Introduction
Gliomas, or glial tumors, are the most common type
of primary brain tumor and account for ~81% of all malignant brain
tumors (1). Although relatively
rare, gliomas cause marked morbidity and mortality (2). Glioblastoma (GBM) is the most
aggressive and common (45%) tumor of all six glioma types and
grades and presents with a median survival time of ~15 months
(1). However, no significant
progress has been made in glioma treatment over the last decade
(3). The absence of effective
treatment can be attributed to the numerous strategies used by
cancer cells to escape the immune system (4). Notably, the immunosuppressive
microenvironment of glioma involves checkpoints such as programmed
death-ligand 1 and CTLA-4 that further contribute to the poor
prognosis (5,6).
In the context of neuroanatomy, glioma presents
particular difficulties as the brain is enclosed within a hard
skull. This raises concerns regarding the possible effects of
increasing edema on the brain by GBM, including increased
intracranial pressure (ICP), compression, tension and other
mechanical stresses. Thus, compression of brain tissue by the
primary tumor mass is a typical feature and a key cause of the
clinical symptoms observed in patients with brain cancer. As the
tumor expands inside the skull, it must be pushed away from the
surrounding tissue. This tumor growth-induced brain deformation can
lead to severe disabilities and is associated with poor prognosis
(7). In particular, cranial midline
shift is a prevalent feature among patients diagnosed with GBM.
Such individuals typically have considerable brain compression,
which is associated with rapid development and severe neurological
deficits (8). Furthermore, a
previous study discovered a negative correlation between midline
shift and the survival of patients who survived a biopsy but not in
those who were able to undergo resection (8), indicating that resection and the
ensuing reduction in compressive forces brought on by tumor growth
may enhance treatment (8,9). Despite the significance of mechanical
compression in brain tumors and ongoing research on its impact on
other tumor types (10–12), to the best of our knowledge, no
study has examined its direct effects. Furthermore, the optimal
model for mimicking the physical conditions of GBM has not yet been
developed (13).
Therefore, the present study aimed to investigate
how glioma senses mechanical forces and responds to stress, as well
as to identify the molecules involved in this process. To simulate
the GBM microenvironment, such as compressive forces, glass
coverslips placed on non-metastatic H4 glioma cells were used. In
the present study, it was investigated whether Piezo1, a
stretch-activated calcium channel, or growth differentiation factor
15 (GDF15) might be involved in this system as mechanosensors.
Additionally, it was explored whether the immune checkpoint
protein, CTLA4, could be regulated by signaling induced by altered
mechanical forces. Targeting compression-induced tumor progression
is particularly important, as identifying the processes that drive
signal transduction could reveal new therapeutic targets for
treating patients with brain tumors.
Materials and methods
Cell culture and pharmacological
reagents
The human neuroglioma H4 cell line was obtained from
Professor Sangmyung Rhee (Chung-Ang University, Seoul, Korea), and
the A172 cell line was purchased from the American Type Culture
Collection (ATCC). U87MG, a GBM cell line of unknown origin cell
line, was also purchased from ATCC (ATCC no. HTB-14). The cells
were maintained in high-glucose Dulbecco's Modified Eagle's Medium
(cat. no. LM001-05; Welgene, Inc.) containing 10% fetal bovine
serum (cat. no. US-FBS-500; GW Vitek) and 1%
penicillin/streptomycin antibiotics (cat. no. 15140-122; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2. The cells were incubated with or without 10 µM
BAPTA-AM (cat. no. 2787; Tocris Bioscience), an intracellular
calcium chelator, for 30 min prior to pressure application.
Mycoplasma testing was performed on all cell lines using an
eMyco™ VALiD kit (cat. no. 25239; LiliF Diagnostics;
iNtRON Biotechnology).
Compression device
To apply mechanical compression to brain cancer cell
monolayers, a commonly used physical contact procedure was followed
(14,15). Briefly, 1–2×105 H4 cells
were cultured for ~24 h in a 6-well plate (35-mm diameter; cat. no.
30006; SPL Life Sciences) at 37°C in a humidified incubator. A 2%
low melting agarose cushion was placed on top of the cells to
prevent any direct contact between the glass weights and the cells,
and to provide a uniform distribution of the applied force. The
initial pressure ranges examined in this study varied from a
minimum of 0.15 mmHg to a maximum of 3 mmHg, achieved by regulating
the number of glass layers. However, pressures that were too low or
too high (assessed based on their effects on cellular viability and
motility) were excluded from further analysis. Consequently, this
study focused solely on the pressures of 0.75 and 1.5 mmHg. Each
pressure was applied on top of the agarose gel to compress the
cells for 12 h (16). The cells
covered only with an agarose cushion served as the control.
Cell motility analysis
To quantify cell motility, the culture plate was
placed under an inverted fluorescence microscope (ECLIPSE Ti2;
Nikon Corporation) and motility was observed using phase-contrast
imaging. Images were obtained by selecting a specific location on
the culture plate and capturing an image every 3 min. The process
was carried out for a total of 5 h in an environmental chamber
maintained at 37°C and 5% CO2. The cells were tracked in
time-lapse image sequences using the manual tracking plug-in for
Fiji (ImageJ; http://imagej.net/Fiji), which
comprises a passive tracking log. Cells in 1 field were analyzed by
selecting an independent field with at least 20 non-overlapping
cells and translating them into 0.33 µm/pixel images. The travel
distance and speed of each cell were determined using the
Chemotaxis and Migration Tool Version 2.0 software (ibidi
GmbH).
Wound healing
Cells were seeded into a 6-well plate and allowed to
reach a confluency of >90%. Then, scratches were made using 1-ml
sterile tips, and the cell debris were removed by washing with PBS.
After changing to medium containing 5% FBS (17), the cells were then compressed for 12
h as aforementioned, whereas the control samples were only covered
with an agarose cushion. Prolonged exposure of cells to pressure
for 12 h can induce significant stress, potentially leading to cell
death. Therefore, in this assay, the serum concentration was
reduced to 5% for the wound healing assay, but the cells were not
serum-starved. Images of the wounded area were collected at 0, 12
and 24 h, using a digital camera with bright field (IX-81; Olympus
Corporation). The wound area was measured using ImageJ and wound
closure was calculated using the following formula: Wound closure
(%)=[(width of the wound at 0 h-width of the wound at 24 h)/width
of the wound at 0 h] ×100.
Analysis of datasets for human samples
in The Cancer Genome Atlas (TCGA)
Data on differentially expressed genes in GBM [tumor
(T), n=163; normal (N), n=207] were collected using the Gene
Expression Profiling Interactive Tool (GEPIA; http://gepia.cancer-pku.cn/), an interactive gene
expression profiling web server. The RNA-sequencing datasets used
by GEPIA are based on the UCSC Xena project (http://xena.uscs.edu), which are computed by a
standard pipeline. Box plots were constructed to visualize the
expression of GDF15 and Piezo1 in tumors vs. matched normal
tissues.
Immunohistochemistry
Paraffin-embedded brain tissue slides from the tumor
and normal regions of patients with GBM were provided by the Korean
Brain Bank (Korea Brain Research Institute; Table SI). The experimental procedures
were approved by the Institutional Review Board of the Chung-Ang
University (Seoul, South Korea; approval no.
1041078-202209-HR-199). Briefly, slides were deparaffinized and
rehydrated with xylene three times for 4 min and 100, 95, 70 and
50% ethanol for 5 min each. Then, the slides were treated with
sodium citrate buffer (pH 6) for 15 min at 125°C in a pressure
cooker (Bio SB, Inc.) and washed in distilled water for 5 min. For
permeabilization, the slides were washed three times with 0.1%
TritonX-100 in PBS for 10 min, and a circle was drawn around the
tissue on the slide using a hydrophobic barrier pen. Subsequently,
the sections were blocked with 5% bovine serum albumin (cat. no.
A7030; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, and
then incubated with antibodies against GDF15 (1:100; monoclonal
antibody; cat. no. sc-377195; Santa Cruz Biotechnology, Inc.) and
Piezo1 (1:100; polyclonal antibody; cat. no. 15939-1-AP;
Proteintech Group, Inc.) for 1 h at room temperature (25–26°C).
Based on the manufacturer's recommended protocol,
immunohistochemistry was performed using a Vector Laboratories
VECTASTAIN Elite ABC University kit (cat. no. PK-7200; Vector
Laboratories, Inc.) and DAB staining (cat. no. K3468; Dako; Agilent
Technologies, Inc.). The slides were washed with water and
cover-slipped with EcoMount (cat. no. BRR897L; Biocare Medical,
LLC). The images were captured using an inverted microscope
equipped with a camera (Leica DFC320; Leica Microsystems GmbH).
Reverse transcription-quantitative PCR
(qPCR)
Total RNA was extracted from H4 cells using the
RNeasy Mini Kit (cat. no. 74004; Qiagen GmbH) according to the
manufacturer's instructions. cDNAs were synthesized from 1–2 µg of
total RNA using the Maxima First Stand cDNA synthesis kit (cat. no.
K1642; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. qPCR was performed using Power SYBR Green
PCR Master Mix reagent (cat. no. 4367659; Applied Biosystems;
Thermo Fisher Scientific, Inc.) and primers specific to the target
genes [PIEZO1, GDF15, matrix metalloproteinase 9 (MMP9),
CTLA4 and GAPDH] in a StepOnePlus™ Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The sequences of the primers used in the qPCR are listed in
Table SII. The 40-cycle PCR
consisted of two steps: Denaturation step, 15 sec at 95°C; and
combined annealing and extension step, 30 sec at 55°C. To calculate
the fold change, the quantification cycles (Cq) were determined
using the StepOne software version 2.3 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The mRNA expression was normalized to
GAPDH. The relative gene expression ratios were analyzed
using the 2−ΔΔCq method (18). All fold changes are expressed
relative to those in the control group.
Western blotting
Whole cells were harvested by washing twice with
ice-cold PBS (cat. no. LB001-01; Welgene, Inc.) and then lysed in
RIPA lysis buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.)
supplemented with a phosphatase and protease inhibitor cocktail
(cat. no. P3100-001; cat. no. 3200-001; GenDEPOT, LLC). Total
protein content was quantified using a Bradford protein assay kit
(cat. no. 5000006; Bio-Rad Laboratories, Inc.) following the
manufacturer's instructions. Next, ~40 µg of total protein from
each sample was separated by 8–10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The proteins were then
transferred to a nitrocellulose membrane. The membranes were
blocked in 5% (w/v) skimmed milk for 1 h at room temperature and
then incubated with anti-Piezo1 (1:1,000; cat. no. 15939-1-AP;
polyclonal antibody; Proteintech Group, Inc.), anti-GDF15 (1:1,000;
cat. no. sc-377195; monoclonal antibody; Santa Cruz Biotechnology,
Inc.) and anti-β-actin (1:1,000; cat. no. sc-47778; monoclonal
antibody; Santa Cruz Biotechnology, Inc.) overnight at 4°C with
gentle shaking. Then, horseradish peroxidase-conjugated mouse
anti-rabbit (1:5,000; cat. no. sc-2357; Santa Cruz Biotechnology,
Inc.) and goat anti-mouse IgG (1:5,000; cat. no. GTX 213111-01;
GeneTex, Inc.) secondary antibodies were applied for 1 h at room
temperature. The signals were detected with a chemiluminescent
reagent using an enhanced chemiluminescence imaging system
(ImageQuant™ LAS 4000; Cytiva).
Small interfering (si)RNA
transfection
Transient siRNA transfection was performed using
DharmaFECT1 (cat. no. T-2001-02; GE Healthcare Dharmacon, Inc.)
according to the manufacturer's instructions. In preparation for
siRNA transfection, the H4 cells were plated at 70% confluency with
1.5×105 cells per well in a 6-well plate. The following
day SMARTpool siRNAs against GDF15 (cat. no.
J-019875-05-0002), PIEZO1 (cat. no. J-020870-09-0002) and
control siRNA (cat. no. D-001210-01-05), all from GE Healthcare
Dharmacon, Inc., were transfected at a final concentration of 25 nM
at 37°C for 24 h. Experiments were then performed with or without
pressure.
Statistical analysis
All data were analyzed using GraphPad Prism version
10.2.3 software (Dotmatics) and are present as the mean ± standard
error of the mean. Differences between two groups were analyzed
using unpaired Student's t-test, whereas those among three or more
treatment groups were assessed using one-way ANOVA and Tukey's
multiple comparison method. P<0.05 was considered to indicate a
statistically significant difference.
Results
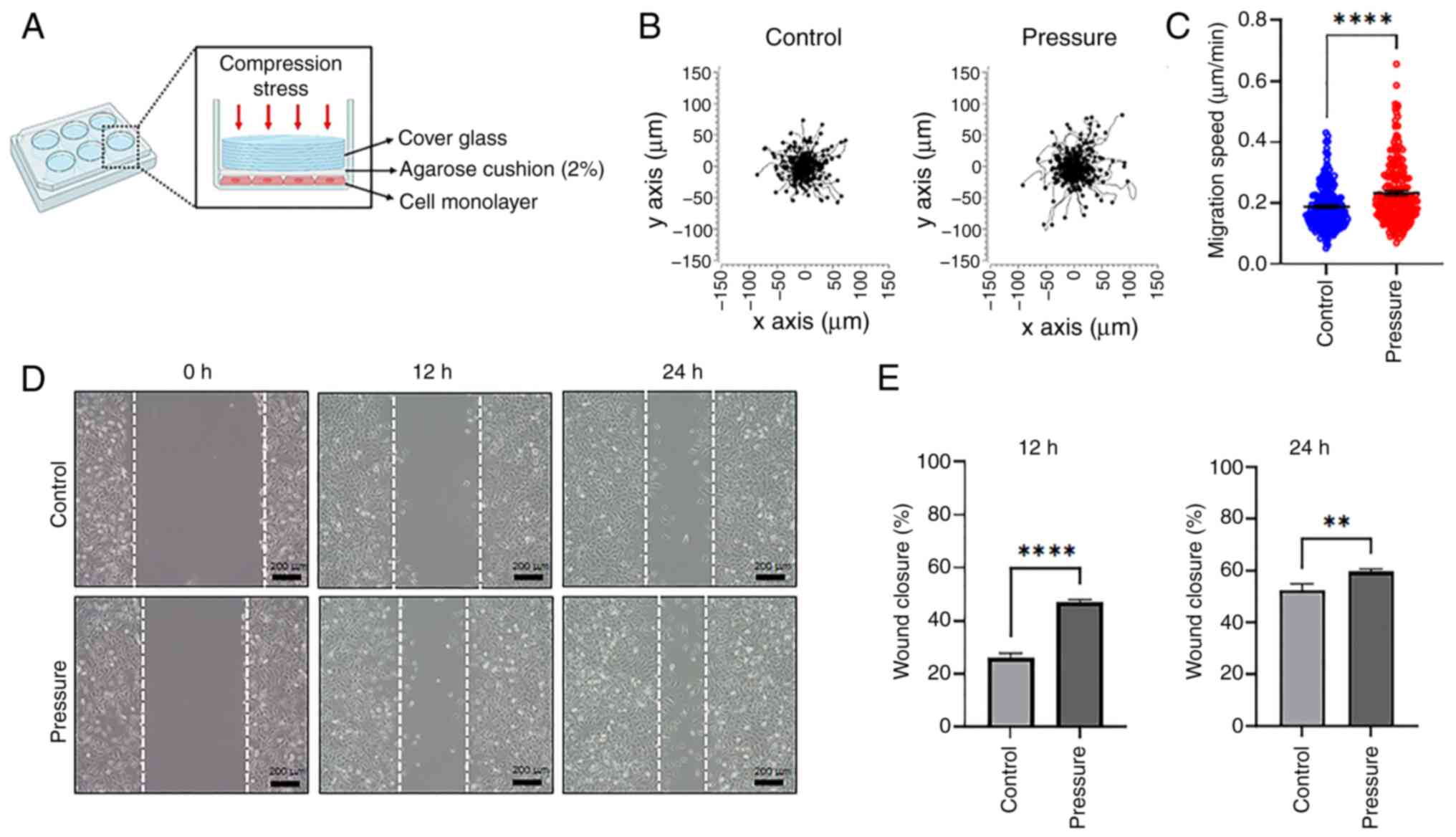
Mechanical compression promotes the
migration of human brain glioma cells
Brain glioma cells experience compression stress
within the confined space of the skull. In the present study,
pressure was applied to mimic the brain tumor microenvironment, as
illustrated in Fig. 1A. To
determine the optimal pressure for compressing glioma cells without
causing cell death, different pressures were applied to the human
non-metastatic H4 glioma cell line for 12 h, before releasing the
pressure. The control was subjected to no pressure. Pressure
equivalent to 0.75, 1.5 and 0 mmHg was applied on top of the cells
for 12 h using an agarose cushion. It was found that under 1.5 mmHg
pressure, the cell population decreased (Fig. S1). Therefore, the applied pressure
was set at 0.75 mmHg. To determine whether compression affects cell
motility, the cells were cultured for 12 h under 0.75 or 0 mmHg
pressure. Cell motility was then monitored for 5 h using time-lapse
imaging (Datas S1 and 2). When the movement path and location of
the cells were plotted, the cells to which pressure was applied
were found to move over long distances (Fig. 1B). The average migration speed of
cells in the control was 0.18±0.005 µm/min and that of cells
exposed to pressure was 0.23±0.007 µm/min (Fig. 1C). These results indicated a
significant increase in cell motility under pressure. Additionally,
wound closure was assessed using a wound healing assay to verify
whether pressure affects cell migration. The cell monolayer was
wounded, placed under pressure for 12 h and the wound closure was
examined at 0, 12 and 24 h (Fig.
1D). At 12 h, the cells under pressure showed a higher closure
rate (46.98±0.97%) than the control group (26.07±1.72%), which
persisted at 24 h (Fig. 1E). These
results suggest that mechanical compression enhances the migration
of human brain cancer cells.
Compressive solid stress enhances the
expression of the GDF15 and Piezo1 mechanosensors in glioma
cells
As shown in Fig. 1,
compressive stress increased the migration of glioma cells. We
hypothesized that mechanosensors present in the cell membrane that
sense mechanical force could initiate signal transduction and
ultimately affect motility. Therefore, these mechanosensors were
identified according to our previous study (19). Notably, TCGA profiling through GEPIA
confirmed that the expression of GDF15 and PIEZO1 was
significantly higher in GBM tissues than in normal tissues
(Fig. 2A). Additionally, the
expression of GDF15 and Piezo1 was examined in human GBM tissues
using immunohistochemistry. The expression of these proteins was
markedly enhanced in GBM tissues compared with the adjacent
non-tumor tissues (Fig. 2B and C).
The changes in gene expression in H4 cells with or without
compressive stress for 12 h were also examined. The gene expression
levels of GDF15 and PIEZO1 significantly increased
2-fold and 1.5-fold, respectively, under compressive solid stress.
Pressure-induced GDF15 and PIEZO1 expression was also
observed in the A172 and U87MG glioma cell lines (Fig. S2). Furthermore, the expression of
MMP9, which plays an important role in cancer progression,
including extracellular matrix (ECM) remodeling and metastasis,
also increased by ~2-fold (Fig. 2D)
in H4 cells. Piezo1 and GDF15 protein expression increased under
pressure at 12 h and was further enhanced after exposure for 24 h
in a time-dependent manner (Fig.
2E). Notably, enhanced expression of GDF15 and
PIEZO1 was gradually reduced in H4 cells and returned to
basal level 24 h after the pressure was removed (Fig. S3). In summary, consistent with
observations in the samples from patients with GBM (Fig. 2B and C), mechanical compression
stress increased the expression of the mechanical sensors GDF15 and
Piezo1 in H4 cells.
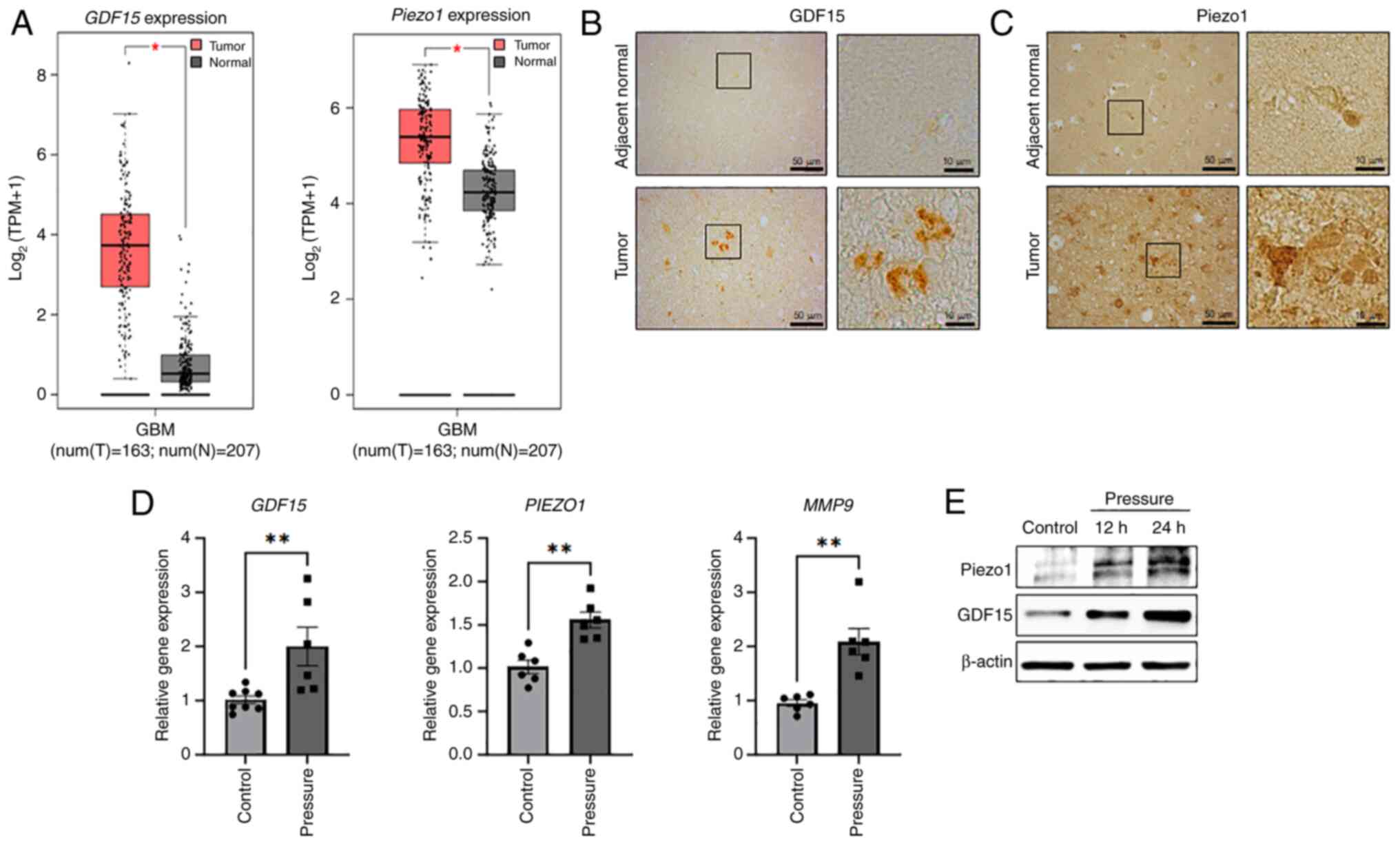 | Figure 2.Compressive solid stress increases
the expression of the GDF15 and Piezo1 mechanosensors. (A)
Comparisons of GDF15 and PIEZO1 gene expression
between human GBM and normal brain tissues using data from The
Cancer Genome Atlas and GTEx, analyzed with GEPIA. The red box
plots represent GBM tissues (n=163) and the gray box plots
represent non-GBM tissue (n=207). The figure was obtained from
GEPIA. The expression levels of (B) GDF15 and (C) Piezo1 in human
GBM tissue were compared with normal brain tissue by
immunohistochemistry. The tissues were obtained from the Korea
Brain Bank Network. Scale bars represent 50 µm and 10 µm. (D) H4
cells were subjected to compression for 12 h, and the gene
expression levels of GDF15, PIEZO1 and MMP9 were
evaluated using reverse transcription-quantitative PCR (n=3
independent experiments). (E) Piezo1 and GDF15 protein expression
in H4 cells exposed to pressure for 12 and 24 h measured via
immunoblotting analysis. *P<0.05, **P<0.01. GBM, glioblastoma
multiforme; GDF15, growth differentiation factor 15; MMP9, matrix
metalloproteinase 9; N, normal tissue; T, tumor tissue; TPM,
transcripts per million. |
Piezo1-mediated mechanotransduction
promotes the motility of glioma cells through GDF15 regulation
To determine whether the changes in glioma cells
caused by mechanical compression were initiated by GDF15 and
Piezo1, their expression was suppressed using respective siRNAs
(Figs. 3E and S4). When pressure was applied to cells
transfected with control siRNA, cell motility significantly
increased, as observed in the previous experiment. However, when
pressure was applied to cells in which GDF15 expression was
suppressed using siRNA, there was no increase in motility (Fig. 3A and B). Furthermore, there was no
increase in motility when pressure was applied after the knockdown
of PIEZO1 using siRNA (Fig. 3C
and D). Gene expression was confirmed with or without pressure
applied to PIEZO1-knockdown cells. PIEZO1 expression
increased under pressure, but in siPiezo1, the expression of
PIEZO1 did not increase whether pressure was applied or not
(Fig. 3E). Similarly, GDF15
expression increased with pressure, but was not enhanced regardless
of the presence or absence of pressure when Piezo1 was ablated
(Fig. 3F). Furthermore, the
intracellular calcium chelator, BAPTA-AM, was applied to H4 cells
during pressure exposure, as Piezo1 is a stretch-activated calcium
channel (20). Consequently, when
intracellular calcium levels were suppressed, GDF15
expression was not significantly changed, even in the presence of
pressure (Fig. 3G and H). These
results suggest that Piezo1 acts as a mechanosensor for compressive
forces and regulates GDF15 expression as an upstream
regulator. Overall, it can be hypothesized that mechanical pressure
in the GBM microenvironment promotes the progression of glioma
cells through the Piezo1-GDF15 axis.
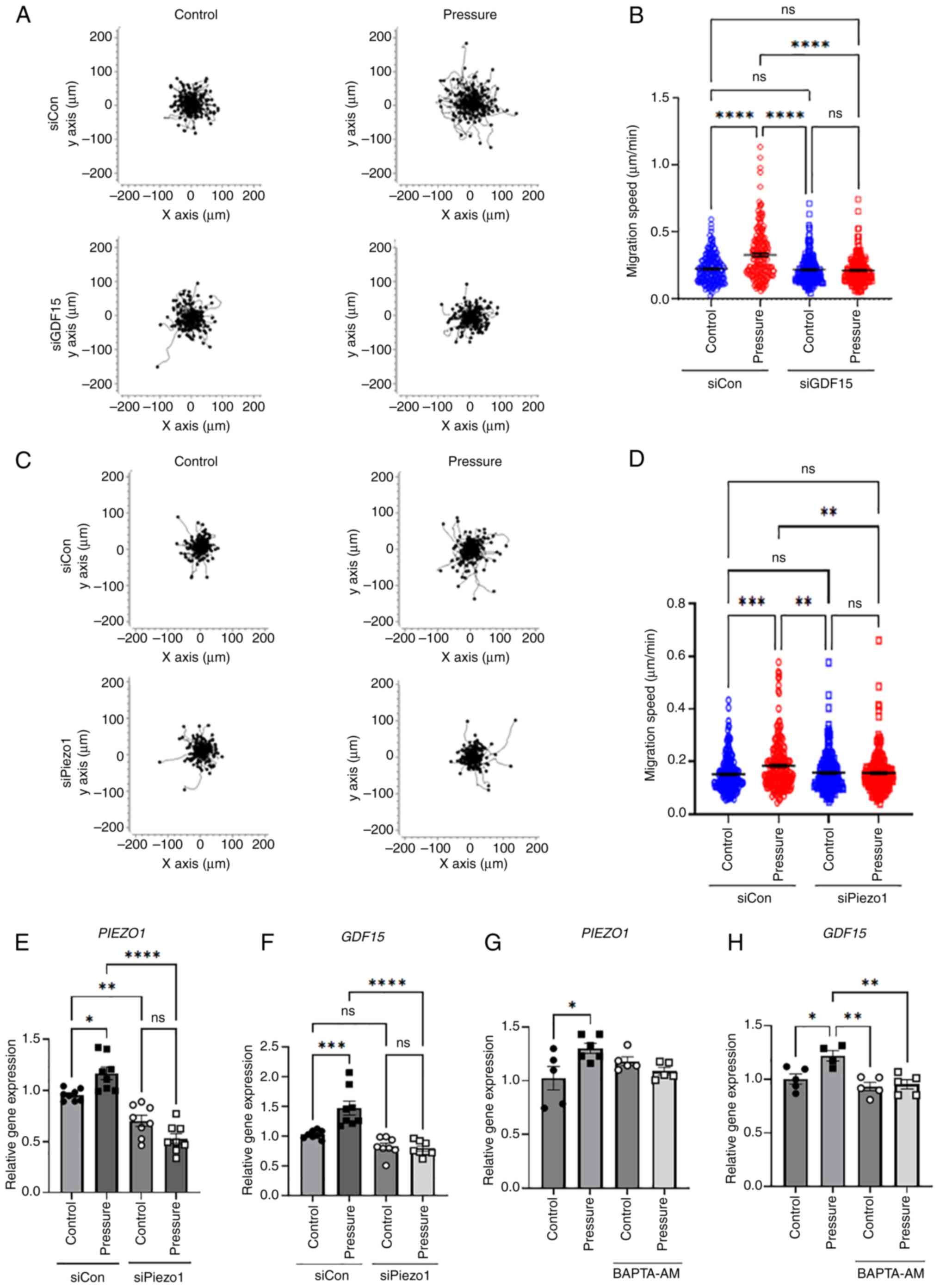 | Figure 3.Knockdown of GDF15 and
PIEZO1 using siRNA significantly reduces cell motility under
pressure. (A) Motility plots of siCon or siGDF15 transfected cells
subjected to pressure for 12 h and then observed for 5 h under a
time-lapse imaging microscope. Plots depict the motility of
individual cells in 1 representative experiment. (B) Quantification
of the migration speed of individual cells (n=200). (C) Motility
plots of siCon or siPiezo1 transfected cells subjected to pressure
for 12 h and then observed for 5 h under a time-lapse imaging
microscope. Plots depict the motility of individual cells in 1
representative experiment. (D) Quantification of the migration
speed of individual cells (n=200). Cells with or without
PIEZO1 knockdown were subjected to pressure for 12 h, and
the gene expression levels of (E) PIEZO1 and (F)
GDF15 were evaluated using RT-qPCR (n=3 independent
experiments for each gene). Cells were subject to pressure with or
without the addition of 10 µM BAPTA-AM for 12 h, and the gene
expression levels of (G) PIEZO1 and (H) GDF15 were
assessed using RT-qPCR (n=3 independent experiments for each gene).
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, determined
using one-way ANOVA followed by Tukey's test. Con, control; GDF15,
growth differentiation factor 15; ns, not significant; RT-qPCR,
reverse transcription-quantitative PCR; siRNA, small interfering
RNA. |
Mechanical compression induces CTLA4
expression in glioma cells via Piezo1 and GDF15
CTLA4, an immune checkpoint protein, contributes to
immune evasion via antitumor activity. High CTLA4 expression has
also been reported to be correlated with the poor prognosis of
patients with GBM (21). To
determine whether CTLA4 expression was increased in the
brain tumor microenvironment system, the gene expression of
CTLA4 was examined. When a pressure of 0.75 mmHg was applied
to the cells for 12 h, the expression of CTLA4 increased
1.5-fold (Fig. 4A). To confirm its
relationship with the mechanical sensors, the gene expression of
CTLA4 was measured when GDF15 expression was knocked
down by siRNA. Application of pressure to cells transfected with
siGDF15 failed to increase CTLA4 expression (Fig. 4B). Additionally,
PIEZO1-knockdown suppressed CTLA4 expression under
solid compressive stress (Fig. 4C).
These results confirm that ICP in brain glioma cells increases the
expression of CTLA4, and mechanosensors such as Piezo1 and
GDF15 affect the regulation of CTLA4 expression.
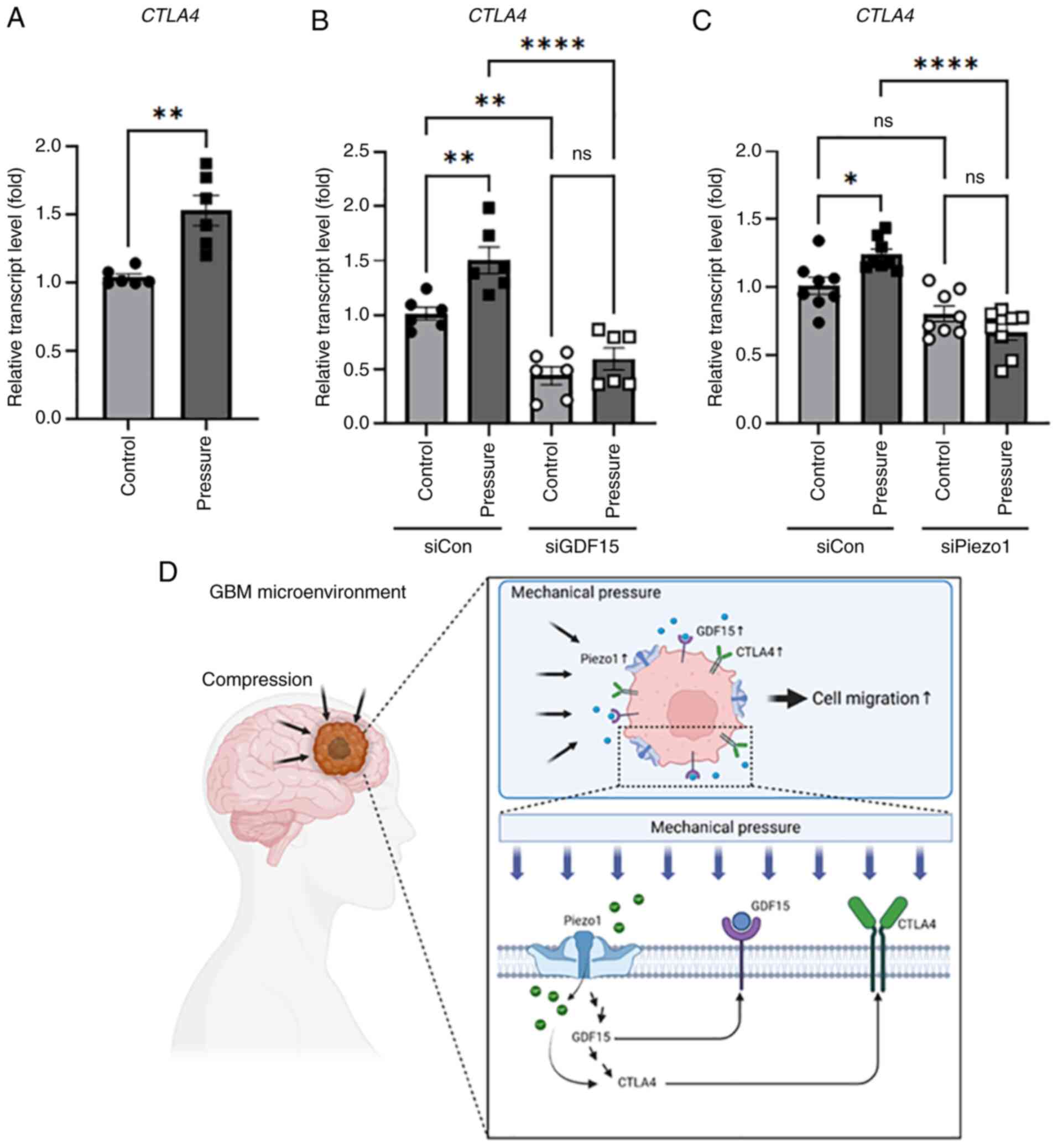 | Figure 4.Compressive stimuli regulate the
expression of CTLA4 in neuroglioma cells. (A) H4 cells were
subjected to 12 h of compressive stimuli and the expression of
CTLA4 was analyzed using RT-qPCR. n=6 independent
experiments; **P<0.01, determined by unpaired t-test. Cells
transfected with (B) siCon or siGDF15 and (C) siCon or siPiezo1
were subjected to compressive stimulation for 12 h, and expression
of CTLA4 was analyzed using RT-qPCR. n=3 independent
experiments for each transfection procedure; *P<0.05,
**P<0.01, ****P<0.0001 determined by one-way ANOVA followed
by Tukey's test. (D) A proposed mechanism that promotes glioma
progression through a mechanosensor that detects mechanical
pressure in the brain tumor microenvironment. As GBM grows in a
limited space, pressure builds between cells and surrounding
tissues. The expression of Piezo1, a mechanosensor present in the
cell membrane, increases, followed by the expression of GDF15.
Subsequently, the immune checkpoint protein, CTLA4, is upregulated,
enhancing the poor prognosis of glioma. Con, control; GBM,
glioblastoma; GDF15, growth differentiation factor 15; ns, not
significant; RT-qPCR, reverse transcription-quantitative PCR;
siRNA, small interfering RNA. |
Discussion
In the tumor microenvironment, mechanical forces and
matrix stiffness are two distinct biomechanical anomalies (22,23).
However, while extensive research has explored the effect of matrix
stiffness on tumor progression, the impact of mechanical stress on
cancer cell behavior, particularly brain cancer cell migration and
progression, remains largely unexplored. Thus, to mimic the brain
microenvironment in the present study, compression forces were
applied to non-metastatic glioma H4 cells. To investigate the
effect of compressive stress on glioma, an agarose cushion was used
to apply pressure to the glioma cells. In the present study,
agarose cushions were used for both the control and pressure
groups. A previous report demonstrated that chondrocytes embedded
in agarose gel maintained a survival rate >95% after 24 h of
incubation, which was maintained up to 72 h (24), suggesting that applying pressure via
agarose gel does not significantly affect cell viability. The
results of the present study demonstrated that compressive solid
stress increased the motility of glioma cells and the protein
expression of Piezo1 and GDF15 in H4 cells. When PIEZO1
expression was reduced using siRNA, GDF15 expression was
also suppressed, and an increase in cellular motility under a
compression force was no longer observed. This suggested that
Piezo1 regulates GDF15 expression, followed by cellular motility,
in response to mechanical stimuli. Furthermore, the use of
BAPTA-AM, a calcium chelator, inhibited the expression of
GDF15, indicating that Piezo1 channels, activated by
pressure, increase intracellular calcium levels, which in turn
regulates GDF15 expression. Notably, compression highly enhanced
CTLA4 expression in H4 cells, which may be regulated by
Piezo1-GDF15 signaling.
Regarding the enhanced motility of glioma H4 cells
observed in the present study, Kalli et al (25) reported that, through actin
remodeling by ras homolog family member B GTPase and Rac family
small GTPase 1, compressive forces (2 or 4 mmHg) promote cellular
motility in non-metastatic H4 glioma cells, but not in metastatic
A172 glioma cells. As 1.5 mmHg of pressure decreased the density of
H4 cells after 12 h of incubation in the present study, its effect
on glioma was not test any further. The lower pressure of 0.75 mmHg
used in the present study was considered sufficient to increase
cellular motility and glioma progression. Notably, different levels
of compressive stress have been applied to gliomas in various
studies, with reported values of 2.8–6.1 (10), 4–28 (26), 28–120 (27) and 3.7–16.0 (28) mmHg. Depending on the measurement
method, the equivalent compressive solid stress is 0.02 kPa (0.15
mmHg) in ex vivo measurements but reaches 0.1 kPa (0.75
mmHg) in in situ measurements. Furthermore, the precise
magnitude of pressure or solid stress exerted within the brain by a
tumor remains unknown and is challenging to study, as it is highly
dependent on the location and size of the tumor as well as the
stage of progression, which is a limitation of the present study.
However, what is important is that pressure or solid stress is a
key factor in the progression of brain cancer. This suggests that
the effect of compressive force on the tumor is more significant
than the absolute value of the pressure itself. In the present
study, it was demonstrated that the initial compressive force could
induce cell migration through the Piezo1-GDF15-CTLA4 pathway during
glioma progression.
In the present study, Piezo1 expression was
increased by pressure in glioma cells, suggesting that Piezo1 acts
as a mechanosensor. Piezo1 is a member of a non-selective cationic
mechanosensitive channel family expressed in mammalian cells
(29). In vivo, Piezo
channels respond to a number of various forces including laminar
flow and cellular compression (30–32)
and are highly expressed in the bladder where they respond to
mechanical stretching (33). The
ablation of the PIEZO1 gene is lethal during early embryonic
development (32), and vascular
abnormalities occur due to the targeted deletion of the
PIEZO1 gene in the endothelium shortly after the onset of
cardiac activity (20). In our
previous study, Piezo1 was found to sense interstitial fluid-like
flow and initiate signal transduction of the Src-Yes associated
transcriptional regulator axis to promote cancer progression and
metastasis in the tumor microenvironment of prostate cancer
(19). Furthermore, the expression
of Piezo1 in GBM has been reported by a number of other groups.
Notably, Piezo1 has been reported to be sensitively activated by
the stiffness of the brain matrix in GBM, and further ECM
remodeling is followed by integrin-FAK signaling, which accelerates
GBM progression (34). Overall,
these results suggest that mechanical pressure, which is a unique
physical stimulus in GBM, activates the Piezo1 channel in the
membrane of glioma cells and initiates a signaling cascade in GBM
through calcium influx, followed by GBM progression.
In the present study, it was also demonstrated that
GDF15 expression was regulated by Piezo1 activation. GDF15
is a tumor suppressor in the early stages of tumor formation
(35,36), but subsequently promotes the growth
of high-grade tumors (37,38). In particular, GDF15 controls immune
evasion and cell proliferation in glioma (39). GDF15 has also been linked to poor
prognosis and is thought to be an oncogenic factor in glioma
(40). Notably, the results of the
present study showed that GDF15 affected the expression of
CTLA4, and that compressive force-induced CTLA4
expression was significantly reduced by knocking down GDF15
expression using siRNA. Although CTLA4 has been almost exclusively
studied in terms of the T cell lineage, certain studies have shown
that its expression is not limited to T cells and is found in other
cells, including solid tumors (41,42).
In the present study, mechanical stress directly increased the
expression of CTLA4 in H4 cells, which was reduced by
PIEZO1 or GDF15 knockdown. Studies exploring the
transcriptional regulation of CTLA4 are limited; however, a report
has indicated that transcription factors such as NFAT, STAT1, Fox
and Myc may promote CTLA4 transcription (43). The involvement of those
transcription factors in the system were not investigated in the
present study. Further research is necessary to fully understand
the molecular mechanisms underlying CTLA4 regulation, as epigenetic
regulation, direct regulation through microRNAs and transcription
factors are additional mechanisms that govern this protein.
Furthermore, intracellular calcium levels may regulate CTLA4
expression. Linsley et al (44) reported that increasing intracellular
calcium levels rapidly increased the cell surface expression of
CTLA4 in T cells, indicating that CTLA4 expression may be increased
by intracellular calcium levels through Piezo1 activation.
Overall, the findings of the present study indicate
that mechanical pressure in the glioma microenvironment enhances
glioma aggression through the Piezo1-GDF15-CTLA4 axis (Fig. 4D). Piezo1 likely senses the
compressive pressure from outside the glioma and initiates signal
transduction, such as GDF15 activation, which in turn enhances
glioma motility. Although the role of CTLA4 expression in glioma
remains to be elucidated, increased CTLA4 expression in H4 cells
might drive immune evasion or poor prognosis in glioma, similar to
that in other diseases. To further investigate the detailed role of
CTLA4 in gliomas, we plan to further verify these findings in
animal models to explore potential therapeutic drugs. Thus, the
present study demonstrates the molecular mechanisms by which
physical stimuli originating in the GBM microenvironment increase
the aggressiveness of cancer cells and will help to develop
strategies to target these molecules for the treatment of GBM in
the future.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Human brain tissues were provided by the Korea Brain
Bank Network through the National Brain Bank Project and Korean
Brain cluster promotion project funded by the Ministry of Science
and ICT (23-BR-09-01).
Funding
This research was financially supported by the National Research
Foundation of Korea (NRF) grants funded by the Korean government
(grant nos. 2023R1A2C2006894 and 2021R1A6A3A01088243) and the
Chung-Ang University Young Scientist Scholarship in 2021.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
OHK, IJT and HJL conceived and designed the
experiments. OHK, IJT, HK and ESC performed the experiments. OHK
and IJT analyzed the data. OHK and HJL wrote the manuscript. HJL
revised the manuscript. OHK and HJL confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The experiments using human brain tissue were
performed according to the guidelines of the Institutional Review
Board (approval no. 1041078-202209-HR-199) of Chung-Ang University
(Seoul, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A ‘state of the
science’ review. Neuro Oncol. 16:896–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin D, Wang M, Chen Y, Gong J, Chen L, Shi
X, Lan F, Chen Z, Xiong T, Sun H and Wan S: Trends in intracranial
glioma incidence and mortality in the United States, 1975–2018.
Front Oncol. 11:7480612021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeo AT and Charest A: Immune checkpoint
blockade biology in mouse models of glioblastom. J Cell Biochem.
118:2516–2527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bausart M, Préat V and Malfanti A:
Immunotherapy for glioblastoma: The promise of combination
strategies. J Exp Clin Cancer Res. 41:352022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parry RV, Chemnitz JM, Frauwirth KA,
Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB
and Riley JL: CTLA-4 and PD-1 receptors inhibit T-cell activation
by distinct mechanisms. Mol Cell Biol. 25:9543–9553. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wintterle S, Schreiner B, Mitsdoerffer M,
Schneider D, Chen L, Meyermann R, Weller M and Wiendl H: Expression
of the B7-related molecule B7-H1 by glioma cells: A potential
mechanism of immune paralysis. Cancer Res. 63:7462–7467.
2003.PubMed/NCBI
|
|
7
|
Seano G, Nia HT, Emblem KE, Datta M, Ren
J, Krishnan S, Kloepper J, Pinho MC, Ho WW, Ghosh M, et al: Solid
stress in brain tumours causes neuronal loss and neurological
dysfunction and can be reversed by lithium. Nat Biomed Eng.
3:230–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gamburg ES, Regine WF, Patchell RA,
Strottmann JM, Mohiuddin M and Young AB: The prognostic
significance of midline shift at presentation on survival in
patients with glioblastoma multiforme. Int J Radiat Oncol Biol
Phys. 48:1359–1362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreth FW, Berlis A, Spiropoulou V, Faist
M, Scheremet R, Rossner R, Volk B and Ostertag CB: The role of
tumor resection in the treatment of glioblastoma multiforme in
adults. Cancer. 86:2117–2123. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng G, Tse J, Jain RK and Munn LL:
Micro-environmental mechanical stress controls tumor spheroid size
and morphology by suppressing proliferation and inducing apoptosis
in cancer cells. PLoS One. 4:e46322009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paul CD, Mistriotis P and Konstantopoulos
K: Cancer cell motility: Lessons from migration in confined spaces.
Nat Rev Cancer. 17:131–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tse JM, Cheng G, Tyrrell JA,
Wilcox-Adelman SA, Boucher Y, Jain RK and Munn LL: Mechanical
compression drives cancer cells toward invasive phenotype. Proc
Natl Acad Sci USA. 109:911–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grossen A, Smith K, Coulibaly N, Arbuckle
B, Evans A, Wilhelm S, Jones K, Dunn I, Towner R, Wu D, et al:
Physical forces in glioblastoma migration: A systematic review. Int
J Mol Sci. 23:40552022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calhoun MA, Cui Y, Elliott EE, Mo X, Otero
JJ and Winter JO: MicroRNA-mRNA interactions at low levels of
compressive solid stress implicate mir-548 in increased
glioblastoma cell motility. Sci Rep. 10:3112020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JW, Lee KS, Nahm JH and Kang YG:
Effects of compressive stress on the expression of M-CSF, IL-1β,
RANKL and OPG mRNA in periodontal ligament cells. Korean J Orthod.
39:248–256. 2009. View Article : Google Scholar
|
|
16
|
Kalli M, Papageorgis P, Gkretsi V and
Stylianopoulos T: Solid stress facilitates fibroblasts activation
to promote pancreatic cancer cell migration. Ann Biomed Eng.
46:657–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YC, Fu YS, Tsai SW, Wu PK, Chen CM,
Chen WM and Chen CF: IL-1b in the secretomes of MSCs seeded on
human decellularized allogeneic bone promotes angiogenesis. Int J
Mol Sci. 23:153012022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak K and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim OH, Choi YW, Park JH, Hong SA, Hong M,
Chang IH and Lee HJ: Fluid shear stress facilitates prostate cancer
metastasis through Piezo1-Src-YAP axis. Life Sci. 308:1209362022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy
SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, et al: Piezo1,
a mechanically activated ion channel, is required for vascular
development in mice. Proc Natl Acad Sci USA. 111:10347–10352. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu F, Huang J, Liu X, Cheng Q, Luo C and
Liu Z: CTLA-4 correlates with immune and clinical characteristics
of glioma. Cancer Cell Int. 20:72020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilkes DM and Wirtz D: Tumour
mechanopathology: Cutting the stress out. Nat Biomed Eng.
1:00122017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalli M and Stylianopoulos T: Defining the
role of solid stress and matrix stiffness in cancer cell
proliferation and metastasis. Front Oncol. 8:552018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guaccio A, Borselli C, Oliviero O and
Netti PA: Oxygen consumption of chondrocytes in agarose and
collagen gels: Acomparative analysis. Biomaterials. 29:1484–1493.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalli M, Voutouri C, Minia A, Pliaka V,
Fotis C, Alexopoulos LG and Stylianopoulos T: Mechanical
compression regulates brain cancer cell migration through MEK1/Erk1
pathway activation and GDF15 expression. Front Oncol. 9:9922019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piek J, Plewe P and Bock WJ:
Intrahemispheric gradients of brain tissue pressure in patients
with brain tumours. Acta Neurochir (Wien). 93:129–132. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Helmlinger G, Netti PA, Lichtenbeld HC,
Melder RJ and Jain RK: Solid stress inhibits the growth of
multicellular tumor spheroids. Nat Biotechnol. 15:778–783. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stylianopoulos T, Martin JD, Chauhan VP,
Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR,
Hornicek FJ, Boucher Y, et al: Causes, consequences, and remedies
for growth-induced solid stress in murine and human tumors. Proc
Natl Acad Sci USA. 109:15101–15108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coste B, Mathur J, Schmidt M, Earley TJ,
Ranade S, Petrus MJ, Dubin AE and Patapoutian A: Piezo1 and Piezo2
are essential components of distinct mechanically activated cation
channels. Science. 330:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brohawn SG, Campbell EB and MacKinnon R:
Physical mechanism for gating and mechanosensitivity of the human
TRAAK K+ channel. Nature. 516:126–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee W, Leddy HA, Chen Y, Lee SH, Zelenski
NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, et al:
Synergy between Piezo1 and Piezo2 channels confers high-strain
mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA.
111:E5114–E5122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Hou B, Tumova S, Muraki K, Bruns A,
Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al: Piezo1
integration of vascular architecture with physiological force.
Nature. 515:279–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyamoto T, Mochizuki T, Nakagomi H, Kira
S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M and
Tominaga M: Functional role for Piezo1 in stretch-evoked
Ca2+ influx and ATP release in urothelial cell cultures.
J Biol Chem. 289:16565–16575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X, Wanggou S, Bodalia A, Zhu M, Dong
W, Fan JJ, Yin WC, Min HK, Hu M, Draghici D, et al: A feedforward
mechanism mediated by mechanosensitive ion channel PIEZO1 and
tissue mechanics promotes glioma aggression. Neuron.
100:799–815.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cekanova M, Lee SH, Donnell RL,
Sukhthankar M, Eling TE, Fischer SM and Baek SJ: Nonsteroidal
anti-inflammatory drug-activated gene-1 expression inhibits
urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer
Prev Res (Phila). 2:450–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Husaini Y, Qiu MR, Lockwood GP, Luo XW,
Shang P, Kuffner T, Tsai VW, Jiang L, Russell PJ, Brown DA and
Breit SN: Macrophage inhibitory cytokine-1 (MIC-1/GDF15) slows
cancer development but increases metastases in TRAMP prostate
cancer prone mice. PLoS One. 7:e438332012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li S, Ma YM, Zheng PS and Zhang P: GDF15
promotes the proliferation of cervical cancer cells by
phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp
Clin Cancer Res. 37:802018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vaňhara P, Hampl A, Kozubík A and Souček
K: Growth/differentiation factor-15: Prostate cancer suppressor or
promoter? Prostate Cancer Prostatic Dis. 15:320–328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roth P, Junker M, Tritschler I,
Mittelbronn M, Dombrowski Y, Breit SN, Tabatabai G, Wick W, Weller
M and Wischhusen J: GDF-15 contributes to proliferation and immune
escape of malignant gliomas. Clin Cancer Res. 16:3851–3859. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shnaper S, Desbaillets I, Brown DA, Murat
A, Migliavacca E, Schluep M, Ostermann S, Hamou MF, Stupp R, Breit
SN, et al: Elevated levels of MIC-1/GDF15 in the cerebrospinal
fluid of patients are associated with glioblastoma and worse
outcome. Int J Cancer. 125:2624–2630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laurent S, Queirolo P, Boero S, Salvi S,
Piccioli P, Boccardo S, Minghelli S, Morabito A, Fontana V, Pietra
G, et al: The engagement of CTLA-4 on primary melanoma cell lines
induces antibody-dependent cellular cytotoxicity and TNF-α
production. J Transl Med. 11:1082013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pistillo MP, Carosio R, Grillo F, Fontana
V, Mastracci L, Morabito A, Banelli B, Tanda E, Cecchi F, Dozin B,
et al: Phenotypic characterization of tumor CTLA-4 expression in
melanoma tissues and its possible role in clinical response to
Ipilimumab. Clin Immunol. 215:1084282020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Dai Z, Wu W, Wang Z, Zhang N,
Zhang L, Zeng WJ, Liu Z and Cheng Q: Regulatory mechanisms of
immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer
Res. 40:1842021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Linsley PS, Bradshaw J, Greene J, Peach R,
Bennett KL and Mittler RS: Intracellular trafficking of CTLA-4 and
focal localization towards sites of TCR engagement. Immunity.
4:535–543. 1996. View Article : Google Scholar : PubMed/NCBI
|