Introduction
Acquired severe aplastic anemia (SAA) is regarded as
a result of the immune-mediated destruction of hematopoietic cells
(1). Aplastic anemia (AA) may
co-exist with or appear to evolve to myelodysplastic syndrome (MDS)
(1). MDS is a clonal disorder
characterized by cytopenia arising from ineffective hematopoiesis
and is associated with an increased risk of developing acute
myeloid leukemia (AML) (2).
Crohn's disease (CD) is a chronic inflammatory disorder of the
gastrointestinal tract. CD is characterized by the presence of
transmural inflammation and endoscopic findings of longitudinal
ulcers or skip lesions.
The concurrent presence of MDS and CD has been
reported (3-6).
However, reports of patients treated with allogeneic hematopoietic
stem cell transplantation (allo-HSCT) for MDS in which CD improved
are rare, particularly in Japan. The present study describes the
case of a 56-year-old male with CD who achieved complete remission
(CR) following allogeneic bone marrow transplantation (allo-BMT)
for high-risk MDS.
Case report
A 56-year-old male was admitted to Gifu Municipal
Hospital due to pancytopenia. Laboratory data upon admission were
as follows: White blood cell count, 3.1x109/l with 51.4%
neutrophils, 40.6% lymphocytes, 3.0% eosinophils, 0.0% basophils
and 5.0% monocytes; red blood cell count, 1,780x109/l;
hemoglobin levels, 6.7 g/dl; hematocrit, 19.7%; reticulocytes,
1.49%; and platelet count, 36.0x109/l. Blood
biochemistry revealed mildly increased lactate dehydrogenase levels
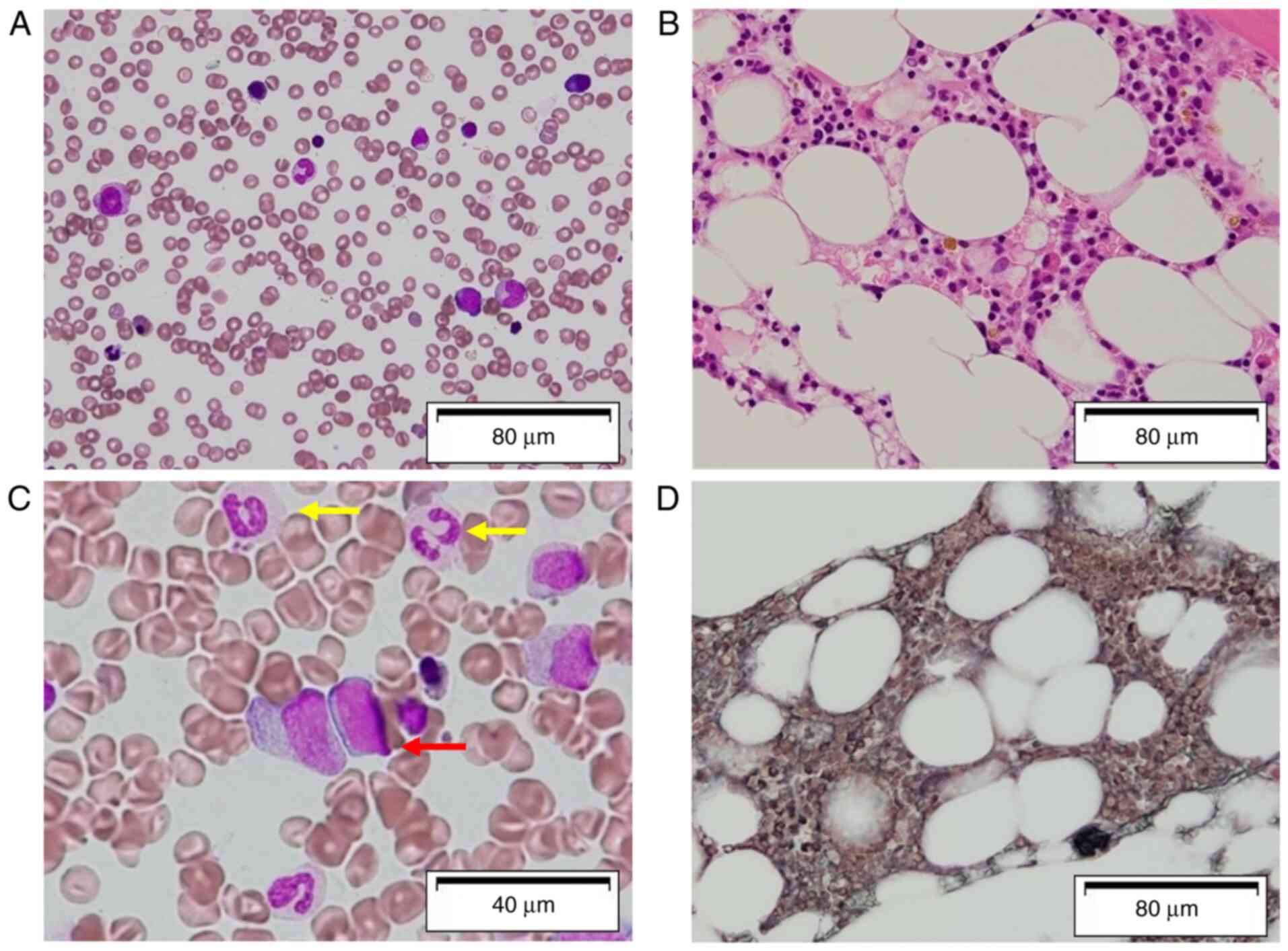
(297 IU/l). Bone marrow biopsy and aspiration revealed a markedly
hypocellular marrow without apparent dysplasia, with no increase
the number of blasts in the bone marrow (Fig. 1A and B). A bone marrow smear was stained
according to the May-Grünwald-Giemsa method. May-Grünwald-Giemsa
staining was performed as briefly described below: The blood smear
was prepared and air-dried. The smear was then fixed for 3 min with
methanol at room temperature and then stained with May-Grünwald
stain (cat. no. 5053; Muto Pure Chemicals Co., Ltd.) diluted with
an equal volume of distilled water for 5 min at room temperature.
The smear was then placed without washing in a 1:3 solution of
Giemsa stain (cat. no. 1500; Muto Pure Chemicals Co., Ltd.) diluted
with distilled water for 10 min at room temperature. The smear was
then washed in distilled water and allowed to dry. Chromosomal
analysis of the bone marrow demonstrated a normal karyotype. For
the detection of PNH-type granulocytes, phycoerythrin (PE)-labeled
anti-CD11b monoclonal antibodies (cat. no. 347557; BD Biosciences),
fluorescein-isothiocyanate (FITC)-labeled anti-CD55 (cat. no.
555693; BD Biosciences), and FITC-labeled anti-CD59 (cat. no.
550976; BD Biosciences) were used in combination with
isotype-matched control mAbs as previously described (7). For the analysis of PNH-type RBCs,
PE-labeled anti-glycophorin A mAb (cat. no. R707801-2; Dako;
Agilent Technologies, Inc.) was used instead of anti-CD11b mAb.
Fresh peripheral blood was diluted to 3% with phosphate-buffered
saline, and 50 µl diluted blood was incubated with PE-labeled
anti-glycophorin A mAb, FITC-labeled anti-CD55, and anti-CD59 mAb
on ice for 25 min. At least 105 CD11b-positive
granulocytes and glycophorin A-positive RBCs within each
corresponding gate were analyzed using a FACScan flow cytometer (BD
Biosciences). Flow cytometric analysis revealed that the red blood
cells and granulocytes were 100% positive for CD55 and CD59; there
were no paroxysmal nocturnal hemoglobinuria (PNH) clones. Magnetic
resonance imaging (MRI) of the spine revealed severely fatty bone
marrow. The patient was diagnosed with acquired SAA.
Immunosuppressive therapy with cyclosporine (CsA, 4 mg/kg, 200
mg/day) was commenced. This was effective and improved his
pancytopenia. However, 1 year and 6 months following the initiation
of CsA treatment, his pancytopenia recurred. Bone marrow aspiration
did not reveal an increased number of blasts; however, it revealed
trilineage dysplasia (Fig. 1C and
D). Azan staining was performed to
confirm the fibrotic changes. Azan staining was performed as
briefly described as follows: The smear was stained in Azocarmine G
solution (cat. no. 40012; Muto Pure Chemicals Co., Ltd.) for 1 h at
room temperature and then washed with running tap water. The smear
was then fixed in 5% phosphomolybdic acid solution for 1 h at room
temperature and washed with running tap water and rinsed using
distilled water. The smear was then stained with aniline
blue-orange G mixture (cat. no. 40051; Muto Pure Chemicals Co.,
Ltd.) for 30 min at room temperature and the slide was wiped with
filter paper to drain off the solution. Chromosomal analysis of the
bone marrow demonstrated der(1;7) with abnormalities on chromosome
1[46, XY 17/20: 46, XY, +1, der(1;7) 3/20]. A diagnosis of a
transformation from AA to MDS with refractory cytopenia with
multilineage dysplasia (RCMD) was made. High-grade fever and lower
abdominal pain developed at approximately the same time. A
colonoscopy revealed a longitudinal ulcer and bleeding from the
colorectal mucosa in the terminal ileum (Fig. 2A and B). A biopsy of these lesions was reported
as suggestive of CD. A pathological examination revealed that
lymphocytes infiltrated with focal irregularity.
Since characteristic features of CD, such as
longitudinal ulcer, fever, abdominal pain and pathological findings
were present, a definitive diagnosis of CD was made. Treatment
consisting of mesalazine (3,000 mg/day) and adalimumab (40
mg/day/every 2 weeks) was commenced. The Revised International
Prognostic Score for MDS (IPSS-R) (8) was high; the IPSS score was
intermediate-2 and the WHO classification-based prognostic scoring
system (WPSS) (9) score was high.
Thus, allo-HSCT was planned. Donor selection was performed with the
aid of the Japanese Association for Marrow Donor Program. Two
courses of azacitidine (AZA; 75 mg/m2/day, days 1-7)
were administered as an induction therapy assuming allo-HSCT.
Following AZA treatment, his pancytopenia improved, and chromosomal
analysis of the bone marrow revealed a normal karyotype. It was
considered that the patient was able to tolerate the pre-transplant
conditioning treatment, and it was decided that allo-BMT should be
performed, that was preceded by a reduced-intensity conditioning
regimen, including fludarabine (30 mg/m2 daily, days-7
to -3), cytarabine (2 g/m2 daily, days-5 to -3) and
cyclophosphamide (50 mg/kg daily, day-2). A total of 2 Gy total
body irradiation were administered on day-8. Tacrolimus (FK506) and
short-term methotrexate were used for prophylaxis against
graft-versus-host disease (GVHD) (10). allo-HSCT was performed with a
HLA-matched sibling as the donor. This conditioning regimen was
used as a prospective study for MDS in Gifu Municipal Hospital. The
administration of adalimumab was continued until 2 months following
engraftment. On day 20, the neutrophil count exceeded
0.5x109/l. FK506 treatment was continued until 4 months
following engraftment. A chromosomal analysis of the bone marrow
revealed 100% donor-derived abnormal karyotype. No severe diarrhea
caused by GVHD was observed during the course of engraftment.
Adalimumab treatment for CD was continued until 3 months following
engraftment. The administration of mesalazine has been continued.
At 1 year and 3 months following allo-BMT, although the amount of
Wilms' tumor 1 mRNA in the peripheral blood was 1x102
copies/g RNA, complete chimerism was confirmed by a 100%
donor-derived karyotype. The digestive symptoms caused by CD were
resolved. A colonoscopy revealed almost normal mucosa in the
terminal ileum (Fig. 2C and
D). The patient has remained in CR
for both MDS and CD.
Discussion
The present study describes the case of a patient
with MDS that developed from AA. The results of bone marrow biopsy
and aspiration revealed the diagnosis of SAA. However, the
possibility of the accurate diagnosis of this patient being
idiopathic cytopenia of uncertain significance (ICUS) should be
considered. This case may have developed as ICUS; hence, CsA may
have become refractory and the patient may have then developed MDS.
Otherwise, the patient may have had MDS from the beginning.
Acquired AA is an immune-mediated disease. AA, MDS
and PNH constitute the bone marrow failure syndrome. These diseases
overlap with each other, and a differential diagnosis may
occasionally be difficult, as these diseases share underlying
mechanisms. Of note, the transformation from AA to MDS has
sometimes been observed (11).
Some cases of MDS are immune-mediated, and in particular, as in the
case presented herein, underlying mechanisms may have included
immune-mediated destruction. Thus, autoantibodies are easily
produced in MDS. Indeed, Saif et al (12) reported that autoantibodies were
observed in 34% of patients with MDS. However, the mechanism of
autoantibody production remains unclear in MDS. Autoantibody
production may occur due to the impaired immune response with the
overproduction of inflammatory cytokines (13,14).
CD is a relapsing inflammatory disease, and it appears to result
from the impaired interaction of the intestinal commensal
microbiota.
Eng et al (4) first reported the concurrent
development of MDS and CD in 1992. A potential role of immune
dysfunction as a common pathogenic factor has also been suggested
(15). MDS is a common disease
affecting the elderly, and CD is a common disease affecting young
individuals; thus, the concurrent development of MDS and CD is
rare. However, some reports have described the concurrent
development of MDS and CD (3-6,16).
Previous studies have demonstrated that the incidence of MDS in
patients with inflammatory bowel disease ranged from
170-550/105 individuals (17,18).
This incidence appears to be higher than that in the general
population. A pathophysiological link between MDS and CD has been
suggested on the basis of a common immunologic impairment (3-6,16).
CD is an autoimmune disease, and there is evidence to indicate that
MDS is also known to be related to autoimmunity (19). Indeed, CD is treated with
immunosuppressive therapy, and some patients with MDS respond well
to immunosuppressive therapy with cyclosporine and/or antithymocyte
globulin (20). The association
between the development of CD and the treatment of AA is not clear.
It is possible that there is an insult that triggers an autoimmune
attack against both the marrow and bowel. The same immunological
trigger may initiate simultaneous attacks on the bone marrow and
bowel. However, it is difficult to identify the exact trigger.
Regardless of the trigger, it may be more reasonable to assume that
general autoimmunity is activated.
The patient in the present study received adalimumab
and mesalazine simultaneously. These agents may have also affected
the clinical course of this patient. Although the administration of
adalimumab and mesalazine was continued, the digestive symptoms
caused by CD remained until allo-HSCT, and these symptoms were
resolved following allo-HSCT. This clinical course revealed that
the clinical effects of adalimumab and mesalazine were limited. In
addition, the benefits of FK506 and MTX as immunosuppressive
therapy for allo-HSCT should be considered. It may be possible that
these immunosuppressive therapies led to the remission of CD.
However, the remission of CD was continued following the
discontinuation of these immunosuppressive therapies; hence, it was
considered that allo-HSCT led the remission of CD.
Hu et al (21) reported a case similar to the one in
the present study. They reported the case of a patient with CD and
MDS who was successfully treated with allo-HSCT. Hu et al
(21) also hypothesized the
mechanisms underlying the of curative effects on CD as follows:
Autoimmune T-cell clonal proliferation and the excessive secretion
of interleukin (IL)-1, IL-6 and tumor necrosis factor α by
lymphocytes have been observed in patients with MDS. These
cytokines may play an important role in the development of CD
(21). Another hypothesis to
explain the pathophysiological link between MDS and CD is based on
chromosomal abnormalities. Eng et al (4) reported 3 cases of the concurrent
development of MDS and CD with clonal abnormalities of chromosome
20. However, it remains unknow as to how these chromosomal
abnormalities are related to CD. Such chromosomal abnormalities
were not found in the case in the present study. The third
hypothesis is that the phagocytosis and killing capacity of
neutrophils are decreased in patients with MDS. Local infection of
the mucosa may play an important role in the development of CD
(21). Thus, CD may develop
following a local infection in an immunocompromised host with MDS.
The final hypothesis is that the association of MDS and CD may be
coincidental; however, this last hypothesis appears unlikely.
Case-control studies are thus required to rule out these
possibilities.
A thorough review of the recent literature revealed
that reports of MDS combined with CD treated with HSCT were
extremely rare (21). Generally,
allo-HSCT is increasingly used as a curative treatment option for
hematological malignancies (22).
The IPSS-R is an age-adjusted risk score based on the percentage of
marrow blasts, modified cytogenetic risk groups and the severity of
cytopenia (8). In the case
presented herein, the patient was classified as high-risk. Thus,
allo-HSCT was selected. When allo-HSCT is performed, the immune
system of the patient is transiently destroyed by the conditioning
regimen, and a new immune system derived from the donor is
reconstructed. The new reconstructed immune system may restore the
autoimmunity of CD (23).
Ditschkowski et al (24)
reported that 10 out of 11 patients remained free of inflammatory
bowel disease following allo-HSCT for hematological malignancies.
These results demonstrate that allo-HSCT may be a promising
treatment strategy for patients with combined MDS and CD. However,
CD is listed in the hematopoietic stem cell transplantation
specific comorbidity index (25).
Chronic inflammation of the gastrointestinal mucosa by CD may
destroy the defensive function of the mucosa. The infection risk of
the gastrointestinal tract may be increased due to chronic
inflammation. In addition, this chronic inflammation may increase
the risk of aggravation of CD. The indications for allo-HSCT for
patients with combined MDS and CD should thus be carefully
considered.
In conclusion, allo-HSCT may be a promising
treatment strategy for patients with combined MDS and CD. However,
its use should be carefully considered.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG, TH, KF, TT and HT prepared the manuscript. HG,
KF, TT, TH and HT performed the literature search. HG, HT, YK, KY,
YS and SK conceived and designed the study. HG and TH drafted the
manuscript for important intellectual content. HG KF and TT
prepared the figures. All authors gave final approval of the
version to be published. HG and HT confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The patient provided written informed consent for
his participation in this case report.
Patient consent for publication
Written informed consent for publication of their
clinical details and clinical images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Young NS, Calado RT and Scheinberg P:
Current concepts in the pathophysiology and treatment of aplastic
anemia. Blood. 108:2509–2519. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tefferi A and Vardiman JW: Myelodysplastic
syndromes. N Engl J Med. 361:1872–1885. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang Z, Zhou Y and Liu Y: Concurrent
inflammatory bowel disease and myelodysplastic syndrome: Report of
nine new cases and a review of the literature. Dig Dis Sci.
53:1929–1932. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eng C, Farraye FA, Shulman LN, Peppercorn
MA, Krauss CM, Connors JM and Stone RM: The association between the
myelodysplastic syndromes and Crohn disease. Ann Intern Med.
117:661–662. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Castellote J, Porta F, Tuset E and Salinas
R: Crohn's disease and the myelodysplastic syndrome. J Clin
Gastroenterol. 24:286–287. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bosch X, Bernadich O and Vera M: The
association between Crohn disease and the myelodysplastic
syndromes. Report of 3 cases and review of the literature. Medicine
(Baltimore). 77:371–377. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang H, Chuhjo T, Yamazaki H, Shiobara S,
Teramura M, Mizoguchi H and Nakao S: Relative increase of
granulocytes with a paroxysmal nocturnal haemoglobinuria phenotype
in aplastic anaemia patients: The high prevalence at diagnosis. Eur
J Haematol. 66:200–205. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Malcovati L, Germing U, Kuendgen A, Della
Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B,
Bernasconi P, Knipp S, et al: Time-dependent prognostic scoring
system for predicting survival and leukemic evolution in
myelodysplastic syndromes. J Clin Oncol. 25:3503–3510.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hiraoka A, Ohashi Y, Okamoto S, Moriyama
Y, Nagao T, Kodera Y, Kanamaru A, Dohy H and Masaoka T: Japanese
FK506 BMT(Bone Marrow Transplantation) Study Group. Phase III study
comparing tacrolimus (FK506) with cyclosporine for
graft-versus-host disease prophylaxis after allogeneic bone marrow
transplantation. Bone Marrow Transplant. 28:181–185.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun L and Babushok DV: Secondary
myelodysplastic syndrome and leukemia in acquired aplastic anemia
and paroxysmal nocturnal hemoglobinuria. Blood. 136:36–49.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saif MW, Hopkins JL and Gore SD:
Autoimmune phenomena in patients with myelodysplastic syndromes and
chronic myelomonocytic leukemia. Leuk Lymphoma. 43:2083–2092.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Braun T and Fenaux P: Myelodysplastic
syndromes (MDS) and autoimmune disorders (AD): cause or
consequence? Best Pract Res Clin Haematol. 26:327–336.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimamoto T and Ohyashiki K:
Immunosuppressive treatments for myelodysplastic syndromes. Leuk
Lymphoma. 44:593–604. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hanauer SB, Wong KK, Frank PH, Sweet DL
and Kirsner JB: Acute leukemia following inflammatory bowel
disease. Dig Dis Sci. 27:545–548. 1982.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nahas SC, Nahas CS, Marques CF, Borba MR,
Helito AS and Odoni V: Concurrent development of Crohn disease and
myelodysplastic syndrome in a child: Case report and literature
review. Pediatr Hematol Oncol. 23:477–483. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Harewood GC, Loftus EV Jr, Tefferi A,
Tremaine WJ and Sandborn WJ: Concurrent inflammatory bowel disease
and myelodysplastic syndromes. Inflamm Bowel Dis. 5:98–103.
1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hebbar M, Kozlowski D, Wattel E, Mastrini
S, Diévart M, Duclos B, Bonaz B, d'Almagne H, Belaiche J, Colombel
JF and Fenaux P: Association between myelodysplastic syndromes and
inflammatory bowel diseases. Report of seven new cases and review
of the literature. Leukemia. 11:2188–2191. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hamblin T: Immunologic abnormalities in
myelodysplastic syndromes. Hematol Oncol Clin North Am. 6:571–586.
1992.PubMed/NCBI
|
|
20
|
Molldrem JJ, Jiang YZ, Stetler-Stevenson
M, Mavroudis D, Hensel N and Barrett AJ: Haematological response of
patients with myelodysplastic syndrome to antithymocyte globulin is
associated with a loss of lymphocyte-mediated inhibition of CFU-GM
and alterations in T-cell receptor Vbeta profiles. Br J Haematol.
102:1314–1322. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu C, Lv L, Liu D and Huo J: Treatment of
Crohn's disease complicated with myelodysplastic syndrome via
allogeneic hematopoietic stem cell transplantation: Case report and
literature review. Clin J Gastroenterol. 7:299–304. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Passweg JR, Baldomero H, Bregni M, Cesaro
S, Dreger P, Duarte RF, Falkenburg JH, Kröger N, Farge-Bancel D,
Gaspar HB, et al: Hematopoietic SCT in Europe: Data and trends in
2011. Bone Marrow Transplant. 48:1161–1167. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Duijvestein M, van den Brink GR and Hommes
DW: Stem cells as potential novel therapeutic strategy for
inflammatory bowel disease. J Crohns Colitis. 2:99–106.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ditschkowski M, Einsele H, Schwerdtfeger
R, Bunjes D, Trenschel R, Beelen DW and Elmaagacli AH: Improvement
of inflammatory bowel disease after allogeneic stem-cell
transplantation. Transplantation. 75:1745–1747. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sorror ML, Maris MB, Storb R, Baron F,
Sandmaier BM, Maloney DG and Storer B: Hematopoietic cell
transplantation (HCT)-specific comorbidity index: A new tool for
risk assessment before allogeneic HCT. Blood. 106:2912–2919.
2005.PubMed/NCBI View Article : Google Scholar
|