AMT/mTOR signaling pathway, decreasing OC
differentiation. M-CSF inhibition also reduces lung
adenocarcinoma-mediated interactions between OCs and osteoblasts,
thereby decreasing the occurrence of this ‘vicious circle’ and
osteolytic metastases (43). This
feedback loop plays a crucial role in lung cancer BM. Another
cellular study demonstrated that increased OC activity in non-small
cell lung cancer cells via the cyclic effects of parathyroid
hormone-related peptide (PTHrP)/IL-8 interference with osteoblasts
and OCs increased the incidence of BM in NSCLC (45). In a cellular experiment, co-culture
of kidney cancer cells with osteoblasts demonstrated that
osteoblasts promote tumor cell proliferation, suggesting that
osteoclasts create a more favorable environment for tumor survival.
In addition, BM occurrence could be effectively reduced by
inhibiting the growth of OCs (46).
M-CSF is an important factor in inducing OC differentiation;
therefore, it is hypothesized that it is a potential target for
treating or preventing BM in renal cell carcinoma.
GM-CSF is a 22 kDa glycosylated secretory protein
that promotes the proliferation and maturation of neutrophils,
eosinophils and macrophages from bone marrow progenitor cells
(28). In addition, GM-CSF acts
synergistically with other cytokines as a growth factor for
erythroid and megakaryocyte progenitors. In addition, it modulates
progenitor cells and interacts with erythropoietin to stimulate the
in vitro formation of eosinophil and megakaryocyte colonies
(9). GM-CSF promotes bi-directional
differentiation of hematopoietic stem cells to granulocytes and
macrophages by binding to GM-CSFR and activating signaling pathways
such as JAK2/STAT5, MAPK and PI3K/AKT (28). GM-CSF has been associated with
inflammatory responses, activating neutrophil activity in the human
body (47). It has been observed
that GM-CSF and G-CSF can induce endothelial cell proliferation and
migration, thereby promoting angiogenesis (48). These properties indicate that GM-CSF
may have potential applications in adjuvant tumor therapy (49). Angiogenesis not only promotes cancer
cell growth but significantly increases distance metastasis and
advances tumor stages more quickly (50). In addition, serum GM-CSF receptor
levels are elevated in patients with advanced cancer and distant
metastases (15). Studies have
shown that GM-CSF promotes the development of tumors such as lung,
breast, pancreatic, prostate, skin, colon, rectal, head and neck
squamous cell carcinomas (16,17,51–55).
In vivo and in vitro research shows that GM-CSF
overexpression elevates tumor cell migration and invasion (56). In 1999, a study reported that GM-CSF
could promote the invasiveness of lung cancer cells (20). In addition, a mouse tumor model
revealed that GM-CSF gene expression is correlated with tumor
metastasis in mice (57). GM-CSF
plays a dual role in tumor development, both promoting and
inhibiting tumor growth, depending on the context. GM-CSF has a
complex role in the tumor microenvironment, either suppressing
tumors by enhancing anti-tumor immune responses or promoting tumor
growth and metastasis by promoting the activity of tumor-associated
macrophages (58).
A significant association between GM-CSF and BM has
been observed. A study revealed that CTNND1 gene knockdown
accelerated the differentiation of immature bone marrow cells and
promoted BM development. This may be due to CTNND1 knockdown
enhancing PI3K/AKT/HIF-1α/CXCR4 pathway expression, promoting EMT
in tumor cells. When the CTNND1 knockdown tumor cells reach
the bone, they secrete more GM-CSF and IL-8, enhancing immature
bone marrow cells (especially neutrophils) and promoting BM
development (59). In a mouse model
of breast cancer BM, GM-CSF promotes the metastatic ability of
cancer cells and BM (60). For
nasopharyngeal cancer, bone is the most common metastatic site.
Among patients with advanced nasopharyngeal cancer, 64–67% carry BM
and the most common BM type in nasopharyngeal cancer is osteolytic
(61). GM-CSF secreted by cancer
cells can promote BM by promoting the secretion of IGF-1 from OCs,
which in turn promotes the proliferation of nasopharyngeal
carcinoma cells via the IGF-1/IGF-1R signaling pathway (61). In addition, metastatic breast cancer
was also associated with arthritis progression, indicating a
‘vicious cycle’ (62). The M-CSF
and GM-CSF are important pro-inflammatory factors associated with
rheumatoid arthritis (63).
Therefore, it is suggested that M-CSF and GM-CSF may contribute to
the development of breast cancer BM by influencing the course of
arthritis.
IL-3, also called multi-CSF, is a hematopoietic
factor produced by activated T-cells and NK-cells. In addition, it
promotes the growth and differentiation of bone-marrow-derived
T-cells in the immune response. Similarly, it increases the
formation of fibroblasts, granulocytes, macrophages,
megakaryocytes, eosinophils and mast cell colonies (9). IL-3 is recognized as an essential
early hematopoietic growth factor that regulates hematopoiesis.
IL-3 mainly acts on early hematopoietic progenitor cells to promote
their proliferation and differentiation. In the later stages, IL-3
acts in conjunction with hematopoietic growth factors such as
erythropoietin, GM-CSF and thrombopoietin to promote the
proliferation and differentiation of myeloid hematopoietic stem
cells. IL-3 stimulates the formation of progenitor cell colonies of
granulocytes, monocytes, erythrocytes and macrophages; enhances
macrophage phagocytosis; promotes hematopoietic stem cell
proliferation; and facilitates the proliferation and
differentiation of mast cells, basophils and eosinophils (64,65).
IL-3 and GM-CSF were first reported in 1988 to co-stimulate
hematopoiesis in primates (66).
Evidence suggests that IL-3 is involved in the onset and
progression of various hematological diseases, including acute
myeloid leukemia, chronic myeloid leukemia and myelodysplastic
syndromes (64). IL-3 has been
identified as a potential marker for the severity and mortality of
COVID-19-associated pneumonia during SARS-CoV-2 infection. Thus,
IL-3 serves as a predictive marker for the severity of SARS-CoV-2
infection and a potential therapeutic target for
COVID-19-associated pneumonia (67). In addition, since IL-3 can influence
the development of basophils and mast cells, it is often associated
with allergies, asthma, inflammation and other diseases. Extensive
data suggest that IL-3 is closely associated with various types of
cancer. For instance, IL-3 levels were significantly higher in the
serum of patients with colorectal cancer than in healthy subjects
(68). Therefore, it is inferred
that IL-3 influences cancer development in several ways. IL-3
alters the tumor microenvironment by affecting basophils, thereby
promoting cancer development (69).
Further, IL-3 promotes cancer cell proliferation by inducing
angiogenesis (70). In cellular
assays, it is found that osteoclast differentiation could be
inhibited by adding benzyl isothiocyanate with zoledronic acid to a
breast cancer-conditioned medium. Osteoclast inhibition is
accompanied by a significant increase in IL-3 (71). Immunohistochemical analysis of
patients with prostate cancer reveal that IL-3 is implicated in the
development of prostate cancer BM (72). However, the relationship between
IL-3 and BM requires further experimental validation.
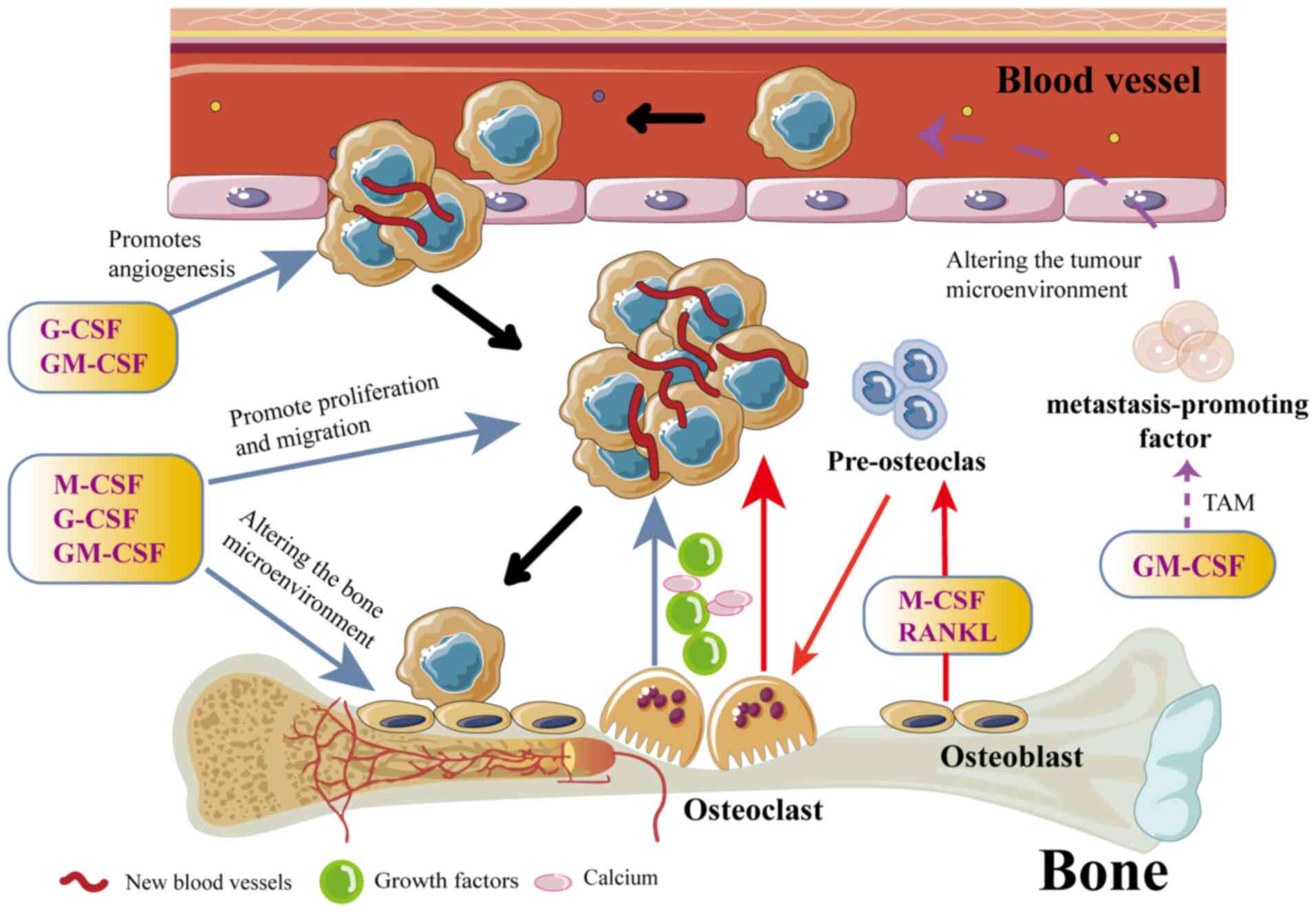
CSFs play complex and diverse roles in developing
and progressing BM in various types of cancer. They affect not only
the hematopoietic system but also the skeletal microenvironment and
the behavior of tumor cells through various mechanisms (Fig. 3). First, CSF promotes the bone
metastatic potential of tumor cells. CSF increases the metastatic
potential of cancer cells by promoting neoangiogenesis and
enhancing their invasiveness (73).
Studies have shown that G-CSF and GM-CSF can enhance the BM in
tumor cells through multiple pathways. For example, G-CSF promotes
tumor cell growth and metastasis by increasing angiogenesis and
improving nutrient and oxygen availability to tumor cells (74). In addition, G-CSF can improve the
tolerance of tumor cells to chemotherapeutic drugs, making it
easier for them to survive and proliferate in the bone (75,76).
GM-CSF enhances the pro-metastatic properties of the tumor
microenvironment by promoting the generation and activation of
TAMs, which are capable of secreting a variety of pro-angiogenic
and pro-metastatic factors (for example, VEGF, MMPs and IL-10),
further promoting BM of tumor cells (73).
Second, CSF indirectly affects the occurrence and
development of BM by regulating cellular components and factor
secretion in the bone marrow microenvironment. Bone is a common
site of metastasis for a number of cancers due to the high
expression of specific chemokines and growth factors in its
microenvironment, such as stromal cell-derived factor 1, which
promotes tumor metastasis (77). It
is considered that before the primary tumor reaches the bone, it
alters the bone microenvironment to promote cancer cell
proliferation and colonization. In addition, tumor cells induce
metastasis by forming ‘pre-metastatic niches’ in the bone,
comprising clusters of bone marrow-derived cells, creating a
favorable environment for the subsequent invasion and growth of
tumor cells (78). Osteolytic
lesions caused by OCs are significantly associated with BM. In
osteolytic BM, OCs activating factors act on OCs to induce its
formation. CSF and RANKL are important activators of OCs. In
addition, osteoclastogenesis is primarily modulated by the
interaction of CSF-1R, M-CSF, as well as RANK and RANKL (79). Therefore, M-CSF is essential for OC
generation and increased OC activity promotes osteolytic BM. M-CSF
is a key factor affecting OC generation and can influence OC
precursor's survival and development. Similarly, M-CSF induces
cytoskeletal rearrangement of OCs by activating c-Src and
phosphocreatine 3-kinase (80). The
M-CSF directly affects OCs by binding to M-CSFR, which attracts a
signaling complex comprising phosphorylated dDNA-activated protein
12 and the non-receptor tyrosine kinase Syk. In addition, it also
activates ERK/growth factor receptor-binding protein 2 and Akt/PI3K
signaling to regulate the proliferation, differentiation and growth
of OCs and their precursor cells (80). In addition, M-CSF can induce
osteoclastogenesis by increasing the OCs precursor RANK expression
via RANKL binding (81). In a
number of cancer models, tumor cells have been observed to secrete
cytokines that stimulate osteoblasts, such as PTHrP, VEGF-A and
hepatocyte growth factor. This increases the expression of RANKL
and M-CSF, stimulating osteoclastogenesis and altering the bone
microenvironment, thereby promoting BM development. M-CSF affects
osteoclastogenesis and influences other members of the CSF family
on OCs (2–7). In 1993, it was reported that M-CSF,
GM-CSF and IL-3 could promote osteoclastogenesis (82). In addition, GM-CSF is stimulated by
increased levels of NF-kB, resulting in increased OC activity,
which in turn leads to bone destruction and highly metastatic tumor
growth (15).
Third, CSF also plays an important role in
regulating the immune system, ultimately affecting BM. G-CSF
inhibits T-cell activity and reduces the killing effect of the
immune system on tumor cells, making it easier for tumor cells to
grow and metastasize in the bone (83).
BM often has a poor prognosis and high mortality due
to delayed diagnosis and limited treatment options. Bone is a
common site of metastasis in breast cancer; however, BM in
early-stage breast cancer is difficult to detect. As a result, BM
in breast cancer is often diagnosed late, leading to delayed
treatment. BM has been reported to occur in ~70% of patients with
advanced breast cancer. The median overall survival following BM
diagnosis was 40 months, indicating a high mortality rate (84). Similarly, BM has been reported in
>70% of patients with advanced prostate cancer (1). Epidemiological data from the United
States show that lung cancer now has a higher mortality rate than
breast and prostate cancers, ranking as the leading cause of
cancer-related death (85).
Recently, nanoparticles have provided new directions in lung cancer
treatment (86,87). Distant metastasis is the primary
cause of death in patients with lung cancer. Bone is a common site
of metastasis in advanced lung cancer and in advanced patients with
lung cancer, 40% develop BM (88).
The median survival of patients with lung cancer BM is often <6
months (89). Therefore, developing
effective treatments for BM remains a significant challenge for
clinicians and researchers.
CSFs are widely used in patients with cancer after
chemotherapy to elevate critically low leukocyte levels (9). The clinical role of CSFs has been
widely publicized. In 1990, with the availability of recombinant
mouse CSF experimental, it was revealed that subcutaneous
injections of G-CSF into mice increased blood leukocyte levels.
High doses of G-CSF have been associated with erythrocyte
suppression in the bone marrow (90). In addition, a 1987 study revealed
that injection of recombinant GM-CSF in mice does not significantly
increase blood leukocytes; however, it markedly increases the
macrophage numbers and activity (91). The most significant increase in the
blood of mice injected with recombinant multi-CSF was in
eosinophils, followed by neutrophils and monocyte levels (92). Further research is needed to
determine whether prolonged use of high-dose CSF has additional
side effects. Transgenic mice overexpressing GM-CSF indicates
blindness and various inflammatory lesions. In addition, a number
of transgenic mice overexpressing GM-CSF died at 2–4 months due to
macrophage activation, leading to muscle atrophy (93). In IL-3 overexpressing mice, there
were increased progenitor cells in the spleen and peritoneum and
decreased bone marrow progenitor cells. In addition, 80% of the
mice succumbed within 5 weeks (94). Mice overexpressing G-CSF cause the
proliferation of granulocytes and progenitor cells but does not
cause severe tissue damage (95).
The role of different cytokines in influencing the immunogenicity
of tumor cells and the vaccination properties of murine tumor cells
have been investigated in an animal experiment. Tumor cells
expressing mouse GM-CSF have the most significant stimulatory
effect on anti-tumor immunity following irradiation (96). GM-CSF is used in the treatment of
melanoma (97).
The first clinical trials of CSF, published in 1988,
demonstrated a significant increase in neutrophils when recombinant
human G-CSF was administered to patients with cancer prior to
chemotherapy (98). In addition,
G-CSF-associated side effects are minimal the most common bring
bone pain, which might be due to increased bone marrow cell counts
(99). A phase II clinical trial at
the Cancer Research Institute in Australia found that treatment
with recombinant human GM-CSF in 21 patients with advanced cancer
resulted in a 10-fold increase in leukocytosis. The trial also
observed increases in circulating neutrophils, eosinophils,
monocytes and lymphocytes. In addition, side effects, such as bone
pain, myalgia, rash and hepatic dysfunction, are observed with high
doses of rhGM-CSF (100). The
safety and efficacy of GM-CSF in combination with Ipilimumab
(Yervoy) for treating metastatic malignant melanoma is in a phase
II clinical trial (NCT01363206). In another clinical trial
(NCT02156388), G-CSF was administered in patients with advanced
metastatic cancer following chemotherapy, which revealed increased
neutrophil counts and activity and shorter duration and acute
symptoms of neutropenia. This minimized the incidence of serious
infections, reflecting improved efficacy and a longer half-life.
The therapeutic effect of G-CSF with trastuzumab in metastatic
breast cancer has also been studied in a randomized phase II
clinical trial (NCT00169104).
CSF has been widely used in treating rheumatoid
arthritis, coronary atherosclerosis and various inflammatory and
autoimmune diseases (28). A number
of preclinical trials have demonstrated that CSF is closely
associated with mechanisms of BM in various types of cancer.
However, more evidence is required to establish its efficacy in
treating BM. It is hypothesized that targeted therapies against
M-CSF or its signaling pathway may have clinical applications in BM
treatment. The effectiveness of combining CSF with existing
anti-bone metastatic drugs, such as bisphosphonates or RANKL
inhibitors, in inhibiting BM progression warrants further
investigation. Therefore, CSF might serve as a therapeutic target
for treating BM in different cancers.
Cancer is the leading cause of death and the
incidence of distant metastases significantly reduces survival in
patients with cancer. Bone is a common site of metastasis in
patients with cancer, often leading to serious complications such
as pain and hypercalcemia. In addition, BM significantly reduces
quality of life and imposes a substantial economic burden on
patients and healthcare systems. In addition, the limited treatment
options for BM contribute to significant psychological distress and
anxiety in affected patients. A number of studies have indicated
that changes in the bone microenvironment are closely related to
BM, suggesting that modulating the bone microenvironment may help
alleviate metastasis. In BM, cancer cells first undergo EMT, invade
the blood and lymphatic vessels and migrate into bone tissue. Prior
to entering bone tissue, cancer cells secrete cytokines that modify
the bone microenvironment, facilitating their colonization and
proliferation. For example, tumor cells can release factors that
induce osteoblast formation during BM. This enhances the
osteoblast-mediated bone formation while increasing the resorption
of mineralized bone by OCs, which severely disrupts normal bone
homeostasis (2). CSF has been shown
to stimulate the differentiation and proliferation of hematopoietic
cells. In addition, the relationship between CSF and BM has been
extensively studied. Further, M-CSF is essential for promoting the
differentiation of OC precursors. Drugs targeting OCs, such as
bisphosphonates and the RANK ligand inhibitor denosumab, have been
established to treat BM. It was also observed that administering OC
inhibitors such as bisphosphonates, OPG and RANKL antagonists
before tumor inoculation reduces the incidence of BM (101,102). However, the clinical application
of CSF in treating BM remains underexplored. The treatment of BM
remains a significant challenge for oncologists and further
clinical trials are required to determine whether CSF could serve
as a therapeutic target for BM.
Not applicable.
The present study was supported by Hubei Province Key Laboratory
of Molecular Imaging (grant no. 2023fzyx025 to HJB), Jingzhou 2023
Medical Health Science and Technology Plan Project (grant no.
2023HC07 to HJB), Hubei Provincial Natural Science Foundation
(grant no. 2023AFB969 to HJB). Jingzhou Science and Technology
Bureau Project (grant no. 2022HC78 to PXC) and Wujieping Medical
Foundation digestive tract cancer research fund (grant no.
320.6750.2024–10-3 to PXC).
Not applicable.
XP and JH designed and supervised the study. YH and
YW reviewed the references. YH, YW and XP wrote the manuscript. YQ,
DC, and TL contributed to tables and figures and XP, JH and WW
revised the manuscript. XP and JH acquired funding. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chaoying L, Chao M, Xiangrui Y, Yingjian
H, Gang Z, Yunhan R and Yu G: Risk factors of bone metastasis in
patients with newly diagnosed prostate cancer. Eur Rev Med
Pharmacol Sci. 26:391–398. 2022.PubMed/NCBI
|
|
2
|
Clézardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Udagawa N, Koide M, Nakamura M, Nakamichi
Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C
and Tsuda E: Osteoclast differentiation by RANKL and OPG signaling
pathways. J Bone Miner Metab. 39:19–26. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guise TA, Mohammad KS, Clines G, Stebbins
EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L
and Chirgwin JM: Basic mechanisms responsible for osteolytic and
osteoblastic bone metastases. Clin Cancer Res. 12:6213s–6216s.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barreda DR, Hanington PC and Belosevic M:
Regulation of myeloid development and function by colony
stimulating factors. Dev Comp Immunol. 28:509–554. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Metcalf D: The colony-stimulating factors
and cancer. Nat Rev Cancer. 10:425–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wakefield PE, James WD, Samlaska CP and
Meltzer MS: Colony-stimulating factors. J Am Acad Dermatol.
23:903–912. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradley TR and Metcalf D: The growth of
mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci.
44:287–299. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ichikawa Y, Pluznik DH and Sachs L: In
vitro control of the development of macrophage and granulocyte
colonies. Proc Natl Acad Sci USA. 56:488–495. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartung T: Immunomodulation by
colony-stimulating factors. Rev Physiol Biochem Pharmacol.
136:1–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hareng L and Hartung T: Induction and
regulation of endogenous granulocyte colony-stimulating factor
formation. Biol Chem. 383:1501–1517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Qiao L, Hu P, Deng G and Zhang J,
Liang N, Xie J and Zhang J: The effect of granulocyte and
granulocyte-macrophage colony stimulating factors on tumor
promotion. J BUON. 22:21–28. 2017.PubMed/NCBI
|
|
16
|
Mueller MM, Peter W, Mappes M, Huelsen A,
Steinbauer H, Boukamp P, Vaccariello M, Garlick J and Fusenig NE:
Tumor progression of skin carcinoma cells in vivo promoted by
clonal selection, mutagenesis, and autocrine growth regulation by
granulocyte colony-stimulating factor and granulocyte-macrophage
colony-stimulating factor. Am J Pathol. 159:1567–1579. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gutschalk CM, Herold-Mende CC, Fusenig NE
and Mueller MM: Granulocyte colony-stimulating factor and
granulocyte-macrophage colony-stimulating factor promote malignant
growth of cells from head and neck squamous cell carcinomas in
vivo. Cancer Res. 66:8026–8036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CH, Lin SH, Chang SF, Chang PY, Yang
ZP and Lu SC: Extracellular signal-regulated kinase 2 mediates the
expression of granulocyte colony-stimulating factor in invasive
cancer cells. Oncol Rep. 30:419–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braun B, Lange M, Oeckler R and Mueller
MM: Expression of G-CSF and GM-CSF in human meningiomas correlates
with increased tumor proliferation and vascularization. J
Neurooncol. 68:131–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pei XH, Nakanishi Y, Takayama K, Bai F and
Hara N: Granulocyte, granulocyte-macrophage, and macrophage
colony-stimulating factors can stimulate the invasive capacity of
human lung cancer cells. Br J Cancer. 79:40–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek
T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al:
Granulocyte-colony stimulating factor promotes lung metastasis
through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci
USA. 107:21248–21255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mroczko B, Szmitkowski M and Czygier M:
Granulocyte colony stimulating factor (G-CSF) in diagnosis and
monitoring of non-small-cell lung cancer (NSCLC). Pol Arch Med
Wewn. 103:163–168. 2000.(In Polish). PubMed/NCBI
|
|
23
|
Fukuta K, Daizumoto K, Takahashi M, Mori
H, Otomi Y, Uehara H, Fukawa T, Yamamoto Y, Yamaguchi K and
Kanayama HO: Granulocyte colony-stimulating factor producing
retroperitoneal leiomyosarcoma. IJU Case Rep. 4:75–78. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yip RKH, Rimes JS, Capaldo BD, Vaillant F,
Mouchemore KA, Pal B, Chen Y, Surgenor E, Murphy AJ, Anderson RL,
et al: Mammary tumour cells remodel the bone marrow vascular
microenvironment to support metastasis. Nat Commun. 12:69202021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ushach I and Zlotnik A: Biological role of
granulocyte macrophage colony-stimulating factor (GM-CSF) and
macrophage colony-stimulating factor (M-CSF) on cells of the
myeloid lineage. J Leukoc Biol. 100:481–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hume DA and MacDonald KP: Therapeutic
applications of macrophage colony-stimulating factor-1 (CSF-1) and
antagonists of CSF-1 receptor (CSF-1R) signaling. Blood.
119:1810–1820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moore MA and Warren DJ: Synergy of
interleukin 1 and granulocyte colony-stimulating factor: In vivo
stimulation of stem-cell recovery and hematopoietic regeneration
following 5-fluorouracil treatment of mice. Proc Natl Acad Sci USA.
84:7134–7138. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamilton JA: Colony-stimulating factors in
inflammation and autoimmunity. Nat Rev Immunol. 8:533–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dougherty ST, Eaves CJ, McBride WH and
Dougherty GJ: Role of macrophage-colony-stimulating factor in
regulating the accumulation and phenotype of tumor-associated
macrophages. Cancer Immunol Immunother. 44:165–172. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han Y, Ma FY, Tesch GH, Manthey CL and
Nikolic-Paterson DJ: c-fms blockade reverses glomerular macrophage
infiltration and halts development of crescentic anti-GBM
glomerulonephritis in the rat. Lab Invest. 91:978–991. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaposhnik Z, Wang X and Lusis AJ:
Arterial colony stimulating factor-1 influences atherosclerotic
lesions by regulating monocyte migration and apoptosis. J Lipid
Res. 51:1962–1970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Manthey CL, Johnson DL, Illig CR, Tuman
RW, Zhou Z, Baker JF, Chaikin MA, Donatelli RR, Franks CF, Zeng L,
et al: JNJ-28312141, a novel orally active colony-stimulating
factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor
tyrosine kinase inhibitor with potential utility in solid tumors,
bone metastases, and acute myeloid leukemia. Mol Cancer Ther.
8:3151–3161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McDermott RS, Deneux L, Mosseri V,
Védrenne J, Clough K, Fourquet A, Rodriguez J, Cosset JM, Sastre X,
Beuzeboc P, et al: Circulating macrophage colony stimulating factor
as a marker of tumour progression. Eur Cytokine Netw. 13:121–127.
2002.PubMed/NCBI
|
|
36
|
Scholl SM, Lidereau R, de la Rochefordiere
A, Le-Nir CC, Mosseri V, Noguès C, Pouillart P and Stanley FR:
Circulating levels of the macrophage colony stimulating factor
CSF-1 in primary and metastatic breast cancer patients. A pilot
study. Breast Cancer Res Treat. 39:275–283. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang J, Choi YJ, Seo BY, Jo U, Park SI,
Kim YH and Park KH: A Selective FGFR inhibitor AZD4547 suppresses
RANKL/M-CSF/OPG-dependent ostoclastogenesis and breast cancer
growth in the metastatic bone microenvironment. Sci Rep.
9:87262019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liverani C, Mercatali L, Spadazzi C, La
Manna F, De Vita A, Riva N, Calpona S, Ricci M, Bongiovanni A,
Gunelli E, et al: CSF-1 blockade impairs breast cancer
osteoclastogenic potential in co-culture systems. Bone. 66:214–222.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borzone FR, Giorello MB, Martinez LM,
Sanmartin MC, Feldman L, Dimase F, Batagelj E, Yannarelli G and
Chasseing NA: Senescent mesenchymal stem/stromal cells in
pre-metastatic bone marrow of untreated advanced breast cancer
patients. Oncol Res. 31:361–374. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee C, Whang YM, Campbell P, Mulcrone PL,
Elefteriou F, Cho SW and Park SI: Dual targeting c-met and VEGFR2
in osteoblasts suppresses growth and osteolysis of prostate cancer
bone metastasis. Cancer Lett. 414:205–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao E, Wang L, Dai J, Kryczek I, Wei S,
Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET and Zou W:
Regulatory T cells in the bone marrow microenvironment in patients
with prostate cancer. Oncoimmunology. 1:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ide H, Hatake K, Terado Y, Tsukino H,
Okegawa T, Nutahara K, Higashihara E and Horie S: Serum level of
macrophage colony-stimulating factor is increased in prostate
cancer patients with bone metastasis. Hum Cell. 21:1–6. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsai YM, Chong IW, Hung JY, Chang WA, Kuo
PL, Tsai MJ and Hsu YL: Syringetin suppresses osteoclastogenesis
mediated by osteoblasts in human lung adenocarcinoma. Oncol Rep.
34:617–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fujita H, Gomori A, Fujioka Y, Kataoka Y,
Tanaka K, Hashimoto A, Suzuki T, Ito K, Haruma T, Yamamoto-Yokoi H,
et al: High potency VEGFRs/MET/FMS triple blockade by TAS-115
concomitantly suppresses tumor progression and bone destruction in
tumor-induced bone disease model with lung carcinoma cells. PLoS
One. 11:e01648302016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hung JY, Chang WA, Tsai YM, Hsu YL, Chiang
HH, Chou SH, Huang MS and Kuo PL: Tricetin, a dietary flavonoid,
suppresses benzo(a)pyrene-induced human non-small cell lung cancer
bone metastasis. Int J Oncol. 46:1985–1993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spadazzi C, Recine F, Mercatali L,
Miserocchi G, Liverani C, De Vita A, Bongiovanni A, Fausti V and
Ibrahim T: mTOR inhibitor and bone-targeted drugs break the vicious
cycle between clear-cell renal carcinoma and osteoclasts in an in
vitro co-culture model. J Bone Oncol. 16:1002272019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Suzuki K, Hino M, Hato F, Tatsumi N and
Kitagawa S: Cytokine-specific activation of distinct
mitogen-activated protein kinase subtype cascades in human
neutrophils stimulated by granulocyte colony-stimulating factor,
granulocyte-macrophage colony-stimulating factor, and tumor
necrosis factor-alpha. Blood. 93:341–349. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mann A, Breuhahn K, Schirmacher P and
Blessing M: Keratinocyte-derived granulocyte-macrophage colony
stimulating factor accelerates wound healing: Stimulation of
keratinocyte proliferation, granulation tissue formation, and
vascularization. J Invest Dermatol. 117:1382–1390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu FPK, Westphal JR, Hoekman K, Mels AK,
Statius Muller MG, de Waal RW, Beelen RHJ, van Leeuwen PAM, Meijer
S and Cuesta MA: The effects of surgery, with or without rhGM-CSF,
on the angiogenic profile of patients treated for colorectal
carcinoma. Cytokine. 25:68–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mueller MM and Fusenig NE: Friends or
foes-bipolar effects of the tumour stroma in cancer. Nat Rev
Cancer. 4:839–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller
G and Bar-Sagi D: Oncogenic Kras-induced GM-CSF production promotes
the development of pancreatic neoplasia. Cancer Cell. 21:836–847.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vilalta M, Rafat M, Giaccia AJ and Graves
EE: Recruitment of circulating breast cancer cells is stimulated by
radiotherapy. Cell Rep. 8:402–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Obermueller E, Vosseler S, Fusenig NE and
Mueller MM: Cooperative autocrine and paracrine functions of
granulocyte colony-stimulating factor and granulocyte-macrophage
colony-stimulating factor in the progression of skin carcinoma
cells. Cancer Res. 64:7801–7812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lahm H, Wyniger J, Hertig S, Yilmaz A,
Fischer JR, Givel JC and Odartchenko N: Secretion of bioactive
granulocyte-macrophage colony-stimulating factor by human
colorectal carcinoma cells. Cancer Res. 54:3700–3702.
1994.PubMed/NCBI
|
|
55
|
Oshika Y, Nakamura M, Abe Y, Fukuchi Y,
Yoshimura M, Itoh M, Ohnishi Y, Tokunaga T, Fukushima Y, Hatanaka
H, et al: Growth stimulation of non-small cell lung cancer
xenografts by granulocyte-macrophage colony-stimulating factor
(GM-CSF). Eur J Cancer. 34:1958–1961. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gutschalk CM, Yanamandra AK, Linde N,
Meides A, Depner S and Mueller MM: GM-CSF enhances tumor invasion
by elevated MMP-2, -9, and -26 expression. Cancer Med. 2:117–129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Takeda K, Hatakeyama K, Tsuchiya Y,
Rikiishi H and Kumagai K: A correlation between GM-CSF gene
expression and metastases in murine tumors. Int J Cancer.
47:413–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kumar A, Taghi Khani A, Sanchez Ortiz A
and Swaminathan S: GM-CSF: A double-edged sword in cancer
immunotherapy. Front Immunol. 13:9012772022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin Q, Fang X, Liang G, Luo Q, Cen Y, Shi
Y, Jia S, Li J, Yang W, Sanders AJ, et al: Silencing CTNND1
mediates triple-negative breast cancer bone metastasis via
upregulating CXCR4/CXCL12 axis and neutrophils infiltration in
bone. Cancers (Basel). 13:57032021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee SK, Park KK, Kim HJ, Park J, Son SH,
Kim KR and Chung WY: Human antigen R-regulated CCL20 contributes to
osteolytic breast cancer bone metastasis. Sci Rep. 7:96102017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang K, Hu Y, Feng Y, Li K, Zhu Z, Liu S,
Lin Y and Yu B: IGF-1R mediates crosstalk between nasopharyngeal
carcinoma cells and osteoclasts and promotes tumor bone metastasis.
J Exp Clin Cancer Res. 43:462024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Das Roy L, Pathangey LB, Tinder TL,
Schettini JL, Gruber HE and Mukherjee P: Breast-cancer-associated
metastasis is significantly increased in a model of autoimmune
arthritis. Breast Cancer Res. 11:R562009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fuentelsaz-Romero S, Cuervo A,
Estrada-Capetillo L, Celis R, Garcia-Campos R, Ramirez J, Sastre S,
Samaniego R, Puig-Kröger A and Cañete JD: GM-CSF expression and
macrophage polarization in joints of undifferentiated arthritis
patients evolving to rheumatoid arthritis or psoriatic arthritis.
Front Immunol. 11:6139752021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Varricchi G, Poto R, Marone G and
Schroeder JT: IL-3 in the development and function of basophils.
Semin Immunol. 54:1015102021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yadav P, Vats R, Bano A and Bhardwaj R:
Hematopoietic stem cells culture, expansion and differentiation: An
insight into variable and available media. Int J Stem Cells.
13:326–334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Donahue RE, Seehra J, Metzger M, Lefebvre
D, Rock B, Carbone S, Nathan DG, Garnick M, Sehgal PK, Laston D, et
al: Human IL-3 and GM-CSF act synergistically in stimulating
hematopoiesis in primates. Science. 241:1820–1823. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bénard A, Jacobsen A, Brunner M, Krautz C,
Klösch B, Swierzy I, Naschberger E, Podolska MJ, Kouhestani D,
David P, et al: Interleukin-3 is a predictive marker for severity
and outcome during SARS-CoV-2 infections. Nat Commun. 12:11122021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mroczko B, Szmitkowski M,
Wereszczynska-Siemiatkowska U and Okulczyk B: Stem cell factor
(SCF) and interleukin 3 (IL-3) in the sera of patients with
colorectal cancer. Dig Dis Sci. 50:1019–1024. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Marone G, Gambardella AR, Mattei F,
Mancini J, Schiavoni G and Varricchi G: Basophils in tumor
microenvironment and surroundings. Adv Exp Med Biol. 1224:21–34.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dentelli P, Rosso A, Olgasi C, Camussi G
and Brizzi MF: IL-3 is a novel target to interfere with tumor
vasculature. Oncogene. 30:4930–4940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hahm ER, Kim SH, Pore SK, Mathan SV, Singh
RP and Singh SV: Mechanism of synergistic inhibitory effect of
benzyl isothiocyanate and zoledronic acid combination on breast
cancer induction of osteoclast differentiation. Mol Carcinog.
63:301–313. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sugihara A, Maeda O, Tsuji M, Tsujimura T,
Nakata Y, Akedo H, Kotake T and Terada N: Expression of cytokines
enhancing the osteoclast activity, and parathyroid hormone-related
protein in prostatic cancers before and after endocrine therapy: An
immunohistochemical study. Oncol Rep. 5:1389–1394. 1998.PubMed/NCBI
|
|
73
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Aliper AM, Frieden-Korovkina VP, Buzdin A,
Roumiantsev SA and Zhavoronkov A: A role for G-CSF and GM-CSF in
nonmyeloid cancers. Cancer Med. 3:737–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY,
Kim CH, Park HG, Han SI and Kang HS: Induction of metastasis,
cancer stem cell phenotype, and oncogenic metabolism in cancer
cells by ionizing radiation. Mol Cancer. 16:102017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Luo S, Li P, Zhang A, Meng L, Huang L, Wu
X, Cheng H, Tu H and Gong X: G-CSF improving combined whole brain
radiotherapy and immunotherapy prognosis of non-small cell lung
cancer brain metastases. Int Immunopharmacol. 130:1117052024.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Psaila B and Lyden D: The metastatic
niche: Adapting the foreign soil. Nat Rev Cancer. 9:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Győri DS and Mócsai A: Osteoclast signal
transduction during bone metastasis formation. Front Cell Dev Biol.
8:5072020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Boyce BF: Advances in the regulation of
osteoclasts and osteoclast functions. J Dent Res. 92:860–867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Povolny BT and Lee MY: The role of
recombinant human M-CSF, IL-3, GM-CSF and calcitriol in clonal
development of osteoclast precursors in primate bone marrow. Exp
Hematol. 21:532–537. 1993.PubMed/NCBI
|
|
83
|
Ray AL, Saunders AS, Nofchissey RA, Reidy
MA, Kamal M, Lerner MR, Fung KM, Lang ML, Hanson JA, Guo S, et al:
G-CSF is a novel mediator of T-cell suppression and an
immunotherapeutic target for women with colon cancer. Clin Cancer
Res. 29:2158–2169. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Brook N, Brook E, Dharmarajan A, Dass CR
and Chan A: Breast cancer bone metastases: Pathogenesis and
therapeutic targets. Int J Biochem Cell Biol. 96:63–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu Y, Cheng W and Xin H, Liu R, Wang Q,
Cai W, Peng X, Yang F and Xin H: Nanoparticles advanced from
preclinical studies to clinical trials for lung cancer therapy.
Cancer Nanotechnol. 14:282023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lv T, Meng Y, Liu Y, Han Y, Xin H, Peng X
and Huang J: RNA nanotechnology: A new chapter in targeted therapy.
Colloids Surf B Biointerfaces. 230:1135332023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cheng D, Wang J, Wang Y, Xue Y, Yang Q,
Yang Q, Zhao H, Huang J and Peng X: Chemokines: Function and
therapeutic potential in bone metastasis of lung cancer. Cytokine.
172:1564032023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Al Husaini H, Wheatley-Price P, Clemons M
and Shepherd FA: Prevention and management of bone metastases in
lung cancer: A review. J Thorac Oncol. 4:251–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Molineux G, Pojda Z and Dexter TM: A
comparison of hematopoiesis in normal and splenectomized mice
treated with granulocyte colony-stimulating factor. Blood.
75:563–569. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Metcalf D, Begley CG, Williamson DJ, Nice
EC, De Lamarter J, Mermod JJ, Thatcher D and Schmidt A: Hemopoietic
responses in mice injected with purified recombinant murine GM-CSF.
Exp Hematol. 15:1–9. 1987.PubMed/NCBI
|
|
92
|
Metcalf D, Begley CG, Johnson GR, Nicola
NA, Lopez AF and Williamson DJ: Effects of purified bacterially
synthesized murine multi-CSF (IL-3) on hematopoiesis in normal
adult mice. Blood. 68:46–57. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lang RA, Metcalf D, Cuthbertson RA, Lyons
I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK,
Gonda TJ, et al: Transgenic mice expressing a hemopoietic growth
factor gene (GM-CSF) develop accumulations of macrophages,
blindness, and a fatal syndrome of tissue damage. Cell. 51:675–686.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chang JM, Metcalf D, Lang RA, Gonda TJ and
Johnson GR: Nonneoplastic hematopoietic myeloproliferative syndrome
induced by dysregulated multi-CSF (IL-3) expression. Blood.
73:1487–1497. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chang JM, Metcalf D, Gonda TJ and Johnson
GR: Long-term exposure to retrovirally expressed
granulocyte-colony-stimulating factor induces a nonneoplastic
granulocytic and progenitor cell hyperplasia without tissue damage
in mice. J Clin Invest. 84:1488–1496. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dranoff G, Jaffee E, Lazenby A, Golumbek
P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D and Mulligan
RC: Vaccination with irradiated tumor cells engineered to secrete
murine granulocyte-macrophage colony-stimulating factor stimulates
potent, specific, and long-lasting anti-tumor immunity. Proc Natl
Acad Sci USA. 90:3539–3543. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Conlon KC, Miljkovic MD and Waldmann TA:
Cytokines in the treatment of cancer. J Interferon Cytokine Res.
39:6–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gabrilove JL, Jakubowski A, Fain K, Grous
J, Scher H, Sternberg C, Yagoda A, Clarkson B, Bonilla MA, Oettgen
HF, et al: Phase I study of granulocyte colony-stimulating factor
in patients with transitional cell carcinoma of the urothelium. J
Clin Invest. 82:1454–1461. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Renwick W, Pettengell R and Green M: Use
of filgrastim and pegfilgrastim to support delivery of
chemotherapy: Twenty years of clinical experience. BioDrugs.
23:175–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lieschke GJ, Maher D, Cebon J, O'Connor M,
Green M, Sheridan W, Boyd A, Rallings M, Bonnem E, Metcalf D, et
al: Effects of bacterially synthesized recombinant human
granulocyte-macrophage colony-stimulating factor in patients with
advanced malignancy. Ann Intern Med. 110:357–364. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Boissier S, Ferreras M, Peyruchaud O,
Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM and
Clézardin P: Bisphosphonates inhibit breast and prostate carcinoma
cell invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|
|
102
|
Gao L, Deng H, Zhao H, Hirbe A, Harding J,
Ratner L and Weilbaecher K: HTLV-1 Tax transgenic mice develop
spontaneous osteolytic bone metastases prevented by osteoclast
inhibition. Blood. 106:4294–4302. 2005. View Article : Google Scholar : PubMed/NCBI
|