Introduction
The incidence of prostate cancer (PCa) is increasing
yearly, and in 2022, PCa was the second most common cancer in men,
with >1 million new cases and >390,000 deaths worldwide
(1). Localized PCa can be treated
by surgery or radiotherapy and has a good prognosis. Although the
treatment of metastatic PCa is improving, the 5-year survival rate
of metastatic PCa is only 32% (2).
PCa is an androgen-dependent tumor and androgens can strongly
promote PCa cell proliferation and metastasis. Thus, androgen
deprivation therapy (ADT) is a modality that has been used to treat
PCa for decades and has become one of the most effective treatment
options for patients with metastatic hormone-sensitive PCa (mHSPC)
(3). Recently, a number of studies
have shown that ADT combined with docetaxel or androgen receptor
pathway inhibitors (ARPIs) can improve the overall survival (OS) of
patients with mHSPC (4–8), and this new treatment regimen has
become the standard treatment for this disease (9). Unfortunately, despite the advances in
therapeutic drugs, HSPC can gradually lose sensitivity to treatment
and eventually develop into metastatic castration-resistant PCa
(mCRPC) (10). First-line treatment
of mCRPC includes ARPIs, docetaxel, sipuleucel-T, Ipatasertib and
poly (ADP-ribose) polymerase inhibitors combined with an ARPI
(11). All patients with mCRPC who
receive first-line treatment will eventually experience disease
progression (11).
Subsequent treatment options for mCRPC include
several novel agents. For instance, antibody-drug conjugates
(ADCs), consisting of monoclonal antibodies (mAbs) and potent
cytotoxic payloads conjugated by chemical linkers, are a
therapeutic option that has been rapidly developing in recent years
(12). The basic principle of ADCs
is the specific combination of antigens and antibodies. Antitumor
drugs can specifically bind to antigens on the surface of cancer
cells and eliminate these cells through antibody delivery, which
reduces the toxicity of antitumor drugs (13). The enhanced antitumor efficacy of
ADCs leads to an improved quality of life of the patient and
reduced toxicity compared with conventional chemotherapies
(14). Additionally, ADCs targeting
the same molecular pathway may retain efficacy in patients who have
developed resistance to specific targeted therapies, thereby
expanding their potential indications for antitumor treatment
(15). Human epidermal growth
factor receptor 2 (HER2) serves as a crucial therapeutic target for
advanced breast cancer, and in cases where patients with metastatic
breast cancer develop resistance to anti-HER2 targeted agents,
anti-HER2 ADCs can still effectively exert antitumor activity
(16). Notably, the anti-HER2 ADC
trastuzumab deruxtecan has demonstrated efficacy even in patients
with advanced breast cancer and low HER2 expression (17). ADCs are also effective in targeting
novel therapeutic targets. For instance, sacituzumab govitecan
(SG), which specifically targets trophoblast cell-surface antigen 2
(TROP-2), offers additional treatment alternatives for advanced
triple-negative breast cancer management (18).
At present, patients with advanced PCa, such as
mCRPC, have few treatment options and the emergence of ADCs brings
new alternatives for these patients. Although ADCs were developed
decades ago for treating patients with advanced solid tumors, their
use in PCa treatment is still in the clinical drug development
stages. Therefore, how to further utilize ADCs in the field of PCa
treatment is the current clinical challenge, including reducing
adverse events (AEs) and improving effectiveness. In the treatment
of PCa, several therapeutic targets of ADCs have been developed,
including prostate-specific membrane antigen (PSMA), STEAP family
member 1 (STEAP1), TROP-2, CD46, B7-H3, tissue factor (TF) and
delta-like protein 3 (DLL3).
In the present review, first, the developmental
course, structural composition and mechanism of action of ADCs is
illustrated. Second, the clinical outcomes of ADCs in the field of
PCa treatment as well as the ongoing clinical trials are
systematically described. Finally, the ongoing challenges in ADC
development and the corresponding strategies to overwhelm them are
discussed.
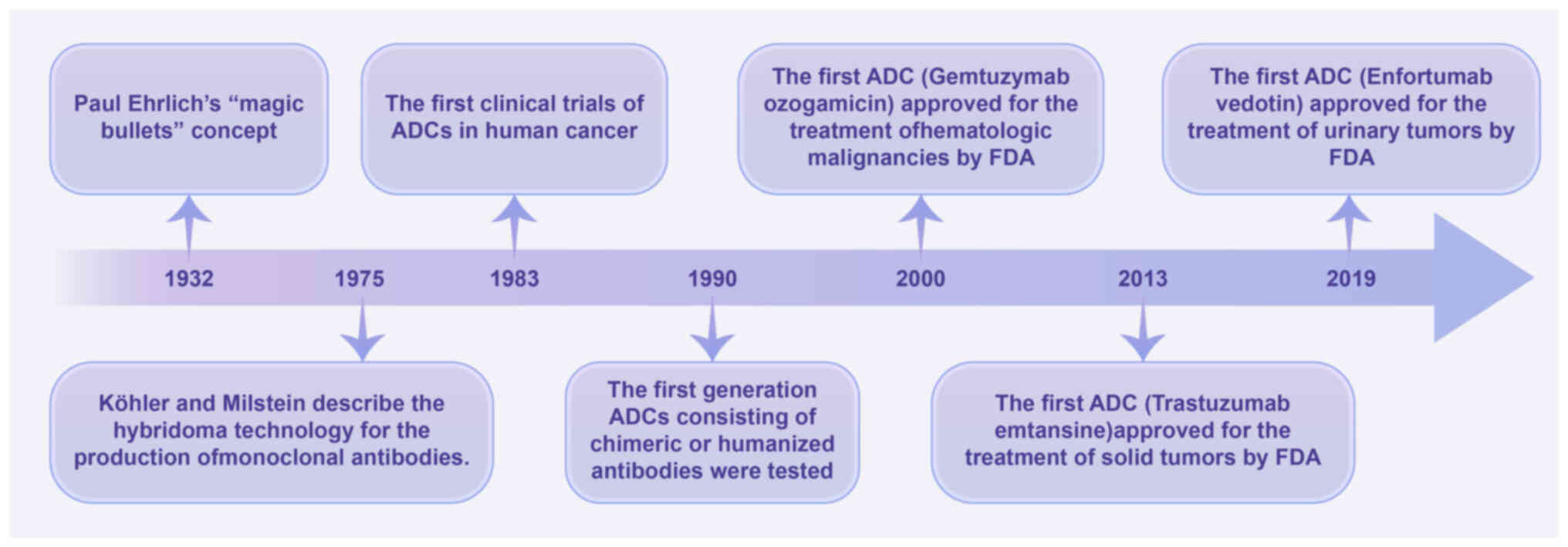
Development of ADCs
ADCs originate from the idea by Paul Ehrlich in 1913
that ‘antibodies are in a way magic bullets that identify their
target themselves without harming the organism’ (19). In 1975, Köhler and Milstein
(20) developed hybridoma
technology and successfully produced mAbs, which was an important
step in turning this ‘idea’ into a treatment reality. In 1983, ADCs
were first used in clinical trials; however, clinical efficacy was
not successful due to the inclusion of murine mAbs (19). This prompted researchers to further
develop ADCs containing humanized or chimeric antibodies that can
eliminate cancer cells through multiple mechanisms, such as
antibody-dependent cytotoxicity, interference with signaling,
complement-dependent cytotoxicity and immune regulation (21,22).
In 1990, novel ADCs containing chimeric or humanized antibodies
were tested in clinical trials. Ultimately, ADCs passed clinical
trials and were approved by the United States Food and Drug
Administration (FDA) in 2000. The first ADC approved for use in
hematological malignancies was gemtuzumab ozogamicin, which was
approved by the FDA on May 17, 2000, for the treatment of
CD33+ acute myelogenous leukemia (23). Furthermore, the FDA granted approval
to trastuzumab emtansine on February 22, 2013, marking the first
authorization of an ADC for the treatment of solid tumors in
patients with HER2+ advanced breast cancer (24,25).
On December 18, 2019, enfortumab vedotin was approved by the FDA
for the treatment of metastatic urothelial carcinoma, which was the
first ADC approved for the treatment of urological malignancies
(Fig. 1) (26). At present, >10 ADCs have been
approved by the FDA and ~200 ADCs are being evaluated in clinical
trials. ADCs have changed the treatment strategy of numerous
malignant tumors and have expanded the treatment options for
patients with advanced tumors; however, to the best of our
knowledge, there are still no approved ADCs for PCa.
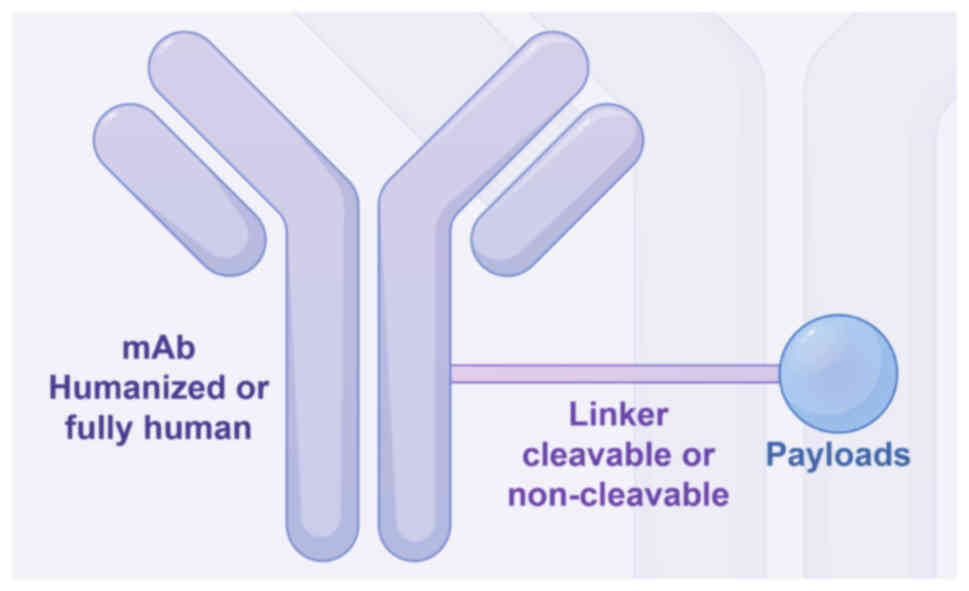
Components and mechanism of action of
ADCs
ADCs are developed based on enhanced antibody
technology and comprise three essential components: A mAb that
specifically targets tumor surface antigens, a potent cytotoxic
payload engineered to induce damage to DNA or tubulin within the
targeted cancer cells and a linker that firmly attaches the
antibody to the payload (Fig. 2)
(27). The general mechanism of
action of ADCs is shown in Fig. 3.
Briefly, once introduced into the bloodstream, ADCs specifically
recognize and bind to antigens that are upregulated on the surface
of cancer cells. Subsequently, these bound ADCs undergo rapid
internalization into the cells, where they are then processed by
intracellular lysosomes for the release of the cytotoxic payloads
(28). After being released from
the antibody within the cell, a portion of the hydrophobic payload
has the ability to diffuse beyond the target cell and effectively
eliminate surrounding antigen-negative cancer cells or
non-malignant cells. This phenomenon, referred to as the bystander
killing effect, has been extensively studied, and research
indicates that this effect can significantly augment the antitumor
activity of ADCs (29–31).
The antibody component of ADCs, which serves a
crucial role in specifically binding to target cell surface
antigens, has undergone significant advancements and refinements in
recent decades (28). The targets
of ADCs (cell surface antigens) should ideally be upregulated on
cancer cells while maintaining low expression on normal cells. This
specificity enables the specific binding of ADCs and the subsequent
release of toxic payloads, thereby minimizing the cytotoxic effects
on normal cells (32). The
identification of two distinct types of tumor surface antigens,
namely tumor-associated antigens (TAAs) and tumor-specific antigens
(TSAs), has been accomplished (33). TAAs are upregulated in cancer cells
and downregulated in normal cells, whereas TSAs are exclusively
expressed on the surface of cancer cells rather than normal cells.
Consequently, TSAs can serve as markers for discriminating between
tumor and normal cells (34). The
use of a TSA as the target of an ADC will greatly improve the
effectiveness of the ADC and reduce the toxicity to normal cells.
However, most of the ADC targets in current clinical trials are
TAAs (35).
The linker, a crucial component of ADCs, has a
pivotal role in determining the toxicity and efficacy of ADCs. Once
introduced into the bloodstream, it is imperative for the linker to
maintain stability throughout circulation to prevent premature
detachment of toxic payloads from the ADCs (36). The two primary types of linkers in
current use are cleavable and non-cleavable linkers. At present,
the majority of ADCs employ cleavable linkers that incorporate
chemical triggers capable of efficient cleavage, leading to the
release of cytotoxic payloads (37). These linkers are designed to be
cleaved after internalization into cancer cells, ensuring optimal
efficacy by releasing toxic loads at the desired site. However,
premature cleavage of these cleavable linkers in circulation
results in the early release of effective payloads, thereby
increasing blood toxicity and diminishing the effectiveness of ADCs
(38). Non-cleavable linkers
consist of stable bonds and are resistant to protease degradation.
These linkers are an integral part of the payload, and the release
of the cytotoxic payload occurs only after complete internalization
of ADCs into the cell. This mechanism enhances the efficacy while
reducing the toxicity of ADCs (37). At present, research is focused on
the development of novel linkers with improved stability,
solubility and release characteristics.
The cytotoxic payload is the primary component
responsible for the eradication of cancer cells by ADCs, which is
released subsequent to ADC internalization into the cellular milieu
(39). The in vitro toxicity
levels of commonly used chemotherapies are in the micromolar range,
whereas the payloads utilized for ADCs exhibit sub-nanomolar or
even picomolar levels of cytotoxicity in vitro (40). The current repertoire of cytotoxic
payloads utilized for ADCs primarily encompass potent tubulin
inhibitors, DNA damage agents and recently developed
immunomodulators (41). Tubulin
inhibitors function by disrupting microtubule-dependent mitosis.
Prominent tubulin inhibitors include monomethyl auristatin (MMA)E,
MMAF and their maytansine derivatives, DM1 and DM4 (42). The half-maximal inhibitory
concentration (IC50) values of microtubule inhibitors
typically fall within the nanomolar range, whereas DNA damaging
agents can exhibit IC50 values as low as picomolar
(43). Therefore, ADCs are
occasionally more effective when conjugated to DNA damaging agents,
even when targeting cells with low surface antigen expression
(43). The currently utilized DNA
damaging agents include calicheamicins and pyrrolobenzodiazepine
dimers (PBDs), as well as topoisomerase I inhibitors such as SN-38
and the exatecan derivative (DXd). The field of ADCs is witnessing
a surge in the development of novel payloads, with small molecule
immunomodulators, also known as immunostimulatory antibody
conjugates, emerging as a promising class of cytotoxic agents
(44). This innovative payload
holds great potential in enhancing the efficacy of ADCs and
extending patient survival.
ADCs in PCa
PSMA
PSMA is a transmembrane protein consisting of 750
amino acids that is located on the surface of prostate epithelial
cells (45). The expression of PSMA
is typically low in normal prostate tissue, but it undergoes a
notable increase of >1,000-fold in PCa. Moreover, the
upregulation of PSMA expression is positively correlated with a
higher tumor grade, castration resistance and tumor metastasis
(46). Therefore, PSMA has emerged
as a commonly employed target for the diagnosis and treatment of
PCa. At present, four PSMA-targeting ADCs, namely MLN2704, PSMA
ADC, MEDI3726 and ARX517, have been developed for clinical
trials.
MLN591 is a deimmunized anti-PSMAext mAb,
which exhibits rapid internalization and high affinity for the
external domain of PSMA, and MLN2704 is an ADC consisting of MLN591
linked to DM1, a potent chemotherapeutic drug that acts against
microtubules (47). MLN2704
exhibits rapid internalization upon binding to PSMA, resulting in
the intracellular release of DM1 and the subsequent eradication of
PCa cells. MLN2704 has shown dose-and schedule-dependent antitumor
activity in a PCa xenograft model (48). The initial human trial of MLN2704,
aimed at assessing the safety profile of doses ranging 18–343
mg/m2 administered every 4 weeks, encompassed a cohort
of 23 patients with mCRPC. Among this cohort, 3 individuals
experienced grade 3 drug-related AEs, and while no grade 4
toxicities were observed, 8 patients reported neuropathy. Notably,
a prostate-specific antigen (PSA)50 response was
detected in ~22% of the participants. These findings unequivocally
established the favorable safety profile of MLN2704 (Table I) (49). The PSA50 response is
defined as a ≥50% decrease from the initial level of serum PSA,
which is confirmed by a measurement taken at least 4 weeks after
treatment. In a subsequent phase I/II trial including 62 patients
with mCRPC, only 8% (5 out of 62) of patients demonstrated a
PSA50 response, while an equal proportion of patients
(8%) exhibited PSA stability lasting for ≥90 days. However, the
majority of patients (71%) experienced peripheral neuropathy and a
small percentage (10%) encountered grade 3/4 AEs (47). Due to the limited clinical efficacy
of MLN2704 and its narrow therapeutic range, further clinical
studies were not conducted.
 | Table I.The clinical trial data of ADCs in
PCa. |
Table I.
The clinical trial data of ADCs in
PCa.
| First author/s,
year | Drug | Target | Conditions | Treatment | Phase | Trial number | Efficacy | Safety | (Refs.) |
|---|
| Ma et al,
2006 | MLN2704 | PSMA | mCRPC | Single agent | 1 | NCT00052000 | 22%
PSA50 response. | 13% Study
drug-related G 3 toxicities | (50) |
| Milowsky et
al, 2016 | MLN2704 | PSMA | mCRPC | Single agent | 1/2 | NCT00070837 | 8% PSA50
response; 8% PSA stabilization ≥90 days. | 71% exhibited
peripheral neuropathy: 10% had G 3/4. | (47) |
| Petrylak et
al, 2020 | PSMA ADC | PSMA | mCRPC progressing
on ARSI or docetaxel | Single agent | 1 | NCT01414283 | 15%
PSA50 response. | Most common
toxicities: fatigue (40%), neutropenia (33%). | (52) |
| Cho et al,
2018 | PSMA ADC | PSMA | mCRPC progressing
on ARSI or docetaxel | Single agent | 2 | NCT01695044 | 14%
PSA50 response; 78% CTC declined ≥50%. | Most common G ≥3
TRAEs: neutropenia, fatigue; most common serious AEs: dehydration,
hyponatremia. | (53) |
| Shen et al,
2023 | MEDI3726 | PSMA | mCRPC progressing
on ARSI or docetaxel | Single agent | 1 | NCT02991911 | 6.1%
PSA50 response. | 45%G3/4TRAEs. | (55) |
| Doronina et
al, 2003 | DSTP3086S | STEAP family member
1 | mCRPC | Single agent | 1 | NCT01283373 | 17.7%
PSA50 response. | Most common Gr ≥3
TRAEs: hyperglycaemia; hyperglycaemia and hypophosphataemia were
dose limiting. | (59) |
| Zang and Allison,
2022 | FOR46 | CD46 | mCRPC progressing
on ARSI | Single agent | 1a/1b | NCT03575819 | 45.5%
PSA50 response. | Most common Gr ≥3
TRAEs: neutropenia (77%) | (68) |
| Shenderov et
al, 2021 | MGC018 | B7-H3 | mCRPC progressing
on ARSI or docetaxel | Single agent | 1 | NCT03729596 | 55.5%
PSA50 response. | At least 1
treatment emergent AEs in 87% with commonest being
neutropenia. | (73) |
| Corti et al,
2023 | Tisotumab
vedotin | Tissue factor | Advanced solid
tumours including mCRPC | Single agent | 1/2 | NCT02001623 | None | Most common Gr ≥ 3
AEs: fatigue | (80) |
| Fuentes-Antras
et al, 2023 | Rovalpituzumab
tesirine | Delta-like protein
3 | Neuroendocrine
including carcinomas neuroendocrine PCa | Single agent | 1/2 | NCT02709889 | 76% clinical
benefit rate | Most common G3/4
AEs: anemia,. thrombocytopenia | (86) |
The PSMA ADC is prepared by conjugating a fully
human anti-PSMA mAb with MMAE through a valine-citrulline linker
(50). The PSMA ADC undergoes rapid
internalization upon binding to PSMA, leading to the release of
MMAE within the cell and the subsequent induction of cell cycle
arrest and apoptosis (50). The
initial 2019 phase I clinical trial of PSMA ADC involved dose
escalation and included patients with mCRPC who had previously
undergone taxane-based chemotherapy (51). The aforementioned study aimed to
determine the maximum tolerated dose of PSMA ADC and evaluate its
safety, tolerability and pharmacokinetics (51). The PSMA ADC exhibited encouraging
antitumor efficacy and tolerable toxicity in this phase I trial.
The findings prompted a subsequent phase II trial involving 119
patients with mCRPC, wherein 14% achieved a PSA50
response, >75% experienced at least a 50% reduction in
circulating tumor cell (CTC) counts and 65% transitioned from an
unfavorable to a favorable CTC status (52). The most frequently observed
treatment-related AEs (TRAEs) of grade ≥3 in this study included
neutropenia, fatigue, electrolyte imbalance, anemia and neuropathy
(52). Serious AEs (SAEs)
encompassed dehydration, hyponatremia, febrile neutropenia and
constipation. Among the subjects who received a dosage of 2.5
mg/kg, 2 succumbed to sepsis.
The ADC, MEDI3726, is composed of an anti-PSMA
antibody conjugated with PBDs (53). MEDI3726 binds to the highly
expressed PSMA on PCa cells and is rapidly internalized, followed
by intracellular cathepsin cleavage, mediating the PBD payload
release. In a phase I trial involving 33 patients with mCRPC who
had experienced disease progression after prior treatment with
abiraterone, enzalutamide or taxane-based chemotherapy, the rate of
PSA50 response or a conversion in CTC count was found to
be 6.1% after treatment, while the rate of composite response was
12.1% (54). TRAEs were observed in
30 patients (90.9%), predominantly presenting as skin toxicities
and effusions, with grade 3/4 TRAEs occurring in 15 patients
(45.5%) and SAE-related discontinuation reported in 11 patients
(33.3%). Therefore, due to the lack of safety and efficacy,
MEDI3726 was not tested in a phase II trial.
The recruitment of patients with mCRPC commenced in
2021 for a phase I clinical trial aimed at evaluating the safety
profile of ARX517, an ADC comprising a human mAb targeting PSMA
that is covalently linked to the microtubule-disrupting toxin,
amberstatin-269. The study enrolled 24 patients with mCRPC who had
received at least one ARPI treatment line by October 2023. The
results demonstrated a PSA50 response in 8 patients and
a significant decline in CTCs in 12 patients. No SAEs were
reported, except for four grade 3 AEs, including thrombocytopenia
and lymphopenia (55). An ongoing
clinical trial (NCT04662580) aims to further verify the safety and
efficacy of ARX517 (Table II).
 | Table II.The ongoing clinical trial data of
antibody-drug conjugates in prostate cancer. |
Table II.
The ongoing clinical trial data of
antibody-drug conjugates in prostate cancer.
| Drug | Target | Conditions | Treatment | Phase | Trial number | Estimated
Completion Date |
|---|
| ARX517 | Prostate-specific
membrane antigen | mCRPC | Single agent | 1 | NCT04662580 | March 2027 |
| Datopotamab | TROP-2 |
Advanced/Metastatic | Single agent
or | 2 | NCT05489211 | August 2026 |
| Deruxtecan |
| solid tumours | with Saruparib |
|
|
|
| (Dato-DXd) |
| including
mCRPC |
|
|
|
|
| Sacituzumab
govitecan (SG) | TROP-2 | mCRPC progressing
on ARSI | Single agent | 2 | NCT03725761 | April 2025 |
| FOR46 | CD46 | mCRPC progressing
on abiraterone | Combination with
enzalutamide | 1b/2 | NCT05011188 | March 2027 |
| MGC018 | B7-H3 | mCRPC progressing
on ARSI or docetaxel | Single agent | 2 | NCT05551117 | May 2027 |
| MGC018 | B7-H3 | Advanced/Metastatic
solid tumours including mCRPC | Combination with
lorigerlimab (MGD019) | 1 | NCT05293496 | March 2026 |
| Ifinatamab
deruxtecan (I-DXd) | B7-H3 | Advanced solid
tumours including mCRPC | Single agent | 1/2 | NCT04145622 | March 2027 |
| XB002 | Tissue factor | Advanced solid
tumours including mCRPC | Single agent or
with nivolumab | 1 | NCT04925284 | October 2024 |
STEAP1
The STEAP1 protein, also known as prostate
six-transmembrane epithelial antigen 1, is recognized for its role
as an ion channel or multi-transmembrane transporter (56). The expression levels of STEAP1 are
5-10-fold higher in human PCa cells compared with other cancer cell
types, thus positioning STEAP1 as a promising therapeutic target
for PCa (57). The DSTP3086S ADC is
designed to target STEAP1, consisting of the anti-STEAP1 mAb,
MSTP2109A, conjugated to MMAE via a cleavable linker (58). The DSTP3086S molecule specifically
binds to the surface of PCa cells, targeting STEAP1, and undergoes
rapid internalization. This leads to the intracellular release of
MMAE, which effectively inhibits the mitotic process in cancer
cells and ultimately induces cell death (59). In a phase I dose-escalation trial of
DSTP3086S, 77 patients with mCRPC and high STEAP1 expression were
enrolled. The dose range administered was escalated from 0.3–2.8
mg/kg every 3 weeks. Among these patients, doses >2 mg/kg were
well tolerated by 62 individuals, and while grade ≥3 AEs occurred
in 26 patients, a PSA50 response was observed in 11 patients
(17.7%) (58). The safety and
efficacy of DSTP3086S need to be further evaluated through phase II
trials.
TROP-2
TROP-2 is a glycoprotein expressed on the surface of
prostate cells, with upregulation observed in PCa cells (60). The majority of patients with mCRPC
treated with androgen receptor signaling inhibitors (ARSIs) who
progressed have been reported to exhibit upregulation of TROP-2
(61). The emergence of this
phenomenon has therefore prompted researchers to develop ADCs that
specifically target TROP-2 for the treatment of mCRPC. SG, an ADC
designed to target TROP-2, consists of a humanized RS7 mAb coupled
with SN-38 via a moderately stable carbonate bond (62). An ongoing open-label phase II trial
(NCT03725761) is currently enrolling patients with mCRPC who have
experienced disease progression following treatment with ARSIs. At
the time of the interim analysis, 20 patients had been enrolled,
demonstrating a 6-month radiographic progression-free survival rate
(PFS) of 45%. Among the observed AEs, neutropenia was the most
frequently reported (63).
The compound, Datopotamab Dxd (Dato-DXd), is an ADC
that specifically targets TROP-2 and is a humanized mAb linked to a
topoisomerase 1 inhibitor (64).
The safety and efficacy of Dato-DXd in patients with advanced solid
tumors, including mCRPC, are currently being investigated through
an ongoing phase II clinical trial (NCT05489211).
CD46
The membrane protein, CD46, is widely expressed in
human cell membranes, with the exception of red blood cells. CD46
has a crucial role in regulating the deposition of C3b/C4b on
various cell populations and serves as a target for numerous
pathogens (65). CD46 is
upregulated in mCRPC cells, and its expression is significantly
elevated in patients with mCRPC undergoing abiraterone and
enzalutamide treatment. Genomic analysis revealed that 45% of
patients with abiraterone-resistant mCRPC had acquired the CD46
gene (66). The ADC, FOR46,
consists of a mAb that specifically targets CD46 and is conjugated
with MMAE. In a phase Ia/Ib dose-escalation trial involving 33
patients with mCRPC who had experienced disease progression while
receiving ARPI, neutropenia emerged as the sole significant grade 3
AE, with 45.2% of the evaluable patients (14 out of 31) achieving a
PSA50 response (67). The efficacy
of FOR46 in patients with mCRPC necessitates further investigation,
and patient recruitment is currently underway for a phase Ib/II
trial evaluating the combination of FOR46 with enzalutamide in
patients with mCRPC who have experienced disease progression
following prior abiraterone therapy (NCT05011188).
B7-H3
B7-H3, an immunomodulatory protein, is also known as
CD276 (68). Studies have
demonstrated a correlation between the expression of B7-H3 on the
surface of PCa cells and an unfavorable prognosis following
surgical intervention, including early postoperative PSA recurrence
as well as the development of CRPC (69,70).
Moreover, the majority of patients with CRPC exhibit significant
upregulation of B7-H3 expression (93%) (71). The humanized ADC, MGC018,
specifically targets and eliminates cancer cells that upregulate
B7-H3. MGC018 exhibits favorable pharmacokinetic properties and has
demonstrated safety in preclinical tumor models (72). The recruitment of patients is
currently ongoing in a phase I trial evaluating the efficacy and
safety of MGC108 in mCRPC. As of May 3, 2021, 26 patients with
mCRPC had been enrolled, of which 11/22 evaluable patients showed a
PSA50 response, while the most frequently reported AEs included
neutropenia, fatigue, weakness and headache (73). At present, two clinical trials of
MGC018 in solid tumors, including mCRPC, are recruiting patients
(NCT05551117 and NCT05293496).
Ifinatamab Dxd (I-Dxd) is an additional ADC that
targets B7-H3. I-Dxd consists of a mAb that specifically binds to
B7-H3, which is linked to the topoisomerase I inhibitor, Dxd, via a
cleavable tetrapeptide linker (74). Enrollment is currently ongoing in a
phase I/II clinical trial (NCT04145622) aimed at investigating the
safety and efficacy of I-Dxd in patients diagnosed with advanced
solid tumors, including mCRPC.
TF
TF is alternatively referred to as thromboplastin,
factor III or CD142 (75). TF is a
transmembrane glycoprotein and, under physiological conditions, TF
activation marks the start of the exogenous coagulation pathway
(76). TF binds to its
physiological ligand, FVIIa, triggering the activation of
protease-activated receptor 2 and initiating an intracellular
signaling cascade that can be exploited by tumors to facilitate
tumor angiogenesis, enhance cancer cell survival and growth as well
as facilitate metastasis (77). The
expression of TF is upregulated in numerous solid malignancies,
including pancreatic, lung, breast, bladder and PCa (75). Tisolumab vedotin, an ADC composed of
a fully human mAb specific to TF conjugated with MMAE, has been
developed to target tumors that express TF (78). The InnovaTV 201 trial was a phase
I/II open-label study that involved dose-escalation and
dose-expansion in 147 patients with advanced solid tumors,
including PCa. During the dose-expansion phase, fatigue emerged as
the most frequently reported grade ≥3 AE (79). Among the patients with PCa included
in the study, none exhibited radiographic changes or a PSA response
(79). It is necessary to continue
evaluating the antitumor activity of TF in the context of PCa.
XB002 is an additional ADC that specifically targets
TF, consisting of a mAb directed against TF coupled with the
tubulin inhibitor, MMAE, via a cleavable linker (80). The ongoing clinical trial is a phase
I, open-label, multicenter study involving dose-escalation and
expansion of XB002 or XB002 in combination with nivolumab for
advanced solid tumors, including mCRPC (NCT04925284).
DLL3
The DLL3 ligand is involved in the Notch signaling
pathway and exhibits elevated expression levels in neuroendocrine
tumors, while remaining absent in normal tissues (81). The neuroendocrine carcinomas (NECs)
represent a cluster of poorly differentiated neuroendocrine tumors
characterized by the upregulation of DLL3. Research findings have
indicated that DLL3 is upregulated in 65–74% of large-cell NECs and
77% of castration-resistant neuroendocrine PCa (NEPC) cells
(82). The Rovalpituzumab tesirine
(Rova-T) ADC specifically targets DLL3 and consists of a mAb that
binds to DLL3 that is linked to a DNA damaging agent via a
cleavable linker (83). In a phase
I/II trial involving NEPC and other advanced NECs that upregulate
DLL3, the primary objective of the study was to evaluate the safety
profile of Rova-T. The most frequently observed grade 3/4 AEs
included anemia (17%), thrombocytopenia (15%) and elevated
aspartate aminotransferase levels (8%) (84). Among the 21 patients with NEPC
enrolled in the trial, the clinical benefit rate reached 76.2%,
with an average PFS time of 4.5 months and an average OS time of
5.7 months. The clinical benefit rate was defined as the percentage
of participants with the best overall response (confirmed or
unconfirmed) of complete response, partial response or stable
disease according to the Response Evaluation Criteria in Solid
Tumors v1.1 (85).
Discussion and perspectives
The treatment options for PCa are evolving rapidly
but more effective treatment options are still needed. ADCs
represent a novel class of tumor targeting therapeutic drugs that
have demonstrated both efficacy and safety in the treatment of
solid tumors such as breast cancer. These agents possess the
ability to selectively recognize antigens that are upregulated on
the surface of cancer cells and subsequently deliver cytotoxic
payloads within these cells to effectively target and eliminate
malignant growth. In recent years, there has been notable progress
in the development of ADCs for PCa. However, these therapeutic
agents have also encountered numerous challenges in effectively
treating PCa, including limited efficacy, safety concerns and a
narrow treatment window (86).
The progression of PCa is driven by androgen
signaling, resulting in a limited response to conventional
chemotherapeutic agents due to slow growth kinetics and activation
of the androgen receptor (87). The
cytotoxic payloads in most ADCs designed for the treatment of PCa
exert their action by suppressing cancer cell division, which could
be one contributing factor to the relatively lower efficacy of ADCs
in PCa compared with other malignancies. However, chemotherapy
drugs currently used for PCa, such as docetaxel and cabazitaxel,
have shown significant efficacy against, and mainly act by
affecting tubulin and then inhibiting the mitotic process (88,89).
The potential difference may stem from the specific expression of
targeted antigens on the surface of cancer cells, as ADCs are
designed to selectively target these antigens, distinguishing them
from conventional chemotherapy drugs. The efficacy and toxicity of
ADCs are also influenced by the inherent structure of the drug and
enhancing any component of the ADC will ameliorate these
issues.
The selection of appropriate targets is pivotal for
the efficacy of ADCs. At present, most antigens targeted in PCa
treatment with ADCs are TAAs, such as PSMA, STEAP1, TROP-2 and
CD46. The development of TSA-targeted ADCs is currently in the
preclinical research stage, with no clinical trials conducted thus
far, to the best of our knowledge. However, it is anticipated that
TSA targeting ADCs may receive approval in the future, offering
potential enhancements in efficacy and a reduction in the
off-target side effects associated with ADCs. In addition to the
cancer cell surface antigen expression profiles, the
internalization and turnover rates of ADCs have a notable impact on
efficacy (90). Thus, optimizing
the binding affinity between antigens and antibodies is a key step
in enhancing the efficacy of ADCs. However, an excessively high
binding affinity can lead to the retention of ADC molecules on the
surface of cancer cells, thereby impeding their internalization
capacity, a phenomenon referred to as the binding site barrier
effect (91).
Optimizing the antibody structure is also a viable
approach to enhance efficacy and to mitigate the toxicity of ADCs
(92). Conditional antibody ADCs
and bispecific ADCs are novel design ideas (13,93).
The activation of conditional antibody ADCs is restricted to cancer
cells, thereby significantly augmenting the efficacy of ADCs and
preventing off-target toxicity (13). In addition, the advancement of
bispecific antibody technology also presents enhanced prospects for
ADC innovation since these antibody ADCs exhibit heightened
internalization efficiency and improved tumor specificity (39). For instance, the utilization of
bispecific ADCs enables the targeting of distinct epitopes on a
single antigen, thereby enhancing both the antigen and antibody
binding affinity while facilitating internalization of ADCs
(94). Similarly, the development
of dual-payload ADCs incorporating diverse cytotoxic payloads
allows for the controlled delivery to cancer cells by manipulating
the drug ratio, resulting in improved efficacy and reduced drug
resistance (95).
Most ADC toxicities originate from off-target,
non-tumor toxicities that are caused by premature detachment of
ADCs in the systemic circulation and subsequent release of the
payload (96). The released payload
can infiltrate neighboring non-malignant cells and induce the
bystander killing effect through passive diffusion or
transporter-mediated uptake (97,98).
Certain ADCs that use microtubule inhibitors as the payload have
off-target toxicity associated with peripheral neuropathy. For
instance, the majority of patients with PCa (71%) who received
MLN2704, a microtubule inhibitor with M1 as the payload,
experienced peripheral neuropathy and were unable to proceed to the
next phase of clinical trials due to excessive toxicity (47). The exploration of novel compounds as
potential payloads for ADCs is imperative to mitigate off-target
toxicity and achieve comparable or superior efficacy in cancer cell
eradication.
The premature release of the payload in the systemic
circulation is primarily responsible for off-target toxicity, as
aforementioned. Therefore, maintaining stability in the systemic
circulation is crucial for linkers to minimize off-target toxicity
of ADCs (99). The ideal linker
should exhibit stability in the bloodstream and selectively release
cytotoxic payloads upon internalization of ADCs into cancer cells.
Nevertheless, existing linkers often demonstrate non-specific
payload release in circulation, inevitably resulting in off-target
toxicity (100). Among the ADCs
designed to treat PCa, hematological toxicities, including
neutropenia, thrombocytopenia, leukopenia and anemia, were observed
as the most prevalent AEs. The occurrence of hematotoxicity may be
attributed to the premature release of cytotoxic payloads into the
systemic circulation (101). The
current requirement is to further advance the development of novel
linkers to ensure the stability of ADCs within the bloodstream and
their targeted release of effective payloads upon internalization
into cancer cells. This will effectively minimize off-target
toxicity and enhance the overall efficacy of ADCs (37).
It has been suggested that combining ADCs with other
therapies possessing distinct mechanisms of action and minimal
overlapping toxic effects may be an effective approach for
mitigating AEs and enhancing efficacy (102). The utilization of ADCs in
conjunction with immune checkpoint inhibitors (ICIs) has emerged as
a promising therapeutic approach for patients with advanced PCa in
recent years (103). In several
studies, the combination of ADCs and ICIs has been demonstrated to
significantly augment efficacy (104,105). ICIs enhance the capacity of the
immune system to eliminate cancer cells (106), while ADCs selectively target and
eradicate cancer cells (107). The
combination of ADCs and ICIs can therefore augment the cytotoxicity
of the immune system against cancer cells, facilitate targeted
elimination of cancer cells and notably enhance clinical efficacy
(102). Personalized studies of
ADCs in combination with ICIs is currently undergoing clinical
trials, indicating its potential as a promising alternative
treatment for PCa in the future.
At present, ADCs are mainly used to treat patients
with mCRPC. Due to excessive AEs and insufficient efficacy, the
development of ADCs for PCa therapy is still at the phase I/II
clinical trial stage. However, several studies are ongoing and
researchers are also developing new ADCs and improving their
structure. It is conceivable that additional ADCs will receive
approval for the management of PCa in the foreseeable future.
Although most of the ongoing clinical trials focus on patients with
mCRPC, if ADCs show considerable efficacy in more clinical trials,
their potential application for the treatment of patients with
early-stage PCa is likely to materialize.
In conclusion, although there are numerous treatment
options for advanced PCa such as mCRPC, more treatment options are
currently needed. ADCs represent a new hope in the treatment of
PCa. A number of clinical trials investigating ADCs for the
treatment of PCa have been prematurely terminated due to inadequate
efficacy or excessive toxicity. Moving forward, it is imperative to
develop more stable linkers, highly specific antibodies and more
potent payloads to further enhance the pharmacological properties
of ADCs and expand the therapeutic options for patients with
PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82360603).
Availability of data and materials
Not applicable.
Authors' contributions
CY, CG and LW were the major contributors in writing
and editing the manuscript. HL, ZT and MD provided direction and
guidance throughout the preparation of this manuscript. DL, YH, JL,
WC and SF analyzed and organized the data. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calabrese M, Saporita I, Turco F,
Gillessen S, Castro E, Vogl UM, Di Stefano RF, Carfì FM, Poletto S,
Farinea G, et al: Synthetic lethality by co-inhibition of androgen
receptor and polyadenosine diphosphate-ribose in metastatic
prostate cancer. Int J Mol Sci. 25:782023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamada Y and Beltran H: The treatment
landscape of metastatic prostate cancer. Cancer Lett. 519:20–29.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong AJ, Szmulewitz RZ, Petrylak DP,
Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi
T, Shore ND, et al: ARCHES: A randomized, phase III study of
androgen deprivation therapy with enzalutamide or placebo in men
with metastatic hormone-sensitive prostate cancer. J Clin Oncol.
37:2974–2986. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi KN, Agarwal N, Bjartell A, Chung BH,
de Santana Gomes AJ, Given R, Soto ÁJ, Merseburger AS, Özgüroğlu M,
Uemura H, et al: Apalutamide for metastatic, castration-sensitive
prostate cancer. N Engl J Med. 381:13–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis ID, Martin AJ, Stockler MR, Begbie
S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath
LG, et al: Enzalutamide with standard first-line therapy in
metastatic prostate cancer. N Engl J Med. 381:121–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gillessen S, Armstrong A, Attard G, Beer
TM, Beltran H, Bjartell A, Bossi A, Briganti A, Bristow RG, Bulbul
M, et al: Management of patients with advanced prostate cancer:
Report from the advanced prostate cancer consensus conference 2021.
Eur Urol. 82:115–141. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verry C, Vincendeau S, Massetti M,
Blachier M, Vimont A, Bazil ML, Bernardini P, Pettré S and Timsit
MO: Pattern of clinical progression until metastatic
castration-resistant prostate cancer: An epidemiological study from
the European prostate cancer registry. Target Oncol. 17:441–451.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tilki D, van den Bergh RCN, Briers E, Van
den Broeck T, Brunckhorst O, Darraugh J, Eberli D, De Meerleer G,
De Santis M, Farolfi A, et al: EAU-EANM-ESTRO-ESUR-ISUP-SIOG
guidelines on prostate cancer. Part II-2024 update: Treatment of
relapsing and metastatic prostate cancer. Eur Urol. 86:164–182.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dumontet C, Reichert JM, Senter PD,
Lambert JM and Beck A: Antibody-drug conjugates come of age in
oncology. Nat Rev Drug Discov. 22:641–661. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drago JZ, Modi S and Chandarlapaty S:
Unlocking the potential of antibody-drug conjugates for cancer
therapy. Nat Rev Clin Oncol. 18:327–344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beck A, Goetsch L, Dumontet C and Corvaia
N: Strategies and challenges for the next generation of
antibody-drug conjugates. Nat Rev Drug Discov. 16:315–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trail PA, Dubowchik GM and Lowinger TB:
Antibody drug conjugates for treatment of breast cancer: Novel
targets and diverse approaches in ADC design. Pharmacol Ther.
181:126–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al:
Trastuzumab deruxtecan in previously treated HER2-low advanced
breast cancer. N Engl J Med. 387:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bardia A, Hurvitz SA, Tolaney SM, Loirat
D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak
AB, et al: Sacituzumab govitecan in metastatic triple-negative
breast cancer. N Engl J Med. 384:1529–1541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strebhardt K and Ullrich A: Paul Ehrlich's
magic bullet concept: 100 years of progress. Nat Rev Cancer.
8:473–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carter P: Improving the efficacy of
antibody-based cancer therapies. Nat Rev Cancer. 1:118–129. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schrama D, Reisfeld RA and Becker JC:
Antibody targeted drugs as cancer therapeutics. Nat Rev Drug
Discov. 5:147–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sievers EL: Efficacy and safety of
gemtuzumab ozogamicin in patients with CD33-positive acute myeloid
leukaemia in first relapse. Expert Opin Biol Ther. 1:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amiri-Kordestani L, Blumenthal GM, Xu QC,
Zhang L, Tang SW, Ha L, Weinberg WC, Chi B, Candau-Chacon R, Hughes
P, et al: FDA approval: Ado-trastuzumab emtansine for the treatment
of patients with HER2-positive metastatic breast cancer. Clin
Cancer Res. 20:4436–4441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghose A, Lapitan P, Apte V, Ghosh A,
Kandala A, Basu S, Parkes J, Shinde SD, Boussios S, Sharma A, et
al: Antibody drug conjugates in urological cancers: A review of the
current landscape. Curr Oncol Rep. 26:633–646. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang E, Weinstock C, Zhang L, Charlab R,
Dorff SE, Gong Y, Hsu V, Li F, Ricks TK, Song P, et al: FDA
approval summary: Enfortumab vedotin for locally advanced or
metastatic urothelial carcinoma. Clin Cancer Res. 27:922–927. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li K, Xie G, Deng X, Zhang Y, Jia Z and
Huang Z: Antibody-drug conjugates in urinary tumors: Clinical
application, challenge, and perspectives. Front Oncol.
13:12597842023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuchikama K and An Z: Antibody-drug
conjugates: Recent advances in conjugation and linker chemistries.
Protein Cell. 9:33–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giugliano F, Corti C, Tarantino P,
Michelini F and Curigliano G: Bystander effect of antibody-drug
conjugates: Fact or fiction? Curr Oncol Rep. 24:809–817. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khera E, Dong S, Huang H, de Bever L, van
Delft FL and Thurber GM: Cellular-Resolution imaging of bystander
payload tissue penetration from antibody-drug conjugates. Mol
Cancer Ther. 21:310–321. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Staudacher AH and Brown MP: Antibody drug
conjugates and bystander killing: Is antigen-dependent
internalisation required? Br J Cancer. 117:1736–1742. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mjaess G, Aoun F, Rassy E, Diamand R,
Albisinni S and Roumeguere T: Antibody-drug conjugates in prostate
cancer: Where are we? Clin Genitourin Cancer. 21:171–174. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trail PA, King HD and Dubowchik GM:
Monoclonal antibody drug immunoconjugates for targeted treatment of
cancer. Cancer Immunol Immunother. 52:328–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Cozzi PJ and Russell PJ: Promising
tumor-associated antigens for future prostate cancer therapy. Med
Res Rev. 30:67–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Y, Schladetsch MA, Huang X, Balunas MJ
and Wiemer AJ: Stepping forward in antibody-drug conjugate
development. Pharmacol Ther. 229:1079172022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Conilh L, Sadilkova L, Viricel W and
Dumontet C: Payload diversification: A key step in the development
of antibody-drug conjugates. J Hematol Oncol. 16:32023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su Z, Xiao D, Xie F, Liu L, Wang Y, Fan S,
Zhou X and Li S: Antibody-drug conjugates: Recent advances in
linker chemistry. Acta Pharm Sin B. 11:3889–3907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baah S, Laws M and Rahman KM:
Antibody-drug conjugates-a tutorial review. Molecules. 26:29432021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu Z, Li S, Han S, Shi C and Zhang Y:
Antibody drug conjugate: The ‘biological missile’ for targeted
cancer therapy. Signal Transduct Target Ther. 7:932022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Samantasinghar A, Sunildutt NP, Ahmed F,
Soomro AM, Salih ARC, Parihar P, Memon FH, Kim KH, Kang IS and Choi
KH: A comprehensive review of key factors affecting the efficacy of
antibody drug conjugate. Biomed Pharmacother. 161:1144082023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diamantis N and Banerji U: Antibody-drug
conjugates-an emerging class of cancer treatment. Br J Cancer.
114:362–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaur R, Kaur G, Gill RK, Soni R and
Bariwal J: Recent developments in tubulin polymerization
inhibitors: An overview. Eur J Med Chem. 87:89–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheung-Ong K, Giaever G and Nislow C:
DNA-damaging agents in cancer chemotherapy: Serendipity and
chemical biology. Chem Biol. 20:648–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ackerman SE, Pearson CI, Gregorio JD,
Gonzalez JC, Kenkel JA, Hartmann FJ, Luo A, Ho PY, LeBlanc H, Blum
LK, et al: Immune-stimulating antibody conjugates elicit robust
myeloid activation and durable antitumor immunity. Nat Cancer.
2:18–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahbar K, Afshar-Oromieh A, Jadvar H and
Ahmadzadehfar H: PSMA theranostics: Current status and future
directions. Mol Imaging. 17:15360121187760682018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun M, Niaz MJ, Niaz MO and Tagawa ST:
Prostate-Specific membrane antigen (PSMA)-targeted radionuclide
therapies for prostate cancer. Curr Oncol Rep. 23:592021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Milowsky MI, Galsky MD, Morris MJ, Crona
DJ, George DJ, Dreicer R, Tse K, Petruck J, Webb IJ, Bander NH, et
al: Phase 1/2 multiple ascending dose trial of the
prostate-specific membrane antigen-targeted antibody drug conjugate
MLN2704 in metastatic castration-resistant prostate cancer. Urol
Oncol. 34:530 e515–530 e521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Henry MD, Wen S, Silva MD, Chandra S,
Milton M and Worland PJ: A prostate-specific membrane
antigen-targeted monoclonal antibody-chemotherapeutic conjugate
designed for the treatment of prostate cancer. Cancer Res.
64:7995–8001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galsky MD, Eisenberger M, Moore-Cooper S,
Kelly WK, Slovin SF, DeLaCruz A, Lee Y, Webb IJ and Scher HI: Phase
I trial of the prostate-specific membrane antigen-directed
immunoconjugate MLN2704 in patients with progressive metastatic
castration-resistant prostate cancer. J Clin Oncol. 26:2147–2154.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma D, Hopf CE, Malewicz AD, Donovan GP,
Senter PD, Goeckeler WF, Maddon PJ and Olson WC: Potent antitumor
activity of an auristatin-conjugated, fully human monoclonal
antibody to prostate-specific membrane antigen. Clin Cancer Res.
12:2591–2596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Petrylak DP, Kantoff P, Vogelzang NJ, Mega
A, Fleming MT, Stephenson JJ Jr, Frank R, Shore ND, Dreicer R,
McClay EF, et al: Phase 1 study of PSMA ADC, an antibody-drug
conjugate targeting prostate-specific membrane antigen, in
chemotherapy-refractory prostate cancer. Prostate. 79:604–613.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Petrylak DP, Vogelzang NJ, Chatta K,
Fleming MT, Smith DC, Appleman LJ, Hussain A, Modiano M, Singh P,
Tagawa ST, et al: PSMA ADC monotherapy in patients with progressive
metastatic castration-resistant prostate cancer following
abiraterone and/or enzalutamide: Efficacy and safety in open-label
single-arm phase 2 study. Prostate. 80:99–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cho S, Zammarchi F, Williams DG, Havenith
CEG, Monks NR, Tyrer P, D'Hooge F, Fleming R, Vashisht K, Dimasi N,
et al: Antitumor activity of MEDI3726 (ADCT-401), a
pyrrolobenzodiazepine antibody-drug conjugate targeting PSMA, in
preclinical models of prostate cancer. Mol Cancer Ther.
17:2176–2186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Bono JS, Fleming MT, Wang JS, Cathomas
R, Miralles MS, Bothos J, Hinrichs MJ, Zhang Q, He P, Williams M,
et al: Phase I study of MEDI3726: A prostate-specific membrane
antigen-targeted antibody-drug conjugate, in patients with mCRPC
after failure of abiraterone or enzalutamide. Clin Cancer Res.
27:3602–3609. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen J, Pachynski R, Nordquist LT, Adra N,
Bilen MA, Aggarwal R, Reichert Z, Schweizer M, Iravani A, Aung S,
et al: 1804P APEX-01: First-in-human phase I/II study of ARX517 an
anti-prostate-specific membrane antigen (PSMA) antibody-drug
conjugate (ADC) in patients (pts) with metastatic
castration-resistant prostate cancer (mCRPC). Ann Oncol.
34:S974–S975. 2023. View Article : Google Scholar
|
|
56
|
Gomes IM, Maia CJ and Santos CR: STEAP
proteins: From structure to applications in cancer therapy. Mol
Cancer Res. 10:573–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rocha SM, Nascimento D, Coelho RS, Cardoso
AM, Passarinha LA, Socorro S and Maia CJ: STEAP1 knockdown
decreases the sensitivity of prostate cancer cells to paclitaxel,
docetaxel and cabazitaxel. Int J Mol Sci. 24:66432023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Danila DC, Szmulewitz RZ, Vaishampayan U,
Higano CS, Baron AD, Gilbert HN, Brunstein F, Milojic-Blair M, Wang
B, Kabbarah O, et al: Phase I study of DSTP3086S, an antibody-drug
conjugate targeting six-transmembrane epithelial antigen of
prostate 1, in metastatic castration-resistant prostate cancer. J
Clin Oncol. 37:3518–3527. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Doronina SO, Toki BE, Torgov MY,
Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee
TD, Siegall CB, et al: Development of potent monoclonal antibody
auristatin conjugates for cancer therapy. Nat Biotechnol.
21:778–784. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trerotola M, Ganguly KK, Fazli L, Fedele
C, Lu H, Dutta A, Liu Q, De Angelis T, Riddell LW, Riobo NA, et al:
Trop-2 is up-regulated in invasive prostate cancer and displaces
FAK from focal contacts. Oncotarget. 6:14318–14328. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sperger JM, Helzer KT, Stahlfeld CN, Jiang
D, Singh A, Kaufmann KR, Niles DJ, Heninger E, Rydzewski NR, Wang
L, et al: Expression and therapeutic targeting of TROP-2 in
treatment-resistant prostate cancer. Clin Cancer Res. 29:2324–2335.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Starodub AN, Ocean AJ, Shah MA, Guarino
MJ, Picozzi VJ Jr, Vahdat LT, Thomas SS, Govindan SV, Maliakal PP,
Wegener WA, et al: First-in-Human trial of a novel anti-trop-2
antibody-sn-38 conjugate, sacituzumab govitecan, for the treatment
of diverse metastatic solid tumors. Clin Cancer Res. 21:3870–3878.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lang J, Tagawa ST, Slovin S, Emamekhoo H,
Rathkopf D, Abida W, Autio K, Xiao H, Molina AM, Eickhoff J, et al:
1406P Interim results of a phase II trial of sacituzumab govitecan
(SG) in patients (Pts) with metastatic castration resistant
prostate cancer (mCRPC) progressing on androgen receptor signaling
inhibitors (ARSI). Ann Oncol. 33:S11882022. View Article : Google Scholar
|
|
64
|
Corti C, Antonarelli G, Valenza C, Nicolò
E, Rugo H, Cortés J, Harbeck N, Carey LA, Criscitiello C and
Curigliano G: Histology-agnostic approvals for antibody-drug
conjugates in solid tumours: Is the time ripe? Eur J Cancer.
171:25–42. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Elvington M, Liszewski MK and Atkinson JP:
CD46 and oncologic interactions: Friendly fire against cancer.
Antibodies (Basel). 9:592020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Su Y, Liu Y, Behrens CR, Bidlingmaier S,
Lee NK, Aggarwal R, Sherbenou DW, Burlingame AL, Hann BC, Simko JP,
et al: Targeting CD46 for both adenocarcinoma and neuroendocrine
prostate cancer. JCI Insight. 3:e1214972018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Aggarwal RR, Vuky J, VanderWeele DJ,
Rettig M, Heath EI, Beer TM, Huang J, Pawlowska N, Sinit R, Abbey
J, et al: Phase 1a/1b study of FOR46, an antibody drug conjugate
(ADC), targeting CD46 in metastatic castration-resistant prostate
cancer (mCRPC). J Clin Oncol. 40:3001. 2022. View Article : Google Scholar
|
|
68
|
Zang X and Allison JP: The B7 family and
cancer therapy: Costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bonk S, Tasdelen P, Kluth M, Hube-Magg C,
Makrypidi-Fraune G, Möller K, Höflmayer D, Rico SD, Büscheck F,
Minner S, et al: High B7-H3 expression is linked to increased risk
of prostate cancer progression. Pathol Int. 70:733–742. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mendes AA, Lu J, Kaur HB, Zheng SL, Xu J,
Hicks J, Weiner AB, Schaeffer EM, Ross AE, Balk SP, et al:
Association of B7-H3 expression with racial ancestry, immune cell
density, and androgen receptor activation in prostate cancer.
Cancer. 128:2269–2280. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guo C, Figueiredo I, Gurel B, Neeb A, Seed
G, Crespo M, Carreira S, Rekowski J, Buroni L, Welti J, et al:
B7-H3 as a therapeutic target in advanced prostate Cancer. Eur
Urol. 83:224–238. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Scribner JA, Brown JG, Son T, Chiechi M,
Li P, Sharma S, Li H, De Costa A, Li Y, Chen Y, et al: Preclinical
development of MGC018, a duocarmycin-based antibody-drug conjugate
targeting B7-H3 for solid cancer. Mol Cancer Ther. 19:2235–2244.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shenderov E, Mallesara GHG, Wysocki PJ, Xu
W, Ramlau R, Weickhardt AJ, Zolnierek J, Spira A, Joshua AM,
Powderly J, et al: 620P MGC018, an anti-B7-H3 antibody-drug
conjugate (ADC), in patients with advanced solid tumors:
Preliminary results of phase I cohort expansion. Ann Oncol.
32:S657–S659. 2021. View Article : Google Scholar
|
|
74
|
Belluomini L, Sposito M, Avancini A,
Insolda J, Milella M, Rossi A and Pilotto S: Unlocking new horizons
in small-cell lung cancer treatment: The onset of antibody-drug
conjugates. Cancers (Basel). 15:53682023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Breij EC, de Goeij BE, Verploegen S,
Schuurhuis DH, Amirkhosravi A, Francis J, Miller VB, Houtkamp M,
Bleeker WK, Satijn D and Parren PW: An antibody-drug conjugate that
targets tissue factor exhibits potent therapeutic activity against
a broad range of solid tumors. Cancer Res. 74:1214–1226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chu AJ: Tissue factor, blood coagulation,
and beyond: An overview. Int J Inflam. 2011:3672842011.PubMed/NCBI
|
|
77
|
Versteeg HH: Tissue factor: Old and new
links with cancer biology. Semin Thromb Hemost. 41:747–755. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Markham A: Tisotumab vedotin: First
approval. Drugs. 81:2141–2147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
de Bono JS, Concin N, Hong DS,
Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH,
Nielsen D, Windfeld K, et al: Tisotumab vedotin in patients with
advanced or metastatic solid tumours (InnovaTV 201): A
first-in-human, multicentre, phase 1–2 trial. Lancet Oncol.
20:383–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Corti C, Bielo LB, Schianca AC, Salimbeni
BT, Criscitiello C and Curigliano G: Future potential targets of
antibody-drug conjugates in breast cancer. Breast. 69:312–322.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Saunders LR, Bankovich AJ, Anderson WC,
Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang
A, et al: A DLL3-targeted antibody-drug conjugate eradicates
high-grade pulmonary neuroendocrine tumor-initiating cells in vivo.
Sci Transl Med. 7:302ra1362015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Puca L, Gavyert K, Sailer V, Conteduca V,
Dardenne E, Sigouros M, Isse K, Kearney M, Vosoughi A, Fernandez L,
et al: Delta-like protein 3 expression and therapeutic targeting in
neuroendocrine prostate cancer. Sci Transl Med. 11:eaav08912019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rudin CM, Pietanza MC, Bauer TM, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA III,
Robert F, et al: Rovalpituzumab tesirine, a DLL3-targeted
antibody-drug conjugate, in recurrent small-cell lung cancer: A
first-in-human, first-in-class, open-label, phase 1 study. Lancet
Oncol. 18:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mansfield AS, Hong DS, Hann CL, Farago AF,
Beltran H, Waqar SN, Hendifar AE, Anthony LB, Taylor MH, Bryce AH,
et al: A phase I/II study of rovalpituzumab tesirine in delta-like
3-expressing advanced solid tumors. NPJ Precis Oncol. 5:742021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fuentes-Antras J, Genta S, Vijenthira A
and Siu LL: Antibody-drug conjugates: In search of partners of
choice. Trends Cancer. 9:339–354. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lohiya V, Aragon-Ching JB and Sonpavde G:
Role of chemotherapy and mechanisms of resistance to chemotherapy
in metastatic castration-resistant prostate cancer. Clin Med
Insights Oncol. 10:57–66. 2016.PubMed/NCBI
|
|
88
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
de Goeij BE, Satijn D, Freitag CM,
Wubbolts R, Bleeker WK, Khasanov A, Zhu T, Chen G, Miao D, van
Berkel PH and Parren PW: High turnover of tissue factor enables
efficient intracellular delivery of antibody-drug conjugates. Mol
Cancer Ther. 14:1130–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Thurber GM, Schmidt MM and Wittrup KD:
Antibody tumor penetration: Transport opposed by systemic and
antigen-mediated clearance. Adv Drug Deliv Rev. 60:1421–1434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ruan DY, Wu HX, Meng Q and Xu RH:
Development of antibody-drug conjugates in cancer: Overview and
prospects. Cancer Commun (Lond). 44:3–22. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Autio KA, Boni V, Humphrey RW and Naing A:
Probody therapeutics: An emerging class of therapies designed to
enhance on-target effects with reduced off-tumor toxicity for use
in immuno-oncology. Clin Cancer Res. 26:984–989. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Andreev J, Thambi N, Bay AE, Delfino F,
Martin J, Kelly MP, Kirshner JR, Rafique A, Kunz A, Nittoli T, et
al: Bispecific antibodies and antibody-drug Conjugates (ADCs)
bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs.
Mol Cancer Ther. 16:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tang F, Yang Y, Tang Y, Tang S, Yang L,
Sun B, Jiang B, Dong J, Liu H, Huang M, et al: One-pot
N-glycosylation remodeling of IgG with non-natural
sialylglycopeptides enables glycosite-specific and dual-payload
antibody-drug conjugates. Org Biomol Chem. 14:9501–9518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Colombo R and Rich JR: The therapeutic
window of antibody drug conjugates: A dogma in need of revision.
Cancer Cell. 40:1255–1263. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tarantino P, Ricciuti B, Pradhan SM and
Tolaney SM: Optimizing the safety of antibody-drug conjugates for
patients with solid tumours. Nat Rev Clin Oncol. 20:558–576. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhu Y, Liu K, Wang K and Zhu H:
Treatment-related adverse events of antibody-drug conjugates in
clinical trials: A systematic review and meta-analysis. Cancer.
129:283–295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Donaghy H: Effects of antibody, drug and
linker on the preclinical and clinical toxicities of antibody-drug
conjugates. MAbs. 8:659–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tumey LN and Han S: ADME considerations
for the development of biopharmaceutical conjugates using cleavable
linkers. Curr Top Med Chem. 17:3444–3462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mahalingaiah PK, Ciurlionis R, Durbin KR,
Yeager RL, Philip BK, Bawa B, Mantena SR, Enright BP, Liguori MJ
and Van Vleet TR: Potential mechanisms of target-independent uptake
and toxicity of antibody-drug conjugates. Pharmacol Ther.
200:110–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen YF, Xu YY, Shao ZM and Yu KD:
Resistance to antibody-drug conjugates in breast cancer: Mechanisms
and solutions. Cancer Commun (Lond). 43:297–337. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Piombino C, Tonni E, Oltrecolli M, Pirola
M, Pipitone S, Baldessari C, Dominici M, Sabbatini R and Vitale MG:
Immunotherapy in urothelial cancer: Current status and future
directions. Expert Rev Anticancer Ther. 23:1141–1155. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
O'Malley DM, Matulonis UA, Birrer MJ,
Castro CM, Gilbert L, Vergote I, Martin LP, Mantia-Smaldone GM,
Martin AG, Bratos R, et al: Phase Ib study of mirvetuximab
soravtansine, a folate receptor alpha (FRalpha)-targeting
antibody-drug conjugate (ADC), in combination with bevacizumab in
patients with platinum-resistant ovarian cancer. Gynecol Oncol.
157:379–385. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Uliano J, Nicolo E, Corvaja C, Salimbeni
BT, Trapani D and Curigliano G: Combination immunotherapy
strategies for triple-negative breast cancer: Current progress and
barriers within the pharmacological landscape. Expert Rev Clin
Pharmacol. 15:1399–1413. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Thana M and Wood L: Immune checkpoint
inhibitors in genitourinary malignancies. Curr Oncol. 27:S69–S77.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bakhtiar R: Antibody drug conjugates.
Biotechnol Lett. 38:1655–1664. 2016. View Article : Google Scholar : PubMed/NCBI
|