Introduction
Central nervous system (CNS) infections encompass a
range of neurological symptoms and are caused by various agents,
including bacterial, viral, fungal, parasitic and prion infections
(1-4).
These infections typically present with typical signs, such as
fever, headache and vomiting. They can progress to more severe
symptoms, including seizures, altered consciousness, neurological
deficits, sensory disturbances and visual impairment (3-5).
From 1990 to 2016, there were an estimated 389 cases of CNS
infections per 100,000 individuals worldwide, with a higher
incidence in low- and middle-income countries compared to
high-income countries, particularly Southeast Asia (3).
CNS infections are severe and potentially
life-threatening conditions, resulting in neurological and
cognitive complications, behavioral changes and mental disorders
(2). These infections are
associated with high mortality and morbidity rates, leading to
prolonged durations of hospitalization and significant financial
burdens (1,4). Establishing a prompt and accurate
diagnosis and providing appropriate therapy is critical for
preventing long-term complications (4). However, the current approach to the
treatment of CNS infections primarily focuses on targeting the
causative pathogen rather than addressing the underlying
pathogenesis mechanisms. Therefore, it is essential to explore the
use of appropriate adjuvant therapies that can reduce morbidity,
improve cure rates and alleviate the burden of disease caused by
CNS infections (6).
Citicoline emerges as a promising candidate for CNS
infection with its established use in various neurological
disorders, such as head trauma, Alzheimer's disease and stroke
(6-13).
Citicoline, or CDP-choline, is a mononucleotide consisting of
ribose, choline, pyrophosphate and cytosine (9,10,12).
In ischemic conditions such as stroke, citicoline may improve
symptoms by restoring neuronal cell membrane integrity, promoting
the regeneration of axons and interneuron synapses, enhancing
acetylcholine production and increasing cerebral blood flow through
vasodilatory effects (9,10,14).
Inflammation occurs in CNS infections due to active disease, and
other complications,s such as cytokine overproduction, endothelial
activation, blood-brain barrier disruption and free radical
release, which may damage neurons (6,10-13).
Thus, citicoline has the potential as an adjuvant therapy in CNS
infections due to its pleiotropic effects as an anti-inflammatory,
antiviral, antioxidant, neuroprotector and neurorestorative agent
(9,10,12,13).
Notably, citicoline demonstrates minimal
side-effects and a favorable safety profile, highlighting its
potential for use as a therapeutic option for CNS infections
(9,10). However, it is essential to note
that, to the best of our knowledge; there are no studies available
summarizing preclinical and clinical research on the use of
citicoline as an adjuvant therapy in CNS infections, despite its
use in other neurological conditions, such as head trauma and
stroke (9,10). The present study describes the case
of a patient with concurrent CNS tuberculosis (TB) infection and
COVID-19, and summarizes and discusses the relevant evidence
regarding the use of citicoline, as presented in the
literature.
Case report
A 50-year-old male presented to the emergency room
of Tebet Subdistrict Public Hospital (Jakarta, Indonesia) with a
progressive decline in consciousness over a period of 1 day. He
also reported experiencing shortness of breath and cough with green
phlegm for the past 2 weeks. Prior to his admission, the patient
had received a diagnosis of pulmonary TB at a community health
center and had been undergoing a standard anti-tuberculosis
treatment regimen for 5 days. This regimen included an oral
fixed-dose combination of Rifampicin at 150 mg, Isoniazid at 75 mg,
Pyrazinamide at 400 mg and Ethambutol at 275 mg. During the last 3
months, the patient had experienced significant weight loss, with
his weight decreasing from 47 to 30 kg, and he frequently felt
weak. The increasing number of COVID-19 cases during the second
wave of pandemic in Indonesia (between June and September, 2021)
influenced his reluctance to seek treatment. Upon his arrival, the
patient exhibited a Glasgow Coma Scale (GCS) score of 11 (eye
movement, 3; verbal response, 3; motor response, 5), a respiratory
rate of 42 breaths per minute, a pulse rate of 140 beats per
minute, a blood pressure of 130/80 mmHg, and an oxygen saturation
(SaO2) of 65% in room air. The body mass index (BMI) of
the patient was 13 kg/m2, and his upper arm
circumference measured 17 cm.
Routine blood tests revealed several abnormalities,
including a decreased platelet count (130,000/µl), an elevated
erythrocyte sedimentation rate (127 mm/h) and a high neutrophil
count (77%). Additionally, there was an elevation in aspartate
transaminase levels to 122 U/l and in alanine aminotransferase
levels to 90 U/l. Blood gas analysis indicated respiratory
acidosis. Cerebrospinal fluid analysis revealed the presence of
leukocytes (480 cells/mm³), with a predominance in lymphocytes
(72%). The analysis of lumbar puncture also revealed no
erythrocytes, low glucose levels (1.6 mmol/l) and elevated protein
levels (4.14 g/l). The results of the cytological analysis
confirmed the presence of TB infection. The Xpert MTB/RIF test
indicated moderate Mycobacterium tuberculosis (M.tb) levels
and no resistance to rifampicin. Chest X-rays (images not available
as these were obtained at another center) revealed bilateral
infiltrates, suggesting a co-infection of TB with viral pneumonia,
confirmed as COVID-19 through positive antigen and PCR results
(details of the laboratory test results of the patient are
presented in Table SI).
The patient presented with symptoms of shortness of
breath and decreased consciousness. The initial diagnosis indicated
CNS TB, with additional considerations of septic shock. The patient
also had active pulmonary TB and viral pneumonia caused by
COVID-19. The CURB-65 score was 2, positive for disorientation and
tachypnea. The qSOFA score was 2, and the SOFA score was 5,
suggesting the possibility of septic shock. However, due to the
unavailability of a lactate test and the fact that the blood
pressure and mean arterial pressure (MAP) of the patient were
within normal limits, the diagnosis of septic shock could not be
confirmed. Due to the limited resources of the hospital, the
patient could not be tested for procalcitonin, a critical marker
for sepsis guiding antibiotic treatment (15,16).
However, due to the normal plasma leukocyte count despite severe
infection, along with the predominant presence of lymphocytes,
decreased glucose levels, and increased protein levels in the
cerebrospinal fluid, suggest TB as the likely infection source.
Upon arrival, the patient received immediate
treatment, including 10 liters per minute (lpm) of oxygen, which
increased the SaO2 to 99%. Fluid resuscitation was
performed with NaCl 0.9% at a dose of 30 ml/kg body weight over a
period of 3 h, along with ampicillin/sulbactam at a dose of 1.5 g
every 8 h. The following day, the antibiotics were changed to
meropenem at 1 g every 8 h due to the worsening of the patient's
condition. Anti-TB treatment consisting of rifampicin 150 mg,
isoniazid 75 mg, pyrazinamide 400 mg and ethambutol 275 mg was
administered at one tablet twice daily for the intensive phase.
Additionally, the patient received adjunct treatments, such as
citicoline at a dose of 1,000 mg once daily, azithromycin at a
dosage of 500 mg once daily, favipiravir (1,600 mg twice on the
first day and 600 mg twice on the second day), omeprazole IV at a
dose of 40 mg twice daily, ondansetron IV at a dose of 4 mg,
N-acetylcysteine at a dose of 200 mg three times daily, pyridoxine
at a dose of 10 mg twice daily, ursodeoxycholic acid at a dose of
250 mg twice daily, curcuma at a dose of one tablet three times
daily, and bisoprolol at a dose of 2.5 mg once daily. The
administration of citicoline in this case is still in line with the
Systematic Review study by Cochrane Stroke Group (17) and the International Citicoline
Trial in Acute Stroke (ICTUS) protocol, which recommended a dose of
1,000 mg twice daily for the first 3 days intravenously, continued
with 500 mg twice daily for the following 6 weeks (18,19).
The nutritional needs of the patient were met through an oral diet
of 1,600 kcal/day administered via a nasogastric tube. Although
lactate and procalcitonin measurements were unavailable, blood
samples were taken for culture before the patient was transferred
to another hospital. Following the admission to the intensive care
unit for hemodynamic stabilization, the patient continued to
experience difficulties in breathing and communication, and also
exhibited delirium. The patient exhibited a respiratory rate of 36
breaths/min, an SaO2 of 99% with oxygen at 10 lpm, and
norepinephrine was administered at a dose of 0.05 µg/kg.
Ultimately, following 12 h of stabilization, the patient was
referred to another hospital.
Discussion
As regards the management of cases of CNS TB and
COVID-19, the authors were interested in investigating whether
citicoline, as an adjuvant therapy, can improve the recovery of
consciousness and neurological function in CNS infections. The
subsequent sections will delve into the mechanisms of action of
citicoline in CNS repair.
Role of citicoline in CNS infections:
Proposed mechanisms of action
The mechanisms of action of citicoline in
alleviating neurological issues in CNS infections are summarized in
Fig. 1 (6,7,9-13,20-34).
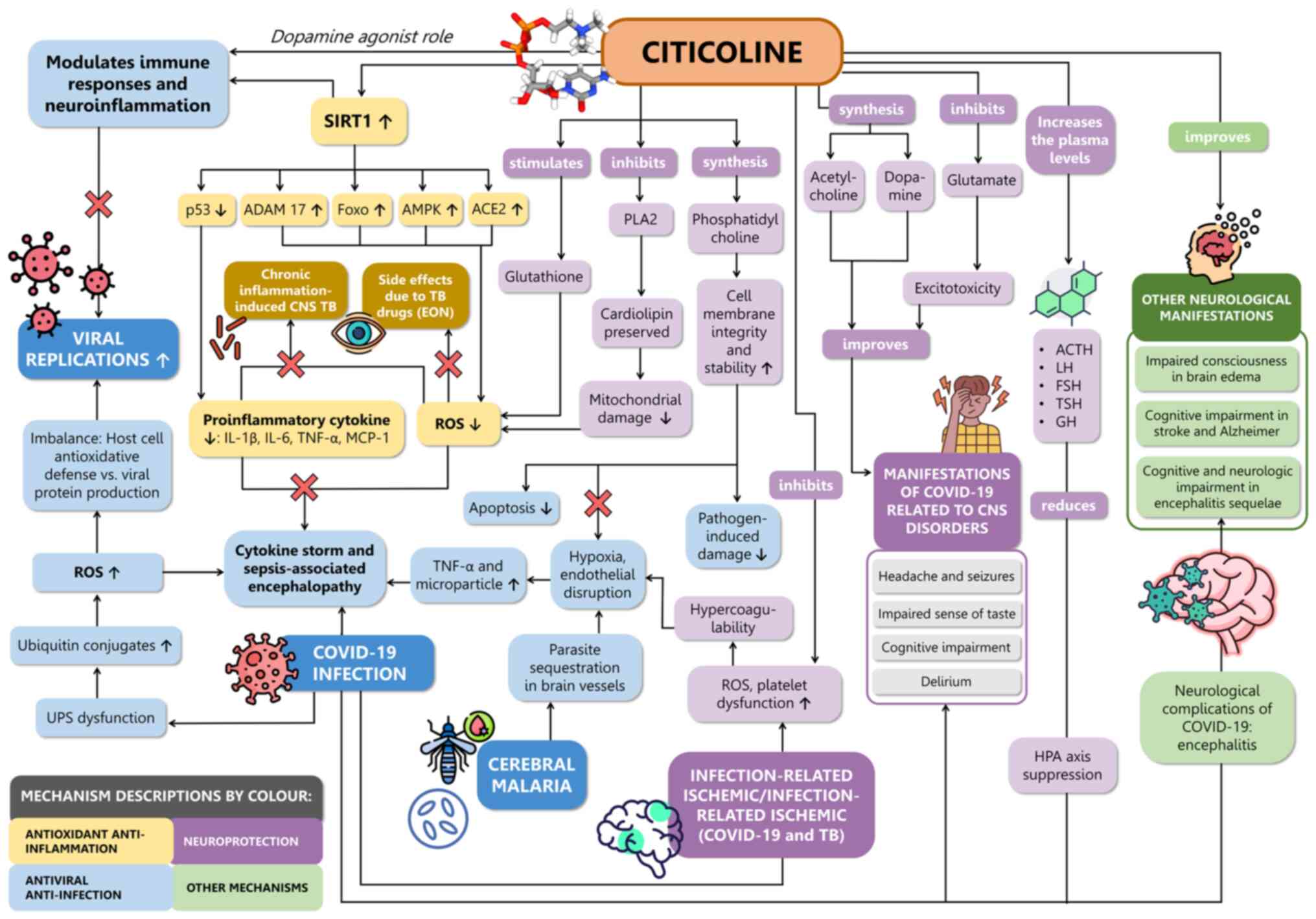 | Figure 1The proposed role and mechanisms of
action of citicoline in CNS infection reviewed based on scientific
evidence. Citicoline acts as an antiviral and anti-infection agent
through inhibition of the UPS and as a dopamine agonist,
potentially modulating immune response and neuroinflammation,
crucial for reducing viral replication. Furthermore, citicoline
possesses antioxidant and anti-inflammatory properties that may
enhance SIRT1, suppress the release of pro-inflammatory cytokines,
and reduce oxidative stress. This potential mechanism may help
prevent cytokine storms associated with COVID-19 infection,
sepsis-associated encephalopathy, cerebral malaria,
infection-related ischemic conditions, CNS TB with chronic
inflammation, and EON. Additionally, citicoline exerts
neuroprotective effects by stimulating glutathione production,
inhibiting PLA2, promoting phosphatidylcholine synthesis,
inhibiting platelet dysfunction, and increasing levels of
glutathione, acetylcholine, dopamine and hormones such as ACTH, LH,
FSH, TSH and GH. Moreover, citicoline has shown promise in
improving neurological manifestations associated with various
conditions, including brain edema, stroke, Alzheimer's disease, and
encephalitis. ACE2, angiotensin receptor 2; ACTH,
adrenocorticotropic hormone; ADAM-17, A-disintegrin and
metalloprotease-17; ampk, adenosine monophosphate protein kinase;
eon, ethambutol-induced Optic Neuropathy; FFA, free fatty acid;
Foxo, forkhead box O; FSH, follicle-stimulating hormone; GH, growth
hormone; HPA, hypothalamic-pituitary-adrenal; LH, luteinizing
hormone; MCP-1, monocyte chemoattractant protein-1; PLA2,
phospholipase A2; ROS, reactive oxygen species; TNF-α, tumor
necrosis factor-α; TSH, thyroid stimulating hormone; UPS, ubiquitin
proteasome system. |
Anti-infection. One of the anti-infection
mechanisms of citicoline is through the ubiquitin-proteasome system
(UPS). It primarily regulates protein degradation and turnover
within cells, playing essential roles in various cellular
processes, including immune responses (35). However, while alterations in this
system can affect immune function, there is limited direct evidence
linking the anti-infection properties of citicoline specifically to
its modulation of the UPS. The UPS plays a crucial role in the
early stages of viral replication, particularly in endocytosis and
viral maturation (11). Viral
infections, such as COVID-19 can manipulate the ubiquitin system by
producing de-ubiquitinated proteins and accumulating ubiquitin
conjugates (11). As a regulator
of UPS activity, citicoline can impede UPS function and thus hinder
viral replication through various mechanisms, including inhibiting
protein synthesis, inducing endoplasmic reticulum stress and
promoting cell death (11). While
citicoline shows promise as a potential treatment, its
effectiveness as an anti-infection agent, and particularly as an
antibacterial agent remains controversial, and requires further
exploration in future studies.
Another facet of the mechanism of action of
citicoline as an anti-infective is as an anti-inflammatory agent.
Citicoline has been shown to modulate inflammation inherent to
infections. A recent study demonstrated that applying citicoline
chitosan-coated liposomes gel to diabetic ulcers in rat models of
diabetes accelerated the healing process through reducing
inflammation. Citicoline could increase the secretion of vascular
endothelial growth factor (VEGF), which regulates angiogenesis and
collagen deposition, thereby facilitating wound healing (22). Chitosan-coated liposomes containing
citicoline exhibit notable antibacterial properties against
Gram-negative and Gram-positive bacteria, a crucial aspect in wound
healing (22). Notably, in cases
of flap-ischemia-reperfusion injury associated with diabetic
ulcers, citicoline demonstrates the ability to reduce inflammation,
prevent ischemic damage to the flap and decrease lipid peroxidation
(22). Flap ischemia-reperfusion
injury occurs when blood flow to the tissue flap is temporarily
restricted during surgery and then is subsequently restored,
leading to oxidative stress and inflammation (22). Further research is required to
explore the potential role of citicoline as an antibacterial agent
and its efficacy in the treatment of TB.
Additionally, the anti-infective mechanism of
citicoline involves its role in phospholipid synthesis, crucial for
maintaining cell membrane integrity and stability. This is vital to
shield cells from pathogen-induced damage. By enhancing
phospholipid metabolism, citicoline ensures optimal cell membrane
function, essential for neural communication and overall brain
health (11). In conditions such
as cerebral ischemia or low choline levels, citicoline mitigates
phospholipid hydrolysis by phospholipase A2 (PLA2), thereby
reducing the release of harmful compounds, such as reactive oxygen
species and lipid peroxides that harm the CNS (11,36,37).
Although not directly inhibiting PLA2, the involvement of
citicoline in phosphatidylcholine synthesis modulates PLA2 activity
and rectifies age-related changes in neuronal membranes. This is
also relevant in Streptococcus pneumoniae bacterial
meningitis, where phosphatidylcholine synthesis is inhibited
through the inhibition of choline phosphotransferase. In a previous
study using a murine model of meningitis, with the administration
of citicoline, hippocampal apoptosis was shown to be significantly
reduced (P#x003C;0.05) compared to the untreated animals (38). Additionally, citicoline preserves
key mitochondrial components, addressing neurological issues
observed in conditions like COVID-19(11).
Citicoline also has the potential to modulate immune
responses. This means that it can enhance the ability of the body
to fight infections by activating immune cells, such as macrophages
and T-cells (13). Moreover,
although not directly related to its role in fighting infections,
citicoline also has neuroprotective effects. These effects
indirectly support immune function by preserving the function of
neurons and facilitating communication between the nervous and
immune systems (11). Another
hypothesis is also that the role of citicoline as an indirect
dopamine agonist is relevant to its potential as an anti-infection
agent for the CNS (39,40). Dopamine plays a crucial role in the
immune system, particularly in the CNS, where it can modulate
neuroinflammation and immune responses (39). By indirectly increasing dopamine
levels, citicoline may help regulate immune function within the
CNS, potentially contributing to its anti-infection properties
(40). However, this hypothesis is
still debatable, as dopamine was found in previous studies may lead
to increased viral seeding and the exacerbation of inflammation in
the CNS, as observed particularly among patients with
HIV-associated neurocognitive-disorders (40,41,42).
Furthermore, the indirect activation of dopamine
receptors by citicoline in the CNS hypothetically suggests a
potential role in mitigating sepsis-induced inflammation (40,41,42).
Dopamine, particularly via D1-like receptors, exerts
anti-inflammatory effects, making it a target in sepsis management.
The ability of citicoline to modulate dopamine levels may regulate
immune responses, offering therapeutic potential. Nevertheless,
further research on citicoline, its dopaminergic role, potential
for anti-infection and sepsis management is warranted in order to
clarify its mechanisms of action and applications in the treatment
of CNS infections (40).
Antioxidant. Citicoline functions as an
antioxidant by inhibiting the accumulation of free fatty acids
(FFA), free radicals, lipid peroxidation and sphingomyelin damage
(9,10). In ischemic conditions, nerve cell
damage and death result in the deposition of FFA, glycerol and
arachidonic acid in the lesion, with the subsequent accumulation of
metabolites, such as prostaglandins and thromboxane causing further
damage over time. Citicoline also stimulates glutathione synthesis
and enhances glutathione reductase activity, essential antioxidants
that help prevent cell damage (9,10,12).
These actions inhibit lipid peroxidation and the activation of
PLA2, suppressing inflammation and neuronal cell death caused by
oxidative stress. This was confirmed in another study, where
citicoline was shown to reduce the biomarker of oxidative stress,
malondialdehyde (12). In
COVID-19, there is a decrease in choline levels, which is a source
of phospholipid synthesis. Citicoline, a source of choline, can
prevent PLA2 activity on the mitochondrial membrane and reduce
phospholipid hydrolysis (11).
However, the referred studies are reviews; hence, the level of
evidence is not very high (9-12).
Additionally, some studies did not solely focus on CNS infections
and lacked the descriptions of their literature search strategies
and critical appraisal specifications (9-12).
Anti-inflammatory. Citicoline possesses
anti-inflammatory properties, inhibiting pro-inflammatory cytokines
(TNF-α, IL-1β, IL-6 and monocyte chemoattractant protein-1/MCP-1)
while enhancing the generation of anti-inflammatory cytokines, such
as IL-10, IFN-γ and TGF-β (11,12).
Additionally, citicoline contributes to the UPS, which is impaired
in COVID-19, resulting in increased ubiquitin conjugates, the
inhibition of protein synthesis and stress on the endoplasmic
reticulum (11). Citicoline
functions as a proteasome inhibitor that prevents the formation of
the 26S proteasome complex and subsequently inhibits the
inflammatory response, protein synthesis, endoplasmic reticulum
stress and cell damage (11).
Eventually, citicoline is predicted to suppress the replication of
the COVID-19 virus, although this was only proposed as a hypothesis
by Longhitano et al (24)
and lacked supporting studies.
In CNS TB, M. tuberculosis can penetrate the
blood-brain barrier through two mechanisms: Bacterial adhesion to
laminin-1 and 2 on brain endothelial cells or via infected
neutrophils and macrophages (Trojan horse mechanism), leading to
the formation of rich foci (23,25,26).
Neutrophils secrete neutrophil extracellular traps containing
destructive enzymes, and together with TB-infected microglia, they
release pro-inflammatory cytokines, such as IL-1β, TNF-α, CCL2,
CCL5 and CXCL-10 (23,25,26).
The rupture of rich foci can result in the release of bacteria into
the sub arachnoid space and meninges, producing a thick exudate
(23,25,26).
These conditions lead to hydrocephalus, vascular and cytotoxic
edema and vasospasm, ultimately causing increased intracranial
pressure and ischemia (23,25,26).
The neutrophil expulsion of matrix metalloproteinase-9 can also
cause damage to endothelial cells of the blood-brain barrier and
the ejection of leukocytes into the brain, resulting in brain edema
and chronic CNS infection (23,25,27).
As the pathological mechanism is similar to other ischemic
conditions, further studies are required to confirm the role of
citicoline in CNS TB.
In sepsis, systemic inflammation can cause end-organ
damage by decreasing perfusion (septic shock), and particularly in
the CNS, sepsis-associated encephalopathy. As previously
demonstrated, in a murine model of septic shock, induced by cecal
ligation-incision, citicoline intravenous injection at a dose of
100 mg/kg at the 180th min after shock induction is capable of
reversing hypotension and recovering arterial pressure to control
levels in 60 min after the injection. This appeared to be due to
the attenuation in TNF-α, IL-1β and IL-6 levels (43). Cecal ligation and puncture was also
used in cyclophilin D knockout (CypD KO) mice in order to research
whether the mitochondrial permeability transition pore (MPTP),
whose induction sensitivity is controlled by CypD, causes neuronal
dysfunction, apoptosis and cell death in sepsis-associated
encephalopathy (SAE). It was discovered that CypD KO mice have
increased concentrations of reduced glutathione and citicoline
compared to the sham group, which was associated with decreased
rates of sepsis-induced hypothermia [wild-type (WT): 29.8±1.3˚C vs.
KO: 32.0±1.7˚C; P=0.008], death (WT, no survival after 46 h vs.
>50% survival rate past 70 h; P=0.004), and the death of
parietal cortex and hippocampal neurons, thus proving the
protective effects of citicoline and the role of MPTP in SAE in
this animal model (44).
Neuroprotection. Citicoline plays an
essential role in the formation of cell membrane components,
including mitochondria, endoplasmic reticulum, nucleus and myelin
sheath (7). It enhances the
synthesis of phosphatidylcholine, phosphatidylethanolamine and
phosphatidylserine, all which are crucial for axonal and synaptic
regeneration (10). Impaired
phosphatidylcholine synthesis can decrease phospholipid levels in
ischemic stroke and brain trauma (7-9).
Citicoline also exhibits neuroprotection by preserving cardiolipin,
an integral constituent of mitochondrial membranes, thereby
maintaining mitochondrial function and sphingomyelin for nerve cell
signal transduction (9,10). Moreover, citicoline promotes
Na+/K+-ATPase activity, supporting cellular
energy production and electrolyte balance, ultimately protecting
nerve cells from ATP loss (11).
Citicoline also has the potential to activate the
forkhead box O (Foxo) transcription factor, which is co-activated
by sirtuin-1 (SIRT1). SIRT1 is a protein involved in cellular
processes, such as metabolism, the regulation of oxidative stress,
inflammatory response and aging (45). Possible mechanisms on the role of
SIRT1 and citicoline (itself an activator of SIRT1) in the
activation of the Foxo transcription factor include epigenetic
regulation, neuroprotection, mitochondrial support and
anti-inflammatory properties (12). As a SIRT1 activator, citicoline
also suppresses the expression of A disintegrin and
metalloprotease-17 and tissue metalloproteinase inhibitor-3,
thereby inhibiting the production of pro-inflammatory cytokines,
such as IL-1β, IL-6 and TNF-α (12). It is also involved in neurogenesis,
gliogenesis, neural plasticity and the prevention of cognitive
decline in degenerative conditions (11) Additionally, SIRT1 activates
adenosine monophosphate protein kinase, which may suppress
inflammation and neuronal cell damage caused by oxidative stress
through this mechanism (12).
Increased oxidative stress is also observed in CNS TB, where
neutrophils produce reactive oxygen species, leading to damage in
the blood-brain barrier, brain edema and tissue damage (23).
Furthermore, in the context of COVID-19 infection,
it has been suggested that SIRT1 activity is decreased, while p53
protein expression is elevated, leading to increased oxidative
stress, mitochondrial damage and the hyper-inflammation of nerve
cells (11,46,47).
Furthermore, citicoline modulates angiotensin-converting enzyme 2
expression, thus suppressing inflammation and nerve cell damage
(12). Additionally, citicoline
exerts anti-apoptotic effects by reducing procaspase activity and
caspase expression, while increasing the expression of BCL-2 and
SIRT1 proteins (11). In
vivo studies have demonstrated that citicoline upregulates
SIRT1 expression, potentially mitigating neuroinflammation. By
activating SIRT1, citicoline could aid in cognitive preservation
and neuroprotection, offering benefits in COVID-19 and other
neurological conditions (48,49).
During COVID-19 infection, glutamate expression is increased, which
can cause neurotoxicity due to hyper-inflammation and oxidative
stress (8,11,12).
This glutamate neurotoxicity can lead to specific symptoms such as
headaches, seizures, and an impaired sense of taste and anosmia
(12). Citicoline prevents
excessive glutamate release and excitotoxicity by impeding
glutamate transporter reversal and enhancing excitatory amino acid
transporters (11). Additionally,
citicoline regulates tight junction proteins, which helps restore
brain cell endothelial damage and reduce brain edema (11,12).
Chronic inflammation in infectious cases may be
associated with brain ischemia (28) secondary to atherosclerotic plaque
formation, eventually leading to ischemic stroke (30,31).
Conversely, brain cell damage in ischemic conditions also induces
inflammation and infection due to impaired neuronal resilience,
local immune status, electrolyte imbalance, hypoxia, elevated body
temperature, impaired blood-brain barrier permeability, and
acidosis) (28). Infections may
also provoke thrombosis by activating extrinsic factors, decreasing
thrombomodulin and inhibiting fibrinolysis (28). This is particularly evident in
COVID-19 and tuberculous meningitis, where hypercoagulability,
endothelial damage, severe inflammation and mitochondrial damage
initiate a cytokine storm. This can contribute to platelet
dysfunction, microglia activation, thrombotic plaque formation,
and, ultimately, ischemic stroke (30,31,50).
In tuberculous meningitis, there are alterations to the blood
vessels in the circle of Willis due to the inflammatory response
and thick exudates that cause vasospasm and thrombosis (23,25,26,32).
Citicoline also helps restore cell membrane
integrity and stabilize the vascular endothelium in cerebral
malaria. During this infection, parasites are sequestrated in
erythrocytes, platelets and leukocytes within the blood vessels in
the brain. This leads to excessive cytokine release, resulting in
hypoxia, endothelial activation, the disruption of the blood-brain
barrier, and eventually, in increased levels of microparticles and
TNF-α (6,13). Nevertheless, it is essential to
consider that this in vivo study used mice as subjects;
hence, the results may differ if applied to humans (6). Another study on pediatric patients
with encephalitis demonstrated significant improvements in patients
receiving citicoline, particularly in domains such as expressive
language, receptive language, social development, bowel control and
academic performance (7). However,
that study has several limitations, including a small sample size
(40 children), no control group, and potential bias due to having
only one author who was also the principal investigator.
Similarly, another study using citicoline in
patients with reversible ethambutol-induced optic neuropathy (EON)
demonstrated the amelioration of visual impairment with immediate
discontinuation of ethambutol and simultaneous administration of
citicoline and zinc (20).
However, this was a single case report; hence, the findings cannot
be generalized to the broader population. This finding was
supported by evidence demonstrating that the administration of
citicoline to rats with EON resulted in the preservation of a
significantly larger number of retinal ganglion cells compared to
the control group (51), as well
as thicker ganglion layers and a higher protein expression of BCL-2
and a lower expression of the apoptotic enzyme, caspase-3(52).
Neuromodulator, neurorestoration and
neuroregeneration. Citicoline exhibits multifaceted benefits in
neurological recovery and cognitive enhancement. Studies have
highlighted its efficacy in aiding the recovery of consciousness
and neurological function. In children with encephalitis,
citicoline was shown to lead to improvements in various
neurological symptoms, including language ability, social
development, bladder and bowel control and intellectual function
(7). Moreover, research indicates
that citicoline can restore consciousness, leading to enhancements
in electroencephalogram readings, behavior, clinical symptoms and
the reduction of brain edema (53). This positive outcome may be
attributed to the role of citicoline in phospholipid synthesis, a
crucial component of nerve cell membranes (7).
Furthermore, the neuroprotective effects of
citicoline extend to enhancing neurotransmitter synthesis, glucose
metabolism and blood flow in the brain (7). Clinical research on elderly patients
with chronic cerebrovascular disease has demonstrated that
administering citicoline for 30-60 days can correct memory
impairment (10). Moreover, as
cited in these narrative reviews, citicoline also increases
dopamine levels promotes neuron repair and regeneration in the
substantia nigra of mice, leading to improvements in cognition,
coordination and motor function (9,10).
Citicoline also shows promise in preventing
neurological deterioration caused by COVID-19 by upregulating SIRT1
and inhibiting PLA2 expression (11). Its antioxidant effects help
mitigate memory impairments by inhibiting oxidative stress during
neuron activation (12).
Additionally, the effects of citicoline on sensory information
processing may stem from its ability to increase the surface area
of the nervous system through the augmentation of dendrite length
and branches (9). Moreover,
studies summarized by narrative reviews cited in this sentence have
indicated that citicoline administration mitigates behavioral
changes in rats subjected to chronic hypoxia by inducing the
vasodilation of blood vessels and increasing cerebral blood flow
(9,10).
Pharmacological effects of citicoline
in the CNS: An emphasis on infectious cases
Citicoline is water-soluble and almost completely
absorbed, with 92% bioavailability (10,12,54).
It is formed by connecting cytidine and choline through a
pyrophosphate bridge (54).
Following breakdown, citicoline is hydrolyzed in the intestine into
cytidine and choline, which are then absorbed and metabolized in
the liver (9,10,12,54).
Subsequently, citicoline is metabolized into phosphatidylcholine,
facilitated by the CDP-choline-(1,2-diacylglycerol choline-)
phosphotransferase enzyme (54).
Citicoline is widely distributed in the liver, kidney and brain,
with the brain accounting for the most significant proportion of
distribution (62.8%) (54). It
enters the brain as choline and uridine, with uridine being
converted to cytidine triphosphate within cells (9). The re-phosphorylation of the
pyrophosphate bridges in the brain then re-forms citicoline
(54). Citicoline is primarily
excreted through respiration as CO2, followed by urinary
excretion, with only 1% excreted in feces (9,10).
An adequate dose of citicoline ranges from 500-2,000
mg/day or 7-29 mg/kg body weight/day (12,54).
It should be noted that, to the best of our knowledge, no published
study to date has yet investigated the most effective dose and
route of administration of citicoline in infectious diseases,
particularly CNS infections. Previous trials, such as ICTUS, which
used citicoline as a neuroprotector in acute ischemic stroke,
employed a dose of 1,000 mg twice daily intravenously, followed by
500 mg twice daily for 6 weeks (18,19).
However, conflicting results were observed with the dose used for
stroke cases at 1,000 mg twice daily intravenously, followed by 500
mg twice daily for 6 weeks, with some patients exhibiting no
benefit (55,56) and others indicating statistically
significant enhancements in cognition and prognosis (57-59).
Given these research gaps, further studies are warranted to
determine the optimal dosage, route of administration and
effectiveness of citicoline in CNS infections.
Citicoline is available in oral and parenteral
forms, with similar metabolism and bioavailability between the two
forms of administration (54). In
most countries, citicoline is available in various forms, including
capsules/tablets (100, 200, 500 and 1,000 mg), solutions/syrups
(100 mg/ml, 100 mg/5 ml), and parenteral injections (62.5, 125 and
250 mg/m;), which can be administered as an intramuscular or
intravenous injection (slow bolus or drip) (information accessible
online from: https://verification.fda.gov.ph/med_mental_illnesseslist.php).
However, in Indonesia, citicoline preparations are limited to 500
and 1,000 mg capsules and 125 mg/ml injections; 500 mg of
citicoline taken orally yields 107 mg choline and 250 mg cytidine
(information accessible online from Drug Bank Online, MIMS Generic
Medicine Info, and Drugs.com entries on citicoline).
Although, to the best of our knowledge, there is no
specific study yet available on the cost-effectiveness of
citicoline in CNS infection, pharmaco-economic studies in other
neurological disorders have shown promising results. The addition
of citicoline to conventional therapy has demonstrated superiority
over placebo in ischemic stroke. The number of patients reporting
benefits was found to range from 50-99 per 1,000 cases, with cost
savings per patient reaching up to €101.20 (~$110.65) compared to
€126.40 (~$138.20) (60).
Similarly, a Russian study revealed a cost-effectiveness ratio of
citicoline to placebo of 435,368.00 RUB (~$5,204.82) compared to
513,099.20 RUB (~$6,134.10), respectively, with significant savings
in treatment costs totaling ~1,719,610.00 RUB (~$20,557.94)
(61).
Safety profile of citicoline
Citicoline is renowned for its excellent safety
record, rendering it appropriate for children and older individuals
(10,12,62).
The reported adverse effects are generally mild, including
gastrointestinal disorders such as diarrhea and abdominal pain
(10,62). Side-effects, such as headache, a
tingling sensation and numbness are usually transient and resolve
independently (62). A recent
meta-analysis did not support previous concerns about the effects
of citicoline on psychiatric episodes and interactions with
psychiatric drugs (57). The
administration of CDP-choline, a combination of choline and
cytidine, can reduce toxicity by up to 20-fold (14). Citicoline has no significant
toxicity in acute, sub-acute, or chronic use (39). In a study involving older
individuals with moderate-severe neurological deficits due to
ischemic stroke, administering 2 g/day of citicoline did not cause
significant side-effects (63).
Experimental animal studies indicated minor effects, such as a
slight increase in serum creatinine in male rats and renal tubular
mineralization in female rats after citicoline use at specific
doses, likely due to increased phosphorus intake (64). Citicoline has also demonstrated no
toxicity in pregnant women and fetuses (39). Although rare drug interactions have
been reported, one study suggested that citicoline can
significantly reduce the dose of levodopa and its associated
side-effects (39).
Relevance to the case presented and
recommendations
In the case described in the present study, the
patient presented symptoms of pulmonary TB and the suspected
involvement of the central nervous system alongside COVID-19
pneumonia. Despite previous treatments yielding no improvement and
worsening his condition, the administration of citicoline was
considered due to its favorable safety profile and absence of
contraindications. Based on available evidence, citicoline exhibits
potential to improve neurological disorders in CNS infections. It
has anti-infective properties, beneficial for both TB and viral
infections such as COVID-19 (11,22).
CNS TB and COVID-19 in the patient described herein can both cause
brain oxidative stress and inflammation, leading to neuronal
damage. The antioxidant and anti-inflammatory properties of
citicoline may reduce tissue damage and improve neurological
outcomes (9-12,25-29).
Additionally, its neuroprotective, neuromodulatory and
neurorestorative properties may aid in brain tissue repair, restore
normal neural signaling and neurotransmitter balance, and enhance
cognitive and motor functions caused by these infections (7,9-12).
Considering the current evidence supporting the safety and multiple
beneficial properties of citicoline, it may serve as a valuable
adjuvant treatment for patients, such as in the case presented
herein. However, the careful assessment and monitoring of the
patient's response to citicoline are essential. Further research
and clinical trials investigating the efficacy of citicoline in CNS
infections would be beneficial to strengthen its role in treatment
strategies.
In conclusion, in this case report, the use of
citicoline as an adjuvant therapy was investigated in an aim to
enhance consciousness and neurological function in a patient with
pulmonary TB and suspected CNS infection with co-infection with
COVID-19. Although specific studies on such cases are lacking,
considering the potential of citicoline as an adjuvant therapy in
CNS infection is warranted based on its effectiveness in stroke and
head trauma cases. Citicoline offers various mechanisms to improve
neurological and cognitive disorders associated with CNS
infection.
The findings underscore the uniqueness of using
citicoline in conjunction with conventional treatments, offering
potential benefits in reducing viral replication, inhibiting
oxidative stress, suppressing inflammatory responses, and promoting
neuronal repair and regeneration. The positive outcomes observed in
various neurological disorders and ischemic conditions further
support the therapeutic potential of citicoline. However, further
research is necessary, including preclinical studies, animal
studies, human studies, and randomized clinical trials, to provide
more substantial evidence regarding the benefits of citicoline as
an adjuvant therapy in CNS infections. Another limitation of the
present study is the absence of specialized sophisticated
diagnostic tools, such as blood tests for procalcitonin and
lactate. This deficiency hampers the process of diagnosing and
assessing the severity of the condition. It is preferable to
monitor future cases in a more advanced medical facility to ensure
that no crucial data is overlooked.
While the present study provides valuable insight
into the potential benefits of citicoline in CNS infections, the
authors acknowledge the limitation of evidence and literature
discussing this topic, as it represents a relatively novel
direction in neurology. To address this limitation, a pragmatic
approach was adopted, incorporating articles with varying levels of
evidence in the review and discussion. Further clinical research is
required to validate and expand upon the findings discussed herein,
in order to provide more robust evidence on the efficacy of
citicoline in the management of CNS infections.
Supplementary Material
Laboratory examination results for
complete blood count, white blood cell count, blood chemistry,
blood gas analysis, lumbar puncture analysis, COVID-19 test, and
tuberculosis examination of the patient at the time of admission
(reference range is based on the hospital laboratory).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH conceptualized the study and was involved in data
curation. MH was also involved in funding acquisition, obtaining
resources, utilizing software, data validation and visualization.
MH, SS and WKS were involved in the formal analysis. MH, SS and WKS
were also involved in reviewing the literature discussed in this
study. MH was involved in the treatment of this patient and was
responsible for this patient. MH and SS were involved in project
administration, and in the writing, reviewing and editing of the
manuscript. MH, SS and WKS were involved in the writing and
preparation of the first draft of the manuscript. MH and WKS
confirm the authenticity of the presented raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The patient has given his consent and permission for
his participation in the study by concealing the identity details
according to the principles of the Declaration of Helsinki. The
CARE guidelines were followed in the writing of the present case
report.
Patient consent for publication
The patient has given his consent and permission for
publication by concealing the identity details according to the
principles of the Declaration of Helsinki. The CARE guidelines were
followed in the writing of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Z, Song Y, Kang J, Duan S, Li Q,
Feng F and Duan J: Epidemiology of patients with central nervous
system infections, mainly neurosurgical patients: A retrospective
study from 2012 to 2019 in a teaching hospital in China. BMC Infect
Dis. 21(826)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
John CC, Carabin H, Montano SM, Bangirana
P, Zunt JR and Peterson PK: Global research priorities for
infections that affect the nervous system. Nature. 527:S178–S186.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Robertson FC, Lepard JR, Mekary RA, Davis
MC, Yunusa I, Gormley WB, Baticulon RE, Mahmud MR, Misra BK,
Rattani A, et al: Epidemiology of central nervous system infectious
diseases: A meta-analysis and systematic review with implications
for neurosurgeons worldwide. J Neurosurg. 130:1107–1126.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Giovane RA and Lavender PD: Central
nervous system infections. Prim Care. 45:505–518. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gajurel BP, Giri S, Rayamajhi S, Khanal N,
Bishowkarma S, Mishra A, Karn R, Rajbhandari R and Ojha R:
Epidemiological and clinical characteristics of central nervous
system infections in a tertiary center: A retrospective study.
Health Sci Rep. 6(e1099)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
El-Assaad F, Combes V, Grau GER and Jambou
R: Potential efficacy of citicoline as adjunct therapy in treatment
of cerebral malaria. Antimicrob Agents Chemother. 58:602–605.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Warsiki E: The effect of citicoline
injections on children with organic brain syndrome caused by
infection. Paediatr Indones. 33:87–94. 2019.
|
|
8
|
Mallah K, Couch C, Borucki DM, Toutonji A,
Alshareef M and Tomlinson S: Anti-inflammatory and neuroprotective
agents in clinical trials for CNS disease and injury: Where do we
go from here? Front Immunol. 11(2021)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qureshi SS, Gupta JK and Mishra P:
Citicoline: A potential breakthrough in cerebrovascular disorder.
Austi J Pharmacol Ther. 4(1077)2016.
|
|
10
|
Qureshi I and Endres JR: Citicoline: A
novel therapeutic agent with neuroprotective, neuromodulatory, and
neuroregenerative properties. Nat Med J. 2:11–25. 2010.
|
|
11
|
Turana Y, Nathaniel M, Shen R, Ali S and
Aparasu RR: Citicoline and COVID-19-related cognitive and other
neurologic complications. Brain Sci. 12(59)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Al-Kuraishy HM, Al-Buhadily AK, Al-Gareeb
AI, Alorabi M, Hadi Al-Harcan NAH, El-Bouseary MM and Batiha GES:
Citicoline and COVID-19: Vis-à-vis conjectured. Naunyn
Schmiedebergs Arch Pharmacol. 395:1463–1475. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jambou R, El-Assaad F, Combes V and Grau
GE: Citicoline (CDP-choline): What role in the treatment of
complications of infectious diseases. Int J Biochem Cell Biol.
41:1467–1470. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
D'Orlando KJ and Sandage BW Jr: Citicoline
(CDP-choline): Mechanisms of action and effects in ischemic brain
injury. Neurol Res. 17:281–284. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Samsudin I and Vasikaran SD: Clinical
utility and measurement of procalcitonin. Clin Biochem Rev.
38:59–68. 2017.PubMed/NCBI
|
|
16
|
Vijayan AL, Vanimaya V, Ravindran S,
Saikant R, Lakshmi S, Kartik R and Manoj E: Procalcitonin: A
promising diagnostic marker for sepsis and antibiotic therapy. J
Intensive Care. 5(51)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martí-Carvajal AJ, Valli C, Martí-Amarista
CE, Solà I, Martí-Fàbregas J and Bonfill Cosp X: Citicoline for
treating people with acute ischemic stroke. Cochrane Database Syst
Rev. 8(CD013066)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dávalos A, Alvarez-Sabín J, Castillo J,
Díez-Tejedor E, Ferro J, Martínez-Vila E, Serena J, Segura T, Cruz
VT, Masjuan J, et al: Citicoline in the treatment of acute
ischaemic stroke: An international, randomised, multicentre,
placebo-controlled study (ICTUS trial). Lancet. 380:349–357.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Overgaard K: The effects of citicoline on
acute ischemic stroke: A review. J Stroke Cerebrovas Dis.
23:1764–1769. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nusanti S, Putri RL and Dearaini D:
Reversible ethambutol-induced optic neuropathy: Report of a rare
case. Universa Med. 41:271–276. 2022.
|
|
21
|
National Center for Biotechnology
Information: PubChem Compound Summary for CID 13804, Citicoline.
National Library of Medicine, Bethesda, MD, 2023.
|
|
22
|
Eid HM, Ali AA, Ali AMA, Eissa EM, Hassan
RM, Abo El-Ela FIA and Hassan AH: Potential use of tailored
citicoline chitosan-coated liposomes for effective wound healing in
diabetic rat model. Int J Nanomedicine. 17:555–575. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Poh XY, Loh FK, Friedland JS and Ong CWM:
Neutrophil-mediated immunopathology and matrix metalloproteinases
in central nervous system-tuberculosis. Front Immunol.
12(788976)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Longhitano L, Tibullo D, Giallongo C,
Lazzarino G, Tartaglia N, Galimberti S, Li Volti G, Palumbo GA and
Liso A: Proteasome inhibitors as a possible therapy for SARS-CoV-2.
Int J Mol Sci. 21(3622)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Manyelo CM, Solomons RS, Walzl G and
Chegou NN: Tuberculous meningitis: Pathogenesis, immune responses,
diagnostic challenges, and the potential of biomarker-based
approaches. J Clin Microbiol. 59:e01771–20. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Davis AG, Rohlwink UK, Proust A, Figaji AA
and Wilkinson RJ: The pathogenesis of tuberculous meningitis. J
Leukoc Biol. 105:267–280. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Romero-Adrian TB, Leal-Montiel J,
Fernández G and Valecilo A: Role of cytokines and other factors
involved in the Mycobacterium tuberculosis infection. World
J Immunol. 5:16–50. 2015.
|
|
28
|
Chamorro A, Urra X and Planas AM:
Infection After Acute Ischemic Stroke: A manifestation of
brain-induced immunodepression. Stroke. 38:1097–1103.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Grabska K, Gromadzka G and Członkowska A:
Infections and ischemic stroke outcome. Neurol Res Int.
2011(691348)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chavda V, Chaurasia B, Fiorindi A, Umana
GE, Lu B and Montemurro N: Ischemic stroke and SARS-CoV-2
infection: The bidirectional pathology and risk morbidities. Neurol
Int. 14:391–405. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dmytriw AA, Dibas M, Phan K, Efendizade A,
Ospel J, Schirmer C, Settecase F, Heran MKS, Kühn AL, Puri AS, et
al: Acute ischaemic stroke associated with SARS-CoV-2 infection in
North America. J Neurol Neurosurg Psychiatry. 93:360–368.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang S, Liu L, Xie Z, He Y, Zhang Y, Xie
Y, Chen S, Liu Y, Wei Y and Liang Z: Acute Ischemic stroke in
tubercular meningitis patients without conventional vascular risk
factors: A retrospective case control study. J Inflamm Res.
15:6617–6627. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee HR, Yoo JE, Choi H, Han K, Jung JH,
Park J, Lee H and Shin DW: Tuberculosis and risk of ischemic
stroke: A nationwide cohort study. Stroke. 53:3401–3409.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huaman MA, Henson D, Ticona E, Sterling TR
and Garvy BA: Tuberculosis and cardiovascular disease: Linking the
epidemics. Trop Dis Travel Med Vaccines. 1(10)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang J and Maldonado MA: The
ubiquitin-proteasome system and its role in inflammatory and
autoimmune diseases. Cell Mol Immunol. 3:255–261. 2006.PubMed/NCBI
|
|
36
|
Hemalika DVD: Phospholipase enzymes as
potential biomarker for SARS CoV-2 virus. Int J Sci Res Pub.
11:189–197. 2021.
|
|
37
|
Müller C, Hardt M, Schwudke D, Neuman BW,
Pleschka S and Ziebuhr J: Inhibition of cytosolic phospholipase
A2α impairs an early step of coronavirus replication in
cell culture. J Virol. 92:e01463–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zweigner J, Jackowski S, Smith SH, van der
Merwe M, Weber JR and Tuomanen EI: Bacterial inhibition of
phosphatidylcholine synthesis triggers apoptosis in the brain. J
Exp Med. 200(99)2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Secades JJ and Gareri P: Citicoline:
Pharmacological and clinical review, 2022 update. Rev Neurol.
75:S1–S89. 2022.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
40
|
Channer B, Matt SM, Nickoloff-Bybel EA,
Pappa V, Agarwal Y, Wickman J and Gaskill PJ: Dopamine, immunity,
and disease. Pharmacol Rev. 75:62–158. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nolan RA, Muir R, Runner K, Haddad EK and
Gaskill PJ: Role of macrophage dopamine receptors in mediating
cytokine production: Implications for neuroinflammation in the
context of HIV-associated neurocognitive disorders. J Neuroimmune
Pharmacol. 14:134–156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Calderon TM, Williams DW, Lopez L, Eugenin
EA, Cheney L, Gaskill PJ, Veenstra M, Anastos K, Morgello S and
Berman JW: Dopamine increases CD14+CD16+
monocyte transmigration across the blood brain barrier:
Implications for substance abuse and HIV neuropathogenesis. J
Neuroimmune Pharmacol. 12:353–370. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sevim Ç, Altinbaş B, Yalçin M, Inan S,
Özyiğit MÖ, Arican İ and Yilmaz MS: Protective effect of
CDP-choline on hypotension and tissue injury in septic shock model.
Ankara Üniv Vet Fak Derg. 64:103–110. 2017.
|
|
44
|
Kobayashi T, Uchino H, Elmér E, Ogihara Y,
Fujita H, Sekine S, Ishida Y, Saiki I, Shibata S and Kawachi A:
Disease outcome and brain metabolomics of cyclophilin-D knockout
mice in sepsis. Int J Mol Sci. 23(961)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang Y, Liu Y, Wang Y, Chao Y, Zhang J,
Jia Y, Tie J and Hu D: Regulation of SIRT1 and its roles in
inflammation. Front Immunol. 13(831168)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bordoni V, Tartaglia E, Sacchi A, Fimia
GM, Cimini E, Casetti R, Notari S, Grassi G, Marchioni L, Bibas M,
et al: The unbalanced p53/SIRT1 axis may impact lymphocyte
homeostasis in COVID-19 patients. Int J Infect Dis. 105:49–53.
2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hemmat N, Asadzadeh Z, Ahangar NK,
Alemohammad H, Najafzadeh B, Derakhshani A, Baghbanzadeh A, Baghi
HB, Javadrashid D, Najafi S, et al: The roles of signaling pathways
in SARS-CoV-2 infection; Lessons learned from SARS-CoV and
MERS-CoV. Arch Virol. 166:675–696. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hurtado O, Hernández-Jiménez M, Zarruk JG,
Cuartero MI, Ballesteros I, Camarero G, Moraga A, Pradillo JM, Moro
MA and Lizasoain I: Citicoline (CDP-choline) increases Sirtuin1
expression concomitant to neuroprotection in experimental stroke. J
Neurochem. 126:819–826. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Grieb P: Neuroprotective properties of
citicoline: Facts, doubts and unresolved issues. CNS Drugs.
28:185–193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Swain SK, Das S and Lenka S: Sudden
sensorineural hearing loss among COVID-19 patients-our experiences
at an Indian teaching hospital. Siriraj Med J. 73:77–83. 2021.
|
|
51
|
Kinoshita J, Iwata N, Maejima T, Kimotsuki
T and Yasuda M: Retinal function and morphology in monkeys with
ethambutol-induced optic neuropathy. Invest Ophthalmol Vis Sci.
53:7052–7062. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nusanti S, Sari RI, Siregar NC and Sidik
M: The effect of citicoline on ethambutol optic neuropathy:
Histopathology and immunohistochemistry analysis of retina ganglion
cell damage level in rat model. J Ocul Pharmacol Ther. 38:584–589.
2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yasuhara M and Naito H: Characteristic
actions of CDP-choline on the central nervous system. Curr Ther Res
Clin Exp. 16:346–374. 1974.PubMed/NCBI
|
|
54
|
Faiq MA, Wollstein G, Schuman JS and Chan
KC: Cholinergic nervous system and glaucoma: From basic science to
clinical applications. Prog Retin Eye Res.
72(100767)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shaukat N, Nisar S, Jan OA, Sajid U,
Mahmood A and Zahid H: The role of citicoline in neuroprotection
and neuro repair in acute stroke. Pak Armed Forces Med J.
73:1161–1164. 2023.
|
|
56
|
Agarwal A, Vishnu VY, Sharma J, Bhatia R,
Garg A, Dwivedi S, Upadhyay A, Goyal V, Singh MB, Gupta A, et al:
Citicoline in acute ischemic stroke: A randomized controlled trial.
PLoS One. 17(e0269224)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rana D: Evaluation of therapeutic efficacy
of citicoline in acute ischemic stroke patients: A meta analysis.
Int J Med Pharm Res. 4:39–51. 2023.
|
|
58
|
Premi E, Cantoni V, Benussi A, Gilberti N,
Vergani V, Delrio I, Gamba M, Spezi R, Costa A, Padovani A, et al:
Citicoline treatment in acute ischemic stroke: A randomized,
single-blind TMS study. Front Neurol. 13(915362)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kuryata OV, Kushnir YS, Nedzvetsky VS,
Korsa VV and Tykhomyrov AA: Serum levels of the biomarkers
associated with astrocytosis, neurodegeneration, and demyelination:
Neurological benefits of citicoline treatment of patients with
ischemic stroke and atrial fibrillation. Neurophysiology. 53:2–12.
2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Casado A, Secades JJ, Ibarz R, Herdman M
and Brosa M: Cost-effectiveness of citicoline versus conventional
treatment in acute ischemic stroke. Expert Rev Pharmacoecon
Outcomes Res. 8:151–157. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ryazhenov VV and Gorokhova SG:
Pharmacoeconomic benefits of citicoline in the treatment of acute
ischemic stroke in Russia. Value Health. 16:A519–A520. 2013.
|
|
62
|
Gareri P, Castagna A, Cotroneo AM,
Putignano S, De Sarro G and Bruni AC: The role of citicoline in
cognitive impairment: Pharmacological characteristics, possible
advantages, and doubts for an old drug with new perspectives. Clin
Interv Aging. 10:1421–1429. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Putignano S, Gareri P, Castagna A, Cerqua
G, Cervera P, Cotroneo AM, Fiorillo F, Grella R, Lacava R, Maddonni
A, et al: Retrospective and observational study to assess the
efficacy of citicoline in elderly patients suffering from stupor
related to complex geriatric syndrome. Clin Interv Aging.
7:113–118. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Schauss AG, Somfai-Relle S, Financsek I,
Glavits R, Parent SC, Endres JR, Varga T, Szücs Z and Clewell A:
Single- and repeated-dose oral toxicity studies of citicoline
free-base (choline cytidine 5'-pyrophosphate) in Sprague-Dawley
rats. Int J Toxicol. 28:479–487. 2009.PubMed/NCBI View Article : Google Scholar
|















