Introduction
Head and neck tumors (HNTs) are a diverse group of
tumors that develop due to a variety of genetic and environmental
risk factors (1). HNTs commonly
occur in the larynx, oral cavity, sinonasal tract and nasopharynx
(2). Risk factors, such as
smoking, excessive alcohol consumption, and long-term exposure to
ultraviolet radiation, viral infections and asbestos have been
documented to lead to the development of HNTs (1). In developing countries, tobacco and
alcohol, which contribute to inflammation, are responsible for
>90% of HNT cases. Moreover, in developed countries, human
papillomavirus infection with oncogenic features accounts for
>70% of cases (3,4). Although recent progress has been made
in the diagnosis and treatment of head and neck cancer (HNC), there
have been minimal improvements in life expectancy over the past few
decades (5). Surgery, radiation
therapy and chemotherapy are the currently available treatment
options (6). However, local
recurrence and metastasis occur in approximately half of patients
with HNC (7).
According to Owusu-Afriyie et al (8), the incidence of HNC varies greatly
across sub-Saharan Africa, with the incidence in Ghana being 0.8
per 100,000 individuals, compared to 11.1 per 100,000 individuals
in South Africa. Biomarkers including p53, epidermal growth factor
receptor, p16 and cyclin-D1 have been studied in Ghana to assess
their prognostic significance (8-10).
However, alternative therapeutic markers remain unexplored.
Nuclear factor κB (NF-κB), a transcription factor
with five family members, namely p105/p50 (NF-κB1), p100/p52
(NF-κB2), p65 (RELA), c-REL and RELB, has been linked to chronic
inflammation and malignancies, including HNC. The increased
expression of NF-κB target genes has been shown to be associated
with tumorigenesis (11,12). NF-κB is normally inactive in the
cytosol, bound to the inhibitor of κB (IκB). The IκB kinase (IKK)
stimuli-mediated phosphorylation degrades IκB, freeing NF-κB to
translocate to the nucleus to perform transcriptional functions and
modulate biological roles (13).
Studies have shown that NF-κB activation is a key phenomenon that
contributes to tumorigenesis and the survival of cancer cells, as
observed in pancreatic nd breast cancer (14,15).
While typically regulated by negative feedback loops, there is a
malfunction in NF-κB activity in cancer cells due to mutations or
chronic exposure to activating stimuli, leading to its
overexpression (13,16). TNF-α, IL-1 and IL-17 produced by
immune cells infiltrating the gastrointestinal mucosa can increase
NF-κB activity, thereby increasing risk of colon cancer (17). The activation of the NF-κB pathway
also promotes metastasis by regulating epithelial-mesenchymal
transition (18) and matrix
metalloproteinases-9(19). The
increased expression of NF-κB (p65) has been demonstrated to be
associated with tumor grade in breast cancer (20). Currently, the clinical value of
NF-κB (p65) in tumor specimens is unclear and remains
underexplored. Despite advancements being made in cancer research
in Africa, including Ghana, to the best of our knowledge, no study
available to date has explored the prognostic value of NF-κB (p65)
in HNTs. The present study thus aimed to fill this gap by
evaluating the prognostic relevance of NF-κB (p65) in patients with
HNTs at the Cape Coast Teaching Hospital (CCTH) in Cape Coast,
Ghana.
Patients and methods
Study design, ethical approval and
sample collection
The present cross-sectional study was approved by
the Institutional Review Board of CCTH (Approval no.
CCTHERC/EC/2023/183). Laboratory analyses were conducted at the
Kumasi Center for Collaborative Research (KCCR), Kumasi, Ghana. The
inclusion criteria were patients with histologically confirmed
HNTs, available archived formalin-fixed paraffin-embedded (FFPE)
tissues and complete medical records. The exclusion criteria were
patients with a history of no preoperative chemotherapy and no
concurrent cancers from 2017 to 2022. The patients who qualified
were purposively sampled at the ACT Pathology Consult, the
consulting pathology firm of CCTH. Clinicopathological data
including age, sex, tumor location, type of biopsy, type of tumor,
perineural invasion, tumor node metastasis (TNM), laterality, tumor
grade and lymphovascular invasion were collected from the
laboratory request forms of the patients. Hematoxylin and eosin
staining was used to confirm the pathological classification of the
HNTs prior to the selection of the tissues. A total of 112 tumor
tissues were selected for analysis.
Immunohistochemistry
FFPE sections (4-µm-thick) were attached to
positively charged slides. These portions underwent several
preparatory procedures, such as deparaffinization and rehydration
with xylene (two changes for 10 min each and various ethanol
concentrations (100, 90 and 70% for 3 min each), respectively. A
Biocare decloaking chamber (Biocare Medical System, Inc.) set at
95˚ for 30 min was used for antigen retrieval using Novocastra
buffer (pH 9.0) (lot no. 6093858, Leica Microsystems, Ltd.),
followed by a 35-min cooling phase in the same buffer and washing
with reaction buffer from Ventana (lot no. H18324, Ventana Medical
Systems, Inc.).
A protein blocker (Biocare Medical System, Inc.) and
3% hydrogen peroxide were used to inhibit non-specific protein
binding and hydrogen peroxidase in the tissues. The tissues were
then exposed to appropriately diluted (1:400) NF-κB (p65) rabbit
polyclonal antibody IgG (cat. no. 10745-1-AP, Proteintech Group,
Inc.) for 2 h at room temperature. The tissues were then rinsed and
treated with MACH 1 Universal horse radish peroxidase (HRP)
polymer, as a secondary antibody (lot no. 092822A, Biocare Medical
System, Inc.) at room temperature for 45 min (no dilution was
done). 3,3-Diaminobenzidine (DAB) chromogen (lot no. 010722A-4,
Biocare Medical System, Inc.) and substrate (lot no. 071422A-2,
Biocare Medical System, Inc.) were added following a second round
of washing, and Mayer's hematoxylin (Abcam) was employed for
counterstaining at room temperature for 1-2 min. Before mounting
the dyed tissues with coverslips using para-mount, the tissues were
dehydrated and cleared. Of note, two pathologists then used an
Opto-Edu brand [Opto-Edu (Beijing) Co., Ltd.] digital microscope to
analyze the slides and read the protein expression of NF-κB (p65)
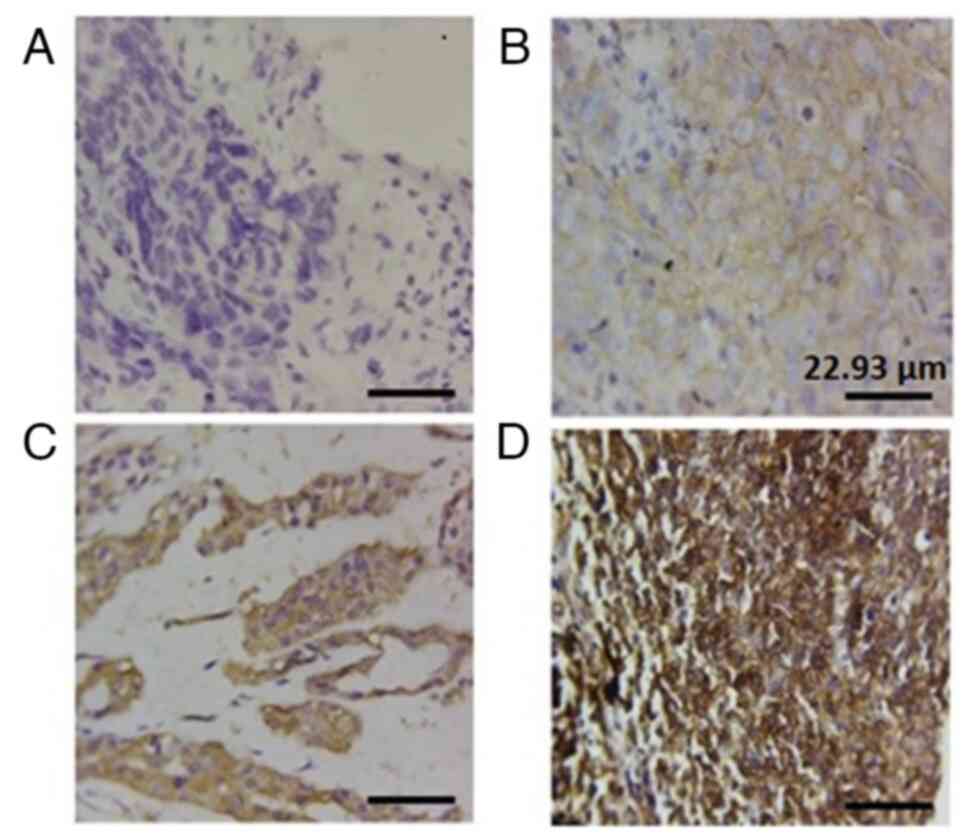
in the HNTs. A brownish-yellow colour observed in the cytoplasm was
regarded as positive staining. The semi-quantitative approach,
which combines the staining intensity (0, no staining; 1, mild
staining; 2, moderate staining; and 3, strong staining) and the
percentage of the tumors stained (0, negative; 1, <25%; 2,
25-50%; 3, 51-75%; 4, 76-100%), were used to calculate the final
cytoplasmic stain of NF-κB (p65), as previously described (21). Tissues with the product of staining
intensity and percentage scores of 0-6 were considered as having a
low expression and those with scores of 7-12 were considered as
having a high expression. At a magnification of x400, three to four
images were obtained.
Statistical analysis
Categorical variables are expressed as frequencies
and percentages. Pearson's Chi-square test and Fisher's exact test
were used to determine the associations between clinicopathological
variables using SPSS version 25 software (IBM Corp.). P-values
<0.05 were considered to indicate statistically significant
differences. Logistic regression analysis was conducted for the
associated variables and diagnostic performance was assessed using
receiver operation characteristic analysis with Xlstat (https://www.xlstat.com/en/).
Results
Characteristics of patients with
HNTs
The present study utilized FFPE tissue blocks from
112 patients with various HNTs, aged between 1 and 86 years. The
average age of the patients was 42.71±18.85 years. There was an
equal distribution of male [56 (50%)] and female [56 (50%)]
participants. The analysis of the age distribution revealed that 30
patients (26.8%) were of 1-29 years of age, 55 patients (49.1%) of
30-57 years of age and 27 (24.1) of 58-86 years of age. As regards
biopsy types, 54 cases (48.2%) were excisional biopsies, 46 cases
(41.1%) were incisional biopsies, 8 cases (7.1%) were punch
biopsies and 2 cases (1.8%) were free needle core biopsies.
Additionally, there was an equal representation of curettage and
ultrasound-guided core biopsies (n=1; 0.9%). Of the 112 tumors
analyzed, 62 (55.4%) were benign and 50 (44.6%) were malignant. The
majority of the tumors were located in the nasal cavity 24 (21.4%)
and the larynx 16 (14.3%). Other tumor sites included the ear [6
(5.4%)], oral cavity [10 (8.9)], salivary gland [7 (6.3%)], eye [5
(4.5%)], face [7 (6.3%)], mandible [9 (8.0%)], maxillary region [7
(6.3%)], nasopharynx [13 (11.6%)] and neck [8 (7.1%)]. Laterality
data revealed that 52 cases (46.4%) had no specific side
designation, while 32 cases (28.6%) were from the left side and 28
cases (25.0%) were from the right side. The detailed patient
characteristics are presented in Table
I.
 | Table IAssociation between NF-κB (p65) status
and characteristics of patients with head and neck tumors. |
Table I
Association between NF-κB (p65) status
and characteristics of patients with head and neck tumors.
| | NF-κB (p65)
expression status | |
|---|
| Variables, n (%) | Total (n=112) | Low (n=64) | High (n=48) |
χ2/Fisher's | P-value |
|---|
| Sex | | | | 7.146 | 0.013 |
|
Male | 56 (50.0) | 25 (44.6) | 31 (55.4) | | |
|
Female | 56 (50.0) | 39 (69.6) | 17 (30.4) | | |
| Age group, years | | | | 1.386 | 0.500 |
|
1-29 | 30 (26.8) | 19 (29.7) | 11 (22.9) | | |
|
30-57 | 55 (49.1) | 32 (50.0) | 23 (47.9) | | |
|
58-86 | 27 (24.1) | 13 (20.3) | 14 (29.2) | | |
| Types of biopsy | | | | 3.707a | 0.670a |
|
Excision | 54 (48.2) | 34 (63.0) | 20 (37.0) | | |
|
Incision
biopsy | 46 (41.1) | 24 (52.2) | 22 (47.8) | | |
|
Punch
biopsy | 8 (7.1) | 4 (50.0) | 4 (50.0) | | |
|
Free needle
core biopsy | 2 (1.8) | 1 (50.0) | 1 (50.0) | | |
|
Curettage | 1 (0.9) | 1 (100.0) | 0 (0) | | |
|
Ultrasound
guided core biopsy | 1 (0.9) | 0 (0) | 1 (100.0) | | |
| Type of tumor | | | | 10.839 | 0.001 |
|
Benign | 62 (55.4) | 44 (71.0) | 18 (29.0) | | |
|
Malignant | 50 (44.6) | 20 (40.0) | 30 (60.0) | | |
| Tumor location | | | | 15.600a | 0.099a |
|
Ear | 6 (5.4) | 5 (83.3) | 1 (16.7) | | |
|
Oral
cavity | 10 (8.9) | 5 (50.0) | 5 (50.0) | | |
|
Salivary | 7 (6.3) | 3 (42,9) | 4 (57.1) | | |
|
Eye | 5 (4.5) | 4 (80.0) | 1 (20.0) | | |
|
Face | 7 (6.3) | 7 (100.0) | 0 (0) | | |
|
Larynx | 16 (14.3) | 7 (43.8) | 9 (56.3) | | |
|
Mandible | 9 (8.0) | 6 (66.7) | 3 (33.3) | | |
|
Maxillary
region | 7 (6.3) | 3 (42.9) | 4 (57.1) | | |
|
Nasal
cavity | 24 (21.4) | 16 (66.7) | 8 (33.3) | | |
|
Nasopharynx | 13 (11.6) | 4 (30.8) | 9 (69.2) | | |
|
Neck | 8 (7.1) | 4 (50.0) | 4 (50.0) | | |
| Laterality | | | | 1.317 | 0.518 |
|
Left | 32 (28.6) | 21 (65.6) | 11 (34.4) | | |
|
Right | 28 (25.0) | 15 (53.6) | 13 (46.4) | | |
|
N/A | 52 (46.4) | 28 (53.8) | 24 (46.2) | | |
NF-κB (p65) protein expression in
HNTs, as demonstrated using immunohistochemistry
NF-κB (p65) protein expression was observed
exclusively in the cytoplasm, as depicted in Fig. 1. In the benign tumors, NF-κB (p65)
expression analysis revealed that 18 (29%) of the tumors exhibited
a high expression, while 44 (71%) exhibited a low expression.
Conversely, immunostaining in the malignant tumors demonstrated
that 30 (60%) of the tissues exhibited a high NF-κB (p65)
expression, whereas 20 cases (40%) exhibited a low expression, as
detailed in Table I.
Association of NF-κB (p65) expression
with clinicopathological characteristics of patients with HNTs
Despite the equal number of males and females, there
was a significant association between NF-κB (p65) expression and
sex (P=0.013), with males exhibiting higher levels of NF-κB (p65)
protein expression compared to females (n=31, 55.4% vs. n=17,
30.4%). Conversely, a low expression of NF-κB (p65) was more
prevalent in female tumors (n=39; 69.6%) compared to male tumors 25
(44.6%). Additionally, a significant association (P=0.001) was
observed between tumor type (benign or malignant) and NF-κB (p65)
expression. A high protein expression of NF-κB (p65) was twice as
common in malignant tumors compared to benign tumors (n=30, 60.0%
vs. n=18, 29.0%). However, NF-κB (p65) protein expression in HNTs
was not significantly associated with age, type of biopsy, location
of tumor and laterality (Table
I).
Logistic regression analysis revealed a significant
decrease in odds for NF-κB (p65) expression when comparing sex
[odds ratio (OR), 0.352; 95% confidence interval (CI), 0.162-0.764;
P-value=0:008] and tumor type (OR, 0.273, 95% CI, 0.124-0.600;
P-value=0.001) (Table II). When
the data were stratified to examine the associations between benign
tumors and NF-κB (p65) expression (Table III), no significant associations
were observed with any of the clinicopathological variables. When
examining malignant tumors and NF-κB (p65) expression, tumor grade,
perineural invasion, lymphovascular invasion, laterality, type of
biopsy and tumor location, no significant associations were
observed. Notably, sex and TNM stage were significantly associated
with NF-κB (p65) expression (Table
IV).
 | Table IILogistic regression analysis of NF-κB
(p65) expression vs. sex and type of tumor. |
Table II
Logistic regression analysis of NF-κB
(p65) expression vs. sex and type of tumor.
| | NF-κB (p65)
status | |
|---|
| Variables, n
(%) | Total (n=112) | Low (n=64) | High (n=48) | OR (95% CI) | P-value |
|---|
| Sex | | | | | 0.008 |
|
Male | 56 (50.0) | 25 (44.6) | 31 (55.4) | 1 | |
|
Female | 56 (50.0) | 39 (69.6) | 17 (30.4) | 0.352
(0.162-0.764) | |
| Type of tumor | | | | | 0.001 |
|
Benign | 62 (55.4) | 44 (71.0) | 18 (29.0) | 1 | |
|
Malignant | 50 (44.6) | 20 (40.0) | 30 (60.0) | 0.273
(0.124-0.600) | |
 | Table IIIAssociation between NF-κB (p65)
expression status and benign head and neck tumors. |
Table III
Association between NF-κB (p65)
expression status and benign head and neck tumors.
| | NF-κB (p65)
expression status | |
|---|
| Variable, n
(%) | Total (n=62) | Low (n=44) | High (n=18) |
χ2/Fisher's | P-value |
|---|
| Sex | | | | 0.677 | 0.572 |
|
Male | 26 (41.9) | 17 (65.4) | 9 (34.6) | | |
|
Female | 36 (58.1) | 27 (75.0) | 9 (25.0) | | |
| Age group,
years | | | | 0.389a | 0.899a |
|
1-29 | 20 (32.3) | 15 (34.1) | 5 (27.8) | | |
|
30-57 | 38 (61.3) | 26 (59.1) | 12 (66.7) | | |
|
58-86 | 4 (6.4) | 3 (6.8) | 1 (5.6) | | |
| Type of biopsy | | | | 3.201a | 0.332a |
|
Excision | 40 (64.5) | 29 (72.5) | 11 (27.5) | | |
|
Incision
biopsy | 18 (29.0) | 12 (66.7) | 6 (33.3) | | |
|
Punch
biopsy | 3 (4.8) | 3 (100.0) | 0 (0) | | |
|
Free needle
core biopsy | 1 (1.6) | 0 (0) | 1 (100.0) | | |
| Tumor location | | | | 10.900a | 0.302a |
|
Ear | 5 (8.1) | 4 (80.0) | 1 (20.0) | | |
|
Oral
cavity | 6 (9.7) | 4 (66.7) | 2 (33,3) | | |
|
Salivary | 2 (3.2) | 1 (50.0) | 1 (50.0) | | |
|
Eye | 4 (6.5) | 3 (75.0) | 1 (25.0) | | |
|
Facial | 7 (11.3) | 7 (100.0) | 0 (0) | | |
|
Larynx | 5 (8.1) | 3 (60.0) | 2 (40.0) | | |
|
Mandible | 8 (12.9) | 5 (62.5) | 3 (37.5) | | |
|
Maxillary | 4 (6.5) | 2 (50.0) | 2 (50.0) | | |
|
Nasal
cavity | 16 (25.8) | 13 (81.3) | 3 (18,8) | | |
|
Nasopharynx | 2 (3.2) | 0 (0) | 2 (100.0) | | |
|
Neck | 3 (4.8) | 2 (66.7) | 1 (33.3) | | |
| Laterality | | | | 0.642a | 0.727a |
|
Left | 17 (27.4) | 12 (70.6) | 5 (29.4) | | |
|
Right | 17 (27.4) | 11 (64.7) | 6 (35.3) | | |
|
N/A | 28 (45.2) | 21 (75.0) | 7 (25.0) | | |
 | Table IVNF-κB (p65) status in malignant head
and neck tumors. |
Table IV
NF-κB (p65) status in malignant head
and neck tumors.
| | NF-κB (p65)
status | |
|---|
| Variable, n
(%) | Total (n=50) | Low (n=20) | High (n=30) |
χ2/Fisher's | P-value |
|---|
| Sex | | | | 5.556 | 0.038 |
|
Male | 30 (60.0) | 8 (40.0) | 22 (73.3) | | |
|
Female | 20 (40.0) | 12 (60.0) | 8 (26.7) | | |
| Age group,
years | | | | 0.420a | 0.551a |
|
1-29 | 7 (14.0) | 2 (10.0) | 5 (16.7) | | |
|
30-57 | 20 (40.0) | 8 (40.0) | 12 (40.0) | | |
|
58-86 | 23 (46.0) | 10 (50.0) | 13 (43.7) | | |
| Type of biopsy | | | | 4.361a | 0.516a |
|
Excision | 14 (28.0) | 5 (25.0) | 9 (30.0) | | |
|
Incision
biopsy | 28 (56.0) | 12 (60.0) | 16 (53.3) | | |
|
Punch
biopsy | 5 (10.0) | 1 (5.0) | 4 (13.3) | | |
|
Free needle
core biopsy | 1 (2.0) | 1 (5.0) | 0 (0) | | |
|
Curettage | 1 (2.0) | 1 (5.0) | 0 (0) | | |
|
Ultrasound
guided core biopsy | 1 (2.0) | 0 (0) | 1 (3.3) | | |
| Tumor location | | | | 5.177a | 0.928a |
|
Ear | 1 (2.0) | 1 (5.0) | 0 (0) | | |
|
Oral
cavity | 4 (8.0) | 1 (5.0) | 3 (10.0) | | |
|
Salivary | 5 (10.0) | 2 (10.0) | 3 (10.0) | | |
|
Eye | 1 (2.0) | 1 (5.0) | 0 (0) | | |
|
Larynx | 11 (22.0) | 4 (20.0) | 7 (23.3) | | |
|
Mandible | 1 (2.0) | 1 (5.0) | 0 (0) | | |
|
Maxillary | 3 (6.0) | 1 (5.0) | 2 (6.7) | | |
|
Nasal
cavity | 8(16) | 3 (15.0) | 5 (16.7) | | |
|
Nasopharynx | 11(22) | 4 (20.0) | 7 (23.3) | | |
|
Neck | 5 (10.0) | 2 (10.0) | 3 (10.0) | | |
| Tumor grade | | | | 0.940 | 0.828 |
|
Well | 15 (30.0) | 6 (30.0) | 9 (30.0) | | |
|
Moderate | 16 (32.0) | 5 (25.0) | 11 (36.7) | | |
|
Poor | 19 (38.0) | 9 (45.0) | 10 (33.3) | | |
| PNI | | | | 1.531 | 0.216 |
|
Present | 1 (2.0) | 1(5.0) | 0 (0) | | |
|
Absent | 49 (98.0) | 19(95) | 30 (100.0) | | |
| LVI | | | | 0.000 | 0.999 |
|
Present | 5 (10.0) | 2 (10.0) | 3 (10.0) | | |
|
Absent | 45 (90.0) | 18 (90.0) | 27 (90.0) | | |
| Laterality | | | | 3.734 | 0.156 |
|
Left | 15 (30.0) | 9 (45.0) | 6 (20.0) | | |
|
Right | 11 (22.0) | 4 (20.0) | 7 (23.3) | | |
|
N/A | 24 (48.0) | 7 (35.0) | 17 (56.7) | | |
| TNM stage | | | | 6.551 | 0.010 |
|
I-II | 33 (66.0) | 9 (45.0) | 24 (80.0) | | |
|
III-IV | 17 (34.0) | 11 (55.0) | 6 (20.0) | | |
Diagnostic performance of NF-κB (p65)
as a marker in HNTs in CCTH
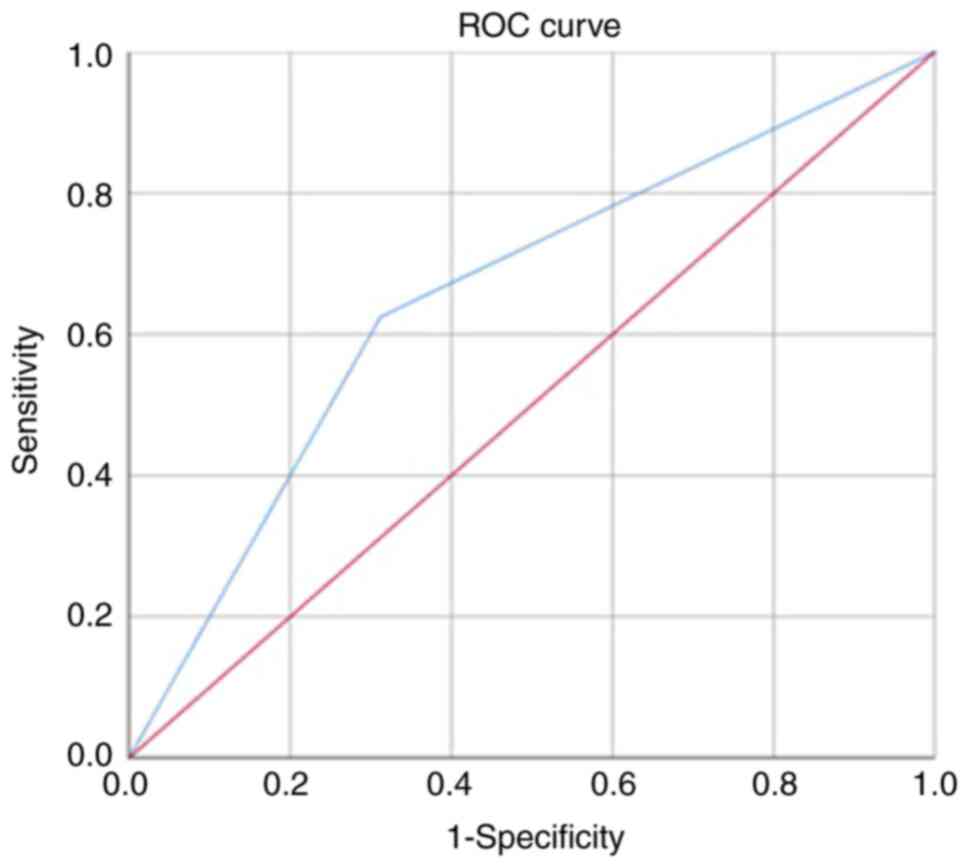
The diagnostic performance of NF-κB (p65) protein
expression in HNTs was determined by drawing receiver operating
characteristic curves with Xlstat. Sensitivity, specificity,
positive predictive (PPV) and negative predictive (NPV) values were
determined and the results are presented in Fig. 2 and Table V. The overall sensitivity was
62.5%, with a specificity of 68.8%. The PPV and NPV were 60.0 and
71.1% respectively. Using a cut-off value ≥0.600, the area under
the curve was calculated as 0.656 (P<0.0001).
 | Table VDiagnostic performance of NF-κB (p65)
in head and neck tumors. |
Table V
Diagnostic performance of NF-κB (p65)
in head and neck tumors.
| Protein | Cut-off value | Sensitivity (%) 95%
(CI) | Specificity (%) 95%
(CI) | PPV (%) | NPV (%) | AUC | P-value |
|---|
| NF-κB (p65) | 0.600 | 62.5 | 68.8 | 60.0 | 71.0 | 0.656 | P<0.0001 |
| | | (48.374.8) | (56.5-78.8) | (49.5-69.6) | (62.1-78.5) | (0.57-0.75) | |
Discussion
A number of inflammatory mediators and signaling
pathways have been linked to the development of HNCs (22). However, NF-κB, a key driver and
inducer of inflammatory mediators that promote cell survival and
therapeutic resistance (23), has
not been extensively investigated in numerous African countries,
including Ghana. Additionally, there is a global paucity of data on
this topic. Therefore, the present study was designed to provide
pertinent information on NF-κB (p65) expression and its association
with the clinicopathological characteristics of patients with
HNTs.
In the present study, only cytoplasmic
immunostaining was observed in all the HNTs examined. Consistent
with the findings of the present study, cytoplasmic NF-κB (p65) has
been reported as a good prognostic marker in triple-negative breast
cancer (24). Moreover, Al-Mutairi
and Habashy (25) demonstrated
that cytoplasmic NF-κB (p65) expression was associated with tumor
size and high grade. Furthermore, Barnes et al (20) demonstrated that cytoplasmic NF-κB
(p65) was linked to tumor grade. Conversely, NF-κB (p65) expression
has been found in the nucleus (26). The findings of the present study
suggest that NF-κB (p65) may be restrained in the cytosol,
preventing its nuclear translocation and subsequent transcriptional
regulation through DNA binding. This finding indicates the
dysfunction of NF-κB (p65) signaling in HNTs, which may provide
insight into the pathophysiology of these cancers and may highlight
its potential for use as a prognostic biomarker and therapeutic
target.
One of the main findings of the present study was
the substantial association between sex and NF-κB (p65) expression
in HNC. According to Pasquali et al (27), the tumor microenvironment involving
NF-κB is influenced by hormonal and inflammatory dynamics, which
may vary by sex. This suggests that sex hormones have the potential
to modulate NF-κB (p65) events. Moreover, the data of the present
study revealed that males expressed higher levels of NF-κB (p65)
than females, with a statistically significant difference
(P=0.013). This notable sex difference in NF-κB (p65) expression
levels may have significant clinical ramifications, influencing
tumor behavior and therapeutic responses. It also indicates that
NF-κB (p65) regulation in these tumors may be influenced by
sex-specific differences in the immune response and inflammation.
Generally, it is well-established that females mount stronger
immune responses and have chronic inflammation at lower levels
compared to males (28). The exact
mechanism(s) underpinning this link is unknown; thus, further
investigations are required to clarify the pathogenesis and
hormonal elements causing this sex-based difference. The analysis
of malignant tumors revealed that sex and TNM stage were
significantly associated with NF-κB (p65) protein expression. This
is consistent with the findings of other studies on other types of
cancer, where NF-κB (p65) was shown to be significantly associated
with TNM stage (29,30). The sex-specific association
observed in malignant tumors underscores the importance of
considering sex-related factors, such as hormonal receptor
expressions in future studies, on NF-κB (p65) and HNC. These
findings also suggest that sex-specific therapeutic approaches
should be explored when targeting NF-κB (p65)-related pathways in
the treatment of HNC. Furthermore, the association between NF-κB
(p65) protein expression and TNM stage in malignant tumors
highlights the critical role of NF-κB (p65) signaling in cancer
progression and metastasis. Elevated NF-κB (p65) levels are
typically linked to more advanced disease stages and poorer
clinical outcomes.
Another main result of the present study was the
association between NF-κB (p65) protein expression and tumor types
(benign vs. malignant). Malignant tumors expressed significantly
high NF-κB (p65) similar to previous studies (20,25).
This finding raises the possibility that the NF-κB (p65) may be
involved in the pathophysiology of malignant transformation in head
and neck cancer. Numerous pro-tumorigenic mechanisms, such as
inflammation, cell survival and proliferation, have been connected
to NF-κB (p65) activation. Therefore, its increased expression in
malignant tumors may indicate a role in promoting tumor growth.
The present study found no significant links between
NF-κB (p65) protein expression and age, biopsy type, tumor site,
laterality (in both benign and malignant tumors), tumor grade,
perinueral invasion and lymphovascular invasion in malignant
tumors. However, while age and declining immunity has been shown to
be associated with NF-κB (31),
similarly, the present study found a higher expression of NF-κB
(p65) among older populations. However, the results of the present
study may have been influenced by its retrospective design, limited
clinicopathological variables analyzed and immunohistochemistry
scoring methods.
The overall diagnostic performance of NF-κB (p65) in
distinguishing between benign and malignant HNTs was moderate. The
moderate sensitivity and specificity indicates that while NF-κB
(p65) may not be sufficient as a stand-alone prognostic tool, it
could be combined with other markers or clinical data to enhance
predictive value and improve patient prognosis. To establish NF-κB
(p65) as a reliable prognostic marker for HNTs, additional studies
with larger and more diverse patient populations are required.
Further studies are required to better determine the factors
influencing NF-κB (p65) expression and refine its utility as a
diagnostic and a prognostic tool.
In conclusion, the present study emphasizes that
NF-κB (p65) expression levels may indeed be controlled by sex and
TNM stage, with males usually exhibiting a higher NF-kB (p65)
activity than females in HNC. These results highlight the need for
further studies to elucidate the molecular mechanisms regulating
NF-κB (p65) expression, advanced disease stages and sex
disparities, which may involve genetic, hormonal and environmental
factors. Investigating its potential as a prognostic biomarker and
therapeutic target is crucial to improving the outcomes and
wellbeing of patients.
Acknowledgements
The authors would like to thank Mr. Samuel
Mingyigilougu Apewe Ka-Chungu of the Department of Pathology at
Komfo Anokye Teaching Hospital, Kumasi, Ghana for assisting with
tissue sectioning.
Funding
Funding: The Directorate of Research, Innovation and Consultancy
(RSG/GRP/CoHAS/2022/104), University of Cape Coast (Cape Coast,
Ghana) funded this study with additional support from Samuel and
Emelia Brew-Butler/Graduate Studies research grant, University of
Cape Coast.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PB and ROS were involved in the conceptualization
and design of the study, as well as in data collection, data
analysis, project administration, manuscript writing and funding
acquisition. AM, LDK, EAd, EAg, ESY, BA, EGI, GA, PKA, FHL, KD,
SVN, DOY were involved in the laboratory investigation, literature
review, data analysis and the drafting of the manuscript. All
authors have read, edited and approved the final manuscript. PB and
EAd confirm the authenticity of the raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Cape Coast Teaching Hospital (reference no.
CCTHERC/EC/2023/183). Consent to participate was waived as it was a
retrospective study which did not violate patient privacy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Sarwar S, Mulla M, Mulla M, Tanveer R,
Sabir M, Sultan A and Malik SA: Human papillomavirus, tobacco, and
poor oral hygiene can act synergetically, modulate the expression
of the nuclear factor kappa B signaling pathway for the development
and progression of head and neck cancer in the Pakistani
population. Chin Med J (Engl). 135:1829–1836. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
D'cruz A, Lin T, Anand AK, Atmakusuma D,
Calaguas MJ, Chitapanarux I, Cho BC, Goh BC, Guo Y, Hsieh WS, et
al: Consensus recommendations for management of head and neck
cancer in Asian countries: A review of international guidelines.
Oral Oncol. 49:872–877. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kobayashi K, Hisamatsu K, Suzui N, Hara A,
Tomita H and Miyazaki T: A review of HPV-related head and neck
cancer. J Clin Med. 7(241)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: Response to correspondence on the molecular biology of head and
neck cancer. Nat Rev Cancer. 11:382. 2011.
|
|
5
|
Adeyi A and Olugbenga S: The challenges of
managing malignant head and neck tumors in a tropical tertiary
health center in Nigeria. Pan Afr Med J. 10(31)2011.PubMed/NCBI
|
|
6
|
Cramer JD, Burtness B, Le QT and Ferris
RL: The changing therapeutic landscape of head and neck cancer. Nat
Rev Clin Oncol. 16:669–683. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alterio D, Marvaso G, Ferrari A, Volpe S,
Orecchia R and Jereczek-Fossa BA: Modern radiotherapy for head and
neck cancer. Semin Oncol. 46:233–245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Owusu-Afriyie O, Owiredu WKBA,
Owusu-Danquah K, Komarck C, Foltin SK, Larsen-Reindorf R,
Acheampong E, Quayson SE, Prince MEP, McHugh JB, et al: Expression
of immunohistochemical markers in non-oropharyngeal head and neck
squamous cell carcinoma in Ghana. PLoS One.
13(e0202790)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barnes P, Yeboah F, Zhu J, Saahene RO,
Akakpo P and Ephrraim RK: Prognostic significance of epidermal
growth factor receptor (EGFR) in head and neck tumours at some
selected hospital In Ghana, 2019.
|
|
10
|
Barnes P, Yeboah FA, Zhu J, Saahene RO,
Obirikorang C, Adinortey MB, Amoani B, Kyei F, Akakpo P and Awuku
YA: Prognostic worth of epidermal growth factor receptor (EGFR) in
patients with head and neck tumors. J Cancer Epidemiol.
2020(5615303)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morgan EL, Chen Z and Van Waes C:
Regulation of NFκB signalling by ubiquitination: A potential
therapeutic target in head and neck squamous cell carcinoma?
Cancers (Basel). 12(2877)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
El-Rayes BF, Ali S, Ali IF, Philip PA,
Abbruzzese J and Sarkar FH: Potentiation of the effect of erlotinib
by genistein in pancreatic cancer: The role of Akt and nuclear
factor-kappaB. Cancer Res. 66:10553–10559. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Biswas DK, Shi Q, Baily S, Strickland I,
Ghosh S, Pardee AB and Iglehart JD: NF-kappa B activation in human
breast cancer specimens and its role in cell proliferation and
apoptosis. Proc Natl Acad Sci USA 10137-10142, 2004.
|
|
16
|
Kim HJ, Hawke N and Baldwin AS: NF-kappa B
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chou YC, Sheu JR, Chung CL, Chen CY, Lin
FL, Hsu MJ, Kuo YH and Hsiao G: Nuclear-targeted inhibition of
NF-kappaB on MMP-9 production by N-2-(4-bromophenyl) ethyl
caffeamide in human monocytic cells. Chem Biol Interact.
184:403–412. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barnes P, Mensah A, Derkyi-Kwarteng L,
Adankwa E, Agbo E, Yahaya ES, Amoani B, Adjei G, Ka-Chungu SMA,
Akakpo PK, et al: Prognostic significance of nuclear factor kappa B
(p65) among breast cancer patients in cape coast teaching hospital.
Med Princ Pract. 33:1–11. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Adankwah E, Danquah K, Gyamfi D, Sampene
P, Ossei P and Asiamah E: Nuclear localisation of autophagic p62
and associated cytoplasmic Beclin-1 and Bcl-2 expressions in
adenomas and adenocarcinomas of the colorectal regions. J Carcinog
Mutagen. 9(2)2018.
|
|
22
|
Yang X, Cheng H, Chen J, Wang R, Saleh A,
Si H, Lee S, Guven-Maiorov E, Keskin O, Gursoy A, et al: Head and
neck cancers promote an inflammatory transcriptome through
coactivation of classic and alternative NF-κB pathways. Cancer
Immunol Res. 7:1760–1774. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baba M, Takahashi M, Yamashiro K, Yokoo H,
Fukai M, Sato M, Hosoda M, Kamiyama T, Taketomi A and Yamashita H:
Strong cytoplasmic expression of NF-κB/p65 correlates with a good
prognosis in patients with triple-negative breast cancer. Surgery
Today. 46:843–851. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al-Mutairi MS and Habashy HO: Nuclear
factor-κB clinical significance in breast cancer: An
immunohistochemical study. Med Princ Pract. 32:33–39.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yan M, Xu Q, Zhang P, Zhou XJ, Zhang ZY
and Chen WT: Correlation of NF-kappaB signal pathway with tumor
metastasis of human head and neck squamous cell carcinoma. BMC
Cancer. 10(437)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pasquali D, Giacomelli L, Pedicillo MC,
Conzo G, Gentile G, De Stefano IS, Angelillis F, Santoro A, Miele
F, Digitale Selvaggio L, et al: Tumor inflammatory microenvironment
of the thyroid cancer: Relationship between regulatory T-Cell
imbalance, and p-NFΚB (p65) Expression-A preliminary study. J Clin
Med. 12(6817)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roved J, Westerdahl H and Hasselquist D:
Sex differences in immune responses: Hormonal effects, antagonistic
selection, and evolutionary consequences. Horm Behav. 88:95–105.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou XL, Fan W, Yang G and Yu MX: The
clinical significance of PR, ER, NF-κB, and TNF-α in breast cancer.
Dis Markers. 2014(494581)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pyo JS and Kim EK: Clinicopathological
significance and prognostic implication of nuclear factor-κB
activation in colorectal cancer. Pathol Res Pract.
215(152469)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Songkiatisak PS, Rahman MT, Aqdas M and
Sung MH: NF-κB, a culprit of both inflamm-ageing and declining
immunity? Immun Ageing. 19(20)2022.PubMed/NCBI View Article : Google Scholar
|