Introduction
Malignant melanoma is one of the most common types
of cancer in the US as well as worldwide. It is an aggressive
disease with high metastatic potential and resistance to many
cytotoxic agents (1). Melanoma
cells have low levels of spontaneous apoptosis, and
chemotherapeutic drugs function by inducing apoptosis (1). Many dietary agents, including kinase
inhibitors, have been inversely correlated to the spread of
malignant melanoma (2–4). The alkylating agent dacarbazine is
currently being used in clinical trials in combination with novel
therapeutic agents (4). Quercetin
(3,5,7,3′,4′-tetrahydroxyflavone) is a flavonoid isolated from
onion, whereas sulforaphane
[1-isothiocyanato-4-(methylsulfinyl)-butane] is a member of an
isothiocyanate family of chemopreventive agents isolated from
broccoli. The anti-cancer properties of these compounds have been
demonstrated in a number of malignancies, including prostate,
breast, skin and liver cancers (5–9).
Previous reports suggest that quercetin is an anti-oxidant and
anti-inflammatory compound (10,11).
Others have indicated that sulforaphane acts as a potential HDAC
inhibitor and an inducer of various pro-apoptotic molecules
(12–14). Sulforaphane was also found to
inhibit prostate tumor growth and lung metastasis in a melanoma
model (15,16).
Matrix metalloproteinases (MMPs) are extracellular
matrix proteins known to play a crucial role in normal
physiological processes, such as embryogenesis, wound healing,
morphogenesis, reproduction, tissue resorption and remodeling, and
in pathological processes, such as inflammation, arthritis, cancer,
cardiovascular and pulmonary diseases; hence, they are considered
therapeutic targets (17,18). Certain MMPs act as tumor
suppressors, whereas others act as tumor promoters. MMP-9 is
expressed mostly by stromal cells in a tumor environment, although
cancer cells do express MMP-9 at low levels (19,20).
MMP-9 efficiently degrades native type IV and V collagens,
fibronectin, ectactin and elastin. The regulation of MMP-9
activation is more complex than that of other MMPs, as most cells
in general do not express the constitutively active form of MMP-9.
Rather, its activity is induced by different stimuli depending on
cell type, thereby contributing to specific pathological events
(21,22). Previous reports have demonstrated
that transgenic mice lacking MMP-9 exhibit reduced keratinocyte
hyperproliferation and a decreased incidence of invasive tumors
(23). The imbalance between MMP
activity and the inhibitory action of tissue inhibitors of
metalloproteinase is implied in the regulation of multiple types of
diseases. MMP-9 plays a crucial role in the modulation of cytokines
and proteases, and in the degradation of the serine protease
inhibitor α1-anti-trypsin, which leads to lung destruction. The
early stage of a clinical trial has shown promising results using
an MMP-9 inhibitor in multiple sclerosis. These observations
suggest the hypothesis that MMP-9 is a potential drug target for
both chronic obstructive pulmonary diseases and multiple sclerosis.
Thus, the further development of highly potent and specific MMP-9
inhibitor is warranted (24,25).
In the present study, we report for the first time that quercetin
and sulforaphane, when used in combination as opposed to alone, are
more effective in suppressing cell migration and tumor growth
through the down-regulation of MMP-9 expression, in in vitro
as well as in vivo melanoma models.
Materials and methods
Statement of ethics
Animals were maintained in the experimental animal
facilities of the National Center for Cell Science (NCCS), India,
in accordance with the Institutional Animal Care and Use Committee
(IACUC) guidelines (approval no. NCCS/IACUC/2007/B-107).
Cell culture and reagents
Mouse melanoma (B16F10) cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and maintained
in Dulbecco's modified Eagle's medium supplemented with 10% FBS
(Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin and 2
mM glutamine in a humidified atmosphere with 5% CO2 at
37°C. Quercetin (purity >98%) was purchased from HIMEDIA
(India). D,L-sulforaphane (purity >99%) was a generous gift from
Professor S.V. Singh of the University of Pittsburgh School of
Medicine, PA, USA. Trypan blue dye was from Cambrex (Walkersville,
MD, USA). Gelatin was from ICN (Aurora, OH, USA). Goat polyclonal
anti-mouse MMP-9, anti-actin antibodies, horseradish
peroxidase-conjugated IgG and Ultra-Cruz mounting media containing
4′,6-diamidino-2-phenylindole (DAPI) were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The Cy2-conjugated anti-goat
IgG was obtained from Calbiochem (La Jolla, CA, USA).
Cell viability assay
The effect of quercetin and sulforaphane on cell
proliferation either alone or in combination was studied using the
trypan blue dye exclusion assay as described previously (26). Briefly, B16F10 cells
(1×104) were seeded in 24-well plates and treated with
quercetin (50 μM) or sulforaphane (20 μM) or
combinations of two doses of quercetin and sulforaphane (25 and 10
μM or 50 and 20 μM, respectively) and then incubated
for 16 h. The number of viable cells were counted, analyzed and
represented as the percentage of cell viability vs. treatment. Each
of the experiments was performed in triplicate, and data were
analyzed by one-way ANOVA and represented in the form of a bar
graph.
Wound migration assay
The wound migration assay was performed as described
previously (27). Briefly, B16F10
cells were seeded and grown to confluency. Wounds with a constant
diameter were made using sterile tips, and the cells were treated
with quercetin and sulforaphane either individually or in
combination under similar conditions as described above. The wound
images were captured at 0 and 16 h using a phase contrast
microscope (Nikon). The wound assay data were analyzed, quantified
and represented in the form of a bar graph using Image Pro-plus 6.0
software (Nikon) and one-way ANOVA.
In vivo tumorigenicity and
immunofluorescence experiments
The tumorigenicity and immunohistochemistry
experiments were performed as described previously (28,29).
B16F10 cells (1.5×106) were injected subcutaneously into
the right flanks of male C57 BL6 mice (4–6 weeks of age). Animals
were randomly separated into 5 groups (6 mice/group). Quercetin (15
mg/kg body weight) or sulforaphane (3.5 mg/kg body weight) was
injected thrice a week into the peripheral sites of tumors for 3
weeks. In seperate experiments, a combination of quercetin (7.5
mg/kg body weight) and sulforaphane (1.75 mg/kg body weight) was
injected into the peripheral sites of tumors. In another
experiment, a combination of quercetin (15 mg/kg body weight) and
sulforaphane (3.5 mg/kg body weight) was injected. Animals were
maintained in the experimental animal facilities of the NCCS,
India, in accordance with IACUC guidelines. At the termination of
the experiments, all animals were sacrificed, tumors were
photographed, excised and weighed, and the volumes [0.5 (length ×
breadth2)] were calculated. Paraffin-embedded tumor
sections were used for immunofluorescence analysis. Briefly,
sections were deparafinized, rehydrated, used for heat-induced
antigen retrieval and quenched. The sections were blocked with 2%
bovine serum albumin and incubated with an anti-MMP-9 antibody
(1:20). The sections were washed and incubated with Cy2-conjugated
anti-goat IgG and counterstained with mounting media containing
DAPI and analyzed using a confocal microscope (Zeiss).
Western blot analysis and zymography
experiment
Western blotting was performed as previously
described (30). Tumor samples
were lysed in RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM
NaCl, 1% Nonidet P-40, 1% Triton X-100, 1% sodium deoxycholate,
0.2% SDS, 5 mM iodoacetamide containing 1 mM DTT and 2 mM PMSF),
and the protein concentration in the lysates was measured using the
Bio-Rad protein assay. The samples containing an equal amount of
total proteins (40 μg) were resolved by 7.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrotransferred onto nitrocellulose membranes. The membranes
were incubated with goat anti-MMP-9 antibody (1:2,000), further
incubated with horseradish peroxidase-conjugated anti-goat IgG
(1:4,000) and detected by Western blotting using a luminol reagent
(Santa Cruz Biotechnology).
The gelatinolytic activity was measured as described
previously (31). To assess the
effect of quercetin and sulforaphane on MMP-9 expression, an equal
amount of total proteins from tumor lysates was mixed with sample
buffer in the absence of a reducing agent, incubated for 30 min and
separated by 7.5% zymography-SDS-PAGE containing gelatin (0.5
mg/ml). The gel was washed with 0.25% Triton X-100 and incubated in
incubation buffer [50 mM Tris-HCl (pH 7.5) containing 100 mM
CaCl2, 1 μM ZnCl2, 1% Triton X-100,
0.02% (w/v) NaN3]. Negative staining indicated the zones
of gelatinolytic activity.
Results
Effect of quercetin or sulforaphane alone
or in combination on B16F10 cell proliferation
Previous reports have revealed that quercetin or
sulforaphane inhibit cancer cell proliferation in an independent
manner (9,32). We therefore examined whether a
combination of quercetin and sulforaphane confers a significant
effect on the proliferation of B16F10 cells. Accordingly, B16F10
cells were treated with quercetin (50 μM) or sulforaphane
(20 μM) either alone or in a combination of two different
doses as described in the Materials and methods, and cell
proliferation assays were performed using the trypan blue dye
exclusion test. The results indicated that quercetin and
sulforaphane in combination had a stronger inhibitory effect on the
growth of B16F10 cells than either compound alone (Fig. 1A). The data were analyzed by
one-way ANOVA and represented in the form of a bar graph
(p<0.05).
Effect of quercetin or sulforaphane alone
or in combination on B16F10 melanoma cell motility
To ascertain whether quercetin and sulforaphane
exhibit a combinatorial effect on B16F10 cell motility, a wound
migration assay was performed. Cells were grown in monolayer to
attain cobblestone morphology and were treated with quercetin (50
μM) or sulforaphane (20 μM), or with a combination of
two different doses for 16 h as described above. The data indicated
that a combination of quercetin and sulforaphane inhibited melanoma
cell migration more significantly compared to treatment with each
agent individually (Fig. 1B). The
data were analyzed using Image Pro plus 6.0 software (Nikon) and
one-way ANOVA. Fig. 1C shows a
graphical representation of the percentage of wound migration with
respect to the control (p<0.05).
Quercetin and sulforaphane in combination
suppress melanoma growth in C57 BL6 mice
Our in vitro results prompted us to
investigate the combined effect of quercetin and sulforaphane on
melanoma growth in a mouse model. Accordingly, B16F10 cells were
injected into the right flanks of C57 BL6 mice. After 1 week,
quercetin or sulforaphane, either alone or in combination, were
injected at the peripheral site of the tumors thrice a week for 3
weeks. The data revealed that a combination of quercetin and
sulforaphane is more effective in suppressing melanoma growth than
each agent used alone (Fig. 2A).
Mice were sacrificed by cervical dislocation. Tumors were excised
and weighed, and the volume was calculated as described in
Materials and methods. The tumor volumes and weights were analyzed
by one-way ANOVA (p<0.05) and represented in the form of bar
graphs (Fig. 2B and C).
Quercetin and sulforaphane reduce tumor
growth through the down-regulation of MMP-9 expression
To elucidate the molecular mechanism of tumor growth
suppression in response to quercetin and sulforaphane,
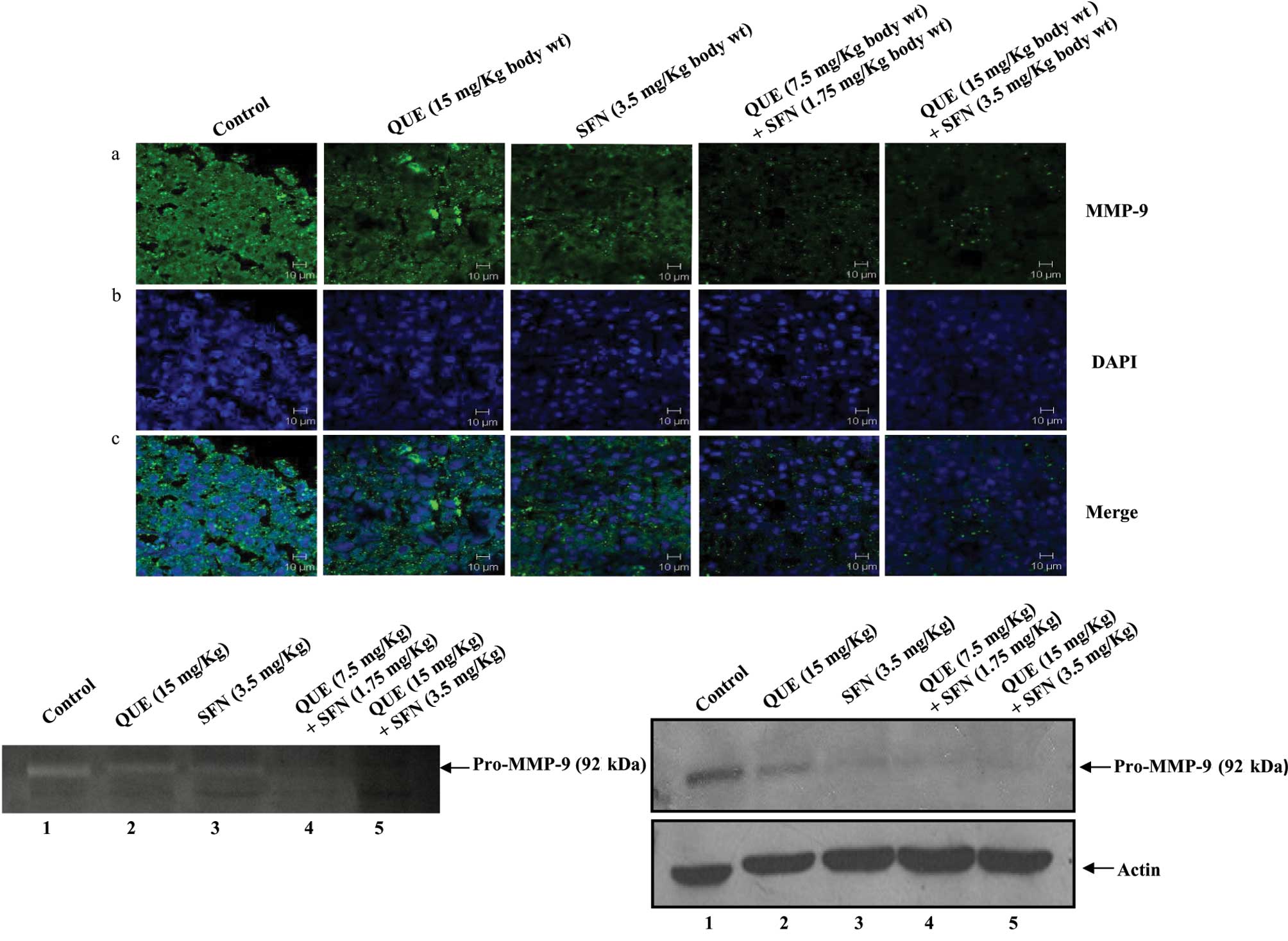
formalin-fixed tumor sections were analyzed by immunofluorescence
using an anti-MMP-9 antibody. The data revealed that the expression
of MMP-9 in tumor sections from the mice treated with a combination
of quercetin and sulforaphane was significantly decreased as
compared to tumors from the control mice or mice treated with
quercetin or sulforaphane alone (Fig.
3A, panel a). Panels b and c show nuclear staining by DAPI and
merged images, respectively.
The expression of MMP-9 in tumor lysates was further
confirmed by Western blot analysis and gelatin zymography. Tumor
tissues were lysed, and an equal amount of total proteins in the
lysates were analyzed by gelatin zymography. The zymography data
showed that the expression of pro-MMP-9 in tumors from the mice
treated with quercetin and sulforaphane in combination was
dramatically reduced as compared to tumors from the control mice or
mice treated with either compound alone (Fig. 3B). This was further confirmed by
Western blot analysis using an anti-MMP-9 antibody (Fig. 3C). Taken together, these data
strongly suggest that quercetin and sulforaphane in combination
inhibit pro-MMP-9 expression, which in turn suppresses melanoma
progression in a mouse model.
Discussion
Historically, plant-derived compounds have been used
as traditional medicines in Asian countries as well as worldwide
(33). Many of these plant
products have shown great potential to act as anti-carcinogenic,
anti-inflammatory and anti-diabetic agents (33). However, higher concentrations of
these compounds are required to show significant inhibitory
effects. Therefore, combination studies using two or more such
pharmacologically active compounds may increase the efficacy of the
drug and overcome the hurdle of drug resistance.
The present study demonstrated the
anti-carcinogenic/anti-tumor activity of a plant-derived flavonoid,
quercetin, and an isothiocyanate, sulforaphane, on melanoma
progression when used in combination. Previous reports have
revealed that quercetin and sulforaphane act as anti-cancer agents
in various cancer models when used individually (5–9). Lin
et al showed that quercetin inhibits tumor cell motility and
invasion through PKCδ/ERK/AP-1-dependent MMP-9 activation in breast
cancinoma cells (34). Our in
vitro data suggest that a combination of quercetin and
sulforaphane inhibits melanoma cell proliferation more effectively
than each used independently. The combination treatment also
significantly inhibits melanoma cell motility. Our results revealed
that the use of quercetin and sulforaphane at the ratio 2.5:1 is
quite effective against the proliferation and migration of B16F10
cells.
Our in vitro results prompted us to examine
the effects of quercetin and sulforaphane in an in vivo
melanoma model. The B16F10 cells were injected subcutaneously into
the right flanks of C57 BL6 mice. Tumors were generated, and
quercetin and sulforaphane, either independently or in combination,
were injected into the peripheral site of the tumors. Notably,
quercetin and sulforaphane in combination drastically suppressed
melanoma growth as compared to the agents used independently in a
mouse isograft model. However, the molecular mechanism by which
quercetin and sulforaphane inhibit melanoma growth has not yet been
fully understood. Zhang et al reported that quercetin
inhibits the invasion of murine melanoma B16BL6 cells by decreasing
pro-MMP-9 via the PKC pathway (35). Therefore, we examined the status of
pro-MMP-9 expression in the tumors treated with quercetin and
sulforaphane in combination. As shown using immunofluorescence,
zymography and Western blotting, the level of expression of MMP-9
was reduced significantly in tumors treated with lower and higher
doses of the two compounds as compared to the control or their
individual use. These data demonstrated that combination therapy is
more effective than the use of a single compound for the treatment
of melanoma growth.
In summary, for the first time we report that
quercetin and sulforaphane in combination are much more effective
in regulating melanoma progression through the down-regulation of
MMP-9 expression than each compound used alone. Thus, inhibiting
MMP-9 expression by quercetin and sulforaphane in combination may
be a novel therapeutic strategy for the prevention of melanoma
progression.
Abbreviations:
|
QUE
|
quercetin
|
|
SFN
|
sulforaphane
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
DAPI
|
4′6-diamidino-2 phenylindole
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
Acknowledgements
We thank Professor S.V. Singh of the
University of Pittsburgh School of Medicine, PA, USA, for providing
the sulforaphane. The study was partially supported by the Council
of Scientific and Industrial Research, Government of India, India
(to R. Mishra and P. Sharma).
References
|
1.
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Verhoeven DT, Goldbohm RA, van Poppel G,
Verhagen H and van den Brandt PA: Epidemiological studies on
brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers
Prev. 5:733–748. 1996.PubMed/NCBI
|
|
3.
|
Zhang SM, Hunter DJ, Rosner BA,
Giovannucci EL, Colditz GA, Speizer FE and Willett WC: Intakes of
fruits, vegetables, and related nutrients and the risk of
non-Hodgkin's lymphoma among women. Cancer Epidemiol Biomarkers
Prev. 9:477–485. 2000.
|
|
4.
|
Bedikian AY, Millward M, Pehamberger H,
Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey
P, Kirkwood JM and Haluska FG: Bcl-2 antisense (oblimersen sodium)
plus dacarbazine in patients with advanced melanoma: the Oblimersen
Melanoma Study Group. J Clin Oncol. 24:4738–4745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Musonda CA and Chipman JK: Quercetin
inhibits hydrogen peroxide (H2O2)-induced
NF-kappaB DNA binding activity and DNA damage in HepG2 cells.
Carcinogenesis. 19:1583–1589. 1998.PubMed/NCBI
|
|
6.
|
Avila MA, Velasco JA, Cansado J and
Notario V: Quercetin mediates the down-regulation of mutant p53 in
the human breast cancer cell line MDA-MB468. Cancer Res.
54:2424–2428. 1994.PubMed/NCBI
|
|
7.
|
Vijayababu MR, Arunkumar A, Kanagaraj P,
Venkataraman P, Krishnamoorthy G and Arunakaran J: Quercetin
downregulates matrix metalloproteinases 2 and 9 proteins expression
in prostate cancer cells (PC-3). Mol Cell Biochem. 287:109–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Choi S and Singh SV: Bax and Bak are
required for apoptosis induction by sulforaphane, a cruciferous
vegetable-derived cancer chemopreventive agent. Cancer Res.
65:2035–2043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhang Y, Tang L and Gonzalez V: Selected
isothiocyanates rapidly induce growth inhibition of cancer cells.
Mol Cancer Ther. 2:1045–1052. 2003.PubMed/NCBI
|
|
10.
|
Zhang W and Zhang F: Effects of quercetin
on proliferation, apoptosis, adhesion and migration, and invasion
of HeLa cells. Eur J Gynaecol Oncol. 30:60–64. 2009.PubMed/NCBI
|
|
11.
|
Braganhol E, Zamin LL, Canedo AD, Horn F,
Tamajusuku AS, Wink MR, Salbego C and Battastini AM:
Antiproliferative effect of quercetin in the human U138MG glioma
cell line. Anticancer Drugs. 17:663–671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dashwood RH and Ho E: Dietary histone
deacetylase inhibitors: from cells to mice to man. Semin Cancer
Biol. 17:363–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Juge N, Mithen RF and Traka M: Molecular
basis for chemoprevention by sulforaphane: a comprehensive review.
Cell Mol Life Sci. 64:1105–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Singh AV, Xiao D, Lew KL, Dhir R and Singh
SV: Sulforaphane induces caspase-mediated apoptosis in cultured
PC-3 human prostate cancer cells and retards growth of PC-3
xenografts in vivo. Carcinogenesis. 25:83–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Myzak MC, Tong P, Dashwood WM, Dashwood RH
and Ho E: Sulforaphane retards the growth of human PC-3 xenografts
and inhibits HDAC activity in human subjects. Exp Biol Med.
232:227–234. 2007.PubMed/NCBI
|
|
16.
|
Thejass P and Kuttan G: Antimetastatic
activity of sulforaphane. Life Sci. 78:3043–3050. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Parks WC, Wilson CL and Lopez-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pyke C, Ralfkiaer E, Tryggvason K and Dano
K: Messenger RNA for two type IV collagenases is located in stromal
cells in human colon cancer. Am J Pathol. 142:359–365.
1993.PubMed/NCBI
|
|
20.
|
Zeng ZS and Guillem JG: Colocalisation of
matrix metalloproteinase-9-mRNA and protein in human colorectal
cancer stromal cells. Br J Cancer. 74:1161–1167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Houde M, de Bruyne G, Bracke M,
Ingelman-Sundberg M, Skoglund G, Masure S, van Damme J and
Opdenakker G: Differential regulation of gelatinase B and
tissue-type plasminogen activator expression in human Bowes
melanoma cells. Int J Cancer. 53:395–400. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor inducing kinase plays crucial role in osteopontin
induced MAPK/IKK dependent nuclear factor κB-mediated promatrix
metalloproteinase-9 activation. J Biol Chem. 279:38921–38935.
2004.PubMed/NCBI
|
|
23.
|
Coussens LM, Tinkle CL, Hanahan D and Werb
Z: MMP-9 supplied by bone marrow-derived cells contributes to skin
carcinogenesis. Cell. 103:481–490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Muroski ME, Roycik MD, Newcomer RG, van
den Steen PE, Opdenakker G, Monroe HR, Sahab ZJ and Sang QX: Matrix
metalloproteinase-9/gelatinase B is a putative therapeutic target
of chronic obstructive pulmonary disease and multiple sclerosis.
Curr Pharm Biotechnol. 9:34–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ram M, Sherer Y and Shoenfeld Y: Matrix
metalloproteinase-9 and autoimmune diseases. J Clin Immunol.
26:299–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Xiao D, Choi S, Johnson DE, Vogel VG,
Johnson CS, Trump DL, Lee YJ and Singh SV: Diallyl
trisulfide-induced apoptosis in human prostate cancer cells
involves c-Jun N-terminal kinase and extracellular-signal regulated
kinase-mediated phosphorylation of Bcl-2. Oncogene. 23:5594–5606.
2004. View Article : Google Scholar
|
|
27.
|
Behera R, Kumar V, Lohite K, Karnik S and
Kundu GC: Activation of JAK2/STAT3 signaling by osteopontin
promotes tumor growth in human breast cancer cells. Carcinogenesis.
31:192–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Philip S, Bulbule A and Kundu GC:
Osteopontin stimulates tumor growth and activation of promatrix
metalloproteinase-2 through nuclear factor-kappa B-mediated
induction of membrane type 1 matrix metalloproteinase in murine
melanoma cells. J Biol Chem. 276:44926–44935. 2001. View Article : Google Scholar
|
|
29.
|
Chakraborty G, Jain S and Kundu GC:
Osteopontin promotes vascular endothelial growth factor-dependent
breast tumor growth and angiogenesis via autocrine and paracrine
mechanisms. Cancer Res. 68:152–161. 2008. View Article : Google Scholar
|
|
30.
|
Jain S, Chakraborty G and Kundu GC: The
crucial role of cyclooxygenase-2 in osteopontin-induced protein
kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate
tumor progression and angiogenesis. Cancer Res. 66:6638–6648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Philip S and Kundu GC: Osteopontin induces
nuclear factor kappa B-mediated promatrix metalloproteinase-2
activation through I kappa B alpha /IKK signaling pathways, and
curcumin (diferulolylmethane) down-regulates these pathways. J Biol
Chem. 278:14487–14497. 2003. View Article : Google Scholar
|
|
32.
|
Jeong JH, An JY, Kwon YT, Rhee JG and Lee
YJ: Effects of low dose quercetin: cancer cell-specific inhibition
of cell cycle progression. J Cell Biochem. 106:73–82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008.PubMed/NCBI
|
|
35.
|
Zhang X-M, Huang S-P and Xu Q: Quercetin
inhibits the invasion of murine melanoma B16BL6 cells by decreasing
pro-MMP-9 via the PKC pathway. Cancer Chemother Pharmacol.
53:82–88. 2004. View Article : Google Scholar : PubMed/NCBI
|