Introduction
Idiopathic pulmonary fibrosis (IPF) is a fatal
interstitial lung disease characterized by rapidly progressive
scarring of the lung tissue, exacerbated fibroblast proliferation,
excessive deposition of matrix proteins and destruction of alveolar
architecture (1,2), which may result in loss of lung
function and ultimately respiratory failure. The exact etiology of
IPF remains unclear. Current treatments for IPF primarily include
anti-inflammatory, immunosuppressive or anti-fibrotic methods,
which exhibit a limited capacity to prevent the progression of the
disease or to improve patient quality of life (3). In order to obtain optimal therapeutic
results there is a requirement for novel therapeutic methods for
the treatment of IPF.
Flavonoids are diphenyl propanoids present in edible
plants and are categorized as flavonols, flavones, catechins,
flavanones, anthocyanidins and isoflavonoids. Flavonoids exert
anti-proliferative and anti-inflammatory properties (4–6).
Apigenin (4,5,7-trihydroxyflavone) is a non-toxic and non-mutagenic
flavone that exists abundantly in numerous herbs and vegetables,
including chamomile, thyme, parsley and broccoli, and displays
cytostatic and cytoprotective activity (7–9).
However, at present it remains unclear how apigenin affects the
lung fibrosis process, although it harbors anti-proliferative and
anti-inflammatory properties. The aim of the present study was to
investigate the potential of apigenin to treat lung fibrosis using
a bleomycin-induced rat lung fibrosis model.
Materials and methods
Animals
A total of 80 male Wistar rats (age, 8 weeks;
weight, 200–240 g) were obtained from Vital River Laboratories
(Beijing, China). The animals were fed a standard rat chow and
water ad libitum and housed in a temperature-controlled
environment (20–22°C) with an alternating cycle of 12-h light and
dark. All rats were allowed to acclimatize for one week prior to
the initiation of any experiments. The experimental design and
procedures were approved by the Ethical Committee for Animal Care
and Use at Pingmei Shenma Medical Group General Hospital
(Pingdingshan, China).
Experimental model of
bleomycin-induced lung fibrosis
Rats were randomized into 5 groups, each containing
16 animals. Group 1 consisted of saline-treated control rats and
group 2 comprised bleomycin-treated rats. Animals in groups 3, 4
and 5 were treated with 10, 15 and 20 mg/kg/day apigenin,
respectively, starting concurrently with the induction of lung
fibrosis by the administration of bleomycin and continued until the
end of the experiment. Single doses of bleomycin (5.0 mg/kg in 1.0
ml phosphate-buffered saline; Tianjin Taihe Pharmaceutical Co.,
Ltd., Tianjin, China) were injected into the rat lung
intratracheally. Control animals received an identical volume of
intratracheal saline instead of bleomycin. Apigenin (dissolved in
olive oil; Sigma-Aldrich, St. Louis, MO, USA) was administered
orally via a feeding tube. The day of bleomycin injection was
defined as day 0 and the weight of the animals was recorded every
3–4 days.
Lung preparation and biochemical
assays
Following the treatment, on days 7 and 28, the rats
were anesthetized using 3% pentobarbital sodium and sacrificed by
exsanguination at the cephalic artery. The lungs were removed and
weighed and washed twice with cold saline. Then each lung was
divided into two sections: The right section was fixed in 10%
formalin solution for histological examination and the left section
was prepared for biochemical assay and cytokine detection as
described below.
The lung samples were prepared as 10% homogenates in
0.9% saline by homogenizer on ice according to their respective
weight. The resulting homogenate was centrifuged, and the
supernatant was collected and stored at −20°C until assayed. The
assays of superoxide dismutase (SOD; A001-3), myeloperoxidase (MPO;
A044) and hydroxyproline (A030-2) levels were all purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and
performed according to the manufacturer's instructions.
Histological examination of
fibrosis
Lung specimens were fixed in 10% formalin solution
for 24 h, dehydrated in ethyl alcohol and embedded in paraffin.
Subsequently, 5-µm sections were stained with hematoxylin and eosin
(H&E) and Masson trichrome for histological evaluation of lung
injury and fibrosis using an Olympus CKX41 light microscope
(Olympus Corporation, Tokyo, Japan).
Measurement of tumor necrosis factor
(TNF)-α and transforming growth factor (TGF)-β
Lung homogenate (10%) was centrifuged at 12,000 × g
for 30 min at 4°C to remove cell debris, and the supernatants were
used for TNF-α and TGF-β determination. The levels of TNF-α were
determined using an ELISA kit from Assaypro, LLC (St. Charles, MO,
USA). TGF-β levels were assayed using a commercially available
TGF-β ELISA kit (TGF-β E max ImmunoAssay System; Promega
Corporation, Madison, WI, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). Data are
presented as the mean ± standard deviation. Statistical differences
were analyzed using one-way analysis of variance, followed by
post-hoc multiple comparison tests (least significant difference).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Differences in the body weight and
lung index
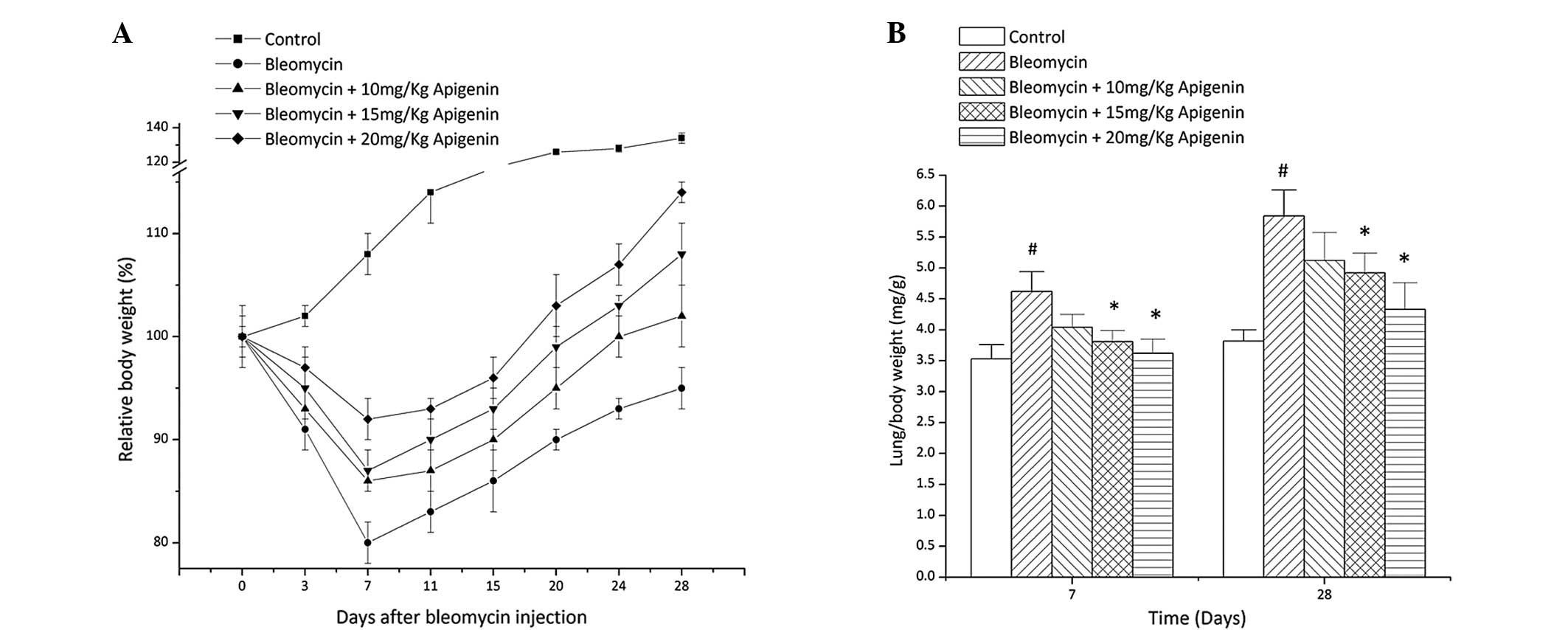
By contrast to the control group, the body weight of
the bleomycin-treated group decreased gradually and reached the
lowest level at day 7 after bleomycin injection (Fig. 1A). Apigenin treatment provided a
dose-dependent protective effect on the body weight loss caused by
bleomycin (Fig. 1A).
Lung index [weight of wet lung (mg)/body weight] is
a parameter used for evaluating lung edema. In contrast with body
weight loss, the lung index of the bleomycin-treated rats increased
due to lung weight gain at days 7 and 28 after bleomycin injection
compared with that of the normal control rats (Fig. 1B). Apigenin treatment inhibited the
increase of the lung index of the bleomycin-treated rats in a
concentration-dependent manner (Fig.
1B).
Effect of apigenin on hydroxyproline
content, SOD activity and MPO activity
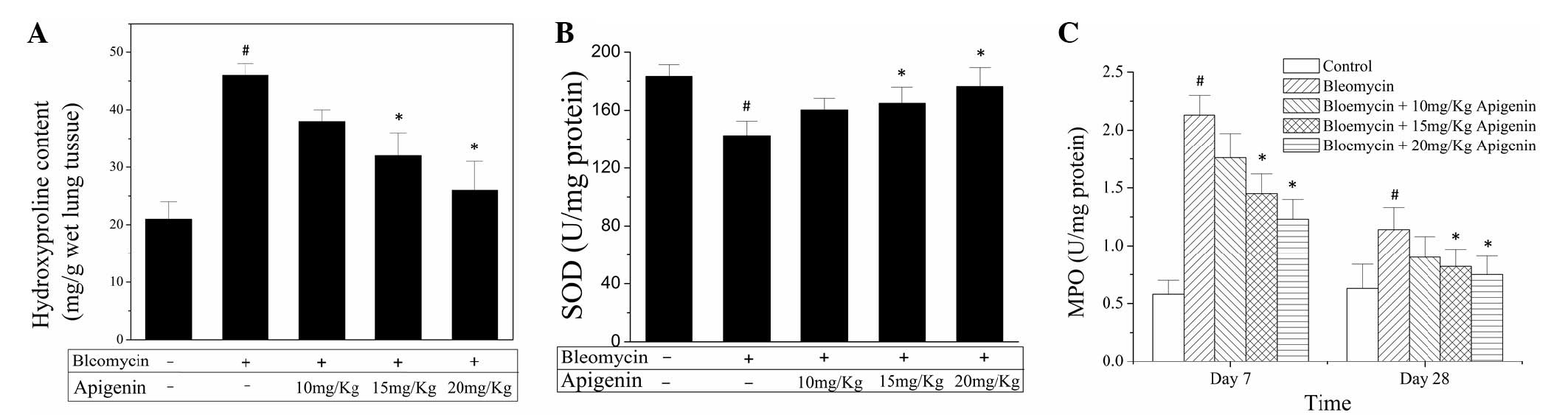
In order to assess the extent of fibrosis, the lung
hydroxyproline levels were determined. As shown in Fig. 2A, at day 28 after bleomycin the lung
hydroxyproline level in the bleomycin-treated rats was increased
markedly compared with that of the normal control rats.
Administration of apigenin reduced the hydroxyproline level in the
lung homogenates in a dose-dependent manner (Fig. 2A).
The depletion of SOD indirectly indicates the
production of oxygen free radicals following bleomycin injection.
As shown in Fig. 2B, administration
of bleomycin significantly decreased the SOD activity in the lung
homogenates at day 28 after bleomycin injection. Treatment with
apigenin concentration-dependently inhibited the decline of SOD
activity caused by bleomycin administration (Fig. 2B).
MPO activity has been used as an index of leukocyte
adhesion and accumulation in a range of tissues (10). Treatment with bleomycin elicited a
profound increase in MPO activity in the lung homogenates at days 7
and 28 after bleomycin injection compared with that in the control
group (Fig. 2C). Administration of
apigenin led to a significant reduction in MPO activity, in a
dose-dependent manner (Fig. 2C).
Effect of apigenin on pro-inflammatory
and pro-fibrotic cytokine production in lung homogenates
In order to identify the function of cytokines in
lung fibrosis, TNF-α and TGF-β levels in lung homogenates were
determined. As shown in Fig. 3A, at
day 7 after bleomycin administration the level of TNF-α increased
markedly compared with those in normal control rats. Apigenin
suppressed the increase of TNF-α level dose-dependently (Fig. 3A). In addition, treatment of rats
with bleomycin elicited a notable increase in TGF-β levels in the
lung homogenates at days 7 and 28 after bleomycin injection
compared with that in normal control rats (Fig. 3B). Administration of apigenin
inhibited the increase of TGF-β level caused by bleomycin treatment
in a concentration-dependent manner (Fig. 3B).
Histological differences
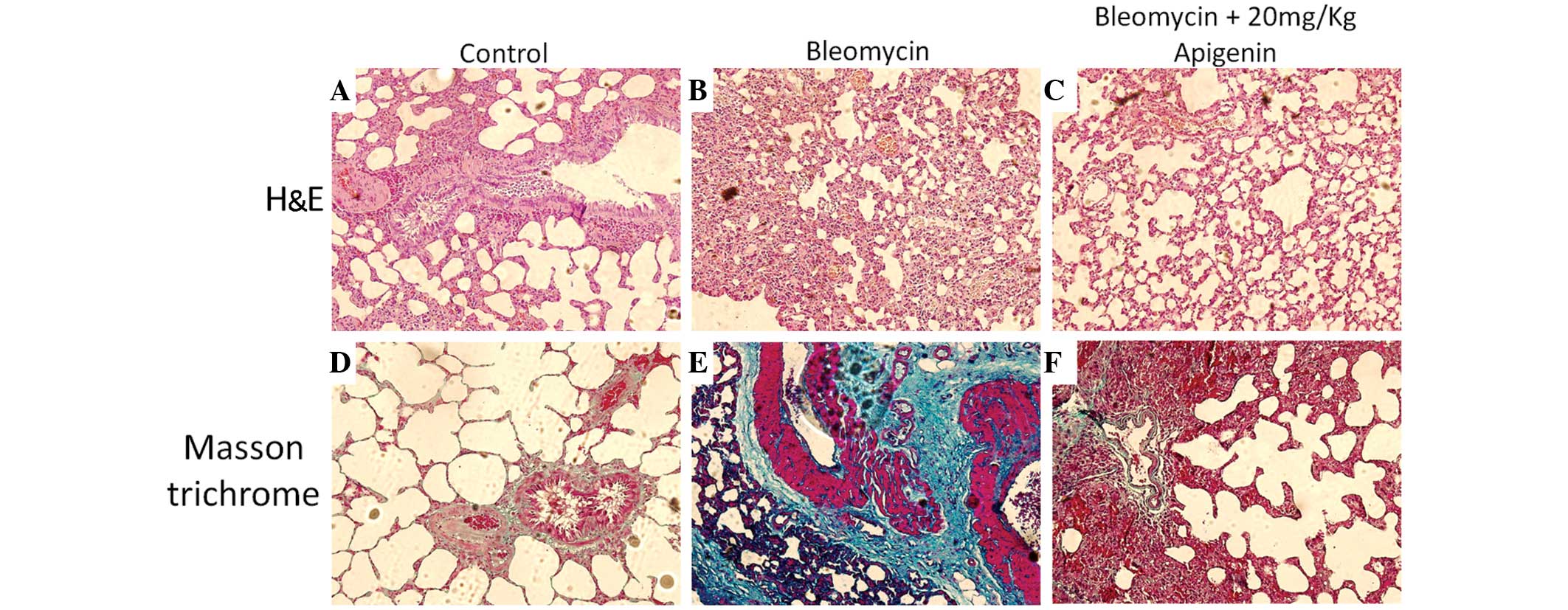
Rat lungs were examined histologically on day 7
after bleomycin injection (Fig. 4).
The lung architecture in the control group appeared unaffected
(Fig. 4A), while the
bleomycin-treated rats exhibited collapsed and narrowed alveoli,
marked thickening of the interalveolar septa and dense interstitial
infiltration by inflammatory cells (Fig.
4B). In addition, Masson trichrome staining results
demonstrated that there was excessive collagen deposition in the
lung tissues of the bleomycin-treated rats (Fig. 4E) compared with the control rats
(Fig. 4D). In contrast to the
bleomycin-treated rats (Fig. 4B),
the rats treated with bleomycin and 20 mg/kg apigenin presented
with a significant suppression of inflammatory cell infiltration,
reduced thickening of the interalveolar septa and increased
inflation of the alveoli (Fig. 4C).
Furthermore, 20 mg/kg apigenin treatment markedly reduced collagen
deposition in the lung tissues of the bleomycin-treated rats, as
indicated by Masson trichrome staining (Fig. 4F).
Discussion
Previous studies have demonstrated the
anti-inflammatory activity of apigenin under a range of
pathophysiological conditions. Apigenin has been shown to block
lipopolysaccharide (LPS)-induced lethality in vivo and the
expression of proinflammatory cytokines by inactivating NF-κB via
the suppression of p65 phosphorylation (10). In endothelial cells, apigenin
provides a protective effect against LPS-induced inflammation by
decreasing caspase-3 activation and modulating mitochondrial
function (11). Apigenin exerts
anti-inflammatory activity via multiple mechanisms (12), including the induction of apoptosis
and the regulation of MAPK signaling pathways in mouse macrophage
ANA-1 cells (13). Other mechanisms
include the inhibition of inflammatory cytokine production.
Apigenin has demonstrated the ability to suppress the expression of
interleukin (IL)-1β and tumor necrosis factor (TNF)-α at the
genetic level in J774.2 macrophages (14). Apigenin has also been shown to
inhibit the expression of IL-6, IL-8 and intracellular adhesion
molecule 1 in bis(2-ethylhexyl)phthalate-stimulated human umbilical
vein endothelial cells and in vivo (15). In cystic fibrosis bronchial cells,
the expression of the pro-inflammatory IL-8 gene was suppressed by
apigenin (16). In addition,
apigenin has been indicated to exert a protective effect against
oxidative stress (17–19). Furthermore, apigenin inhibits
TGF-β1-induced collagen production and fibroblast-to-myofibroblast
transition in human lung fibroblast populations (20,21).
Although apigenin exhibits numerous activities that are potentially
preventative of fibrotic disease, the impact of apigenin on lung
fibrosis processes previously remained unclear.
In the present study, the protective effect of
apigenin on bleomycin-induced lung fibrosis was investigated in
rats. Bleomycin administration resulted in body weight loss, lung
index increase, progressive and significant inflammation,
exacerbated fibrosis and severe alveolar destruction in rat lungs.
In addition, significant reductions in SOD activity and elevation
of hydroxyproline content, MPO activity, TNF-α and TGF-β1 levels
were detected in the rat lung tissues following bleomycin exposure.
However, apigenin treatment prevented bleomycin-induced lung
fibrosis and inflammation, decreased the TNF-α and TGF-β1 levels
and attenuated the reduction of SOD activity in rat lungs. Overall,
the present results suggest that apigenin exerts a protective
effect against bleomycin-induced lung fibrosis in rats.
Bleomycin-induced animal lung injury has been widely
used as a model of human lung fibrosis as certain biochemical and
functional changes in the early stages in animals resemble those
observed in humans. The inflammatory responses observed following
bleomycin administration include leukocytes infiltration.
Infiltrated leukocytes are able to synthesize and secrete various
cytokines, chemokines, reactive oxygen species and proteases, that
may sustain injury/repair processes (22,23). MPO
activity has been used as an index of leukocyte infiltration. As
shown in Fig. 2C, apigenin inhibited
the bleomycin-induced increase of MPO activity, suggesting that
apigenin inhibited leukocyte infiltration.
A number of studies have demonstrated that redox
state and oxidant-antioxidant balance serve a crucial function in
the pathogenesis of lung fibrosis in animal models, and potentially
in humans (24,25). High levels of oxidants enhance TGF-β
production (26), activate protease
and promote the fibrotic response. Certain antioxidants, including
SOD, have been observed to inhibit collagen deposition and protect
the lungs in a range of animal models (27). As shown in Fig. 2B, apigenin was able to reverse the
bleomycin-induced reduction of SOD activity and thus provide a
protective effect.
Cytokines are involved in lung fibrosis. TNF-α
functions as a chemokine in inflammatory cells, in addition to
promoting the synthesis of fibronectin, prostaglandin and TGF-β.
Depletion of TNF-α by antibodies has demonstrated a beneficial
effect in the inhibition of bleomycin-induced lung injury (28,29). In
the present study, a marked increase in TNF-α levels in the lungs
occurred following bleomycin exposure, which was suppressed by
apigenin treatment in a dose-dependent manner (Fig. 3A). TGF-β is a key cytokine associated
with the induction of lung injury, and it contributes to lung
fibrosis via the induction of collagen gene expression or synthesis
by stimulation of fibroblast proliferation (30–33). In
the present study, TGF-β production in the lung homogenates was
increased following bleomycin injection, while apigenin treatment
inhibited TGF-β production, retaining it at a basal level
comparable with that detected in the normal control group (Fig. 3B). Thus, the reduced hydroxyproline
production (Fig. 2A) and lung injury
observed in the apigenin-treated rats may be partially attributed
to the capacity of apigenin to inhibit TGF-β production.
In summary, apigenin demonstrated protective effects
against bleomycin-induced lung fibrosis in rats. The beneficial
effect of apigenin may be associated with its anti-inflammatory,
antioxidative and cytokine inhibition capacities.
References
|
1
|
Gross TJ and Hunninghake GW: Idiopathic
pulmonary fibrosis. N Engl J Med. 345:517–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dempsey OJ and Miller D: Idiopathic
pulmonary fibrosis. BMJ. 347:f65792013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahendran S and Sethi T: Treatments in
idiopathic pulmonary fibrosis: Time for a more targeted approach?
QJM. 105:929–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villar A, Gasco MA and Alcaraz MJ:
Anti-inflammatory and anti-ulcer properties of
hypolaetin-8-glucoside, a novel plant flavonoid. J Pharm Pharmacol.
36:820–823. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerdin B and Svensjö E: Inhibitory effect
of the flavonoid O-(beta-hydroxyethyl)-rutoside on increased
microvascular permeability induced by various agents in rat skin.
Int J Microcirc Clin Exp. 2:39–46. 1983.PubMed/NCBI
|
|
6
|
Agarwal OP: The anti-inflammatory action
of nepitrin, a flavonoid. Agents Actions. 12:298–302. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clere N, Faure S, Martinez MC and
Andriantsitohaina R: Anticancer properties of flavonoids: Roles in
various stages of carcinogenesis. Cardiovasc Hematol Agents Med
Chem. 9:62–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czyz J, Madeja Z, Irmer U, Korohoda W and
Hülser DF: Flavonoid apigenin inhibits motility and invasiveness of
carcinoma cells in vitro. Int J Cancer. 114:12–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shukla S and Gupta S: Apigenin-induced
cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt and
loss of cyclin D1 associated retinoblastoma dephosphorylation in
human prostate cancer cells. Cell Cycle. 6:1102–1114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicholas C, Batra S, Vargo MA, Voss OH,
Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E and Doseff AI:
Apigenin blocks lipopolysaccharide-induced lethality in vivo and
proinflammatory cytokines expression by inactivating NF-kappaB
through the suppression of p65 phosphorylation. J Immunol.
179:7121–7127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duarte S, Arango D, Parihar A, Hamel P,
Yasmeen R and Doseff AI: Apigenin protects endothelial cells from
lipopolysaccharide (LPS)-induced inflammation by decreasing
caspase-3 activation and modulating mitochondrial function. Int J
Mol Sci. 14:17664–17679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Wang G, Gurley EC and Zhou H:
Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory
response through multiple mechanisms in macrophages. PloS One.
9:e1070722014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao Y, Shen W, Kong G, Lv H, Tao W and Bo
P: Apigenin induces the apoptosis and regulates MAPK signaling
pathways in mouse macrophage ANA-1 cells. PloS One. 9:e920072014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kowalski J, Samojedny A, Paul M, Pietsz G
and Wilczok T: Effect of apigenin, kaempferol and resveratrol on
the expression of interleukin-1beta and tumor necrosis factor-alpha
genes in J774.2 macrophages. Pharmacol Rep. 57:390–394.
2005.PubMed/NCBI
|
|
15
|
Wang J, Liao Y, Fan J, Ye T, Sun X and
Dong S: Apigenin inhibits the expression of IL-6, IL-8 and ICAM-1
in DEHP-stimulated human umbilical vein endothelial cells and in
vivo. Inflammation. 35:1466–1476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lampronti I, Borgatti M, Vertuani S,
Manfredini S and Gambari R: Modulation of the expression of the
proinflammatory IL-8 gene in cystic fibrosis cells by extracts
deriving from olive mill waste water. Evid Based Complement
Alternat Med. 2013:9606032013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT,
Li CC, Wang TS and Chen HW: Protection by chrysin, apigenin and
luteolin against oxidative stress is mediated by the Nrf2-dependent
up-regulation of heme oxygenase 1 and glutamate cysteine ligase in
rat primary hepatocytes. Arch Toxicol. 87:167–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han JY, Ahn SY, Kim CS, Yoo SK, Kim SK,
Kim HC, Hong JT and Oh KW: Protection of apigenin against
kainate-induced excitotoxicity by anti-oxidative effects. Biol
Pharm Bull. 35:1440–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin BH, Qian LB, Chen S, Li J, Wang HP,
Bruce IC, Lin J and Xia Q: Apigenin protects endothelium-dependent
relaxation of rat aorta against oxidative stress. Eur J Pharmacol.
616:200–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ricupero DA, Poliks CF, Rishikof DC, Kuang
PP and Goldstein RH: Apigenin decreases expression of the
myofibroblast phenotype. FEBS Lett. 506:15–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wojcik KA, Skoda M, Koczurkiewicz P, Sanak
M, Czyż J and Michalik M: Apigenin inhibits TGF-β1 induced
fibroblast-to-myofibroblast transition in human lung fibroblast
populations. Pharmacol Rep. 65:164–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tarnell EB, Oliver BL, Johnson GM, Watts
FL and Thrall RS: Superoxide anion production by rat neutrophils at
various stages of bleomycin-induced lung injury. Lung. 170:41–50.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oury TD, Thakker K, Menache M, Chang LY,
Crapo JD and Day BJ: Attenuation of bleomycin-induced pulmonary
fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir
Cell Mol Biol. 25:164–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinnula VL, Fattman CL, Tan RJ and Oury
TD: Oxidative stress in pulmonary fibrosis: A possible role for
redox modulatory therapy. Am J Respir Crit Care Med. 172:417–422.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuwano K, Nakashima N, Inoshima I,
Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K
and Hara N: Oxidative stress in lung epithelial cells from patients
with idiopathic interstitial pneumonias. Eur Respir J. 21:232–240.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bellocq A, Azoulay E, Marullo S, Flahault
A, Fouqueray B, Philippe C, Cadranel J and Baud L: Reactive oxygen
and nitrogen intermediates increase transforming growth
factor-beta1 release from human epithelial alveolar cells through
two different mechanisms. Am J Respir Cell Mol Biol. 21:128–136.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salvemini D, Riley DP and Cuzzocrea S: SOD
mimetics are coming of age. Nat Rev Drug Discov. 1:367–374. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piguet PF, Ribaux C, Karpuz V, Grau GE and
Kapanci Y: Expression and localization of tumor necrosis
factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am J
Pathol. 143:651–655. 1993.PubMed/NCBI
|
|
29
|
Yara S, Kawakami K, Kudeken N, Tohyama M,
Teruya K, Chinen T, Awaya A and Saito A: FTS reduces
bleomycin-induced cytokine and chemokine production and inhibits
pulmonary fibrosis in mice. Clin Exp Immunol. 124:77–85. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin TR and Matute-Bello G: Experimental
models and emerging hypotheses for acute lung injury. Crit Care
Clin. 27:735–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anscher MS: Targeting the TGF-beta1
pathway to prevent normal tissue injury after cancer therapy.
Oncologist. 15:350–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilson MS, Madala SK, Ramalingam TR,
Gochuico BR, Rosas IO, Cheever AW and Wynn TA: Bleomycin and
IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp
Med. 207:535–552. 2010. View Article : Google Scholar : PubMed/NCBI
|