Introduction
The heart is a specialised pump that functions by
regular and continuous contractions for delivery of blood
throughout the body (1). The pumping
action is caused by a flow of electricity through the heart that
repeats itself in a cycle, known as heart rate (HR) or heart pulse.
HR is the speed of the heartbeat measured by the number of
contractions per unit of time (2), a
measure determined by calculating the heart rate variability (HRV)
from electrocardiogram (ECG) recordings.
The understanding of the significance of HRV is
ongoing. However, it has been suggested that HRV is an important
method for assessing cardiovascular autonomic parameters that are
partially under the regulatory control of innervations from the
sympathetic and parasympathetic systems (3,4).
Notably, these two components of the autonomic nervous system (ANS)
balance between them affects the consistency in the time between
heart beats. Consequently, HRV reflects oscillations in the heart
cycle duration over time and is generally considered the measure of
regulatory influences, mainly of the activity of ANS to regulate
the function of the cardiovascular system. Previous findings have
identified the potential use of HRV for recognizing healthy and
diseased states since the vagal-mediated HRV indices were inversely
associated with several risk factors for diabetes, glucose
intolerance, insulin resistance, central obesity, dyslipidemia and
hypertension (3).
However, while HRV was largely applied to predict
sudden cardiac death and diabetic neuropathies in assessing disease
progression (5,6), recent studies demonstrated the
application of HRV in exercise training. Their findings supported
the use of HRV as a marker to reflect the cardiac modulation of the
sympathetic and vagal component of the ANS (7), and suggested that monitoring indices of
HRV may be useful for tracking the time course of training
adaptation/maladaptation in order to set optimal training loads
that lead to improved performances (3,7–9). Specifically, the aim of all training is
the use of enough physical training loads to the body to transfer
homeostasis and the whole autonomic balance. Athletes may better
adapt their physiology during a sufficient recovery of the body
(10). However, how long- and
short-term effects of training and the condition of athletes, as
well as the efficiency of the training, and the recovery level to
maximize the benefits of training remain to be determined.
Nevertheless, studies (2,11,12)
comparing ANS between sedentary and active subjects or athletes of
different sports modalities have shown different HRV profiles,
suggesting the possibility of monitoring HRV indices for improving
physical and physiological states.
The present review examined the possible role of HRV
in sports physiology. Particularly, we examined the autonomic
regulation of the heart and its relationship with HRV, as well as
the relevance of the use of HRV in sport training with
consideration of the intensity of the training, the level of
athletes, the gender and age of athletes and, the use of HRV to
monitor and improve sports physiology.
Heart rate variability (HRV)
Autonomic innervations of the
heart
The heart and circulatory system are primarily
controlled by the higher brain center (central command) and by the
cardiovascular control area located in the brain stem, through the
activity of the ANS. The ANS comprises the sympathetic and
parasympathetic nerves (vagal nerves) outflow to the heart and
blood vessels, which are primarily regulated by the medulla
(13). Particularly, the nucleus
tractus solitarius in the medulla receives sensory input and
stimulates cardiovascular responses for emotion and physical
stress. From the medulla, the parasympathetic vagus nerve
innervates the heart to the sympathetic nerve fibers. The right and
left vagus nerves innervate the sinus atrial (SA) and
atrioventricular nodes, respectively. The atria are also innervated
by vagal efferent, whereas the ventricular myocardium is sparsely
innervated by the vagal efferent. Sympathetic efferent nerves are
present throughout the atria, particularly in the SA node and
ventricles. Sympathetic stimulation increases the HR, and
contractility and conduction velocity through the mediation of α
and β adrenoreceptors. Parasympathetic stimulation has the opposite
effects through the muscarinic receptor. Autonomic control of the
cardiovascular system is also affected by baroreceptors,
chemoreceptor, muscle afferents, local tissue metabolism,
circulating hormones, and environmental behaviour (14–16 as well as
ethnic group (17,18). Although sympathetic and
parasympathetic systems are active at rest, the parasympathetic
fibers release acetylcholine, which acts to retard the pacemaker's
potential of the SA node and thus reduce the HR (19).
ANS regulation of HRV
HRV refers to the beat-to-beat alteration of the
heart. The ECG of a healthy individual measured under resting
conditions shows periodic variation consisting of a rhythmic
phenomenon known as respiratory sinus arrhythmia (RSA). RSA
fluctuates with the phase of respiration with cardio-acceleration
during inspiration and cardio-deceleration during expiration. Vagal
efferent trafficking to the sinus node occurred primarily in the
phase with expiration, and absent or attenuated during inspiration.
These data identify RSA as predominantly mediated by respiratory
gating of parasympathetic efferent activity to the heart; referring
HRV as a marker of dynamic and cumulative loads. As a dynamic
marker of loads, HRV appears to be sensitive and responsive to
acute stress. Experimentally, it has been shown that mental load
(i.e., making complex decisions, public speaking tasks) decrease
HRV. As a marker of cumulative wear and tear, HRV is also reduced
with the aging process. Significant resting HR does not change with
aging (20) and a reduction of HRV
was attributed to a decrease in efferent vagal tone and reduced
β-adrenergic responsiveness. By contrast, regular physical activity
retards the aging process, increasing HRV, presumably by increasing
vagal tone. Therefore, HRV is considered a marker of frequent
activation (short dips in HRV in response to acute stress) and the
inadequate response (long-term vagal withdrawal, resulting in the
over-activity of the counter-regulatory system), leading to the
sympathetic control of cardiac rhythm.
Basic measurement of HRV parameters
and interpretations
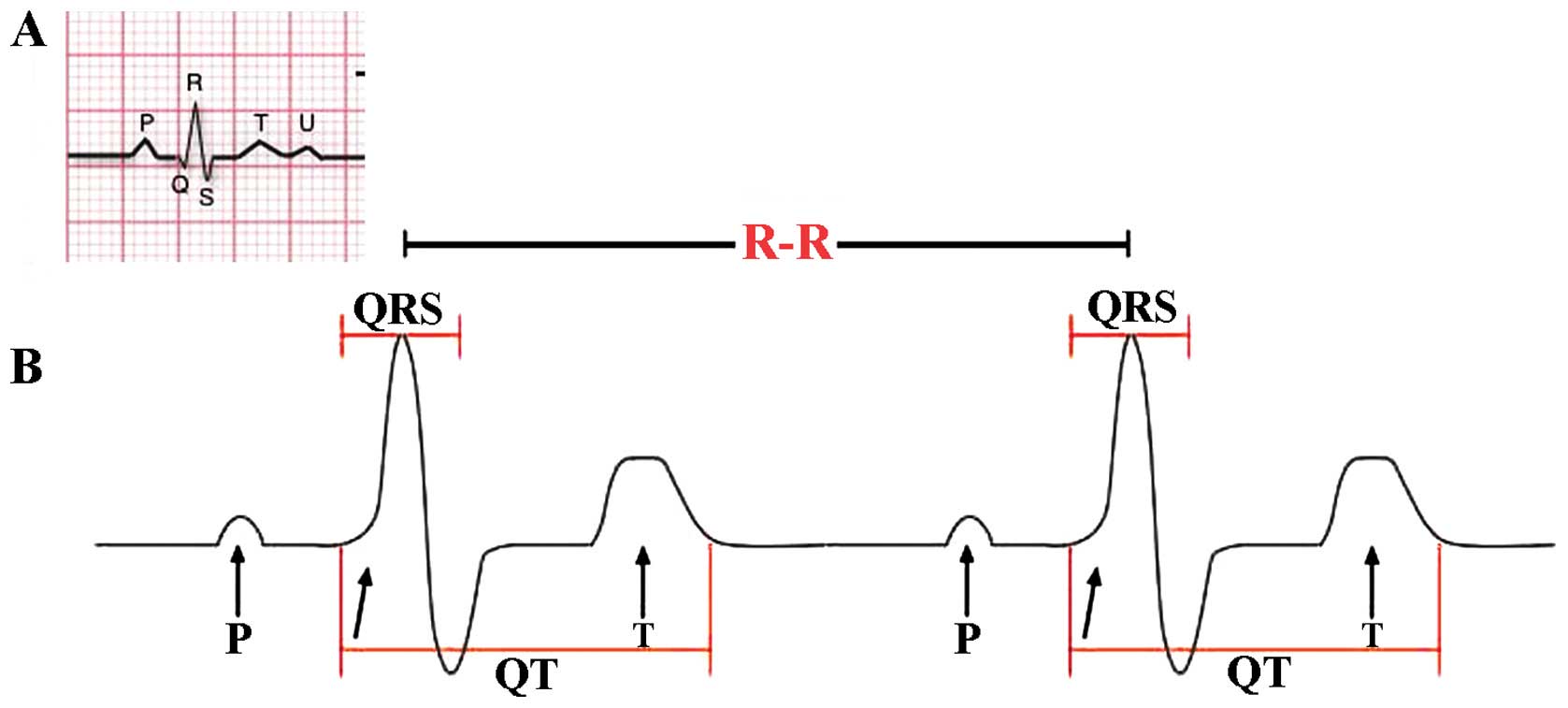
HRV may be evaluated by a variety of complex
methods. The most common method is standard ECG, considering the
temporal variation between the sequences of consecutive heart beats
(Fig. 1). The lengths of successive
R peaks (R-R) in the QRS complex can be described mathematically.
R-R is not consistent between successive R peaks. Of note, during
the onset of physical activity, R-R intervals become shorter and
more uniform, resulting from increased sympathetic activity and
parasympathetic withdrawal. R-R can provide actionable information
regarding the physiological stress and fatigue levels during and
after training (21). Thus, despite
the complexity of the type of mathematics involved in the
calculation of HRV, a variety of algorithmic models that represent
R-R intervals are widely available, and autonomic activation can be
evaluated by analysing HRV to estimate the sympathetic-vagal
balance (22,23). In addition, the period between the
QRS complex resulting from sinus node depolarisations is termed the
normal-normal (N-N) interval. HRV is the measurement of the
variability of the N-N intervals (24). Additionally, frequency-domain
analysis describes high and low frequency (LV) rates of the
variability changes, corresponding to the activity of different
branches of ANS. By applying these frequency range differences in
HRV analysis, the individual contribution of parasympathetic and
sympathetic systems were identified. Parameters LF referring to
modulation of the R-R interval changes between 0.04 and 0.15 Hz
corresponds to the sympathetic and parasympathetic activity
together. High frequency (HF) modulation (0.15–0.4 Hz) of R-R
interval changes is primarily regulated through innervations of the
heart by the parasympathetic (vagal) nerve (25). LF and HF parameters are provided as
normalized (LFn and HFn) by calculating the fraction of LF or HF
relative to the total minus LF (26). Another crucial element for the
analysis of HRV is the time-domain parameters reflecting the
standard deviation (SD) of all N-N intervals (SDNN) that reproduce
the total variability and the root mean square of SDs between
adjacent N-N intervals (RMSSD), which reflect parasympathetic
activity (2,16,27).
Another parameter that may be considered is the Pointcaré plot,
calculated as follows: an individual's R-R intervals plotted over
time and SD used to interpret changes are evident in the plot
(28). The standard descriptor 1
(SD1) is the fast beat-to-beat variability in the R-R intervals,
while the standard descriptor 2 (SD2) describes the longer-term
variability (29). SD1 reflects
mainly the parasympathetic input to the heart, while SD2 reflects
the sympathetic and parasympathetic contributions to the heart.
However, as mentioned earlier, respiration greatly
affects HRV, thereby increasing HRV when respiratory frequency
decreases, rendering difficult the proper interpretation of HRV
data. Thus, investigators have accepted various respiratory
frequency ranges (i.e., 6–15 beats/min) and admitted self-organized
respiratory pattern to be maintained during the recording period
(17,23), in order to have interpretable
results.
HRV and sports physiology
Application of HRV on sports
training
Although there are notable physical and
physiological differences between athletes training for different
sporting activities (15), HRV is
becoming one of the most used training and recovery monitoring
tools in sport sciences (31,32). The
possibility of applying HRV on such variety is based on the fact
that cardiovascular autonomic regulation is an important
determinant of training adaptations, before also being responsive
to training effects (33). In
concordance with these observations, an ANS comparison between
sedentary subjects and recreationally active subjects or athletes
of different sports modalities have demonstrated that athletes
exhibit a different HRV profile to sedentary control subjects, with
an overall increase in HRV and parasympathetic cardiac modulation
(12), while evidence suggests that
high-intensity training can chronically lead to a shift vagal to
sympathetic cardiac modulation. In recreational marathon athletes,
the progressive sympathetic predominance at peak training load may
predict performances in the race (13). Thus, a transition from vagal to
sympathetic predominance in cardiovascular autonomic modulation,
ranging from low intensities to peak training loads on world-class
rowers has been reported (2,34,35). It
has also been shown that endurance and team athletic activities
induce an elevated parasympathetic modulation over the 24 h
recording period (higher RMSDD, PNN50 and HF, and lower LF/HF
ratio) (36). Thus, endurance elite
athletes have an elevated parasympathetic tone compared to
recreational athletes and non-athletes; confirming that athletic
conditioning is an important variable that influences the autonomic
control of the heart.
Application of HRV on the intensity of
sports training
The HR varies relative to the body's physical needs,
such as the need to absorb oxygen and excrete carbon dioxide,
physical exercise, sleep, anxiety, stress, illness, ingesting and
drugs (37–39). In athletes, the sympathovagal balance
is altered in response to different intensities and duration of
aerobic training. This was evidenced by changes in HRV variables
measured, including LF, HF and the total power of frequency domain,
in an intensity-dependent manner (13). At baseline, the LFn was slightly
greater than that of the HFn. The ratio LF/HF increased over time
and reflected the sympathetic tone (increased LFnu) and the
decrease in the parasympathetic tone (decreased HFnu) (13). The results of HRV spectra indicated
by LFn and HFn in exercise vary in function to ventilatory
thresholds (VTs) (40,41) and identified a sympathetic
predominance (indicated by an increased LF/HF ratio) during
relatively less intense exercise and parasympathetic predominance
(indicated by a decreased LF/HF ratio) during relatively more
intense exercise.
Investigation of regional elite triathletes (~15
years old) challenged with moderate-intensity test (below VT power)
or high-intensity test (above VT power) until exhaustion, Cottin
et al (40) measured the R-R
interval together with VO2, VCO2 (42) and blood lactate levels.
The authors found higher absolute LF, HF, and TP
values at moderate-intensity compared to high-intensity cycling.
Normalization showed LFn was significantly higher in cycling below
VT, indicating predominance of the sympathetic input while the
opposite was observed during heavy exercise, indicating a
predominance of the parasympathetic input (40). These data may be explained by a
change in the breathing rate combined with disappearance of the
cardiac autonomic control that occurs with heavy exercise
conditions. Furthermore the LF/HF ratio was always >1 for
cycling below VT and <1 for cycling above VT (40). This gradual switch ratio suggests the
overstepping of the VT and presents be a reliable index of VT
detection from the HRV.
Application of HRV on the effects of
gender and age on training
The beneficial effects of physical exercise on
enhancing vagal tone have been identified (43). Using SD1 normalized (SD1n) for
average R-R intervals on subjects from different age groups and
conditioning showed that SD1n was significantly higher at rest in
the young subjects (24–34 years old) compared with the middle-aged
(35–46 years) or elderly (47–64 years). These age-related
differences in cardiac vagal activity were not significant after
exercise (44). The age-matched
subjects with good, average and poor VO2 peaks showed no difference
in SD1n at rest, but were significantly different in the low- to
moderate-intensity levels. These findings suggest that poor
physical fitness was associated with impairment in cardiac vagal
function during physical exercise. Enhanced vagal activity in youth
was more evident at rest (45).
However, endurance athletes have a higher parasympathetic tone than
sedentary subjects and the effects of resistance training on
athletes showed gender differences resulting from training
(45). Indeed, significant
differences in selected domains between genders, including NFnm,
LFnm, LH, LHnu and PNN50 indicated that the autonomic control of
the heart is not merely a question of conditioning (45). Thus aging, gender and athletic
conditioning are important variables that influence the autonomic
control of the heart in athletes.
Application of HRV on the effects of
sports training
Scientific data have shown that HRV taken
immediately after exercise during recovery reflects characteristic
responses indicating the body is to exercise, which is correlated
with athletic fitness (46).
Accordingly, an analysis of HRV identified that HFn, SD1, SD2 and
SDNN were decreased on the first day after maximal exercise
compared to the pre-exercise value in 10 healthy male cross-country
skiers of ~36 years of age during a 75-km race. On the second day,
HFn returned to the baseline level, with LFn been reduced to near
or below pre-exercise levels on the second day after the race,
revealing a reduction in the parasympathetic tone after a
cross-country skiing performance and demonstrating recovery time
inversely correlated with the VO2 max measured during a bicycle
test prior to the race. The reduced vagal outflow appeared to be
blunted after prolonged exercise dependent on the cardiorespiratory
fitness of the athlete. An accentuated rebound of altered
regulation was found on the second day after prolonged exercise. By
linking HRV measurements to VO2 max, the intensity of the exercise
(VO2 max) was correlated with the HRV measurements. In addition,
HRV indices derived from the traditional time-consuming methods
have been shown to be sensitive effects in team sports players
(11,47,48),
using single measure per period of assessment. Averaging multiple
measures per week to increase the confidence of data and
sensitivity to detect changes associated with variations in
training loads (12,49,50) have
been suggested. In this sense, shorter data acquisition procedures
and simplified monitoring tools to quantify data, such as the
ultra-short-term HRV (HRV measured in 1 min post-1-min
stabilization) were recently introduced to allow the detection of
training adaptations in Futsal player (51). This suggests that ultra short-term
HRV is sensitive to training effects in team sports players, thus
of relevance in sport physiology.
In addition, the determination of HRV immediately
post-exercise in different training regiment resulted in different
changes in HRV. HF values during the first hour after exercise were
higher after constant intensity training when compared with
interval training. The early recovery of autonomic modulation from
5 min to 1 h after termination of exercise were greatly dependent
on the allocation of training of exercise load. In particular,
athletes showed a slower return of parasympathetic activity during
short-term recovery after an interval (intensive) method of
training relative to constant intensity of exercise (13). This suggests that the slower return
may be due to withdrawal of the parasympathetic activity or for a
more pronounced sympathetic involvement during the interval method
relative to the stable method. By contrast, the late recovery of 24
and 48 h after termination of exercise did not depend on the type
of exercise (interval training or constant identifying of
exercise). Although it was concluded that the total exercise load
(volume) determined long-term HRV recovery, it was found that
trained athletes have different HRV post-exercise than less trained
athletes (52). Changes could be
identified at 2 min post-exercise recovery.
The use of HRV for monitoring and
improving sports physiology
Optimal training depends on matching the specific
ability of an athlete, such as muscle, strength, endurance,
explosiveness, flexibility and adaptability to the individual's
aerobic capacity, training load and recovery. For this purpose, the
use of HRV is a suitable solution since it reflects the major
regulatory processes after physical exercise. The use of HRV to
detect which measures are altered versus physical exercise, type
and intensity have been extended to demonstrate how monitoring
physical fitness during exercise and post-exercise periods can be
applied to athletic training more broadly in the future (33,53).
Long-term HRV changes during a prolonged period, over 4 weeks of
exercise has been shown to be a particularly good indicator of
physiological adaptation in athletes able to assist in the planning
training programs. Previous findings showed that daily exercise
intensity based on the HRV of the athlete, and lowering the
intensity based on HRV decreased maintained fitness levels
comparatively to the control groups (54,55),
indicating the importance of HRV use in sports physiology.
The usefulness of HRV measurements in prescribing
exercise training in moderately active people have also been
identified (56) within the
prescription of standard training or HRV-guided training, including
2 months of moderate training (70% max HR) or vigorous training
(85% max HR). Additionally, the utility of HRV in daily endurance
exercise prescription in 26 moderately fit males during a 4-week
training period showed similarly beneficial outcomes in individuals
who were prescribed lower-intensity exercise with decreased HRV,
consistent with previous studies (57). However, these data were not
reproducible in non-trained athletes or subjects with lower levels
of physical ability (2,55). Additionally, in athletes, HRV
monitoring is frequently applied to prevent and diagnose
overtraining (OT) syndrome (58),
which is associated with numerous syndromes such as ANS dysfunction
and imbalance (58). The test to
diagnose fluctuations in ANS and the OT state (33,59) is
based on the measurement of the orthostatic HR that occurs between
sitting and standing. Athletes in an OT state may show a
significant decrease in frequency domain (TP, LF and HF) and time
domain (RMSSDD and SDNN) variables. Additional observations
(60) have yielded information
revealing hyper-responsiveness in the frequency and time domain in
OT athletes. Changes in HR do not occur in OT athletes with
short-term training (i.e., 6 days) or long-term (6 months) OT
(58,61). Other findings have shown a
predominance for LF (sympathetic) or HF (vagal) parameters
(61,62). This shift from vagal to sympathetic
predominance has been reported in female athletes assayed for HRV
in the supine rest position after a 6- to 9-week high-intensity
training (63).
In junior cross-country skiers with reduced
performance in competitions, early training breathlessness and
central fatigue (64) were reported
on the effects of OT. Power spectral analysis of the HRV at rest,
during OT, and after recovery, showed that HF and TP were higher in
the supine position compared to before and after OT, suggesting an
extensive parasympathetic modulation during OT, in contrast to the
sympathetic predominance identified during the 6 days of intensive
boot camp received by these athletes and others in the OT state
described in previous studies (7,21,63).
In the same registry, HRV change in 27 male and 30
female elite Nordic-skiers, examined between 2004 and 2008, were
undertaken to characterize different types of ‘fatigue’.
R-R intervals recorded at rest during 8 min supine
followed by 7 min standing for the analysis of LF, HF, LF+HF and
HR, revealed that supine HRV patterns were independently sorted
according to differently paired changes in the two postures. This
characterization may be useful for further understanding of the
autonomic rearrangement in different ‘fatigue’ conditions (21). HRV is used in the detection of the
non-functional overreaching (NFOR) states, which are periods when
athletic performance is substantially decreased, due to prolonged
intensive training. Change in 34 elite female wrestlers (~23 years
old) were detected (60) using
supine HRV analysis performed weekly at the same time of day, using
the time and frequency domain before 11 competitions in the course
of the study. Seven athletes were found in the NFOR state and 2
athletes in the FOR state (60).
The hypethesis behind the early detection of NFOR,
OT and fatigue is the possibility of good recovery (35), involving the get of rest before
training continues to allow repair to the body and to strengthen it
between workouts. By allowing such a recovery to pre-training based
on the constantly changing dynamic of the athlete and the amount of
further training encountered or near pre-training levels, the
recovery supports an optimizing future performance. The performance
begins to decline if the recovery is not totally achieved as shown
in the OT syndrome. In the OT syndrome, the training for an event
or the event itself pushes beyond the body's ability to
recover.
Thus, findings indicate the relevance of HRV
measurements for the early identification of overreaching states
and fatigues in professional athletes, which is of interest for
sports physiology.
Conclusion
HRV is a non-invasive method used to obtain valuable
data concerning physiological changes that occur in the response to
physical activity. Findings resulting from multiple studies
(7) suggest that HRV parameters are
relevant in the analysis of stress that the body experiences during
training and to increase insight into physiological recovery after
training. Referring to athletes, changes in the patterns of their
ANS reflected by altered HRV may serve as useful parameters for
managing their physical fatigue and establishing their exercise
intensity. Information regarding the extent to which the body
recovers after training may provide useful data for the
personalization of sports training, training loads and recovery
times, targeting goal of improvement, avoiding OT and NFOR by
keeping the athlete in a efficient frame work, which isimportant
for sports physiology. However, standard protocols and methods for
research with athletes should be established considering the
intensity, the duration of exercise as well as the position of the
body during recording and the duration of recording (14) for the use of HRV in sports
physiology. Accordingly, the ultra-short-term HRV measurement
method, demanding only 1 min of data acquisition after the
stabilization period, may arguably improve the practicality of
cardiac autonomic activity monitoring on a daily basis. This is
relevant because the optimization of recovery requires the
monitoring of HRV following workouts, which is crucial for the
prevention of the extreme accumulation of physical fatigue during
preparation or competition.
References
|
1
|
Boudoulas KD, Paraskevaidis IA, Boudoulas
H and Triposkiadis FK: The left atrium: From the research
laboratory to the clinic. Cardiology. 129:1–17. 2014.PubMed/NCBI
|
|
2
|
Acharya Rajendra U, Paul Joseph K,
Kannathal N, Lim CM and Suri JS: Heart rate variability: A review.
Med Biol Eng Comput. 44:1031–1051. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hemingway H, Shipley M, Brunner E, Britton
A, Malik M and Marmot M: Does autonomic function link social
position to coronary risk? The Whitehall II study. Circulation.
111:3071–3077. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boullosa DA, Tuimil JL, Leicht AS and
Crespo-Salgado JJ: Parasympathetic modulation and running
performance in distance runners. J Strength Cond Res. 23:626–631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khandoker AH, Jelinek HF and Palaniswami
M: Identifying diabetic patients with cardiac autonomic neuropathy
by heart rate complexity analysis. Biomed Eng Online. 8:32009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tereshchenko LG, Cygankiewicz I, McNitt S,
Vazquez R, Bayes-Genis A, Han L, Sur S, Couderc JP, Berger RD, de
Luna AB, et al: Predictive value of beat-to-beat QT variability
index across the continuum of left ventricular dysfunction:
Competing risks of noncardiac or cardiovascular death and sudden or
nonsudden cardiac death. Circ Arrhythm Electrophysiol. 5:719–727.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amano M, Kanda T, Ue H and Moritani T:
Exercise training and autonomic nervous system activity in obese
individuals. Med Sci Sports Exerc. 33:1287–1291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oliveira RS, Leicht AS, Bishop D,
Barbero-Álvarez JC and Nakamura FY: Seasonal changes in physical
performance and heart rate variability in high level futsal
players. Int J Sports Med. 34:424–430. 2013.PubMed/NCBI
|
|
9
|
Plews DJ, Laursen PB, Kilding AE and
Buchheit M: Heart rate variability in elite triathletes, is
variation in variability the key to effective training? A case
comparison. Eur J Appl Physiol. 112:3729–3741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaikkonen P, Hynynen E, Mann T, Rusko H
and Nummela A: Heart rate variability is related to training load
variables in interval running exercises. Eur J Appl Physiol.
112:829–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vesterinen V, Häkkinen K, Hynynen E,
Mikkola J, Hokka L and Nummela A: Heart rate variability in
prediction of individual adaptation to endurance training in
recreational endurance runners. Scand J Med Sci Sports. 23:171–180.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mourot L, Bouhaddi M, Tordi N, Rouillon JD
and Regnard J: Short- and long-term effects of a single bout of
exercise on heart rate variability: Comparison between constant and
interval training exercises. Eur J Appl Physiol. 92:508–517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Triposkiadis F, Karayannis G, Giamouzis G,
Skoularigis J, Louridas G and Butler J: The sympathetic nervous
system in heart failure physiology, pathophysiology, and clinical
implications. J Am Coll Cardiol. 54:1747–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aubert AE, Seps B and Beckers F: Heart
rate variability in athletes. Sports Med. 33:889–919. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bosquet L, Papelier Y, Léger L and Legros
P: Night heart rate variability during overtraining in male
endurance athletes. J Sports Med Phys Fitness. 43:506–512.
2003.PubMed/NCBI
|
|
16
|
Malpas SC and Purdie GL: Circadian
variation of heart rate variability. Cardiovasc Res. 24:210–213.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hill LK, Hu DD, Koenig J, Sollers JJ III,
Kapuku G, Wang X, Snieder H and Thayer JF: Ethnic differences in
resting heart rate variability: A systematic review and
meta-analysis. Psychosom Med. 77:16–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Makivić B, Nikić M.D, Willis MS, Education
P and Parovića: Heart rate variability (HRV) as a tool for
diagnostic and monitoring performance in sport and physical
activities. Volume 16 Number. 3:103–131. 2013.
|
|
19
|
Campbell GD, Edwards FR, Hirst GD and
O'Shea JE: Effects of vagal stimulation and applied acetylcholine
on pacemaker potentials in the guinea-pig heart. J Physiol.
415:57–68. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogliari G, Mahinrad S, Stott DJ, Jukema
JW, Mooijaart SP, Macfarlane PW, Clark EN, Kearney PM, Westendorp
RG, de Craen AJ, et al: Resting heart rate, heart rate variability
and functional decline in old age. CMAJ. 187:E442–E449. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmitt L, Regnard J, Parmentier AL, Mauny
F, Mourot L, Coulmy N and Millet GP: Typology of ‘Fatigue’ by Heart
Rate Variability Analysis in Elite Nordic-skiers. Int J Sports Med.
36:999–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stein PK, Bosner MS, Kleiger RE and Conger
BM: Heart rate variability: A measure of cardiac autonomic tone. Am
Heart J. 127:1376–1381. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Ravenswaaij-Arts CM, Kollée LA, Hopman
JC, Stoelinga GB and van Geijn HP: Heart rate variability. Ann
Intern Med. 118:436–447. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reed MJ, Robertson CE and Addison PS:
Heart rate variability measurements and the prediction of
ventricular arrhythmias. QJM. 98:87–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aubert AE, Seps B and Beckers F: Heart
rate variability in athletes. Sports Med. 33:889–919. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mainardi LT, Bianchi AM, Baselli G and
Cerutti S: Pole-tracking algorithms for the extraction of
time-variant heart rate variability spectral parameters. IEEE Trans
Biomed Eng. 42:250–259. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pagani M, Lombardi F, Guzzetti S, Rimoldi
O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S and
Piccaluga E: Power spectral analysis of heart rate and arterial
pressure variabilities as a marker of sympatho-vagal interaction in
man and conscious dog. Circ Res. 59:178–193. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malik M and Camm AJ: Components of heart
rate variability - what they really mean and what we really
measure. Am J Cardiol. 72:821–822. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tulppo MP, Mäkikallio TH, Takala TE,
Seppänen T and Huikuri HV: Quantitative beat-to-beat analysis of
heart rate dynamics during exercise. Am J Physiol. 271:H244–H252.
1996.PubMed/NCBI
|
|
30
|
Strano S, Lino S, Calcagnini G, Di
Virgilio V, Ciardo R, Cerutti S, Calcagnini G and Caselli G:
Respiratory sinus arrhythmia and cardiovascular neural regulation
in athletes. Med Sci Sports Exerc. 30:215–219. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Oliveira, Ottone V, de Castro Magalhães
F, de Paula F, Avelar NC, Aguiar PF, da Matta Sampaio PF, Duarte
TC, Costa KB, Araújo TL, Coimbra CC, et al: The effect of different
water immersion temperatures on post-exercise parasympathetic
reactivation. PLoS One. 9:e1137302014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Plews DJ, Laursen PB, Stanley J, Kilding
AE and Buchheit M: Training adaptation and heart rate variability
in elite endurance athletes: Opening the door to effective
monitoring. Sports Med. 43:773–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hottenrott K, Hoos O and Esperer HD: Heart
rate variability and physical exercise. Current status. Herz.
31:544–552. 2006.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaikkonen P, Rusko H and Martinmäki K:
Post-exercise heart rate variability of endurance athletes after
different high-intensity exercise interventions. Scand J Med Sci
Sports. 18:511–519. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaikkonen P, Nummela A and Rusko H: Heart
rate variability dynamics during early recovery after different
endurance exercises. Eur J Appl Physiol. 102:79–86. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vanderlei LC, Silva RA, Pastre CM, Azevedo
FM and Godoy MF: Comparison of the Polar S810i monitor and the ECG
for the analysis of heart rate variability in the time and
frequency domains. Braz J Med Biol Res. 41:854–859. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee S, Lee MS, Choi JY, Lee SW, Jeong SY
and Ernst E: Acupuncture and heart rate variability: A systematic
review. Auton Neurosci. 155:5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Posadzki P, Kuzdzal A, Lee MS and Ernst E:
Yoga for Heart Rate Variability: A Systematic Review and
Meta-analysis of Randomized Clinical Trials. Appl Psychophysiol
Biofeedback. 40:239–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kemp AH, Quintana DS, Gray MA, Felmingham
KL, Brown K and Gatt JM: Impact of depression and antidepressant
treatment on heart rate variability: A review and meta-analysis.
Biol Psychiatry. 67:1067–1074. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cottin F, Médigue C, Leprêtre PM, Papelier
Y, Koralsztein JP and Billat V: Heart rate variability during
exercise performed below and above ventilatory threshold. Med Sci
Sports Exerc. 36:594–600. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pichon AP, de Bisschop C, Roulaud M,
Denjean A and Papelier Y: Spectral analysis of heart rate
variability during exercise in trained subjects. Med Sci Sports
Exerc. 36:1702–1708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chwyczko T, Sterliński M, Maciag A, Firek
B, Labecka A, Jankowska A, Kośmicki M, Kowalik I, Malczewska B and
Szwed H: Impact of cardiac resynchronisation therapy on adaptation
of circulatory and respiratory systems to exercise assessed by
cardiopulmonary exercise test in patients with chronic heart
failure. Kardiol Pol. 66:406–412; discussion 413–414.
2008.PubMed/NCBI
|
|
43
|
He X, Zhao M, Bi X, Sun L, Yu X, Zhao M
and Zang W: Novel strategies and underlying protective mechanisms
of modulation of vagal activity in cardiovascular diseases. Br J
Pharmacol. 172:5489–5500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tulppo MP, Mäkikallio TH, Seppänen T,
Laukkanen RT and Huikuri HV: Vagal modulation of heart rate during
exercise: Effects of age and physical fitness. Am J Physiol.
274:H424–H429. 1998.PubMed/NCBI
|
|
45
|
Berkoff DJ, Cairns CB, Sanchez LD and
Moorman CT III: Heart rate variability in elite American
track-and-field athletes. J Strength Cond Res. 21:227–231. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hautala A, Tulppo MP, Mäkikallio TH,
Laukkanen R, Nissilä S and Huikuri HV: Changes in cardiac autonomic
regulation after prolonged maximal exercise. Clin Physiol.
21:238–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Freitas VH, Pereira LA, de Souza EA,
Leicht AS, Bertollo M and Nakamura FY: Sensitivity of the Yo-Yo
Intermittent Recovery Test and cardiac autonomic responses to
training in futsal players. Int J Sports Physiol Perform.
10:553–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Soares-Caldeira LF, de Souza EA, de
Freitas VH, de Moraes SM, Leicht AS and Nakamura FY: Effects of
additional repeated sprint training during preseason on
performance, heart rate variability, and stress symptoms in futsal
players: A randomized controlled trial. J Strength Cond Res.
28:2815–2826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Flatt AA and Esco MR: Smartphone-Derived
Heart-Rate Variability and Training Load in a Women's Soccer Team.
Int J Sports Physiol Perform. 10:994–1000. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plews DJ, Laursen PB, Le Meur Y,
Hausswirth C, Kilding AE and Buchheit M: Monitoring training with
heart rate-variability: How much compliance is needed for valid
assessment? Int J Sports Physiol Perform. 9:783–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakamura FY, Flatt AA, Pereira LA,
Ramirez-Campillo R, Loturco I and Esco MR: Ultra-Short-Term Heart
Rate Variability is Sensitive to Training Effects in Team Sports
Players. J Sports Sci Med. 14:602–605. 2015.PubMed/NCBI
|
|
52
|
Seiler S, Haugen O and Kuffel E: Autonomic
recovery after exercise in trained athletes: Intensity and duration
effects. Med Sci Sports Exerc. 39:1366–1373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Buchheit M, Millet GP, Parisy A, Pourchez
S, Laursen PB and Ahmaidi S: Supramaximal training and postexercise
parasympathetic reactivation in adolescents. Med Sci Sports Exerc.
40:362–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pichot V, Busso T, Roche F, Garet M,
Costes F, Duverney D, Lacour JR and Barthélémy JC: Autonomic
adaptations to intensive and overload training periods: A
laboratory study. Med Sci Sports Exerc. 34:1660–1666. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pichot V, Roche F, Gaspoz JM, Enjolras F,
Antoniadis A, Minini P, Costes F, Busso T, Lacour JR and Barthélémy
JC: Relation between heart rate variability and training load in
middle-distance runners. Med Sci Sports Exerc. 32:1729–1736. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kiviniemi AM, Hautala AJ, Kinnunen H,
Nissilä J, Virtanen P, Karjalainen J and Tulppo MP: Daily exercise
prescription on the basis of HR variability among men and women.
Med Sci Sports Exerc. 42:1355–1363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kiviniemi AM, Hautala AJ, Kinnunen H and
Tulppo MP: Endurance training guided individually by daily heart
rate variability measurements. Eur J Appl Physiol. 101:743–751.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mourot L, Bouhaddi M, Perrey S, Cappelle
S, Henriet MT, Wolf JP, Rouillon JD and Regnard J: Decrease in
heart rate variability with overtraining: Assessment by the
Poincaré plot analysis. Clin Physiol Funct Imaging. 24:10–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hynynen E, Uusitalo A, Konttinen N and
Rusko H: Heart rate variability during night sleep and after
awakening in overtrained athletes. Med Sci Sports Exerc.
38:313–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tian Y, He ZH, Zhao JX, Tao DL, Xu KY,
Earnest CP and McNaughton LR: Heart rate variability threshold
values for early-warning nonfunctional overreaching in elite female
wrestlers. J Strength Cond Res. 27:1511–1519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hedelin R, Kenttä G, Wiklund U, Bjerle P
and Henriksson-Larsén K: Short-term overtraining: Effects on
performance, circulatory responses, and heart rate variability. Med
Sci Sports Exerc. 32:1480–1484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hedelin R, Wiklund U, Bjerle P and
Henriksson-Larsén K: Cardiac autonomic imbalance in an overtrained
athlete. Med Sci Sports Exerc. 32:1531–1533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Uusitalo AL, Uusitalo AJ and Rusko HK:
Heart rate and blood pressure variability during heavy training and
overtraining in the female athlete. Int J Sports Med. 21:45–53.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Quintana DS, Heathers JA and Kemp AH: On
the validity of using the Polar RS800 heart rate monitor for heart
rate variability research. Eur J Appl Physiol. 112:4179–4180. 2012.
View Article : Google Scholar : PubMed/NCBI
|