Introduction
The immune response has been shown to play an
essential role in the protection of the human body against invading
pathogens. However, an exaggerated immune response can result in
inflammation that negatively affects the human body and this is
known as hypersensitivity reactions (1). In fact, a variety of xenoantigens can
be involved in hypersensitivity reactions. For instance, pollen,
pollutants, food and medications have been observed to participate
in type I hypersensitivity inductions (2). Exposure to the xenoantigens can lead
to the development of anaphylaxis. Type I hypersensitivity
reactions have been shown to be caused by the exaggerated secretion
of immunoglobulin E (IgE) (3).
This immune reaction can be induced by exposing the body to an
antigen. The development of hypersensitivity diseases are indeed
associated with various risk factors. These risk factors can be
genetic or environmental factors (4-7).
Presented antigens by antigen-presenting cells (APCs) can be
recognized by T-cells that lead to the exaggerated production of
IgE. Subsequently, IgE antibodies bind with their receptors on mast
cells and eosinophils, and accordingly, release histamine and other
mediators. Subsequently, these mediators induce high vasodilation
that can lead to elevate mucous secretions in the lungs and
bronchospasm (8,9). As a result, inflammatory reactions
and lung edema in the pulmonary interstitial space can occur and
may lead to severe pulmonary dysfunctions (8,10).
Platelets have been shown to play an essential role
in pulmonary inflammation via activating neutrophil recruitment
into lung tissue (10,11). It has demonstrated that platelet
distribution width (PDW) may induce the activation of platelets and
may be used as a marker of hypercoagulation and pulmonary
inflammation in chronic obstructive pulmonary disease (COPD).
Moreover, red blood cell distribution width (RDW) has also been
used as a marker to predict the severity of COPD (12,13).
Consolidation is well-known as a pathogenic
condition for lung tissue that can occur via the exudation of
inflammatory cells and fluids into alveoli. The infiltration
materials can be inflammatory cells, blood or inhaled water, and
this condition can lead to lung dysfunction (14). The hallmark of lung inflammation is
the recruitment of pro-inflammatory immune cells into pulmonary
inflamed sites. It has been shown that neutrophils are main immune
cells that can be recruited to the site of pulmonary disease under
various conditions. For instance, it has been found that
neutrophils play an essential role in COVID-19-induced pulmonary
infection (15), septic-induced
lung infection (16,17) and systemic lung inflammation
associated with acute pancreatitis (18,19).
The infiltration of pro-inflammatory immune cells to lung tissue
can lead to an increase in the viscosity of pulmonary mucus
secretion, and can subsequently impair pulmonary gas exchange and
cause systemic hypoxia (20).
Thus, in the present study, neutrophils were measured as indicators
of lung inflammation linked to hypersensitivity.
Subjects and methods
Design of study
The present study was performed on patients who
suffered from type 1 hypersensitivity and aimed to study the link
between infiltrated neutrophils and lung inflammation in type 1
hypersensitivity. The included patients attended the Al-Sader
Teaching Hospital in Amarah, Maysan, Iraq during the period from
October, 2022 to April, 2023. Ethics approval for the current study
was obtained and authorized by the Ethics Committee of Al-Sader
Teaching Hospital (Approval no. 2021-02). Written informed consent
was obtained from all the participants.
Study subjects
The present study included 128 patients who were
identified with type 1 hypersensitivity, as deemed by shortness of
breath, wheezing, and high levels of IgE and basophils. In
addition, 40 healthy individuals with no etiology of type 1
hypersensitivity served as the controls in the present study. Blood
and sputum samples were collected from the patients and the
controls to analyze serum IgE, blood neutrophils, basophil, PDW,
RDW and sputum neutrophils. Moreover, the patients and controls
were also subjected to chest radiographs in the unit of x-ray in
Al-Sader Hospital by specialist radiologist staff and the existence
or absence of consolidation were analyzed in a blinded manner.
The inclusion criteria for the study were as
follows: i) Shortness of breath; ii) wheezing; iii) high IgE
levels; iv) a high number of basophils; v) patients aged ≥18 years.
However, the exclusion criteria were as follows: i) Patients who
suffered from chronic illnesses; ii) patients who inhaled
corticosteroids; iii) those who had pulmonary fibrosis; iv)
patients with other diseases.
Measurement of IgE levels
A commercial kit (Human IgE ELISA kit, cat. no.
89-022-695, Thermo Fisher Scientific, Inc.;) was used to measure
the levels of IgE in the serum of the patients and controls. This
was performed as per the manufacturer's instructions.
Identification of sputum
neutrophils
Sputum cells were identified as previously described
(21). The induction of sputum was
performed using 200 µg albuterol (Merck KGaA) that was administered
to the study subjects 10 min prior through a metered-dose inhaler
(MDI). The subjects were then nebulized with 3.5% saline using
ultrasonic nebulizer at a rate of 3 ml/min for a total of 10 min.
Deep cough was performed by subjects at intervals of 3 min for a
total of 10 min and subjects were requested to perform mouth
washing at each interval. In order to release infiltrated cells
from pulmonary mucin, sputum samples were homogenized using 0.1%
DTT, which contained 3% BSA (Merck KGaA) to protect the infiltrated
cells. The mixture was then vortexed and placed on a tube rocker at
room temperature for 10 min. The mixture was then subjected to
filtration using a 48-µm nylon filter. Thereafter, centrifugation
was performed to the filtrated mixture at 500 x g for 10 min at
4˚C. Cold PBS (Thermo Fisher Scientific, Inc.) was added
to the cell pellet and the cell pellet was then stained with
Leishman stain (Micromaster Laboratories Pvt. Ltd.) as previously
described (22). Briefly, for 5
min at room temperature, Leishman's stain was applied to the
slides. Methanol, which was part of the dye preparation, was used
to fix the cells. Before diluting the solution with an equivalent
volume of pH 7.0 buffered water, the slides were kept for 2 to 3
min. Using a plastic Pasteur pipette, the buffered water was
gradually added. These slides were then held at room temperature
for 7 min. The surplus stain was then removed using buffered water.
Once the slides had dried completely, neutrophils were identified
under a light microscope (Olympus CX21, Olympus Corporation) using
x100 high power fields.
Measurement of neutrophils, basophils,
PDW and RDW in blood samples
After harvesting the blood samples from the patients
and controls, CELL-DYN (Abbott Pharmaceutical Co. Ltd.) was used to
count and classify white blood cells using MAPSS technology
(https://www.gmi-inc.com/product/abbott-cell-dyn-3200-automated-hematology-analyzer/).
The device also uses optical laser light scatter analysis to
determine the PDW and RDW.
X-ray images analysis
According to regional protocols, all chest
radiographs were obtained as digital radiographs in the X-ray Unit
of Al-Sader Hospital. Specialist radiologist staff who were blinded
to the patient data analyzed the existence or absence of
consolidation. A homogeneous opacification that conceals the blood
vessels and airway walls was described as consolidation.
Statistical analysis
The data were analyzed using SigmaStat 10.0
software. The t-test and linear regression were used for
statistical analysis. The data are presented as the mean values ±
standard error of the mean. P#x003C;0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics of patients with
hypersensitivity type 1
The data from 128 patients with hypersensitivity
type 1 were obtained. There were 71 males and 57 females with ages
ranging from 18 to 53 years and a mean ± SE age of 33.039±0.844
years. There were 89.84% of hypersensitivity type 1 patients who
suffered from shortness of breath. Additionally, 87.5% of patients
with hypersensitivity type 1 were suffered from wheezing (Table I).
 | Table ICharacteristics of patients with
hypersensitivity type 1. |
Table I
Characteristics of patients with
hypersensitivity type 1.
| Characteristics | Patients (n=128) |
|---|
| Age, years | |
|
Mean ±
SE | 33.039±0.844) |
|
Range | 18-53 |
| Sex (%) | |
|
Male | 55.47 |
|
Female | 44.53 |
| Shortness of breath
(%) | |
|
Yes | 89.84 |
|
No | 10.16 |
| Wheezing (%) | |
|
Yes | 87.5 |
|
No | 12.5 |
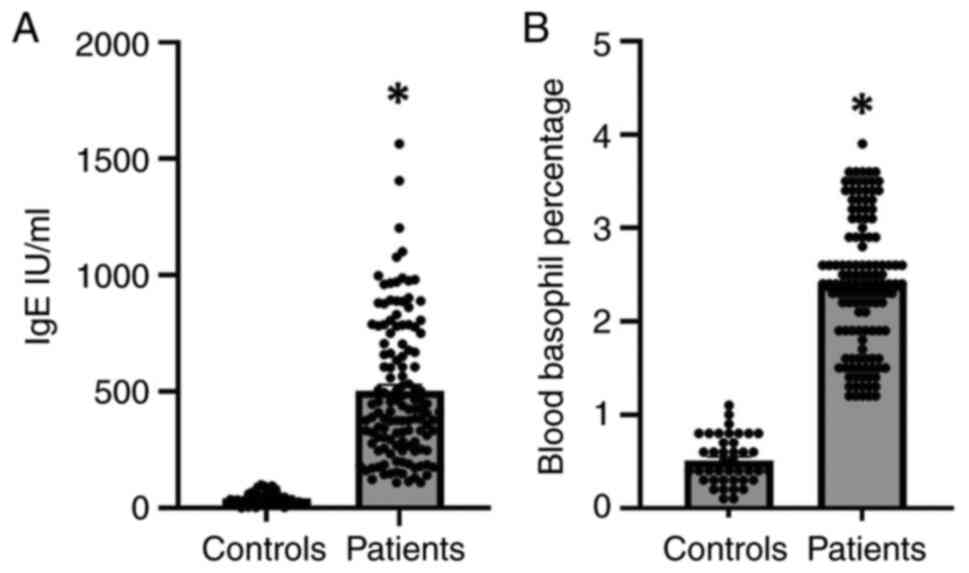
Levels of IgE and basophils in patient
with hypersensitivity and the controls
It is well known that IgE has been used as a common
indicator for the diagnosis of type 1 hypersensitivity (4). Moreover, basophils have been
established to play a critical role in type 1 hypersensitivity
(23). In the present study, type
1 hypersensitivity was identified in patients and healthy controls
based on the measurement of the levels of IgE and basophils
(Fig. 1). The results revealed
that the levels of IgE were significantly increased by 7-fold in
the serum of patients with type 1 hypersensitivity compared to the
controls (Fig. 1A). In addition,
the results demonstrated that the levels of basophils were
substantially elevated in the blood of patients with
hypersensitivity reactions as compared to the controls (Fig. 1B). Accordingly, elevated levels of
IgE and basophils may explain the induction of type 1
hypersensitivity reactions.
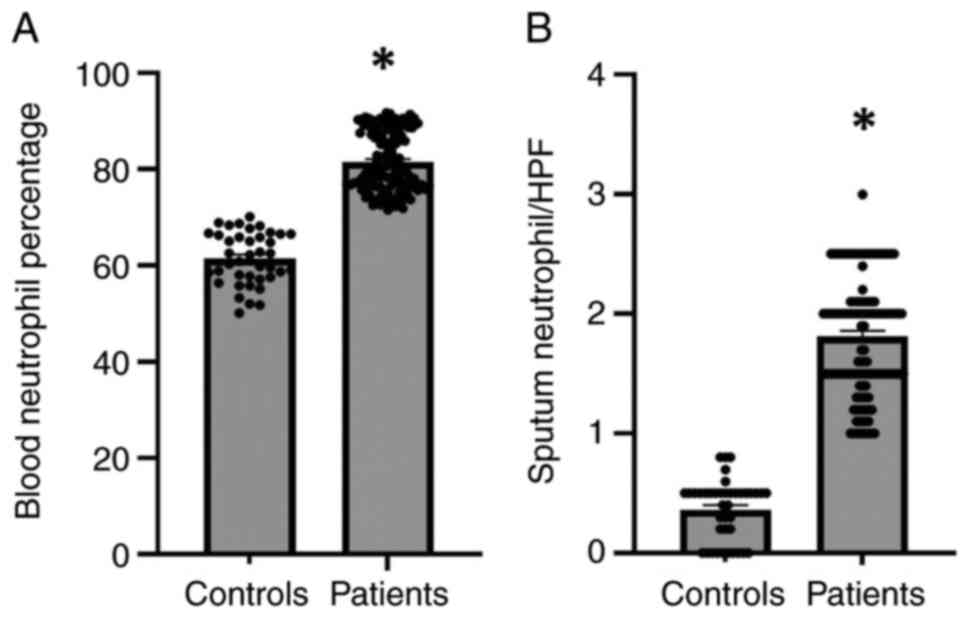
Estimation of neutrophils in the blood
and sputum of patients with hypersensitivity and the controls
Infiltrated neutrophils have been shown to play an
essential role in the inflammatory response (24). The present study first examined the
percentage of neutrophils in blood of patients with
hypersensitivity and the controls (Fig. 2). The results revealed that the
neutrophil percentage was significantly elevated (P#x003C;0.01) in
the blood of patients with hypersensitivity compared with the
controls (Fig. 2A). Furthermore,
we explored the infiltration of neutrophil into lung tissue through
examining the number of neutrophils in the sputum of patients and
control groups (Fig. 2B). It was
also observed that the number of infiltrated neutrophils was
substantially increased by 5-fold in the sputum of patients
compared with the control group (Fig.
2B). Consistently, the high levels of neutrophils could explain
the lung inflammation and exudates into the pulmonary airways that
result in shortness of breath in patients with hypersensitivity
reactions.
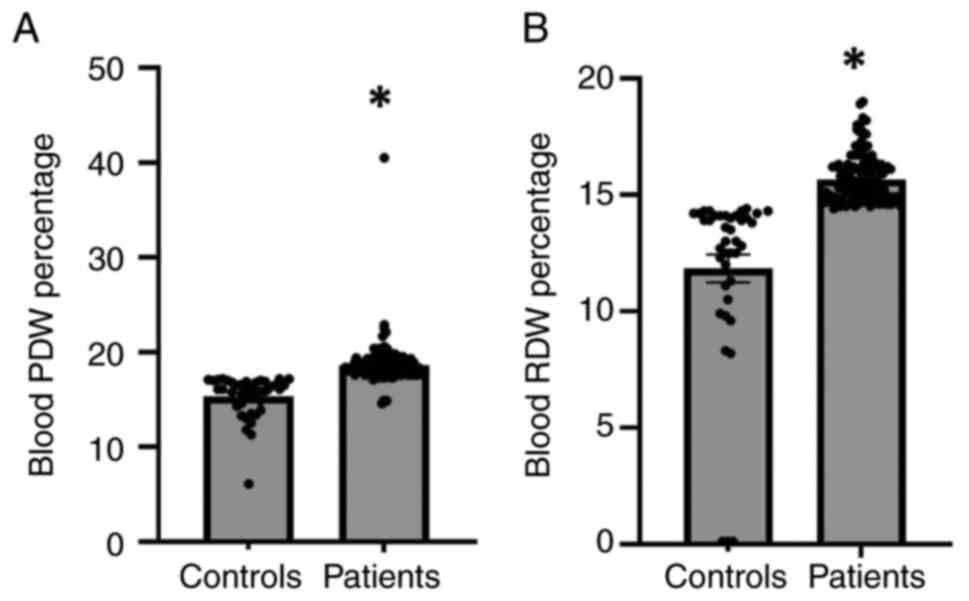
PDW and RDW in patients with
hypersensitivity and the controls
Subsequently, the present study examined the PDW and
RDW in patients with hypersensitivity and the controls (Fig. 3). The results of statistical
analysis revealed that the PDW was substantially increased in
patients with hypersensitivity compared with the controls (Fig. 3A). Moreover, the results
demonstrated that the RDW was markedly elevated in patients with
hypersensitivity compared with the controls (Fig. 3B). Thus, an increased PDW and RDW
may indicate, at least partly, the inflammation and disease
severity in patients with hypersensitivity reactions.
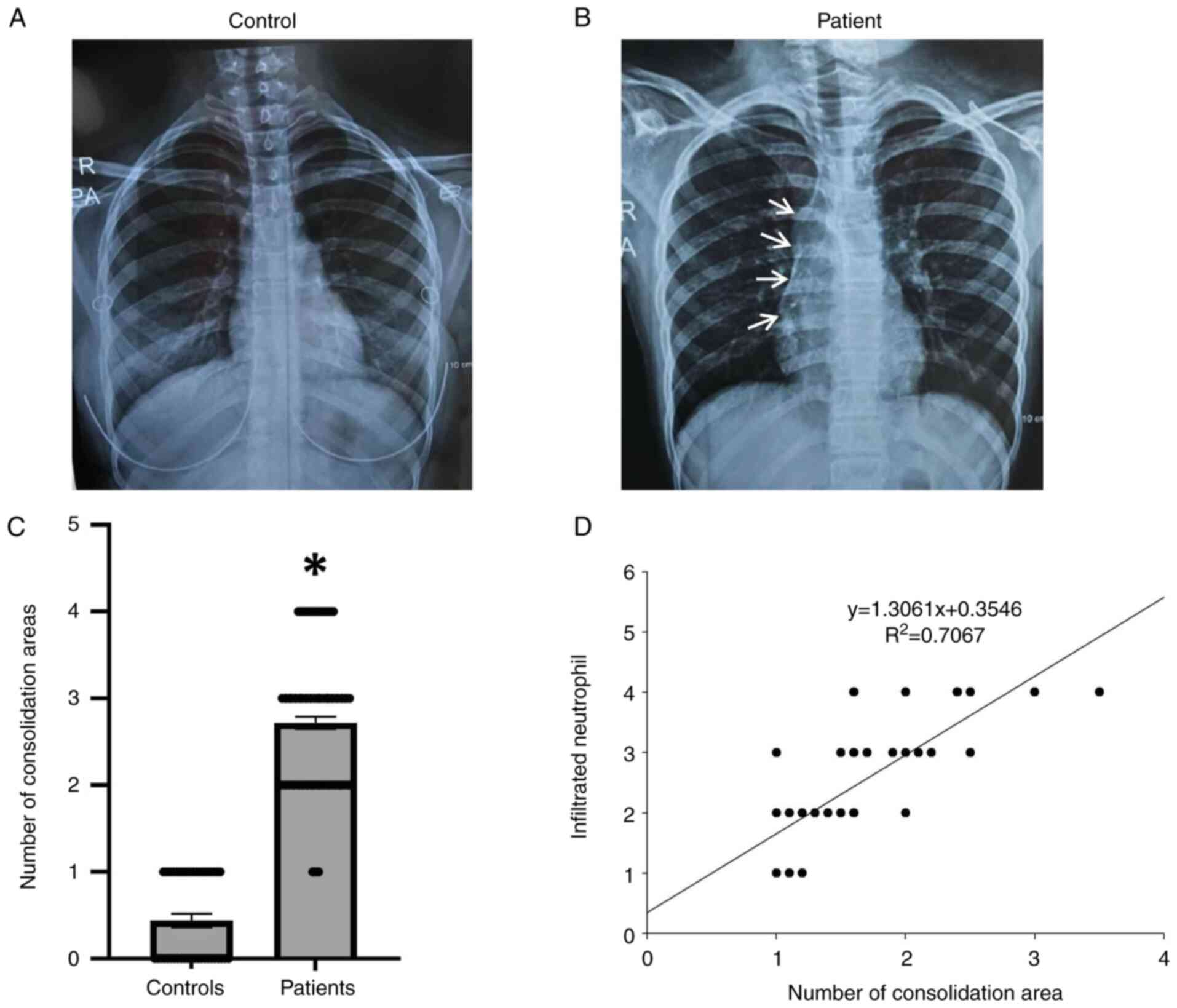
Neutrophil navigation and lung
inflammation in patients with hypersensitivity
Consolidation is a pathogenic condition that affects
lung functions. The present study examined the area of
consolidation in the lung tissue of patients with hypersensitivity
and healthy controls using X-rays (Fig. 4A-C). The results indicated that the
consolidation area was substantially increased by 70% in the lung
tissues of patients with hypersensitivity reactions compared with
the controls (Fig. 4A-C).
Subsequently, the present study also examined the association
between infiltrated neutrophils and lung inflammation in patients
with hypersensitivity reactions. The results revealed a significant
association (P#x003C;0.001) between neutrophil recruitment and lung
inflammation with respect to the consolidation area in the lung
tissues of patients with hypersensitivity reactions (Fig. 4D). Therefore, the association
between infiltrated neutrophils and consolidation areas may reflect
the levels of inflammation and secreted fluids in the lungs of
patients with hypersensitivity reactions.
Discussion
Neutrophil infiltration has been demonstrated to be
linked to lung inflammation. In the present study, the results
demonstrated that the number of neutrophils was substantially
increased in the blood and sputum of patients with hypersensitivity
reactions. Moreover, the RDW and PDW were also elevated.
Furthermore, the consolidation area was increased in the lungs of
patients with hypersensitivity reactions. Therefore, targeting
neutrophil navigation may decrease lung inflammation in patients
with hypersensitivity reactions.
The immune system is crucial for maintaining bodily
health and protecting against pathogen invasion. However, the same
mechanism can also lead to heightened inflammatory and
immunological reactions, which have adverse effects and are known
as hypersensitive reactions (1).
Antigen-presenting cells (APCs) pass allergens on to T-cells during
the sensitization stage of hypersensitivity type I. The T-cells
will signal once the B-cells have been induced to produce IgE
antibodies, which bind to the Fc receptors on mast cells and
basophils (8). The present study
demonstrated that the serum levels of IgE were significantly
increased (P#x003C;0.001) in patients with hypersensitivity
reactions (Fig. 1A). Moreover, the
results of the present study revealed a marked increase in the
levels of basophil in patients with hypersensitivity reactions
(Fig. 1B). These results are in
line with data form a recent study demonstrating that IgE antibody
mediates type 1 hypersensitivity (25). In fact, the binding of IgE
antibodies to mast cells and basophils can lead to the stimulation
of degranulation and produce mediators, such as histamine,
proteolytic enzymes, cytokines and platelet-activating factors.
Subsequently, the produced mediators can cause inflammation in the
lung tissue and this could explain the link between increased
levels of IgE and lung inflammation in patients with
hypersensitivity reactions.
It is considered that neutrophils contribute to
pulmonary inflammation (26).
Moreover, increased levels of pulmonary neutrophils, which can emit
a variety of pro-inflammatory cytokines and chemokines (27), as well as proteases that contribute
to the emphysema development, are a defining characteristic of lung
inflammation (28,29). A non-invasive approach for
determining the amount of neutrophils in the airway spaces is
induced sputum (30-32).
In the present study, it was observed that the levels of
infiltrated neutrophils were markedly elevated in the sputum of
patients with hypersensitivity reactions. A previous study found
that neutrophils play a critical role in IgE-mediated
hypersensitivity reactions (33),
and these findings were indeed in line with the results of the
present study. Infiltrated neutrophils to the lung tissue can
release reactive oxygen species that subsequently damage pulmonary
tissue. Moreover, infiltrated neutrophils have been shown to
produce neutrophil extracellular traps (NETs), which can lead to an
increase the viscosity of pulmonary mucus secretion and can
subsequently impair pulmonary gas exchange and cause systemic
hypoxia (18,24). Moreover, neutrophils have been
shown to play an essential role in various lung infections. For
instance, neutrophils have been demonstrated to resist the invading
pathogens in the lungs. However, it has been suggested that
neutrophils may also contribute to exuberate pulmonary inflammation
by producing different proteases, as well as expelling web-like
structures known as NETs (17,19).
The accumulation of NETs in the pulmonary airways causes lung
tissue damage and increases mucus viscosity, leading to shortness
of breath. In fact, since neutrophils are suspected to play an
essential role in pathophysiology (34,35),
the measurement of infiltrated neutrophils can be easily performed
in the target organ using a non-invasive method (4,5);
thus, measuring induced sputum neutrophils fulfills some of the
ideal criteria for a biomarker of lung inflammation linked to
hypersensitivity reactions. Future studies are required however, in
order to examine the potential utility of this biomarker in
hypersensitivity in further detail. It should be mentioned that PDW
and RDW have been documented to be connected to lung inflammation.
The present study found that the PDW and RDW were substantially
increased in patients with hypersensitivity reactions. Moreover,
the findings of the present study revealed that consolidation area
was significantly increased in patients with hypersensitivity
reactions. Notably, the present study also demonstrated a
substantial association between infiltrated neutrophils in the
sputum and the consolidation area in the lung tissue, which could
explain the pulmonary inflammation in patients with
hypersensitivity reactions.
In conclusion, the results of the present study
demonstrate a substantial association between neutrophil navigation
into lung airways and lung inflammation in patients with
hypersensitivity reactions. In addition, the findings presented
herein reveal that consolidation areas are strongly linked to
neutrophil infiltration. In fact, targeting the navigation of
neutrophils may reduce the inflammation and the viscosity of mucus
secretion in the pulmonary airways and may subsequently enhance
pulmonary gas exchange in patients with hypersensitivity reactions.
This strategy may indeed have potential implications in the
clinical management of such patients. However, the present study
has certain limitations which should be mentioned. The authors were
not able to obtain consent from patients to illustrate the
infiltration of neutrophils in the lung tissues using hematoxylin
and eosin staining. Moreover, neutrophils are essential immune
cells for protecting the body against pathogens, and targeting them
results in disorders in the immune response in real clinical
practice. Thus, future studies are required to explore the exact
role of neutrophils in hypersensitivity reactions. Additionally,
further studies are also warranted to determine the signaling
mechanisms involved in the navigation of neutrophils into lung
tissue in patients with hypersensitivity reactions.
Acknowledgements
The authors would like to thank Mr. Hasan Mahdi and
Mr. Sameh Riyad Faisal for their assistance with the collection of
blood samples.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KIJ and RM conceived the study. RM, AA, MCM and KIJ
were involved in the study methodology. RM and AA were involved in
the investigative aspects of the study. RM, AA, MCM and KIJ were
involved in data analysis. RM, AA, MCM and KIJ were involved in the
writing and preparation of the original draft of the manuscript,
and in the writing, review and editing of the manuscript. RM, AA
and MCM were involved in preparation of the figures. RM supervised
the study. RM and AAL were involved in project administration. All
authors have read and approved the final manuscript. RM and AA
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The Maysan Health Department/Al-Sadr Teaching
Hospital in the province of Maysan, Iraq was the authorizing
committee that approved the present study. Written informed consent
was obtained from all the participants (patients and controls).
Patient consent for publication
Written informed consent was obtained from the
participants (patients and controls) for the publication of the
X-ray images depicted in Fig.
4.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warrington R, Watson W, Kim HL and
Antonetti FR: An introduction to immunology and immunopathology.
Allergy Asthma Clin Immunol. 7 (Suppl 1)(S1)2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Witkowski M, Grajeta H and Gomułka K:
Hypersensitivity reactions to food additives-preservatives,
antioxidants, flavor enhancers. Int J Environ Res Public Health.
19(11493)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Justiz Vaillant AA, Vashisht R and Zito
PM: Immediate hypersensitivity reactions. In: StatPearls. Treasure
Island (FL): StatPearls Publishing, 2023.
|
|
4
|
Justiz Vaillant AA, Vashisht R and Zito
PM: Immediate hypersensitivity reactions (archived). In: StatPearls
[Internet]. Treasure Island (FL): StatPearls Publishing, 2024.
|
|
5
|
Yang KD, Wu CC, Lee MT, Ou CY, Chang JC,
Wang CL, Chuang H, Kuo HC, Chen CP and Hsu TY: Prevalence of infant
sneezing without colds and prediction of childhood allergy diseases
in a prospective cohort study. Oncotarget. 9:7700–7709.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bach JF: The effect of infections on
susceptibility to autoimmune and allergic diseases. N Engl J Med.
347:911–920. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weiss ST: Eat dirt-the hygiene hypothesis
and allergic diseases. N Engl J Med. 347:930–931. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rosado Ingelmo A, Doña Diaz I, Cabañas
Moreno R, Moya Quesada MC, García-Avilés C, García Nuñez I,
Martínez Tadeo JI, Mielgo Ballesteros R, Ortega-Rodríguez N, Padial
Vilchez MA, et al: Clinical practice guidelines for diagnosis and
management of hypersensitivity reactions to contrast media. J
Investig Allergol Clin Immunol. 26:144–155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Gonzales JN, Lucas R and Verin AD: The
acute respiratory distress syndrome: Mechanisms and perspective
therapeutic approaches. Austin J Vasc Med. 2(1009)2015.PubMed/NCBI
|
|
11
|
Madhi R, Rahman M, Taha D, Linders J,
Merza M, Wang Y, Mörgelin M and Thorlacius H: Platelet IP6K1
regulates neutrophil extracellular trap-microparticle complex
formation in acute pancreatitis. JCI Insight: e129270, 2019 (Epub
ahead of print).
|
|
12
|
Kalemci S, Akin F, Sarihan A, Sahin C,
Zeybek A and Yilmaz N: The relationship between hematological
parameters and the severity level of chronic obstructive lung
disease. Polish Arch Intern Med. 128:171–177. 2018.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
13
|
Tertemiz KC, Ozgen Alpaydin A, Sevinc C,
Ellidokuz H, Acara AC and Cimrin A: Could ‘red cell distribution
width’ predict COPD severity? Rev Port Pneumol (2006). 22:196–201.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee KS, Han J, Chung MP and Jeong YJ:
Consolidation. Radiology Illustrated: Chest Radiology, pp221-233,
2014.
|
|
15
|
Madhi R: Consideration of nebulized
lidocaine for treatment of Covid19 severity via targeting
neutrophil extracellular traps. Anaesth Surg Open Access J.
2(000538)2020.
|
|
16
|
Park I, Kim M, Choe K, Song E, Seo H,
Hwang Y, Ahn J, Lee SH, Lee JH, Jo YH, et al: Neutrophils disturb
pulmonary microcirculation in sepsis-induced acute lung injury. Eur
Respir J. 53(1800786)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Y, Luo L, Braun OÖ, Westman J, Madhi
R, Herwald H, Mörgelin M and Thorlacius H: Neutrophil extracellular
trap-microparticle complexes enhance thrombin generation via the
intrinsic pathway of coagulation in mice. Sci Rep.
8(4020)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Madhi R, Rahman M, Mörgelin M and
Thorlacius H: c-Abl kinase regulates neutrophil extracellular trap
formation, inflammation, and tissue damage in severe acute
pancreatitis. J Leukoc Biol. 106:455–466. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Madhi R, Rahman M, Taha D, Mörgelin M and
Thorlacius H: Targeting peptidylarginine deiminase reduces
neutrophil extracellular trap formation and tissue injury in severe
acute pancreatitis. J Cell Physiol. 234:11850–11860.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Singh D, Edwards L, Tal-Singer R and
Rennard S: Sputum neutrophils as a biomarker in COPD: Findings from
the ECLIPSE study. Respir Res. 11(77)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Efthimiadis A, Spanevello A, Hamid Q,
Kelly MM, Linden M, Louis R, Pizzichini MMM, Pizzichini E, Ronchi
C, Van Overveld F and Djukanovic R: Methods of sputum processing
for cell counts, immunocytochemistry and in situ hybridisation. Eur
Respir J. 20:19S–23S. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mathur A, Tripathi AS and Kuse M: Scalable
system for classification of white blood cells from Leishman
stained blood stain images. J Pathol Inform. 4
(Suppl)(S15)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mukai K, Matsuoka K, Taya C, Suzuki H,
Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T,
et al: Basophils play a critical role in the development of
IgE-mediated chronic allergic inflammation independently of T cells
and mast cells. Immunity. 23:191–202. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Linders J, Madhi R, Mörgelin M, King BC,
Blom AM and Rahman M: Complement component 3 is required for tissue
damage, neutrophil infiltration, and ensuring NET formation in
acute pancreatitis. Eur Surg Res. 61:163–176. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pritchard DI, Falcone FH and Mitchell PD:
The evolution of IgE-mediated type I hypersensitivity and its
immunological value. Allergy. 76:1024–1040. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Madhi R: Platelet-neutrophil crosstalk and
neutrophil extracellular traps in acute pancreatitis. Jap J
Gastroenterol Res. 2(1084)2022.
|
|
27
|
Chan L, Karimi N, Morovati S, Alizadeh K,
Kakish JE, Vanderkamp S, Fazel F, Napoleoni C, Alizadeh K, Mehrani
Y, et al: The roles of neutrophils in cytokine storms. Viruses.
13(2318)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gaffney E, Murphy D, Walsh A, Connolly S,
Basdeo SA, Keane J and Phelan JJ: Defining the role of neutrophils
in the lung during infection: Implications for tuberculosis
disease. Front Immunol. 13(984293)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bordon J, Aliberti S, Fernandez-Botran R,
Uriarte SM, Rane MJ, Duvvuri P, Peyrani P, Morlacchi LC, Blasi F
and Ramirez JA: Understanding the roles of cytokines and neutrophil
activity and neutrophil apoptosis in the protective versus
deleterious inflammatory response in pneumonia. Int J Infect Dis.
17:e76–e83. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guiot J, Demarche S, Henket M, Paulus V,
Graff S, Schleich F, Corhay JL, Louis R and Moermans C: Methodology
for sputum induction and laboratory processing. J Vis Exp.
(56612)2017.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Koc-Günel S, Schubert R, Zielen S and
Rosewich M: Cell distribution and cytokine levels in induced sputum
from healthy subjects and patients with asthma after using
different nebulizer techniques. BMC Pulm Med.
18(115)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maestrelli P, Richeldi L, Moretti M and
Fabbri LM: Analysis of sputum in COPD. Thorax. 56:420–422.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Polak D, Hafner C, Briza P, Kitzmüller C,
Elbe-Bürger A, Samadi N, Gschwandtner M, Pfützner W, Zlabinger GJ,
Jahn-Schmid B and Bohle B: A novel role for neutrophils in
IgE-mediated allergy: Evidence for antigen presentation in
late-phase reactions. J Allergy Clin Immunol. 143:1143–1152.e4.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Loh JT and Lam KP: Neutrophils in the
pathogenesis of rheumatic diseases. Rheumatol Immunol Res.
3:120–127. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tang J, Yan Z, Feng Q, Yu L and Wang H:
The roles of neutrophils in the pathogenesis of liver diseases.
Front Immunol. 12(625472)2021.PubMed/NCBI View Article : Google Scholar
|