1. Introduction
Obesity is characterized by excessive fat
accumulation in adipose cells and is typically identified by a body
mass index (BMI) of ≥30 kg/m² (1).
Ethnic-specific waist circumference cut-points, which are
determined by sex and body lipid percentage through various body
composition methods, are used for overall and abdominal obesity
assessments (2). Obesity and
overweight are substantial risk factors associated with heightened
mortality and morbidity throughout the lifespan of an individual
(3). In women, these conditions
serve as precursors to pregnancy-related diabetes and postpartum
complications, which contribute to pediatric obesity (4). During puberty, obesity is a notable
risk factor for illness and disability. The likelihood of mortality
increases proportionally with BMI, that is, individuals with a low
BMI (<18 kg/m²) are expected to face an exceptionally high risk
of diseases and death (5).
Physiological mechanisms of
obesity
Insulin resistance, diet and inflammation-induced
obesity primarily originate from excessive nutrient intake,
malfunctioning adipocytes and tissue hypoxia (6). Hypoxia involves hypoxia-inducible
factor (HIF-1) and other signaling molecules, which may stimulate
the release of free fatty acids and inhibit glucose uptake in
adipose tissues (7). Upon
activation, nuclear factor kappa B (NFκB1) in the cytoplasm
translocates into the nucleus and regulates numerous genes that
encode cytokines, such as tumor necrosis factor-alpha (TNF-α),
interleukin-1 (IL-1) and IL-6. These cytokines play a crucial role
in the inflammation of adipose tissues. Cytokines affect specific
metabolic processes; in particular, IL-6 and TNF-α regulate glucose
transporter type 4 and influence insulin-stimulated glucose uptake
by inhibiting lipoprotein lipase (8).
Cellular and tissue responses to hypoxia,
particularly in adipocytes, are a complex process. When subjected
to hypoxia, adipocytes initially experience swelling, which
increase their size. This phenomenon induces hypoxic conditions
within the adipose tissue and potentially leads to the generation
of new blood vessels through adipogenesis-derived angiogenesis when
required (4). Following the
crucial expansion of adipose tissue mass, the demand for cardiac
output and blood flow in the vessels to the tissue increases to aid
in responses against hypoxia (9).
Genetic studies on obesity and
hypoxia
Genetic studies are important in understanding the
relationship between hypoxia and obesity and elucidate the
molecular mechanisms that connect the two conditions. Hypoxia could
be a consequence of obesity, that is, obese individuals have lower
rates of blood flow in adipose tissues and muscles than non-obese
individuals. Elevated body mass index (BMI) can lead to an increase
in red blood cells (RBCs), which may be attributed to hypoxia in
obese individuals. Genetic analyses revealed the causal association
between elevated RBCs and risk of type 2 diabetes (T2D), that is,
RBCs may mediate the pathogenesis of obesity-induced T2D. Higher
BMI has been linked to an increased risk of myocardial infarction
(MI), highlighting the importance of identifying the genetic basis
of obesity-related complications, such as T2D and MI (10-13).
Understanding the genetic underpinnings of obesity within the
context of hypoxia is crucial to advance scientific knowledge and
develop personalized interventions for individuals at high risk.
Genetic investigations, such as Mendelian randomization, provide
valuable insights into the causal effects of obesity on various
health outcomes, such as cardiovascular diseases. By decoding
genetic factors that influence susceptibility to obesity under
hypoxic conditions, researchers aim to identify novel targets for
intervention and therapeutic strategies to address the complex
interplay among genetics, hypoxia and obesity (10,12,13).
Blood flow decreases as the tissue size increases
until angiogenesis occurs, leading to the development of new
vessels. This process is instrumental in combating hypoxia
(10). The progression of hypoxia
is contingent on the elevation of inflammatory response levels
within the hypoxic adipose tissue in obesity. Other contributors to
inflammation development include oxidative stress and endoplasmic
reticulum stress (4). The surge in
obesity rates has a profound effect on global populations and
adversely influences psychosocial, physical and professional
well-being. In Malaysia, obesity is a significant contributor to
the declining health status of the general population (11). The present review provided a basis
to explore the intricate interplay of lifestyle, physical activity
and genetic factors in obesity and elucidate diverse patterns of
anthropometric measurements across various populations. Emphasizing
the need for supplementary genetic studies, it aimed to contribute
data that complement initiatives, such as the Human Variome Project
(12) and the HapMap Project
(13) and offer valuable insights
for a comprehensive understanding of obesity.
2. Obesity
Obesity is characterized by excessive fat
accumulation in adipose cells and often identified by BMI >30
kg/m² (14). Treatment and
prevention play crucial roles in comprehensively understanding
biological functions related to obesity and overweight (15,16).
A high BMI may indicate overall inactivity, weakening muscles and
reduced aerobic capacity. The association between obesity and
functional limitations elevates the risk of diseases linked to high
BMI and directly influences the likelihood of disability (17). Obesity is connected to certain
health behavior and practices. A distinct relationship exists
between smoking and body size; slimmer individuals are more likely
to smoke, whereas obese individuals are more inclined to abstain
from smoking (18,19). Cigarette smoking reduces body fat
through various mechanisms, including decreasing caloric and fat
intake and increasing metabolic rate and energy expenditure
(20,21). Hormonal and neurological signals,
which are orchestrated and processed in the brain, govern energy
balance. The central nervous system (CNS) is influenced by leptin,
a hormone released by adipocytes and present in specific quantities
corresponding to the number of adipose tissues (22-24).
Leptin binds to receptors in the CNS and activates neurons in the
appetite control center of the brain. This interaction is pivotal
in regulating food intake (25).
The global increase in obesity cases is a growing
concern and it is estimated that it will affect more than one
billion individuals by 2030(26).
Obesity has emerged as a significant public health issue and
contributes to insulin resistance and associated comorbidities,
such as metabolic syndrome, cardiovascular disease (CVD) and T2D
(27). The prevalence of obesity
has increased since the 1960s in advanced countries. In 1980, 2.9%
of the United States population was extremely obese and 34.9%
individuals were overweight (28).
By 2000s, approximately half of the population had BMI >25
kg/m2, with one-third having BMI >30
kg/m2. The total economic cost of obesity in the United
States is estimated at US$ 60 billion per year, a significant
portion of which is attributed to obesity-related T2D. The risk of
obesity tends to increase significantly when calories and fat are
widely accessible to populations (15).
The storage of surplus energy derived from the
breakdown of food molecules that exceed the body's immediate needs,
such as fat, is a significant evolutionary adaptation (29). Throughout history, humans have
encountered diverse situations and periods marked by food scarcity
and excess nutritional resources (30). Developed countries benefit from
advanced technologies, food preservation techniques, efficient
transportation, rapid westernization and urbanization, to
facilitate extensive and cost-effective food storage (31). This practice has diminished the
likelihood of returning to eras characterized by food scarcity and
minimal nutritional value (32).
The global statistics indicate that more than 1.9 billion adults
worldwide are classified as overweight; according to the World
Health Organisation (WHO) in 2016(33), 13% of individuals were obese. The
prevalence of obesity worldwide is detailed in Table I.
 | Table IPrevalence of obesity in the world
according to WHO. |
Table I
Prevalence of obesity in the world
according to WHO.
| First author/s,
year | Obesity in the
world | (Refs.) |
|---|
| Chooi et al,
2019 | >1.9 billion
adults (18-year age) and older were overweight | (116) |
| Chooi et al,
2019 | 39% of men and 40%
of women aged 18 or over living with overweight 13% living with
obesity. | (116) |
| Bluher et
al, 2019 | 38.2 million
children under the age of 5 years were overweight or obese. | (117) |
| Wang et al,
2020 | ~770 million adults
globally were affected by obesity | (118) |
| WHO, 2023 | Overweight and
obesity (BMI ≥25 kg/m²) over 2.6 billion in 2020 compared with 1.9
billion in 2015 | https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight |
According to the estimates of WHO, the number of
individuals with diabetes in Malaysia is projected to increase to
2.48 million by 2030 compared with 0.94 million in 2000,
representing a substantial increase of 164%. This surge in diabetes
cases is associated with the increasing prevalence of obesity
(34,35). The likelihood of individuals with
diabetes experiencing cardiovascular disease is two to three times
higher than that of non-diabetics. Obesity has emerged as a primary
public and clinical health concern that considerably affects
healthcare services in Malaysia (35). Individuals have decreased physical
activities and most of them engage in sedentary behavior, such as
using cars and public transport, including trains and buses and
relying on technological innovations, such as television,
smartphones and computers (36).
The proliferation of Internet resources has contributed to a
sedentary lifestyle characterized by reduced physical activity and
increased time spent on sedentary behavior, such as watching TV,
sitting at work or driving. This shift in lifestyle is linked to an
elevated risk of obesity and overweight (37).
3. Role of genetics in obesity
The genetic pattern of obesity is subdivided into
three types; inclusive monogenic syndromic obesity (Mendelian),
monogenic non-syndromic obesity and polygenic obesity (38). Mendelian obesity depends on
abnormalities in chromosomes (X-linked) and is polygenic. Monogenic
is not common and is caused by high-risk variants. Polygenetic
obesity is multifactorial; variants have minimal effects
individually and can synergistically cause obesity (39).
A number of genes and their mutations can cause
obesity phenotype in animals and humans. These genes are components
of energy balance system regulation. For example, leptin (OB
protein) is a hormone from adipose tissues and has a number of
physiological roles; it involves brain receptors that manage energy
expenditure and food intake (3).
The levels of leptin depend on body fat fluctuation, which may
decrease or increase food intake volume (15). Humans become greatly obese if
mutations occur in the OB protein or leptin in the hypothalamus,
peripheral tissues and brain. Any damage in hypothalamus neurons
with leptin receptors can cause massive obesity (40).
Syndromic obesity is distinguished by the presence
of various associated clinical phenotypes, including organ-specific
developmental abnormalities, intellectual disability (ID) and
dysmorphic features. Over 25 syndromic forms of obesity have been
identified, such as Bardet-Biedl and Prader-Willi syndrome
(41). Non-syndromic obesity is
primarily characterized by genetic associations with
leptin/melanocortin disorders and results in hyperphagic obesity
(42). Polygenic obesity involves
the collective effect of multiple genes. For instance, fat mass and
obesity-associated protein (FTO) are significant genes in polygenic
obesity (43).
4. Role of hypoxia in obesity
Hypoxia is associated with decreases in the normal
levels of tissue oxygen concentration. It may cause cell death if
it is prolonged or severe (44).
Scientists study hypoxia because of its disruption in responses to
radiotherapy. Radiation can destroy target cells by producing free
radicals in the hypoxic tissue and inducing cell death (45). Tumor cells can survive and continue
proliferating within hypoxic areas in comparison with cells in
perfusion-limited areas (46).
Nordsmark et al (47)
fabricated oxygen electrodes related to a tumor oxygen supply and
demonstrated that hypoxia is associated with human tumors. They
proved the association between poor survival metastasis and low
oxygen tension in patients suffering from tumors of the breast,
cervical or neck and head (48).
Hypoxia can activate a number of genes and
transcription factors, such as hypoxia-inducible factors
(HIFs) and nuclear factor kappa subunit B (NF-κB),
which play a central role in the activation of other genes and in
the release of several inflammatory chemokines and cytokines
(49,50). Cytokines have important roles in
inflammation responses, such as macrophage permeation into adipose
tissues. Yin et al (7)
focused on the production of chronic inflammation caused by
obesity-induced hypoxia. Hypoxia reduced free fatty acid (FFA)
uptake and lipolysis was increased in adipocytes in adipose
tissues; hence, hypoxia has a role in increasing chronic
inflammation.
The diagnostic or prognostic roles of hypoxia are
related physiologically (51). A
number of studies examined cellular responses and molecular
signaling pathways under acute and severe hypoxia. Several pathways
are involved in hypoxia and cells have various biological responses
to hypoxic conditions. The first pathway involves the transition of
hypoxic cells from aerobic to anaerobic metabolism (52). Hypoxia enhances tyrosine
hydroxylase synthesis in neural cells, contributes to catecholamine
production and stimulates the production of erythropoietin by renal
cells, leading to increased red blood cell production and enhanced
hemoglobin oxygen transport (53).
Another pathway for hypoxic response is the production and
activation of growth factors that induce angiogenesis; this pathway
induces the production of new blood vessels during acute and
chronic vascular diseases, cancer, pulmonary disease and tissue
injuries (54).
In cancer, the affected tissue becomes hypoxic
depending on the level of angiogenesis and may die; tumor cells
exhibit adaptive and genetic changes, which allow them to
proliferate and survive in the hypoxic microenvironment (55). Human tumors can grow around blood
vessels and utilize oxygen molecules 180 µm away from the blood
vessels, which is the maximum distance for oxygen molecules between
the capillary and the cells (55,56).
Chronic hypoxia is caused by the uncontrolled
production of tumor cells to outgrow the limited oxygen supplied by
blood. Another type of hypoxia, known as ‘perfusion-limited’ or
‘acute’ hypoxia, occurs when blood vessels are blocked and can
cause reverse blood flow (57).
Blocked vessels can be treated by inverting the supply of
oxygenated blood to the hypoxic tissue; this phenomenon, also
called oxygenation injury, can increase the number of free-radical
molecules and activate stress-response genes and tissue damages
(58). Continued hypoxia occurs in
individuals who stay in high-altitude areas and conditional hypoxia
occurs in patients with sleep apnea who have blocked airways while
sleeping, leading to a rapid decline in blood oxygen pressure in a
process called hypoxemia (59).
Obese individuals exhibit augmented size and volume
of adipose tissues and fat accrual within white adipose cells
induces chronic hypoxia in the adipose tissue. This phenomenon
occurs because cells with expanded size have inadequate oxygen
provision (60,61). Adipose cells respond to hypoxia
differently compared with other cell types. The nature and pace of
the response is contingent on the rate of decline in oxygen
concentration (62). The initial
effect of hypoxia on adipose tissues involves the enlargement of
adipose cells. However, the tissue requires more oxygen than that
currently available, thereby increasing the hypoxia levels
(10). Adipose tissue consists of
macrophages, mast cells, dendritic cells and vascular endothelial
cells. Macrophages are important in the primary inflammatory
reaction and prevent tissues from developing obesity (63). Obesity-induced inflammation is
recognized by the elevated inflammatory level in adipose tissues
and plasma and macrophages in adipose tissues are implicated in
inflammation and obesity (64).
HIF-1 is the main transcription factor in response
to decreases in oxygen rate. Other crucial transcription factors
that contribute to adipose tissue responses to hypoxia are glucose
transporter 1, leptin, vascular endothelial growth factor (VEGF),
matrix metalloproteinase-2, matrix metalloproteinase-9, IL-6, IL-4
and plasminogen activator inhibitor-1(65). The role of hypoxia is not yet fully
understood but has been discovered in a number of essential
processes, such as insulin resistance, glucose intolerance and
inflammation with induced angiogenesis (66). A study reported on other nuclear
partners involved in oxygen level pathways [HIF-2, NF-κB,
peroxisome proliferator-activated receptors (PPARγ) and cAMP
response element binding (CREB)] (67).
5. Role of inflammation in obesity and lipid
metabolism
The major reasons for insulin resistance,
diet-induced obesity and inflammatory-induced obesity are excess
nutrients, adipocyte dysfunction and tissue hypoxia (66). Hypoxia involves hif-1α and other
signaling molecules that might promote free fatty acid release and
prevent glucose uptake in adipose tissues (68). Hypoxia induces the expression of
NFκB1, which regulates a number of genes that encode
cytokines, such as TNF-α, IL-1 and IL-6 and has an important role
in adipose tissue inflammation (6). Metabolic processes, such as
lipoprotein lipase inhibition by IL-6 and TNF-α, are affected.
TNF-α also regulates insulin-stimulated glucose uptake via glucose
transporter 4(69). A strong
association has been found between obesity and chronic inflammatory
response and it is characterized by the management of
pro-inflammatory signaling pathways and uncontrolled production of
adipokines, which induces the expression of biological markers of
inflammation (70). Inflammation
affects lipids, energy metabolism and glucose in adipose tissues
during obesity (71). Various
pathways associated with obesity contribute to inflammation.
Examples include the activation of protein kinase C or JNK by fatty
acid derivatives (such as ceramide or diglycerides), activation of
Toll-like receptor (TLR) 4 by fatty acids, induction of endoplasmic
reticulum stress, high levels of reactive oxidative species and
activation of macrophages by adipocyte death (72). The catabolic and anabolic pathways
for lipid storage require specific transporters, such as
chylomicron for diet in lipids, triglycerides (TG), very
low-density lipoprotein and albumin (73).
Physical activity reduces percentage and mass of
body fat, visceral and subcutaneous adipose tissue, BMI and TG
levels and increases high-density lipoprotein cholesterol (74). Decreases in triglycerides and total
cholesterol affect body shape, especially BMI and fat mass
(2). Adipose tissue is the main
endocrine organ that generates hormones, such as resistin,
adiponectin, leptin, apelin and acute phase serum amyloid A
(A-SAA); it controls body metabolism and regulates whole-body
components and weight (75). A-SAA
has a necessary role in fat mobilization and systemic inflammation
and produces inflammatory markers, such as IL-8, which are
associated with a number of diseases, including atherosclerosis and
insulin resistance. Other inflammatory markers that have an
important role in metabolic diseases are TNF-α and IL-6, which
result from oxidative stress and hypoxia (76).
The association between metabolic syndrome and
obesity is linked to an increased risk of T2D and cardiovascular
diseases. A key component of metabolic syndrome is insulin
resistance, which is accompanied by dysregulation of glucose and
blood pressure and altered lipid metabolism (77). Abdominal obesity is an important
component of metabolic syndrome; determining fat distribution in
obese body and its effects on body health is important (78). Abdominal hip and waist
circumference and their ratio (W/H) are used to classify obesity
into ganoid (peripheral) and android (central) types. Peripheral
fat plays a main role in metabolic risk processes and these indices
are used to differentiate metabolic syndrome and obesity. The
mechanisms underlying the effects of metabolic risk on obesity are
not fully understood and all fat indices are only predictors of
diabetes and heart diseases (79).
6. HIF and obesity
HIF-1. is crucial to maintain oxygen
homeostasis and regulate biological processes, including protein
translation, post-translational modification, gene transcription,
angiogenesis, glucose, energy metabolism, erythropoiesis, iron
homeostasis, cell proliferation, survival, cell death and autophagy
(71-73).
The HIF-1 activator complex activates several
hypoxia-responsive genes, such as hypoxia-response elements (HRE),
VEGF and nuclear factor kappa βeta (NFκB1) (80). The HIF-1 activator complex
is composed of two protein subunits: i) HIF-1 aryl hydrocarbon
receptor nuclear translocator (HIF-1β/ARNT); and ii)
HIF-1α, which are present in cells but activated only under
hypoxia. The HIF-1α subunit is synthesized and degraded
under normoxic situations while it accumulates upon exposure to
lack or low oxygen levels (81).
According to the description of HIF and its regulatory
functions, these genes produce chemokines and interleukins, such as
TNF-α, which can activate transcriptional factors, such as
NFκB1 (38).
HIF-1 gene
The HIF-1 gene is located in chromosome 14 at
locus q23.2 and comprises 14 exons and 13 introns with a direct
orientation. The chromosome assembly reveals the initiation and
termination points of the HIF-1 gene at 61,695,401 and
61,748,259 base pairs, respectively, encompassing a total size of
456,821 bases and consisting of 152,273 codons for translation
(82,83).
Single-nucleotide polymorphisms (SNPs)
in HIF-1 gene
Numerous polymorphisms within the HIF-1 gene have
been identified because of their significant involvement in hypoxia
and angiogenesis. A selection of these SNPs is provided in Table II.
 | Table IISNP of HIF-1 gene. |
Table II
SNP of HIF-1 gene.
| First author/s,
year | SNP | Alleles | (Refs.) |
|---|
| Nagy et al
2009 | rs11549465 | C/T | (85) |
| Lu et al
2012 | rs2057482 | C/T | (105) |
| He et al
2011 | rs17039192 | C/T | (114) |
| Bradbury et
al 2017 | rs9340 | C/T | (119) |
| Emanuele et
al 2010 | rs2010963 | C/G | (120) |
| Rockwell et
al 2009 | rs1870377 | A/T | (48) |
Association of HIF-1 (rs11549465)
variant with obesity
HIF-1 is associated with various reported SNPs,
which play crucial roles in different diseases and pathways
(11,84). These SNPs are implicated in
conditions such as elite endurance in athletes, T1 and T2D,
prostate cancerand knee osteoarthritis (85). One specific HIF-1 gene
variant, rs11549465, is located at 61,740,839 bp, approximately
45,438 bp upstream of the translation start site of the HIF-1 gene.
This variant has the T allele as an alternative and the C allele as
a reference allele (11). The
characteristics of the rs11549465 variant are summarized in
Table III.
 | Table IIIHIF 1 gene (rs11549465)
variant characterization. |
Table III
HIF 1 gene (rs11549465)
variant characterization.
| First author/s,
year |
Characterization | rs11549465 | (Refs.) |
|---|
| Nagy et al,
2009 | Position | 61740839
(Chromosome assembly) | (85) |
| Nagy et al,
2009 | Risk Allele | ‘T’ | (85) |
| Nagy et al,
2009 | Normal Allele | ‘C’ | (85) |
| Nagy et al,
2009 | Chromosome | 14 | (85) |
| Nagy et al,
2009 | Orientation | Plus (Direct) | (85) |
| Nagy et al,
2009 | Stabilization | Plus (Direct) | (85) |
| Nagy et al,
2009 | Reference | GRCh38.p7 Genome
reference consortium human build 38 patch release 12 | (85) |
The present review investigated the rs11549465
(Pro582Ser) variant of HIF-1 because of its significance and role
under specific conditions, considering the sample type and
population. Table IV outlines
diseases associated with the HIF-1 (rs11549465) variant.
 | Table IVDisease association of HIF 1
variant (rs11549465). |
Table IV
Disease association of HIF 1
variant (rs11549465).
| First author/s,
year | Disease | Association | Method | (Refs.) |
|---|
| Nagy et al,
2009 | Type 1 and type 2
diabetes (hypoxia) | Significant | Restriction
fragment analysis (RFLP) and reverse transcription PCR | (85) |
| Döring et
al, 2010 | Hypoxia-inducible
factor-1alpha gene in elite endurance athletes | Significant | Reverse
transcription PCR | (84) |
| Fernández-Torres
et al, 2015 | Development of knee
osteoarthritis | Significant | Reverse
transcription PCR | (121) |
| Jacobs et
al, 2008 | Angiogenesis and
Prostate Cancer | Significant | Reverse
transcription PCR | (86) |
| Drozdovska et
al, 2013 | Athlete status in
Ukrainians (Hypoxia) | Significant | PCR | (87) |
A study explored the link between HIF-1-α gene and
T1 and T2D in a Caucasian (Hungarian) population. The findings
revealed a statistically significant reduction in TT and CT
genotypes, indicating that the T allele had a protective effect
against T2D and T1D (85). Döring
et al (84) discovered the
association between the HIF-1 variant and maximum volume of oxygen
(V˙O2) before and after workout training of elderly
Swedish men. The homozygous pro variant of HIF-1A (Pro582Ser) was
implicated.
A pilot study investigated the polymorphism of the
HIF-1A gene rs11549465 variant and demonstrated its potential
contribution to the development of knee osteoarthritis in Mexico
(82). A significant association
was found between the HIF-1 gene polymorphism rs11549465 and the
risk of osteoarthritis, particularly in the C allele. This variant
is known to protect articular cartilage in the population (80). In a similar study in Georgia that
focused on angiogenesis genes with prostate cancer, HIF-1A
rs11549465 SNP, also known as P582S, could significantly reduce the
risk of developing prostate cancer (86). A study, ‘The association of
(HIF-1A rs11549465, ACE I/D, PPARA intron 7
G/C, NOS3-786 T/C, PPARGC1B Ala203Pro and PPARG
Pro12Ala) gene polymorphisms with athlete status in Ukrainians
population’ (87) found that
NOS3 T, HIF-1A Ser and PPARG Ala alleles are
related to the strength of Ukrainian athletes.
Heterozygosity of HIF-1 (rs11549465)
variant
Heterozygosity is the percentage of heterozygous
genotypes compared with the whole sample of genotypes and reflects
the entire heterozygosity of the population (88). The HIF-1 (rs11549465) variant has
been studied in different populations to determine the level of
heterozygosity. The highest level of heterozygosity has been
reported in the Sub-Saharan African population. Table V summarises the heterozygosity of
the HIF-1 (rs11549465) variant in different populations.
 | Table VStudies of HIF 1 gene
rs11549465 heterozygosity (dpSNP database, 2018, https://www.ncbi.nlm.nih.gov/snp/). |
Table V
Studies of HIF 1 gene
rs11549465 heterozygosity (dpSNP database, 2018, https://www.ncbi.nlm.nih.gov/snp/).
| ss
Polymorphism | Population | Heterozygosity,
% |
|---|
| ss1351283416 | EAS | 4.5 |
| | EUR | 10.0 |
| | AFR | 3.2 |
| | AMR | 8.6 |
| | SAS | 11.9 |
| ss1691508589 | EXAC | 9.5 |
| ss226605360 |
Pilot-1-YRI-low-coverage-panel | 11.9 |
| ss236568433 |
Pilot-1-CEU-low-coverage-panel | 8.9 |
| ss242997447 |
Pilot-1-CHB+JPT-low-coverage-panel | 2.5 |
| ss24616754 | AFD-EUR | 12.5 |
| | AFD-AFR | 13.0 |
| | AFD-CHN | 12.5 |
| ss342388769 |
ESP-Cohort-Population | 15.0 |
| ss48419058 | AGI-ASP
Caucasian | 15.7 |
| ss491688417 | CSAgilent | 20.9 |
| ss66538519 | R24 | 6.0 |
| ss69159438 | HapMap-CEU | 15 |
| | HapMap-HCB | 4.4 |
| | HapMap-JPT | 4.4 |
| | HapMap-YRI | 5.0 |
| ss7689800 | HapMap-CEU
(European) | 13.2 |
| | HapMap-HCB
(Asian) | 4.6 |
| | HapMap-JPT
(Asian) | 6.9 |
| | HapMap-YRI
(Sub-Saharan African) | 5.3 |
| HIF-1
(rs11549465) heterozygosity average | 16.3±-0.234% |
7. NF-κB and obesity
NF-κB is a transcription factor that comprises a
protein complex including NFκB1 (p50/p105), NFKB2
(p52/p100), RelA (p65), RelB and c-Rel. NFκB1 plays a
crucial role in cellular responses to hypoxia, oxidative stress,
cytokines, ultraviolet irradiation, free radicals and bacterial
antigens (89). NFκB1, an
IκB-bound compound in the cytoplasm, is inactive under normal
conditions. NFκB1 can be activated through two pathways,
namely, canonical (classical) and non-canonical (alternative)
(90). The common regulatory step
in both pathways is the activation of an IκB kinase (IKK) compound,
a sensing protein termed NFκB1 essential modulator (NEMO).
As such, NFκB1 dimers are activated through IKK-mediated
phosphorylation-induced degradation of the IκB inhibitor, which
leads to the entry of the NFκB1 dimers to the nucleus from
the cytoplasm to activate specific target genes (91). NFκB1 is a transcription
factor localized in the cytoplasm of every cell and is translocated
to the nucleus upon activation. NFκB1/IκB regulates the
expression of more than 200 target genes (for example, TNF-α, IL-1
and IL-6) and most of which are related to immune responses
(91).
NF-KB 1 gene
The NFκB1 gene is located at chromosome
number 4 at locus 4q24. The gene has 24 exons and 23 introns with
direct orientation. The chromosome assembly shows the starting gene
at 102,501,329 base pair and ends at 102,617,302 base pair with a
total size of 115,974 base pairs and 38,658 codons for translation
(92,93).
SNPs in NFκB1 gene
NFκB1 exhibits cellular responses to the body's
reaction to stimuli, such as hypoxia, oxidative stress cytokines,
ultraviolet irradiation, free radicals and bacterial antigens
(94). It regulates more than 200
target genes involved in immune regulation. A number of
polymorphisms are reported in the NFκB1 gene related to
inflammation and symptomatic diseases, such as certain types of
tumor (95). Table VI summarizes some of these
polymorphisms.
 | Table VISNP of the NFκB1 gene. |
Table VI
SNP of the NFκB1 gene.
| First author/s,
year | SNP | Alleles | Genotypic assembly
location | Location | (Refs.) |
|---|
| Zhou et al,
2009 | rs28362491 | ±ATTG | 102500998 | Promoter | (122) |
| Bauman- et
al, 2019 | rs4648090 | A/G | 102605911 | Intron variant | (112) |
| Chen et al,
2015 | rs4648068 | A/G | 102597148 | Intron variant | (123) |
| Chen et al,
2015 | rs4648065 | C/T | 102596306 | Intron variant | (123) |
| Bauman-Fortin et
al, 2019 | rs230511 | A/G | 102553611 | Intron variant | (112) |
| Curtin et
al, 2010 | rs4648110 | A/T | 102612664 | Intron variant | (124) |
Evidence suggests that polymorphisms in the promoter
region can affect transcription regulation and result in expression
and differential expression of the NFκB1 gene (96,97).
Association of NFκB1 (rs28362491)
variant with obesity
The NFκB1 gene (rs28362491) variant is
located between 102500998 and 102501001 base pair as four base
pairs in the chromosome 4 assembly, with the ATTG insertion allele
as an alternative allele or the deletion of the ATTG allele as a
reference allele.
A number of variants of the nuclear factor-kappa β 1
(NFκB1) gene have been reported, but the present review
focused on the NFκB1 (rs28362491) variant only given its
roles in a number of diseases and conditions, such as diabetic
nephropathy, morbid obesity, coronary artery disease, rheumatoid
arthritis and acute myeloid leukemia. Table VII summarizes previous studies on
this variant and its association in different populations.
 | Table VIIDisease association of NFκB1
(rs28362491) variant. |
Table VII
Disease association of NFκB1
(rs28362491) variant.
| First author/s,
year | Disease | Method | Signification | (Refs.) |
|---|
| Gautam et
al, 2017 | Inflammatory
biomarkers and susceptibility to diabetic nephropathy | PCR-restriction
fragment length polymorphism and ELISA | Significant | (98) |
| Soydas et
al, 2016 | Morbid obesity | PCR-restriction
fragment length polymorphism | Significant | (99) |
| Yang et al,
2014 | Coronary artery
disease | Standard reverse
transcription PCR | Significant | (100) |
| López-Mejías et
al, 2012 | Cardiovascular
disease in patients with rheumatoid arthritis | Reverse
transcription PCR | Significant | (101) |
| Zhang et al,
2013 | Congenital heart
disease | PCR polyacrylamide
gel electrophoresis | Significant | (102) |
| Rybka et al,
2016 | The risk of acute
myeloid leukemia | reverse
transcription PCR | Significant | (125) |
| Pereire et
al, 2021 | Sarcopenia | PCR | Significant | (126) |
Gautam et al (98) conducted a study on ‘The association
of NFκB1 gene (rs28362491) SNP with levels of inflammatory
biomarkers and susceptibility to diabetic nephropathy in Asian
Indians’. The NFκB1 variant rs28362491 was significantly
associated with increased levels of urinary monocyte
chemoattractant protein-1 and plasma TNF-α, thereby increasing the
risk of diabetic nephropathy. The -94 ATTG Ins/Ins variant might be
related to an increased risk of developing nephropathy among Asian
Indians (98). Another study
investigated the relationship between NFκB1 and TLR2
variants with the risk of morbidity obesity in the Turkish
population. The insertion allele, especially Ins/Ins of the
(rs28362491) variant genotype, was significantly higher in patients
with morbidity obesity than in the control group (99). Yang et al (100) studied ‘the association between
NFκB1 gene rs28362491 variant and coronary artery disease
(CAD) in the Chinese population, especially in Han and Uyger
women’; the frequency of (rs28362491) variant genotypes was
significantly different between CAD and control subjects. The
NFκB1 Del\Del genotype of SNP rs28362491 has been proposed
as a new genetic marker for CAD in the Chinese population.
Another study in Spain determined the relationship
between the risk of coronary heart disease (CHD) and the
NFκB1 variant in Caucasian patients with rheumatoid
arthritis (RA); the result showed that the Ins/Del genotype of the
NFκB1 gene (rs28362491) variant is associated with CHD in
patients with RA (101). In
addition, the frequencies of the II (Insertion/Insertion) genotype
in patients with VSD and ASD were significantly higher than those
in controls; hence, the NFκB1 rs28362491 polymorphism in the
promoter region is associated with CHD (102).
8. Relationship between HIF 1 and
NFκB1 SNPs and their association with diseases
SNPs in the HIF-1A gene are linked to several
diseases; for example, rs11549465 is linked to multiple types of
cancer, including colorectal, gastric, prostate, oral, breast, lung
and renal cell carcinoma (103).
Preeclampsia is linked to (s11549467) (104). In patients with aggressive
hepatocellular carcinoma, rs2057482 predicts clinical outcomes
(105). Chronic obstructive
pulmonary disease, CVD, skin disorders, diabetic complications,
preeclampsia, T1 and T2D, lumbar disc degeneration and other
conditions have been linked to 1772 C/T and 1790 G/A polymorphisms
(103-105).
Studies have examined the relationships between these SNPs in the
HIF-1A gene and various disorders and emphasized the significance
of genetic differences in determining disease risk. rs230530,
rs230529, rs230525, rs35680095, rs230494, rs170731, rs230528 and
rs4698858 are found in the NFκB1 gene; they are related to
several diseases, including multiple myeloma, severe influenza A,
atopic asthma, lung disease, cytokine modulation, liver cancer,
alcohol addiction and inflammatory reactions in lymphedema
following breast cancer therapy (106-108).
NFκB1 polymorphisms can affect the risk and severity of
several diseases by altering immunological responses and gene
expression. Further investigation is required to elucidate the
influence of these SNPs on illness susceptibility and
progression.
9. HIF-1 gene (rs11549465) and
NFκB1 gene (rs28362491) variations
HIF-1 and NFκB1 are not the only genes linked
to obesity. Human obesity has been linked to variations in genes
relevant to innate immunity. The following genes have been linked
to obesity: Turkish TLR2 rs5743708 is related to an increased risk
of morbid obesity; Argentinian TLR4 rs11536889 protects against
overweight; TLR4 rs4986790 + rs4986791 is involved in enhanced
visceral and total body fat in in habitants of Caucasus mountains;
TLR4 rs1928295 is related to a high BMI in Europeans; TLR5
rs5744168 protects against obesity among Saudi Arabians and leads
to low BMI; LRRFIP1 rs11680012 is linked to higher levels of
abdominal obesity and inflammatory indicators in Canadians; CD14
rs2569190 lead to a high BMI in Koreans; NLRC3 rs758747 is related
to high obesity rates in Pima Indians, Pakistanis and Europeans;
NLRP3 rs10754558 protects Brazilians against obesity; and FFAR4
rs116454156 indicates high probability to be obese among Europeans.
The selection of the rs11549465 SNP in the HIF-1 gene and the
rs28362491 SNP in the NFκB1 gene was based on their
well-documented roles in inflammation-induced obesity and their
associations with various diseases and conditions, including morbid
obesity, diabetic nephropathy, coronary artery disease and
rheumatoid arthritis. These SNPs have been extensively studied and
are significantly associated with different health outcomes,
particularly obesity (109,110). The present review aimed to
provide focused analysis of the intricate relationship between
these SNPs, obesity and related inflammatory and metabolic
processes. Additionally, it highlighted the importance of further
research on these variants in diverse populations and geographical
locations to broaden our understanding of their effect on obesity
and related conditions. By discussing the two SNPs, it is intended
to lay a foundation for future studies to delve deeper into genetic
mechanisms contributing to obesity. The present review recognized
the importance of expanding the investigation to include additional
genetic variations and pathways in future research endeavors.
10. Molecular network involving HIF-1 and
NFκB1
The molecular network involving HIF-1 and
NFκB1 encompasses a complex interplay of signaling pathways
and regulatory mechanisms that orchestrate cellular responses to
various stimuli, including hypoxia and inflammation. HIF-1 and
NFκB1 are pivotal signaling molecules that interact with a
myriad of other proteins, transcription factors and regulatory
elements to modulate gene expression and cellular functions
(111). Under hypoxic conditions,
HIF-1 plays a crucial role as a master transcriptional regulator
and controls adaptive responses to oxygen deprivation by activating
genes involved in angiogenesis, glycolysis and cell survival. HIF-1
collaborates with proteins, such as HIF-1β and coactivators, such
as p300/CBP, to form active transcriptional complexes that bind to
HREs in target gene promoters. This activation leads to the
upregulation of genes crucial for tissue protection and adaptation,
including those involved in metabolically adapting tissues to
oxygen deprivation and anaerobic ATP synthesis. HIF stabilization
during hypoxia can dampen tissue inflammation and promote repair
processes, highlighting its significance in managing various
conditions, such as cancer and chronic diseases (112). The molecular network involving
HIF-1 and NFκB1 exhibits a complex interplay with mutual
regulation and crosstalk between their signaling pathways.
Hypoxia-induced stabilization of HIF-1 can affect NFκB1
activity through various mechanisms, including direct
protein-protein interactions or regulation of shared target genes
involved in inflammatory responses. Inflammatory signals mediated
by NFκB1 can influence HIF-1 activity through pathways
involving reactive oxygen species or cytokines. This intricate
relationship between HIF-1 and NFκB1 is crucial under
various conditions, such as cancer, inflammation and immune
responses, thereby highlighting the importance of understanding
their interplay for potential therapeutic interventions (113).
HIF 1 involves several biological activities,
including post-translational modification, gene transcription,
protein translation and oxygen molecule homeostasis. The
transcriptional factor HIF-1 is currently the most important
regulator of oxygen homeostasis (11,111). HIF-1 is activated by hypoxia as
part of a broad transcriptional cascade. HIF-1 regulates numerous
genes that are involved in several processes, including autophagy,
angiogenesis, cell division and proliferation. Hypoxia-responsive
genes, including NFκB1 (112), VEGF and HREs, are activated by
the HIF-1 activator complex. HIF-1 Aryl hydrocarbon Receptor
Nuclear Translocator (HIF-1β/ARNT) and HIF-1α are two protein
subunits that are typically present in cells but only activated
under hypoxia-level conditions and can form the HIF-1 activator
complex. Under normoxic conditions, the HIF-1α subunit is
synthesized and destroyed, but it accumulates when exposed to
conditions with low or no oxygen. The regulatory roles of HIF
include the production of specific chemokines and interleukins,
such as TNF, which activate certain transcription factors, such as
NFκB1. Activated HIF-1 and NFκB1 regulate genes
involved in vascular reactivity and remodeling, angiogenesis,
glucose, energy metabolism, erythropoiesis, iron homeostasis, cell
proliferation and survival, causing the disruption or dysfunction
in insulin metabolism and glycolysis and regulating the storage of
glucose and production of fat NFKB (6,11,94,113). This phenomenon leads to the
development of obesity and intolerance of losing weight with diets
and sports.
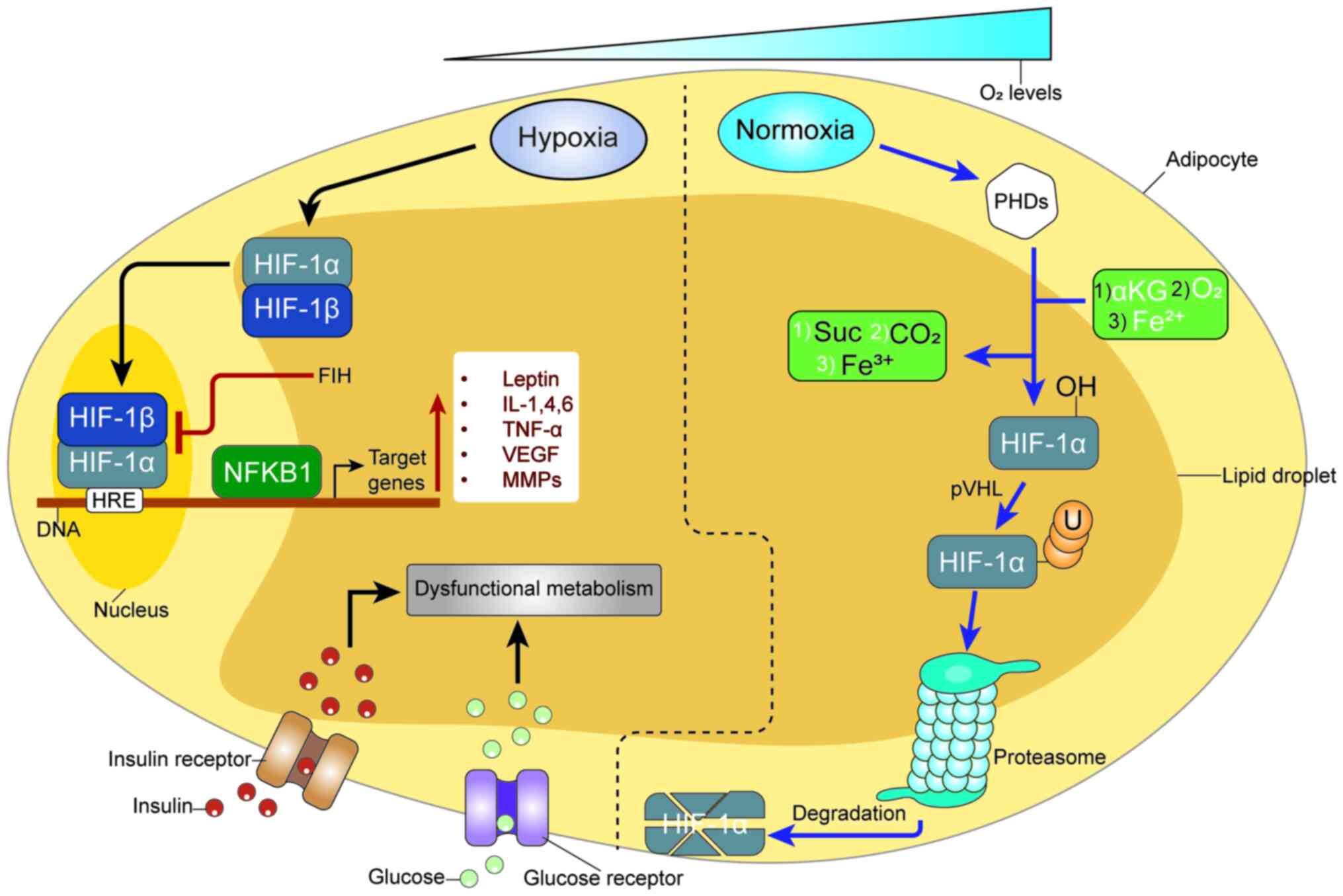
Fig. 1 shows the
hypoxia pathway and inflammatory-induced obesity. HIF-1a and NF-kB
crosstalk regulate essential inflammatory cytokines in adipose
tissue hypoxia. HIF-1α is activated in response to low oxygen
levels. In the context of obesity, the activation of HIF-1α in
adipose tissue is associated with inflammatory responses and
insulin resistance (114). The
hypoxic environment in adipose tissue activates various pathways,
including HIF-1α and NF-κB, leading to the secretion of
pro-inflammatory cytokines. Chronic low-grade inflammation and
insulin resistance are hallmarks of obesity-related metabolic
disorders (115).
11. Conclusion
The present review highlighted the significance of
two genetic (HIF-1 rs11549465 and NFκB1 rs28362491) variants
in inflammation-induced obesity and emphasized the potential
association between these mutations and obesity. Results indicated
the importance of further research on these variants and the
exploration of their associations in diverse populations, races and
countries. Researchers should investigate these genetic variants to
elucidate their role in obesity. Moreover, further studies should
provide a basis for formulation of public health policies aimed at
promoting healthy lifestyle choices to mitigate the prevalence of
obesity.
The significance of HIF-1 rs11549465 and
NFκB1 rs28362491 variations with obesity through the hypoxia
pathway and inflammatory-induced obesity provides a foundation for
further research on genetics and inflammatory-induced obesity; the
combination of the two pathways increases obesity status.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization was by OMA and TD. Writing and
preparation of original draft was by OMA and MA. Writing reviewing
and editing the final manuscript was by OMA, SA, NA, IM, MA, TD and
AM. All authors read and approved the final manuscript. Data
authentication is not applicable
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID
Omar Mahmoud Alshajrawi: https://orcid.org/0009-0006-2458-3580
References
|
1
|
Piché ME, Poirier P, Lemieux I and Després
JP: Overview of epidemiology and contribution of obesity and body
fat distribution to cardiovascular disease: An update. Prog
Cardiovasc Dis. 61:103–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yunus NFM, Omar M, Mohan V, Noor KM and
Jathin R: Effect of indoor rowing exercise on body composition,
blood glucose, and lipid profile among obesity: A pilot study. In:
Regional conference on science, technology and social sciences
(RCSTSS 2016). Springer, pp697-704, 2018.
|
|
3
|
Boulangé CL, Neves AL, Chilloux J,
Nicholson JK and Dumas ME: Impact of the gut microbiota on
inflammation, obesity, and metabolic disease. Genome Med.
8(42)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Van Greevenbroek MM, Schalkwijk CG and
Stehouwer CD: Obesity-associated low-grade inflammation in type 2
diabetes mellitus: Causes and consequences. Neth J Med. 71:174–187.
2013.PubMed/NCBI
|
|
5
|
Thota P, Perez-Lopez FR, Benites-Zapata
VA, Pasupuleti V and Hernandez AV: Obesity-related insulin
resistance in adolescents: A systematic review and meta-analysis of
observational studies. Gynecol Endocrinol. 33:179–184.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
MN I, AB A, Mai A, Om AS, Ariff TM and
Simbak N: Rising trends of obesity in Malaysia; Role of
inflammation and inflammatory markers in obesity related insulin
resistance: A nuclear factor kappa B (Nfkb) perspective. Curr
Trends Biomedical Eng Biosci. 10(CTBEB.MS.ID.555593)2017.
|
|
7
|
Yin J, Gao Z, He Q, Zhou D, Guo Z and Ye
J: Role of hypoxia in obesity-induced disorders of glucose and
lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab.
296:E333–E342. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ng ZY, Veerapen MK, Hon WM and Lim RLH:
Association of leptin/receptor and TNF- α gene variants with
adolescent obesity in Malaysia. Pediatr Int. 56:689–697.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Egea J, Fabregat I, Frapart YM, Ghezzi P,
Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG,
Olaso-Gonzalez G, et al: European contribution to the study of ROS:
A summary of the findings and prospects for the future from the
COST action BM1203 (EU-ROS). Redox Biol. 13:94–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Trayhurn P: Hypoxia and adipose tissue
function and dysfunction in obesity. Physiol Rev. 93:1–21.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Shajrawi OM, Basit E and Baig AA: HIF1
(rs11549465) and NFKB1 (rs28362491) variants association with
obesity in Malaysia. Meta Gene. 25(100753)2020.
|
|
12
|
Burn J and Watson M: The Human Variome
Project. Hum Mutat. 37:505–507. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
National Library of Medicine: HapMap.
https://www.ncbi.nlm.nih.gov/probe/docs/projhapmap/.
|
|
14
|
Powell-Wiley TM, Poirier P, Burke LE,
Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland
IJ, Sanders P, et al: Obesity and cardiovascular disease: A
scientific statement from the American Heart Association.
Circulation. 143:e984–e1010. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Friedman JM: Obesity: Causes and control
of excess body fat. Nature. 459:340–342. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baker JS, Supriya R, Dutheil F and Gao Y:
Obesity: Treatments, conceptualizations, and future directions for
a growing problem. Biology (Basel). 11(160)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Capodaglio P, Castelnuovo G, Brunani A,
Vismara L, Villa V and Maria Capodaglio E: Functional limitations
and occupational issues in obesity: A review. Int J Occup Saf
Ergon. 16:507–523. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Owolabi EO: Prevalence and associated
factors of obesity among South African adults: A cross-sectional
study. Online Journal of Health and Allied Sciences. 16(2)2017.
|
|
19
|
Dare S, Mackay DF and Pell JP:
Relationship between smoking and obesity: A cross-sectional study
of 499,504 middle-aged adults in the UK general population. PLoS
One. 10(e0123579)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Himes CL: Obesity, disease, and functional
limitation in later life. Demography. 37:73–82. 2000.PubMed/NCBI
|
|
21
|
Gastaldelli A, Folli F and Maffei S:
Impact of tobacco smoking on lipid metabolism, body weight and
cardiometabolic risk. Curr Pharm Des. 16:2526–2530. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
de Luca C, Kowalski TJ, Zhang Y, Elmquist
JK, Lee C, Kilimann MW, Ludwig T, Liu SM and Chua SC Jr: Complete
rescue of obesity, diabetes, and infertility in db/db mice by
neuron-specific LEPR-B transgenes. J Clin Invest. 115:3484–3493.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Enriori PJ, Evans AE, Sinnayah P, Jobst
EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M,
Nillni EA, et al: Diet-induced obesity causes severe but reversible
leptin resistance in arcuate melanocortin neurons. Cell Metab.
5:181–194. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu S, Cheng H, François M,
Qualls-Creekmore E, Huesing C, He Y, Jiang Y, Gao H, Xu Y and
Zsombok A: Preoptic leptin signaling modulates energy balance
independent of body temperature regulation. Elife.
15(e33505)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ulyanova A, To XV, Asad A, Han W and
Chuang KH: MEMRI detects neuronal activity and connectivity in
hypothalamic neural circuit responding to leptin. Neuroimage.
147:904–915. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nikooyeh B, Abdollahi Z, Salehi F,
Nourisaeidlou S, Hajifaraji M, Zahedirad M, Shariatzadeh N, Kalay
A, Balderlou FB, Salmas JZ, et al: Prevalence of obesity and
overweight and its associated factors in urban adults from West
Azerbaijan, Iran: The national food and nutritional surveillance
program (NFNSP). Nutr Food Sci Res. 3:21–26. 2016.
|
|
27
|
Phillips CM: Metabolically healthy
obesity: Definitions, determinants and clinical implications. Rev
Endocr Metab Disord. 14:219–227. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Garrouste-Orgeas M, Troché G, Azoulay E,
Caubel A, de Lassence A, Cheval C, Montesino L, Thuong M, Vincent
F, Cohen Y and Timsit JF: Body mass index: An additional prognostic
factor in ICU patients. Intensive Care Med. 30:437–443.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Van Soest PJ: Nutritional ecology of the
ruminant. Cornell university press, 2018.
|
|
30
|
Mouritsen OG: Deliciousness of food and a
proper balance in fatty acid composition as means to improve human
health and regulate food intake. Flavour. 5(1)2016.
|
|
31
|
Black E: Globalization of the food
industry: Transnational food corporations, the spread of processed
food, and their implications for food security and nutrition.
2016;.
|
|
32
|
Adeyeye SAO: The role of food processing
and appropriate storage technologies in ensuring food security and
food availability in Africa. Nutr Food Sci. 47:122–139. 2017.
|
|
33
|
Ahmed B and Konje JC: The epidemiology of
obesity in reproduction. Best Pract Res Clin Obstet Gynaecol.
89(102342)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Boney CM, Verma A, Tucker R and Vohr BR:
Metabolic syndrome in childhood: Association with birth weight,
maternal obesity, and gestational diabetes mellitus. Pediatrics.
115:e290–e296. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Akhtar S, Nasir JA, Ali A, Asghar M,
Majeed R and Sarwar A: Prevalence of type-2 diabetes and
prediabetes in Malaysia: A systematic review and meta-analysis.
PLoS One. 17(e0263139)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Felson M and Boba RL: Crime and everyday
life. Sage, 2010.
|
|
37
|
Ghosh S, Paul M, Mondal KK, Bhattacharjee
S and Bhattacharjee P: Sedentary lifestyle with increased risk of
obesity in urban adult academic professionals: An epidemiological
study in West Bengal, India. Sci Rep. 13(4895)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kaur Y, de Souza RJ, Gibson WT and Meyre
D: A systematic review of genetic syndromes with obesity. Obes Rev.
18:603–634. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Loos RJF and Janssens ACJW: Predicting
polygenic obesity using genetic information. Cell Metab.
25:535–543. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL
and Kalra PS: Interacting appetite-regulating pathways in the
hypothalamic regulation of body weight. Endocr Rev. 20:68–100.
1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Geets E, Meuwissen MEC and Van Hul W:
Clinical, molecular genetics and therapeutic aspects of syndromic
obesity. Clin Genet. 95:23–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vos N, Oussaada SM, Cooiman MI,
Kleinendorst L, ter Horst KW, Hazebroek EJ, Romijn JA, Serlie MJ,
Mannens MMAM and van Haelst MM: Bariatric surgery for monogenic
non-syndromic and syndromic obesity disorders. Curr Diab Rep.
20(44)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sokhi J: Analysis of SNPs in PI3K IRS1 INS
INSR KCNJ11 PP1GG and ABCC8 genes and their susceptibility to type
2 diabetes in different population groups of Punjab. PhD thesis,
Guru Nanak Dev University, Amritsar, Punjab, 2016. http://hdl.handle.net/10603/160793.
|
|
44
|
Sendoel A and Hengartner MO: Apoptotic
cell death under hypoxia. Physiology (Bethesda). 29:168–176.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li Y, Zhao L and Li XF: Hypoxia and the
tumor microenvironment. Technol Cancer Res Treat.
20(15330338211036304)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nordsmark M, Bentzen SM, Rudat V, Brizel
D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, et
al: Prognostic value of tumor oxygenation in 397 head and neck
tumors after primary radiation therapy. An international
multi-center study. Radiother Oncol. 77:18–24. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rockwell S, Dobrucki IT, Kim EY, Marrison
ST and Vu VT: Hypoxia and radiation therapy: Past history, ongoing
research, and future promise. Curr Mol Med. 9:442–458.
2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
D'Ignazio L and Rocha S: Hypoxia induced
NF-κB. Cells. 5(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
D'Ignazio L, Bandarra D and Rocha S: NF-κB
and HIF crosstalk in immune responses. FEBS J. 283:413–424.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zachary JF and McGavin MD: Pathologic
basis of veterinary disease expert consult. 6th Edition. Elsevier,
2016.
|
|
52
|
Michiels C: Physiological and pathological
responses to hypoxia. Am J Pathol. 164:1875–1882. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Semenza GL: Oxygen homeostasis. Wiley
Interdiscip Rev Syst Biol Med. 2:336–361. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Greenberg DA and Jin K: From angiogenesis
to neuropathology. Nature. 438:954–959. 2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Muz B, de la Puente P, Azab F and Azab AK:
The role of hypoxia in cancer progression, angiogenesis,
metastasis, and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447.
2004.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Quaegebeur A and Carmeliet P: Oxygen
sensing: A common crossroad in cancer and neurodegeneration. Curr
Top Microbiol Immunol. 345:71–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hool LC and Corry B: Redox control of
calcium channels: From mechanisms to therapeutic opportunities.
Antioxid Redox Signal. 9:409–435. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bhutta BS, Alghoula F and Berim I:
Hypoxia. StatPearls, 2022.
|
|
60
|
Hodson L, Humphreys SM, Karpe F and Frayn
KN: Metabolic signatures of human adipose tissue hypoxia in
obesity. Diabetes. 62:1417–1425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu F, He J, Wang H, Zhu D and Bi Y:
Adipose morphology: A critical factor in regulation of human
metabolic diseases and adipose tissue dysfunction. Obes Surg.
30:5086–5100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mahat B, Chassé É, Mauger JF and Imbeault
P: Effects of acute hypoxia on human adipose tissue lipoprotein
lipase activity and lipolysis. J Transl Med. 14(212)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ghigliotti G, Barisione C, Garibaldi S,
Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G,
Palombo D, et al: Adipose tissue immune response: Novel triggers
and consequences for chronic inflammatory conditions. Inflammation.
37:1337–1353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mraz M and Haluzik M: The role of adipose
tissue immune cells in obesity and low-grade inflammation. J
Endocrinol. 222:R113–R127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Palazon A, Goldrath AW, Nizet V and
Johnson RS: HIF transcription factors, inflammation, and immunity.
Immunity. 41:518–528. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rasouli N: Adipose tissue hypoxia and
insulin resistance. J Investig Med. 64:830–832. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Chen L, Chen R, Wang H and Liang F:
Mechanisms linking inflammation to insulin resistance. Int J
Endocrinol. 2015(508409)2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sun K, Kusminski CM and Scherer PE:
Adipose tissue remodeling and obesity. J Clin Invest.
121:2094–2101. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Coppack SW: Pro-inflammatory cytokines and
adipose tissue. Proc Nutr Soc. 60:349–356. 2001.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chung HY, Cesari M, Anton S, Marzetti E,
Giovannini S, Seo AY, Carter C, Yu BP and Leeuwenburgh C: Molecular
inflammation: Underpinnings of aging and age-related diseases.
Ageing Res Rev. 8:18–30. 2009.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kojta I, Chacińska M and
Błachnio-Zabielska A: Obesity, bioactive lipids, and adipose tissue
inflammation in insulin resistance. Nutrients.
12(1305)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ye J: Emerging role of adipose tissue
hypoxia in obesity and insulin resistance. Int J Obes (Lond).
33:54–66. 2009.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Desvergne A, Michalik L and Wahli W:
Transcriptional regulation of metabolism. Physiol Rev. 86:465–514.
2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Watras AC, Buchholz AC, Close RN, Zhang Z
and Schoeller DA: The role of conjugated linoleic acid in reducing
body fat and preventing holiday weight gain. Int J Obes (Lond).
31:481–487. 2007.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Al-suhaimi EA and Shehzad A: Leptin,
resistin and visfatin: The missing link between endocrine metabolic
disorders and immunity. Eur J Med Res. 18(12)2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ouchi N, Parker JL, Lugus JJ and Walsh K:
Adipokines in inflammation and metabolic disease. Nat Rev Immunol.
11:85–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pozza C and Isidori AM: What's behind the
obesity epidemic. In: Imaging in bariatric surgery. Springer,
pp1-8, 2018.
|
|
78
|
Sperling LS, Mechanick JI, Neeland IJ,
Herrick CJ, Despres JP, Ndumele CE, Vijayaraghavan K, Handelsman Y,
Puckrein GA, Araneta MR, et al: The cardiometabolic health
alliance: Working toward a new care model for the metabolic
syndrome. J Am Coll Cardiol. 66:1050–1067. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Goossens GH: The metabolic phenotype in
obesity: Fat mass, body fat distribution, and adipose tissue
function. Obes Facts. 10:207–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Chachami G, Kalousi A, Papatheodorou L,
Lyberopoulou A, Nasikas V, Tanimoto K, Simos G, Malizos KN and
Georgatsou E: An association study between hypoxia inducible
factor-1alpha (HIF-1α) polymorphisms and osteonecrosis. PLoS One.
8(e79647)2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Marxsen JH, Stengel P, Doege K, Heikkinen
P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P and Metzen E:
Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by
induction of HIF-α-prolyl-4-hydroxylases. Biochem J. 381:761–767.
2004.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Nagy G, Kovacs-Nagy R, Kereszturi E,
Somogyi A, Szekely A, Nemeth N, Hosszufalusi N, Panczel P, Ronai Z
and Sasvari-Szekely M: Association of hypoxia inducible factor-1
alpha gene polymorphism with both type 1 and type 2 diabetes in a
Caucasian (Hungarian) sample. BMC Med Genet. 10(79)2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Semenza GL, Rue EA, Iyer NV, Pang MG and
Kearns WG: Assignment of the hypoxia-inducible factor 1α gene to a
region of conserved synteny on mouse chromosome 12 and human
chromosome 14q. Genomics. 34:437–439. 1996.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Döring F, Onur S, Fischer A, Boulay MR,
Pérusse L, Rankinen T, Rauramaa R, Wolfarth B and Bouchard C: A
common haplotype and the Pro582Ser polymorphism of the
hypoxia-inducible factor-1α (HIF1A) gene in elite endurance
athletes. J Appl Physiol (1985). 108:1497–1500. 2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Nagy G, Kovacs-Nagy R, Kereszturi E,
Somogyi A, Szekely A, Nemeth N, Hosszufalusi N, Panczel P, Ronai Z
and Sasvari-Szekely M: Association of hypoxia inducible factor-1
alpha gene polymorphism with both type 1 and type 2 diabetes in a
Caucasian (Hungarian) sample. BMC Med Genet. 10(79)2009.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Jacobs EJ, Hsing AW, Bain EB, Stevens VL,
Wang Y, Chen J, Chanock SJ, Zheng SL, Xu J, Thun MJ, et al:
Polymorphisms in angiogenesis-related genes and prostate cancer.
Cancer Epidemiol Biomarkers Prev. 17:972–977. 2008.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Drozdovska SB, Dosenko VE, Ahmetov II and
Ilyin VN: The association of gene polymorphisms with athlete status
in Ukrainians. Biol Sport. 30:163–167. 2013.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Herráez DL, Bauchet M, Tang K, Theunert C,
Pugach I, Li J, Nandineni MR, Gross A, Scholz M and Stoneking M:
Genetic variation and recent positive selection in worldwide human
populations: Evidence from nearly 1 million SNPs. PLoS One.
4(e7888)2009.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Foti DP and Brunetti A: Editorial:
‘Linking Hypoxia to Obesitz’. Front Endocrinol (Lausanne).
8(34)2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Li P, Cao Q, Shao PF, Cai HZ, Zhou H, Chen
JW, Qin C, Zhang ZD, Ju XB and Yin CJ: Genetic polymorphisms in
HIF1A are associated with prostate cancer risk in a Chinese
population. Asian J Androl. 14:864–869. 2012.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684.
2006.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Edenberg HJ, Koller DL, Xuei X, Wetherill
L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev
F, et al: Genome-wide association study of alcohol dependence
implicates a region on chromosome 11. Alcohol Clin Exp Res.
34:840–852. 2010.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Ota N, Nakajima T, Shirai Y and Emi M:
Isolation and radiation hybrid mapping of a highly polymorphic CA
repeat sequence at the human nuclear factor kappa-beta subunit 1
(NFKB1) locus. J Hum Genet. 44:129–130. 1999.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Yenmis G, Oner T, Cam C, Koc A, Kucuk OS,
Yakicier MC, Dizman D and Sultuybek GK: Association of NFKB1 and
NFKBIA polymorphisms in relation to susceptibility of Behçet's
disease. Scand J Immunol. 81:81–86. 2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wang D, Xie T, Xu J, Wang H, Zeng W, Rao
S, Zhou K, Pei F and Zhou Z: Genetic association between NFKB1-94
ins/del ATTG Promoter Polymorphism and cancer risk: A meta-analysis
of 42 case-control studies. Sci Rep. 6(30220)2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ju Z, Zheng X, Huang J, Qi C, Zhang Y, Li
J, Zhong J and Wang C: Functional characterization of genetic
polymorphisms identified in the promoter region of the bovine PEPS
gene. DNA Cell Biol. 31:1038–1045. 2012.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Song S, Chen D, Lu J, Liao J, Luo Y, Yang
Z, Fu X, Fan X, Wei Y, Yang L, et al: NFκB1 and NFκBIA
polymorphisms are associated with increased risk for sporadic
colorectal cancer in a southern Chinese population. PLoS One.
6(e21726)2011.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Gautam A, Gupta S, Mehndiratta M, Sharma
M, Singh K, Kalra OP, Agarwal S and Gambhir JK: Association of
NFKB1 gene polymorphism (rs28362491) with levels of
inflammatory biomarkers and susceptibility to diabetic nephropathy
in Asian Indians. World J Diabetes. 8:66–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Soydas T, Karaman O, Arkan H, Yenmis G,
Ilhan MM, Tombulturk K, Tasan E and Sultuybek GK: The correlation
of increased CRP levels with NFKB1 and TLR2 polymorphisms in the
case of morbid obesity. Scand J Immunol. 84:278–283.
2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yang YN, Zhang JY, Ma YT, Xie X, Li XM,
Liu F, Chen BD, Dong XH, Zheng YY, Pan S, et al: -94 ATTG
insertion/deletion polymorphism of the NFKB1 gene is
associated with coronary artery disease in Han and Uygur women in
China. Genet Test Mol Biomarkers. 18:430–438. 2014.PubMed/NCBI View Article : Google Scholar
|
|
101
|
López-Mejías R, García-Bermúdez M,
González-Juanatey C, Castañeda S, Miranda-Filloy JA, Gómez-Vaquero
C, Fernández-Gutiérrez B, Balsa A, Pascual-Salcedo D, Blanco R, et
al: NFKB1-94ATTG ins/del polymorphism (rs28362491) is associated
with cardiovascular disease in patients with rheumatoid arthritis.
Atherosclerosis. 224:426–429. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zhang D, Li L, Zhu Y, Zhao L, Wan L, Lv J,
Li X, Huang P, Wei L and Ma M: The NFKB1-94 ATTG insertion/deletion
polymorphism (rs28362491) contributes to the susceptibility of
congenital heart disease in a Chinese population. Gene.
516:307–310. 2013.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gladek I, Ferdin J, Horvat S, Calin GA and
Kunej T: HIF1A gene polymorphisms and human diseases: Graphical
review of 97 association studies. Genes Chromosomes Cancer.
56:439–452. 2017.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Harun-Or-Roshid M, Ali MB and Jesmin and
Mollah MNH: Association of hypoxia inducible factor 1-Alpha gene
polymorphisms with multiple disease risks: A comprehensive
meta-analysis. PLoS One. 17(e0273042)2022.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Lu R, Gao X, Chen Y, Ni J, Yu Y, Li S and
Guo L: Association of an NFKB1 intron SNP (rs4648068 ) with gastric
cancer patients in the Han Chinese population. BMC Gastroenterol.
12(87)2012.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kuba A, Raida L, Mrazek F, Schneiderova P,
Kriegova E, Fürst T, Furstova J, Faber E, Ambruzova Z and Papajik
T: NFKB1 gene single-nucleotide polymorphisms (SNPs): Protection
against acute and chronic graft-versus-host disease (GvHD) in
allografted patients. Biol Blood Marrow Transplant.
22(S404)2016.
|
|
107
|
Yue M, Tian T, Wang C, Fan H, Wu J, Wang
J, Li J, Xia X, Zhang A, Yu R, et al: Genetic mutations in NF-κB
pathway genes were associated with the protection from hepatitis C
virus infection among Chinese Han population. Sci Rep.
9(10830)2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Mirzo SM, Kumar A, Sharma NK, Li L,
Balshaw R, Plummer FA, Luo M and Liang B: NFκB1 polymorphisms are
associated with severe influenza A (H1N1) virus infection in a
Canadian Population. Microorganisms. 10(1886)2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Jin SY, Luo JY, Li XM, Liu F, Ma YT, Gao
XM and Yang YN: NFKB1 gene rs28362491 polymorphism is associated
with the susceptibility of acute coronary syndrome. Biosci Rep.
39(BSR20182292)2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Li XD, Zi H, Fang C and Zeng XT:
Association between HIF1A rs11549465 polymorphism and risk of
prostate cancer: A meta-analysis. Oncotarget. 8:44910–44916.
2017.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Giaccia AJ: HIF-2: The missing link
between obesity and cardiomyopathy. J Am Heart Assoc.
2(e000710)2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Bauman-Fortin J, Ma DWL, Mutch DM,
Abdelmagid SA, Badawi A, El-Sohemy A and Fontaine-Bisson B: The
association between plasma Omega-6/Omega-3 ratio and anthropometric
traits differs by Racial/Ethnic groups and NFKB1 genotypes in
healthy young adults. J Pers Med. 9(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Ilyas MN, AB A, Al-Hatamleh MAI,
Al-Shajrawi OM, Ariff TM and Simbak N: Rising trends of obesity in
Malaysia; role of inflammation and inflammatory markers in obesity
related insulin resistance: A nuclear factor kappa B (Nfkb)
perspective. Exp Clin Endocrinol Diabetes. 109:S135–S148. 2017.
|
|
114
|
He Q, Gao Z, Yin J, Zhang J, Yun Z and Ye
J: Regulation of HIF-1{alpha} activity in adipose tissue by
obesity-associated factors: Adipogenesis, insulin, and hypoxia. Am
J Physiol Endocrinol Metab. 300:E877–E885. 2011.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Fujisaka S, Usui I, Ikutani M, Aminuddin
A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K
and Tobe K: Adipose tissue hypoxia induces inflammatory M1 polarity
of macrophages in an HIF-1α-dependent and HIF-1α-independent manner
in obese mice. Diabetologia. 56:1403–1412. 2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Chooi YC, Ding C and Magkos F: The
epidemiology of obesity. Metabolism. 92:6–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Blüher M: Obesity: Global epidemiology and
pathogenesis. Nat Rev Endocrinol. 15:288–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Wang Y, Beydoun MA, Min J, Xue H, Kaminsky
LA and Cheskin LJ: Has the prevalence of overweight, obesity and
central obesity levelled off in the United States? Trends,
patterns, disparities, and future projections for the obesity
epidemic. Int J Epidemiol. 49:810–823. 2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Bradbury KE, Guo W, Cairns BJ, Armstrong
MEG and Key TJ: Association between physical activity and body fat
percentage, with adjustment for BMI: A large cross-sectional
analysis of UK Biobank. BMJ Open. 7(e011843)2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Emanuele E, Bertona M and Geroldi D: A
multilocus candidate approach identifies ACE and HIF1A as
susceptibility genes for cellulite. J Eur Acad Dermatol Venereol.
24:930–935. 2010.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Fernández-Torres J, Hernández-Díaz C,
Espinosa-Morales R, Camacho-Galindo J, Galindo-Sevilla NDC,
López-Macay Á, Zamudio-Cuevas Y, Martínez-Flores K,
Santamaría-Olmedo MG, Pineda C, et al: Polymorphic variation of
hypoxia inducible factor-1 a (HIF1A) gene might contribute to the
development of knee osteoarthritis: A pilot study. BMC
Musculoskelet Disord. 16(218)2015.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Zhou B, Qie M, Wang Y, Yan L, Zhang Z,
Liang A, Wang T, Wang X, Song Y and Zhang L: Relationship between
NFKB1-94 insertion/deletion ATTG polymorphism and susceptibility of
cervical squamous cell carcinoma risk. Ann Oncol. 21:506–511.
2010.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Chen Y, Lu R, Zheng H, Xiao R, Feng J,
Wang H, Gao X and Guo L: The NFKB1 polymorphism (rs4648068) is
associated with the cell proliferation and motility in gastric
cancer. BMC Gastroenterol. 15(21)2015.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Curtin K, Wolff RK, Herrick JS, Abo R and
Slattery ML: Exploring multilocus associations of inflammation
genes and colorectal cancer risk using hapConstructor. BMC Med
Genet. 11(170)2010.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Rybka J, Gębura K, Wróbel T, Wysoczańska
B, Stefanko E, Kuliczkowski K and Bogunia-Kubik K: Variations in
genes involved in regulation of the nuclear factor-κB pathway and
the risk of acute myeloid leukaemia. Int J Immunogenet. 43:101–106.
2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Pereira EEB, de Carvalho DC, Leitão LPC,
Rodrigues JCG, Modesto AAC, de Sousa EC, Dos Santos SEB, Fernandes
MR and Dos Santos NPC: Influence of Polymorphism on the NFkB1 Gene
(rs28362491) on the susceptibility to sarcopenia in the elderly of
the Brazilian Amazon. J Pers Med. 11(1045)2021.PubMed/NCBI View Article : Google Scholar
|