Introduction
Occurring in ~90% of patients during their illness
(1), pain is one of the most common
symptoms associated with cancer. Cancer pain is a severe clinical
problem that oncologists continue to encounter, for which the most
common current treatment is analgesics, which are often
insufficient or addictive. The neurotransmitter system in the brain
is an effective regulator of pain perception (2), and the majority of previous studies
associated with pain transmission and modulation have focused on
the dorsal horn of the spinal cord (3,4). Other
studies have demonstrated that neurons in the forebrain, including
neurons in the rostral anterior cingulate cortex (rACC), are
associated with chronic pain and pain-related perception (5,6). In a
mouse model of neuropathic pain, a previous study demonstrated that
peripheral nerve injury may trigger both long-term presynaptic and
postsynaptic enhancement of excitatory synaptic transmission in
layer II/III neurons within the rACC (7). Furthermore, long-term increases in the
synaptic insertion of glutamate receptor (GluR)1 subunits in rACC
neurons may be induced by peripheral inflammation, which
subsequently increases synaptic transmission during chronic pain
(8). These results suggest that
long-term changes in the plasticity of rACC neurons may have
important roles in the development and maintenance of bone
cancer-associated pain.
One major feature of long-term plasticity changes in
rACC neurons is that it requires the activation of N-methyl
D-aspartate (NMDA) receptors. Previous studies have demonstrated
that chronic pain is selectively enhanced in mice overexpressing
GluN2B-containing NMDA receptors (GluN2B) (9,10),
suggesting that forebrain GluN2B receptors are critical for the
development of chronic pain. Following persistent inflammation, the
GluN2B component in NMDA receptor-mediated excitatory postsynaptic
currents (EPSCs) was increased due to the increased expression of
GluN2B receptors in the rACC (11).
Furthermore, the behavioral responses induced by peripheral
inflammation during a mechanical allodynia test were inhibited by
microinjection into the rACC or systemic administration of GluN2B
receptor with selective antagonists (11). These results have been corroborated
by genetic studies, in which overexpression of GluN2B in the
forebrain of mice selectively enhanced inflammation-related
persistent pain, without significantly altering acute pain
(12). The antiallodynic effects of
GluN2B receptor antagonists have also been demonstrated in other
animal models of chronic pain (11).
Collectively, these findings suggest that GluN2B receptors within
rACC neurons are involved in the development and maintenance of
bone cancer-associated pain.
RNA interference (RNAi) is a powerful tool that
induces loss-of-function phenotypes by silencing
post-transcriptional gene expression (13,14).
Long-term knockdown of gene expression has previously been achieved
using lentiviral vector constructs that express short hairpin
(sh)RNAs in vector-infected cells, including non-dividing cells
(15,16). These studies suggest that
rACC-specific reductions in GluN2B receptor expression levels may
ameliorate debilitating pain, and thus may contribute to the
treatment of bone cancer pain. However, to the best of our
knowledge, no evidence for relieving the debilitating pain of bone
cancer by selectively decreasing GluN2B expression levels in the
rACC has yet been reported. In the present study, a rat model of
bone cancer-associated pain was used to investigate whether
intra-rACC administration of lentiviral-mediated small interfering
RNAs targeting GluN2B (LV-GluN2B) successfully decreased the
expression levels of GluN2B receptors in the rACC, and if this
could relieve debilitating pain. The results demonstrated that
rACC-specific reductions in GluN2B receptor expression levels
successfully attenuated the debilitating pain associated with bone
cancer.
Materials and methods
GluN2B siRNA sequence design and
construction of the siRNA-expressing lentiviral vector
siRNA sequences targeting the GluN2B gene (GenBank:
NM_012574) were designed and synthesized as follows by GeneChem Co.
(Shanghai, China): Sense, 5′-TCAGAGAGGAATATCCGTAATA-3′ and
antisense, 5′-TATTACGGATATTCCTCTCTGTTTTTTC-3; and the negative
control (NC) siRNA sequences were as follows: Sense,
5′-TCGAGAAAAAACAGAGAGGAATATCCGTAATA-3′ and antisense,
5′-TATTACGGATATTCCTCTCTGA-3. The sequences were subsequently cloned
into respective pFU-GW-iRNA expression vectors (GeneChem Co.),
containing a green fluorescence protein (GFP) reporter gene and an
ampicillin resistance gene.
The lentiviruses were generated as previously
described (17) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA),
a recombinant work vector and package plasmids, which were
co-transfected into 293T cells (Shanghai Bioleaf Biotech Co., Ltd.,
Shanghai, China). The culture medium (Dulbecco's modified Eagle's
medium; Biowest, Nuaillé, France) was collected over 48 h,
concentrated, aliquoted, and subsequently stored at −80°C until
required. The lentiviral titer was determined by the number of
cells expressing GFP multiplied by the corresponding dilutions,
using a hole-by-dilution titer assay (18). The final titer of the recombinant
virus was 1×109 transducing units/ml. The efficiency of
LV-GluN2B in decreasing GluN2B expression levels was examined in
vitro by transfecting neurons (data not shown).
Experimental animals
Male Sprague Dawley rats, weighing 220–250 g, were
provided by the Experimental Animal Center of Shandong University
(Jinan, China) and were maintained in the following identical
conditions: A controlled temperature of 22±1°C, a 12 h light/dark
cycle and ad libitum access to food and water. The rats were
randomly divided into three groups (n=7/group): Normal saline (NS)
group, LV-GluN2B group and LV-NC group. The NS group received no
treatment. The LV-GluN2B and LV-NC groups were models of bone
cancer pain, the LV-NC group were treated with LV-NC, and the
LV-GluN2B group were treated with LV-GluN2B. All experiments were
approved by the Committee for Animal Experimentation at Shandong
University (Jinan, China) and they complied with the ethical
guidelines of the International Association for the Study of
Pain.
Cell culture and implantation
Osteosarcoma NCTC 2472 cells, purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA) were
incubated and subcultured in NCTC 135 medium (Sigma-Aldrich, St.
Louis, MO, USA) supplemented with 10% horse serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air using a
Forma™ incubator (Thermo Fisher Scientific). The cells
were passaged twice a week according to ATCC recommendations. A rat
model of bone cancer-associated pain was generated as previously
described by Schwei et al (19). At day 0, the rats were anesthetized
via intraperitoneal injection of 40–50 mg/kg pentobarbital sodium
[1% in NS], and a superficial incision was made in the right leg in
order to expose the tibial plateau. Following this, a 27-gauge
needle was used to drill a hole into the medullary cavity, and 10
µl phosphate-buffered saline (PBS) supplemented with
2×106 osteosarcoma NCTC 2472 cells was subsequently
injected using a 29-gauge needle coupled to a Hamilton syringe. The
injection site was closed with tissue glue, thoroughly irrigated
with sterile saline in order to prevent the leakage of cells
outside the bone, and the wound was closed with skin sutures.
Sham-operated controls underwent the same surgical procedure, with
the exception that PBS alone was injected into the medullary
cavity.
Intra-rACC catheter implantation and
administration of viral stocks
The rats were anesthetized via intraperitoneal
injection of 40–50 mg/kg pentobarbital sodium, and were securely
placed into a stereotaxic device with the bregma and lambda at a
horizontal level. According to the atlas outlined by Paxinos et
al (20), a 30-gauge stainless
steel cannula with a 26-gauge stainless steel stylet plug was
bilaterally implanted 0.5 mm above the rACC injection site
(anteroposterior, 2.6; mediolateral, 0.6; and dorsoventral, 2.5
from bregma). The rats recovered and gained weight for 7 days prior
to the initiation of the experiments, and three rats presenting
with neurological deficits following the surgical procedure were
excluded from the study.
Lentiviral stocks were dissolved in NS and over a
3-min period, a total volume of 0.6 µl per hemisphere of NS
(vehicle), or lentiviral stocks (1×108 PFU) was infused
into the rats. The injection syringe was left in place for an
additional 1 min in order to minimize the spread of the drug along
the injection track. At the end of the behavioral test, the rats
received a 0.6 µl infusion of 4% methylene blue in order to verify
the location of the injection site and the extent of the
infusion.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
A total of 7 days after vector administration, three
different brain regions from each rat: The rACC, the caudal
anterior cingulate cortex (cACC), and the ventromedial prefrontal
cortex (vmPFC), were harvested using a blunt-end 17-gauge syringe
needle (inner diameter, 1 mm), according to the atlas outlined by
Paxinos et al (20), and
snap-frozen in liquid nitrogen until all remaining dissections were
collected. Total RNA (1 µg) was isolated from the homogenized
tissues using TRIzol® (Invitrogen; Thermo Fisher Scientific Inc.),
and cDNA was subsequently synthesized using a PrimeScript RT
Reagent kit (Takara Bio, Inc.). RT-qPCR was performed on 100 ng/ml
cDNA using a LightCycler® 480 system (Roche Diagnostics, Basel,
Switzerland) using a SYBR® Premix Ex Taq™ (Tli
RNaseH Plus; Toyobo Co., Ltd., Osaka, Japan) under the following
cycle conditions: 95°C for 30 sec, 40 cycles of 95°C for 5 sec and
60°C for 30 sec, followed by melting at 95°C for 5 sec and 60°C for
60 sec, prior to cooling at 50°C for 30 sec. The oligonucleotide
primers for GluN2B were designed using the NCBI GenBank (NM_012574)
as follows: Forward, 5′-TGGCTATCCTGCAGCTGTTTG-3′ and reverse,
5′-TGGCTGCTCATCACCTCATTC-3′ (Takara Bio, Inc.). β-actin served as a
control for normalization, and the primers were as follows:
Forward, 5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. The relative mRNA expression levels
were calculated using the 2−∆∆Cq method (21).
Western blot analysis
Once defined survival times were achieved, the rats
were sacrificed via an overdose of 50 mg/kg pentobarbital, and
their ACC tissues were subsequently harvested and maintained at
80°C. The frozen tissue was homogenized in a lysis solution (10
volumes PBS containing 1 mM Mg2+, 0.5 mM
Ca2+, 1% Triton X-100, 1 mM phenylmethylsulfonyl
fluoride). Lysates were centrifuged at 8,000 × g for 5 min at 4°C.
Protein concentration was determined using the Bradford method
(Shanghai Yapei Biotechnology Co., Ltd., Shanghai, China). The
extract samples (5.0 mg/ml, 10 µl) were separated by 12% SDS-PAGE,
and electroblotted onto polyvinylidene difluoride membranes
(Shanghai Resun Biotechnology Co., Ltd., Shanghai, China) using a
transfer system (Tanon Science and Technology Co., Ltd., Shanghai,
China). Following this, the membranes were blocked with 5% milk in
PBS supplemented with 0.1% Tween-20 for 1 h at room temperature and
subsequently incubated overnight at 4°C with the antibodies against
GluN2B (1:400; cat. no. 256) or β-actin (1:1,000; cat. no.
BYK-0061R) (both Shanghai Yua Han Biotechnology Co., Ltd.,
Shanghai, China), respectively. The blots were then washed and
incubated with horseradish peroxidase-conjugated donkey anti-rabbit
immunoglobulin G (IgG; 1:1,500; Pierce Biotechnology, Inc.,
Rockford, IL, USA) for 2 h at 4°C, prior to visualization using
enhanced chemiluminescence detection reagent (cat. no. 34096;
Pierce Biotechnology Inc.). Blots were quantified by measuring and
comparing the optical density of the bands using Quantity One
version 4.6.2 software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
GluN2B immunofluorescence
A total of 7 days after vector administration, the
rats were anaesthetized with 40–50 mg/kg pentobarbital prior to the
perfusion of 100 ml NS through the ascending aorta. The rACC
tissues were immediately removed and snap-frozen in liquid nitrogen
until all the samples were collected. The tissues were subsequently
cut into7 µm sections on a cryostat, and all sections were prefixed
with acetone, blocked with goat serum at 37°C and incubated with
mouse anti-GluN2B antibody (cat. no. ab93610; 1:100; Abcam,
Cambridge, UK) overnight at 4°C. Following this, the tissue
sections were washed and incubated for 2 h in
tetramethylrhodamine-conjugated Affinipure goat anti-mouse IgG
secondary antibody solution (cat. no. SSA004, 1:50, ZSGB-BIO,
Beijing China) in a dark room. The stained sections were scanned
and images were subsequently captured using an inverted fluorescent
microscope (Nikon Corporation, Tokyo, Japan).
Measurements of pain-related
behaviors
Experiment 1: Assessment of pain-related
behaviors in tumor-bearing rats
Rats were randomly placed into three groups: Naive,
sham and tumor-bearing; and eight rats were subsequently selected
at random from each group and tested for pain-related behaviors
during a 2-week period: Pre-operative day 0 and on days 3, 5, 7,
10, 14, 21 and 28 following the operation. Mechanical allodynia was
assessed in the rats according to the method described by Chaplan
et al (22), with minor
modifications. The rats were placed in individual transparent
plexiglass cages on a metal mesh floor and the threshold of
mechanical allodynia was measured using a set of von Frey filaments
applied to the right hind paw of each rat. Each von Frey filament
was applied vertically to the plantar surface with sufficient force
for 6 sec, while the filament was gently bent. Paw flinching or
brisk withdrawal were considered to be positive responses. Using
the ‘up and-down’ method, the lowest von Frey filament that induced
>3 positive responses was recorded as the paw withdrawal
mechanical threshold (PWMT). Thermal hyperalgesia was measured
according to the method described by Hargreaves et al
(23), with the paw withdrawal
thermal latency (PWTL) value recorded in response to radiant heat.
Rats were placed in individual transparent plexiglass cages on a 3
mm-thick-glass floor and a radiant thermal stimulator (BME410A;
Institute of Biological Medicine, Academy of Medical Science,
Huaibei, China) was positioned under the glass floor and
subsequently focused on the plantar surface of the rat's hind paw.
PWTL was defined as the time that elapsed between the onset of the
radiant heat and the characteristic lifting or licking of the hind
paw. A cut-off time of 25 sec was imposed in order to avoid tissue
damage. Each rat completed five trials with 5-min intervals, and
the mean PWTL was obtained from the latter three stimuli.
Experiment 2: Effects of intra-rACC
administration of LV-GluN2B on pain behaviors induced by bone
cancer
Tumor-bearing rats, weighing 220–250 g, were
randomly divided into three groups: Normal saline (NS), lentivirus
negative control (LV-NC), and LV-GluN2B; which were respectively
intra-rACC administered with NS, LV-NC, or LV-GluN2B to a total
volume of 0.6 µl per hemisphere. Pain-related behaviors were
assessed prior to administration, which were regarded as baseline,
and at post-administrative days 0, 3, 7, 10 and 14. The locomotor
activity of the rats was measured using perspex boxes (230×280×210
mm) equipped with two parallel infrared beams, one at each end of
the base of the cage.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Rats were randomly assigned to the various treatment
groups. SPSS 19.0 (IBM SPSS, Armonk, NY, USA) was used to
statistically analyze data. Two-way repeated analysis of variance
(ANOVA) was used to detect differences in body weight, PWMT and
PWTL among the groups; whereas one-way ANOVA with
Student-Newman-Keuls post hoc analysis was used to detect
differences in the numbers of GluN2B-immune-positive cells, and the
mRNA and protein expression levels of GluN2B among the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tumor-bearing rats exhibit increased
mechanical allodynia and thermal hyperalgesia
In order to induce ongoing bone cancer pain
behaviors in the rat model, osteosarcoma cells were inoculated into
the intramedullary space of the right tibia. No significant
differences in body weight gain were demonstrated among the naive,
sham-operated, and tumor-bearing groups (Fig. 1A, P>0.05) in the time period
examined. Tumor-bearing rats demonstrated increased mechanical
allodynia and thermal hyperalgesia from postoperative days 10–28,
as compared with the naive and sham-operated groups. A progressive
decrease of PWMT on the ipsilateral side of the tumor-bearing rats
was demonstrated on postoperative day 10 (9.2±1.4 g), which was
significantly less than the threshold observed in the naive
(11.8±2.1 g) or sham-operated rats (12.1±2.4 g), and this persisted
for the remainder of the study (Fig.
1B). Furthermore, tumor-bearing rats demonstrated a reduction
in PWTL on the ipsilateral side at postoperative day 10, which
gradually decreased along with the development of bone
cancer-associated pain (Fig.
1C).
Specificity and efficiency of GluN2B
deletions within the rACC following intra-rACC administration of
LV-GluN2B
In order to investigate whether LV-GluN2B
selectively decreased the expression levels of GluN2B within the
rACC, intra-rACC administration of lentiviral RNAs was performed in
tumor-bearing rats. Detection of GluN2B mRNA expression levels in
the rats demonstrated that GluN2B knockdown was selectively limited
to the rACC, and was statistically significant (P<0.05).
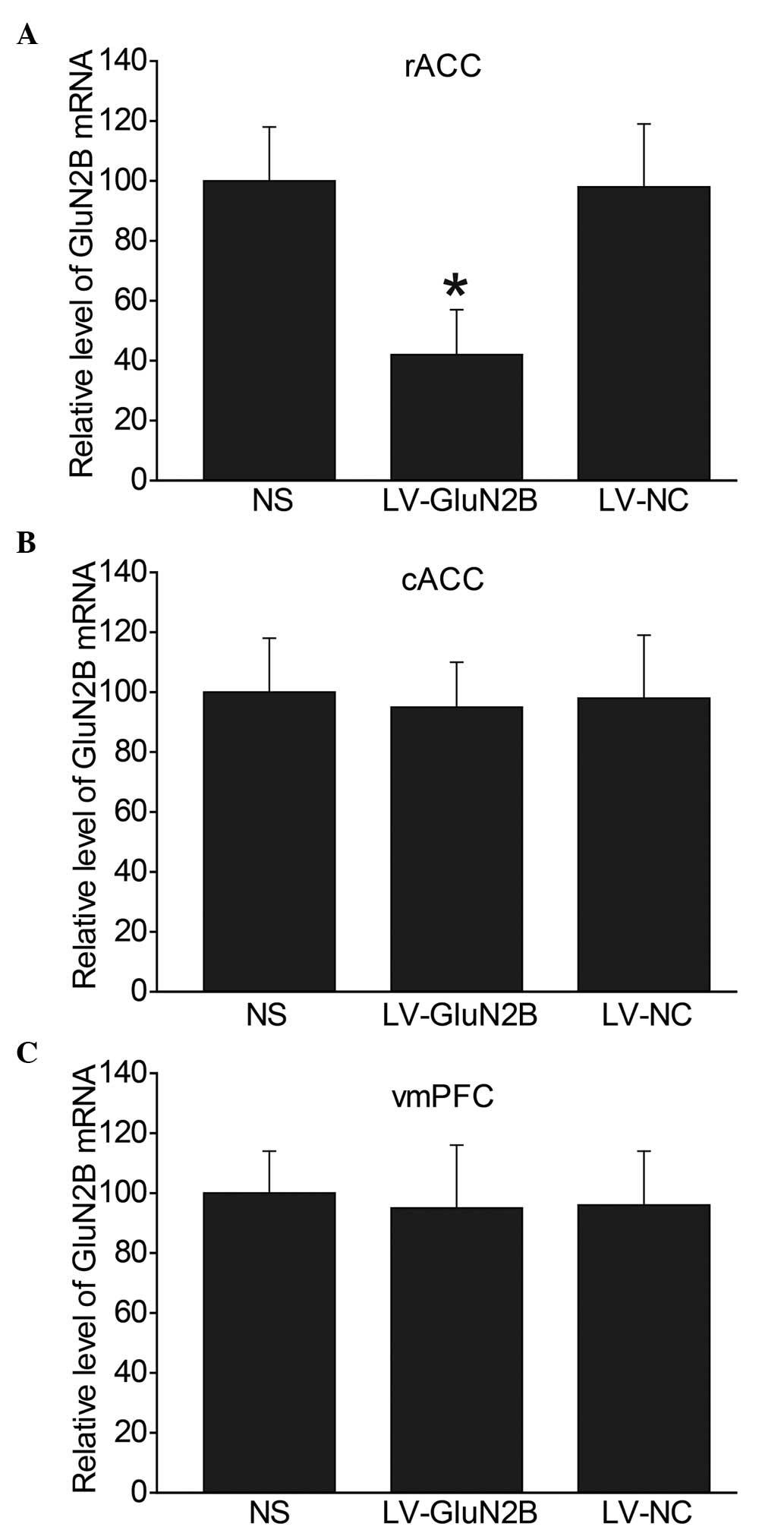
Fig 2 demonstrates the decrease in
GluN2B expression levels within the rACC following LV-GluN2B
infection. In the NS, LV-GluN2B and LV-NC groups, the relative mRNA
expression levels of GluN2B within the rACC were 100, 42 and 98,
respectively (Fig. 2A). Since GluN2B
is also abundantly distributed in other forebrain areas, the mRNA
expression levels of GluN2B in the cACC and vmPFC were also
examined, and no difference in expression was detected (Fig. 2B and C).
Intra-rACC injection of LV-GluN2B
specifically decreases GluN2B protein expression levels in the
rACC
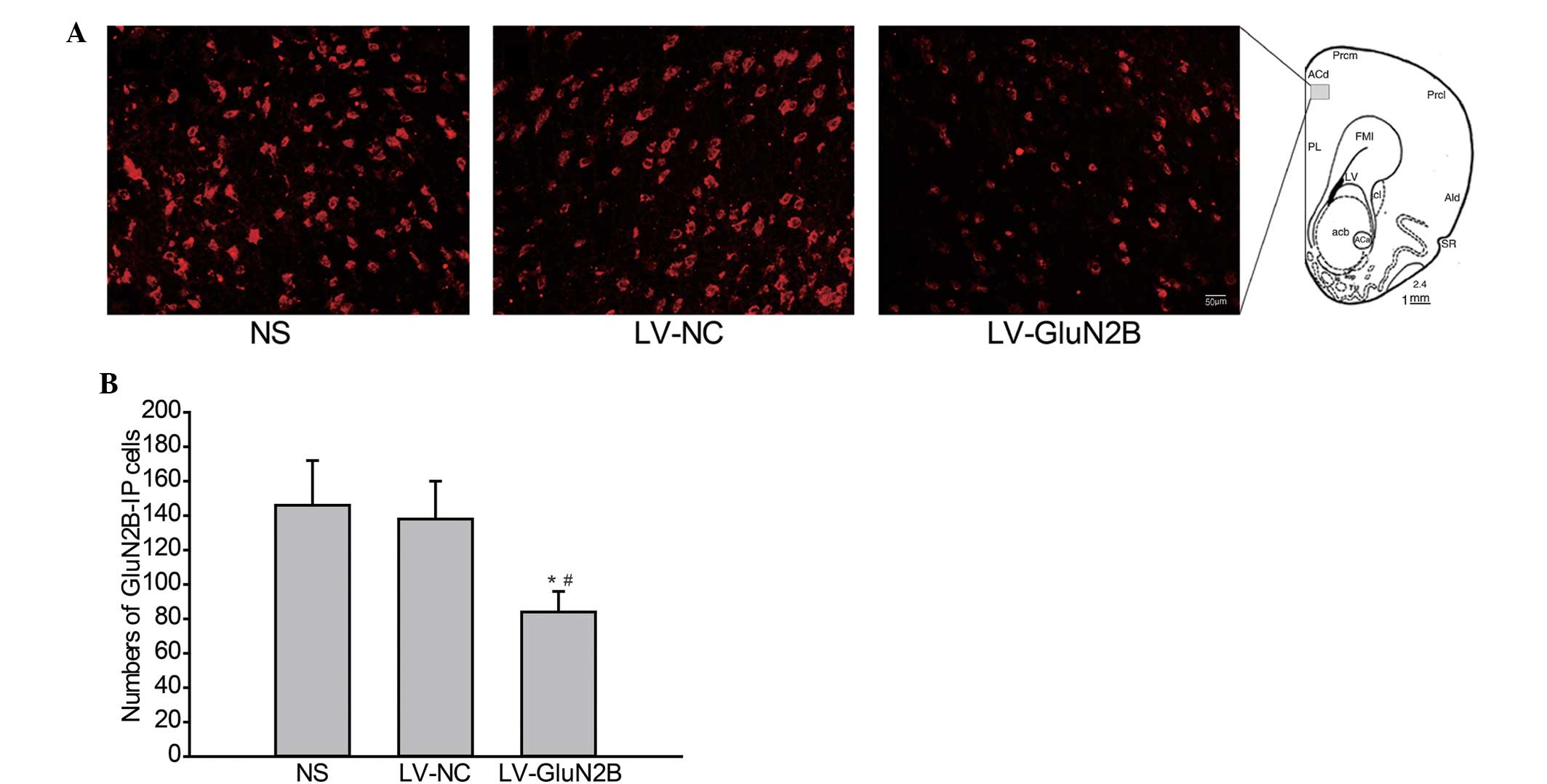
The effects of intra-rACC delivery of LV-GluN2B on
the protein expression levels of GluN2B in rACC neurons were also
analyzed via immunofluorescence staining. As outlined in Fig. 3, following intra-rACC injection of
LV-GluN2B, the expression of GluN2B protein in the rACC neurons
decreased. Furthermore, the number of GluN2B-positive cells in the
rACC were significantly decreased in rats treated with LV-GluN2B,
as compared with that of the NS or LV-GluN2B controls (82±16 vs.
146±22 or 138±20; Fig. 3A and B;
P<0.05).
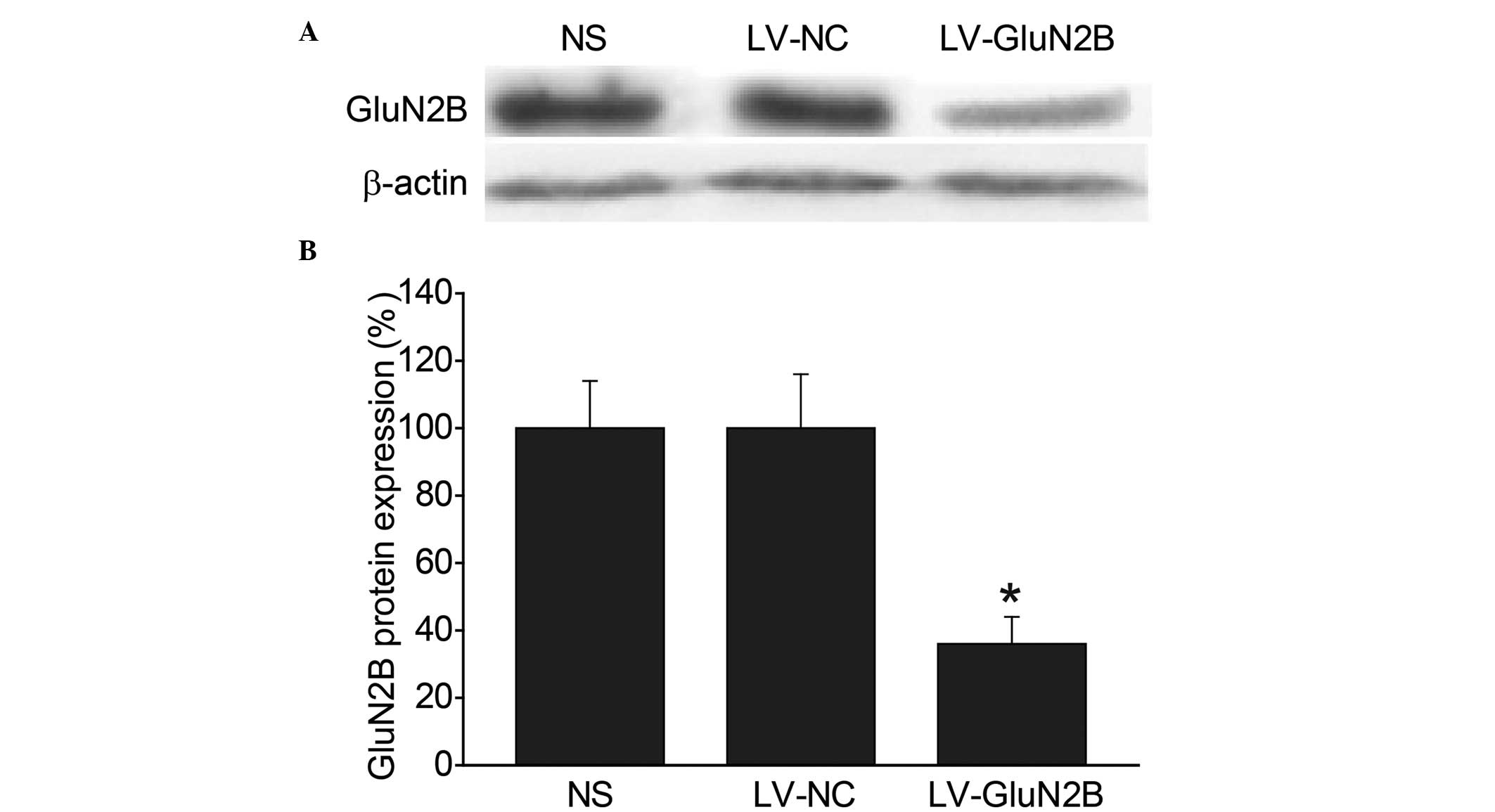
Western blot analysis was subsequently performed in
order to compare the expression levels of the GluN2B subunits in
the rACC among the groups. Consistent with the immunohistochemical
results, intra-rACC injection of LV-GluN2B resulted in a 49.6%
reduction in GluN2B protein expression levels, as compared with the
LV-NC and blank control groups (P<0.05) (Fig. 4A and B).
Intra-rACC injection of LV-GluN2B
reduces tactile allodynia and thermal hyperalgesia in a model of
bone cancer-associated pain, without altering locomotor
activity
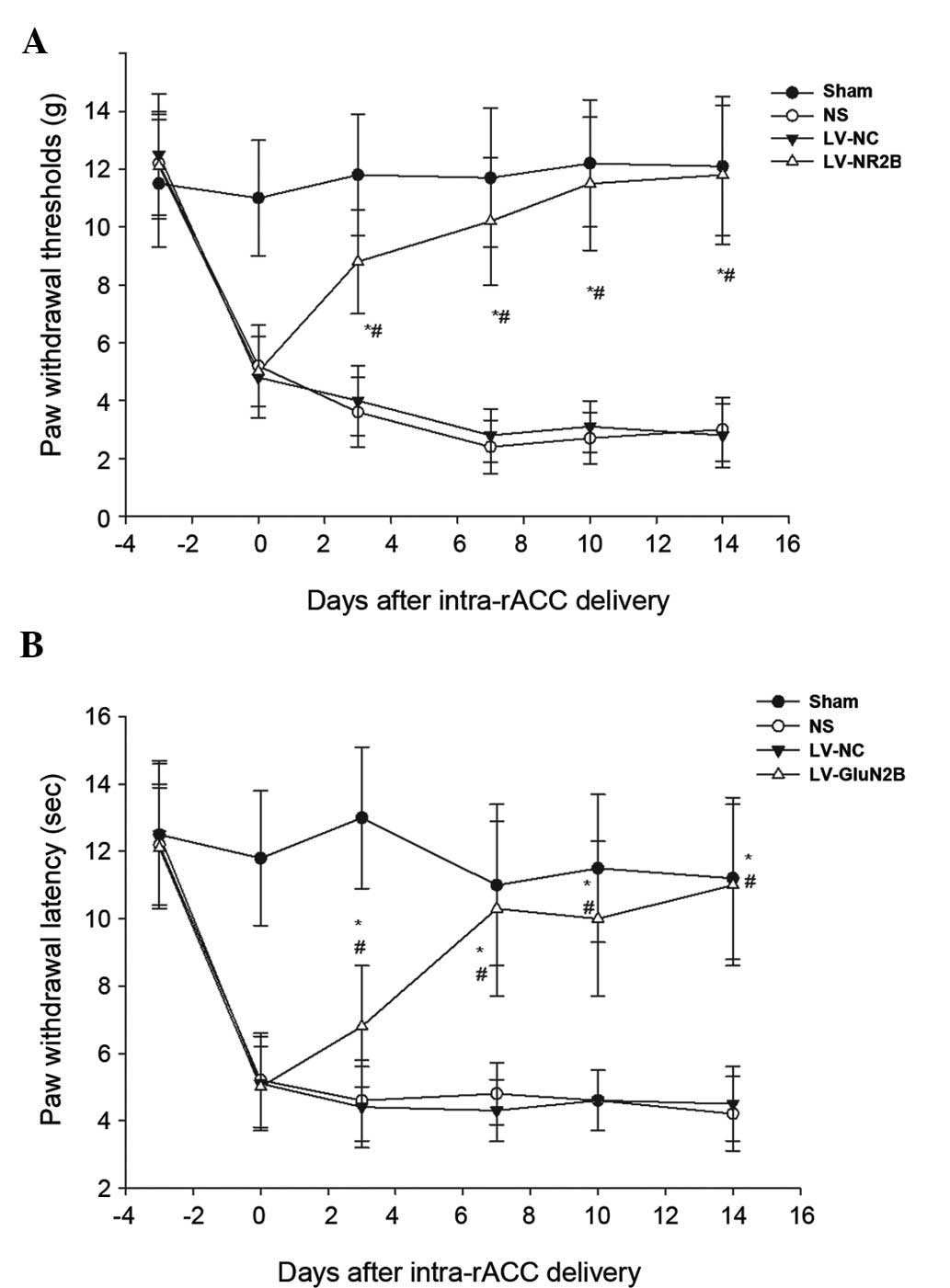
To investigate whether the downregulation of
LV-GluN2B in the rACC could attenuate bone cancer-associated pain,
the pain-related behaviors of the rats were observed for 14 days
following the injection of the lentiviral vectors. The rats
exhibited a normal appearance, level of activity and regular
appetite following intra-rACC injection of the lentiviral vectors.
Furthermore, no significant differences in body weight were
demonstrated among the sham, NS, LV-NC or LV-GluN2B groups.
Notably, all tumor-bearing rats demonstrated increased mechanical
allodynia and thermal hyperalgesia at day 10 following osteosarcoma
inoculation; whereas intra-rACC injection of LV-GluN2B improved
tactile allodynia in the tumor-bearing rats by day 3. Following
administration, the PWMT of the ipsilateral side of the
tumor-bearing rats significantly increased from the baseline
(5.50±1.4 g) to 8.3±2.8 g (P<0.05), and gradually recovered to
10.5±3.2 g by day 7 and baseline by day 14 (Fig. 5A). Similarly, the PWTL of the
tumor-bearing rats recovered to 10.3±2.6 sec by post-administrative
day 7, and had returned to the baseline by day 14 (Fig. 5B). Conversely, intra-rACC
administration of NS or LV-NC had no notable effect on tactile
allodynia or thermal hyperalgesia in the tumor-bearing rats
(Fig. 5).
To examine the effects of intra-rACC injection of
LV-GluN2B on the locomotion of the rats, baseline locomotion was
measured as the total number of beam breaks in 2 h using perspex
boxes (230×280×210 mm) equipped with two parallel infrared beam
arrays. No difference in baseline locomotor activity was determined
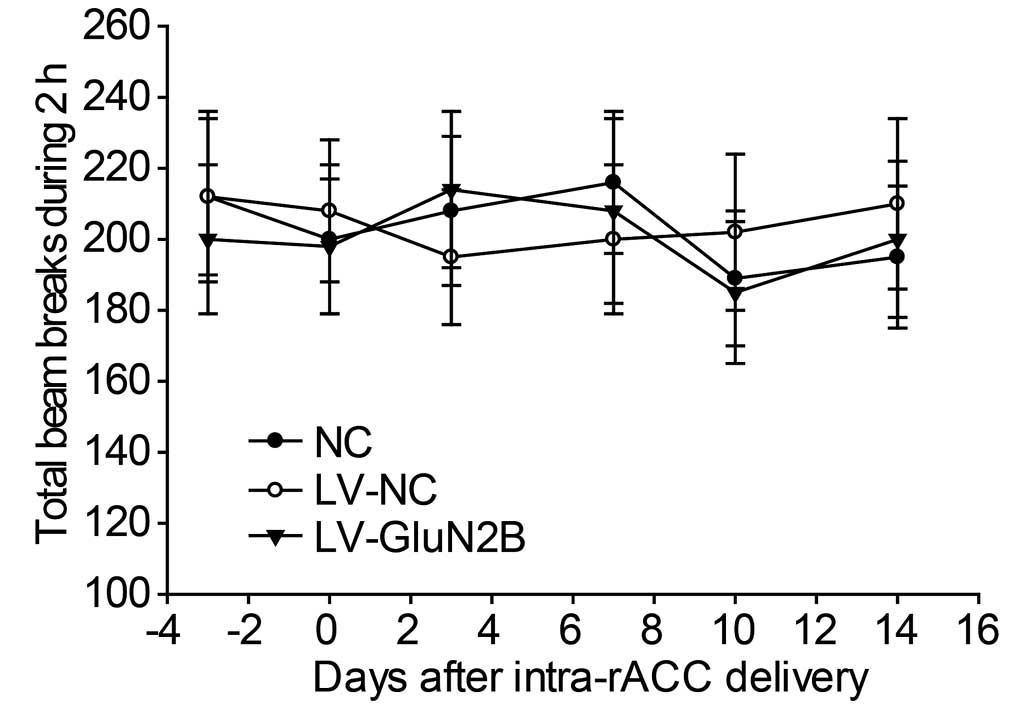
among the NS, LV-NC and LV-GluN2B groups (Fig. 6).
Discussion
The present study demonstrated that GluN2B within
the rACC may be a potential RNAi target for the treatment of pain
associated with bone cancer. Furthermore, intra-rACC delivery of
lentiviral vectors was effective at silencing GluN2B in rACC
neurons and providing a considerable antinociceptive therapeutic
effect for bone cancer-associated pain.
Previous animal models of cancer pain have been
developed in order to examine the mechanisms that underlie
tumor-evoked pain and hyperalgesia. Using models in which
osteolytic fibrosarcoma cells are implanted into the humerus,
femur, tibia, or calcaneus bone, researchers have begun to
elucidate the pathophysiological processes by which cancer produces
pain (24), and evaluate novel
approaches for the treatment of cancer pain (19). In the present study, a rat model of
bone cancer-associated pain was created by injecting osteosarcoma
cells into the intramedullary space of the femur. The rats that
received the osteosarcoma injection exhibited mechanical allodynia
and thermal hyperalgesia by post-operative day 10; whereas those
receiving the sham injection exhibited no detectable pain-related
behavior throughout the observation period. These pain-related
behaviors remained unchanged, as assessed on post-operative days
14, 21 and 28. Therefore, these results suggested that the rat bone
cancer pain model used in the present study may be pathologically
and physiologically applicable to the intended clinical
situation.
RNAi-mediated gene silencing is generated through
the expression of a vector-mediated RNAi anywhere in the genome
(25), and this technique has been
tested in clinical trials as a potential novel therapy for various
diseases (26). Viral delivery of
shRNA expression cassettes allows efficient transduction in tissues
such as the brain and liver. For example, recombinant
adeno-associated viral vectors expressing shRNA have been used in a
previous study to knockdown a target gene associated with
Huntington's disease (27) and
Anesti et al (28)
demonstrated that herpes simplex virus may be used to efficiently
deliver RNAi into peripheral neurons in vivo. Therefore,
viral-based vectors have been developed as an alternative
therapeutic strategy. Lentiviral vectors were selected as a gene
delivery tool in the present study due to their relatively large
cloning capacity and their ability to induce limited inflammation,
express shRNA, and induce stable and long-term gene silencing in
both dividing and non-dividing cells. In the present study, siRNA
sequences targeting the GluN2B gene were cloned into a pFU-GW-iRNA
lentiviral vector, and the recombinant lentiviral vectors were
subsequently injected into the rACC of rats. Following intra-rACC
administration of LV-GluN2B, RT-qPCR and western blot analysis
demonstrated a marked decrease in the mRNA and protein expression
levels of GluN2B in the rACC. Furthermore, quantitative analyses of
the mRNA expression levels following the injection of LV-GluN2B
into rACC revealed that the reduced GluN2B expression levels
demonstrated in the present study were statistically significant
and largely limited to the rACC. These results indicated that the
lentiviral vector delivery strategy employed in the present study
may be a promising novel approach for the treatment of bone
cancer-associated pain.
In the present study, the specific knockdown of
GluN2B receptors in the rACC successfully alleviated the tactile
allodynia and thermal hyperalgesia induced in the rat model of bone
cancer-associated pain. These results corroborate a previous study,
which demonstrated that microinjection of a selective metabotropic
GluR agonist, tACPD, into the rACC produced facilitation of the
tail-flick reflex (29). It has been
demonstrated that spinal nociception is subject to descending
modulation from supraspinal structures, including neurons in the
ACC and insular cortex. The ACC forms part of the affective pain
response system and contributes to various cortical functions,
including the perception of pain and the learning processes
associated with the prediction or avoidance of noxious stimuli
(30). A previous study has
demonstrated that neurons in the ACC respond to acute noxious
stimuli or nerve injury (31), and
lesions in the ACC have been demonstrated to affect the behavioral
response of rats in the hot-plate test (4). Furthermore, it has previously been
reported that efferents from the ACC innervate the periaqueductal
gray (30). A previous study using
afferent vagal stimulation or stimulation in the rostral
ventromedial medulla demonstrated that the latency for facilitatory
modulation of the spinal sensory neurons is longer than that for
inhibition, and the involvement of the rostral loop has been
suggested (32). These findings
suggested that neuronal activity in the ACC may affect spinal
nociception through descending modulatory systems.
The results of the present study suggested that
GluN2B within the rACC is a potential RNAi target for the treatment
of pain associated with bone cancer. A previous study demonstrated
that, in mice genetically engineered to overexpress GluN2B, chronic
pain was selectively enhanced, whereas acute or physiological pain
were unaffected (12); providing the
first genetic evidence that NMDA GluN2B receptors in the forebrain
are associated with chronic pain. In another previous study using a
complete Freund's adjuvant-induced animal model for chronic
inflammation, the expression levels of GluN2B receptors in the ACC
were increased over a long period of time following persistent
inflammation, thus increasing the GluN2B component in NMDA
receptor-mediated EPSCs (12).
Furthermore, a previous study utilized the behavioral allodynia
test and demonstrated that microinjection into the ACC or systemic
administration of GluN2B receptor with selective antagonists
inhibited behavioral responses to peripheral inflammation (11). These results are consistent with
genetic studies, which have demonstrated that mice with GluN2B
forebrain overexpression selectively enhanced inflammation-related
persistent pain without significant changes in acute pain. The
antiallodynic effects of NMDA GluN2B receptor antagonists have also
been reported in other animal models of chronic pain (33). These results indicated that the
development and maintenance of bone cancer-associated pain is
associated with the GluN2B subunit of NMDA receptors in the
rACC.
In addition to the rACC, GluN2B is abundantly
distributed in other areas of the forebrain. Therefore, the
locomotion of the rats was observed in order to determine whether
intra-rACC injection of LV-GluN2B produced any notable effects on
motor activity. No significant differences were determined among
the groups during the 14-day monitoring period. Subsequent RT-qPCR
and western blot analysis demonstrated that intra-injection of
LV-GluN2B specifically decreased GluN2B expression levels within
the rACC and had no obvious effects on the other areas of the
forebrain. These results further support the hypothesis that
RNAi-mediated gene silencing is a potential therapeutic tool for
the treatment of various nervous diseases.
In conclusion, the present study demonstrated that
cancer pain-related behaviors may be ameliorated by intra-rACC
administration of LV-GluN2B and the effects of LV-GluN2B may be
associated with a reduction in the expression of GluN2B in rACC
neurons. Furthermore, the present study may help elucidate the
central mechanism of bone cancer pain and provide a novel
therapeutic strategy for the prevention and/or treatment of bone
cancer pain by focusing on the plasticity alterations occurring at
the ACC synapses.
Acknowledgements
The present study was supported by the National
Science Council of China (grant no. 30872433) and the Science and
Technology Foundation of Shandong Province (grant no.
2010GWZ2023P).
References
|
1
|
Peng WL, Wu GJ, Sun WZ, Chen JC and Huang
AT: Multidisciplinary management of cancer pain: A longitudinal
retrospective study on a cohort of end-stage cancer patients. J
Pain Symptom Manage. 32:444–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuner R: Central mechanisms of
pathological pain. Nat Med. 16:1258–1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Price DD: Psychological and neural
mechanisms of the affective dimension of pain. Science.
288:1769–1772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pastoriza LN, Morrow TJ and Casey KL:
Medial frontal cortex lesions selectively attenuate the hot plate
response: Possible nocifensive apraxia in the rat. Pain. 64:11–17.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogt BA: Pain and emotion interactions in
subregions of the cingulate gyrus. Nat Rev Neurosci. 6:533–544.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuo M: Cortical excitation and chronic
pain. Trends Neurosci. 31:199–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI,
Kim SS, Steenland HW and Zhuo M: Presynaptic and postsynaptic
amplifications of neuropathic pain in the anterior cingulate
cortex. J Neurosci. 28:7445–7453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bie B, Brown DL and Naguib M: Increased
synaptic GluR1 subunits in the anterior cingulate cortex of rats
with peripheral inflammation. Eur J Pharmacol. 653:26–31. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW,
Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ and Zhuo M: Genetic
elimination of behavioral sensitization in mice lacking
calmodulin-stimulated adenylyl cyclases. Neuron. 36:713–726. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YP, Shimizu E, Dube GR, Rampon C,
Kerchner GA, Zhuo M, Liu G and Tsien JZ: Genetic enhancement of
learning and memory in mice. Nature. 401:63–69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J,
Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, et al: Upregulation of
forebrain NMDA NR2B receptors contributes to behavioral
sensitization after inflammation. J Neurosci. 25:11107–111016.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei F, Wang GD, Kerchner GA, Kim SJ, Xu
HM, Chen ZF and Zhuo M: Genetic enhancement of inflammatory pain by
forebrain NR2B overexpression. Nat Neurosci. 4:164–169. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dorn G, Patel S, Wotherspoon G,
Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A,
Ganju P, et al: siRNA relieves chronic neuropathic pain. Nucleic
Acids Res. 32:e492004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naldini L, Blömer U, Gallay P, Ory D,
Mulligan R, Gage FH, Verma IM and Trono D: In vivo gene delivery
and stable transduction of nondividing cells by a lentiviral
vector. Science. 272:263–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manjunath N, Wu H, Subramanya S and
Shankar P: Lentiviral delivery of short hairpin RNAs. Adv Drug
Deliv Rev. 61:732–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coleman JE, Huentelman MJ, Kasparov S,
Metcalfe BL, Paton JF, Katovich MJ, Semple-Rowland SL and Raizada
MK: Efficient large-scale production and concentration of
HIV-1-based lentiviral vectors for use in vivo. Physiol Genomics.
12:221–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Déglon N, Tseng JL, Bensadoun JC, Zurn AD,
Arsenijevic Y, de Almeida Pereira L, Zufferey R, Trono D and
Aebischer P: Self-inactivating lentiviral vectors with enhanced
transgene expression as potential gene transfer system in
Parkinson's disease. Hum Gene Ther. 11:179–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwei MJ, Honore P, Rogers SD,
Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR and Mantyh
PW: Neurochemical and cellular reorganization of the spinal cord in
a murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999.PubMed/NCBI
|
|
20
|
Paxinos G, Watson CR and Emson PC:
AChE-stained horizontal sections of the rat brain in stereotaxic
coordinates. J Neurosci Methods. 3:129–149. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Aanlysis of
relative gene expression data using real-time quantitative PCR and
2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wacnik PW, Eikmeier LJ, Ruggles TR,
Ramnaraine ML, Walcheck BK, Beitz AJ and Wilcox GL: Functional
interactions between tumor and peripheral nerve: Morphology,
algogen identification, and behavioral characterization of a new
murine model of cancer pain. J Neurosci. 21:9355–9366.
2001.PubMed/NCBI
|
|
25
|
Zhai Z, Sooksa-nguan T and Vatamaniuk OK:
Establishing RNA interference as a reverse-genetic approach for
gene functional analysis in protoplasts. Plant Physiol.
149:642–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shrey K, Suchit A, Nishant M and Vibha R:
RNA interference: Emerging diagnostics and therapeutics tool.
Biochem Biophys Res Commun. 386:273–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Franich NR, Fitzsimons HL, Fong DM,
Klugmann M, During MJ and Young D: AAV vector-mediated RNAi of
mutant huntingtin expression is neuroprotective in a novel genetic
rat model of Huntington's disease. Mol Ther. 16:947–956. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anesti AM, Peeters PJ, Royaux I and Coffin
RS: Efficient delivery of RNA interference to peripheral neurons in
vivo using herpes simplex virus. Nucleic Acids Res. 36:e862008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calejesan AA, Kim SJ and Zhuo M:
Descending facilitatory modulation of a behavioral nociceptive
response by stimulation in the adult rat anterior cingulate cortex.
Eur J Pain. 4:83–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogt BA and Gabriel M: Neurobiology of
cingulate cortex and limbic thalamus: A comprehensive handbook.
XIII (Birkhäuser, Boston). 6391993.
|
|
31
|
Talbot JD, Marrett S, Evans AC, Meyer E,
Bushnell MC and Duncan GH: Multiple representations of pain in
human cerebral cortex. Science. 251:1355–1358. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren K, Randich A and Gebhart GF:
Electrical stimulation of cervical vagal afferents. I. central
relays for modulation of spinal nociceptive transmission. J
Neurophysiol. 64:1098–1114. 1990.PubMed/NCBI
|
|
33
|
Salte K, Lea G, Franek M and Vaculin S:
Baclofen reversed thermal place preference in rats with chronic
constriction injury. Physiol Res. Oct 08–2015.(Epub ahead of
print). PubMed/NCBI
|