Introduction
The 2020 Spring Festival was unusual for China, and
a large-scale novel coronavirus pneumonia outbreak occurred in
Wuhan, Hubei, a city in South Central China on the Yangtze River
(1,2). This novel coronavirus was named
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and
the associated pneumonia was designated as COVID-19 by the World
Health Organization (WHO). Since the first 4 cases were reported on
December 29, 2019, the virus rapidly spread throughout China and
numerous countries worldwide (3,4).
SARS-CoV-2 is a β coronavirus (βCoV) that is usually
polymorphous with a diameter varying from approximately 60 to 140
nm. The genome has a 89% nucleotide identity to bat
SARS-like-CoVZXC21 and a 82% identity to human SARS-CoV (5). The virus spreads from individual to
individual by close contact and via respiratory droplets. Based on
the current epidemiological surveys, the incubation period is 1-14
days, mostly 3-7 days. For confirmed SARS-CoV-2 infections, the
reported illnesses range from asymptomatic or mild to severe and
even fatal. Symptoms include fever, fatigue and cough. The
progression of the illness in some patients is rapid, and they
exhibit shortness of breath, hypoxemia, acute respiratory distress
syndrome (ARDS), metabolic acidosis, renal failure and coagulation
dysfunction (6,7).
Guangdong Province was also threatened by the
SARS-CoV-2 infection. Geographically, Chaozhou is an Eastern city
in Guangdong and is >1,000 km away from Wuhan. In the present
study, the first cluster of SARS-CoV-2 infection was investigated
in a family derived from Wuhan.
Case reports
The study subjects were a family of 5 members
(husband, 36 years old; wife, 31 years old; mother-in-law, 53 years
old; son and daughter, aged 8 years and 2 months, respectively) who
visited relatives in Chaozhou by driving in a car from Wuhan on
January 25, 2020 and arrived in Chaozhou on the same day, and then
checked into a hotel. None of the family members had a contact
history with the South China Seafood Whole Sale Market or animals
in Wuhan. In total, 3 family members (husband, mother-in-law and
wife) visited the outpatient clinic at hospital due to respiratory
symptoms on January 29. The husband, wife and mother-in-law were
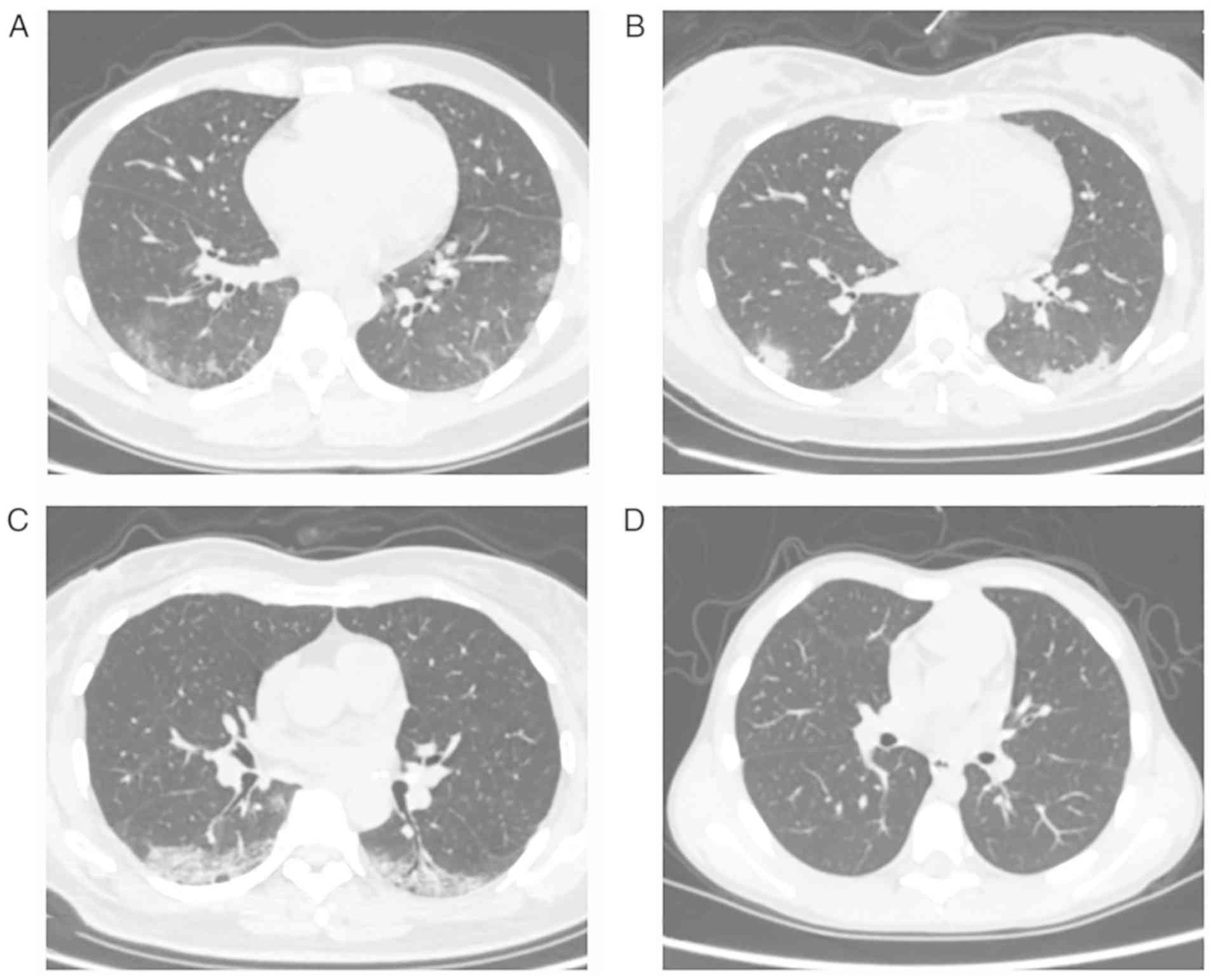
identified with multifocal patchy ground-glass opacity by a chest
CT scan (Fig. 1). Throat swab
specimens from this family were obtained and sent for the detection
of SARS-CoV-2 by reverse transcription-polymerase chain reaction
(RT-PCR) assays; the 3 adults and the boy were found to be
positive. The female infant was negative for SARS-CoV-2 on the same
day, and was subsequently diagnosed with SARS-CoV-2 infection 4
days later. They were admitted to Chaozhou Central Hospital for
isolation and treatment.
The present study was approved by the Institutional
Review Board of Chaozhou Central Hospital. The study-related
information was acquired following consultation with the patients
or their guardians.
Upon admission, the 3 adults had fever accompanied
by a cough and sore throat, whereas the 2 children only exhibited a
mild dry cough. The husband also experienced expectoration,
transient chest pain and general fatigue symptoms. The erythrocyte
sedimentation rate (ESR) and serum glucose levels were increased in
the 3 adults. The wife was currently in the lactation period; her
major clinical manifestation was recurrent and she sustained fever
until the 7th day after admission, and her highest recorded
temperature was 39.4˚C. In addition, 2 RT-PCR tests for SARS-CoV-2
performed on her breast milk were negative.
The laboratory results of the mother-in-law also
displayed elevated levels of creatine kinase and C-reactive
protein; she was not known to suffer from any underlying diseases.
As her oxygen saturation fell to 90%, she was transferred to the
intensive care unit (ICU) and administered non-invasive mechanical
ventilation. On the 18th day following her admission, her oxygen
saturation values improved to 96%, and subsequently, she recovered
and was discharged with a better prognosis.
The infant was breastfeeding before her mother was
diagnosed with SARS-CoV-2 infection. To date, she is the youngest
COVID-19 patient in Guangdong, and her infection source was
possibly the close contact she had with her family members.
The clinical features and laboratory data of these 5
patients with COVID-19 are summarized in Tables I and II. According to the SARS-CoV-2
guidelines (Trial Version 6) (7),
the mother-in-law was categorized as having the severe type, the
husband and the wife had the common type, and the son and daughter
had the mild type of infection. The elder sister of the husband,
who had close contact with this family and 33 other common
contacts, was placed into quarantine and isolated for medical
surveillance. To date, no SARS-CoV-2 infection has been detected in
these contacts.
 | Table IThe physical examination and clinical
symptom data for the 1st day of admission. |
Table I
The physical examination and clinical
symptom data for the 1st day of admission.
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|
| Sex | Male | Female | Female | Male | Female |
| Age | 36 years | 31 years | 53 years | 8 years | 2 months |
| Body temperature
(˚C) | 37.2 | 37.5 | 37.7 | 36.4 | 36.8 |
| Pulse (per min) | 84 | 84 | 92 | 94 | 140 |
| Respiratory rate (per
min) | 18 | 17 | 20 | 22 | 30 |
| Blood pressure
(mmHg) | 132/80 | 120/85 | 132/84 | 100/70 | - |
| Blood oxygen
saturation (%) | 99 | 99 | 99 | 97 | 99 |
| Fever | + | + | + | - | - |
| Cough | + | + | + | + | + |
| Sore throat | + | + | + | - | NA |
| Expectoration | + | - | - | - | - |
| Chest pain | + | - | - | - | - |
| General fatigue | + | - | - | - | NA |
 | Table IISummary of the laboratory results of
the family cluster infected with SARS-CoV-2. |
Table II
Summary of the laboratory results of
the family cluster infected with SARS-CoV-2.
| Characteristic | Reference range | Husband | Wife | Mother-in-law | Boy | Girl |
|---|
| Sex | - | Male | Female | Female | Male | Female |
| Age | - | 36 years | 31 years | 53 years | 8 years | 2 months |
| RT-PCR | | | | | | |
|
ORF1ab | - | +(Ct 36.6) | +(Ct 26.8) | +(Ct 33.6) | +(Ct 36.1) | +(Ct 36.9) |
|
N gene | - | +(Ct 38.0) | +(Ct 29.3) | +(Ct 35.2) | +(Ct 37.0) | +(Ct 37.3) |
| White blood cell
count (109/l) | 3.5-9.5 | 3.69 | 6.73 | 6.26 | 6.03 | - |
| Red blood cell
count (1012/l) |
3.8-5.1a/4.3-5.8b | 4.82 | 4.4 | 4.34 | 4.41 | - |
| Absolute neutrophil
count (109/l) | 1.8-6.3 | 2.21 | 4.72 | 4.76 | 3.44 | - |
| Absolute lymphocyte
count (109/l) | 1.1-3.2 | 1.17 | 1.51 | 1.07 | 1.98 | - |
| Hematocrit |
0.35-0.45a/0.40-0.50b | 0.421 | 0.424 | 0.407 | 0.379 | - |
| Hemoglobin
(g/l) |
115-150a/130-175b | 145 | 141 | 139 | 131 | - |
| Platelet count
(109/l) | 125-350 | 144 | 274 | 145 | 312 | - |
| Calcium
(mmol/l) | 2.08-2.60 | 2.2 | 2.37 | 2.18 | 2.26 | - |
| Sodium
(mmol/l) | 137-147 | 136.4 | 136.4 | 139.2 | 139 | - |
| Potassium
(mmol/l) | 3.5-5.3 | 3.62 | 4.21 | 3.56 | 3.7 | - |
| Chloride
(mmol/l) | 99-110 | 101.8 | 98.2 | 101.7 | 99.2 | - |
| Total carbon
dioxide (mmol/l) | 22-29 | 23.7 | 23.8 | 23.5 | 29.5 | - |
| Glucose
(mmol/l) | 3.90-6.10 | 6.62 | 6.37 | 7.78 | 6 | - |
| Blood urea nitrogen
(mmol/l) | 1.8-8.2 | 2.68 | 2.82 | 2.7 | 3.24 | - |
| Creatinine
(µmol/l) | 41-115a/53-115b | 73 | 68 | 73 | 47 | - |
| Total protein
(g/l) | 65-85 | 74.2 | 86.6 | 78.4 | 79.6 | - |
| Albumin (g/l) | 40-55 | 46 | 48.1 | 44.8 | 46.9 | - |
| Total bilirubin
(µmol/l) | 3.4-26.0 | 7.52 | 3.28 | 4.06 | 4.99 | - |
| Procalcitonin
(ng/ml) | <0.1 | 0.05 | 0.05 | 0.05 | 0.18 | - |
| Alanine
aminotransferase (U/l) | 7-40a/9-50b | 22 | 50 | 18 | 20 | - |
| Aspartate
aminotransferase (U/l) | 13-35a/15-40b | 21 | 42 | 25 | 25 | - |
| Alkaline
phosphatase (U/l) | 50-135a/45-125b | 66 | 162 | 72 | 242c | - |
| Lactate
dehydrogenase (U/l) | 0-247a/0-248b | 184 | 259 | 213 | 217 | - |
| Creatine kinase
(U/l) | 26-174 | 154 | 82 | 205 | 78 | - |
| Creatine kinase
isoenzyme (U/l) | 0-24 | 10.8 | 14.6 | 11.4 | 12.5 | - |
| Myoglobin
(µg/l) | 0-106a/0-154.9b | 30.7 | 16.4 | 30.8 | - | - |
| High sensitive
cardiac troponin-I (µg/l) |
0-0.0156a/0-0.0342b | 0.001 | 0.001 | 0.001 | - | - |
| C-reactive protein
(mg/l) | 0-8.0 | 6.7 | 7.3 | 18.3 | 4.42 | - |
| Fibrinogen
(g/l) | 2-4 | 2.95 | 2.91 | 2.95 | - | - |
| Prothrombin time
(sec) | 9.00-12.8 | 11.8 | 12 | 11.5 | - | - |
| International
normalized ratio | - | 1.07 | 1.09 | 1.04 | - | - |
| D-Dimer
(µg/ml) | <0.5 | 0.11 | 0.07 | 0.15 | - | - |
| Erythrocyte
sedimentation rate (mm/h) | 0-20a/0-15b | 35 | 24 | 32 | - | - |
The 3 adults were administered a combination therapy
of antivirals and antibiotics successively, including Oseltamivir,
kaletra, recombinant human interferon α-2b, thymalfasin and human
immunoglobulin. They were also treated with methylprednisolone, and
no short-term adverse effects were identified. The 2 children
received general care. The medications of the patients are
presented in Table III.
 | Table IIIMedication profiles of the
patients. |
Table III
Medication profiles of the
patients.
| | January 31 -
February 5 | February 6-10 | February 11-15 | February 16-20 | February 21-25 |
|---|
| Family member and
medication | 31 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|
| Husband | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Oseltamivir
(75 mg/bid, p.o.) | x | x | x | x | x | x | x | | | | | | | | | | | | | | | | | | | |
|
Kaletra (500
mg/bid, p.o.) | | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | | | | | | | |
|
Recombinant
human interferon α-2b (5 million IU/bid, uf.) | | | | x | x | x | x | x | x | x | x | x | x | x | x | x | | | | | | | | | | |
|
Human
immunoglobulin (5 g/bid, i.v.gtt.) | | | | | | x | x | x | x | x | x | x | x | x | x | | | | | | | | | | | |
|
Thymalfasin
(1.6 mg/qd, i.h.) | | | | | | | x | x | x | x | x | x | x | x | x | | | | | | | | | | | |
|
Methylprednisolone
(60 mg/qd, i.v.gtt.) | | | | | | x | x | x | x | | | | | | | | | | | | | | | | | |
| Wife | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Oseltamivir
(75 mg/bid, p.o.) | x | x | x | x | x | x | x | | | | | | | | | | | | | | | | | | | |
|
Kaletra (500
mg/bid, p.o.) | | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | | | | | | | |
|
Recombinant
Human Interferon α-2b (5 million IU/bid, uf.) | | | | x | x | x | x | x | x | x | x | x | x | x | x | x | | | | | | | | | | |
|
Human
immunoglobulin (5 g/bid, i.v.gtt.) | | | | | | x | x | x | x | x | x | x | x | x | x | | | | | | | | | | | |
|
Thymalfasin
(1.6 mg/qd, i.h.) | | | | | | | x | x | x | x | x | x | x | x | x | | | | | | | | | | | |
|
Methylprednisolone
(60 mg/qd, i.v.gtt.) | | | | | | x | x | x | x | | | | | | | | | | | | | | | | | |
|
Chloroquine
(250 mg/bid, p.o.) | | | | | | | | | | | | | | | x | x | x | | | | | | | | | |
| Mother-in-law | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Kaletra (500
mg/bid, p.o.) | | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | | | | | | |
|
Recombinant
Human Interferon α-2b (5 million IU/bid, uf.) | | | | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | | | | | |
|
Human
immunoglobulin (5 g/bid, i.v.gtt.) | | | | | | x | x | x | x | x | x | x | x | | | | | | | | | | | | | |
|
Thymalfasin
(1.6 mg/qd, i.h.) | | | | | | | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
|
Methylprednisolone
(60 mg/qd, i.v.gtt.) | | | | | | | x | x | x | x | x | x | x | | | | | | | | | | | | | |
|
Chloroquine
(250 mg/bid, p.o.) | | | | | | | | | | | | | x | x | | | | | | | | | | | | |
|
Human
albumin (10 g/qd, i.v.gtt.) | | | | | | | | | | | x | x | x | x | x | x | | | | | | | | | | |
|
Methylprednisolone
(60 mg/qd, i.v.gtt.) | | | | | | | x | x | x | x | x | x | x | | | | | | | | | | | | | |
Following 2 successive negative results (at
intervals of at least 24 h) for SARS-CoV-2 RNA by throat and anal
swabs, CT imaging showing that the virus infection was no longer
present, and normal temperatures for at least 3 days, they were
discharged from the hospital and returned to their residence for
prolonged isolation under medical surveillance. However, the boy
and the infant tested positive for SARS-CoV-2 by anal swabs on the
4th day following discharge, although their throat swabs were
negative. The son reappeared with a mild fever of 37.6-38˚C,
although no abnormal findings were found in the chest CT and
routine blood tests. They continued to test positive for SARS-CoV-2
RNA by anal swabs for half a month. The results of RT-PCR assays
for the SARS-CoV-2 are presented in Table IV.
 | Table IVResults of RT-PCR testing for the
SARS-CoV-2. |
Table IV
Results of RT-PCR testing for the
SARS-CoV-2.
| | Husband | Wife | Mother-in-law | Boy | Girl |
|---|
| Date | Throat | Anal | Throat | Anal | Throat | Anal | Throat | Anal | Throat | Anal |
|---|
| January 30 | + | | + | | + | NT | - | NT | NT | NT |
| January 31 | NT | NT | NT | NT | NT | NT | + | NT | - | NT |
| February 2 | NT | NT | NT | NT | NT | NT | NT | NT | + | NT |
| February 7 | NT | NT | NT | NT | NT | NT | - | NT | NT | NT |
| February 11 | NT | NT | NT | NT | NT | NT | - | NT | NT | NT |
| February 12 | NT | NT | NT | NT | NT | NT | - | NT | NT | NT |
| February 13 | - | NT | ± | NT | NT | NT | - | NT | NT | NT |
| February 14 | | NT | NT | NT | NT | NT | NT | NT | - | + |
| February 15 | - | NT | - | NT | NT | NT | Discharge | NT | NT |
| February 17 | - | - | - | - | NT | NT | NT | NT | Discharge |
| February 18 | Discharge | Discharge | NT | NT | NT | NT | NT | NT |
| February 19 | NT | NT | NT | NT | - | - | NT | NT | NT | NT |
| February 20 | NT | NT | NT | NT | NT | NT | - | + | NT | NT |
| February 21 | - | - | - | - | - | - | - | + | NT | + |
| February 22 | NT | NT | NT | NT | NT | NT | NT | NT | NT | + |
| February 23 | NT | NT | NT | NT | - | - | - | + | NT | NT |
| February 24 | - | - | - | - | NT | NT | NT | NT | NT | NT |
| February 25 | NT | NT | NT | NT | NT | NT | - | - | NT | NT |
| February 26 | NT | NT | NT | NT | - | - | - | - | NT | NT |
| February 28 | NT | NT | NT | NT | Discharge | - | - | NT | NT |
| March 1 | - | - | - | - | - | - | - | + | - | + |
Discussion
Beginning in December, 2019, multiple cases of
pneumonia caused by an unknown pathogen associated with a market
selling seafood and wild animals at Wuhan were reported. The
pathogen was found to be a novel coronavirus (SARS-CoV-2) (6,8).
Cases have now spread throughout China, including Guangdong
Province. The first case in Guangdong was reported from Shenzhen on
January 19, and a 66-year-old male was confirmed with COVID-19
after visiting Wuhan (9).
The present study reports the first SARS-CoV-2
confirmed family cluster in Chaozhou, Eeastern Guangdong Province.
The family originally resided in Wuhan, China, but claimed that
they had not visited the seafood wholesale market, or any medical
institutions or had any contact with any fever patients during
their stay in Wuhan. Although the origin of this SARS-CoV-2
infection is unknown, the evidence for human-to-human transmission
is clear (6-8,10).
To date, no further cases of SARS-CoV-2 related to this family have
been reported.
Among the 5 cases in this family, the 8-year-old boy
and the 2-month-old girl exhibited only a slight cough, with no
fever or pneumonia. The 3 adults exhibited apparent pneumonia. It
seemed that the younger patient was less likely to be infected and
had mild symptoms of subclinical syndrome. COVID-19 cases in
children have rarely been reported to date. A previous study from
Wuhan described 9 infants infected with SARS-CoV-2, none of whom
required intensive care or mechanical ventilation or had any severe
complications (11). In that
study, 34 cases of children were categorized into 22 (65%) common,
9 (26%) mild and 3 (8.8%) asymptomatic cases in a study from
Shenzhen (11). No severe or
critical cases were identified. The clinical manifestations in
children with SARS-CoV-2 infection are non-specific and are milder
than those in adults (12). To
date, the relatively low infection rate in children may be
explained by the following issues: i) Children are not prone to
SARS-CoV-2 infection, and only a few children are infected when
they encounter SARS-CoV-2; ii) most children with SARS-CoV-2
infection exhibit the mild syndrome or cryptic infection as in the
cases in the present study, and they cannot easily be identified by
health care providers or public health authorities.
The reasons why children would be less severely
affected by SARS-CoV-2 are unclear. One possible reason is related
to their prior exposure to other respiratory viruses, such as
influenza, rendering their immune systems more resilient (13). Another hypothesis that has been
proposed is that young children are not capable of mobilizing a
peak mature immune response, as commonly observed in adults during
the immune dysregulation phase; thus, less organ damage ensues,
with its associated morbidity and mortality (13,14).
Overall, the genome of SARS-CoV-2 has 82% nucleotide
identity with that of human severe acute respiratory syndrome
(SARS)-associated coronavirus (SARS-CoV), which was the causal
agent of the SARS outbreaks in 2003 in Guangdong Province and
Hongkong (5). These 2 types of
coronavirus belong to the same β coronavirus genus (5). Both SARS-CoV (lineage B βCoV) and
SARS-CoV-2 cause severe lower respiratory tract infection and
extrapulmonary manifestations, such as diarrhea, lymphopenia, liver
dysfunction and multiorgan dysfunction. Based on a retrospective
analysis, <10% of SARS-CoV infections occurred in children.
Among the affected children, only 5% required admission to an
intensive care unit, and <1% required mechanical ventilation. No
deaths were reported among the children affected by SARS-CoV
(13). In contrast to its adult
counterpart, the clinical course of affected children is usually
milder, and the time to resolution is shorter. The similarity of
these 2 viruses led us to believe that SARS-CoV-2 is also a
relatively mild disease among children.
The 2 children of the family presented herein were
confirmed to have SARS-CoV-2 infection as they were from Wuhan and
were continually monitored after they arrived at Chaozhou. If they
had not reported their journey, the mildness of their syndromes may
have made them unnoticeable to public health authorities; they
would become SARS-CoV-2 carriers and would have transmitted the
virus to those who came into close contact with them. These cryptic
cases, or walking carriers, may serve as possible infection sources
to propagate the outbreak (7).
Therefore, attention should be paid to asymptomatic cases, which
may play a critical role in the transmission process. It is still
crucial to isolate patients and to trace and quarantine suspected
subjects as early as possible; suspected cases should be isolated
in a single room following doctors' advice, and self-isolation at
home should not be encouraged due to the danger of cluster
infection (7). Further studies on
the epidemiological significance of these asymptomatic cases are
warranted.
The 5 members of the family presented herein were
all infected with SARS-CoV-2, which indicated the potent
transmission capacity of this new virus (15). This virus is a new type of
coronavirus; thus, individuals generally lack immunity to this
virus and are susceptible to infection. A close relative
(sister-in-law) who had taken care of the infant was not infected
in the present study, as confirmed by 2 RT-PCR measurements. The
explanation for this was either that the quantity of the virus from
the infant was not sufficient or that the immune function of the
exposed relative was strong enough to resist infection.
There was a lactating mother in the present study.
Her milk was collected and tested for SARS-CoV-2 infection, and no
viral RNA was detected in the milk by RT-PCR. Either the low level
of the virus could not reach our detection limit (1,000 copies/ml),
or no virus was present in the milk. Further studies are required
to determine whether SARS-CoV-2 is secreted in the milk of infected
mothers and, if so, to determine whether consuming breast milk is
associated with SARS-CoV-2 infection in infants. In the family of
the present study, the infant lived with her mother and was
breast-fed, and the most likely transmission method for the infant
was close contact within the household. The mother was persuaded to
terminate breast feeding after her SARS-CoV-2 infection was
confirmed, and if necessary, theoretically, the pasteurization of
the mother's milk could prevent SARS-CoV-2 transmission if the
infant needed her mother's milk. There were several cases of
pregnancy in the 2003 outbreak of SARS-CoV, and no report has
indicated the presence of the virus in the milk of infected mothers
(16).
The nucleic acids of SARS-CoV-2 were detected in
stool samples in an early study (17). Another study found that 8 of the 11
patients who were positive for the virus by anal swab had a severe
clinical manifestation (18). The
2 children of the present study were detected with SARS-CoV-2 by
anal swabs after they were released from the hospital, although
they had no diarrhea. This suggested 2 possibilities: One
explanation was that the detected targets were decomposed fragments
of dead SARS-CoV-2, which could not cause disease; the other
explanation was that the virus could proliferate in the digestive
tract, was alive in the anal swab, and had transmission potential
in humans (19). The second
explanation appeared more substantial as dead virus RNA could not
persist for a long time after the viruses degraded. This issue
needs to be clarified further.
Despite the worsening trends of COVID-19, no drugs
have been proven to have significant efficacy in the clinical
treatment of patients with COVID-19. Remdesivir was considered the
most promising antiviral agent for COVID-19; it functions by
inhibiting the activity of RNA-dependent RNA polymerase. In a
recent study of adult patients with severe COVID-19, remdesivir was
not associated with any significant clinical benefits. However, it
shortened the time for clinical improvement (20). The protease inhibitor
lopinavir/ritonavir (LPV/RTV) alone is not shown to provide better
antiviral efficacy than standard care (20). However, the regimen of LPV/RTV plus
ribavirin was shown to be effective against SARS-CoV in
vitro. The regimen of LPV/RTV was applied for the 3 adult
patients in the present study, and better results were obtained;
however, the limited number of cases in the present study was not
sufficient to reach a conclusion; thus, the measurement of the
efficacy requires ongoing randomized, placebo-controlled
trials.
Cluster infection is common in SARS-CoV-2 (11,12);
the gathering of individuals was the main form of transmission.
Therefore, vigilant control is warranted at this early stage of the
pandemic. At the time of the writing of this manuscript,
authorities have closed public transit and have canceled outbound
transportation (train, air and long-distance buses) in several
cities of Hubei. Vehicular traffic in large cities, such as Wuhan,
Wenzhou, Hangzhou, Shanghai and Shenzhen was discouraged. China has
also imposed a ban on overseas travel with tour groups and has
suspended the sale of flight and hotel packages. These measures
were shown to be successful as the number of newly confirmed cases
has apparently decreased in China for the past 2 months (21).
Acknowledgements
Not applicable.
Funding
The present study was funded by the Special Research
Plan SARS-CoV-2 of Chaozhou (2020xg01) and the Natural Science
Foundation of Guangdong Province (2016A030307035) to LYY.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
LYY and FL had roles in the study design, data
collection, and writing of the manuscript. WJD, WZC and JYC were
involved in clinical management. YHW contributed to writing of the
report. PBW, SHT, JZW, SQL and DFL provided and interpreted the
data. ZML, JSC, JFL, JWH, JPG and PFX contributed to the data
analysis. All authors reviewed and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Chaozhou Central Hospital. The study-related
information was acquired following consultation with the patients
or their guardians.
Patient consent for publication
Written consent forms were obtained from all
patients, and informed consents were signed or thumb printed by the
participants or their guardians.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
World Health Organization. Pneumonia of
unknown cause-China. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.
Accessed January 5, 2020.
|
|
2
|
WHO. Novel coronavirus-China. January 12,
2020. http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/.
Accessed January 19, 2020.
|
|
3
|
Tan WJ, Zhao X and Ma XJ: A novel
coronavirus genome identified in a cluster of pneumonia cases:
Wuhan, China 2019-2020. China CDC Weekly. 2:61–62. 2020.
|
|
4
|
Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS,
Myoung J, Kim BT and Kim SJ: Current status of epidemiology,
diagnosis, therapeutics, and vaccines for novel coronavirus disease
2019 (COVID-19). J Microbiol Biotechnol. 30:313–324.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan
S and Yuen KY: Genomic characterization of the 2019 novel
human-pathogenic coronavirus isolated from a patient with atypical
pneumonia after visiting Wuhan. Emerg Microbes Infect. 9:221–236.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
China National Health Commission:
Diagnosis and treatment of pneumonitis caused by new coronavirus
(trial version 6). Beijing: China National Health Commission, 2020.
http://www.nhc.gov.cn/xcs/zhengcwj/202002/8334a8326dd94d329df351d7da8aefc2.shtm.
|
|
8
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
http://wsjkw.gd.gov.cn/xxgzbdfk/content/post_2880738.html.
|
|
10
|
Special Expert Group for Control of the
Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive
Medicine Association. An update on the epidemiological
characteristics of novel coronavirus pneumonia (COVID-19). Zhonghua
Liu Xing Bing Xue Za Zhi. 41:139–144. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
Wei M, Yuan J, Liu Y, Fu T, Yu X and Zhang
ZJ: Novel coronavirus infection in hospitalized infants under 1
year of age in China. JAMA. 323:1313–1314. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang XF, Yuan J, Zheng YJ, Chen J, Bao YM,
Wang YR, Wang LF, Li H, Zeng JX, Zhang YH, et al: Retracted:
Clinical and epidemiological characteristics of 34 children with
2019 novel coronavirus infection in Shenzhen. Zhonghua Er Ke Za
Zhi. 58(E008)2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
13
|
Li AM and Ng PC: Severe acute respiratory
syndrome (SARS) in neonates and children. Arch Dis Child Fetal
Neonatal Ed. 90:F461–F465. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hon KL, Leung CW, Cheng WT, Chan PK, Chu
WC, Kwan YW, Li AM, Fong NC, Ng PC, Chiu MC, et al: Clinical
presentations and outcome of severe acute respiratory syndrome in
children. Lancet. 361:1701–1703. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu JT, Leung K and Leung GM: Nowcasting
and forecasting the potential domestic and international spread of
the 2019-nCoV outbreak originating in Wuhan, China: A modelling
study. Lancet. 395:689–697. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shek CC, Ng PC, Fung GP, Cheng FW, Chan
PK, Peiris MJ, Lee KH, Wong SF, Cheung HM, Li AM, et al: Infants
born to mothers with severe acute respiratory syndrome. Pediatrics.
112(e254)2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First case of 2019 novel coronavirus in the United
States. N Engl J Med. 382:929–936. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai
X, Li L, He R, Tan Y, Deng X, et al: Detectable 2019-nCoV viral RNA
in blood is a strong indicator for the further clinical severity.
Emerg Microbes Infect. 9:469–473. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Chen C, Zhu S, Shu C, Wang D,
Song J, Song Y, Zhen W, Feng Z, Wu G, et al: Notes from the field:
Isolation of 2019-nCoV from a stool specimen of a
laboratory-confirmed case of the coronavirus disease 2019
(COVID-19). China CDC Weekly. 2:123–124. 2020.
|
|
20
|
Jean SS, Lee PI and Hsueh PR: Treatment
options for COVID-19: The reality and challenges. J Microbiol
Immunol Infect. 53:436–443. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
National Health Commission's briefing on
the pneumonia epidemic situation. Released on May 4, 2020.
http://www.nhc.gov.cn/xcs/yqfkdt/202005/9f8375d7ac9b4eda83515fcd5fd4adf4.shtml.
|