Introduction
Endometriosis is a chronic gynecological disease
that affects ~10% of females of reproductive age (1). It is characterized by the presence of
endometrium-like tissue outside the uterine cavity, mainly in the
peritoneal cavity, ovaries, bladder and intestine (2). Endometriotic implants respond to sex
steroids, in the same manner as the endometrium, characterizing
endometriosis as a hormone-dependent condition (3).
Since the first description of endometriosis, its
etiopathogenesis has been extensively studied. However, despite
extensive efforts made in this direction over the past decade, none
of the theories described can explain all presentations of the
disease. The most commonly accepted theory is the theory of
retrograde menstruation (the implantation or Sampson theory), which
states that endometriosis is the result of the implantation and
establishment of viable endometrial cells regurgitated through the
fallopian tubes (4). However,
retrograde menstruation occurs in ~80% of females of reproductive
age with pervious tubes (5).
Thus, further research is required to elucidate the
putative role of retrograde menstruation in the pathophysiology of
endometriosis. Retrograde menstruation, a type of menstrual
effluent, involves growth factors, cytokines, and immune and
endometrial cells that can be packed in cell condensates (6,7), but
not endometrial tissue fragments (8). Clonal mesenchymal stem cells can also
be found in menstrual effluent (6,9), and
their role in endometriosis has been discussed since their first
identification (10).
In order to elucidate the mechanisms and to propose
therapeutic approaches for endometriosis, numerous in vitro
and in vivo models of the disease have been proposed
(2,11,12).
Rodent models are the main in vivo models but they are
limited by the species-specific differences (rodents do not develop
endometriosis spontaneously) (2,13,14),
and the tissue explant mice models are limited by the availability
of female tissues and the duration of the cultures by residual
immune response from the host (11,15).
In addition, the results obtained from these models may be related
to the method of endometriosis induction, and the association
between inflammation and disease cannot be adequately addressed
(2). The non-human primate models
are very close to the physiology and immunology observed in
females; however, they raise several ethical concerns and are a
very costly (11,16-19).
Due to all these reasons, the in vitro models became a
suitable and effective alternative for the study of
endometriosis.
The most applied in vitro model is the
monolayer (bi-dimensional) culture of primary, lineages or
immortalized cells (2,11,12).
It provides crucial data on the specific cell types and
cell-to-cell interactions (12);
however, it cannot mimic the complete peritoneal and/or endometrial
environment (11,12,14,18),
lacking the essential feedback that occurs in in vivo tissue
(14). The translation of this
model into clinical practice is very limited (2).
Being closer to in vivo physiology than the
majority of other models, the three-dimensional ones are promising,
and highly novel, for the study of endometriosis pathophysiology,
mirroring cell phenotypes and gene expression, and allowing the
study of different cell interactions and mechanisms (18). The three-dimensional models are
able to mimic the human tissues features as cellular organization
and interaction to extracellular matrix and between cells (20). The matrix dependent
three-dimensional cultures are the most commonly applied
three-dimensional model for the study of endometriosis (21-23).
The first two models developed employed only endometrial epithelial
cells (eECs) (22,23) and most recently, a model also
including endometrial stromal cells (eSCs) was developed (24). Those models are able to resemble
the eutopic endometrium physiology (22-24)
and mimic the interactions between cells and extracellular matrix,
but not the behaviour of endometrial cells in the matrix-free
environment, such as peritoneal fluid (25,26).
According to this, the matrix-free model is the
better choice of model for studying the endometrial cells
regurgitated through the fallopian tubes (26), as the peritoneal cavity is a fluid
environment constantly moving within abdominopelvic compartments
(27,28). eSCs and eECs are able to form
spheroids in matrix-free three-dimensional models resembling cell
condensates found in menstrual effluent (14,29).
Menstrual effluent presents different ratios between epithelial and
stromal cells, with eSCs being abundantly viable than eECs
(30,31). Patients with endometriosis appear
to have an increased number of stromal cells in their menstrual
effluent (31,32).
It is well known that interactions between
co-cultured epithelial and stromal cells can activate epithelial
cells and lead to cell polarization and phenotypic changes
(33). The majority of the cells
shed in menstrual effluent are senescent (32) and are not viable for long periods
of time without an attachment surface. However, a lack of
extracellular matrix has been linked to the switch in endometrial
cells from undergoing apoptosis to undergoing
epithelial-to-mesenchymal transition (EMT) (34), which is a phenotypic cell change
characterized by changes in cell-to-cell contact, cytoskeleton
architecture, cell polarity and motility (35). This switch can be related to the
survival of cells in the peritoneal cavity and the early initiation
of endometriosis (36).
The present study aimed to characterize the
spheroids formed by the two main endometrial cell types, stromal
and epithelial primary cells, and to elucidate the mechanisms
involved in the formation of stable cellular structures in a
peritoneal fluid-like environment. A better understanding of these
mechanisms may contribute to the elucidation of the mechanisms
involved in the early initiation of endometriosis.
Materials and methods
Human tissues and cell culture
The study protocol was approved by the Universidade
Federal de São Paulo Ethics Committee (study no. 1054/2021 part of
the main project no. 0875/2018, São Paulo, Brazil), and informed
written consent was obtained from all patients. Human endometrial
samples were collected from four cycling women (n=4) (details of
the patients are presented in Table
SI) undergoing laparoscopic surgery or at an outpatient clinic
at the Pelvic Pain and Endometriosis Unit of the Federal University
of São Paulo, São Paulo, Brazil. The samples were collected between
August, 2020 and March, 2022. Endometrium biopsies were collected
using a pipelle curette (Laboratoire CCD) from four (n=4) cycling
women aged 18-40 years who were examined at the gynecology
outpatient clinic and met the following inclusion criteria: They
had revised American Society of Reproductive Medicine (rASRM) stage
IV endometriosis, had not taken exogenous hormones and had not
given birth or breastfed over the past 3 months prior to sample
collection. Patients who presented with comorbidities, such as
adenomyosis, uterine fibroids, teratomas, myoma or other
endometrial and pelvic inflammatory diseases were not included in
the study.
Cells were isolated from endometrial biopsies from a
modified protocol based on previously described protocols (33,37).
The endometrial tissue was washed twice with phosphate-buffered
saline pH 7.4 (PBS; MilliporeSigma) and minced into smaller
sections. The tissue fragments were digested in Dulbecco's modified
Eagle's medium with nutrient mixture F12 (DMEM:F12) (Thermo Fisher
Scientific, Inc.) containing 255 units of collagenase type IA
(MilliporeSigma) and incubated for 30 min in a 37˚C water bath
under constant agitation. Following the digestion, the cell and
fragments suspension was filtrated with a 40-µm cell strainer to
separate single eSCs and endometrium epithelial sheets and glands.
eSCs, single cells that have passed through the strainer, were
inoculated in eSC medium: DMEM:F12 pH 7.4, 1% Minimum Essential
Medium non-essential amino acids (Gibco; Thermo Fisher Scientific,
Inc.), 0.1 mmol/l 2-mercaptoethanol (MilliporeSigma), 100 U/ml
penicillin and 100 µg/ml streptomycin and 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) into a 25
cm2 cell culture flask. The epithelial clusters retained
in the 40-µm strainer were inoculated in EC medium: DMEM-F12, pH
7.4, 100 U/ml penicillin and 100 µg/ml streptomycin, 2.5 mM FBS,
2.5 mM Nu-Serum (Corning, Inc.) and 1% of L-glutamine into a
pre-coated collagen type IV (MilliporeSigma) 35 mm dish. The
isolated cells were characterized as stromal and epithelial through
the detection of vimentin [1:100 Vimentin (Alexa 488), cat. no.
562338] and cytokeratin [1:50 Pan-cytokeratin-phycoerythrin (PE);
cat. no. 347204; BD Biosciences] expression by fluorescence
microscopy (Fig. S1). The
detailed staining method is described below.
Living cell co-culture spheroid
assay
Calcein (green or orange) was reconstituted to 1
mg/ml in DMSO (MilliporeSigma) according to the manufacturer's
protocol. The eSCs were labeled with 10 µM calcein-AM (cat. no.
C3100MP, Invitrogen; Thermo Fisher Scientific, Inc.) diluted in eSC
medium and the eECs with 10 µM RedOrange-Calcein-AM (cat. no.
C34851, Invitrogen; Thermo Fisher Scientific, Inc.) diluted in eEC
medium and incubated for 15 min at 37˚C/5% CO2, prior to
cell dissociation for co-culture assay. Cells were dissociated from
the culture flasks and suspended in SP medium (DMEM:F12 pH 7.4, 1%
Minimum Essential Medium non-essential amino acids (Gibco; Thermo
Fisher Scientific, Inc.), 0.1 mmol/l 2-mercaptoethanol
(MilliporeSigma), 100 U/ml penicillin and 100 µg/ml streptomycin
and 2 mM L-glutamine). The cell concentration and viability were
determined using a Countess™ Cell Counter (Thermo Fisher
Scientific, Inc.) with the cells were stained with trypan blue 0.4%
(Thermo Fisher Scientific, Inc.) 1:2 sample dilution factor at room
temperature for 30 sec. The two cell types were mixed at three
different ratios of eSCs:eECs as follows: 9:1 for the highly
stromal (HS) group, 1:1 for the equal ratio (SE) group and 1:9 for
the highly epithelial (HE) group. The mixed cell suspensions were
seeded at a density of 10,000 cells per well into 24-well ultralow
attachment plates (CELLSTAR™, Cell-Repellent Surface;
Greiner Bio-One). Living spheroids were visualized by fluorescence
microscopy on an EVOS™ FL Digital Inverted Microscope
(AMG-Advanced Microscopy Group) using the channels GFP (470 nm
excitation, 525 nm emission) and RFP (531 nm excitation, 593 nm
emission) at 1, 2 and 3 days after seeding.
Spheroid assay
Non-stained eSCs and eECs were dissociated from
culture flasks and suspended in SP medium. The two cell types were
mixed at three different eSC:eEC ratios: 9:1 for the HS group
(highly stromal), 1:1 for the SE group (equal ration) and 1:9 for
the HE group (highly epithelial). The mixed cell suspensions were
seeded at a density of 10,000 cells per well into 24-well ultralow
attachment plates (CELLSTAR®, Cell-Repellent Surface;
Greiner Bio-One). Fresh media SP medium was added every 3-4 days.
Spheroids were visualized using brightfield microscopy with an
AxioVert PrimoVert microscope, and images were taken with Zen 2012
(blue edition) software (Carl Zeiss Microscopy GmbH 2011). Images
were recorded at 3, 7 and 15 days after seeding. The morphological
characteristics of the spheroids were assessed using ImageJ (FIJI)
Macro INSIDIA 2.0, software version 2.9.0; Java 1.8.0_322
(64-bit).
Viability assay
The viability of the spheroids was assessed after 15
days of culture. The spheroids were labelled with 10 µM calcein-AM
(cat. no. C3100MP, Invitrogen; Thermo Fisher Scientific, Inc.) and
1 µg/ml Hoescht 33342 (Sigma-Aldrich, Merck KGaA) added to each
well and incubated at 37˚C 5% CO2 for 15 min. Medium was
partially removed (70% of total medium in the well) and the same
volume of fresh medium added. Spheroids were visualized in
EVOS™ FL Digital Inverted Microscope (AMG-Advanced
Microscopy Group) using the channels GFP (470 nm excitation, 525 nm
emission) for calcein detection (cytoplasm) and DAPI (357 nm
excitation, 447 nm emission) for Hoescht 33342 detection
(nuclei).
Immunofluorescence assay
Spheroids were labeled using direct
immunofluorescence method which applies primary
fluorophore-conjugated antibodies without a secondary antibody
step. Spheroids were fixed with 4% paraformaldehyde (PFA)
(MilliporeSigma) for 15 min and permeabilized with 0.3% Triton
X-100 solution in phosphate buffer (pH 7.4; PBS) for 10 min, at
room temperature. Unspecific sites were blocked with 2% bovine
serum albumin (BSA) solution in PBS for 30 min at room temperature.
Spheroids were stained for 1 h at room temperature in PBS pH 7.4
with 0.2% BSA buffer containing one of the conjugated antibodies
pairs: Vimentin [1:100 Vimentin (Alexa 488); cat. no. 562338] and
pancytokeratin [1:50 Pan-Cytokeratin (PE); cat. no. 347204; BD
Biosciences] or the cell surface glycoprotein MUC18 [1:50
CD146-Fluorescein isothiocyanate (FITC); cat. no. 560846; BD
Biosciences] and integrin-β1 [1:200 CD29 (PE); cat. no. 556049; BD
Biosciences]. The control slides were prepared in the same manner
and stained with the fluorophore pairs used in the testing slides.
The isotype controls used were the following: Immunoglobulin G1
(IgG1) isotype controls conjugated with PE (cat. no. 550617, BD
Biosciences), FITC (cat. no. 555909, BD Biosciences) and Alexa 488
(cat. no. 557702, BD Biosciences) at the same concentrations as the
conjugated antibodies. The nuclei were stained with
4',6-diamidino-2-phenylindol (DAPI) (MilliporeSigma) in PBS pH 7.4
for 15 min at room temperature. Spheroid images were captured using
an EVOS™ FL Digital Inverted Microscope (AMG-Advanced
Microscopy Group) with the following channels: DAPI (357 nm
excitation, 447 nm emission), GFP (470 nm excitation, 525 nm
emission) and RFP (531 nm excitation, 593 nm emission).
Statistical analysis
All cell cultures and experiments were performed in
triplicate. Numeric variables are presented as the mean and
standard deviation. Measurements were compared using the
Kruskal-Wallis test, and pairwise comparisons were performed using
the Mann-Whitney U test. Comparisons over time were calculated
using repeated-measures and mixed effects ANOVA. The reported
P-values for the pairwise comparisons were adjusted by the
Dunn-Bonferroni adjustment. The data were analyzed using IBM SPSS
Statistics version 2. A P-value <0.05 was considered to indicate
a statistically significant difference (95% confidence).
Results
Role of eSCs and eECs in spheroid
assembly and structure
The spheroids had different morphologies and
structural organizations depending on the ratio of eSCs to eECs.
The proportions of eSCs and eECs in the cell aggregates formed on
day 1 were determined for the HS and HE groups (Fig. 1A and G). The seeding of equal amounts of eSCs
and eECs (group SE) resulted in a greater proportion of stromal
cells forming cell aggregates on day 1 (Fig. 1D). Furthermore, none of the seeds
had a spheroid-like structure on day 1.
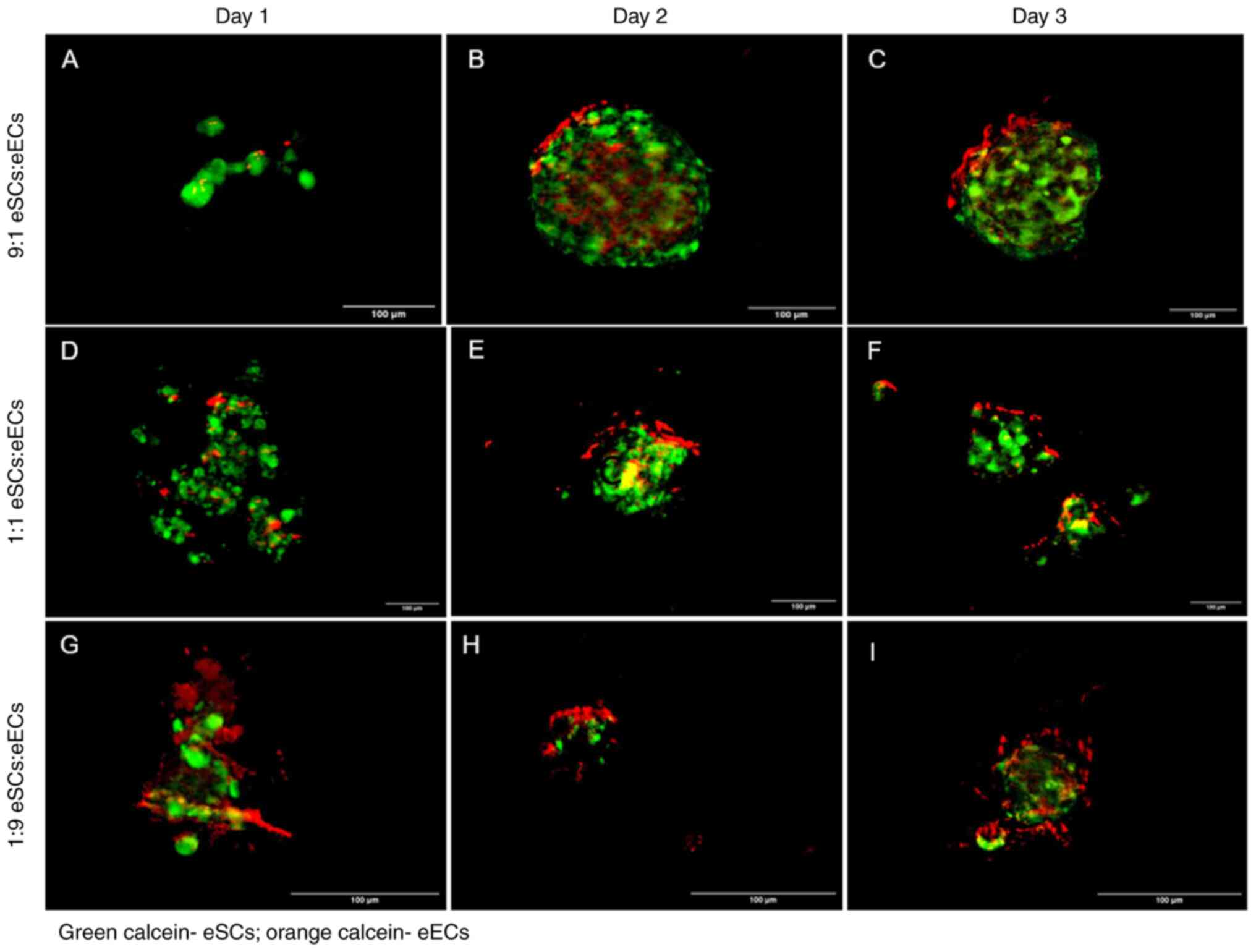 | Figure 1Images of cell aggregates and
spheroids with different eSC and eEC seeding ratios. The eSCs were
labeled with calcein-AM (green), and the eECs are labeled with
RedOrange-Calcein-AM (red). (A-C) Fluorescence microscopy for the
HS group (9:1 eSC:eEC ratio; highly stromal) on days 1, 2 and 3,
respectively; (D-F) fluorescence microscopy for the SE group (1:1
eSC:eEC ratio; equal ratios) on days 1, 2 and 3, respectively;
(G-I) fluorescence microscopy for the HE group (1:9 eSC:eEC ratio;
highly epithelial) on days 1, 2 and 3, respectively. All images
were obtained at the same magnification (x10) and laser powers.
Scale bars, 100 µm. eSCs, endometrial stromal cells; eECs,
endometrial epithelial cells. |
The HS group had a spheroid structure at 2 days
after seeding; eECs were found at the surface of the spheroid, and
eSCs occupied the center (Fig.
1B). Compared with those in the HS group, the spheroids in the
SE and HE groups were smaller (Fig.
1E and H). The distribution of
eECs and eSCs was the same as that in the HS group. Groups SE and
HE formed spheroids with less circularity.
The fluorescent signal of calcein was still faint at
3 days after seeding. The size, structure, cellularity and
circularity remained the same for the HS group (Fig. 1C). The spheroids formed in the SE
group had a non-spherical structure on day 3 (Fig. 1F). The spheroids in the HE group
maintained their general structure and circularity, but the inner
part appeared to be filled with eSCs (Fig. 1I).
Spheroid assembly and evolution over
time
Spheroids with different eEC and eSC contents were
imaged (Fig. 2A-I) and measured at
3, 7 and 15 days after seeding. The main measurement used for
analysis was the radiusE, which was calculated using the optimal
fit ellipse to a single spheroid. At 3 days after seeding, the HS
group formed one spheroid per well with a radius ranging from 30 to
50 µm (mean, 37 µm; SD ±9.90), while the SE group formed 12
spheroids with radii ranging from 18 to 28 µm (mean, 24 µm; SD
±4.17), and the HE group formed 10 spheroids with radii ranging
from 13 to 28 µm (mean, 20 µm; SD ±5.40). Spheroids from the HS
group were significantly larger than those from the HE group
(P=0.008; Fig. 2J).
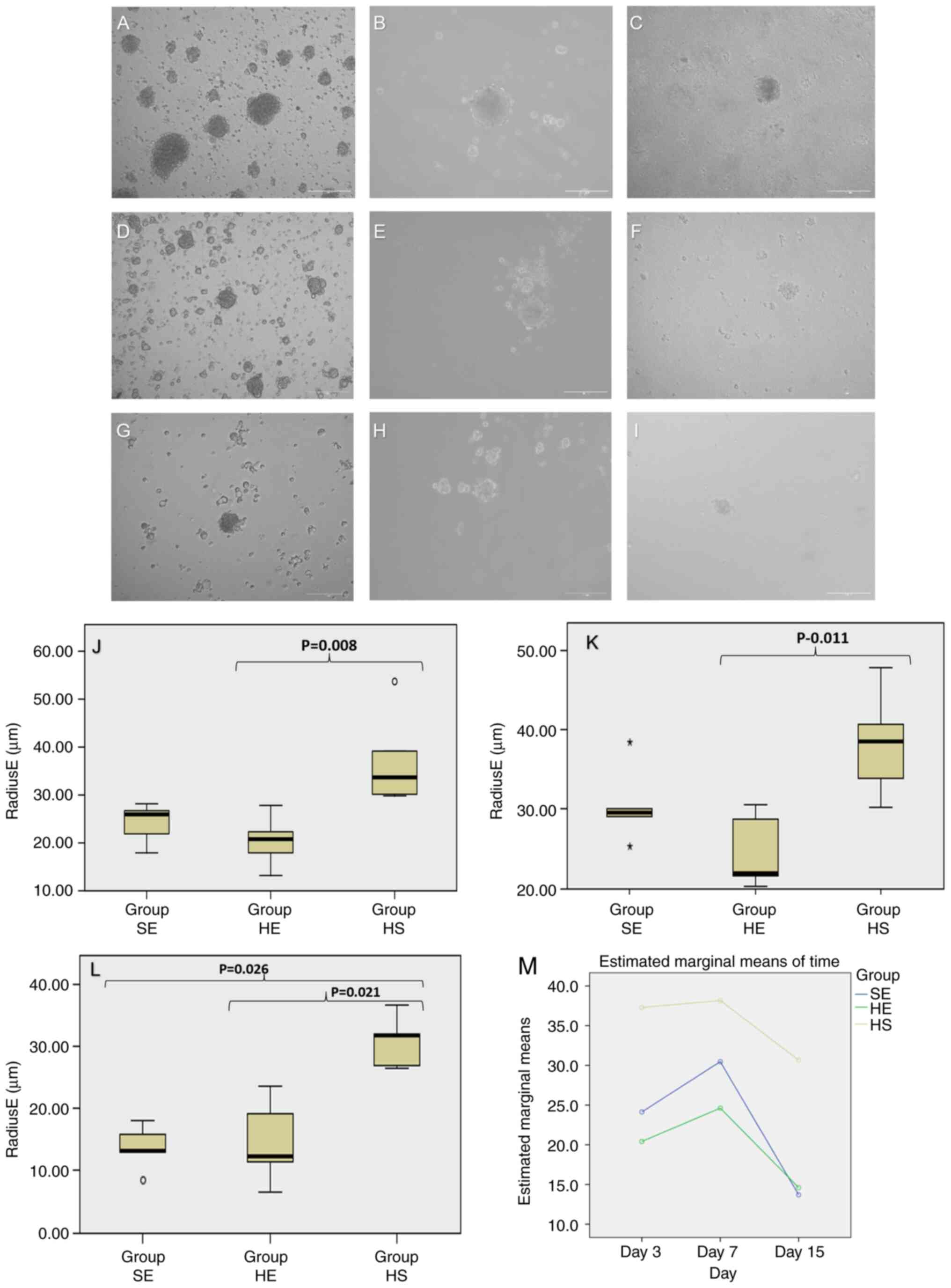 | Figure 2Brightfield microscopy images of the
spheroids at 3, 7 and 15 days after seeding. (A-C) Images from the
HS group (9:1 eSC:eEC ratio; highly stromal); (D-F) images from the
SE group (1:1 eSC:eEC ratio; equal ratios); (G-I) images from the
HE group (1:9 eSC:eEC ratio; highly epithelial). Scale bars, 100
µm. (J) Boxplot representing the RadiusE (µm) from spheroids on day
3. (K) Boxplot representing the RadiusE (µm) from spheroids on day
7. (L) Boxplot representing the RadiusE (µm) from spheroids on day
15; y axis, Radius E in µm; x axis, experimental groups [HS (9:1
eSCs:eECs), SE (1:1 eSCs:eECs) and HE (1:9 eSCs:eECs)]. (M) Changes
in spheroid radius E over time; x axis, time in days (3, 7 and 15
days after seeding); y axis, marginal estimated means for radiusE
calculated using repeated measures ANOVA and mixed effects ANOVA;
green curve, HE group (1:9 eSC:eEC ratio; highly epithelial); blue
curve, SE group (1:1 eSC:eEC ratio; equal ratio); yellow curve, HS
group (9:1eSC:eEC ratio; highly stromal). Mauchly's W=0.729,
P=0.176; within subjects by day (P<0.001) and the day and group
(P=0.598) and between subjects (type) (P<0.001). eSCs,
endometrial stromal cells; eECs, endometrial epithelial cells. |
On day 7, the HS group had spheroids ranging from 30
to 48 µm (mean, 38 µm; SD ±6.71), the SE group had spheroids
ranging from 25 to 38 µm (mean, 30 µm; SD ±4.80) and the HE group
had spheroids ranging from 20 to 31 µm (mean, 25 µm; SD ±4.67)
(P=0.011; Fig. 2K). The spheroids
did not change in radius significantly between days 3 and 7, for
all groups (HS, P=0.999; SE, P=0.640; and HE, P=0.999). The
spheroids from the SE and HE groups became smaller after 15 days of
co-culture (comparison to day 7, P<0.001 and P=0.015,
respectively). Measurements at 15 days followed the patterns
observed at 3 and 7 days of co-culture. The HS group had larger
spheroids (radiusE mean, 31 µm; SD ±4.10) than the SE group (mean,
14 µm; SD ±3.55) and the HE group (mean, 15 µm; SD ±6.80) (P=0.009)
(Fig. 2L). The spheroids from the
SE group became smaller after 15 days of co-culture (comparison to
HE, P=0.999 and HS, P=0.001 and to SE on day 7, P<0.001). Over
time, the spheroids in the HS group were more stable and larger
than the spheroids in the SE and HE groups (P<0.001; Fig. 2M). Spheroids remained viable after
15 days of co-culture (Fig.
S2).
Immunocytofluorescence
characterization of the cells composing the spheroids
The eECs and eSCs were characterized as epithelial
and stromal by the expression of vimentin and cytokeratin,
respectively (Fig. S1). Images of
the cells stained with calcein demonstrated that the spheroids were
composed of both epithelial and stromal cells. The phenotype of the
cells in the spheroids was confirmed by the presence of vimentin,
cytokeratin, CD29 and CD146 in the spheroids on day 7 after
seeding. Vimentin and cytokeratin staining corroborated the calcein
living cell results, as cytokeratin markers were detected in the
cells on the surface of the spheroids, and vimentin was detected in
the inner part of the spheroids (Fig.
3), reinforcing the polarity of the epithelial cells and their
tendency toward the lining.
Notably, the amount of CD146 staining was high even
in the HE group, which was expected to have the smallest quantity
of mesenchymal cells as seeds with a smaller amount of eSCs. The
greater the concentration of eSCs, the greater the expression of
CD146. The cells on the surface of the spheroids expressed not only
cytokeratin (Fig. 3), but also the
mesenchymal markers, CD29 and CD146 (Fig. 4), mainly in the HS and SE groups.
These results indicated that these cells may have changed their
phenotype during cell aggregation. The HE group exhibited a CD29
signal only at the inner part of the spheroid, with no CD146 signal
in the cells at the surface (Fig.
4C).
Discussion
The present study developed a three-dimensional
in vitro model to mimic the Sampson theory for the early
initiation of endometriosis by means of co-culturing endometrial
epithelial and stromal primary cells in a matrix-free culture
system. According to Sampson, endometrial cells are regurgitated
through the fallopian tubes and remain viable in the peritoneal
environment (4). The peritoneal
cavity is a liquid environment and peritoneal fluid is constantly
moving within the abdominopelvic cavity (27,28).
The areas where peritoneal fluid stagnation occurs correspond to
the main areas where endometriotic implants are located, which
corroborates the Sampson theory (28). Retrograde menstruation occurs in
almost every cycle of the endometrium (38); thus, peritoneal disease can be fed
in each cycle and its early initiation can be related to the first
menstrual cycles.
Nevertheless, the Sampson theory does not explain
how endometrial cells remain viable until they reach the stagnation
points or a surface to attach. The present study observed that eECs
were able to form stable structures, spheroids, in a matrix-free
(liquid) environment. Stejskalová et al (39) reported the ability of eSCs only to
form spheroids in hanging-drop assays and described their ability
to form endometriosis lesion-like structures in collagen and
Matrigel matrixes. The spheroids are able to attach to surfaces,
indicating the putative mechanism of the formation of endometriosis
peritoneal implants. The present study included eECs in the stromal
cell model, as menstrual effluent contains all the main cells shed
from the eutopic endometrium, stromal and epithelial cells
(30,31).
The present study observed that the structure,
morphology and viability of spheroids formed by primary endometrial
cells without a supporting matrix was dependent on the proportions
of cocultured eSCs and eECs. The greater the proportion of stromal
cells, the greater the spheroid radius and circularity. The radius
of spheroids seeded with equal amounts of stromal and epithelial
cells decreased with time, as the spheroids seeded with larger
amounts of epithelial cells (1:9 eSCs:eECs) were larger in size on
day 7 compared with day 3, although the size then diminished. The
radius of all spheroids diminished over time. These changes in
radius may be due to cell death or spheroid compaction, and an
increase in spheroid size may be related to cell proliferation or
an increase in the inner cavity. As the present study did not
measure cell proliferation and/or death, the mechanisms behind the
changes in spheroid radius cannot be explained. These findings
indicate a key role for eSCs in cell survival in the peritoneal
environment. Considering that the menstrual effluent from women
with endometriosis have larger amounts of stromal cells (31,32),
these results reinforce the key role of stromal cells in the pelvic
endometriosis pathogenesis.
Three-dimensional models of eECs supported by a
matrix display a gland-like structure, with an empty cavity forming
the organoids (23,40). Only stromal cell spheroids have a
compact structure without a cavity (39). Epithelial cells are characterized
by distinct organelles, membrane domains, and lipid and protein
apical and basal polarity that are necessary for their proper
function (41). Cell polarity is
not only observed in epithelial cells, but is also crucial for stem
cell fate and division (42). Cell
polarity could be clearly observed in the co-cultures described in
the present study work, with stromal cells located in the inner
part of the spheroids and epithelial cells located on the surface
forming a lining. Co-cultures of epithelial and stromal cells
usually display this distribution, as epithelial cells are confined
to the outer layer due to their polarity, and stromal cells
function as a support for epithelial cells and remain within the
sphere (43,44), similar to the structure observed
in vivo (29). This cell
type distribution reflects a dynamic interaction between the cells
and indicates that cell-to-cell interactions are relevant for
survival in a matrix-free environment (29,44).
This polarity of the epithelial cells to the surface
of the spheroid (43) was
expected; of note however, these cells expressed mesenchymal cell
markers (CD146 and CD29). This can indicate a putative transition
to an intermediary stromal phenotype, as the cells were marked with
different living cell markers, and the epithelial phenotype was
detected only on the surface of the spheroids. These intermediary
phenotypes have been described in the endometriosis endometrium and
implants, with the promotion of EMT in endometriotic tissues
(45,46). EMT is suggested to be one of the
key mechanisms for endometriosis initiation and lesion development
(47).
Recently, a model using endometrial cell lines grown
as spheroids achieved similar results for epithelial cell polarity,
but without revealing phenotypic changes (44). Song et al (44), observed the same distribution of
epithelial (surface) and stromal cells (inner part) in the spheroid
structure, but did not report changes in the phenotypic markers as
done in the present study. The present study examined whether the
cells retained the epithelial and stromal markers and observed that
even the cells in the surface of the spheroid expressed
stromal/mesenchymal markers. This observation may be due to the use
of primary cells instead of cell lines. Cell lines, due to
immortalization process, display different metabolic profiles from
primary cells (41,48). Some organ-specific characteristics
and tissue-related phenotypes are lost in the immortalization
process and the cells also carry epigenetic changes (48-50).
The cell physiology mechanisms most affected in the cell lines are
those related to mitochondrial activity, changes in the epigenetic
patterns and the promotion of cell cycle mechanisms (48,49).
Considering that endometriosis cells have been described to have
several cell cycle impairments (51), caution should be used with the use
of cell lines without primary cells for comparisons (48,49,52).
The presence of CD146-positive cells in spheroids
indicates the presence of endometrial mesenchymal stem cells
(eMSCs), which are related to the pathogenesis of endometriosis
(53). eMSCs are abundant in
menstrual effluent (6,9), and their ability to survive in
peritoneal fluid through spheroids can corroborate the retrograde
menstruation theory (10,54). Recently, different cell phenotypes
were identified in the menstrual effluent of endometriosis
patients; the majority of these cells are immune cells, although
there are also changes in the mesenchymal cell content (32). Endometriosis also exhibit
differences in the expression of genes related to TGF-β pathways
and the EMT mechanism SNAIL-1(55). The in vitro physiology
results presented in the present study relate these previous
findings to the ability of eutopic endometriosis cells to survive
in a matrix-free environment. However, further studies on immune
cells, peritoneal fluid and the diversity of cells contained in the
menstrual effluent, as well as the attachment process and secreted
factors that can be related to the implant establishment (e.g.,
VEGF, IL-8, IL-1β and TGF-β) are warranted to confirm the results
presented herein. The authors are currently carrying out some of
these in vitro studies, evaluating the EMT mechanisms
involved in the spheroids formation and attachment including also
mesothelial cells and peritoneal fluid. In addition, the spheroid
model is being applied to test the effect of drugs in the formation
of spheroids, that can indicate putative benefits for pelvic
endometriosis treatment. Additionally, the authors have
standardized the sample to include only peritoneal deep
infiltrating disease; thus, the results can provide insight into
the early initiation of peritoneal implants, but not other
endometriosis presentations, such as endometriomas.
In conclusion, eSCs play essential roles in the
formation of endometrial spheroids. Spheroid assembly may be the
mechanism through which menstrual effluent cells survive in the
peritoneal fluid and avoid immune cell clearance. This model may
aid the elucidation of the mechanisms responsible for the early
initiation of endometriosis.
Supplementary Material
Fluorescence microscopy images of eSCs
and eECs characterized by immunocytofluorescence prior to seeding.
Nuclei were stained with DAPI (blue). (A) eSCs stained with
vimentin-Alexa 488 (green). (B) eECs stained with cytokeratin
(red). Scale bars, 400 μm. eSCs, endometrial stromal cells;
eECs, endometrial epithelial cells.
Fluorescence microscopy image
illustrating the viability of the cells in the spheroid at day 15
after seeding. The cytoplasm was stained with the permeant living
cells dye calcein-AM (green) and nuclei stained with Hoechst 33342
(blue) on day 15 after seeding. Scale bar, 200 μm.
Sample data of the patients in the
present study.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Fundação de Amparo à
Pesquisa do Estado de São Paulo (FAPESP, Brazil; grant no.
2018/11508-9) and Conselho Nacional de Desenvolvimento Científico e
Tecnológico (National Counsel of Technological and Scientific
Development, CNPQ; grant no. 133590/2020-8).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PTN carried out the experiments and was responsible
for the acquisition of data. ALI, ES and MGFS were involved in the
conception and design of the study. ES and AK were involved in the
collection of samples. PTN, ALI, ES, AK and MGFS were involved in
the analysis and interpretation of the data. PTN and ALI confirm
the authenticity of all the raw data. All authors were involved in
the drafting and critical discussion of the manuscript and all
authors have read and approved the final version of the manuscript
to be published.
Ethics approval and consent to
participate
The study protocol was approved by the Universidade
Federal de São Paulo ethical committee (Project no. 1054/2021/CAAE
no. 51541220.8.0000.5505 part of the main project no.
0875/2018/CAAE no. 94562518.4.0000.5505), and informed written
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baranov V, Malysheva O and Yarmolinskaya
M: Pathogenomics of endometriosis development. Int J Mol Sci.
19(1852)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zondervan KT, Becker CM, Koga K, Missmer
SA, Taylor RN and Viganò P: Endometriosis. Nat Rev Dis Primer.
4(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Berkley KJ, Rapkin AJ and Papka RE: The
pains of endometriosis. Science. 308:1587–1589. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sampson JA: Peritoneal endometriosis due
to the menstrual dissemination of endometrial tissue into the
peritoneal cavity. Am J Obstet Gynecol. 14:422–469. 1927.
|
|
5
|
Liu DT and Hitchcock A: Endometriosis: Its
association with retrograde menstruation, dysmenorrhoea and tubal
pathology. Br J Obstet Gynaecol. 93:859–862. 1986.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Patel AN, Park E, Kuzman M, Benetti F,
Silva FJ and Allickson JG: Multipotent menstrual blood stromal stem
cells: Isolation, characterization, and differentiation. Cell
Transplant. 17:303–311. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van der Linden PJ: Theories on the
pathogenesis of endometriosis. Hum Reprod. 11 (Suppl 3):S53–S65.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van der Linden PJ, Dunselman GA, de Goeij
AF, van der Linden EP, Evers JL and Ramaekers FC: Epithelial cells
in peritoneal fluid-of endometrial origin? Am J Obstet Gynecol.
173:566–570. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rodrigues MCO, Lippert T, Nguyen H,
Kaelber S, Sanberg PR and Borlongan CV: Menstrual blood-derived
stem cells: In vitro and in vivo characterization of functional
effects. In: Biobanking and Cryopreservation of Stem Cells.
Advances in Experimental Medicine and Biology. Karimi-Busheri F and
Weinfeld M (eds). Vol 951. Springer International Publishing, Cham,
pp111-121, 2016.
|
|
10
|
Gargett CE, Schwab KE and Deane JA:
Endometrial stem/progenitor cells: The first 10 years. Hum Reprod
Update. 22:137–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grümmer R: Models of endometriosis: in
vitro and in vivo models. In: Endometriosis: Science and Practice.
Giudice LC, Evers JLH and Healy DL (eds). Wiley-Blackwell, Oxford,
pp263-269, 2012.
|
|
12
|
Griffith JS, Rodgers AK and Schenken RS:
In vitro models to study the pathogenesis of endometriosis. Reprod
Sci. 17:5–12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Davies J: Potential advantages of using
biomimetic alternatives. In: Replacing Animal Models. Davies J
(ed). Wiley, pp1-11, 2012.
|
|
14
|
Al-Juboori AAA, Ghosh A, Jamaluddin MFB,
Kumar M, Sahoo SS, Syed SM, Nahar P and Tanwar PS: Proteomic
analysis of stromal and epithelial cell communications in human
endometrial cancer using a unique 3D co-culture model. Proteomics.
19(e1800448)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bruner-Tran KL, McConaha ME and Osteen KG:
odels of endometriosis: Animal models I-rodent-based chimeric mode.
In: Endometriosis: Science and Practice. Giudice LC, Evers JLH and
Healy DL (eds). Wiley-Blackwell, Chichester, pp270-284, 2012.
|
|
16
|
King CM, Barbara C, Prentice A, Brenton JD
and Charnock-Jones DS: Models of endometriosis and their utility in
studying progression to ovarian clear cell carcinoma. J Pathol.
238:185–196. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Braundmeier AG and Fazleabas AT: The
non-human primate model of endometriosis: Research and implications
for fecundity. Mol Hum Reprod. 15:577–586. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Greaves E, Critchley HOD, Horne AW and
Saunders PTK: Relevant human tissue resources and laboratory models
for use in endometriosis research. Acta Obstet Gynecol Scand.
96:644–658. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fazleabas AT: Models of Endometriosis:
Animal models II-non-human primates. In: Endometriosis: Science and
Practice. 1st edition. Giudice LC, Evers JLH and Healy DL (eds).
Wiley Blackwell, Chichester, pp285-291, 2012.
|
|
20
|
Costa EC, de Melo-Diogo D, Moreira AF,
Carvalho MP and Correia IJ: Spheroids formation on non-adhesive
surfaces by liquid overlay technique: Considerations and practical
approaches. Biotechnol J. 13(1700417)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Deane JA, Cousins FL and Gargett CE:
Endometrial organoids: In vitro models for endometrial research and
personalized medicine. Biol Reprod. 97:781–783. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Boretto M, Cox B, Noben M, Hendriks N,
Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie
A, et al: Development of organoids from mouse and human endometrium
showing endometrial epithelium physiology and long-term
expandability. Development. 144:1775–1786. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Turco MY, Gardner L, Hughes J,
Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE,
Brosens JJ, Critchley HO, et al: Long-term, hormone-responsive
organoid cultures of human endometrium in a chemically defined
medium. Nat Cell Biol. 19:568–577. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Gnecco JS, Brown A, Buttrey K, Ives C,
Goods BA, Baugh L, Hernandez-Gordillo V, Loring M, Isaacson KB and
Griffith LG: Organoid co-culture model of the human endometrium in
a fully synthetic extracellular matrix enables the study of
epithelial-stromal crosstalk. Med. 4:554–579.e9. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Malvezzi H, Marengo EB, Podgaec S and
Piccinato CA: Endometriosis: Current challenges in modeling a
multifactorial disease of unknown etiology. J Transl Med.
18(311)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Murphy AR, Campo H and Kim JJ: Strategies
for modelling endometrial diseases. Nat Rev Endocrinol. 18:727–743.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pannu HK and Oliphant M: The subperitoneal
space and peritoneal cavity: Basic concepts. Abdom Imaging.
40:2710–2722. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bricou A, Batt RE and Chapron C:
Peritoneal fluid flow influences anatomical distribution of
endometriotic lesions: Why Sampson seems to be right. Eur J Obstet
Gynecol Reprod Biol. 138:127–134. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wiwatpanit T, Murphy AR, Lu Z, Urbanek M,
Burdette JE, Woodruff TK and Kim JJ: Scaffold-free endometrial
organoids respond to excess androgens associated with polycystic
ovarian syndrome. J Clin Endocrinol Metab. 105:769–780.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koks CA, Dunselman GA, de Goeij AF, Arends
JW and Evers JL: Evaluation of a menstrual cup to collect shed
endometrium for in vitro studies. Fertil Steril. 68:560–564.
1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Warren LA, Shih A, Renteira SM, Seckin T,
Blau B, Simpfendorfer K, Lee A, Metz CN and Gregersen PK: Analysis
of menstrual effluent: Diagnostic potential for endometriosis. Mol
Med. 24(1)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shih AJ, Adelson RP, Vashistha H, Khalili
H, Nayyar A, Puran R, Herrera R, Chatterjee PK, Lee AT,
Truskinovsky AM, et al: Single-cell analysis of menstrual
endometrial tissues defines phenotypes associated with
endometriosis. BMC Med. 20(315)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen JC, Erikson DW, Piltonen TT, Meyer
MR, Barragan F, McIntire RH, Tamaresis JS, Vo KC, Giudice LC and
Irwin JC: Coculturing human endometrial epithelial cells and
stromal fibroblasts alters cell-specific gene expression and
cytokine production. Fertil Steril. 100:1132–1143. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ruiz-Mitjana A, Navaridas R, Vidal-Sabanés
M, Perramon-Güell A, Yeramian A, Felip I, Eritja N, Egea J, Encinas
M, Matias-Guiu X and Dolcet X: Lack of extracellular matrix
switches TGF-β induced apoptosis of endometrial cells to epithelial
to mesenchymal transition. Sci Rep. 12(14821)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kirkwood PM, Gibson DA, Shaw I, Dobie R,
Kelepouri O, Henderson NC and Saunders PTK: Single-cell RNA
sequencing and lineage tracing confirm mesenchyme to epithelial
transformation (MET) contributes to repair of the endometrium at
menstruation. Elife. 11(e77663)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Luckow Invitti A, Schor E, Martins
Parreira R, Kopelman A, Kamergorodsky G, Gonçalves GA and Batista
Castello Girão MJ: Inflammatory cytokine profile of co-cultivated
primary cells from the endometrium of women with and without
endometriosis. Mol Med Rep. 18:1287–1296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Halme J, Hammond MG, Hulka JF, Raj SG and
Talbert LM: Retrograde menstruation in healthy women and in
patients with endometriosis. Obstet Gynecol. 64:151–154.
1984.PubMed/NCBI
|
|
39
|
Stejskalová A, Fincke V, Nowak M, Schmidt
Y, Borrmann K, von Wahlde MK, Schäfer SD, Kiesel L, Greve B and
Götte M: Collagen I triggers directional migration, invasion and
matrix remodeling of stroma cells in a 3D spheroid model of
endometriosis. Sci Rep. 11(4115)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Boretto M, Maenhoudt N, Luo X, Hennes A,
Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I,
et al: Patient-derived organoids from endometrial disease capture
clinical heterogeneity and are amenable to drug screening. Nat Cell
Biol. 21:1041–1051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Inman JL and Bissell MJ: Apical polarity
in three-dimensional culture systems: Where to now? J Biol.
9(2)2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Florian MC and Geiger H: Concise review:
Polarity in stem cells, disease, and aging. Stem Cells.
28:1623–1629. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kosheleva NV, Efremov YM, Shavkuta BS,
Zurina IM, Zhang D, Zhang Y, Minaev NV, Gorkun AA, Wei S, Shpichka
AI, et al: Cell spheroid fusion: Beyond liquid drops model. Sci
Rep. 10(12614)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Song Y, Burns GW, Joshi NR, Arora R, Kim
JJ and Fazleabas AT: Spheroids as a model for endometriotic
lesions. JCI Insight. 8(e160815)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Konrad L, Dietze R, Riaz MA,
Scheiner-Bobis G, Behnke J, Horné F, Hoerscher A, Reising C and
Meinhold-Heerlein I: Epithelial-mesenchymal transition in
endometriosis-when does it happen? J Clin Med.
9(1915)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen M, Zhou Y, Xu H, Hill C, Ewing RM, He
D, Zhang X and Wang Y: Bioinformatic analysis reveals the
importance of epithelial-mesenchymal transition in the development
of endometriosis. Sci Rep. 10(8442)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Matsuzaki S and Darcha C: Epithelial to
mesenchymal transition-like and mesenchymal to epithelial
transition-like processes might be involved in the pathogenesis of
pelvic endometriosis. Hum Reprod. 27:712–721. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pan C, Kumar C, Bohl S, Klingmueller U and
Mann M: Comparative proteomic phenotyping of cell lines and primary
cells to assess preservation of cell type-specific functions. Mol
Cell Proteomics. 8:443–450. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Maqsood MI, Matin MM, Bahrami AR and
Ghasroldasht MM: Immortality of cell lines: Challenges and
advantages of establishment. Cell Biol Int. 37:1038–1045.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kaur G and Dufour JM: Cell lines: Valuable
tools or useless artifacts. Spermatogenesis. 2:1–5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fan H: In-vitro models of human
endometriosis. Exp Ther Med. 19:1617–1625. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cousins FL, O DF and Gargett CE:
Endometrial stem/progenitor cells and their role in the
pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol.
50:27–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nayyar A, Saleem MI, Yilmaz M, DeFranco M,
Klein G, Elmaliki KM, Kowalsky E, Chatterjee PK, Xue X, Viswanathan
R, et al: Menstrual effluent provides a novel diagnostic window on
the pathogenesis of endometriosis. Front Reprod Health.
2(3)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Penariol LBC, Thomé CH, Tozetti PA, Paier
CRK, Buono FO, Peronni KC, Orellana MD, Covas DT, Moraes MEA, Silva
WA Jr, et al: What do the transcriptome and proteome of menstrual
blood-derived mesenchymal stem cells tell us about endometriosis?
Int J Mol Sci. 23(11515)2022.PubMed/NCBI View Article : Google Scholar
|


















