Introduction
Osteoporosis, a systemic skeletal disease, is highly
relevant to age and is characterized by reduced bone mass,
decreased bone mineral composition and bone mineral/matrix ratio,
bone thinning and bone microstructure degeneration, including
reduced trabecular bone, resulting in increased bone brittleness
and bones prone to fracture. Osteoporosis occurs mainly due to the
enhancement of bone resorption, decreased bone formation or both,
with various causes. Bone marrow density, bone remodeling and bone
integrity are regulated by osteoblasts and osteoclasts (1). In normal conditions, these two types of
cells are in a state of equilibrium to ensure the normal
development of the human skeleton and homeostasis of bone
formation. Enhanced osteoclast activity or decreased osteoblast
activity can break the balance, leading to osteoporosis with
reduced bone mass and pathological bone-thinning changes (2); therefore, inhibiting the activity of
osteoclasts by inhibiting osteoclast differentiation is an
important strategy for treating osteoporosis.
Nuclear factor of activated T cells (NFAT) proteins
are a group of proteins that exhibit transcriptional activity and
are found universally in animals. NFATs can regulate cell
proliferation and differentiation at the transcriptional level
(3). There are five major members of
the NFAT family: NFATc1, NFATc2, NFATc3, NFATc4 and NFAT5. Among
them, NFATc1 comprises a group of transcriptional factors that are
regulated by calcineurin and may affect the signaling of T cells
and tissue development. The inactivated form of NFATc1 is located
in the cytoplasm and does not have transcriptional activity. The
activated NFATc1, which exhibits transcriptional activity, can be
translocated into the nucleus where it regulates the expression of
the downstream genes (4). A previous
study found that the specific activation of NFATc1 could induce the
differentiation of osteoclast precursors into mature osteoclasts,
whereas inhibiting the activity of NFATc1 can inhibit osteoclast
differentiation (5). It has also
been found that numerous proteins that are vital for osteoclast
differentiation, including tartrate-resistant acid phosphatase
(TRAP), calcitonin receptor and osteoclast-associated
immunoglobulin-like receptor (OSCAR) are regulated by NFATc1
(6–8). NFATc1 may, therefore, become an
important therapeutic target for treating osteoporosis.
Currently, drugs for osteoporosis contain substances
including estrogen, vitamin D, calcitonin and bisphosphonates.
Biophosphonates are the most commonly used drugs for treating
osteoporosis, with the main mechanism being osteoclast inhibition;
however, the long-term use of bisphosphonates can have adverse
effects. Finding novel therapeutic targets for the inhibition of
osteoclasts is therefore important for the treatment of
osteoporosis.
The aim of the present study was to use a
high-throughout screening system to find a natural product that
could clearly inhibit NFATc1 translocation into the nucleus. Among
the potential natural products was Wogonin, which comes from the
Traditional Chinese Medicine herb, Scutellaria baicalensis;
however, recent research into Scutellaria baicalensis has
mainly focused on its anti-tumor activity, with one study finding
that Wogonin functions as an anti-colon cancer agent by regulating
the Wnt/β-catenin pathway (9,10). To
date, however, there have been no studies regarding the mechanism
underlying the inhibitory effect of Wogonin on NFATc1 and
osteoclasts. The effect of Wogonin on the inhibition of osteoclast
differentiation was, therefore, also investigated.
Materials and methods
Cell culture
Mouse mononuclear macrophage (RAW264.7) cells
(American Type Culture Collection, Manassas, VA, USA) were cultured
in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO,
USA) with 10% fetal bovine serum (FBS; Sigma), 100 U/ml penicillin
and 100 mg/ml streptomycin. Cells were incubated in 5%
CO2 at 37°C. U2OS-EGFP-NFATc1 cells (Bio-Images,
Glasgow, UK) were cultured in DMEM with 10% FBS, 100 U/ml
penicillin, 100 mg/ml streptomycin and 0.5 mg/ml G418.
Osteoclast differentiation
RAW264.7 cells were seeded onto a 24-well plate with
a density of 2×104 cells/well and incubated with DMEM
until the cells were 70% confluent. The cells were washed with
fresh medium, and 100 ng/ml receptor activator of nuclear factor κB
ligand (RANKL; Sigma) was added into the medium, and the cells were
cultured for 3 days for differentiation. The cells were washed with
fresh medium, and cultured in medium with 100 ng/ml RANKL and a
corresponding compound for testing for 2 days. The amount of TRAP
in the cell was detected in accordance with the instructions of the
TRAP detection kit (Beyotime Institute of Biotechnology, Suzhou,
China) in order to determine the effect of the compound on
osteoclast differentiation. The specific method was as follows: The
medium was removed and each well of the plate was washed by
phosphate-buffered saline (PBS) once; 50 ml fixing solution was
added into each well, and the cells were fixed at room temperature
for 5 min. The cells were then washed 3 times and 50 ml TRAP
chromogenic substrate was added and incubated at 37°C for 20–60
min. Osteoclasts were stained in red, and the degree of osteoclast
differentiation was observed under the microscope (X80; Olympus
Corporation, Tokyo, Japan; magnification, x200).
Detection of NFATc1 within and outside
of the nucleus
U2OS cells were stably transfected with EGFP-NFATc1
and cultured until the cells grew confluently. Subculture was
performed at a ratio of 1:3 in a 96-well IN Cell™ Analyzer 1000 (GE
Healthcare Life Sciences, Little Chalfont, UK) and the cells were
cultured overnight. The next day, the natural product for detection
(130 single and small molecules in total, extracted from Chinese
medicine sources; Selleckchem, Houston, TX, USA) or the solvent
(control group) was added. In addition, RANKL was added (100
ng/ml), and the mixture was incubated for 24 h. Following
incubation, the cells were washed twice with PBS, and then 2 mM
Hoechst 33342 was added to stain the nucleus for 15 min. Finally,
the IN Cell Analyzer 1000 was used to capture video images and
software was used to analyze the videos. Three repeatable wells
were established for each treatment and six views were captured for
each well. The ratio of the fluorescent intensity of the nucleus to
the cytoplasm was used to detect the translocation of NFATc1 into
the nucleus.
Quantitative polymerase chain reaction
(qPCR)
RAW264.7 cells were cultured in a six-well plate
until the cells were 60% confluent. RANKL (100 ng/ml) and different
concentrations (5.0, 1.0, 0.1 and 0.0 mM) of Wogonin (Selleckchem)
were added. Dimethyl sulfoxide (DMSO) was used as a control. The
cells were incubated for 24 h and then the total mRNA was
extracted. The specific method was as follows: Cells were washed
three times with PBS, and then 1 ml TRIzol™ (Takara Biotechnology
Co., Ltd., Dalian, China) was added and left for 5 min. The cells
were then collected into a 1.5-ml Eppendorf tube, 200 ml chloroform
was added and the tube was agitated vigorously and left for 3 min.
The tubes were then centrifuged at 15,000 × g for 15 min, and 0.5
ml isopropanol was added into the supernatant and mixed well. The
tubes were left at room temperature for 10 min and then
re-centrifuged at 15,000 × g at 4°C for 15 min. Following
centrifugation, the supernatant was discarded, and the pellet was
washed with 75% ethanol and finally centrifuged at 15,000 × g at
4°C for 15 min. The supernatant was removed and the pellet was
dried at room temperature for 15 min. Diethylpyrocarbonate was
added to dissolve the pellet and the mRNA was obtained. The
contents were then analyzed.
mRNA was reverse transcribed into cDNA according to
the instructions in the kits for reverse transcription (Takara
Biotechnology Co., Ltd.). The specific procedure was as below: mRNA
was quantified, and then random primer, the reverse transcription
enzyme, oligo dT and 1 mg mRNA provided in the kits were added,
prior to cDNA being obtained by reverse transcription at 37°C for
15 min. PCR was performed quantitatively using the real-time
fluorescent PCR kit (Takara Biotechnology Co., Ltd.) with the
following cycle conditions: 94°C for 5 min and 43 cycles; 94°C for
3 sec; 63°C for 10 sec; and 72°C for 60 sec. The primers (Sangon
Biotech Co., Ltd., Shanghai, China) were as follows: Mouse GAPDH
forward sequence, 5′-AGG TCG GTG TGA ACG GAT TTG-3′ and reverse
sequence, 5′-GGG GTC GTT GAT GGC AAC A-3′; mouse OSCAR forward
sequence, 5′-CCT AGC CTC ATA CCC CCA G-3′ and reverse sequence,
5′-CGT TGA TCC CAG GAG TCA CAA-3′; mouse TRAP forward sequence,
5′-CAC TCC CAC CCT GAG ATT TGT-3′ and reverse sequence, 5′-CCC CAG
AGA CAT GAT GAA GTC A-3′; mouse calcitonin receptor forward
sequence, 5′-GAG GTT CCT TCT CGT GAA CAG-3′ and reverse sequence,
5′-AGT CAG TGA GAT TGG TAG GAG C-3′.
Luciferase reporter gene assay
RAW264.7 cells were seeded onto a 24-well plate and
cultured until cells were 60–80% confluent. The culture medium was
changed into medium without serum and antibiotics. Lipofectamine®
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA), luciferase
reporter gene plasmid pRL-SV40 (Promega Corp., Madison, WI, USA)
and pGL3-promoter-NFATc1-response element (RE), which was
constructed by inserting the NFATc1 cis-acting element sequence
(5′-CGC CCA AAG AGG AAA ATT TGT TTC ATA-3′) into the pGL3-promoter
plasmid, were prepared using Opti-MEM®, kept at room temperature
for 5 min and then mixed well, prior to being kept at room
temperature for a further 20 min, added into the medium and mixed
well. This medium was used to culture the cells for 6 h. The medium
was subsequently changed to the complete medium, and RANKL (100
ng/ml) and different concentrations of Wogonin (5.0, 1.0, 0.1 and
0.0 mM) were added. DMSO was added as a control and the cells were
incubated for 24 h. The lysis buffer provided in the luciferase
reporter gene assay kit (Promega Corp.) was used to lyse the cells,
and firefly and Renilla luciferase activity was measured using the
kit.
Statistical analysis
The results were analyzed using SPSS 17.0 software
(SPSS Inc., Chicago, IL, USA), and are expressed as the mean ±
standard deviation. Comparisons between groups were performed using
single-factor analysis of variance; P<0.05 was considered to
indicate a statistically significant difference.
Results
Wogonin inhibits the transcriptional
activity of NFATc1 by inhibiting its translocation into the
nucleus
Research has shown that the transcriptional activity
of NFATc1 is important for osteoclast differentiation. In normal
conditions, NFATc1 is distributed uniformly in the cytoplasm and
lacks the ability to activate transcription; however, upon its
translocation into the nucleus, NFATc1 gains the ability to
activate transcription (11). The
specificity of NFATc1 activation can induce osteoclast precursors
to differentiate into mature osteoclasts; thus, the inhibition of
NFATc1 activation could inhibit osteoclast differentiation and
represent a treatment for osteoporosis (12). Based on the literature, a
high-throughout screening system for measuring the translocation of
NFATc1 into the nucleus was therefore established in the present
study. U2OS cells were stably transcribed with EGFP-NFATc1
(U2OS-EGFP-NFATc1); RANKL was added as a stimulus, and natural
products (130 single and small molecules in total) were added for
screening. The translocation of NFATc1 was observed using the IN
Cell Analyzer 1000 (13). RANKL
could significantly promote the translocation of NFATc1, while the
natural product Wogonin could inhibit the RANKL-induced
translocation of NFATc1 in a concentration-dependent manner
(Fig. 1A and B). These results
showed that Wogonin could inhibit the transcriptional activation
activity of NFATc1 by inhibiting the translocation of NFATc1 into
the nucleus; therefore, a luciferase reporter gene assay was used
to further confirm the effect of Wogonin on the transcriptional
activity of NFATc1. Since mouse mononuclear macrophage (RAW264.7)
cells can differentiate into osteoclasts following stimulation by
RANKL, RAW264.7 cells were transfected with pGL3-promoter-NFATc1-RE
plasmid, and then RANKL (100 ng/ml) and different concentrations of
Wogonin (5.0, 1.0, 0.1 and 0.0 mM) were incubated for 24 h. The
activity of NFATc1-RE was subsequently measured. Wogonin could
inhibit the activation of NFATc1 on its cis-acting element
(NFATc1-RE) in a concentration-dependent manner, suggesting that
Wogonin could inhibit the transcriptional activity of NFATc1 by
decreasing its translocation into the nucleus (Fig. 1C).
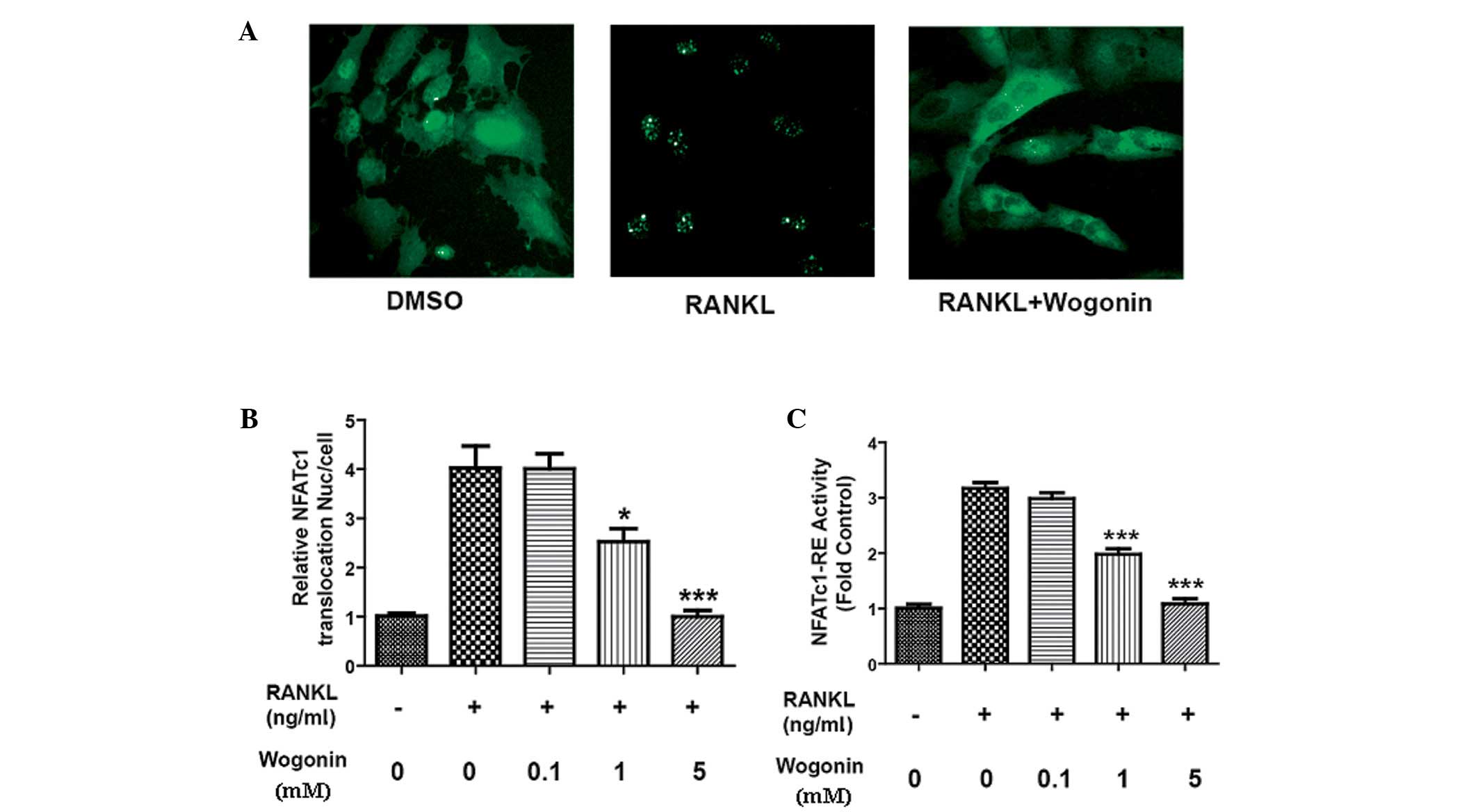 | Figure 1.Effect of Wogonin on the translocation
and transcriptional activity of NFATc1. U2OS cells stably
transfected with EGFP-NFATc1, RANKL (100 ng/ml) and different
concentrations of Wogonin (5.0, 1.0, 0.1 and 0.0 mM) were added and
incubated for 24 h. The IN Cell™ Analyzer 1000 was used to observe
the translocation of NFATc1. (A) DMSO was used as a control. RANKL
(100 ng/ml) significantly stimulated the translocation of NFATc1;
Wogonin (5 mM) significantly decreased the translocation of NFATc1
induced by RANKL (magnification, x200). (B) Statistics showed that
Wogonin could decrease the translocation of NFATc1 into the nucleus
induced by RANKL in a concentration-dependent manner. RAW264.7
cells were transfected with pGL3-promoter-NFATc1-RE, and then RANKL
(100 ng/ml) and different concentrations of Wogonin (5.0, 1.0, 0.1
and 0.0 mM) were added and incubated for 24 h. (C) RANKL
significantly increased the transcriptional activity of NFATc1 and
Wogonin decreased the transcriptional activity of NFATc1 induced by
RANKL in a concentration-dependent manner. n=3; *P<0.05 and
***P<0.001 compared with the RANKL group. NAFTc1, nuclear factor
of activated T cells c1; EGFP, enhanced green fluorescent protein;
DMSO, dimethylsulfoxide, RANKL, receptor activator of nuclear
factor κB ligand; RE, response element. |
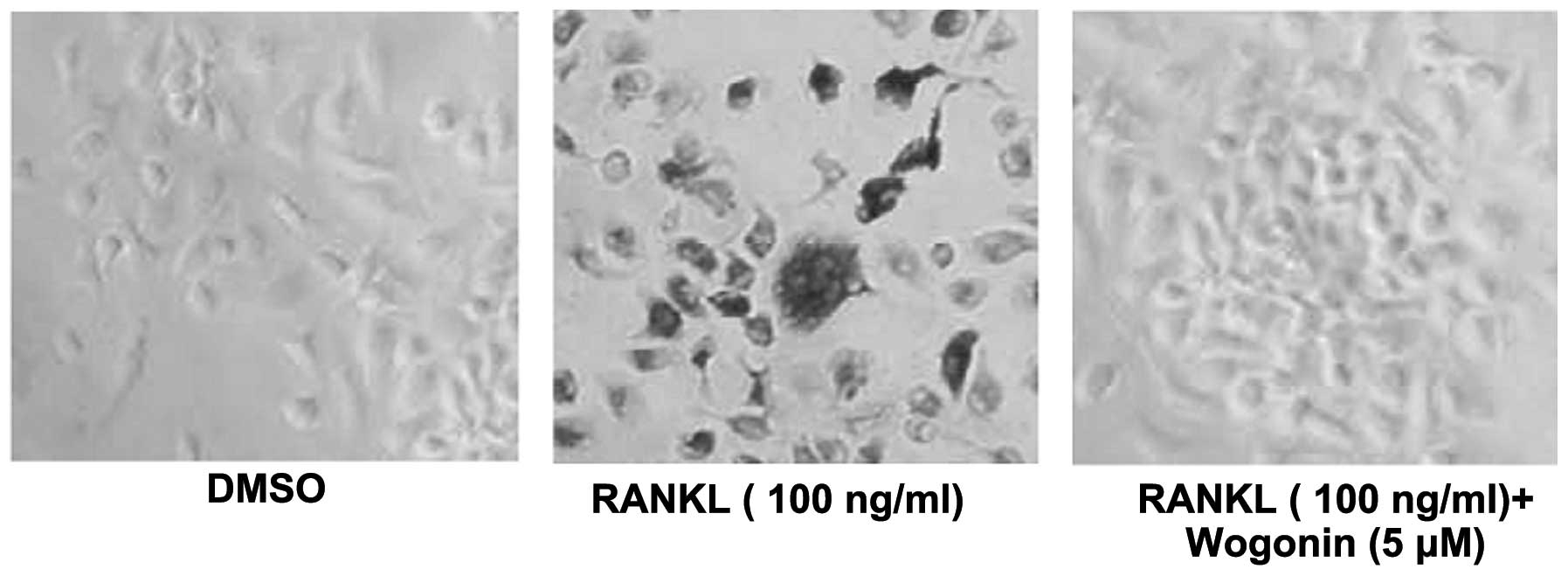
Wogonin inhibits osteoclast
differentiation
Since it was shown that Wogonin could significantly
inhibit the translocation of NFATc1 into the nucleus and its
transcriptional activity, and that inhibiting the transcriptional
activity of NFATc1 could inhibit osteoclast differentiation, mouse
mononuclear macrophage (RAW264.7) cells were stimulated by RANKL to
induce osteoclast differentiation, and the effect of Wogonin on the
RAW264.7 cell differentiation into osteoclasts was assessed.
Wogonin could significantly inhibit the RAW264.7 cell
differentiation into osteoclasts, sugge sting that Wogonin could
inhibit osteoclast differentiation and thus represent a potential
therapeutic agent for treating osteoporosis (Fig. 2).
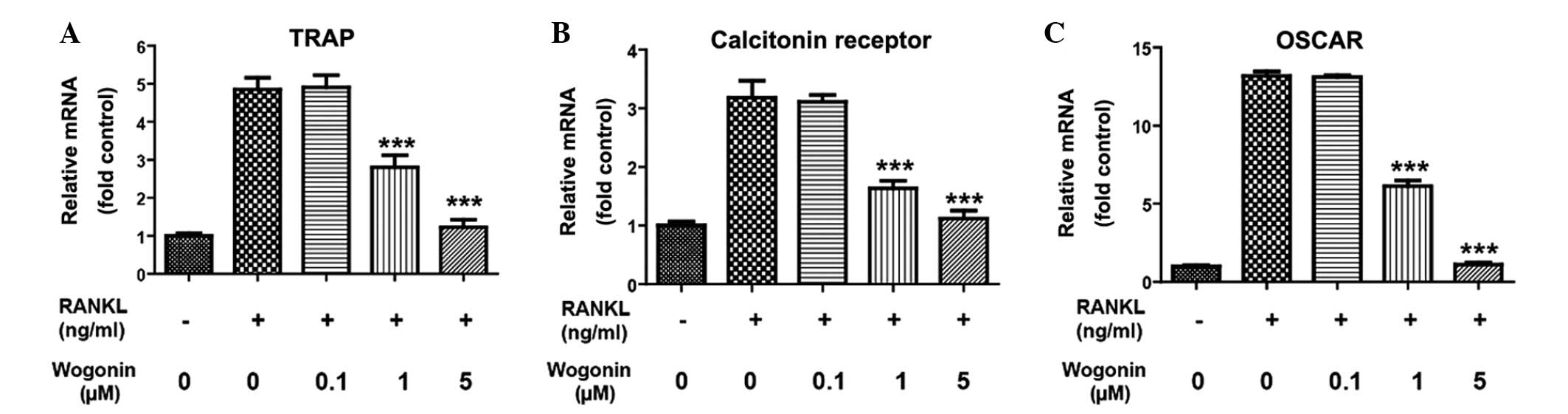
Wogonin inhibits osteoclast
differentiation by inhibiting the transcription activity of
NFATc1
NFATc1 plays a vital role in osteoclast
differentiation. Following its translocation into the nucleus and
its stimulation of transcriptional activity, NFATc1 can enhance
multiple stimuli or activate the transcription of proteins that are
important for osteoclasts, such as TRAP, OSCAR and calcitonin
receptor, thus facilitating osteoclast differentiation. qPCR was
therefore used in the present study to detect the effect of Wogonin
on the transcriptional activity of TRAP, OSCAR and calcitonin
receptor in RAW264.7 cells, and thus further clarify whether
Wogonin could inhibit osteoclast differentiation by inhibiting the
translocation of NFATc1 into the nucleus. As shown in Fig. 3, RANKL could increase the mRNA level
of TRAP, calcitonin receptor and OSCAR in the RAW264.7 cells to
different extents, suggesting that Wogonin could inhibit
transcription of important proteins for osteoclast differentiation
and thus further confirming that Wogonin could inhibit osteoclast
differentiation by inhibiting the transcriptional activity of
NFATc1.
Discussion
Osteoporosis is a systemic bone disease associated
with age. The symptoms are a decrease in bone mass and density,
leading to pain and fractures. Currently, the clinical drugs for
treatment can be classified into two main categories: Calcium
preparations, such as calcium gluconate and vitamin D, which
supplement the calcium content in the body, and agents that inhibit
bone absorption and facilitate bone formation, such as
bisphosphonates. However, calcium preparations can only improve the
relevant symptoms of osteoporosis, and bisphosphonates have toxic
adverse effects; therefore, a novel drug for osteoporosis would be
valuable.
NFATc1 is an important factor involved in the
inhibition of osteoclast differentiation. NFATc1 can translocate
into the nucleus and upregulate the transcription of several
proteins, such as TRAP and OSCAR, which are important for
osteoclast differentiation, following stimulation by RANKL.
Anti-osteoporosis drugs targeting NFATc1 are therefore important
for osteoporosis treatment (14,15). The
present study identified the natural product Wogonin through a
high-throughout screening system. Wogonin could significantly
inhibit the translocation of NFATc1 into the nucleus and thus
inhibit its transcriptional activity. In further investigations, it
was found that Wogonin could significantly decrease the mRNA
expression level of TRAP, OSCAR and calcitonin receptors; these
mRNAs are regulated by NFATc1 and play a vital role in osteoclast
differentiation. Furthermore, Wogonin could inhibit the
RANKL-induced mouse mononuclear macrophage differentiation into
osteoclasts. In combination, these results showed that Wogonin
could inhibit osteoclast differentiation by inhibiting the
transcriptional activity of NFATc1, and thus suggested that Wogonin
could have a therapeutic role in osteoporosis.
Wogonin has a wide range of pharmacological
activities and exerts anti-cancer and anti-inflammatory effects.
Numerous reports have described the role of Wogonin in anti-cancer
treatments (16,17), detailing its significant growth
inhibitory effect on tumor cells (IC50, 15–200 mM). In
addition, in vivo studies have demonstrated its anti-tumor
activity (18,19). Mice were intravenously administered
40 mg/kg Wogonin for 7 days and the inhibition rate for the growth
of S180 transplanted tumors was 53% (18), while an intraperitoneal injection of
200 mg/kg Wogonin could completely inhibit leukemia and CEM cells
(19). The anti-cancer mechanisms of
Wogonin consist of multiple aspects, such as inducing apoptosis and
differentiation, affecting the cell cycle, inhibiting tumor
angiogenesis and inhibiting telomerase (20,21).
In conclusion, the present study has shown that
Wogonin may inhibit osteoclast differentiation by inhibiting the
translocation of NFATc1 into the cell nucleus and represents an
effective small molecule for further study into anti-osteoporosis
drugs. Wogonin also provides a novel mechanism for the Chinese
medicine Scutellaria baicalensis in the treatment of
osteoporosis.
References
|
1
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novack DV and Teitelbaum SL: The
osteoclast: Friend or foe? Annu Rev Pathol. 3:457–484. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horsley V, Aliprantis AO, Polak L,
Glimcher LH and Fuchs E: NFATc1 balances quiescence and
proliferation of skin stem cells. Cell. 132:299–310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei J, Li B, Gao, et al: Fluoride
decreased osteoblastic bone resorption through the inhibition of
NFATc1 gene expression. Environ Toxicol. 29:588–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takayanagi H, Kim S, Koga T, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim K, Kim JH, Lee J, et al: Nuclear
factor of activated T cells c1 induces osteoclast-associated
receptor gene expression during tumor necrosis factor-related
activation-induced cytokine-mediated osteoclastogenesis. J Biol
Chem. 280:35209–35216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim Y, Sato K, Asagiri M, Morita I, Soma K
and Takayanagi H: Contribution of nuclear factor of activated T
cells c1 to the transcriptional control of immunoreceptor
osteoclast-associated receptor but not triggering receptor
expressed by myeloid cells-2 during osteoclastogenesis. J Biol
Chem. 280:32905–32913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He L, Lu N, Dai Q, et al: Wogonin induced
G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway
and inactivating CDK8 in human colorectal cancer carcinoma cells.
Toxicology. 312:36–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XM, Bai Y, Zhong YJ, et al: Wogonin
has multiple anti-cancer effects by regulating c-Myc/SKP2/Fbw7α and
HDAC1/HDAC2 pathways and inducing apoptosis in human lung
adenocarcinoma cell line A549. PLoS One. 8:e792012013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuda ES, Imamura R, Amasaki Y, Arai K
and Arai N: Signalling into the T-cell nucleus: NFAT regulation.
Cell Signal. 10:599–611. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collin-Osdoby P and Osdoby P:
RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Biol. 816:187–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SJ, Ding W, Albrecht B, Green PL and
Lairmore MD: A conserved calcineurin-binding motif in human T
lymphotropic virus type 1 p12I functions to modulate nuclear factor
of activated T cell activation. J Biol Chem. 278:15550–15557. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikeda F, Nishimura R, Matsubara T, et al:
Critical roles of c-Jun signaling in regulation of NFAT family and
RANKL-regulated osteoclast differentiation. J Clin Invest.
114:475–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikeda F, Nishimura R, Matsubara T, Hata K,
Reddy SV and Yoneda T: Activation of NFAT signal in vivo leads to
osteopenia associated with increased osteoclastogenesis and
bone-resorbing activity. J Immunol. 177:2384–2390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Lu N, Zhang H, et al: Wogonin
induced cytotoxicity in human hepatocellular carcinoma cells by
activation of unfolded protein response and inactivation of AKT.
Hepatol Res. 43:890–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chow SE, Chen YW, Liang CA, Huang YK and
Wang JS: Wogonin induces cross-regulation between autophagy and
apoptosis via a variety of Akt pathway in human nasopharyngeal
carcinoma cells. J Cell Biochem. 113:3476–3485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Guo QL, You QD, et al: The
anticancer activities of wogonin in murine sarcoma S180 both in
vitro and in vivo. Biol Pharm Bull. 29:1132–1137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HW, Yang Y, Zhang K, et al: Wogonin
induced differentiation and G1 phase arrest of human U-937 leukemia
cells via PKCD phosphorylation. Eur J Pharmacol. 591:7–12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baumann S, Fas SC, Giaisi M, et al:
Wogonin preferentially kills malignant lymphocytes and suppresses
T-cell tumor growth by inducing PLCC1-and Ca2+-dependent
apoptosis. Blood. 111:2354–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai CF, Yeh WL, Huang SM, Tan TW and Lu
DY: Wogonin induces reactive oxygen species production and cell
apoptosis in human glioma cancer cells. Int J Mol Sci.
13:9877–9892. 2012. View Article : Google Scholar : PubMed/NCBI
|