Introduction
Breast cancer is believed to be the result of
progressive molecular and morphological changes that develop in
ductal epithelial cells. These changes have been studied
extensively. Current detection methods can only identify in
situ or invasive diseases. We know that the vast majority, if
not all, of these cancers have a pre-cancerous phase. Newer
methodologies are evolving to shift this treatment paradigm to
predict which women are at the highest risk of developing breast
cancer and to prevent the development of cancerous lesions.
Widespread screening with mammography and physical
breast examination have been shown to reduce breast cancer
mortality. However, many examinations are false-positive, with
positive predictive values for non-palpable and palpable lesions
ranging from 15–38% (1–3). Prior to the advent of percutaneous
biopsy methods, many women underwent open surgical biopsy. With the
introduction of the percutaneous sampling of non-palpable lesions,
fine needle aspiration (FNA) biopsy and core-needle biopsy have
been used more widely in the evaluation of non-palpable breast
lesions (4–6).
Wrensch et al (7) analyzed nipple aspirate fluid
collected from more than 2,700 women. This study, which had an
average follow-up of 12.7 years, demonstrated that women with
cellular atypia had a relative risk of developing breast cancer
that was 4.9 times greater than that of women who did not have
atypical cells. The 95% confidence interval for this finding was
1.7–13.9.
Women with a first-degree family history of breast
cancer in addition to atypical cells had an 18-fold increased
relative risk; however, the extremely wide confidence interval of
4.6–70.2, determined with a small number of subjects, casts some
doubt on the strength of this association. The presence of atypia
confers an increased risk for the development of breast cancer.
Wrensch et al confirmed these findings in a follow-up study
published in 2001 (8). Fabian
et al (9), using ductal
epithelial cells collected with random periareolar fine-needle
aspiration from 480 high-risk women, demonstrated similar results.
This study, which had a median follow-up of 45 months, found that
high-risk women with atypical cells had a relative risk of
developing breast cancer that was 5 times greater than high-risk
women without atypical cells. The 95% confidence interval ranged
from 2.0 to 12.6.
Until a cure is found, the early detection of breast
cancer is imperative. Improved methods to detect and diagnose
breast cancer early, when it is most curable, are required if a
significant impact on breast cancer-related morbidity and mortality
is to be made. Over 95% of breast cancers begin in the cells lining
the breast ducts (10). It can
take up to 8–10 years before these cells grow into a tumor large
enough to be detected by mammography. Two innovative technologies
to monitor for atypical ductal epithelial cells are ductal lavage
(DL) (11) and nipple aspirate
fluid (NAF) (12), which, in
conjunction with the detection of novel biomarkers linked to breast
cancer, are being investigated for use in the early detection and
diagnosis of breast cancer. However, DL or NAF cytology alone is
not sufficiently sensitive to identify the subgroup of women who
are on a progressive pathway leading to breast cancer (13).
Gene amplification is a characteristic feature of
cancer cells that results in increased production of specific
proteins required for the acquisition and maintenance of the
malignant pheno-type. Amplification of certain oncogenes plays an
important role in the progression of many types of tumors (14–16).
For example, the MYCN oncogene is amplified in neuroblastomas, MYC
and MYCL are amplified in small-cell lung cancer, and HER2/neu is
amplified in breast and ovarian cancers. Detection of such
amplifications may, in some instances, assist in diagnosis and in
prognostic assessment. Gene amplification also contributes to the
development of resistance to cytotoxic drugs. V-Erb-B2
erythroblastic leukemia viral oncogene homolog 2 or the HER2/NEU
(Her-2/neu) oncogene, which codes for a 185-kDa transmembrane
growth factor receptor, is amplified and/or overexpressed in 15–35%
of breast carcinomas (17–20). The association of HER2/neu
amplification and overexpression with rapid proliferation, low
estrogen receptor content and high-grade ductal carcinomas suggests
that this oncogene plays an important role in the progression of
breast cancer. The preoperative detection of HER2/neu amplification
in non-palpable breast lesion patients using FNA may be of
particular relevance and could be useful clinically.
Our objective in this study was to compare the level
of HER2/neu gene amplification in patients with non-palpable breast
lesions with the presence or absence of atypical cells in their
breast cells obtained by employing FNA.
The amplification of the HER2/neu gene is more
frequent in carcinoma in situ than in invasive types
(21). Detection of HER2/neu by a
PCR-based method may aid in the diagnosis of non-palpable breast
lesions in women with unclear or dense mammography images. Since
this gene amplification is related to high proliferation, it may
provide useful preoperative information regarding certain types of
intraductal carcinoma, and may also predict the response to
chemotherapy.
Materials and methods
Breast samples
Twelve patients were enrolled in this pilot project:
9 patients with a non-palpable breast lesion and 3 healthy control
women without breast cancer. FNA was used to obtain a breast
specimen in the non-palpable breast lesion patients and healthy
control women. A written consent form was signed by each patient
prior to the sampling. A general schema of the procedures carried
out in this study is shown in Fig.
1.
Fine needle aspiration technique
The FNAs were performed by a gynecologist in a
preoperative room. In this technique, a thin 21-G needle with a
10-ml syringe was inserted into the breast, and the cells were
aspirated. In non-palpable breast lesion patients, a blind sample
from suspicious breast tissue was isolated. The extracted cells
were used to make two smears on slides which were stained with PAP
stain and examined under an optical microscope. FNA is a safe and
minor surgical procedure and less traumatic than an open surgical
biopsy. Significant complications are usually rare and depend on
the body site. The skin above the insertion area was swabbed with
an antiseptic solution and draped with sterile surgical towels. In
the normal healthy control women, the FNA procedure was performed
in both breasts (two each).
Blood serum sample
A peripheral blood sample (10 ml) was taken from
each patient after FNA. The serum was isolated and maintained at
−20°C until the HER2/neu gene expression ELISA assay was performed
for all of the patient samples.
DNA isolation from breast samples
Genomic DNA was isolated from the blood samples
using Nexttec™ Clean Columns (Nexttect Inc., Leverkusen, Germany).
As opposed to other protocols, no DNA was retained by the column
resin. Instead, proteins, detergents and low-molecular-weight
compounds were retained. The DNA was passed through the column
during a short, one-step purification procedure.
Amplification of the HER2/neu gene by
PCR-based method
Genomic DNA (100 ng) was amplified for the HER2/neu
gene by PCR methodology using Taq DNA polymerase (Promega) followed
by the thermocycling program: after denaturalization at 95°C for 2
min, 35 cycles of denaturalization at 95°C for 30 sec, annealing at
optimal primer temperature for 30 sec, an extension cycle at 72°C
for 30 sec and a final extension at 72°C for 10 min were performed.
The PCR reaction was performed with a specific primer sequence that
codifies for the HER2/neu gene. A parallel PCR reaction was also
performed to amplify β-actin as a control gene amplification.
HER2/neu ELISA assay
The HER2/neu ELISA kit is a sandwich-type enzyme
immunoassay that utilizes two monoclonal antibodies directed to the
extracellular domain (ECD) of HER2/neu. The assay quantifies either
the full-length molecule in tumor tissue (p185) or the ECD (p105)
in serum, plasma, cell cultures and fluids. The capture antibody
was immobilized on the interior surface of the microplate wells. To
perform the assay, an appropriate volume of specimen was incubated
in the coated wells to allow binding of the antigen by the capture
antibody. The immobilized antigen was reacted with the detector
antiserum. The amount of detector antibody bound to the antigen was
measured using a colored reaction product that was quantitated by
spectrophotometry reflecting the amount of protein in the
sample.
Results
Breast fine needle aspiration
cytology
Our main objective in this pilot study was to
determine whether breast FNA is feasible and accurate compared to
breast biopsy in patients with non-palpable breast lesions. Nine
non-palpable breast lesion patients (30–72 years of age) and 3
healthy control patients (28–60 years of age) were studied. FNA was
performed in 12 patients: 9 with non-palpable breast lesions and 3
healthy control women. The efficiency of FNA is documented in
Table I. The FNA results revealed
that 2/9 non-palpable breast lesion patients had acellular material
in their FNA samples, in 1/9 patients FNA was not performed, and in
6/9 patients cellular material was obtained (66.6%). Among the 6
patients for whom cellular material was obtained, the cytopathology
report indicated that 3/6 had benign breast cells (50%), 2 had
cellular atipia (33.3%) and 1 had malignant breast cells (16.7%) in
their FNAs. Of the 3 healthy control women, only 1 exhibited benign
breast epithelial cells in the FNA sample and the remaining
patients had acellular material. Fig.
2A shows an image of an FNA sample from a control female
subject, and B-F are images from the breast cytology. Fig. 2B is a representative image of an
FNA sample with acellular material, and C shows benign breast cells
from a healthy control woman. Fig.
2D shows malignant or tumoral breast cells from a non-palpable
breast lesion from patient no. 1, and E shows an FNA sample of
benign breast epithelial cells from a non-palpable breast lesion
patient. Fig. 2F is a
representative image of an FNA sample with cellular or mild
atipia.
 | Table I.Cytopathology report from breast fine
needle aspirate samples. |
Table I.
Cytopathology report from breast fine
needle aspirate samples.
| Patient no. | ID no. | Age (years) | Family history | Clinical
diagnosis | Breast FNA
sample | Cytopathology
report |
|---|
| 1 | 806200351 | 65 | No | Non-palpable breast
carcinoma | Good cellularity with
blood | Malignant cells |
| 2 | 806200279 | 37 | No | Non-palpable breast
carcinoma | Few epithelial
cells | Cellular atipia |
| 3 | 807010752 | 72 | Yes | Non-palpable breast
carcinoma | Few epithelial
cells | Cellular atipia |
| 4 | 807101541 | 49 | Yes | Non-palpable breast
carcinoma | Good cellularity | Benign breast
epithelial cells |
| 5 | 807280700 | 30 | Yes | Non-palpable breast
carcinoma | Good cellularity | Benign breast
epithelial cells |
| 6 | 807021404 | 64 | Yes | Non-palpable breast
carcinoma | n/p | n/p |
| 7 | 808062023 | 50 | Yes | Non-palpable breast
carcinoma | Acellular
material | Acellular
material |
| 8 | 8101510882 | 58 | No | Non-palpable breast
carcinoma | Acellular
material | Acellular
material |
| 9 | 8101510878 | 62 | No | Non-palpable breast
carcinoma | Good cellularity with
blood | Benign breast
epithelial cells |
| 10 | 8103102312 | 28 | No | Control patient | Acellular
material | Acellular
material |
| 11 | 810290262 | 39 | Yes | Control patient | Acellular
material | Acellular
material |
| 12 | 811042114 | 60 | Yes | Control patient | Few epithelial
cells | Benign breast
epithelial cells |
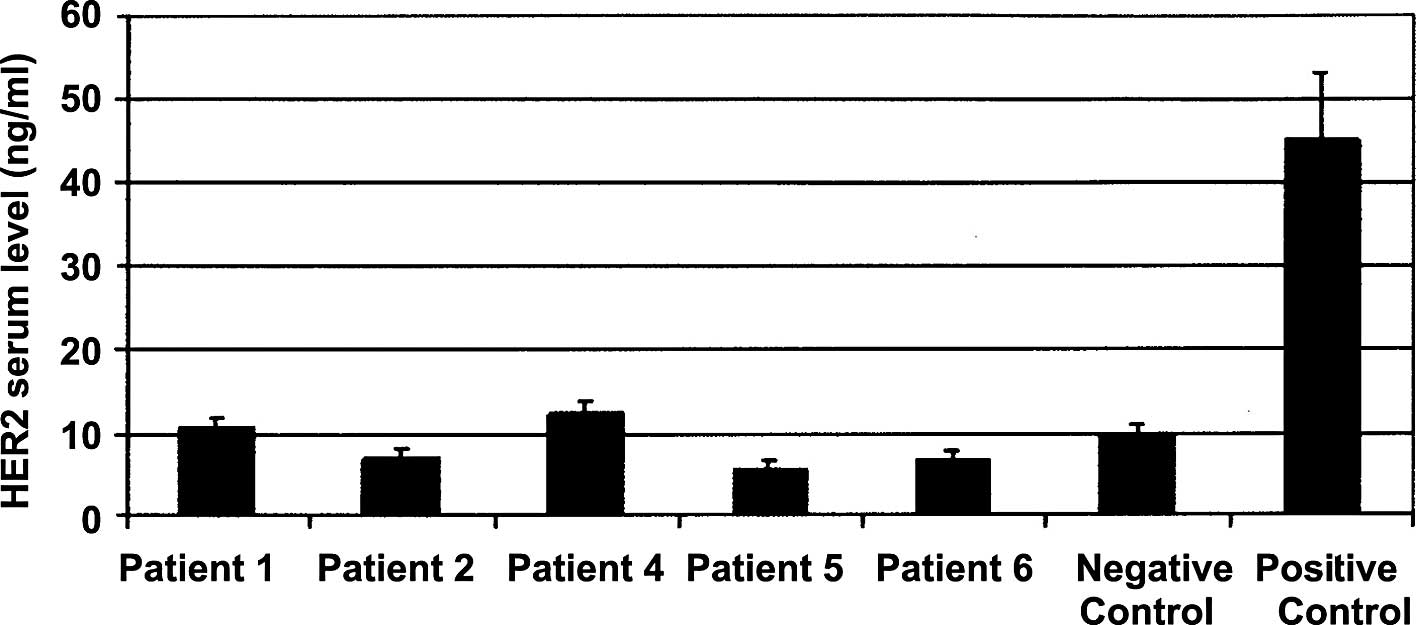
HER2/neu protein expression in serum
samples
The levels of HER2/neu protein expression in serum
from patient nos. 1, 2, 4, 5 and 6 with non-palpable breast lesions
are shown in Fig. 3. One healthy
control woman was included as a negative control, and one invasive
(HER2+) breast carcinoma patient as positive control.
The levels of HER2 protein expression in serum were low in all of
the patients with non-palpable breast lesions, which were
considered HER2−. The serum samples were analyzed from
some of the available patients included in this study.
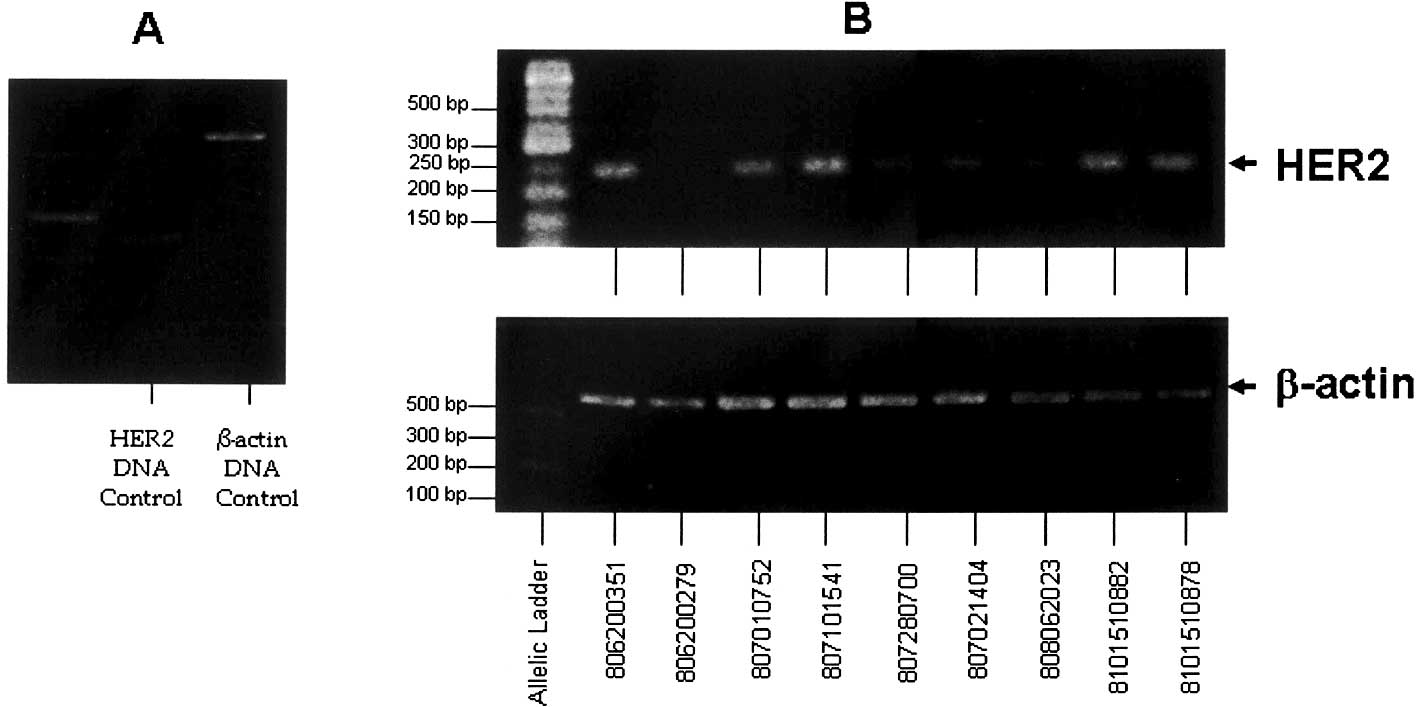
HER2/neu gene expression in blood
samples
HER2/neu gene amplification of the control DNA
sample from a healthy control woman as a control of gene expression
employing the housekeeping gene β-actin is shown in Fig. 4A. The molecular weights
corresponding to HER2/neu and β-actin amplification bands are 198
and 550 bp, respectively. The intensity relationship (R) values
between HER2/neu and β-actin amplification bands were employed to
semi-quantify the HER2/neu expression level in the remaining
patients. HER2/neu and β-actin gene amplification in patient nos.
1–9 is shown in Fig. 4B. The
levels of HER2/neu expression with respect to β-actin in all of the
patients as well as in the DNA control, are represented in Fig. 5A. The value of R (HER2/neu/β-actin)
was considered to be 1.0 in the DNA control. Fig. 5B shows the R values of all of the
patients. As shown in Fig. 5B,
patient nos. 1, 4, 8 and 9 had a >1.5-fold increase in HER2/neu
gene expression with respect to the control. However, patient nos.
2, 5 and 7 had a >2.0-fold lower HER2/neu gene expression with
respect to the control. Patient nos. 3 and 6 demonstrated HER2/neu
gene expression at the same level when compared to the control
DNA.
Discussion
Mammography is generally accepted as a useful
diagnostic clinical tool for characterizing known breast lesions so
that appropriate and timely treatment can be administered. However,
it remains grossly underutilized at what it does best: screening.
Breast FNA is a minimally invasive procedure that allows the
collection of representative breast epithelial cells for diagnosis
and is reliable in the assessment of prognostic and predictive
markers in breast cancer. The sensitivity of mammography in cancer
detection needs to be high, but it is also important in order to
achieve a high diagnostic specificity to avoid the morbidity
associated with unnecessary surgical biopsy. It is in this area
that we envision FNA as a useful tool – to determine whether or not
to perform surgery on a lesion that is non-palpable and without a
clear mammography image. In this study, we demonstrated that FNA
has a very good correlation with breast biopsy. The presence of
atypical hyperplasia in the breast FNA sample plus a suspicious
mammographical image is a powerful tool for defining favorable
treatment options for that patient.
Immunohistochemistry (IHC) provides a useful method
for defining the type of cancer a patient has and the prognosis.
Based on this information, a course of treatment can be determined
according to the factors that the cancerous cells may be receptive
to. A patient bearing cancer with an overexpression of HER2/neu has
a poor prognosis, but is a candidate for treatment with Herceptin.
Thus, IHC yields quick results, is cost effective and is easy to
perform.
Further investigation is warranted to confirm
whether FNA is a suitable method for predicting and analyzing the
development of breast cancer. It is crucial that more samples from
different patients are collected in order to determine whether
there is a correlation between the level of HER2/ neu expression
and breast cancer development. Follow-up is also recommended for a
determined number of years (at least five) to determine whether the
patient develops breast cancer.
These findings provide strong support to a previous
study (8), which demonstrated that
hyperplasia and atypical hyperplasia diagnosed in nipple aspirates
of breast fluid are associated with an increased risk of breast
cancer.
Acknowledgements
We are grateful to Dr Roberto Gentili
from IACA Laboratorios, Bahía Blanca, Argentina for his financial
support which made this research possible.
References
|
1.
|
Spivey GH, Perry BW, Clark VA, et al:
Predicting the risk of cancer at the time of breast biopsy.
Variation in the benign to malignant ratio. Am Surg. 48:326–332.
1982.PubMed/NCBI
|
|
2.
|
Molloy M, Azarow K, Garcia VF, et al:
Enhanced detection of preinvasive breast cancer: combined role of
mammography and needle localization biopsy. J Surg Oncol.
40:152–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Basset LW, Liu TH, Giuliano AE, et al: The
prevalence of carcinoma in palpable vs. impalpable,
mammographically detected lesions. AJR Am J Rowntgenol. 157:21–24.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sickles E: Screening for breast cancer
with mammography. Radiology. 179:463–468. 1991.
|
|
5.
|
Lidbrink E, Elfving J, Fussell J, et al:
Neglected aspects of false positive findings of mammography in
breast cancer screening: analysis of false positive cases from the
Stockholm trial. BMJ. 312:273–276. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Burrell HC, Pinder SE, Wilson AR, et al:
The positive predictive value of mammographic signs: a review of
425 non-palpable breast lesions. Clin Radiol. 51:277–281. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wrensch MR, Petrakis NL, King EB, et al:
Breast cancer incidence in women with abnormal cytology in nipple
aspirates of breast fluid. Am J Epidemiol. 135:130–141.
1992.PubMed/NCBI
|
|
8.
|
Wrensch MR, Petrakis NL, Miike R, et al:
Breast cancer risk in women with abnormal cytology in nipple
aspirates of breast fluid. J Natl Cancer Inst. 93:1791–1798. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fabian CJ, Kimler BF, Zalles CM, et al:
Short-term breast cancer prediction by random periareolar
fine-needle aspiration cytology and the Gail risk model. J Natl
Cancer Inst. 92:1217–1227. 2000. View Article : Google Scholar
|
|
10.
|
Wright T and McGechan A: Breast cancer:
new technologies for risk assessment and diagnosis. Mol Diagn.
7:49–55. 2003.PubMed/NCBI
|
|
11.
|
Dooley WC, Ljung BM, Veronesi U, et al:
Ductal lavage for detection of cellular atypia in women at high
risk for breast cancer. J Natl Cancer Inst. 93:1624–1632. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Petrakis NL: Nipple aspirate fluid in
epidemiologic studies of breast disease. Epidemiol Rev. 15:188–195.
1993.PubMed/NCBI
|
|
13.
|
Wrensch M, Petrakis NL, King EB, et al:
Breast cancer risk associated with abnormal cytology in nipple
aspirates of breast fluid and prior history of breast biopsy. Am J
Epidemiol. 137:829–833. 1993.PubMed/NCBI
|
|
14.
|
Alitalo K and Schwab M: Oncogene
amplification in tumor cells. Adv Cancer Res. 47:235–281. 1986.
View Article : Google Scholar
|
|
15.
|
Schwab M and Amler LC: Amplification of
cellular oncogenes: a predictor of clinical outcome in human
cancer. Genes Chromosomes Cancer. 1:181–193. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Einarsdóttir K, Rosenberg LU, Humphreys K,
et al: Comprehensive analysis of the ATM, CHEK2 and ERBB2 genes in
relation to breast tumour characteristics and survival: a
population-based case-control and follow-up study. Breast Cancer
Res. 8:R672006.PubMed/NCBI
|
|
17.
|
Choi YH, Ahn JH, Kim SB, et al: Tissue
microarray-based study of patients with lymph node-negative breast
cancer shows that HER2/neu overexpression is an important
predictive marker of poor prognosis. Ann Oncol. 20:1337–1343. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Egervari K, Toth J, Nemes Z, et al: An
alternative and reliable real-time quantitative PCR method to
determine HER2/neu amplification in breast cancer. Appl
Immunohistochem Mol Morphol. 17:247–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
D’Alessandro C, Dellapasqua S, Orlando L,
et al: Role of endocrine responsiveness and HER2/neu overexpression
in inflammatory breast cancer treated with multimodality
preoperative therapy. Breast J. 14:435–441. 2008.PubMed/NCBI
|
|
20.
|
Dunn L and Demichele A: Genomic predictors
of outcome and treatment response in breast cancer. Mol Diagn Ther.
13:73–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Motomura K, Koyama H, Noguchi S, et al:
Detection of c-erbB-2 gene amplification in nipple discharge by
means of polymerase chain reaction. Breast Cancer Res. 33:89–92.
1995. View Article : Google Scholar : PubMed/NCBI
|