Introduction
With an estimated annual incidence of >1 million
new cases worldwide, colorectal cancer (CRC) is one of the most
frequently occurring human malignant neoplasms. Approximately 1 in
3 individuals that develop CRC succumb to the disease (1). Studies have demonstrated that aberrant
tyrosine phosphorylation, or activation of signal transducer and
activator of transcription 3 (STAT3), acts as a regulator of
tumorigenesis (2) and that the
expression rates of STAT3 and phosphorylated- (p-)STAT3 in CRC
tissues are significantly higher than those in adjacent normal
intestinal mucosa tissues (3).
Interleukin-6 (IL-6) is a proinflammatory cytokine that is
primarily produced by the cells comprising the tumor
microenvironment: Fibroblasts, myeloid cells and lymphoid cells.
IL-6 plays a key role in promoting the proliferation and inhibition
of apoptosis (4), as it binds to its
receptor (soluble IL-6 receptor) and coreceptor (glycoprotein 130,
or gp130), resulting in the activation of the associated Janus
kinases (JAKs). Activated JAKs phosphorylate gp130, leading to the
recruitment and activation of STAT3 (5). STAT3 is an important transcription
factor that plays an essential role in cell survival and
proliferation (6,7). It is known that the overexpression of
cyclin D1 and B-cell lymphoma-2 (Bcl-2), among others, mediated by
the abnormal activation of IL-6/STAT3, leads to excessive cell
proliferation and apoptosis resistance. This, in turn, may cause
tumorigenesis.
Inhibition of STAT3 transcriptional activity has
been demonstrated to increase the rate of apoptosis in cancer cells
(8). STAT3 has therefore been
validated as a novel anticancer drug target, and targeting the
STAT3 signaling pathway is considered a novel and promising
therapeutic strategy in the treatment of cancer (9). Despite advances in chemotherapy, a
regimen of 5-fluorouracil, in combination with oxaliplatin and
irinotecan, remains one of the most important treatments of CRC
(10); however, the majority of
patients with CRC develop drug resistance and fall subject to
metastasis. This problem has resulted in an increased interest in
natural medicines, with studies in cancer therapeutics revisiting
traditional herbal medicines. A number of herbal extracts or
mixtures based on traditional medicines have exhibited anticancer
effects with fewer or no side effects as compared with other
anticancer therapeutics, including chemical compounds and targeting
antibodies (11–13).
Scutellaria barbata D. Don (SB) is an
important component of numerous medicinal formulas that have
traditionally been used in China to treat a range of types of
cancer, including CRC. We have recently reported that ethanol
extract of SB (EESB) can exert numerous effects: i) Induction of
cancer cell apoptosis by activating the mitochondrion-dependent
pathway; ii) inhibition of tumor angiogenesis via suppression of
Hedgehog signaling; and iii) induction of G1/S arrest in human
colon carcinoma cells by modulating a number of signaling pathways
associated with the cell cycle (14–17). In
order to further elucidate the mechanism underlying the tumoricidal
activity of EESB, the aim of the present study was to explore its
effects on the IL-6-mediated activity in HT-29 human carcinoma
cells, including cell proliferation and apoptosis, the STAT3
phosphorylation level and transcriptional activity, and the
expression of a number of target genes of the IL-6/STAT3 signaling
pathway.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA, TRIzol®
reagent, and caspase-9 and caspase-3 activation kits were purchased
from Invitrogen (Life Technologies, Carlsbad, CA, USA). Monoclonal
antibodies against Bcl-2, Bcl2-associated X protein (Bax), cyclin
D1 and cyclin-dependent kinase 4 (CDK4) and horseradish peroxidase
(HRP)-conjugated secondary antibodies were obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA). SuperScript™ II
reverse transcriptase was obtained from Promega Corp. (Madison, WI,
USA). A bicinchoninic acid (BCA) protein assay kit was purchased
from Tiangen Biotech (Beijing) Co., Ltd. (Beijing, China). Unless
otherwise stated, all other chemicals were obtained from Sigma
Chemical Co. (St. Louis, MO, USA).
Preparation of EESB
The original herb was collected in the Henan region
of China and was identified as SB by Dr Wei Xu at the Department of
Pharmacology, Fujian University of Traditional Chinese Medicine
(Fuzhou, China). The plants were dried and cut into small pieces,
and EESB was prepared as described in a previous study (16). Stock solutions of EESB were prepared
by dissolving the EESB powder in 40% dimethyl sulfoxide (DMSO) to a
concentration of 500 mg/ml, and the solutions were stored at −20°C.
The working concentrations of EESB were made by diluting the stock
solution in the culture medium. The final concentrations of DMSO in
the medium were <0.5%.
Cell culture
HT-29 human colon carcinoma cells were obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The cells were grown as an adherent monolayer in DMEM culture media
containing 10% v/v FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified incubator at 37°C with 5%
CO2.
EESB and IL-6 treatment
HT-29 cells were cultured with DMEM containing 10%
FBS and 1% penicillin/streptomycin. When the cells reached 50%
confluence, the complete medium was changed with FBS-free medium
overnight. The cells were then pretreated with various
concentrations of EESB in complete DMEM for 1 h, followed by
stimulation with 10 ng/ml IL-6 (Sigma Chemical Co.) for the
indicated periods.
Evaluation of cell viability by MTT
assay
The cells were harvested and re-suspended at a final
concentration of 1×105 cells/ml and were seeded into a
96-well plate at 100 µl/well. Following incubation for 24 h at
37°C, the cells were treated with different concentrations of EESB
and/or IL-6 for another 24 h. Subsequently, 100 µl MTT (0.5 mg/ml)
was added to each well. The plates were incubated at 37°C for 4 h,
and 100 µl DMSO was added to dissolve the purple formazan crystals.
The absorbance was then read at 570 nm with an ELISA reader (Model
ELx800; BioTek Instruments, Inc., Winooski, VT, USA).
Observation of morphological
changes
HT-29 cells were seeded into six-well plates in 2 ml
medium at a density of 2.5×105 cells/well. The cells
were treated with various concentrations of EESB and/or IL-6 for 24
h. The cell morphology was observed using a phase-contrast
microscope (Leica, Solms, Germany). Images were captured at a
magnification of x200.
Colony formation
HT-29 cells from the exponentially growing cultures
were seeded into 12-well culture plates at a density of
1×105 cells/well and treated with different
concentrations of EESB and/or IL-6 for 24 h. The cells were then
harvested and seeded into six-well plates at a final concentration
of 1×103 cells/well in 2 ml fresh medium. Following
incubation for 8 days in a humidified incubator at 37°C with 5%
CO2, the formed colonies were fixed in MeOH-HAc (3:1,
v/v) for 10 min, stained with crystal violet and counted. Cell
survival was calculated by normalizing the survival of the control
cells as 100%.
Cell cycle analysis
A total of 2.5×105 HT-29 cells were
seeded into six-well plates in 2 ml medium and treated with the
indicated concentrations of EESB and/or IL-6 for 24 h. The cells
were harvested and adjusted to a concentration of 2×105
cells/ml. The HT-29 cell cycle progression was determined through
flow cytometric analysis using a propidium iodide (PI) staining
cell cycle assay kit (BD Biosciences, Franklin Lakes, NJ, USA). The
cells were fixed in 70% ethanol at 4°C overnight. The fixed cells
were washed twice with cold phosphate-buffered saline (PBS) and
then incubated for 30 min with ribonuclease (8 µg/ml) and PI (10
µg/ml). The fluorescent signal was detected through the FL2
channel, and the proportion of DNA in various phases was analyzed
using ModFit LT version 3.0 (Verity Software House, Inc., Topsham,
ME, USA).
Detection of apoptosis through flow
cytometric analysis with Annexin V/PI staining
A total of 2×105 HT-29 cells were seeded
into six-well plates in 2 ml medium and treated with the indicated
concentrations of EESB and/or IL-6 for 24 h. The apoptosis of the
HT-29 cells was then determined through flow cytometric analysis
using a fluorescence-activated cell sorter (FACSCalibur™; BD
Biosciences) and an Annexin V-fluorescein isothiocyanate/PI kit
(KeyGen Biotech, Nanjing, China). Staining was performed according
to the manufacturer's instructions. In this assay, the Annexin V/PI
double-negative population indicated viable cells, and the Annexin
V-positive/PI-negative or Annexin V/PI double-positive populations
represented cells undergoing early or late apoptosis,
respectively.
Analysis of caspase-9/caspase-3
activation
Caspase-9 and caspase-3 activity was determined via
a colorimetric assay using caspase-9 and caspase-3 activation kits,
following the manufacturer's instructions (Invitrogen). Following
treatment with various EESB concentrations and 10 ng/mL IL-6 for 24
h, the HT-29 cells were lysed with the provided lysis buffer for 30
min on ice. The lysed cells were then centrifuged at 16,000 × g for
10 min. The protein concentration of the clarified supernate was
determined, and 100 µg protein was incubated with 50 µl of the
colorimetric tetrapeptide Leu-Glu-His-Asp-p-nitroaniline
(pNA), a specific substrate of caspase-9, or with
Asp-Glu-Val-Asp-pNA, a specific substrate of caspase-3, at 37°C in
the dark for 2 h. Samples were read at 405 nm in the ELISA reader.
The data were normalized to the activity of the caspases in the
control cells (treated with PBS vehicle) and were represented as
the fold of control.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
A total of 2×105 HT-29 cells were seeded
into six-well plates in 2 ml medium and treated with the indicated
concentrations of EESB and/or IL-6 for 24 h. The total RNA was
isolated with TRIZol reagent. Oligo(dT)-primed RNA (1 µg) was
reverse-transcribed with SuperScript II reverse transcriptase
according to the manufacturer's instructions (Promega Corp.). The
obtained cDNA was used to determine the mRNA expression level of
cyclin D1, (forward, 5-TGG ATG CTG GAG GTC TGC GAG GAA-3 and
reverse, 5-GGC TTC GAT CTG CTC CTG GCA GGC-3, at 57°C), CDK4
(forward, 5-CAT GTA GAC CAG GAC CTA AGC-3 and reverse, 5-AAC TGG
CGC ATC AGA TCC TAG-3, at 58°C), Bcl-2 (forward, 5-CAG CTG CAC CTG
ACG CCCTT-3 and reverse, 5-GCC TCC GTT ATC CTG GAT CC-3, at 55°C)
and Bax (forward, 5-TGC TTC AGG GTT TCA TCC AGG-3 and reverse,
5-TGG CAA AGT AGA AAA GGG CGA-3, at 55°C) by PCR analysis. GAPDH
(forward, 5-GT CAT CCA TGA CAA CTT TGG-3 and reverse, 5-GA GCT TGA
CAA AGT GGT CGT-3′ at 58°C) was used as a reference gene. The PCR
cycling conditions for all sequences were as follows: 35 Cycles of
denaturation at 95°C for 3 min, annealing for 40 sec, and extension
for 45 sec, and a final extension at 72°C for 10 min. Samples were
analyzed using gel electrophoresis (1.5% agarose). The DNA bands
were examined using a gel documentation system (Gel Doc 2000;
Bio-Rad Laboratories, Hercules, CA, USA).
Western blot analysis
HT-29 cells (2×105/ml) were seeded into
flasks and treated with the indicated concentrations of EESB and/or
IL-6 for 24 h. The treated cells were lysed with mammalian cell
lysis buffer containing different protein inhibitors. The total
protein concentrations were determined via BCA assay. Equal
quantities of protein from each cell lysate were subjected to
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were blocked with 5% nonfat dry milk for 2 h and
incubated with the desired primary antibody directed against
p-STAT3, STAT3, cyclin D1, CDK4, Bcl-2, Bax and β-actin, at
dilutions of 1:1,000, overnight at 4°C. To image the
antibody-detected proteins, the appropriate HRP-conjugated
secondary antibodies with chemiluminescence detection were
used.
Statistical analysis
All data are the means of three determinations and
were analyzed using the SPSS package for Windows (version 17.0;
SPSS, Inc., Chicago, IL, USA). Statistical analysis of the data was
performed with a Student's t-test and analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
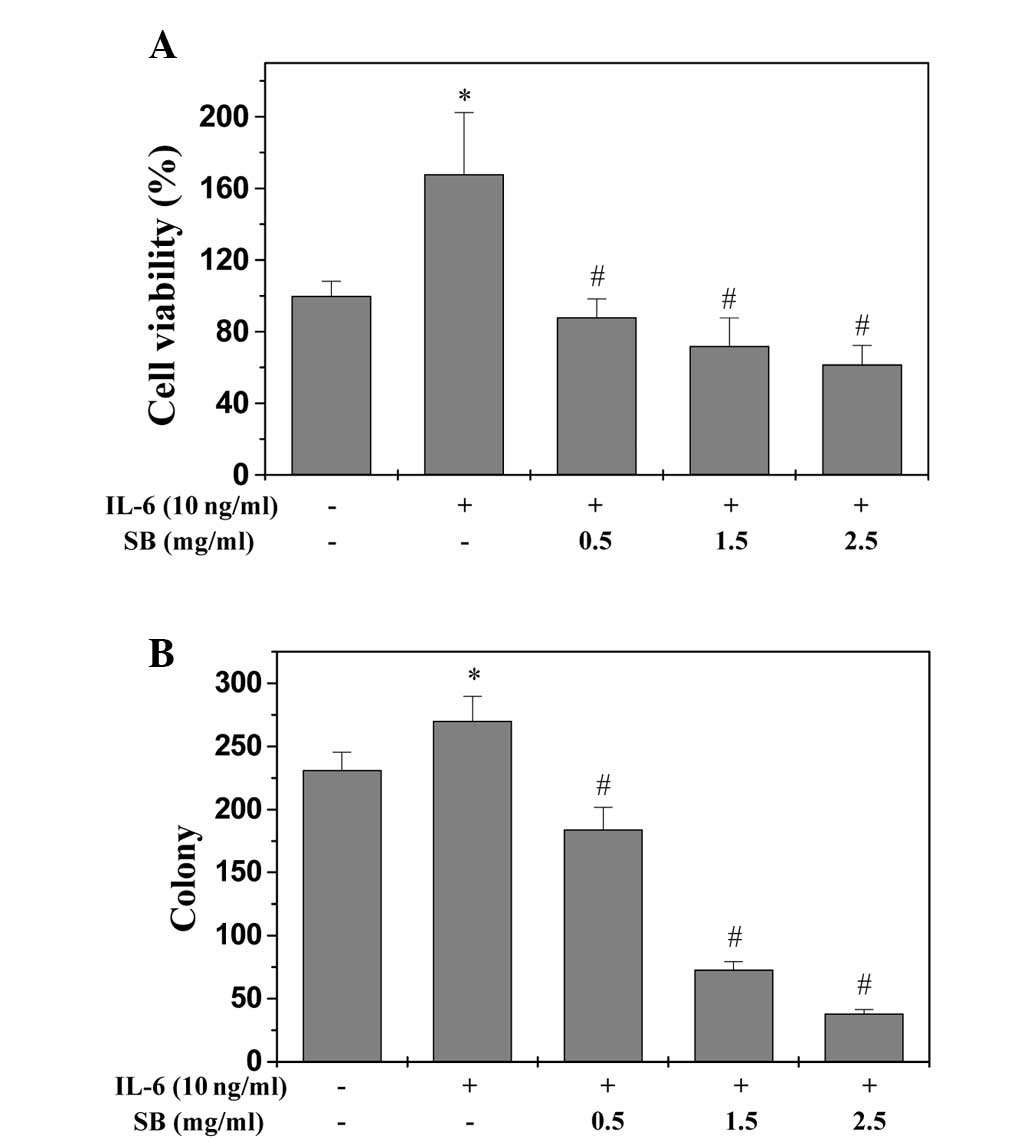
EESB inhibits the growth of HT-29
cells
An MTT assay was used to determine the effect of
EESB on HT-29 cell viability in the presence of IL-6. As shown in
Fig. 1A, IL-6 stimulation increased
the viability of HT-29 cells to 167.89% compared with the control
cells (P<0.05). Treatment with 0.5 to 2.5 mg/ml EESB for 24 h
reduced the cell viability of the IL-6-stimulated cells from 88.06
to 61.72% (P<0.05). Furthermore, the effect of EESB on HT-29
cell survival was examined using a colony formation assay. As shown
in Fig. 1B, treatment with 0.5, 1.5
and 2.5 mg/ml EESB for 24 h could reduce the survival rate of
IL-6-stimulated cells by 20.35, 68.40 and 83.55% (P<0.05),
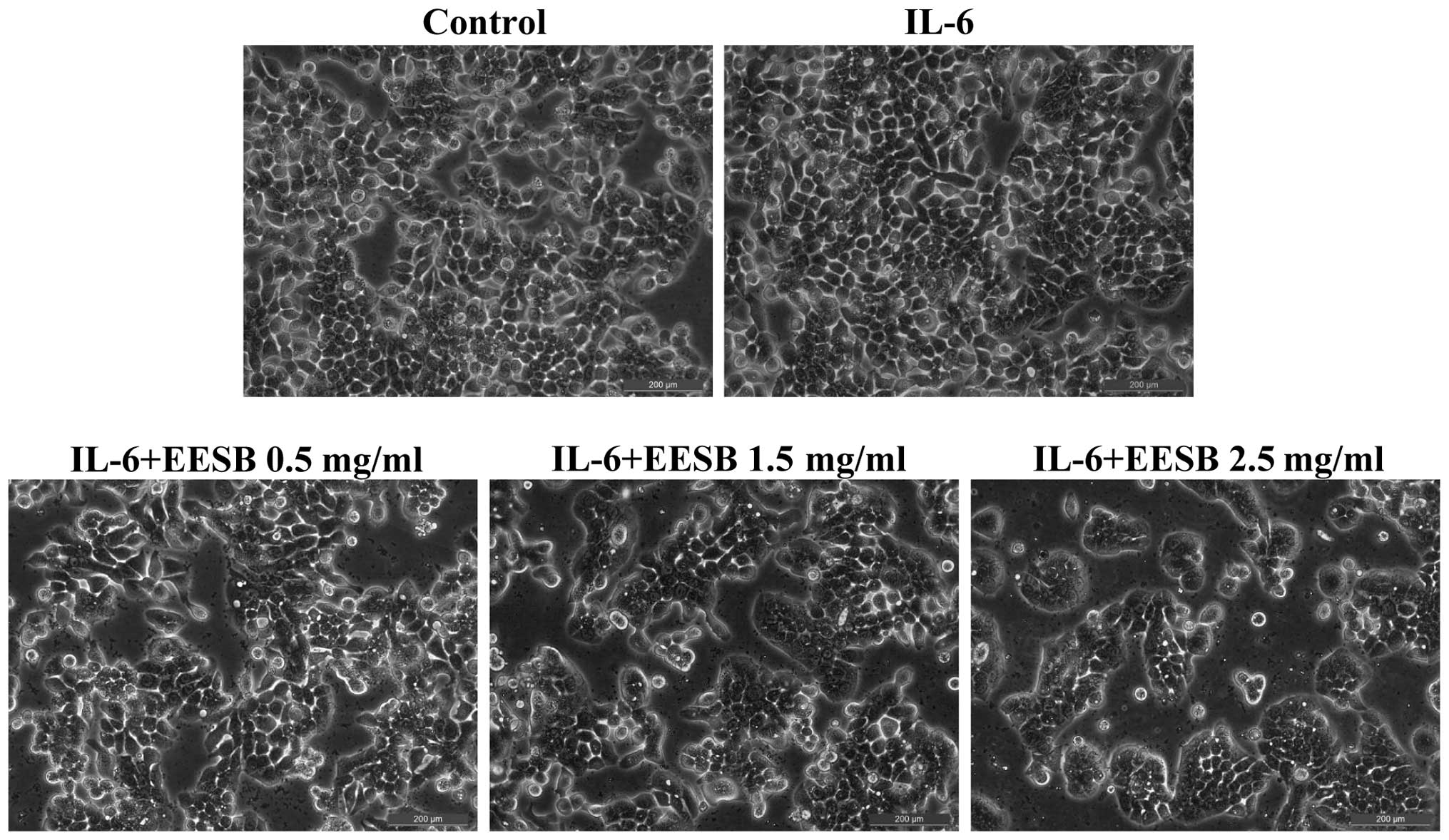
respectively. To further verify these results, the effect of EESB
on HT-29 cell morphology was evaluated via phase-contrast
microscopy, since the morphology of cells in culture is indicative
of the healthy status of the cells. As shown in Fig. 2, it was found that EESB treatment
dose-dependently reduced the density of the HT-29 cells. Taken
together, these data demonstrate that EESB inhibits the growth of
IL-6-stimulated HT-29 cells.
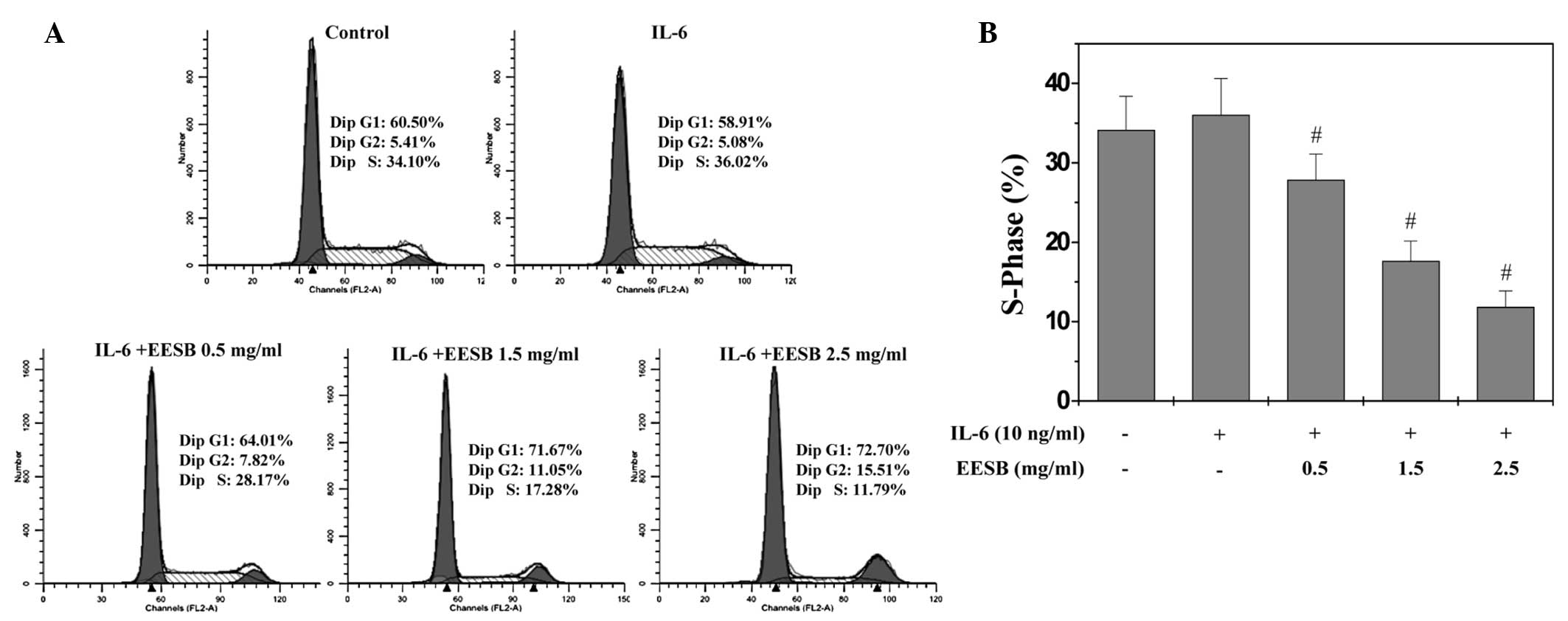
EESB blocks G1/S progression of HT-29
cells
G1/S transition is one of the two main checkpoints
used by a cell to regulate the cell cycle progress and thus the
proliferation of the cell. The effect of EESB on the G1 to S
progression in HT-29 cells was therefore investigated via PI
staining, followed by fluorescence-activated cell sorting analysis.
As shown in Fig. 3, the percentage
proportion of S-phase cells was 34.10% for the untreated control;
36.02% for the IL-6-stimulated HT-29 cells; and 28.17, 17.28 and
11.79% for the IL-6-stimulated HT-29 cells treated with various
EESB concentrations (0.5, 1.5 and 2.5 mg/ml, respectively)
(P<0.05). These results indicate that EESB inhibits the
proliferation of HT-29 cells by blocking the G1- to S-phase
progression of the cell cycle.
EESB induces apoptosis in HT-29
cells
To determine whether EESB could induce cell
apoptosis, Annexin V/PI staining with fluorescence-activated cell
sorting analysis was used to examine the apoptosis of the HT-29
cells. As shown in Fig. 4A and B,
the apoptosis of the HT-29 cells with stimulation of 10 ng/ml IL-6
was not significantly decreased compared with that of the control
cells, which were not stimulated with IL-6 or EESB treatment
(P>0.05); however, EESB treatment increased the percentage of
cells undergoing apoptosis in a dose-dependent fashion (P<0.05,
as compared with the cells stimulated with IL-6 but not treated
with EESB). In the FACS diagram, early apoptosis is shown in the
lower-right quadrant and late apoptosis in the upper right.
 | Figure 4.Effect of EESB on HT-29 cell
apoptosis. Cells were pretreated with various concentration of EESB
for 1 h, followed by stimulation with 10 ng/ml IL-6 for 24 h. (A)
Cells were collected and stained with Annexin V/PI, followed by
fluorescence-activated cell sorting analysis. Double-negative
stained cells indicate the live cell population; Annexin
V-positive/PI-negative stained cells and Annexin V/PI
double-positive stained cells represent early and late apoptosis,
respectively; Annexin V-negative and PI-positive stained cells show
dead cells. (B) Quantification of fluorescence-activated cell
sorting analysis. The data shown are averages with standard
deviation from 3 independent experiments. #P<0.05 vs.
cells treated with IL-6 but not EESB. EESB, ethanol extract of
Scutellaria barbata D. Don; IL-6, interleukin-6; UL, upper
left; UR, upper right; LR, lower right; LL, lower left; PI,
propidium iodide; FITC, fluorescein isothiocyanate. |
EESB induces the activation of
caspase-9 and caspase-3 in HT-29 cells
The activation of caspase-9 and caspase-3 was
examined via colorimetric assay using a specific chromophore.
Caspases, the cytoplasmic aspartate-specific cysteine proteases,
are the key proteins in the apoptotic response. Caspase-9 can
activate caspase-3 so that the specific and vital cellular proteins
are targeted and degraded. Subsequently, the nuclear DNA degrades
and the apoptotic death of the cells occurs; therefore, the
activation of caspases is important to the execution of apoptosis
(14). As shown in Fig. 5, EESB treatment significantly and
dose-dependently induced the activation of both caspase-9 and
caspase-3 in the HT-29 cells (P<0.01, as compared with the cells
stimulated with IL-6 but not treated with EESB). Additionally, IL-6
significantly inhibited the activation of caspase-9 and
caspase-3.
EESB inhibits IL-6-mediated STAT3
activation in HT-29 cells
STAT3 activation was induced in the HT-29 cells via
IL-6, as demonstrated in our previous report (18). Western blotting was performed to
determine the phosphorylation level for STAT3 (p-STAT3) at Tyr705.
As shown in Fig. 6, stimulation with
IL-6 (10 ng/ml) significantly increased the level of p-STAT3, which
was markedly inhibited by EESB in a dose-dependent manner. The
level of non-phosphorylated STAT3 remained unchanged following
treatment with IL-6 and/or EESB (Fig.
6).
EESB downregulates the expression of
cyclin D1, CDK4, Bcl-2 and Bax in HT-29 cells
To further investigate the underlying mechanism of
EESB in HT-29 cells, RT-PCR and western blot analyses were
performed in order to examine the effect of EESB on the expression
of the pro-proliferative cyclin D1 and CDK4, anti-apoptotic Bcl-2
and pro-apoptotic Bax, which are important target genes of the
STAT3 signaling pathway. The results show that the mRNA and protein
expression of cyclin D1, CDK4 and Bcl-2 was increased by IL-6
stimulation (Fig. 7); however, EESB
treatment markedly inhibited the IL-6-induced upregulation of these
genes at both the transcriptional and translational levels. The Bax
mRNA and protein expression was decreased in the presence of IL-6
and increased in IL-6-stimulated HT-29 cells treated with various
concentrations of EESB.
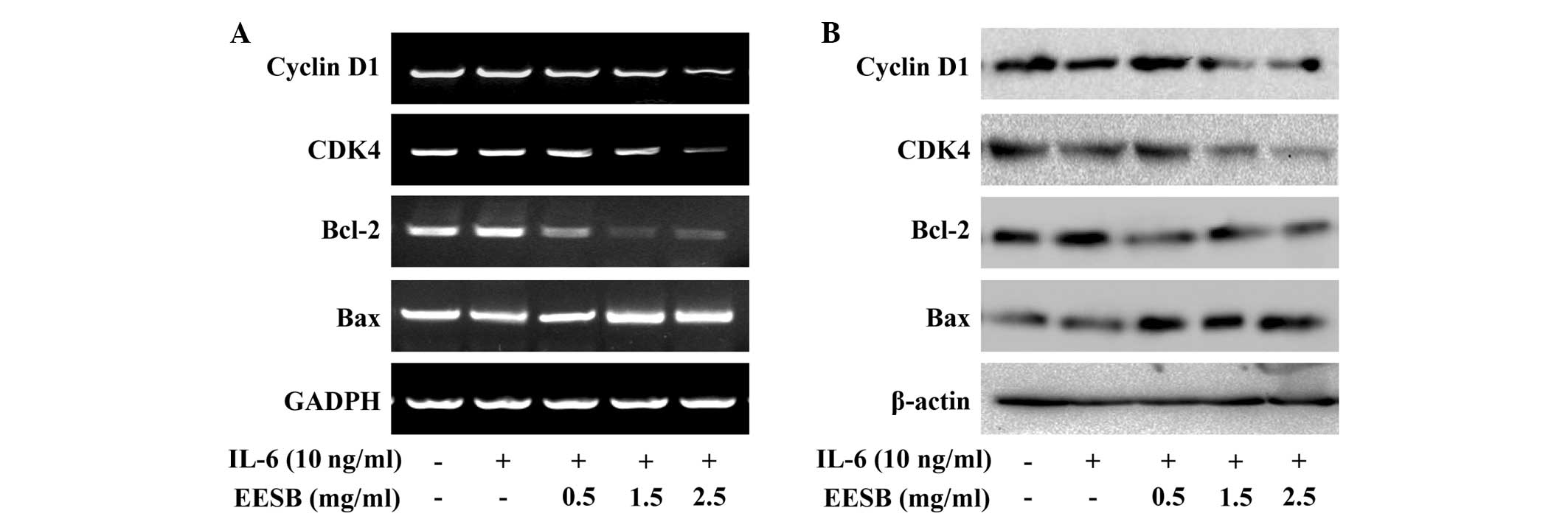 | Figure 7.Effect of EESB on the expression of
cyclin D1, CDK4, Bcl-2 and Bax. Cells were pretreated with various
EESB concentrations for 1 h, followed by stimulation with 10 ng/ml
IL-6 for 24 h. (A) mRNA levels of cyclin D1, CDK4, Bcl-2 and Bax
were determined using RT-PCR. (B) Protein expression levels of
cyclin D1, CDK4, Bcl-2 and Bax were determined using western blot
analysis. GAPDH and β-actin were used as the internal controls for
the RT-PCR and western blot assay, respectively. EESB. EESB,
ethanol extract of Scutellaria barbata D. Don; IL-6,
interleukin-6; Bcl-2, B-cell lymphoma-2; Bax, Bcl2-associated X
protein; CDK4, cyclin-dependent kinase 4; RT-PCR, reverse
transcription polymerase chain reaction. |
Discussion
CRC is the second and third most commonly diagnosed
malignancy in women and men, respectively, with 1.2 million
individual diagnoses and >600,000 mortalities worldwide each
year. Of these patients, 40–50% are likely to relapse or succumb
despite the use of adjuvant chemotherapy (1,19). CRC
is a complex and heterogeneous condition that is associated with
several cellular signal transduction pathways (20–23). As
a result of this, the use of specific inhibitors that only target a
single pathway in complex tumor systems may prove ineffective.
Furthermore, the long-term administration of numerous single-target
agents can lead to the development of drug resistance and unwanted
side effects (24). SB is a major
constituent of numerous traditional medicinal formulas in China
that are used to treat a range of types of cancer, including CRC.
Several traditional herbal medicines are composed of multiple
natural compounds, including SB. Herbal medicines are thus
considered to be multi-component and multi-target agents that exert
their therapeutic functions in a more holistic manner (25).
Numerous studies have shown that STAT3 is
constitutively activated in a diverse range of human tumors
(15,18). Furthermore, it has been suggested
that aberrant STAT3 signaling can promote the initiation and
progression of human cancer through either the inhibition of
apoptosis or the induction of cell proliferation, angiogenesis,
invasion and metastasis. The suppression of STAT3 activation is
associated with the induction of tumor cell apoptosis (26). IL-6/STAT3 signaling regulates the
survival and proliferation of intestinal epithelial cells and plays
an important role in the pathogenesis of inflammatory bowel disease
and CRC. The activation of the intracellular JAK/STAT3 signaling
pathway, triggered by IL-6, leads to the induction of genes
involved in the development of CRC (27). Cell proliferation is governed by the
cell cycle, an order of events that is tightly regulated by a
number of serine/threonine protein kinases known as CDKs and
cyclins, such as CDK4 and cyclin D1 (28). Furthermore, Bax, Bcl-2 and the
activation of caspase-3 and caspase-9 are involved in the apoptosis
of CRC cells (29).
In the present study, HT-29 human colon carcinoma
cells were stimulated with IL-6, and the rapid activation of STAT3
was observed. This led to a significant increase in the
phosphorylation level and transcriptional activity of STAT3. The
IL-6-mediated STAT3 activation was found to be markedly and
dose-dependently inhibited by EESB treatment. Furthermore, EESB
treatment significantly inhibited the mRNA and protein expression
of Bcl-2, cyclin D1 and CDK4. The EESB-induced inhibition of the
IL-6-mediated STAT3 activation and of cyclin D1, CDK4 and Bcl-2
expression led to the suppression of HT-29 cell proliferation and
the induction of cell apoptosis. Furthermore, EESB treatment
increased the expression of Bax.
In conclusion, the results of the present study have
shown that EESB can effectively inhibit the proliferation and
promote the apoptosis of human colon carcinoma cells by modulating
the IL-6/STAT3 signaling pathway and its target genes. Further
studies should be performed in order to fully elucidate the
molecular mechanism underlying the action of EESB in cancer
treatment and to enable the development of more effective
multi-target drugs for cancer therapy.
Acknowledgements
This study was sponsored by the Developmental Fund
of Chen Keji Integrative Medicine (grant nos. CKJ2014013 and
CKJ2015007), the Natural Science Foundation of Fujian Province of
China (grant no. 2013J01333) and the Research Foundation of Fujian
University of Traditional Chinese Medicine (grant no.
X2012012).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong B, Liu Q and Liu Y, Xiong X and Liu
Y: Expressions of STAT3, p-STAT3 and E-cadherin in colorectal
cancer and clinical implications. Zhonghua Wei Chang Wai Ke Za Zhi.
17:594–597. 2014.(In Chinese). PubMed/NCBI
|
|
4
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann NY Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin Q, Lai R, Chirieac LR, Li C, Thomazy
VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K,
et al: Constitutive activation of JAK3/STAT3 in colon carcinoma
tumors and cell lines: Inhibition of JAK3/STAT3 signaling induces
apoptosis and cell cycle arrest of colon carcinoma cells. Am J
Pathol. 167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nielsen DL, Palshof JA, Larsen FO, Jensen
BV and Pfeiffer P: A systematic review of salvage therapy to
patients with metastatic colorectal cancer previously treated with
fluorouracil, oxaliplatin and irinotecan +/- targeted therapy.
Cancer Treat Rev. 40:701–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Wu X, Tan M, Gong J, Tan W, Bian
B, Chen M and Wang Y: Fighting fire with fire: Poisonous Chinese
herbal medicine for cancer therapy. J Ethnopharmacol. 140:33–45.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo- or radio-therapy for cancer. Biosci Trends.
4:297–307. 2010.PubMed/NCBI
|
|
13
|
Cheng HM, Li CC, Chen CY, Lo HY, Cheng WY,
Lee CH, Yang SZ, Wu SL, Hsiang CY and Ho TY: Application of
bioactivity database of Chinese herbal medicine on the therapeutic
prediction, drug development, and safety evaluation. J
Ethnopharmacol. 132:429–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei L, Chen Y, Lin J, Zhao J, Chen X, Xu
W, Liu X, Sferra TJ and Peng J: Scutellaria barbata D. Don
induces apoptosis of human colon carcinoma cell through activation
of the mitochondrion-dependent pathway. J Med Plant Res.
5:1962–1970. 2011.
|
|
15
|
Lin J, Chen Y, Cai Q, Wei L, Zhan Y, Shen
A, Sferra TJ and Peng J: Scutellaria barbata D. Don inhibits
colorectal cancer growth via suppression of multiple signaling
pathways. Integr Cancer Ther. 13:240–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei L, Lin J, Wu G, Xu W, Li H, Hong Z and
Peng J: Scutellaria barbata D. Don induces G1/S arrest via
modulation of p53 and Akt pathways in human colon carcinoma cells.
Oncol Rep. 29:1623–1628. 2013.PubMed/NCBI
|
|
17
|
Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z
and Peng J: Scutellaria barbata D. Don inhibits tumor
angiogenesis via suppression of Hedgehog pathway in a mouse model
of colorectal cancer. Int J Mol Sci. 13:9419–9430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen A, Chen Y, Hong F, Lin J, Wei L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang suppresses IL-6-inducible
STAT3 activation in human colon carcinoma cells through induction
of SOCS3. Oncol Rep. 28:2125–2130. 2012.PubMed/NCBI
|
|
19
|
Scurr M, Ladell K, Besneux M, Christian A,
Hockey T, Smart K, Bridgeman H, Hargest R, Phillips S, Davies M, et
al: Highly prevalent colorectal cancer-infiltrating LAP+
Foxp3− T cells exhibit more potent immunosuppressive
activity than Foxp3+ regulatory T cells. Mucosal
Immunol. 7:428–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Jong PR, Mo JH, Harris AR, Lee J and
Raz E: STAT3: An anti-invasive factor in colorectal cancer. Cancers
(Basel). 6:1394–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sabatino L, Pancione M, Votino C,
Colangelo T, Lupo A, Novellino E, Lavecchia A and Colantuoni V:
Emerging role of the β-catenin-PPARγ axis in the pathogenesis of
colorectal cancer. World J Gastroenterol. 20:7137–7151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vaiopoulos AG, Athanasoula KC and
Papavassiliou AG: NF-κB in colorectal cancer. J Mol Med Berl.
91:1029–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boos G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L and Leung PS: Use of herbal medicines
and natural products: An alternative approach to overcoming the
apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol.
53:224–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
27
|
Yang X, Zhang F, Wang Y, Cai M, Wang Q,
Guo Q, Li Z and Hu R: Oroxylin A inhibits colitis-associated
carcinogenesis through modulating the IL-6/STAT3 signaling pathway.
Inflamm Bowel Dis. 19:1990–2000. 2013.PubMed/NCBI
|
|
28
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis diffusa Willd extracts
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|