Introduction
Infantile hemangioma (IH) is the most common benign
tumor of the skin in infants. The incidence rate of IH in premature
or low birth weight infants is ≤22%, with an increased prevalence
among female infants (~3–5:1, female to male). The life cycle of IH
consists of three stages: Proliferating, involuting and involuted.
The proliferating stage continues for a number of weeks prior to
the involuting or involuted stages. The proliferating phase is
characterized by the presence of small collections of primitive
cells. The stimulus that promotes this growth is unknown. There are
a number of characteristic endothelial markers of the early
proliferating phase, including CD31 and Von Willebrand factor.
Furthermore, other factors have been associated with IH growth in
the later proliferating phase, such as vascular endothelial growth
factor (VEGF) and basic fibroblast growth factor (bFGF). All of
these factors are plausible mechanisms underlying IH proliferation.
IH is divided into three clinical types: Superficial, deep and
mixed. Among these types, superficial IH predominantly occurs on
the skin surface of the head, face and neck, followed by the limbs
and trunk. Traditionally, the standard treatment for superficial or
small-area hemangiomas was to observe the afflicted area and wait
for spontaneous regression; however, certain superficial
hemangiomas are large and the tumors often exhibit rapid growth at
6 months (1,2). Failure to conduct early intervention
may lead to later treatment difficulty, prolong disease duration
and ultimately result in facial defects or dysfunction. There are
currently numerous methods for treating skin hemangioma; however,
these continue to exhibit problems. Among the most effective
treatment options for IH is the application of
90Sr-90Y radiation. Topical timolol maleate
represent novel therapeutic approaches for the treatment of IH,
encouraging selection since it was first used in 2010 (3,4). In the
present study, 90Sr-90Y radiation was
combined with topically applied timolol to treat patients with IH
between September 2012 and December 2013, in order to determine the
value and clinical safety of the combination therapy.
Patients and methods
Patients
In total, 72 patients with IH were recruited from
the 94th Hospital of PLA (Nanchang, China) between September 2012
and December 2013. The patients exhibited no serious heart, lung,
liver or kidney diseases. IH was diagnosed according to the
following criteria: i) Initial manifestation between a few days and
1 month after birth, with red punctate or patchy areas that were
visible to the naked eye and that exhibited varying degrees of
growth; ii) a subcutaneous hemangioma thickness of <3 mm, with
no obvious blood flow signal and no arteriovenous malformation on
color Doppler ultrasound; and iii) exclusion of other skin diseases
following dermatological examination.
The 72 infants were allocated at random into the
observation or control group. The observation group contained 15
males and 22 females, with ages ranging between 1 and 7 months
(mean age, 3.8 months). Among the observation subjects, 17 cases
were ≤3 months of age, while 20 cases were >3 months of age. The
distribution of IH lesions consisted of 14 cases in the head, 10
cases in the face and 13 cases in the limbs and trunk. The tumor
areas ranged between 0.8×0.7 and 18.6×8.0 cm, with 31 cases of
single IH and 6 cases of multiple IH lesions. The control group
consisted of 13 male and 22 female patients, with ages ranging
between 1 and 7 months (mean age, 3.7 months). Among the control
subjects, 15 cases were ≤3 months of age and the remaining 20 cases
were >3 months old. The distribution of the vascular tumors was
as follows: 16 cases in the head and face, 8 cases in the limbs and
11 cases in the trunk. The tumor area ranged between 0.8×1.0 and
15.0×6.3 cm, with 28 cases of single IH and 7 cases of multiple IH.
The demographic data of the two groups of infants can be summarized
as follows: Age ≤3 months, t=0.193 (P>0.05); age >3 months,
t=0.611 (P>0.05); gender, χ2=0.087 (P>0.05);
vascular tumor area, t=0.652 (P>0.05); lesion location,
χ2=0.467 (P>0.05); and lesion count,
χ2=0.174 (P>0.05). No statistically significant
differences were observed in the patient demographics between the
two groups. Treatments were approved by the hospital ethics
committee, and written informed consent was obtained from the
guardians of each patient.
Treatment methods
The observation group received 1–2 courses of
90Sr-90Y contact therapy and local external
application of 0.5% topical timolol solution (Wujing Medicine Co.,
Ltd., Wuhan, China) on the affected area for 3–6 months. Control
group patients received an identical dosage and treatment course of
90Sr-90Y contact therapy, combined with local
topical application of normal saline (NS) for 3–6 months.
The 90Sr-90Y applicator was
produced by Atomic Gaoke Co., Ltd. (Beijing, China) with an
applicator area of 2×2 cm and a surface-absorbed dose rate of 2.2
Gy/min. While in use, the active surface of the applicator was
positioned on the surface of the hemangioma. If the tumor diameter
was larger than the effective diameter of the
90Sr-90Y applicator, the tumor area was
divided into a number of squares of the same size as the
applicator, and the radiation therapy was then administered
gradually (i.e. uniform irradiation) in order to ensure the correct
dose of radiation exposure was received by the patient. Thick
rubber (4–5 mm) was used to shield the normal skin surrounding the
tumor. For the treatment of rapidly growing hemangiomas, the
treatment area was extended to 0.5–1.0 cm beyond the edge of the
tumor. The application of 90Sr-90Y radiation
was conducted following the protocol described in a previous
publication by the Chinese Medical Association (5); however, the dose of
90Sr-90Y was reduced to 10–12 Gy per
treatment course. In cases with a total hemangioma area of <20
cm2, a single course of treatment consisted of a
radiation dose of 2–2.4 Gy, once per day, for 5 consecutive days.
In cases with a total hemangioma area of >20 cm2, a
single course of treatment consisted of a radiation dose of 1–1.2
Gy, once per day, for 10 consecutive days. In the observation
group, 0.5% timolol eye drops were applied evenly to the hemangioma
surface at a dosage of 30 µl/cm2 each morning and night
(6). The hemangioma surface was
divided into a grid of 1×2-cm squares. Each region received a
single 60-µl drop of 0.5% timolol solution, which was gently
smeared over the area by finger for 5–10 sec until the solution was
absorbed. Certain patients exhibited local ‘mild flaking’ following
the administration of the timolol drops, which subsided after
treatment was temporarily discontinued for 3–5 days. The control
group received an identical dose of NS on the hemangioma surface.
The two groups of patients were observed every month, and
alterations in the color, size and texture of the hemangiomas were
recorded. In cases in which the tumor was observed to be healing or
to have significantly subsided, treatment was suspended, but
regular follow-up continued. In all other cases, a second course of
treatment was administered 3 months after the first, but the number
of treatment courses did not exceed 2. The total dose administered
prior to the termination of treatment was <24 Gy, while the
total dose in sensitive areas, such as the lips, eyelids, orbital
cavity and head, did not exceed 15 Gy.
Evaluation of efficacy and adverse
reactions
Treatment efficacy criteria
The criteria for treatment efficacy were as follows
(7): ‘Cure’, the hemangioma subsided
completely and the skin returned to normal or exhibited barely
visible discoloration; ‘excellent’, the color of the hemangioma
faded significantly or the tumor size was reduced by more than
two-thirds; ‘effective’, the hemangioma color faded or the tumor
size was reduced by more than one-third; and ‘invalid’, the
appearance of the hemangioma following treatment exhibited no
significant alteration or the tumor continued to grow. The
treatment response rates were calculated using the following
equations: Effective response rate (%) = (cure cases + excellent
cases + effective cases)/total number of cases × 100; excellent
response rate (%) = (cure cases + excellent cases)/total number of
cases × 100; cure response rate (%) = (cure cases)/total number of
cases × 100.
Adverse reactions
Potential adverse reactions associated with the
90Sr-90Y radiation treatment include
paresthesia (itching, burning, pain), pigmentation (mild
pigmentation, lasting 2–3 months) or depigmentation, skin atrophy,
dry radioactive dermatitis (erythema, rough, dry skin with fine
scales) and wet radioactive dermatitis (skin edema, blisters,
ulceration and infiltration, infection) (7).
In order to evaluate the adverse drug reactions,
blood, liver, kidney, fasting blood glucose and electrocardiogram
(ECG) data were collected from the two groups prior to and
following treatment each month. In the observation group, breathing
rate, heart rate, diet, stool quality and sleep behavior were
recorded within 6 h after the 0.5% timolol solution administration.
If adverse reactions were detected, the patients were administered
the corresponding symptomatic treatment.
Statistical analysis
Data were analyzed using SPSS software, version 18.0
(SPSS, Inc., Chicago, IL, USA). Count data rates were compared
using the χ2 test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Treatment efficacy
In the observation group, 16 cases were classed as
being cured, 1 case showed an excellent response and there were no
effective or invalid responses among the 17 subjects aged ≤3 months
(Table I); among the 20 subjects
aged >3 months, 17 cases were classed as being cured, 3 cases
showed an excellent response and there were no effective or invalid
responses (Table II). In the
control group, 10 cases were classed as being cured, 3 cases showed
an excellent response, 2 cases were effective and no cases were
invalid among the 15 patients aged ≤3 months (Table I); among the 20 patients aged ≤3
months, 12 cases were classed as being cured, 3 cases were classed
as excellent, 5 cases showed an effective response and no cases
were invalid (Table II). The cure
rates after the different treatment courses are shown in Table III. Examples of excellent and cure
responses in variously located superficial hemangioma lesions of
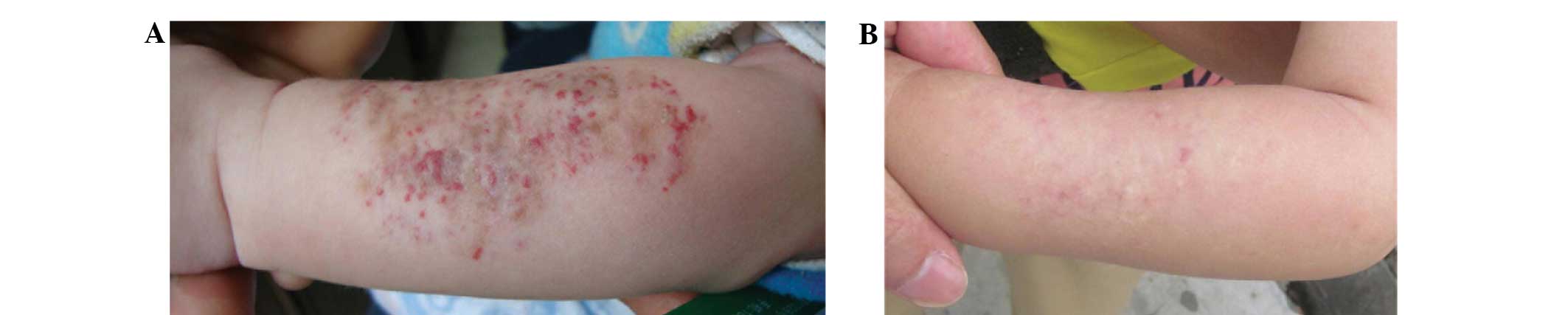
the observation group subjects are shown in Fig. 1 and Figs.
2 and 3, respectively.
 | Table I.Comparison of the treatment efficacy
in patients aged ≤3 months between the observation and control
group. |
Table I.
Comparison of the treatment efficacy
in patients aged ≤3 months between the observation and control
group.
|
|
| Response rate, % (n,
total n) |
|---|
|
|
|
|
|---|
| Group | Patients, n | Cure | Excellent | Effective | Invalid |
|---|
| Observation | 17 | 94.1
(16/17)a | 100
(17/17)b | 100
(17/17)c | 0 (0/17) |
| Control | 15 | 66.7 (10/15) | 86.7 (13/15) | 100 (15/15) | 0 (0/15) |
 | Table II.Comparison of the treatment efficacy
in patients aged >3 months between the observation and control
group. |
Table II.
Comparison of the treatment efficacy
in patients aged >3 months between the observation and control
group.
|
|
| Response rate, % (n,
total n) |
|---|
|
|
|
|
|---|
| Group | Patients, n | Cure | Excellent | Effective | Invalid |
|---|
| Observation | 20 | 85.0
(17/20)a | 100
(20/20)b | 100
(20/20)c | 0 (0/20) |
| Control | 20 | 60.0 (12/20) | 75.0 (15/20) | 100 (20/20) | 0 (0/20) |
 | Table III.Comparison of the efficacy of the
first and second treatment courses between the observation and
control groups. |
Table III.
Comparison of the efficacy of the
first and second treatment courses between the observation and
control groups.
|
|
| First course | Second course |
|---|
|
|
|
|
|
|---|
| Group | Patients, n | Cure rate, % (n,
total n) | Cure time,
months | Cure rate, % (n,
total n) | Cure time,
months |
|---|
| Observation | 33 |
33.3
(11/33)a | 3–4 | 100
(33/33)b | 4–9 |
| Control | 22 | 18.2 (4/22) | 3–4 | 100 (22/22) | 4–9 |
Adverse reactions
Two patients in the observation group exhibited mild
pruritus, although in 1 case the patient did not require any
additional treatment for the reaction and the symptoms subsided
after 3 weeks. The symptoms of the other case were more marked, but
subsided following the external application of Elocon ointment
(Schering-Plough Pharmaceutical Co., Ltd., Shanghai, China) for a
few days. Mild normal skin pigmentation 1 cm from the outer edge of
the lesion was observed in 6 cases after 1–2 weeks of treatment.
Skin color returned to normal after 3 months of treatment with
topical vitamin E gel treatment (Sinopharm Xingsha Pharmaceuticals
Co., Ltd., Xiamen, China).
In 1 case of a large-area (10.8×6.4 cm) forearm
hemangioma, the subject exhibited a fever (38.4°C) after 2 days of
90Sr-90Y application. Analysis revealed that
the patient's leukocyte and neutrophil counts were slightly below
normal, and the lymphocyte ratio was slightly elevated. The fever
subsided following symptomatic treatment for 4 days, and routine
blood tests showed normal results after 1 month. Re-examination of
the blood 1 month later also showed normal results. In 1 case of
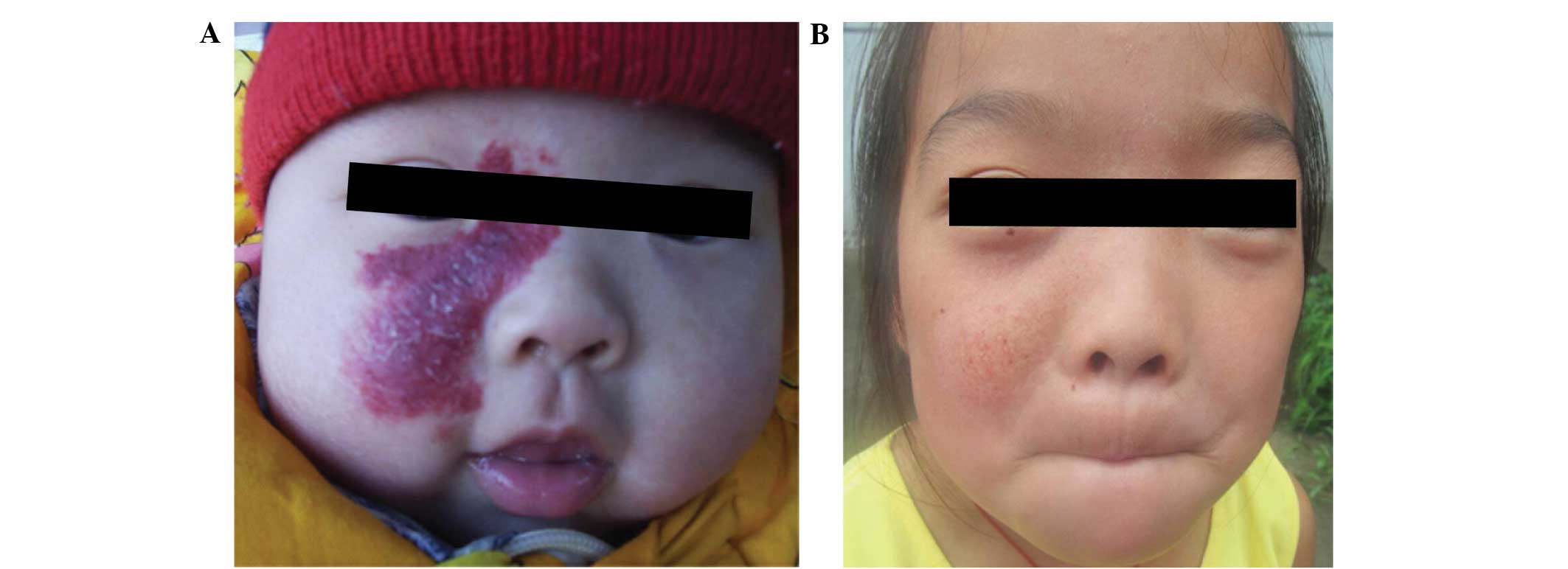
orbital superficial hemangioma (Fig.
1), eyelashes that were adjacent to the tumor were temporarily
lost after 2 weeks of treatment, but normal eyelash growth was
recovered after 6 months of follow-up. Two patients exhibited mild
skin flaking subsequent to receiving the topical timolol
application, but the symptoms disappeared following the temporary
discontinuation of the treatment for 3–5 days. No similar reactions
were observed once the dose was reduced.
In the control group, 3 subjects experienced mild
itching, which subsided after 2 days of symptomatic treatment. Mild
pigmentation of the normal skin adjacent to the tumor edge was
observed in 7 cases, which returned to normal between 3 weeks and 2
months later.
In the observation group, the adverse reaction rate
was 32.4% (12/37), while the control group adverse reaction rate
was 28.6% (10/35), resulting in no significant difference between
the groups (χ2=0.212, P>0.05). During the course of
treatment, there were no obvious blisters, ulceration, exudation,
infection or other wet dermatitis reaction in the two groups. No
significant alterations were detected in respiration and heart
rate, stool quality, diet or sleep habits and no other systemic
adverse reactions were detected. Furthermore, no abnormal changes
were observed in liver and kidney function, fasting blood glucose
and ECG prior to or following treatment.
Discussion
A variety of methods for treating superficial
hemangiomas exist, including lasers, freezing and
90Sr-90Y paste. Laser treatment is convenient
and quick, but is associated with an enhanced risk of hemangioma
proliferation relapse following treatment. Furthermore, only
moderate laser energy should be used, to avoid the formation of
local cicatrices. Cryotherapy may cause pain and can lead to
hypopigmentation. Among the most effective methods of treating IH
are 90Sr-90Y applicators, the use of which
has been reported in a number of previous studies (8,9). The
total cumulative radiation dose must, however, be strictly
controlled, as an excessive dose may result in serious
complications. Potential short-term complications of excessive
radiation include blisters, infection and ulcers, while possible
long-term complications include local pigmentation or
hypopigmentation, scar formation, soft tissue dysplasia and bone
growth inhibition (10,11). In a previous set of experiments (data
not shown), 1 patient with superficial hemangioma received 3
courses of 90Sr-90Y applicator treatment in
January 2008, with a total dose of 50 Gy, >2 times the dose of
the present study. Mild pigmentation was visible at the treatment
site at the 3-year follow-up (Fig.
4). The use of small doses of fractionated irradiation is
currently advocated (5), but which
may prolong cure time and reduce the pathogenicity of certain IHs
with an abundant blood supply.
The application of propranolol was a crucial
development in the treatment of the IH (12). Propranolol is able to effectively
control the proliferation of severe hemangioma, and has provided a
new drug option for the treatment of hemangioma. The disadvantage
of using propranolol is the long cycle of treatment, with countries
generally advocating a treatment duration in infant patients of
>12 months. Patients that do not complete the prescribed
treatment schedule may experience treatment failure or hemangioma
relapse. Furthermore, a limited number of infants are not sensitive
to propranolol and respond negatively. There may be a variety of
adverse responses if propranolol is used to treat small and
superficial lesions that are not particularly suitable, including
bradycardia, hypotension, hypoglycemia, bronchospasm, drowsiness or
insomnia, nightmares, loss of appetite and sweating (13). Recently, Tang et al (7) reported that
90Sr-90Y application combined with
propranolol was more effective than single-use
90Sr-90Y applicator therapy for the treatment
of infants with large-area skin hemangiomas; however, recipients
required close monitoring in order to detect any adverse systemic
reactions.
Following research into the clinical application of
propranolol, it was found that the topical application of
non-selective β-blocker also effectively induced the degradation of
skin hemangioma. Timolol is a non-selective β-adrenergic receptor
blocker, which was initially used for the treatment of glaucoma in
the USA in 1978 (14,15). Timolol treatment has been safely used
in clinical pediatrics and has been employed as a first-line
treatment for glaucoma in the field of pediatric ophthalmology for
30 years (16). According to
previous studies, the pharmacological effects of timolol are 4–10
times more marked than those of propranolol (17–19). Ni
et al (19) applied 0.5%
timolol eye drops, twice per day, to the faces and necks of 4
patients with IH (age range, 3–10 months). The color of the
hemangiomas faded after 2–4 months of treatment, and the affected
area was reduced with no obvious adverse reactions. Wang et
al (6) treated patients with a
0.5% topical application of timolol three times a day, every 8 h,
for 6 consecutive months. The efficacy rate in the treatment group
was 64%, which was significantly higher than that in the control
group (17.65%) (χ2=43.74, P<0.01) (6). The precise mechanism underlying the
timolol-mediated treatment of IH remains unknown; however, we
suggest that it involves a similar mechanism to that of oral
propranolol therapy (20) and that
timolol is able to block the β-receptors of the skin surface in IH
or inhibit the expression of VEGF, leading to the inhibition of the
tumor growth and the eventual eradication of the hemangioma,
concurrent with the induced vasoconstriction.
In the present study, an
90Sr-90Y applicator combined with topical
application of timolol was used to treat superficial IH. The total
cumulative dose of the 90Sr-90Y applicator
radiation and the number of courses (1 or 2) were reduced compared
with the norm (21). The rate of
responses classed as either a cure or effective was notably
increased in the observation group compared with that in the
control group. At 3–4 months after the first course of treatment,
the cure rate in the observation group (33.3%) was significantly
elevated compared with that in the single-use
90Sr-90Y control group (18.2%), which
suggested the combined observation group therapy was more effective
and rapid. It was frequently noted that, for the guardians of the
patients, the desire for an excellent response or cure was
secondary to the desire to observe rapid improvements in the
superficial appearance of the hemangioma, i.e. an effective
response, despite the former two outcomes being more favorable in
the long term.
The dose of the therapeutic intervention determines
the intensity and persistence of adverse reactions. No serious
local or systemic adverse reactions, such as blisters, ulcers,
hypoglycemia or bronchial asthma, were observed in the present
study. The possible reasons for this may be the elevated local drug
concentrations in the hemangioma and the relatively lower systemic
plasma drug concentrations, in addition to the reduced
90Sr-90Y treatment doses and total radiation
exposure. The local area surrounding the lesion exhibited mild
flaking in the observation group, but this subsided following the
temporary discontinuation of the treatment for a number of days.
This reaction did not reappear once the dose of timolol was reduced
appropriately, which may be associated with the individual
sensitivity to the timolol. In 6 cases in the observation group,
mild pigmentation of the normal skin was observable 1 cm from the
outer edge of the lesions after 1–2 weeks of treatment. Skin color
returned to normal following the topical application of vitamin E
gel for 3 months, which may have been due to the vitamin E exerting
antioxidative, free radical-scavenging and whitening effects that
promoted pigment-fading. In 1 case of orbital vascular tumor, the
patient's eyelashes fell off after 1 course of treatment (11 Gy),
but regrew after 6 months. According to previous treatment
experience, the use of a low-dose radiation applicator for the
treatment of hemangiomas in areas with hair covering (orbital
cavity, head) may result in temporary alopecia; however, the
majority of patients will recover hair growth at a later stage. A
variety of topical systemic adverse reactions have been reported as
a result of timolol application, including bronchial spasm, cough,
respiratory failure, nightmares and confusion (22). Close observation is therefore
advisable to prevent the occurrence of adverse reactions during
treatment, particularly in infants that are premature or exhibit
cardiopulmonary dysfunction.
In summary, the results of the present study
indicate that a combined treatment using an
90Sr-90Y applicator and local external
application of 0.5% timolol eye drops exhibits a number of
advantages for the treatment of IH, including rapid onset,
efficacy, convenient administration, low cost and reduced adverse
reactions, particularly for the treatment of superficial lesions on
the face and eye area. The combined treatment described therefore
possesses the potential for clinical application. However, due to
the limited sample size and follow-up period (maximum 1 year) of
the present study, the long-term complications of the therapy
remain unknown. In addition, future studies are required to
determine whether the therapeutic effects observed in the present
study may be achieved using a further-reduced total dose of
90Sr-90Y.
References
|
1
|
Hohenleutner U, Landthaler M, Hamm H and
Sebastian G: Hemangiomas of infancy and childhood. J Dtsch Dermatol
Ges. 5:334–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uihlein LC, Liang MG and Mulliken JB:
Pathogenesis of infantile hemangiomas. Pediatric Annals. 41:1–6.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chakkittakandiyil A, Phillips R, Frieden
IJ, Siegfried E, Lara-Corrales I, Lam J, Bergmann J, Bekhor P,
Poorsattar S and Pope E: Timolol maleate 0.5% or 0.1% gel-forming
solution for infantile hemangiomas: A retrospective, multicenter,
cohort study. Pediatr Dermatol. 29:28–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pope E and Chakkittakandiyil A: Topical
timolol gel for infantile hemangiomas: A pilot study. Arch
Dermatol. 146:564–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou IQ: Radiation treatment of nuclear
applicationThe Operation Standard in Clinical Technology Nuclear
Medicine Fascicule. Chinese Medical Association. People's Military
Medical Press; Beijing: pp. 194–196. 2004
|
|
6
|
Wang YM, Geng F, Zha ZY, Cheng R, Zhang
ZY, Su WJ and Zhang YX: Effectiveness of 0.5% solution of topical
maleate for infantile Hemangiomas. Zu Zhi Gon Cheng Yu Zhong Jian
Wai Ke Za Zhi She. 8:208–212. 2012.(In Chinese).
|
|
7
|
Tang ZW, Huang JH and Tang JH: Clinical
efficacy of 90Sr-90Y applicator combined with
propranolol treatment on large infantile cutaneous hemangiomas.
Zhong Hua He Yi Xue Xa Zhi. 33:49–51. 2013.(In Chinese).
|
|
8
|
Lin TS and Chen WM: Clinical analysis of
90Sr-90Y applicator treatment for 2862
infantile cutaneous capillary hemangiomas. Fu Jian Yi Yao Za Zhi
She. 32:21–22. 2010.(In Chinese).
|
|
9
|
Xi Z, Chen F and Lu L: Effectiveness
analysis of 90Sr/90Y applicator treatment for
107 infantile capillary hemangiomas. Biao Ji Mian Yi Fen Xi Yu Lin
Chuang Bian Ji Bu. 19:247–248. 2012.(In Chinese).
|
|
10
|
Zhang JD, Wang YC, Lv C, et al: Analysis
of nuclide radiation treatment for 1076 infantile hemangiomas. Ren
Min Jun Yi. 54:224–225. 2011.(In Chinese).
|
|
11
|
Fragu P, Lemarchand-Venencie F, Benhamou
S, François P, Jeannel D, Benhamou E, Sezary-Lartigau I and Avril
MF: Long-term effect in skin and thyroid after radiotherapy for
skin angiomas: A French retrospective cohort study. Eur J Cancer.
27:1215–1222. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan ST, Itinteang T and Leadbitter P:
Low-dose propranolol for infantile haemangioma. J Plast Reconstr
Aesthet Surg. 64:292–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiestl C, Neuhaus K, Zoller S, Subotic
U, Forster-Kuebler I, Michels R, Balmer C and Weibel L: Efficacy
and safety of propranolol as fist-line treatment for infantile
hemangiomas. Eur J Pediatr. 170:493–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Buskirk EM and Fraunfelder FT: Timolol
and glaucoma. Arch Ophthalmol. 99:6961981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ciudad Blanco C, Campos Domínguez M,
Moreno García B, Villanueva Álvarez-Santullano CA, Berenguer
Fröhner B and Suárez Fernández R: Episcleral infantile hemangioma
successfully treated with topical timolol. Dermatol Ther. 28:22–24.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coppens G, Stalmans I, Zeyen T and
Casteels I: The safety and efficacy of glaucoma medication in the
pediatric population. J Pediatr Ophthalmol Strabismus. 46:12–18.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lohmöller G and Frohilch ED: A comparison
of timolol and propranolol in essential hypertension. Am Heart J.
89:437–442. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Achong MR, Piafsky KM and Ogilvie RI:
Duration of cardiac effects of timolol and propranolol. Clin
Pharmacol Ther. 19:148–152. 1976.PubMed/NCBI
|
|
19
|
Ni N, Langer P, Wagner R and Guo S:
Topical timolol for periocular hemangioma: Report of further study.
Arch Ophthalmol. 129:377–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Mai HM and Zheng JW: The
mechanisms of propranolol treatment for infantile hemangiomas and
advances in research. Zhong Guo Kou Qiang Zuo Mian Wai Ke Za Zhi
She. 10:342–346. 2012.(In Chinese).
|
|
21
|
Ding SM, Qu W, Wang SJ, Feng J, Zheng XH
and Song C: Clinical observation of hemangioma treated by 90Sr/90Y
application. Zhong Guo Pi Fu Xing Bing Xue Za Zhi She. 11:673–674.
2006.(In Chinese).
|
|
22
|
McMahon P, Oza V and Frieden IJ: Topical
timolol for infantile hemangiomas: Putting a note of caution in
‘cautiously optimistic’. Pediatr Dermatol. 29:127–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|