Introduction
Dragon-pearl tea is a high-quality green tea,
originating from Kai County (Chongqing, China); however, the
effects of Dragon-pearl tea on health have not been well
characterized scientifically (1).
Green tea contains a variety of bioactive
components, including theophylline, polyphenols, tannins, amino
acids, vitamin C and minerals (2).
Purine compounds in green tea, such as theophylline, caffeine and
theobromine, are able to stimulate the central nervous system,
enhance cognitive function and promote the secretion of gastric
acid (3). An excess of gastric acid
is a key cause of gastric ulcers; therefore, overconsumption of
green tea may be harmful for patients with gastric ulcers by
increasing gastric acid. However, black tea may help to prevent
gastric ulcers (4). Through
bioactive components that exert an antioxidative effect, green tea
may improve the efficacy of immune function and inhibit the growth
of Helicobacter pylori, which is an additional common cause
of gastric ulcers (5). The effective
components in green tea have not been fully characterized, and a
curative effect of green tea on gastric ulcers has not been
demonstrated in all cases; however, a previous study indicated that
components of green tea may inhibit the development of gastric
ulcers (6). A number of studies have
demonstrated an association between the antioxidative effects of
plant products and their preventative effect on gastric ulcers,
indicating that antioxidant mechanisms may be crucially involved in
the inhibitory effect of green tea components on gastric ulcers
(7,8). Furthermore, previous studies have
indicated that the polyphenols in green tea exert an improved
antioxidant effect compared with black tea (9). The present study employed a range of
experimental methods to investigate the preventative effect of
Dragon-pearl tea on gastric ulcers. Dragon-pearl tea crude
polyphenols (DTCP) were administered to ICR mice, and the levels of
a number of serum and stomach tissue indices of oxidation were
determined in order to verify the preventative effect of DTCP on
gastric ulcers in mice.
Materials and methods
DTCP extracts
A 500-g sample of Dragon-pearl tea dust was added to
hot water (90°C, 10 litres) for 60 min. Sodium chloride (600 g) was
subsequently added into the extract solution to achieve a mass
fraction of 6%, dialyzing in distilled water for 1.5 h. Following
filtration, 15 g NaHSO3 and 100 ml aluminum sulfate
saturated solution were added to the extract solution. The extract
solution was heated to 80°C and 15% NaHCO3 solution was
added until the pH value of the solution reached a range of
5.0–6.0. A large quantity of precipitates was filtered and washed
three times with 70°C water of the same volume (10 litres). The
precipitates were dissolved in pH 4.0 hydrochloric acid solution
(1.5 L) for 50 min, and a small quantity of gelatinous precipitate
was removed by centrifugation at 25°C for 20 min, 4,000 × g. Next,
9 g NaHSO3 was added to the acid solution, which was
further extracted through administration of ethyl acetate (0.5
times of the solution's volume) three times, with each extraction
occurring for 10 min and the three extractions were mixed. Vitamin
C of 2% of the tea's weight was added to water of 0.4 times the
extraction's volume, and the pH value was adjusted to 3.0 through
the addition of citric acid. The solution was washed twice with the
ethyl acetate extraction, and the DTCP extracts were finally
obtained using rotary evaporation (RE-52A; Shanghai Yarong
Biochemical Instrument Factory, Shanghai, China).
2,2-diphenyl-1-picrylhydrazyl (DPPH)
free radical assay
Samples of 4-ml DTCP solution (25, 50 or 100 µg/ml)
were added to 1.0 ml DPPH methanol solution (1.5×10−4
M). Following storage at room temperature for 30 min, the
absorbance of the solution was determined at 520 nm using a
spectrophotometer (UV-5100; Shanghai Metash Instruments Co. Ltd.,
Shanghi, China), and the remaining DPPH was quantified
[(ODDPPH-ODsample)/ODDPPH]×100%,
where OD is optical density. The results are expressed as the mean
of triplicate experimental values (10).
OH radical assay
A 1.4-ml reaction system was constructed, containing
0.2 ml deoxyribose (6 mM), 0.2 ml sodium phosphate buffer solution
(20 mM, pH 7.4), 0.2 ml anhydrous iron chloride (FeCl3;
400 µM), 0.2 ml FeSO4-ethylenediaminetetraacetic acid
(400 µM), 0.2 ml H2O2 (3 mM), 0.2 ml ascorbic
acid (400 µM) and 0.2 ml DTCP solution (25, 50 or 100 µg/ml).
Following incubation in a water bath for 60 min at 37°C, the
reaction was stopped by adding 1 ml trichloroacetic acid and 1 ml
2-thiobarbituric acid. The solution was subsequently boiled in a
water bath for 20–25 min at 90°C. The absorbance was measured at
532 nm by ultraviolet spectrophotometer (UV-5100). All analyses
were run in triplicate and average values were calculated (10).
Gastric ulcer induction
Institute for Cancer Research mice (male, n=50, 7
weeks old), purchased from Chongqing Medical University (Chongqing,
China), were allocated into five groups: Normal, control and 50,
100 and 200 mg/kg DTCP (n=10 per group). The mice in the normal and
control groups received 0.2 ml distilled water per day by gavage
for 4 weeks. However, the DTCP group mice were orally administered
50, 100 or 200 mg/kg DTCP solutions (0.2 ml) everyday for 4 weeks.
Subsequently, the control, 50 100 and 200 mg/kg DTCP group mice
were administered a single intraperitoneal injection of 10 mg/kg
body weight/day reserpine (Sigma-Aldrich, St. Louis, MO, USA) for 3
days. Following the final injection, all the mice were fasted for
24 h. The mice were sacrificed using CO2 and the stomach
was removed and inflated by injecting 10 ml formalin (1%) for 10
min to fix the tissue walls, and the stomach was opened along the
greater curvature (11). The gastric
ulcer inhibitory index of the hemorrhagic lesions developed in the
stomach was measured using a D550 digital camera (Canon, Tokyo,
Japan) with a square grid, and the images were analyzed using
ImageJ software (National Institutes of Health, Bethesda, MD, USA).
The gastric ulcer inhibitory index was calculated as follows:
Inhibitory index (%) = (gastric ulcer area of the control mice -
gastric ulcer area of the treated mice)/gastric ulcer area of the
control mice. In addition, the gastric secretion volume of each
mouse was measured using a 10-ml measuring cylinder, and the pH of
the gastric juice was measured using a SevenEasy pH meter
(Mettler-Toledo Ltd., Schwerzenbach, Switzerland) following
dilution 10 times with distilled water. Experiments followed a
protocol approved by the Animal Ethics Committee of Chongqing
Medical University (Chongqing, China).
Determination of the serum levels of
superoxide dismutase (SOD), glutathione peroxidase (GSH-Px),
malondialdehyde (MDA), lipid peroxidation (LPO), catalase (CAT),
prostaglandin E2 (PGE2) and nitric oxide (NO)
Serum levels of SOD, GSH-Px, MDA, LPO, CAT, PGE2 and
NO were determined using assay kits for SOD (#A001; WST-1 method),
GSH-Px (#A005; colorimetric method), MDA (#A003; thiobarbituric
acid method), LPO (#A106), CAT (#A007; visible light method), PGE2
(#H099) and NO (#A012; nitrate reductase method), respectively
(Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA from the gastric tissues was isolated
using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer's instructions. The RNA was
digested with RNase-free DNase (Roche Diagnostics, Basel,
Switzerland) for 15 min at 37°C, and purified using an RNeasy kit
(Qiagen GmbH, Hilden, Germany), according to the manufacturer's
instructions. The total RNA (2 µg) was incubated for l h at 37°C
with avian myeloblastosis virus reverse transcriptase (GE
Healthcare Life Sciences, Little Chalfont, UK) and random
hexanucleotides to form cDNA, in accordance with the manufacturer's
instructions. Primers (Thermo Fisher Scientific, Waltham, MA, USA)
for epidermal growth factor (EGF; forward: 5′-GCC AAG CTC AGA AGG
CTA C-3′; reverse: 5′-CAG GCC AGC CTC GTC TCA T-3′), epidermal
growth factor receptor (EGFR; forward: 5′-TCG GTG CTG TGC GAT
TTA-3′; reverse: 5′-TTT CTG GCA GTT GCT CCT C-3′), gastrin (Gas;
forward: 5′-CCT ACT GCC ACA ACA GTT AA-3′; reverse: 5′-CAT CCA TCC
GTA TGC TTC-3′), vascular endothelial growth factor (VEGF; forward:
5′-CCT GGC TTT ACT GCT GTA CCT-3′; reverse: 5′-GTG CCA AAA GGG TCA
TCA TCT C-3′) and vascular endothelial growth factor receptor 1
(Flt-1 or VEGFR-1; forward: 5′-CAA GTG GCC AGA GGC ATG GAG TT-3′;
reverse: 5′-GAT GTA GTC TTT ACC ATC CTG TTG-3′) were used to
specifically amplify the genes. GAPDH was used as a control
(forward: 5′-CGG AGT CAA CGG ATT TGG TC-3′; reverse: 5′-AGC CTT CTC
CAT GGT CGT GA-3′). Amplification was performed in a thermal cycler
(denaturation at 94°C for 1 min, annealing at 56°C for 1 min and
elongation at 72°C for 1 min for 30 cycles; Eppendorf AG, Hamburg,
Germany). The PCR products were separated on a 1.0% agarose gel and
visualized using ethidium bromide staining (12).
Statistical analysis
Analysis of variance (ANOVA) was performed and the
results are presented as the mean ± standard deviation. Differences
between the mean values of the individual groups were assessed
using one-way ANOVA and Duncan's multiple range test. P<0.05 was
considered to indicate a statistically significant difference. SAS
software, version 9.1 (SAS Institute, Inc., Cary, NC, USA) was used
to perform all the statistical analyses.
Results
DPPH and OH radical scavenging
activity
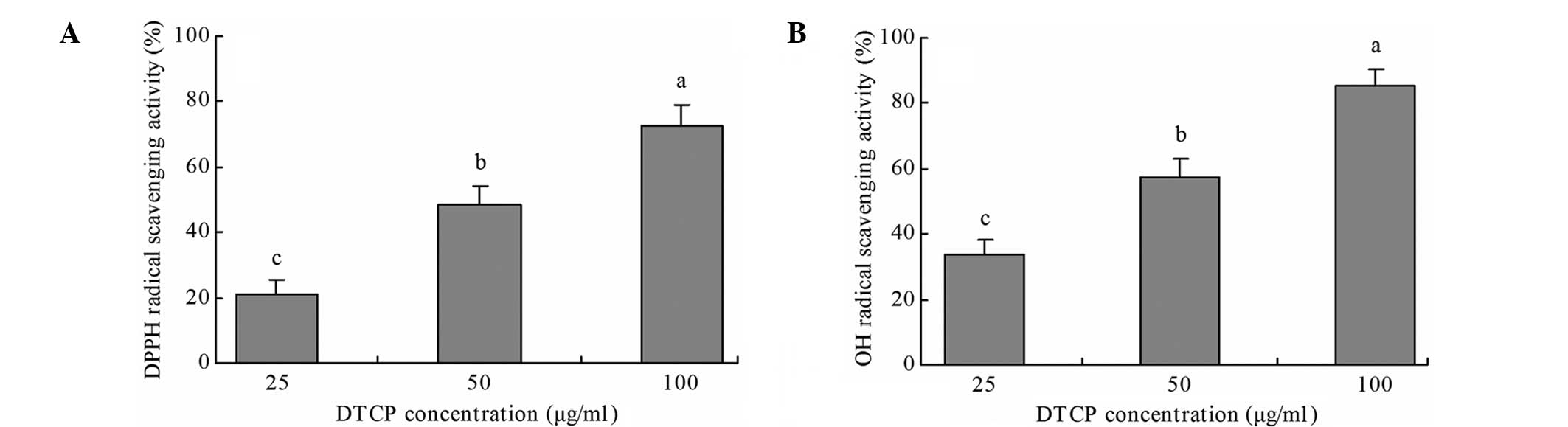
DTCP exhibited notable DPPH and OH
radical-scavenging activity in vitro (Fig. 1). A DTCP concentration of 100 µg/ml
exhibited the highest DPPH and OH radical scavenging activity, at
72.5 and 85.2%, respectively. Furthermore, at doses of 25 and 50
µg/ml, DTCP treatment exhibited 21.3 and 48.7% DPPH radical
scavenging activity and 33.6 and 57.1% OH radical scavenging
activity, respectively.
Gastric ulcer levels
Gastric ulcer levels in the mice from the
experimental groups were shown to decrease following DTCP treatment
(Fig. 2). The gastric ulcer area was
the largest in the untreated control mice at 11.47±3.13
mm2. However, the 50, 100 and 200 mg/kg DTCP-treated
mice presented with reduced gastric ulcer areas of 8.68±2.66,
6.71±1.89 and 3.14±1.53 mm2, while the gastric ulcer
levels were 24.32, 41.50 and 72.63%, respectively.
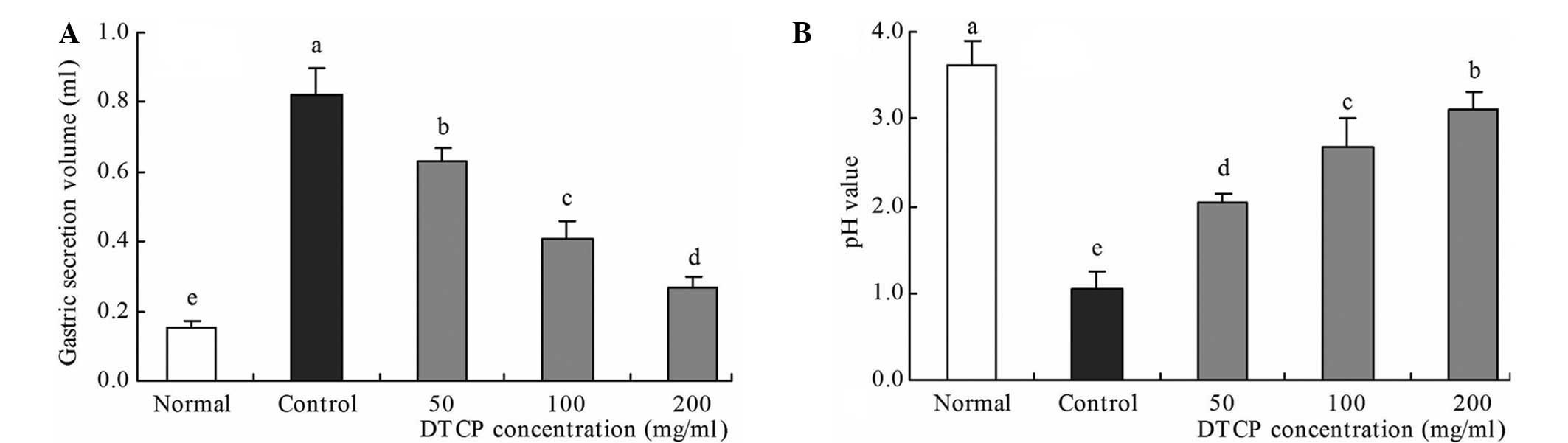
Gastric secretion volume and gastric
juice pH
In the reserpine-induced gastric ulcer control mice,
the highest gastric secretion volume (0.82 ml) and the lowest pH
value (1.04) were observed (Fig. 3).
Conversely, the mice in the normal group exhibited the lowest
gastric secretion volume (0.15 ml) and the highest pH value (3.62).
DTCP treatment was shown to improve these levels, with the 50, 100
and 200 mg/kg DTCP-treated mice exhibiting gastric secretion
volumes of 0.63, 0.41 and 0.27 ml, respectively, and pH values of
2.03, 2.68 and 3.10, respectively.
Serum levels of oxidation-associated
SOD, GSH-Px, MDA, LPO and CAT
Control group mice presented with the lowest levels
of SOD, GSH-Px and CAT, and the highest levels of MDA and LPO
(Table I). Mice in the three DTCP
treatment groups exhibited increasing levels of SOD, GSH-Px and
CAT, and decreasing levels of MDA and LPO levels. The highest
concentration of DTCP (200 mg/kg) produced the maximal change in
these levels in the reserpine-induced gastric ulcer mouse model,
resulting in levels that were comparable with the normal group
mice.
 | Table I.Serum SOD, GSH-Px, MDA, LPO and CAT
levels in the five groups. |
Table I.
Serum SOD, GSH-Px, MDA, LPO and CAT
levels in the five groups.
| Group | SOD (U/ml) | GSH-Px (units) | MDA (nmol/ml) | LPO (µmol/l) | CAT (U/ml) |
|---|
| Normal |
189.62±23.12 |
273.12±31.26 |
7.62±1.37 |
1.28±0.35 |
17.25±3.37 |
| Control |
112.43±16.36 |
138.28±22.18 |
19.66±2.02 |
9.36±1.28 |
5.38±1.64 |
| DTCP (mg/kg) |
|
|
|
|
|
| 50 |
133.10±12.36 |
177.28±19.31 |
16.36±0.78 |
7.08±1.13 |
7.72±1.20 |
| 100 |
141.28±10.06 |
209.73±21.08 |
12.50±1.22 |
5.27±0.49 |
11.76±1.88 |
| 200 |
169.33±11.97 |
238.76±18.49 |
9.64±0.96 |
3.08±0.29 |
14.32±2.03 |
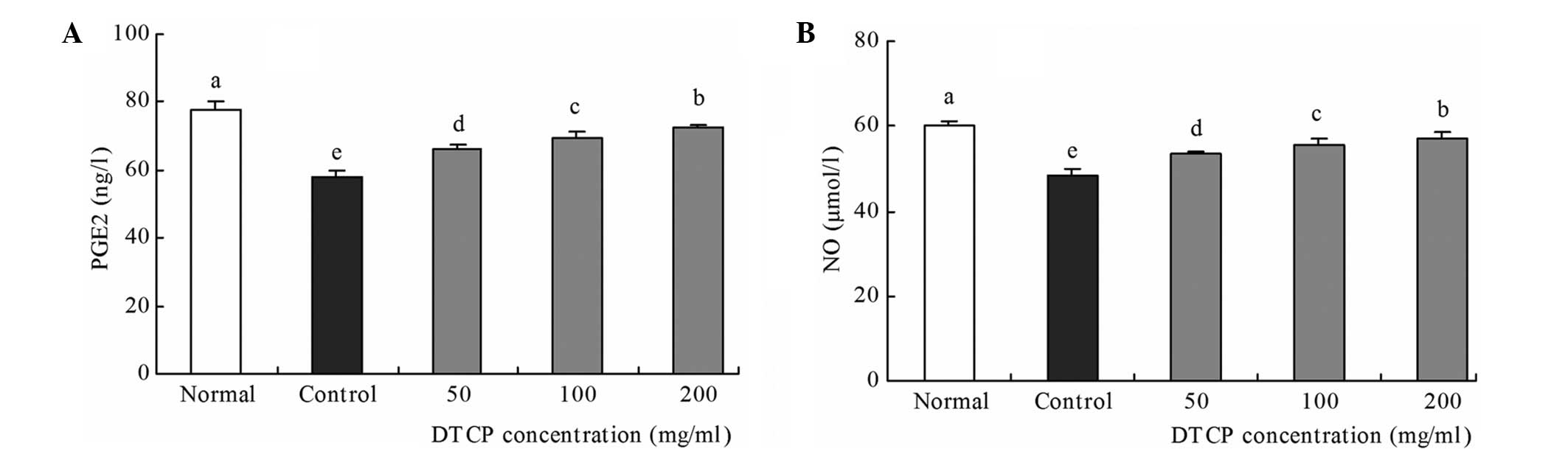
Serum levels of PGE2 and NO,
associated with gastric mucosal protection
Control group mice exhibited the lowest levels of
PGE2 and NO, at 57.80 ng/l and 48.29 µmol/l, respectively (Fig. 4). However, treatment with DTCP was
demonstrated to increase these levels when compared with the
control mice. The 50, 100 and 200 mg/kg DTCP-treated mice exhibited
increasing PGE2 levels of 66.23, 69.38 and 72.65 ng/l,
respectively, and increasing NO levels of 53.47, 55.71 and 57.08
µmol/l, respectively. Among the groups, the PGE2 and NO levels were
highest in the normal group mice, at 77.86 ng/l and 60.26 µmol/l,
respectively.
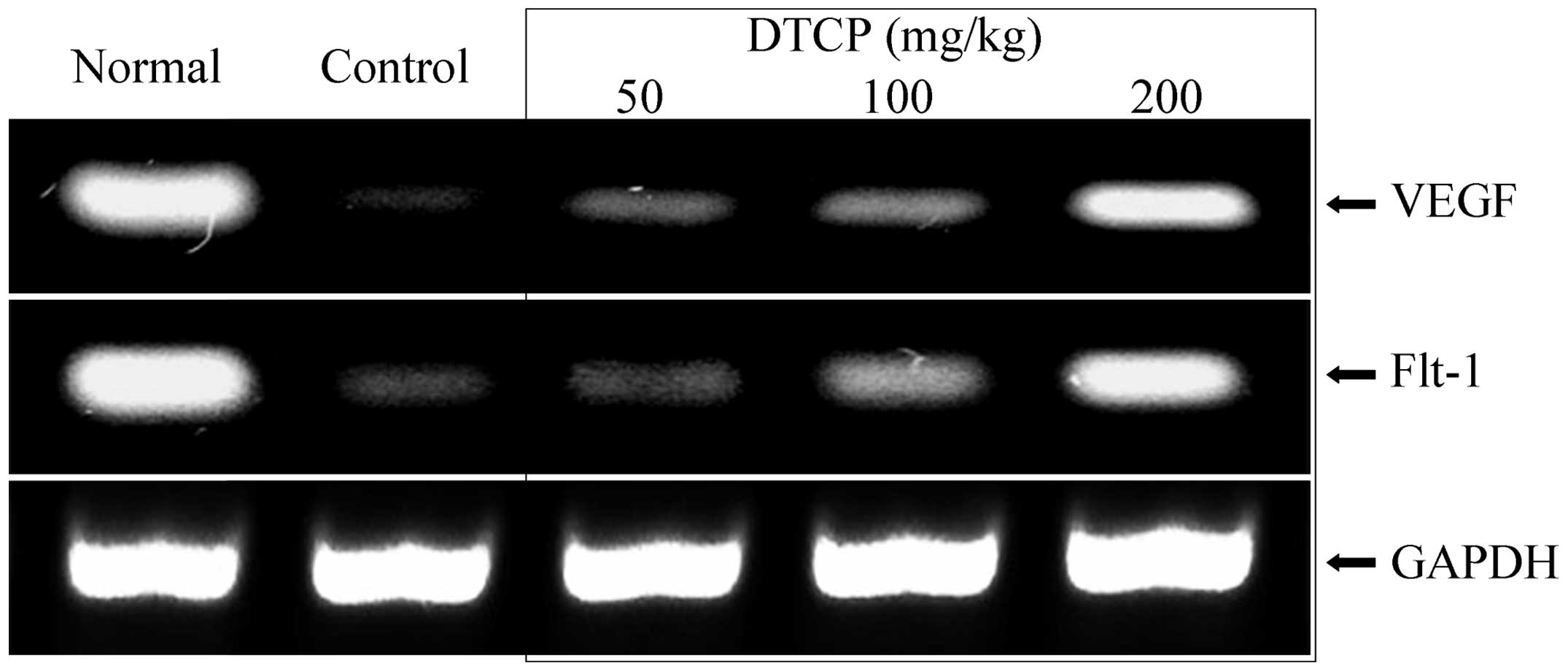
mRNA expression levels of EGF, EGFR,
Gas, VEGF and Flt-1 in the gastric tissue
Expression levels of a number of genes in the
gastric tissue of the mice were determined using RT-qPCR. DTCP was
demonstrated to alter the mRNA expression levels of EGF, EGFR, Gas,
VEGF and Flt-1. DTCP at concentrations of 50, 100 and 200 mg/kg was
shown to increase the mRNA expression levels of the epidermal
growth-related EGF and EGFR (Fig. 5)
Furthermore, DTCP treatment increased the mRNA expression levels of
vascular endothelial growth-related VEGF and Flt-1 (Fig. 6). However, the mRNA expression levels
of Gas were reduced in the mice following DTCP treatment at 50, 100
and 200 mg/kg (Fig. 7). The highest
concentration of DTCP (200 mg/kg) exerted the most marked effect,
resulting in mRNA expression levels of the various genes that were
comparable to those of the normal group mice.
Discussion
DPPH and OH radical assays are important methods for
the determination of antioxidant effects in vitro. In the
present study, the scavenging activity of DTCP on DPPH and OH
radicals was detected, in order to proximately determine the
antioxidative capacity of DTCP in vitro (13).
Gastric ulcers are considered to form as a result of
an imbalance between agressive and defensive factors. Attack
factors include gastric acid, pepsin, H. pylori, drug
toxicity and oxygen free radicals. Among these factors, gastric
acid and pepsin, which are able to damage the gastric mucosa
directly, are the primary attack factors (14). Furthermore, gastric acid may activate
the secretion of pepsin, and erode and damage the gastric mucosa.
With an increase in the gastric mucosa ulcer index and the
secretion of gastric acid and pepsin, the activity of the
H+/K+-ATPase enzyme in the gastric mucosa
parietal cells increases, which subsequently activates the stomach
cells. In turn, these cells enhance the H+ transporting
ability of H+/K+-ATPase; thus, the increased
secretion of gastric acid results in excessive gastric acid
accumulation and high acidity (15).
DTCP may increase the pH value, reduce gastric injury levels and
the gastric juice volume. Therefore, DTCP may be an effective agent
for the prevention of gastric ulcers.
Oxygen free radicals are molecules with uncoupled
electrons, which are intermediate products in normal biochemical
processes. Under normal conditions, free radical metabolites and
their removal system maintain a dynamic balance in the process of
metabolism (16). SOD and GSH-Px are
crucial enzymes for the removal of excess oxygen free radicals in
the body, and a reduction in their activity may result in the
accumulation of oxygen free radical metabolites (17). MDA is the catabolite from the
oxidation of biological membrane polyunsaturated fatty acids by
oxygen free radicals. Thus, enhanced levels of MDA indicate
increased oxygen free radical metabolites in the body (18). The accumulation of oxygen free
radicals in the body results in evident damage to cells, while
peptic ulcers have also been associated with the damage induced by
oxygen free radicals (19). The
activity of SOD and GSH-Px is known to significantly decrease in
high altitude areas, while the content of MDA significantly
increases (20). As a result, the
morbidity of peptic ulcers in high altitude areas is significantly
higher compared with that in plain areas (21). In the blood of patients with gastric
ulcers, MDA levels are increased significantly; however, the
activity levels of SOD and GSH-Px are evidently reduced when
compared with that of healthy individuals (22). GSH is considered to be the primary
physiological scavenger of free radicals in the gastric mucosa. As
free radicals are a key cause underlying the peroxidation of the
gastric mucosa to produce LPO, the levels of GSH and LPO in the
gastric mucosa are associated and exhibit an evident negative
correlation (23). CAT is an enzyme
scavenger that is able to remove hydrogen peroxide from the body to
prevent cells from hydrogen peroxide-induced damage (24). Therefore, the results of the present
study indicate that DCPT exerts strong antioxidative effects in
vitro and induces effective oxidation resistance in
vivo.
PGE2, a crucial factor for cell growth and
regulation, is able to stimulate the secretion of
HCO3− and increase the content of the gastric
mucilage and the flow of mucosal blood, to subsequently enhance the
ability of the gastric mucosa to resist damage (25). NO is a key protective factor for the
gastric mucosa, and can regulate the secretion of gastric acid,
promote the synthesis between the gastric mucus and mucoprotein,
maintain and strengthen the mucous barrier function and clear
oxygen free radicals (26).
Polyphenols in Dragon-pearl tea may increase PGE2 and NO levels in
the serum, improve microcirculation in the gastric mucosa, clear
oxygen free radicals and strengthen the mucous barrier
function.
EGF is an endogenous substance that inhibits the
secretion of gastric acid, promotes epithelial proliferation and
improves the nutrition into the body to prevent gastric mucosa
injury. Furthermore, EGF not only protects the gastric mucosa from
damage factors and maintains the intactness of the stomach mucosa,
but also stimulates the migration and proliferation of cells to
increase the rate of the healing process of gastric ulcers
(27). EGFR and EGF exhibit high
specificity and affinity. Upon reaching target cells, EGF rapidly
binds with EGFR on the cell membrane to regulate cell growth and
differentiation (28). A previous
study demonstrated that the expression levels of EGF and EGFR are
synchronized, which activates the EGF/EGFR system to promote
epithelial cell proliferation and tissue-repair on the gastric
mucosa, in addition to inhibiting the secretion of gastric acid
(29). Excessive gastric acid may be
the primary cause of gastric ulcers, and Gas is the primary factor
that stimulates the secretion of gastric acid. Exogenous and
endogenous Gas can increase the secretion of gastric acid.
Therefore, reducing the secretion of Gas may directly decrease the
secretion of gastric acid caused by illness, subsequently
alleviating gastric ulcers (30).
VEGF is one of the most well-studied and potent angiogenic factors
with a high specificity, which is able to promote the regeneration
of connective tissues and the microvasculature to significantly
reduce damage (31). A previous
study indicated that VEGF serves a function in the protection of
the gastric mucosa and the healing of chronic ulcers (32). VEGF dilutes harmful materials
(chemical toxicants and poisonous animalcules) in the stomach to
protect the gastric mucosa by increasing the permeability of the
microvasculature, and expedites the healing of ulcers by
stimulating the generation of glands and angiogenesis. Insufficient
levels of Flt-1, a receptor of VEGF, may inhibit the generation of
blood vessels in tissues (33).
Thus, ulcer healing may be enhanced by increasing the expression of
VEGF and Flt-1, to subsequently promote the regeneration of
connective tissues, glands and the microvasculature, and dilute the
harmful materials in the stomach to protect the gastric mucosa
(34). The results of the present
study indicate that DTCP may prevent gastric ulcers by increasing
the expression levels of EGF, EGFR, VEGF and Flt-1, and reducing
the expression of Gas.
In conclusion, the in vitro experiments
investigating the antioxidative effects of polyphenols in
Dragon-pearl tea indicated that Dragon-pearl tea exerts a notable
antioxidative effect in vitro. In addition, animal
experiments were conducted to investigate the preventative effect
of DTCP on gastric ulcers in vivo. Study into the effect of
DTCP on the oxidation state of the damaged gastric tissues and
serum in the mice indicated an association between oxidative damage
and gastric ulcer formation. Therefore, the polyphenols present in
Dragon-pearl tea may be able to mitigate oxidative damage and aid
the repair of oxidative-damaged tissues, subsequently preventing
and inhibiting the formation of gastric ulcers.
Acknowledgements
The study was supported by the Program for
Innovation Team Building at the Institutions of Higher Education in
Chongqing (no. KJTD201325).
References
|
1
|
Su GF, Liang SJ, Dai WZ and Huang JC: The
utilization and analyses of nutritive ingredients in Dragon-pearl
tea. Guang Xi Shi Fan Da Xue Xue Bao. 11:63–66. 1993.(In
Chinese).
|
|
2
|
Sharangi AB: Medicinal and therapeutic
potentialities of tea (Camellia sinensis L.) - A review. Food Res
Int. 42:529–535. 2009. View Article : Google Scholar
|
|
3
|
Benowitz NL: Clinical pharmacology of
caffeine. Annu Rev Med. 41:277–288. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maity S, Vedasiromoni JR and Ganguly DK:
Anti-ulcer effect of the hot water extract of black tea (Camellia
sinensis). J Ethnopharmacol. 46:167–174. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Correa P, Fontham ET, Bravo JC, Bravo LE,
Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD and Mera
R: Chemoprevention of gastric dysplasia: Randomized trial of
antioxidant supplements and anti-Helicobacter pylori therapy. J
Natl Cancer Inst. 92:1881–1888. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You
NC, Setiawan VW, Zhou XF, Ding BG, Wang RH, et al: Green tea
drinking and multigenetic index on the risk of stomach cancer in a
Chinese population. Int J Cancer. 116:972–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou F, Shen T, Duan T, Xu YY, Khor SC, Li
J, Ge J, Zheng YF, Hsu S, DE Stefano J, et al: Antioxidant effects
of lipophilic tea polyphenols on
diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis in
rats. In Vivo. 28:495–503. 2014.PubMed/NCBI
|
|
8
|
Yu X, Xu X and Su J: Protective effects of
nanometer selenium on acute gastric mucosal lesion in rats. J Hyg
Res. 37:594–586. 2008.(In Chinese).
|
|
9
|
Fu XC, Shan HL, Bai HB and Hu R:
Protective effect of Jiangbaiweiyan tablet on ethanol-induced
gastric mucosa injury in rats. J Zhejiang Univ (Med Sci).
40:391–394. 2011.(In Chinese).
|
|
10
|
Wang Q, Zhao X, Qian Y and Wang R: In
vitro antioxidative activity of yellow tea and its in vivo
preventive effect on gastric injury. Exp Ther Med. 6:423–426.
2013.PubMed/NCBI
|
|
11
|
Li GJ, Sun P, Wang R, Zhou YL, Qian Y and
Zhao X: Preventive effect of polysaccharide of Larimichthys crocea
swim bladder on reserpine induced gastric ulcer in ICR mice. Korean
J Physiol Pharmacol. 18:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen S, Zhu K, Wang R and Zhao X:
Preventive effect of polysaccharides from the large yellow croaker
swim bladder on HCl/ethanol induced gastric injury in mice. Exp
Ther Med. 8:316–322. 2014.PubMed/NCBI
|
|
13
|
Zhao X, Wang Q, Li GJ, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese Bowl tea. J Funct Foods.
7:590–598. 2014. View Article : Google Scholar
|
|
14
|
Choi SR, Lee SA, Kim YJ, Ok CY, Lee HJ and
Hahm KB: Role of heat shock proteins in gastric inflammation and
ulcer healing = J Physiol Pharmacol. 60:(Suppl 7). 5–17.
2009.PubMed/NCBI
|
|
15
|
Lorentzon P, Bayati A, Lee H and Andersson
K: Selective inhibition of the gastric H+,K(+)-ATPase by omeprazole
and related compounds. Ann N Y Acad Sci. 834:592–599. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J and Wang Y: Effect of different
methods of hypoxic exercise training on free radical oxidation and
antioxidant enzyme activity in the rat brain. Biomed Rep.
1:925–929. 2013.PubMed/NCBI
|
|
18
|
Yu GY, Song XF, Liu Y and Sun ZW: Inhaled
formaldehyde induces bone marrow toxicity via oxidative stress in
exposed mice. Asian Pac J Cancer Prev. 15:5253–5257. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haggag MS, Elsanhoty RM and Ramadan MF:
Impact of dietary oils and fats on lipid peroxidation in liver and
blood of albino rats. Asian Pac J Trop Biomed. 4:52–58. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang W, Li L, Tian X, Yan J, Yang X, Wang
X, Liao G and Qiu G: Astragalus and Paeoniae radix rubra extract
inhibits liver fibrosis by modulating the transforming growth
factor-β/Smad pathway in rats. Mol Med Rep. 11:805–814.
2015.PubMed/NCBI
|
|
21
|
A XR, Zhang XS and Liu LP: Change of free
radicals in levels of machinese in patients with peptic ulcer at
medium altitude. Zhongguo Xian Dai Yi Xue Za Zhi. 16:666–668.
2006.(In Chinese).
|
|
22
|
Nagy L, Mózsik G, Vincze A, Süto G,
Hunyady B, Rinfel J, Past T and Jávor T: Effects of a novel
Hungarian antacid containing Al and Mg (Tisacid) on mucosal
prostaglandin generation and oxygen free radicals in normal rats.
Drugs Exp Clin Res. 16:197–203. 1990.PubMed/NCBI
|
|
23
|
Mohan Kumar M, Joshi MC, Prabha T,
Dorababu M and Goel RK: Effect of plantain banana on gastric
ulceration in NIDDM rats: Role of gastric mucosal glycoproteins,
cell proliferation, antioxidants and free radicals. Indian J Exp
Biol. 44:292–299. 2006.PubMed/NCBI
|
|
24
|
Choi DJ, Kim SL, Choi JW and Park YI:
Neuroprotective effects of corn silk maysin via inhibition of
H2O2-induced apoptotic cell death in SK-N-MC
cells. Life Sci. 109:57–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Demitrack ES, Soleimani M and Montrose MH:
Damage to the gastric epithelium activates cellular bicarbonate
secretion via SLC26A9 Cl(–)/HCO(3)(–). Am J Physiol Gastrointest
Liver Physiol. 299:G255–G264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li WF, Hao DJ, Fan T, Huang HM, Yao H and
Niu XF: Protective effect of chelerythrine against ethanol-induced
gastric ulcer in mice. Chem Biol Interact. 208:18–27. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu KY, Zhu Y and Huang XZ: Effect of
Pongamia pinnata root flavonoids on the quality of ulcer healing
and expression of EGF and TGF-alpha in the rat model of gastric
ulcer induced by acetic acid. Zhongguo Ying Yong Sheng Li Xue Za
Zhi. 28:435–438. 2012.(In Chinese). PubMed/NCBI
|
|
28
|
Alkan Z, Duong FL and Hawkes WC:
Selenoprotein W controls epidermal growth factor receptor surface
expression, activation and degradation via receptor ubiquitination.
Biochim Biophys Acta. 1853:1087–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao MB, Dong L, Chang XM, Zou BC and Qin
B: Effect of Mexican tea herb and pilular adina herb on
concrescence of gastric mucosa in experimental gastric ulcer rats.
Chin J Integr Med. 13:132–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai R, Xu JY, Che MS, Chen HZ, Zhang L,
Zhou J and Xu L: Effects of somatostatin on the serum and gastric
gastrin in pancreatogenous ulcer. Lin Chuang Jun Yi Za Zhi. 24:4–7.
1996.(In Chinese).
|
|
31
|
Chung R, Foster BK and Xian CJ: The
potential role of VEGF-induced vascularisation in the bony repair
of injured growth plate cartilage. J Endocrinol. 221:63–75. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moraes TM, Rozza AL, Kushima H, Pellizzon
CH, Rocha LR and Hiruma-Lima CA: Healing actions of essential oils
from Citrus aurantium and d-limonene in the gastric mucosa: The
roles of VEGF, PCNA and COX-2 in cell proliferation. J Med Food.
16:1162–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khomeriki SG and Zhukov AG: Morphological
features of the gastric mucosa capillary network in patients with
portal hypertension. Arkh Patol. 73:43–47. 2011.(In Russian).
PubMed/NCBI
|
|
34
|
Akimoto M, Hashimoto H, Maeda A, Shigemoto
M and Yamashita K: Roles of angiogenic factors and endothelin-1 in
gastric ulcer healing. Clin Sci (Lond). 103:(Suppl 48). 450S–454S.
2002.PubMed/NCBI
|